Abstract
Long-term cultured pre-B cells are able to differentiate into immunoglobulin (Ig)M-positive B cells (IgM+ cells) when transplanted into severe combined immunodeficient (SCID) mice. Based on previous studies, here we report the development of a reconstitution assay in nonobese diabetic/SCID (NOD/SCID) mice using pre-B cells, which allows us to study the role of calpains (calcium-activated endopeptidases) during B cell development as well as in B cell clonal deletion. Using this model, we show that calpastatin (the natural inhibitor of calpains) inhibits B cell receptor–induced apoptosis in IgM+ cells derived from transplanted mice. We thus hypothesize an important function for calpain in sculpting the B cell repertoire.
Keywords: pre-B cells, calpastatin, calpain, BCR, transplant
Introduction
Several steps are essential for hematopoietic stem cell differentiation to mature B cells 1. Progenitor B cells (pro-B cells, identified by acquisition of B-220, CD43, and c-kit) can rearrange DH to JH segments in the Ig heavy chain locus and are then termed pre-BI cells. Maturation of pre-BI cells occurs when VH and DJH segments join; the μH chain appears in cytoplasm and assembles with the products of the λ5 and V pre-B genes. This mechanism leads to acquisition of the pre-B cell receptor (pre-BCR); at this stage, B cells are called pre-BII cells and become c-kit- and CD43 negative. Later in B cell development, rearrangement begins again, now in the Ig light chain locus; B cells thus express a complete surface IgM molecule and are then denominated immature B cells 1. As the mechanisms that mediate IgH and IgL gene recombination are error prone, a large proportion of B cell precursors fails to express functional IgH and IgL molecules. Those with nonproductive rearrangements die in situ by apoptosis at the pre-BI to pre-BII transition, as they do not receive a survival signal 2.
Another consequence of random V(D)J recombination is the generation of B cells that recognize endogenous self-antigens 3. To avoid autoimmune manifestations, an important mechanism allows the elimination of self-reactive B cells via apoptosis (clonal deletion 3). The apoptotic process is executed by a group of proteases including caspases (cysteinyl aspartate-specific proteinases) and calpains (calcium-activated proteases 4). Implication of both caspases and calpains during B cell clonal deletion have been demonstrated in immature B cells 5 6 7; nevertheless, the biochemical events leading to apoptosis by the BCR are not entirely clear.
B cell development is controlled by IL-7, as indicated by analysis of IL-7 knockout mice 8. Pre-B cells can be cultured in the presence of IL-7 9; when transplanted into SCID mice, these cells differentiate into IgM-positive B cells (IgM+ cells 10). Based on these studies, we developed a reconstitution assay by transplanting long-term cultured pre-B cells in nonobese diabetic/SCID (NOD/SCID) mice, which lack innate and adaptive immunity 11. To track the transplanted pre-B cells in vivo, we transduced them by infection with a Moloney murine leukemia virus retroviral vector (pLZR-IRES/GFP). This vector contains the green fluorescence protein (GFP) under the control of an internal ribosome entry site (IRES), which allows in vivo study of the evolution of these cells. This model thus served to analyze the role of calpains during the B cell differentiation process as well as during B cell clonal deletion. Here we demonstrate that calpastatin (the natural inhibitor of calpains) did not interfere with B cell differentiation in the reconstitution model; however, IgM+ cells expressing calpastatin showed intrinsic resistance to BCR-induced apoptosis. We therefore conclude that calpain has an important function in B cell clonal deletion and in establishing the B cell repertoire.
Materials and Methods
Cell Culture and Animal Facility.
WEHI-231 immature B cells were cultured in RPMI-1640 (BioWhittaker) supplemented with 10% FCS, 2 mM l-glutamine, 10 U/ml penicillin, 10 μg/ml streptomycin, 10 mM HEPES, and 50 μM 2-mercaptoethanol (Sigma-Aldrich) and maintained at 37°C in a humidified atmosphere with 5% CO2. Pre-BI cells were derived from adult BALB/c mouse bone marrow (BM), and cultured in IMDM supplemented with 10 U/ml penicillin, 10 μg/ml streptomycin, 1 mM sodium pyruvate, nonessential amino acids, 50 μM 2-mercaptoethanol, 2 mM l-glutamine, 10% FCS, and 3% supernatant from a murine IL-7–producing cell line. IgM+/mock, IgM+/GFP, and IgM+/calpastatin cells were derived from NOD/SCID mice 2 mo after transplantation and single cell suspensions were prepared from BM. BM cells were flushed from femurs and tibiae and cultured in IMDM supplemented with 10 U/ml penicillin, 2 mM/ml l-glutamine, 10% FCS, and 3% supernatant from a murine IL-7–producing cell line. After 2 d in culture, suspension-growing cells were separated from BM adherent cells. Suspension cells were transferred to new plates in the same medium. Under these conditions, differentiated IgM+ cells proliferate for several weeks in the presence of IL-7 (data not shown). Male and female NOD-Lt/Sz-scid/scid (NOD/SCID) mice, bred in our animal facility, were used at 4 to 6 wk of age. All experiments were performed in compliance with norms of our animal facility committee.
Antibodies and Reagents.
Goat anti–mouse IgM, μ chain specific (10 μg/ml; Jackson ImmunoResearch Laboratories) was used to induce apoptosis. To confirm calpastatin expression, we used goat anti-calpastatin (R-19; Santa Cruz Biotechnology, Inc.). For staining analysis, the following biotin-conjugated antibodies were used: anti-B220 (clone RA3-6B2), anti-CD43 (S7), anti-CD19 (1D3), anti-CD3 ε chain (145-2C11), anti-Ter-119 (TER-119) and anti-Mac-1 (M1/70), anti-CD21 (7G6), anti-CD23 (B3B4), and anti-CD25 (7D4) (all from BD PharMingen); affinity-purified goat anti-IgM (μ chain-specific), and anti-IgD (δ chain-specific; SBA-1) (both from Southern Biotechnology Associates, Inc.). Streptavidin-SpectralRed (SPRD) was from Southern Biotechnology Associates, Inc. Actinomycin D (Act-D) was purchased from Sigma-Aldrich.
