Abstract
Expulsion of the gastrointestinal nematode Trichuris muris is mediated by a T helper (Th) 2 type response involving interleukin (IL)-4 and IL-13. Here we show that Th1 response–associated susceptibility involves prior activation of IL-18 and caspase-1 followed by IL-12 and interferon (IFN)-γ in the intestine. IL-18–deficient mice are highly resistant to chronic T. muris infection and in vivo treatment of normal mice with recombinant (r)IL-18 suppresses IL-13 and IL-4 secretion but does not affect IFN-γ.
In vivo treatment of T. muris–infected IFN-γ–deficient mice with rIL-18 demonstrated that the inhibitory effect of IL-18 on IL-13 secretion is independent of IFN-γ. Hence, IL-18 does not function as an IFN-γ–inducing cytokine during chronic T. muris infection but rather as a direct regulator of Th2 cytokines. These results provide the first demonstration of the critical role of IL-18 in regulating Th cell responses during gastrointestinal nematode infection.
Keywords: Th cell, cytokine, mucosa, nematode, regulation
Introduction
Gastrointestinal nematodes cause some of the most prevalent and chronic human diseases worldwide. The human whip worm (Trichuris trichiura) currently infects ∼1 billion people 1. The naturally occurring mouse counterpart Trichuris muris has provided much information on the immunoregulatory mechanisms underlying resistance and susceptibility to this parasite. Infection of inbred mouse strains with T. muris results in expulsion of the worms and the development of resistance in the majority of mouse strains (e.g., Balb/c and Balb/K). However, certain strains of mice (e.g., AKR/J and B10.BR) fail to expel this parasite and harbor chronic infections. A number of studies have shown that the ability to develop resistance or chronicity is dependent on Th2 and Th1 cells, respectively 2 3 4 5. The Th cell response in mice that develop resistance is dominated by secretion of IL-4, IL-5, IL-9, and IL-13 in the mesenteric lymph node (MLN) tissue and, conversely, the Th cell response in mice that develop chronic infections is dominated by high levels of IFN-γ secretion. Blocking Th2 response development in resistant mice by disrupting the IL-4 gene, blockade of the IL-4 receptor, or administration of IL-12 abrogates resistance and induces chronic infection 3 4 5. Furthermore, administration of IL-4 to susceptible mice induces expulsion of worms 3.
IL-18, originally named IFN-γ–inducing factor 6, is a potent inducer of IFN-γ, particularly when acting in concert with IL-12 6 7 8 9. It acts mainly on Th cells and NK cells but has also been reported to stimulate B cells 10 11 and bone marrow–derived macrophages 12 to synthesize IFN-γ. IL-18 is mainly produced by monocytes/macrophages and dendritic cells but is also produced by other cell types such as osteoblasts 13, keratinocytes, and cells in the adrenal cortex 14. IL-18 is produced in an inactive form that is cleaved by caspase-1 (ICE) to generate functionally active IL-18 15 16, in a similar way to the processing of IL-1β, a close relative in the IL-1 superfamily. IL-18 knockout (KO) mice display reduced IFN-γ production and lower NK cell activity 17. IL-18 is also an important cytokine in the induction of proinflammatory responses in the gut and is upregulated in the intestinal mucosa of patients with inflammatory bowel disorders such as Crohn's disease 18 19. Interestingly, some recent reports suggest a role for IL-18 in promoting Th2 responses, such as increased levels of IgE, IL-4, and IL-13 after in vivo or in vitro stimulation with IL-18 20 21. Therefore, it appears that IL-18 is a pleiotropic cytokine which may have different roles in the immune response depending on where and when it is induced.
In this report we provide new information on the cytokine-mediated initiation of chronic gastrointestinal nematode infection. We show that IL-18 mRNA and protein is expressed in the colonic mucosa during Th1-mediated chronic intestinal nematode infection. Furthermore, we demonstrate that IL-18 KO mice are highly resistant to chronic nematode infection and develop strong Ag-specific Th2 responses. Administration of recombinant (r)IL-18 to naturally resistant mice result in suppression of Ag-specific secretion of IL-13 and IL-4 and the development of chronic infection. Importantly, the levels of IFN-γ were unaffected demonstrating that, rather than inducing IFN-γ in chronic T. muris infection, IL-18 acts as a direct negative regulator of Th2 cytokines such as IL-13 and/or IL-4. This study provides, for the first time, conclusive evidence that IL-18 plays a key pathogenic role in chronic gastrointestinal nematode infections.
Materials and Methods
Animals and Infections.
6–8-wk-old male Balb/c, C57Bl/6, and AKR mice were purchased from Harlan Olac Ltd. Mice in which the IL-18 gene is disrupted (IL-18 KO mice) were as described by Takeda et al. 17. IL-12 KO mice were provided by Jeanne Magram (Hoffman-La Roche, Nutley, NJ; reference 22). IFN-γ KO mice were originally purchased from Jackson ImmunoResearch Laboratories and bred at the animal unit at The University of Manchester. All experiments were performed under the regulations of the Home Office Scientific Procedures Act (1986).
T. muris was maintained as described by Bancrof t et al. 5 and experimental mice were infected by oral gavage on day 0, and the numbers of larvae were counted on day 10 post infection (p.i.) to ensure equivalent establishment of infection in the different groups. T. muris excretory/secretory (ES) Ag was prepared as detailed previously 5.