Flow Cytometry Analysis for Marker Expression.
Cells were washed in PBS containing 2% FCS and 0.1% NaN3 (staining PBS), incubated with biotin-conjugated antibodies (20 min, on ice), then washed with staining PBS, incubated with streptavidin-SPRD, and analyzed on an EPICS XL flow cytometer (Beckman Coulter).
Assessment of Apoptotic Cell Death.
Apoptosis was evaluated by staining cellular DNA with the DNA intercalator propidium iodide (PI) using a semiautomatic procedure (DNA-Prep Reagents; Beckman Coulter), followed by analysis on an EPICS XL flow cytometer. In brief, cells (105–106) were recovered by centrifugation, resuspended in 100 μl of PBS, then permeabilized and stained by addition of 100 μl of detergent reagent followed by 1 ml of PI solution. After mixing, samples were incubated (37°C, 30 min) and analyzed in flow cytometry. Apoptosis was determined as the percentage of DNA located in the hypoploid subG0/G1 peak of the cell cycle.
Western Blot Analysis.
Cells (106) were collected, washed with ice-cold PBS, and resuspended in RIPA lysis buffer (20 mM Tris-HCl, pH 8, 137 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 10% glycerol, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, and protease inhibitors). Lysate protein content was quantified using the DC protein assay (Bio-Rad Laboratories). After SDS-PAGE under reducing conditions, proteins were transferred to nitrocellulose membranes (Bio-Rad Laboratories), which were blocked overnight with 5% nonfat dry milk in TBS buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl). Subsequent antibody incubations and membrane washes were performed in TBS-T buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.2% Tween 20) containing 1% nonfat milk. After 2 h, antibody incubation and washing, PO-conjugated anti–goat was added for 1 h. Blots were washed extensively and developed using the enhanced chemoluminescence (ECL) system (Amersham Pharmacia Biotech).
Cloning of Calpastatin and Retroviral Transduction.
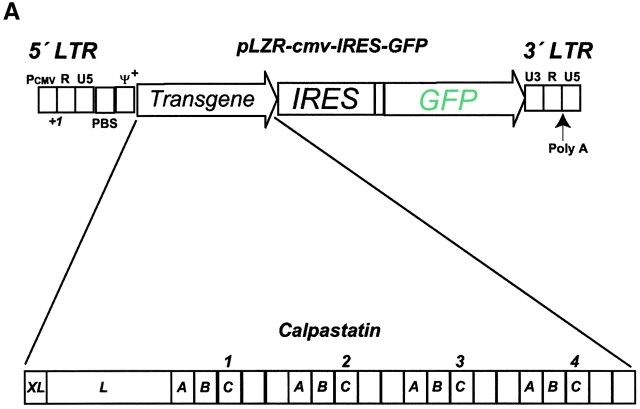
We used a Moloney murine leukemia virus–based retroviral vector (pLZR-IRES/GFP), which was obtained from the pLZR-CMV-gfp plasmid 12 by replacing the enhanced GFP (EGFP) sequence with the IRES/gfp cassette from plasmid pIRES2/EGFP (CLONTECH Laboratories, Inc.). Murine calpastatin cDNA 13 was cloned into the EcoRI site of the pLZR-IRES/GFP vector to generate a pLZR-calpastatin/IRES/GFP construct. GFP+ cells were monitored and sorted in a Beckman Coulter EPICS Altra Hypersort. Retrovirus was produced by transient transfection of 293T cells 12 14. For viral transduction, 105 cells (WEHI-231 or pre-BI cells) were incubated 4 h with 5 μg/ml of protamine sulphate (Sigma-Aldrich) in 1 ml of retroviral supernatant or in virus-free medium. Infection was performed at 37°C and repeated 24 h later under the same conditions.
Calcium Determination.
Changes in intracellular Ca2+ concentration were monitored using the fluorescent probe Indol-AM (Molecular Probes). Cells (107/ml) were washed three times in HBB buffer (1× Hank's balanced salt solution, 0.1% BSA, and 10 mM Hepes, pH 7.5), then incubated with 3 μM Indol-AM (30 min, 37°C). After incubation, cells were washed and resuspended at 0.8 × 106 cells/ml in HBB buffer, then maintained at 4°C until anti-IgM addition. Calcium mobilization in response to 10 μg/ml of anti-IgM was determined at 37°C by fluorimetry.
Results
Calpastatin Prevents BCR-induced Apoptosis in the WEHI-231 Immature B Cell Line.
The immature B cell WEHI-231 has been used as a model for B cell tolerance based on its phenotype (sIgMhighsIgDlow), which parallels that of immature B cells. Although many early signal transduction events through the BCR have now been elucidated, the biochemical events leading to apoptosis are not entirely clear. Molecular dissection of the mechanisms and biochemical pathways involved in these changes showed that calpain activation has an important role in the apoptotic machinery 6 7. The best-characterized calpains are the ubiquitously expressed calpain-1 and calpain-2 (also called catalytic subunits), which are activated by calcium 15. Calpain activity can be regulated by a p30 regulatory subunit with chaperone-like effects on the refolding of the catalytic subunits 16 17, and by calpastatin, which inhibits calpain activation by interacting with both the active site and the calmodulin-like Ca2+-binding domain of calpains 18.
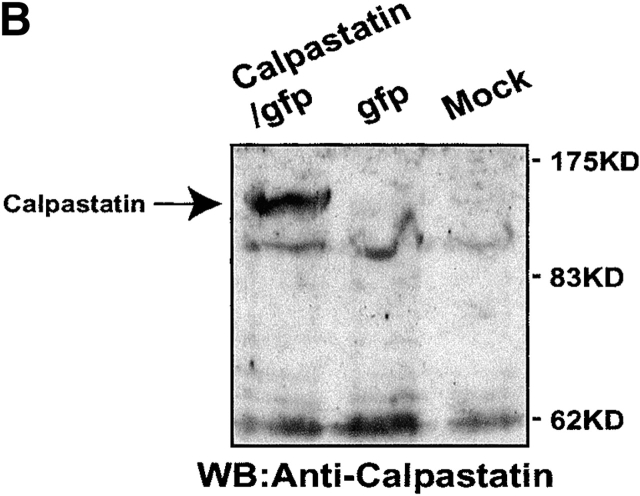
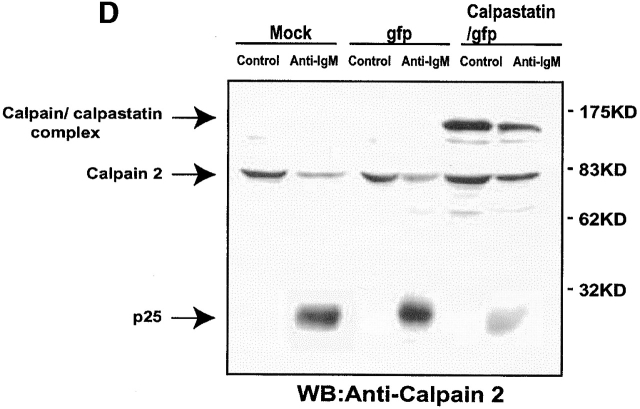
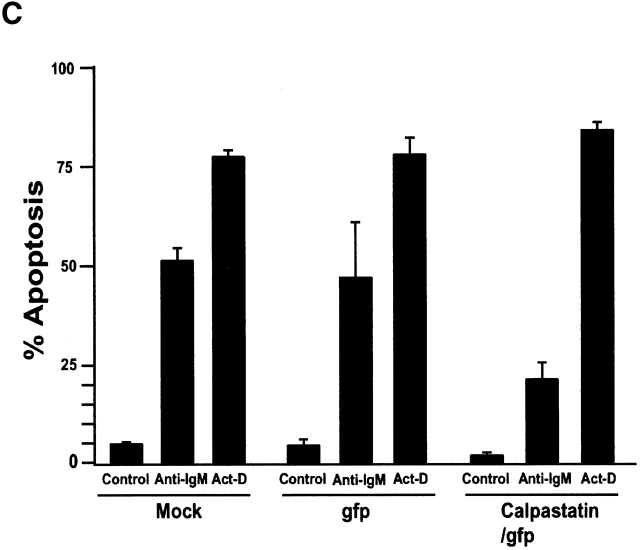
To study the role of calpains during BCR-induced apoptosis, we expressed calpastatin in WEHI-231 cells. We transduced them using the pLZR-IRES/GFP retroviral vector (see Materials and Methods), with GFP under the control of an IRES (Fig. 1 A); this vector is based on others described previously. We cloned murine calpastatin cDNA in the pLZR-IRES/GFP vector to generate a construct (pLZR-calpastatin/IRES/GFP; Fig. 1 A). WEHI-231 cells were then transduced by retroviral infection using both pLZR-IRES/GFP and pLZR-calpastatin/IRES/GFP to generate WEHI-231/GFP and WEHI-231/calpastatin cells (see Materials and Methods); cells were monitored and sorted (see Materials and Methods). After sorting, Western blot analysis for calpastatin expression was performed using total extracts from WEHI-231/GFP and WEHI-231/calpastatin cells, as well as from nontransduced WEHI-231 cells (WEHI-231/mock cells; Fig. 1 B). Apoptosis analysis of these cells showed that calpastatin inhibited BCR-induced apoptosis (Fig. 1 C), confirming the implication of calpains in anti-IgM–induced apoptosis. To clarify whether calpastatin has a general survival effect on WEHI-231 cells, we treated them with Act-D, which induces apoptosis via cytochrome c release from mitochondria. This apoptotic pathway is not found during anti-IgM–induced apoptosis in WEHI-231 cells 6. Act-D–induced apoptosis is not blocked by calpastatin overexpression in these cells (Fig. 1 C), indicating that calpastatin is not a general apoptotic inhibitor, but that its inhibitory effect is cell death stimulus specific. As the presence of autolytic calpain-2 fragments indicate calpain-2 activation 19 20, we performed Western blot analysis for calpain-2 (Fig. 1 D). We detect calpain-2 processing after anti-IgM–induced apoptosis in WEHI-231 cells (Fig. 1 D); nonetheless, calpastatin overexpression inhibits calpain-2 processing incompletely, or possibly the cell compensates for the inhibition by producing additional free calpain-2 (Fig. 1 D). This result may explain why calpastatin does not block apoptosis completely in WEHI-231 cells.
Figure 1.
Calpastatin expression inhibits anti-IgM–induced apoptosis in WEHI-231 cells. (A) Murine calpastatin cDNA (reference 22) was cloned into the EcoRI site of the pLZR-IRES/GFP vector to generate the pLZR-calpastatin/IRES/GFP construct. (B) WEHI-231 cells were transduced by retroviral infection using pLZR-/IRES/GFP and pLZR-calpastatin/IRES/GFP. GFP+ cells were monitored in a Beckman Coulter EPICS Altra Hypersort and analyzed for calpastatin expression. Western blot (WB) analysis was performed using goat anti-calpastatin (R-19) to confirm transgene expression. (C) WEHI-231/mock, WEHI-231/GFP, and WEHI-231/calpastatin cells were cultured (0.25 × 106 cells/ml) in medium alone, with goat anti-IgM antibody (10 μg/ml; 48 h) or with Act-D (1 μg/ml; 12 h) for the apoptosis assay. Apoptosis was evaluated by staining cellular DNA. Values represent the mean of five independent experiments. (D) Western blot analysis for calpain-2 in WEHI-231/mock, WEHI-231/GFP, and WEHI-231/calpastatin cells.
Calpastatin Expression Does Not inhibit IL-7 Deprivation-induced Apoptosis in Long-Term Cultured Pre-BI Cells.