In vivo treatment with rIL-18 was performed by intraperitoneal injections of 200 ng rIL-18 (Peprotech) per mouse daily from days 4–17 after T. muris infection. Control mice received intraperitoneal injections of PBS.
Cell Culture and Cytokine Analysis.
MLN cells were removed from uninfected and infected animals and resuspended in RPMI 1640 supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.05 mM β-mercaptoethanol (all from Life Technologies). MLNs were cultured at 37°C and 5% CO2 in flat-bottomed 96-well plates (Life Technologies) at a final concentration of 5 × 106 cells per milliliter in a final volume of 0.2 milliliter per well. Cells were stimulated with 50 μg/ml T. muris ES Ag, 1 μg/ml LPS (Sigma-Aldrich), or varying doses of recombinant mouse IL-18 (Peprotech). Anti–IL-4 receptor mAb (5 μg/ml M1; from Dr. C. Maliszewski, Immunex Corp., Seattle, WA) was added to cultures to increase detection of IL-4. Cell-free supernatants were harvested after 48 h and stored at −80°C.
Cytokine ELISA.
Cytokine analyses were carried out using sandwich ELISAs for IL-4 (mAb BVC4-1D11 and BVD6-24G2.3; BD PharMingen), IL-5 (TRFK-4 and TRFK-5; BD PharMingen), IFN-γ (R46A2 and XMG1.2; BD PharMingen), and IL-12p40 (C15.6 and C17.8; from Dr. G. Trinchieri, Schering-Plough, Dardilly, France). IL-13, IL-18, and IL-10 were analyzed using antibody pairs from R&D Systems.
Immunohistochemistry.
5-mm sections of caeca from infected and uninfected mice were submerged in liquid nitrogen-cooled OCT embedding compound (Sakura Finetechnical Co.) and stored at −80°C until processed. 5-micron cryostat sections were placed on glass slides and fixed in 3.7% PBS-buffered paraformaldehyde for 15 min at 4°C, washed three times in PBS, and blocked with 10% pig serum in 1% BSA/PBS for 1 h at room temperature. The sections were then incubated with 10 μg/ml of rabbit-anti–mouse IL-18 antibody (Peprotech) or control rabbit IgG (Sigma-Aldrich) for 1 h, washed in PBS three times, and blocked for endogenous peroxidase with MeOH/0.3% H2O2/0.1% NaN3 for 30 min. Bound antibody was detected by incubation with horseradish peroxidase–conjugated swine-anti–rabbit Ig (Dako) followed by 3.3′-diaminobenzidine (Dako) as substrate. The slides were briefly counterstained with Mayers Haemalum (BDH), dehydrated into citroclear, and mounted in DePeX (BDH).
RNase Protection Assay.
Total RNA was extracted from tissue specimens taken from the caecal tip using Trizol (Life Technologies) according to the manufacturer's instructions. A custom-made Riboquant template (BD PharMingen) was used to assay mRNA levels of IL-12, IL-18, IFN-γ, caspase-1, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). An IL-18Rα template was constructed by PCR cloning an IL-18Rα cDNA fragment 11 into pGEM-T Easy Vector (Promega). A clone with the correct insert was confirmed by sequencing (data not shown) and the plasmid purified by ethidium bromide centrifugation and linearized. In vitro transcription with 32[P]-labeled UTP (Amersham Pharmacia Biotech) was performed using a Riboprobe kit (Promega) and SP6 (IL-18Rα) or T7 (IL-12, IL-18, IFN-γ, caspase-1, and GAPDH) polymerase (Promega). 10 μg of RNA from each sample was hybridized with the radiolabeled antisense RNA probe set, digested with RNAses, purified, and the protected probes were resolved on denaturing sequencing gels. Dried gels were exposed to phosphorimaging screens and protected fragments visualized using a Molecular Imager FX System (Bio-Rad Laboratories). All samples were normalized in respect to the housekeeping gene GAPDH to ensure equal input of RNA.
Statistics.
Significant differences (P < 0.05) between experimental groups were determined using the Mann-Whitney U test.
Results
IL-18 mRNA Expression Is Rapidly Upregulated in the Large Intestine During Chronic T. Muris Infection.