IL-7 controls the process of hematopoietic stem cell differentiation to mature B cells 1 8. BM-derived pre-B cells proliferate in vitro in the presence of IL-7, whereas its absence triggers apoptosis 9. To analyze the role of calpastatin as an apoptosis inhibitor during IL-7 deprivation-induced apoptosis, we derived pre-BI cells from adult BALB/c mouse BM and cultured them in IL-7. Long-term cultured pre-BI cells were then transduced by retroviral infection using the pLZR-IRES/GFP and pLZR-calpastatin/IRES/GFP constructs to generate pre-BI/GFP and pre-BI/calpastatin cells. Cells were monitored and sorted (see Materials and Methods), after which pre-BI/GFP and pre-BI/calpastatin cells were cultured in vitro in the presence of IL-7; untransduced pre-BI cells (pre-BI/mock) were used as a control.
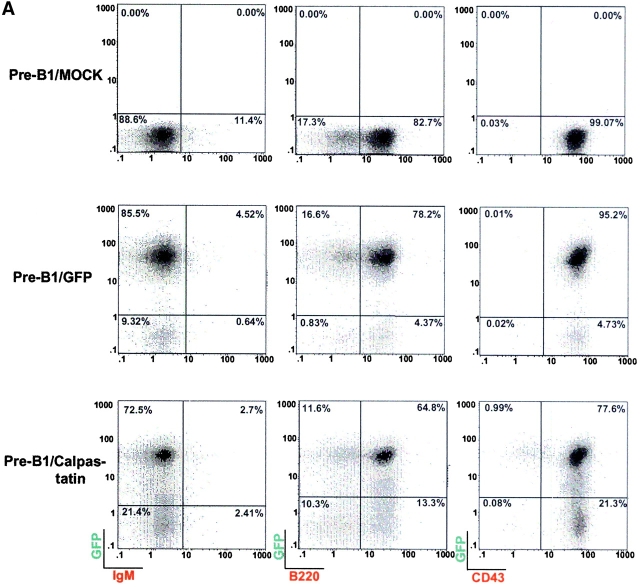
Staining analysis for the surface proteins B220, CD43, and IgM showed that GFP expression in long-term cultured pre-BI cells does not interfere with the pre-BI phenotype (Fig. 2 A). We observed that long-term cultured GFP-positive cells gradually lost GFP protein expression after 2 wk in culture; this effect has been observed in other cell types in which long-term in vivo expression from the viral promoter is not completely satisfactory 21 22. In addition, pre-BI/calpastatin cells lose more GFP expression than do pre-BI/GFP cells. Several studies have been conducted to determine the parameters that influence recognition of the start codon in IRES-dependent translation initiation in bicistronic constructs. These studies show that the secondary structure of the region that separates the 3′ end of the IRES element from the initiator codon play an essential role in start codon recognition 23. The insertion of sequences containing an additional start codon upstream of the IRES element greatly reduce the translation efficiency of the second gene, both in vivo and in vitro 24. Moreover, it has been shown that expression of genes upstream of IRES elements interferes with GFP expression 25 26. Here we made a similar observation in pre-BI cells expressing calpastatin plus GFP; GFP expression is altered in these cells by upstream calpastatin expression.
Figure 2.
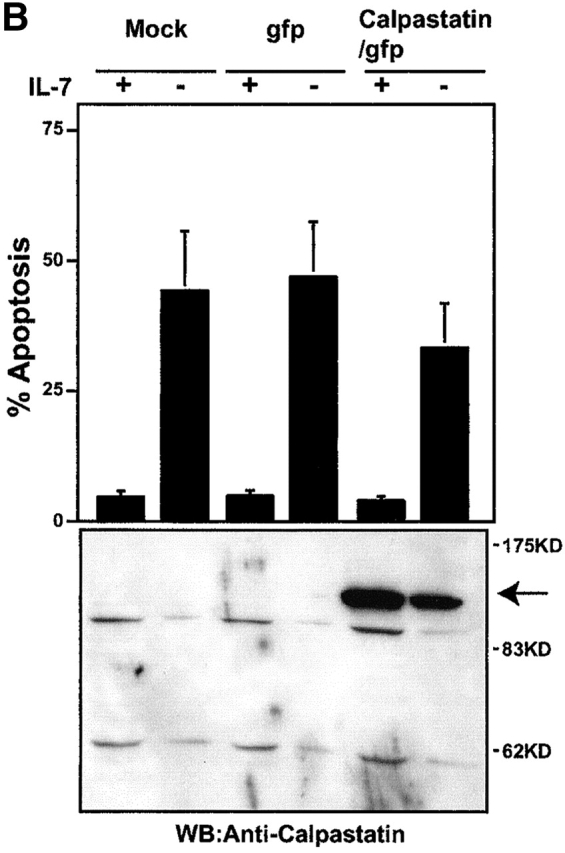
Calpastatin expression does not inhibit IL-7 deprivation-induced apoptosis. (A) Long-term cultured pre-BI cells were transduced using pLZR-/IRES/GFP and pLZR-calpastatin/IRES/GFP. GFP+ cells were monitored and sorted. Pre-BI/mock, pre-BI/GFP, and pre-BI/calpastatin cells (105 each) were collected and washed in ice-cold PBS. Cells were incubated in 30 μl of staining PBS with biotin-conjugated anti-B220, anti-IgM, or anti-CD43 antibody, washed, incubated with streptavidin-SPRD, and analyzed on a flow cytometer. (B) Pre-BI/mock, pre-BI/GFP, and pre-BI/calpastatin cells were cultured (0.25 × 106 cells/ml) in medium with or without IL-7 for the apoptosis assay. Apoptosis was evaluated by staining cellular DNA. Values represent the mean of three independent experiments. Western blot (WB) analysis was performed using goat anti-calpastatin to confirm expression.
Apoptosis analysis after IL-7 deprivation showed that calpastatin expression did not block IL-7 deprivation-induced apoptosis (Fig. 2 B); Western blot analysis for calpastatin was performed as an expression control (Fig. 2 B). These results, together with those from the WEHI-231 cells, demonstrate the contribution of calpains during BCR-induced apoptosis, but not during IL-7 deprivation-induced apoptosis in pre-BI cells.
Characterization of the Reconstitution Assay in NOD/SCID Mice Using Long-Term Cultured Pre-BI Cells.
In vitro–cultured pre-BI cells differentiate into IgM+ cells when transplanted into SCID mice 9 10. This process is regulated by expression of the recombinant protein recombination activating gene (RAG)-2 27. Using long-term cultured pre-BI/GFP cells, which allow in vivo cell tracking, we developed a reconstitution assay in NOD/SCID mice to confirm the ability of pre-BI cells to differentiate into IgM+ cells. Pre-BI/GFP cells (107 cells) were transplanted intravenously into 300 Gy-irradiated NOD/SCID mice. As described previously 10, the dominance of B cell lymphopoiesis in BM is detected between 2 and 4 mo after transplantation (data not shown). Transplanted pre-BI/GFP cells differentiated into IgM+ B cells (IgM+/GFP cells), which were identified by expression of B220+, CD19+, IgM+, and the lack of IgD expression (data not shown). Staining controls for CD3 (for T cells), Mac-1 (macrophages), and Ter-119 (erythrocytes) were negative. In conclusion, these data confirm the ability of long-term cultured pre-BI cells to differentiate into IgM+ cells in BM, as well as the possibility to track these cells in vivo.
Pre-BI Cells Expressing Calpastatin Differentiate into IgM+ B Cells Showing Impaired Clonal Deletion In Vitro.
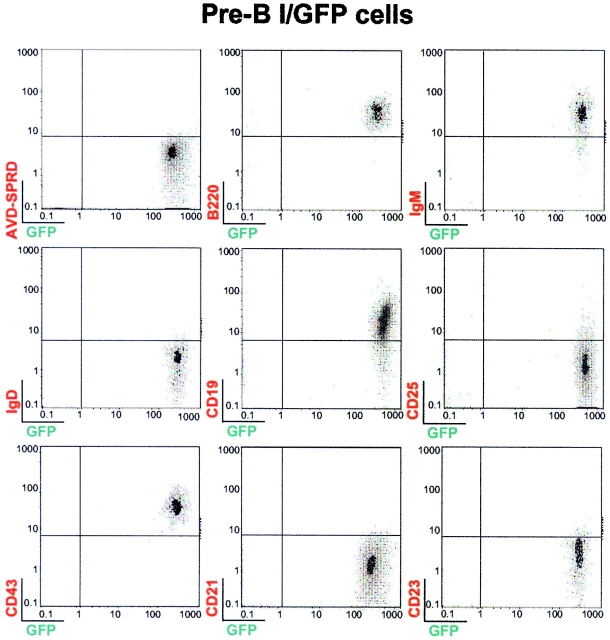
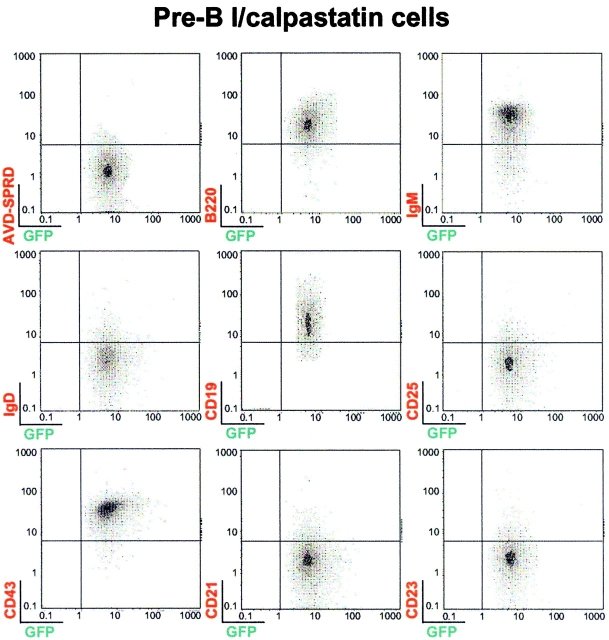
Expression of murine calpastatin prevents BCR-induced apoptosis in WEHI-231 cells, confirming calpain implication during anti-IgM–induced apoptosis in this model of B cell tolerance. We used the reconstitution assay described above to explore the role of calpain in BCR-triggered apoptotic machinery. This assay allowed us to study the impact of calpastatin expression as an inhibitor of BCR-induced apoptosis in IgM+ cells. Pre-BI/mock, pre-BI/GFP, and pre-BI/calpastatin cells were thus transplanted into NOD/SCID mice to derive IgM+/mock, IgM+/GFP, and IgM+/calpastatin cells (see Materials and Methods). To explore whether calpastatin expression has an influence on B cell differentiation, we sorted GFP+ cells from transplanted NOD/SCID mice. FACS® analysis of cells from BM of transplanted pre-BI/GFP and pre-BI/calpastatin NOD/SCID mice indicates that calpastatin does not alter B cell differentiation (Fig. 3). In addition, the results indicate that B cells obtained from transplanted NOD/SCID mice are B220+, IgM+, IgD−, CD19+, CD25−, CD43+, CD21−, and CD23−. Similar results were found when human CD34+ stem cells were transplanted into NOD/SCID mice, generating B cells characterized by expression of the surface markers IgM+, IgD−, CD19+, CD43−, CD21−, CD23−, and Mac-1− 28. Novelli's group identified these as B-1 cells using the reciprocal CD23 and CD43 expression pattern, as B-1 cells are CD23−CD43+, whereas B-2 cells are CD23+CD43− 29 30.
Figure 3.