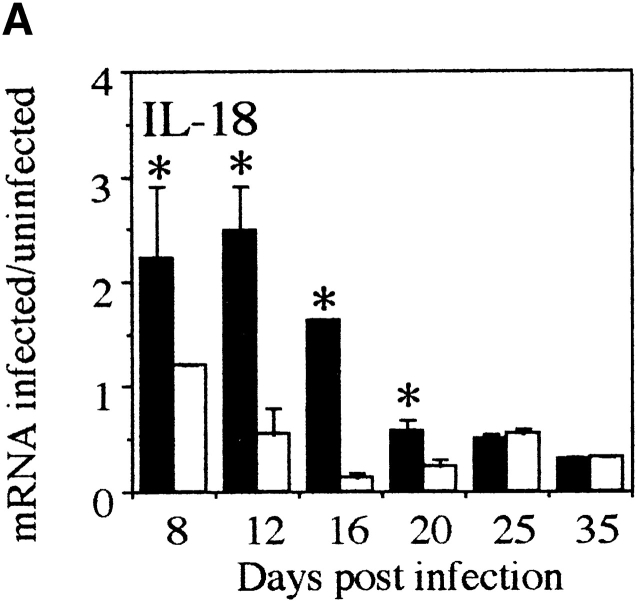
To investigate the kinetics of the proinflammatory response at the site of infection in mice with chronic T. muris infection compared with resistant animals, we analyzed the cytokine mRNA levels in the large intestine by RNase protection assay (RPA). Susceptible AKR mice already show increased expression of IL-18 mRNA on days 8 and 12 p.i. (Fig. 1 A). Thereafter, IL-18 mRNA expression declined to baseline levels. Interestingly, the level of IL-12p40 mRNA displayed a different pattern of kinetics and the peak of expression was later (day 16 p.i.) than the peak of IL-18 mRNA (Fig. 1a and Fig. b). Furthermore, IFN-γ expression did not reach a peak until day 20 p.i. (Fig. 1 C). The mRNA levels of these proinflammatory cytokines were only expressed at low levels during the course of infection in the large intestine of resistant Balb/c mice (Fig. 1a Fig. b Fig. c). These results indicate that IL-18 is an early inducer of the intestinal Th1 response in T. muris–susceptible animals. In addition, elevated intestinal expression of IL-18 mRNA precedes the upregulation of IL-12 and IFN-γ mRNA during chronic T. muris infection.
Figure 1.
IL-18 is expressed earlier than IL-12 and IFN-γ in large intestine during chronic T. muris infection. mRNA for (A) IL-18, (B) IL-12p40, and (C) IFN-γ in the large intestine of susceptible AKR mice (black bar) and resistant Balb/c (white bar) mice was measured by RPA at various timepoints during T. muris infection. mRNA were normalized with respect to the housekeeping gene GAPDH. Results are expressed as fold induction over naive controls and values represent the mean value of 3–5 animals per group ± SEM. *Significantly different between AKR and Balb/c. P < 0.05.
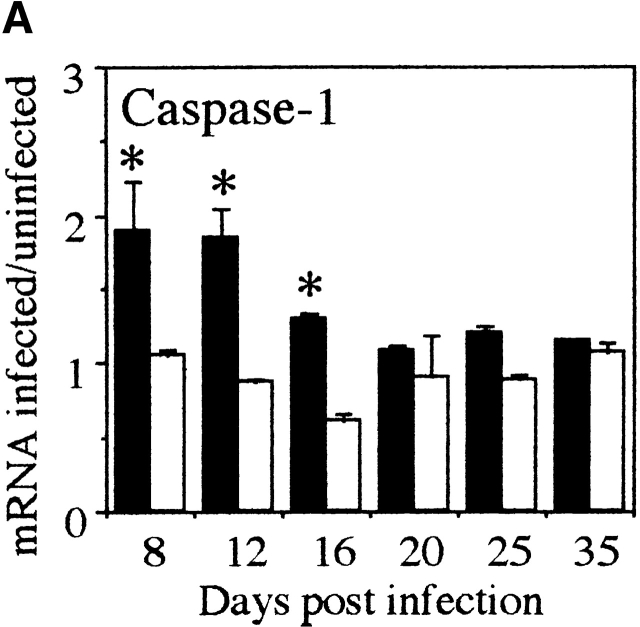
Caspase-1 and IL-18Rα mRNA Expression Is Increased in the Large Intestine During Chronic T. Muris Infection.
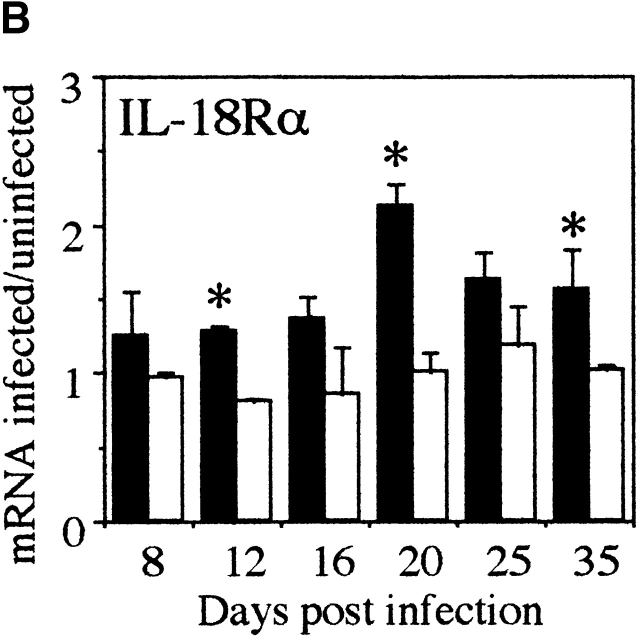
Since IL-18 is cleaved to the bioactive form by caspase-1 we investigated whether caspase-1 mRNA was upregulated in the large intestine of susceptible mice during T. muris infection. As shown in Fig. 2 A, an increase in caspase-1 mRNA could be seen coincidently with the upregulation of IL-18 mRNA at early timepoints (days 8 and 12 p.i.; Fig. 1 A) suggesting that IL-18 is indeed cleaved to a bioactive form in the large intestine. We also investigated the intestinal expression of IL-18Rα chain and found an increase in mRNA expression with a peak at day 20 p.i. (Fig. 2 B).
Figure 2.