Pre-BI/GFP and pre-BI/calpastatin cells differentiate into IgM+ B cells in NOD/SCID mice. Male and female NOD/SCID mice bred in our animal facility were used at 3–4 wk of age for transplantation experiments. Representative results (n = 12) are shown from BM of pre-BI/GFP and pre-BI/calpastatin-transplanted NOD/SCID mice. GFP-positive cells (106) from BM sorted by FACS® analysis were stained with biotin-conjugated anti-B220, anti-IgM, anti-IgD, anti-CD19, anti-CD25, anti-CD43, anti-CD21, or anti-CD23 antibodies. Anti-CD3, anti–Ter-119, and anti–Mac-1 (not shown), as well as streptavidin-SPRD alone were used as controls.
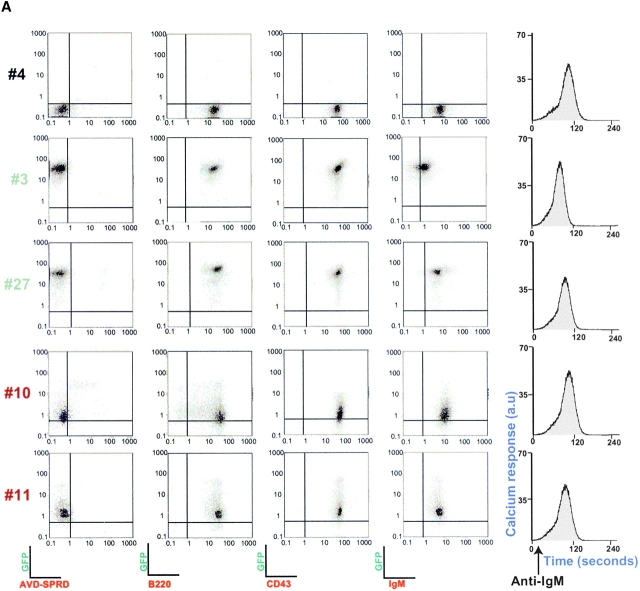
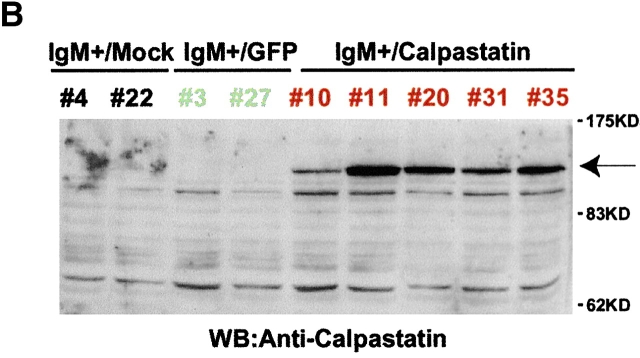
IgM+ cells from reconstituted NOD/SCID mice expressed the BCR and calpastatin, even 2 to 3 wk after isolation from marrow (Fig. 4a and Fig. b). As sustained Ca2+ influx is required for calpain activation 18, we tested whether BCR cross-linking triggered Ca2+ influx in derived IgM+/mock, IgM+/GFP, and IgM+/calpastatin cells (Fig. 4 A). In addition, we analyzed several surface markers in IgM+ cells derived from transplanted NOD/SCID mice, and found no significant differences among IgM+/mock, IgM+/GFP, and IgM+/calpastatin cell lines (Fig. 4 A).
Figure 4.
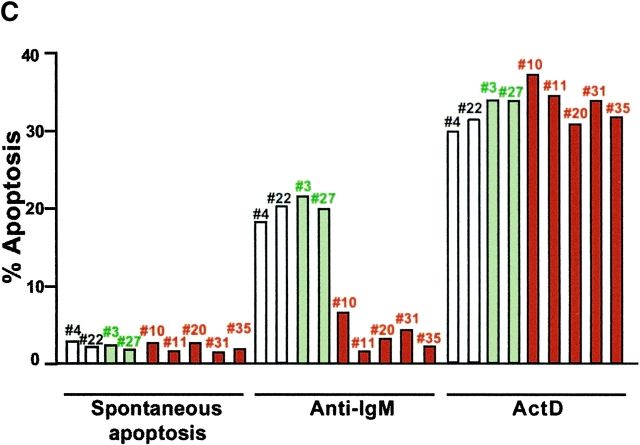
Calpastatin expression inhibits apoptosis in IgM+ B cells. (A) Distinct derived IgM+/mock (mouse #4), IgM+/GFP (#3 and #27), and IgM+/calpastatin (#10 and #11) cells were stained with a biotin-conjugated anti-IgM, anti-B220, or anti-CD43 antibodies, then washed with staining PBS, incubated with streptavidin-SPRD, and analyzed by flow cytometry. Streptavidin-SPRD alone was used as control. In addition, Ca2+ influx was measured as described (see Materials and Methods). (B) Western blot (WB) analysis was performed using goat anti-calpastatin (R-19) to confirm transgene expression in IgM+/mock (#4 and #22), IgM+/GFP (#3 and #27), and IgM+/calpastatin cells (#10, #11, #20, #31, and #35). (C) IgM+/mock, IgM+/GFP, and IgM+/calpastatin cells were cultured (0.25 × 106 cells/ml) in medium alone, with goat anti-IgM antibody (10 μg/ml), or in the presence of 1 μg/ml of Act-D for the apoptosis assay. Apoptosis was evaluated by staining cellular DNA. Values are representative of three independent experiments.
To validate the role of calpastatin as an inhibitor of BCR-induced apoptosis, we analyzed apoptosis at 24 and 48 h after anti-IgM cross-linking. IgM+/calpastatin cells presented intrinsic resistance to BCR-induced apoptosis (Fig. 4 C), confirming calpain implication in this type of apoptosis, indicating an important function for calpains in generating the B cell repertoire.