Caspase-1 and IL-18Rα mRNA is expressed in large intestine during chronic T. muris infection. mRNA for (A) caspase-1 and (B) IL-18Rα in the large intestine of susceptible AKR mice (black bar) and resistant Balb/c (white bar) mice was measured by RPA at various timepoints during T. muris infection. mRNA were normalized with respect to the housekeeping gene GAPDH. Results are expressed as fold induction over naive controls and values represent the mean value of 3–5 animals per group ± SEM. *Significantly different between AKR and Balb/c. P < 0.05.
IL-18 Protein Is Expressed in the Lamina Propria of the Large Intestine During T. Muris Infection.
The cellular source of IL-18 protein at the site of infection was investigated by immunohistochemistry. Sections of large intestine from naive and day 18 infected AKR mice were stained with anti–IL-18 antibody and examined. Large intestine from naive mice showed weak IL-18 staining in the lamina propria (Fig. 3 B), while day 18 infected animals showed strong IL-18 staining (Fig. 3 D). This finding demonstrates that IL-18 protein secretion is still active despite the fact that IL-18 mRNA is already decreased in the large intestine (Fig. 1 A). The time of IL-18 protein expression also correlates with IL-18Rα mRNA expression (Fig. 2 B) suggesting that the IL-18 detected expresses functional signaling capacity. The IL-18 staining of large intestine was confined to the lamina propria with very strong staining of large elongated mononuclear cells, which were likely to be macrophages or dendritic cells. No staining could be detected within the epithelium.
Figure 3.
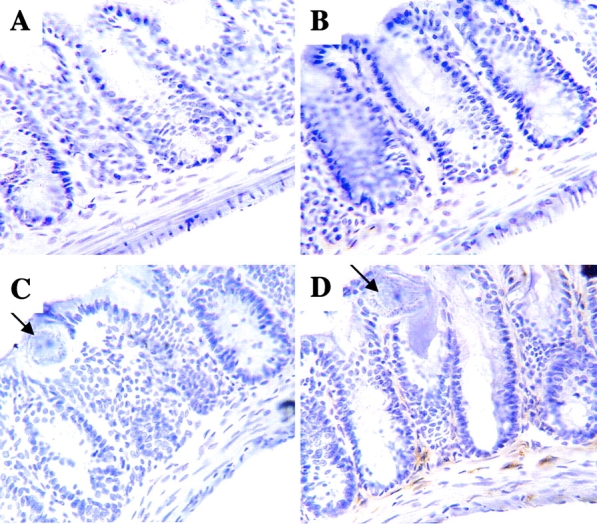
IL-18 is expressed in the lamina propria of T. muris–infected large intestine. Cryosections of large intestine from naive (A and B) or day 18 infected (C and D) AKR mice were stained with rabbit anti–IL-18 antibody (B and D) or control rabbit IgG (A and C). Arrows indicate worms in the day 18 infected animals. A–D, original magnification: ×400.
IL-18 KO Mice Are Resistant to Chronic T. Muris Infection.
To evaluate the functional importance of IL-18 compared with IL-12 in the development of chronic gastrointestinal nematode infection, we infected IL-18 KO and IL-12 KO mice with T. muris. The IL-18 KO and IL-12 KO mice were on a C57Bl/6 background and this strain is usually resistant to high-dose infection 5. Therefore, we utilized a low dose infection protocol enabling us to establish chronic infection in these C57Bl/6 mice 23. IL-18 KO, IL-12 KO, and C57Bl/6 wild-type (WT) mice were infected with 25 embryonated T. muris eggs, and worm burdens were assessed at days 10, 18, and 35 p.i. WT mice developed chronic infection with full worm burdens at day 35 p.i. (Fig. 4). Interestingly, the IL-18 KO mice had already started to expel the worms at day 18 p.i. (P < 0.01) and had completed expulsion by day 35 p.i. (P < 0.01), clearly showing that IL-18 is critical in the induction of chronic gastrointestinal nematode infection. Moreover, IL-12 KO mice infected in the same experiment were also resistant to infection, albeit with a slower expulsion speed than the IL-18 KO mice (Fig. 4, not significant on day 18 p.i. but P < 0.05 on day 35 p.i.), showing that IL-12 is also an important component in chronic T. muris infection in agreement with previously published results 4.
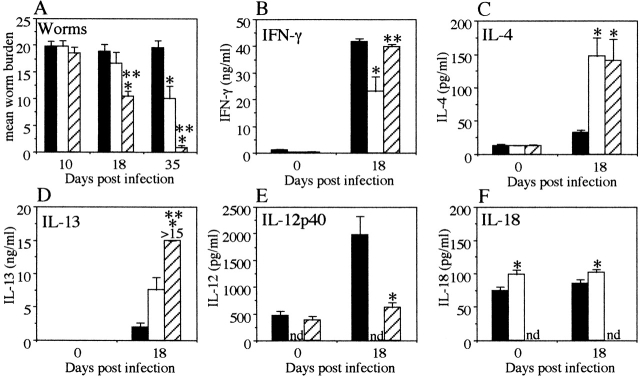
Figure 4.
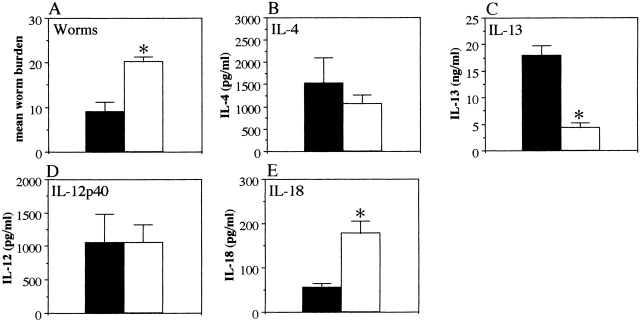
IL-18 KO mice secrete high levels of IL-4 and IL-13 and are resistant to chronic T. muris infection. (A) Worm burdens from T. muris–infected IL-18 KO (striped bar), IL-12p40 KO (white bar), and C57Bl/6 WT (black bar) mice were assessed at days 10, 18, and 35 p.i. MLN cells were removed at day 0 (naive) and day 18 p.i. and stimulated in vitro with T. muris Ag (B–D) or LPS (E and F). Supernatants were analyzed by sandwich ELISA for the presence of (B) IFN-γ, (C) IL-4, (D) IL-13, (E) IL-12p40, and (F) IL-18. Results represent the mean value of 5–7 mice per group ± SEM. *Significantly different from WT. P < 0.05. **Significantly different from other KO. P < 0.05. nd, not detectable.
IL-18 KO Mice Secrete High Levels of both Th1 and Th2 Cytokines During T. Muris Infection.
To investigate the basis for the difference between IL-12 and IL-18 KO mice, we investigated the cytokine profiles from in vitro–stimulated MLN cultures. The results in Fig. 4 B show that IL-18 KO mice secrete similar amounts of Ag-specific IFN-γ to WT mice at day 18 p.i. (IL-18 KO, 39.8 ± 0.98 ng/ml; and WT, 41.9 ± 1.03 ng/ml). The IL-12 KO mice however, secreted twofold lower levels of IFN-γ than both the IL-18 KO and WT mice (IL-12 KO, 23.2 ± 5.42 ng/ml, P < 0.01 as compared with both IL-18 KO and WT at day 18 p.i.; Fig. 4 B). Interestingly, both IL-18 KO and IL-12 KO mice secreted significantly higher levels of IL-4 than the WT mice at day 18 p.i. (IL-18 KO, 140.9 ± 31.8 pg/ml; IL-12 KO, 147.0 ± 27.5 pg/ml; WT, 33.5 ± 2.7 pg/ml, P < 0.01 for both IL-18 KO and IL-12 KO mice as compared with WT at day 18 p.i.; Fig. 4 C). This is despite the fact that only the IL-18 KO mice had started worm expulsion at this timepoint (Fig. 4 A) indicating that the IL-4 secreted in the IL-12 KO mice was not sufficient to induce expulsion at a similar rate as compared with the IL-18 KO mice.
As immune-mediated expulsion of T. muris is IL-13 dependent 5, we analyzed the levels of IL-13 in the supernatants to investigate if the difference in expulsion kinetics between IL-18 KO and IL-12 KO mice was dependent on IL-13 rather than IL-4. IL-18 KO mice secreted significantly higher levels of Ag-specific IL-13 in the culture supernatants than the IL-12 KO mice (IL-18 KO, >15 ng/ml; IL-12 KO, 7.6 ± 1.9 ng/ml; WT, 1.9 ± 0.7 ng/ml, P < 0.05 for IL-18 KO versus IL-12 KO, not significant for IL-12 KO versus WT; Fig. 4 D) suggesting that the slower worm expulsion seen in the IL-12 KO mice was due to lower secretion of IL-13 rather than IL-4.
MLN Cultures from T. Muris–infected IL-12 KO Mice Secrete High Levels of IL-18.
Production of IL-18 and IL-12 protein is readily induced by LPS stimulation of macrophages and/or dendritic cells 6 24 25 26 27. To investigate any differences in the levels of IL-12 and IL-18 secretion between the groups, the supernatants from LPS-stimulated MLN cultures were analyzed. IL-18 KO mice secreted a threefold lower amount of IL-12 in response to LPS stimulation at day 18 p.i. compared with WT mice (IL-12 KO, undetectable; IL-18 KO, 613.5 ± 103.8 pg/ml, WT, 1971.5 ± 355.3 pg/ml, P < 0.01 for IL-18 KO versus WT; Fig. 4 E), while cultures from IL-12 KO mice produced significantly more IL-18 in response to LPS at both day 0 and day 18 p.i. (day 0; IL-12 KO, 98.5 ± 7.2 pg/ml; WT, 74.3 ± 6.2 pg/ml, day 18; IL-12 KO, 102.4 ± 3.4 pg/ml, WT, 85.5 ± 6.0 pg/ml, IL-18 KO, undetectable at all timepoints, P < 0.05 for IL-12 KO versus WT at both timepoints) (Fig. 4 F).
In Vitro Treatment with Exogenous rIL-18 Inhibits Ag-specific IL-13 Secretion in MLN Cultures in the Absence of IL-12 or IFN-γ.