Discussion
Apoptosis is the most common physiological form of cell death; it occurs during embryonic development, tissue remodeling, immune regulation, cell activation, and tumor regression 31. In the hematopoietic system, B cell generation is regulated by a negative selection mechanism, by which B cells undergo a BCR-activated apoptotic process. The apoptotic machinery is activated when the BCR recognizes endogenous self antigens or antigens in the absence of costimulating signals 3. The immature B cell WEHI-231 has been widely used as a model of B cell tolerance, based on its capacity to undergo apoptosis after BCR cross-linking, which induces calpain activation and subsequent apoptosis 6 7. Calpain activation triggers destruction of distinct proteins involved in cell cycle, cytoskeletal modeling, cell spreading, as well as multiple transcription factors, thus causing apoptosis 18; nonetheless, the contribution of calpains to BCR-induced apoptosis is unknown.
Calpain activation takes place after calcium mobilization, which is triggered by BCR cross-linking 18 32. Phospholipase C (PLC-γ) activation by BCR cross-linking leads to phospholipid hydrolysis, yielding inositol 1,4,5-tris phosphate (IP3) and diacylglycerol (DAG). IP3 then binds IP3 receptors located in the endoplasmic reticulum, causing Ca2+ release from internal stores 32. Modulation of BCR activation can be mediated by inhibitory receptors such as FcγRII and paired immunoglobulin-like receptor B (PIR-B; reference 33), whose activation recruits the phosphatase Src homology 2 domain–containing 5′ inositol phosphatase (SHIP), which mediates some inhibitory signaling such as PLC-γ inhibition and subsequent BCR cross-linking–induced Ca2+ mobilization 33. As a consequence of this inhibitory mechanism, FcγRII and PIR-B stimulation prevent BCR-induced apoptosis.
Calpains are implicated in other apoptotic models such as TGF-β–induced apoptosis in B lymphocytes 34, TCR-induced apoptosis in thymocytes 35, or neuronal cell death 36. In neurons, calpain activation induces cleavage of p35 (a neuron-specific activator of cyclin-dependent kinase 5, cdk5) giving rise to p25, which accumulates in the brain of patients with Alzheimer's disease. Conversion of p35 to p25 causes prolonged activation of cdk5, which hyperphosphorylates tau, disrupts the cytoskeleton, and promotes apoptosis 36. In B cells, however, the calpain-driven apoptosis mechanism involves processing and subsequent activation of caspase-7, indicating the distinct induction mechanisms induced by calpain activation 6 7.
Here we analyzed the role of the calpains in B cell clonal deletion by expressing their natural inhibitor, calpastatin, to study the contribution of these proteases to this process. We demonstrate that calpastatin expression inhibited BCR-induced apoptosis in WEHI-231 cells, as well as in IgM+ B cells from reconstituted NOD/SCID mice. The inhibition ability of calpastatin is specific for BCR-induced apoptosis, as calpastatin expression did not block IL-7 deprivation-induced apoptosis in pre-BI cells or Act-D–induced apoptosis in WEHI-231 cells. These data indicate the selective ability of calpastatin as an apoptosis inhibitor, and its capacity to modulate the B cell repertoire by regulating clonal deletion.
Acknowledgments
We would like to thank J. Gutiérrez for technical advice, as well as the technical staff of the department who aid with cell culture and materials preparation, and C. Mark for editorial assistance.
The Department of Immunology and Oncology was founded and is supported by the Spanish National Research Council (CSIC) and by the Pharmacia Corporation.
Footnotes
Abbreviations used in this paper: Act-D, actinomycin D; BCR, B cell receptor; BM, bone marrow; GFP, green fluorescence protein; IRES, internal ribosome entry site; NOD, nonobese diabetic; SPRD, streptavidin-SpectralRed.
References
- Carsetti R. The development of B cells in the bone marrow is controlled by the balance between cell-autonomous mechanisms and signals from the microenvironment. J. Exp. Med. 2000;191:5–8. doi: 10.1084/jem.191.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond D.G. The turnover of B-cell populations. Immunol. Today. 1993;14:34–37. doi: 10.1016/0167-5699(93)90322-C. [DOI] [PubMed] [Google Scholar]
- Nossal G.J. Negative selection of lymphocytes. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Wang K.K. Calpain and caspasecan you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01479-4. [DOI] [PubMed] [Google Scholar]
- Brás A., Ruiz-Vela A., González de Buitrago G., Martínez-A C. Caspase activation by BCR cross-linking in immature B cellsdifferential effects on growth arrest and apoptosis. FASEB J. 1999;13:931–944. doi: 10.1096/fasebj.13.8.931. [DOI] [PubMed] [Google Scholar]
- Ruiz-Vela A., González de Buitrago G., Martínez-A C. Implication of calpain in caspase activation during B cell clonal deletion. EMBO J. 1999;18:4988–4998. doi: 10.1093/emboj/18.18.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brás A., Ruiz-Vela A., García-Domingo D., Martínez-A C. Apoptosis as a scaffold for building up the B cell repertoire. Ann. NY Acad. Sci. 2000;926:13–29. doi: 10.1111/j.1749-6632.2000.tb05595.x. [DOI] [PubMed] [Google Scholar]
- von Freeden-Jeffry U., Vieira P., Lucian L.A., McNeil T., Burdach S.E., Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A., Kudo A., Melchers F. Long-term proliferating early pre-B-cell lines and clones with the potential to develop to surface immunoglobulin-positive, mitogen-reactive B-cells in vitro and in vivo . Biochem. Soc. Trans. 1991;19:275–276. doi: 10.1042/bst0190275. [DOI] [PubMed] [Google Scholar]