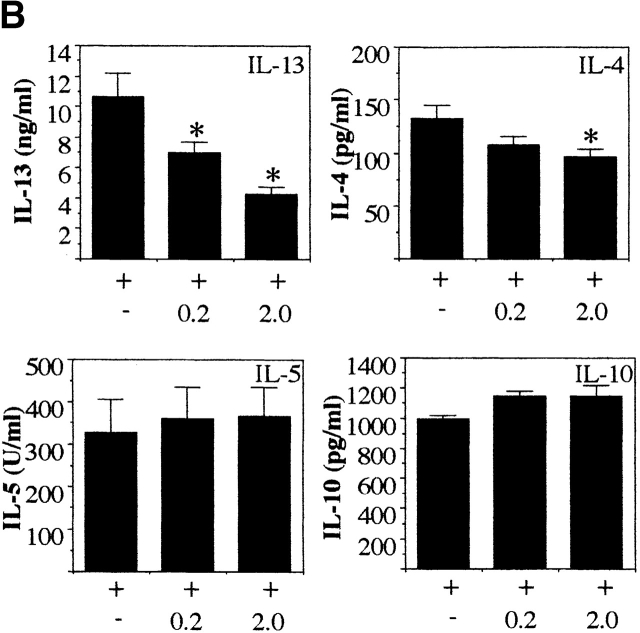
To investigate if IL-18 regulates the secretion of IL-13 and may be responsible for the reduced levels of IL-13 seen in IL-12 KO mice, we restimulated MLN cells from infected IL-12 KO mice with Ag plus different doses of rIL-18. Addition of rIL-18 to the Ag-stimulated cultures reduced the levels of IL-13 secretion in a dose-dependent manner (P < 0.05 for Ag plus 2 ng/ml rIL-18 versus Ag alone; Fig. 5 A). The addition of rIL-18 to the Ag-stimulated cultures had no significant effect on IL-4, IL-5, IL-10, or IFN-γ secretion. Furthermore, to investigate if the downregulatory effect of IL-18 on IL-13 was IFN-γ independent we used MLN cells from T. muris–infected IFN-γ KO mice. Addition of rIL-18 to the Ag-stimulated cultures again significantly downregulated IL-13 secretion (P < 0.05 for Ag plus 0.2 or 2 ng/ml rIL-18 versus Ag alone; Fig. 5 B) while having no effect on IL-5 and IL-10 secretion and a moderate effect on IL-4 secretion. These results demonstrate that IL-18 can specifically inhibit the secretion of IL-13 from already polarized Th2 cells in an Ag-specific manner and that this inhibitory effect is independent of both IL-12 and IFN-γ.
Figure 5.
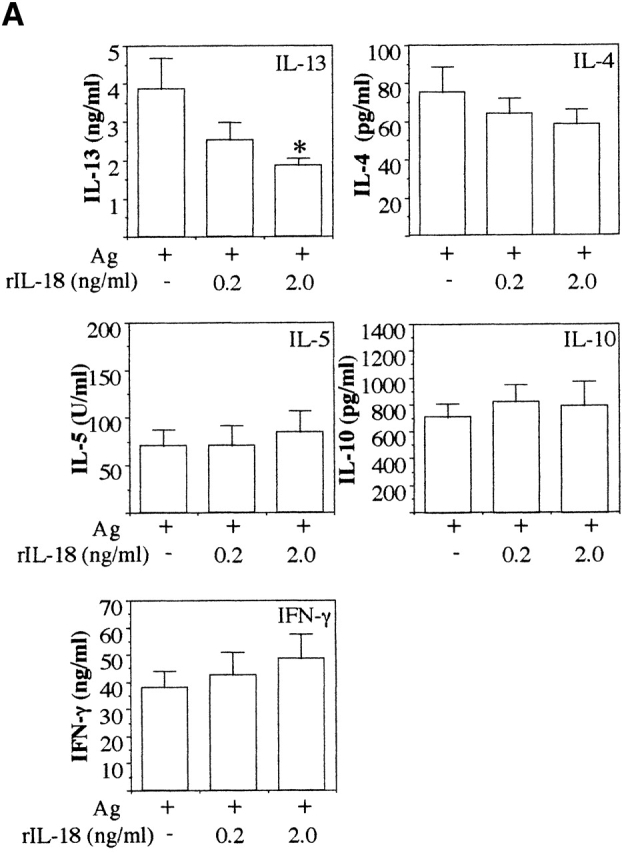
In vitro treatment with exogenous rIL-18 inhibits Ag-induced IL-13 secretion in MLN cultures from T. muris–infected IL-12 and IFN-γ KO mice. MLN cells from day 18 T. muris–infected IL-12p40 KO mice (A) and IFN-γ KO mice (B) were stimulated with T. muris Ag plus indicated doses of rIL-18 in vitro and the supernatant analyzed for IL-13, IL-4, IL-5, IL-10, and IFN-γ secretion by sandwich ELISA. Results represent the mean value of 5–7 mice per group ± SEM. *Significantly different from cultures stimulated with T. muris Ag only. P < 0.05.
In Vivo Treatment of Naturally Resistant Mice with rIL-18 Leads to the Development of Chronic T. Muris Infection and Suppression of Th2 Responses while Th1 Responses Remains Unaffected.
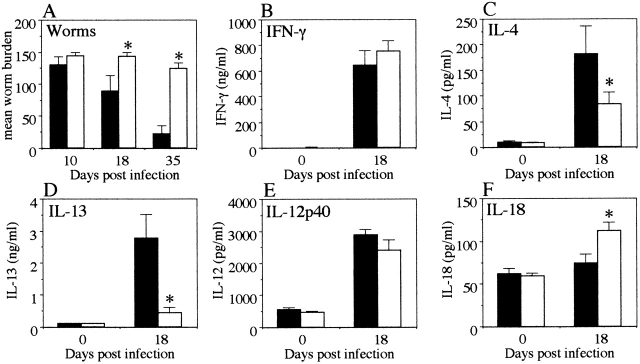
To investigate the effects of IL-18 in vivo, C57Bl/6 mice that are naturally resistant to high dose T. muris infection 5 were treated with daily injections of rIL-18 (200 ng per mouse from days 4–17 p.i.). Control mice that received PBS injections expelled the parasites from the intestine in normal resistant fashion (Fig. 6 A). Mice treated with rIL-18, however, were unable to expel the worms and developed chronic infections (Fig. 6 A). Restimulation of MLN cells with Ag revealed that the in vivo treatment with rIL-18 did not augment Ag-specific IFN-γ secretion (Fig. 6 B) and that the levels of IL-12 were unaffected (Fig. 6 E). However, rIL-18 treatment significantly suppressed Ag-specific IL-4 (PBS treated 181.8 ± 87.4 pg/ml, rIL-18 treated 84.1 ± 23.0 pg/ml, P < 0.05) and IL-13 secretion (PBS treated 2.78 ± 0.73 ng/ml, rIL-18 treated 0.44 ± 0.16 ng/ml, P < 0.05) (Fig. 6C and Fig. D), clearly demonstrating that IL-18 inhibits T. muris–specific Th2 responses in vivo without augmenting Th1 responses.
Figure 6.
In vivo treatment with rIL-18 induces chronic T. muris infection without increasing Th1 responses. T. muris–infected C57Bl/6 mice were injected with rIL-18 (white bar) or PBS (black bar) daily for 14 d. (A) Worm burdens were assessed at days 10, 18, and 35 p.i. MLN cells were removed at day 0 (naive) and day 18 p.i. and stimulated in vitro with T. muris Ag (B–D) or LPS (E and F). Supernatants were analyzed by sandwich ELISA for the presence of (B) IFN-γ, (C) IL-4, (D) IL-13, (E) IL-12p40, and (F) IL-18. Results represent the mean value of 5–7 mice per group ± SEM. *Significantly different from PBS-treated control (P < 0.05).
The Inhibitory Effect of IL-18 on IL-13 Secretion Is Independent of IFN-γ In Vivo.
To confirm that the IFN-γ–independent effects of IL-18 seen in vitro (Fig. 5) also extended to an in vivo situation, we treated T. muris–infected IFN-γ KO mice with daily injections of rIL-18 (200 ng per mouse from day 4–17 p.i). Worm burdens were assessed at day 18 p.i. and found to be significantly higher in the rIL-18–treated animals demonstrating that IL-18 significantly affects worm expulsion in vivo in the absence of IFN-γ (Fig. 7 A). Restimulation of MLN cells with Ag demonstrated that the in vivo treatment with rIL-18 did not affect IL-4 or IL-12p40 secretion (Fig. 7B and Fig. D) but significantly enhanced IL-18 secretion (PBS treated 55.83 ± 8.67 pg/ml, rIL-18 treated 177.27 ± 28.98 pg/ml, P < 0.05; Fig. 7 E). Importantly, the in vivo treatment suppressed Ag-specific IL-13 secretion (PBS treated 17.96 ± 1.85 ng/ml, rIL-18 treated 4.34 ± 0.95 ng/ml, P < 0.05; Fig. 7 C), confirming that IL-18 inhibits T. muris–specific IL-13 responses in vivo independently of IFN-γ.
Figure 7.
In vivo treatment with rIL-18 delays worm expulsion and suppresses Ag-specific IL-13 secretion independently of IFN-γ. T. muris–infected IFN-γ KO mice were injected with rIL-18 (white bar) or PBS (black bar) daily for 14 d. (A) Worm burdens were assessed at day 18 p.i. and MLN cells were stimulated in vitro with T. muris Ag (B and C) or LPS (D and E). Supernatants were analyzed by sandwich ELISA for the presence of (B) IL-4, (C) IL-13, (D) IL-12p40, and (E) IL-18. Results represent the mean value of 4–5 mice per group ± SEM. *Significantly different from PBS-treated control. P < 0.05.
Discussion
The data presented in this report provide the first demonstration that the proinflammatory cytokine IL-18 is a crucial component in the development of chronic gastrointestinal infection. Furthermore, we demonstrate that the effects of IL-18 during T. muris infection of the intestine is mediated through its immunomodulatory effects on Th2 cytokines, most notably IL-13, rather than its ability to induce IFN-γ responses.
Immune-mediated expulsion of T. muris from infected mice is clearly dependent on a Th2 type of response involving IL-4 and IL-13 3 5. However, certain strains of mice are unable to expel the worms and develop chronic infections. This susceptibility is associated with a Th1 type of response but little is known of the events leading up to the initiation of the IFN-γ response in these mice. Importantly, treatment with rIL-12 induces chronic T. muris infection in mouse strains that are normally resistant, demonstrating that IL-12 plays an important role in the development of chronic infection 4.