- Reininger L., Radaszkiewicz T., Kosco M., Melchers F., Rolink A.G. Development of autoimmune disease in SCID mice populated with long-term in vitro proliferating (NZB × NZW)F1 pre-B cells. J. Exp. Med. 1992;176:1343–1353. doi: 10.1084/jem.176.5.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz L.D., Schweitzer P.A., Christianson S.W., Gott B., Schweitzer I.B., Tennent B., McKenna S., Mobraaten L., Rajan T.V., Greiner D.L. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- Yang S., Delgado R., King S.R., Woffendin C., Barker C.S., Yang Z.Y., Xu L., Nolan G.P., Nabel G.J. Generation of retroviral vector for clinical studies using transient transfection. Hum. Gene Ther. 1999;10:123–132. doi: 10.1089/10430349950019255. [DOI] [PubMed] [Google Scholar]
- Takano J., Kawamura T., Murase M., Hitomi K., Maki M. Structure of mouse calpastatin isoformsimplications of species-common and species-specific alternative splicing. Biochem. Biophys. Res. Commun. 1999;260:339–345. doi: 10.1006/bbrc.1999.0903. [DOI] [PubMed] [Google Scholar]
- Naviaux R.K., Costanzi E., Haas M., Verma I.M. The pCL vector systemrapid production of helper-free, high-titer, recombinant retroviruses. J. Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croall D.E., DeMartino G.N. Calcium-activated neutral protease (calpain) systemstructure, function, and regulation. Physiol. Rev. 1991;71:813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T., Sorimachi H., Tomioka S., Ishiura S., Suzuki K. A catalytic subunit of calpain possesses full proteolytic activity. FEBS Lett. 1995;358:101–103. doi: 10.1016/0014-5793(94)01401-l. [DOI] [PubMed] [Google Scholar]
- Hosfield C.M., Elce J.S., Davies P.L., Jia Z. Crystal structure of calpain reveals the structural basis for Ca(2+)-dependent protease activity and a novel mode of enzyme activation. EMBO J. 1999;18:6880–6889. doi: 10.1093/emboj/18.24.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E., Molinari M. Calpaina protease in search of a function? Biochem. Biophys. Res. Commun 247 1998. 193 203[published erratum at 249:572] [DOI] [PubMed] [Google Scholar]
- Crawford C., Brown N.R., Willis A.C. Studies of the active site of m-calpain and the interaction with calpastatin. Biochem. J. 1993;296:135–142. doi: 10.1042/bj2960135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Goll D.E. Binding of calpain fragments to calpastatin. J. Biol. Chem. 1991;266:11842–11850. [PubMed] [Google Scholar]
- Palmer T.D., Rosman G.J., Osborne W.R., Miller A.D. Genetically modified skin fibroblasts persist long after transplantation but gradually inactivate introduced genes. Proc. Natl. Acad. Sci. USA. 1991;88:1330–1334. doi: 10.1073/pnas.88.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahner D., Jaenisch R. Retrovirus-induced de novo methylation of flanking host sequences correlates with gene inactivity. Nature. 1985;315:594–597. doi: 10.1038/315594a0. [DOI] [PubMed] [Google Scholar]
- López de Quinto S., Martínez-Salas E. Parameters influencing translational efficiency in aphthovirus IRES-based bicistronic expression vectors. Gene. 1998;217:51–56. doi: 10.1016/s0378-1119(98)00379-5. [DOI] [PubMed] [Google Scholar]
- López de Quinto S., Martínez-Salas E. Involvement of the aphthovirus RNA sequence located between both functional AUGs in start codon selection. Virology. 1999;255:324–336. doi: 10.1006/viro.1999.9598. [DOI] [PubMed] [Google Scholar]
- Jaleco A.C., Stegmann A.P.A., Heemskerk M.H.M., Couwenberg F., Bakker A.Q., Weijer K., Spits H. Genetic modification of human B-cell development is inhibited by the dominant negative helix loop helix factor Id3. Blood. 1999;94:2637–2646. [PubMed] [Google Scholar]
- Chida D., Miura O., Yoshimura A., Miyajima A. Role of cytokine signaling molecules in erythroid differentiation of mouse fetal liver hematopoietic cellsfunctional analysis of signaling molecules by retrovirus-mediated expression. Blood. 1999;93:1567–1578. [PubMed] [Google Scholar]
- Melamed D., Kench J.A., Grabstein K., Rolink A., Nemazee D. A functional B cell receptor transgene allows efficient IL-7-independent maturation of B cell precursors. J. Immunol. 1997;159:1233–1239. [PubMed] [Google Scholar]
- Novelli E.M., Ramírez M., Leung W., Civin C.I. Human hematopoietic stem/progenitor cells generate CD5+ B lymphoid cells in NOD/SCID mice. Stem Cells. 1999;17:242–252. doi: 10.1002/stem.170242. [DOI] [PubMed] [Google Scholar]
- Lentz V.M., Hayes C.E., Cancro M.P. Bcmd decreases the life span of B-2 but not B-1 cells in A/WySnJ mice. J. Immunol. 1998;160:3743–3747. [PubMed] [Google Scholar]
- Wells S.M., Kantor A.B., Stall A.M. CD43 (S7) expression identifies peripheral B cell subsets. J. Immunol. 1994;153:5503–5515. [PubMed] [Google Scholar]
- Raff M. Cell suicide for beginners. Nature. 1998;396:119–122. doi: 10.1038/24055. [DOI] [PubMed] [Google Scholar]
- Kurosaki T., Maeda A., Ishiai M., Hashimoto A., Inabe K., Takata M. Regulation of the phospholipase C-gamma2 pathway in B cells. Immunol. Rev. 2000;176:19–29. doi: 10.1034/j.1600-065x.2000.00605.x. [DOI] [PubMed] [Google Scholar]
- Ono M., Okada H., Bolland S., Yanagi S., Kurosaki T., Ravetch J.V. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- Brown T.L., Patil S., Cianci C.D., Morrow J.S., Howe P.H. Transforming growth factor beta induces caspase 3-independent cleavage of alphaII-spectrin (alpha-fodrin) coincident with apoptosis. J. Biol. Chem. 1999;274:23256–23262. doi: 10.1074/jbc.274.33.23256. [DOI] [PubMed] [Google Scholar]
- Sarin A., Adams D.H., Henkart P.A. Protease inhibitors selectively block T cell receptor-triggered programmed cell death in a murine T cell hybridoma and activated peripheral T cells. J. Exp. Med. 1993;178:1693–1700. doi: 10.1084/jem.178.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.S., Kwon Y.T., Li M., Peng J., Friedlander R.M., Tsai L.H. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]