IL-18 is an important component in the development of a Th1 response. However, IL-18 alone is unable to initiate a Th1 response and requires the presence of IL-12 in order to do so 6 7 8. In our experiments, quantitative RNase protection analysis of intestinal mRNA expression in T. muris–infected mice revealed that upregulation of IL-18 mRNA at the site of infection occurred earlier than IL-12 mRNA expression in mice that develop chronic infection. This was an unexpected finding since it has previously been reported that IL-12 induces IL-18 and IL-18R expression 7 11 28. However, our data suggest that upregulation of intestinal IL-18 mRNA can be independent of IL-12 and provides the first demonstration of this event during an in vivo infection. Furthermore, the coincident expression of the IL-18–activating enzyme caspase-1 mRNA during the early timepoints of infection suggests that a separate proinflammatory pathway to that of IL-12 was induced in the intestine. The later upregulation of IL-12 mRNA expression correlated with the increased expression of IL-18Rα mRNA which is consistent with the observation that IL-12 upregulates IL-18R expression 7 11 28. IL-12R (both β1 and β2 subunits) mRNA was also upregulated in the intestine of susceptible mice over a similar time frame to that of IL-12 mRNA (data not shown). This indicates that chronic T. muris infection upregulates the signaling pathway for IL-12 at the site of infection in a similar manner to that seen after infection with the parasitic protozoa Leishmania major 29 30.
Immunohistological analysis demonstrated that IL-18 protein is constitutively produced at low levels in the large intestine of naive mice. However, IL-18 protein was greatly increased during T. muris infection in susceptible mice in a similar fashion to that observed in human large intestine during Crohn's disease 18 19. The IL-18 staining was confined to the lamina propria with no staining of the epithelium. This is interesting bearing in mind recent work suggesting that epithelial cell lines are able to produce IL-18 31.
To investigate the functional roles of IL-18 and IL-12 in this infection model we infected IL-18 KO, IL-12 KO, and WT (C57Bl/6) mice with T. muris. By using a low dose protocol it is possible to induce chronic infection in mice that are normally resistant, such as C57Bl/6 23. We successfully established chronic infections in C57Bl/6 WT mice and, consistent with a susceptible phenotype, these mice had high IFN-γ and low IL-4 and IL-13 responses in the MLNs. Interestingly, the IL-18 KO (C57Bl/6) mice rapidly expelled the worms. There was already a significant decrease in worm burden by day 18 p.i. in IL-18 KO mice and almost complete expulsion by day 35 p.i. The IL-12 KO mice infected in the same experiment showed delayed expulsion compared with IL-18 KO mice and had only partially expelled their worm burden by day 35 p.i., a time at which IL-18 KO mice had already completed the process. These results demonstrate that IL-18 is critical for the induction of chronic gastrointestinal nematode infection and that in the absence of IL-12 alone, expulsion takes place but with slower kinetics. One possible explanation for these observations may be related to the increased levels of IL-18 detected in the IL-12 KO mice.
Both IL-12 KO and IL-18 KO mice have previously been shown to display defective Th1 responses in response to various Ags 17 22. Indeed, IL-12/IL-18 double KO mice show almost undetectable Th1 responses 17, demonstrating the important synergistic effects of these cytokines in the induction of most Th1 type responses. Interestingly, cytokine analysis of Ag-stimulated MLN cultures (the lymph nodes that drain the large intestine) from T. muris–infected IL-18 KO mice showed that in the absence of IL-18, the IFN-γ response on day 18 p.i. was equivalent to that seen in WT mice. However, in IL-12 KO mice there was a marked reduction in IFN-γ production despite increased secretion of IL-18, demonstrating that IL-12 alone is sufficient for the induction of IFN-γ in this system. However, it is notable that the high IFN-γ secretion in IL-18 KO mice and the low levels of IFN-γ seen in IL-12 KO mice did not reflect the speed of expulsion. Importantly, these results demonstrate that worm expulsion can take place even in the presence of high levels of IFN-γ. Experiments in IL-18 KO mice using another parasite, the parasitic protozoa L. major, show conflicting results. In some studies, IL-18 KO mice have reduced IFN-γ secretion 32 while in other studies no differences were seen 33 34. Possible reasons for these different results may include variations in the genetic background of the mouse strains studied or strains of L. major parasites used. Taken together with our data it appears that the IFN-γ–inducing ability of IL-18 may depend on the disease, the genetic background of the host, and/or site of infection.
To investigate the basis for the observed differences in kinetics of worm expulsion between WT, IL-12 KO, and IL-18 KO mice, we analyzed the levels of IL-4 and IL-13 protein in the supernatants from Ag-stimulated MLN cultures. The rapid worm expulsion seen in IL-18 KO mice correlated well with high levels of both IL-4 and IL-13 in the MLN supernatants. The IL-12 KO mice however, which suffered from a slower expulsion rate, had high levels of IL-4 but significantly lower levels of IL-13. Since IL-13 is a crucial mediator of T. muris expulsion 5 it is likely that the low levels of IL-13 detected in the IL-12 KO mice are responsible for the slow expulsion rate in these mice. Interestingly, the IL-12 KO mice also had increased levels of IL-18 secretion compared with WT mice. Significantly, when MLN cells from these mice were restimulated with Ag plus exogenous rIL-18 the low levels of secreted IL-13 were decreased further. This effect appears to be IL-13–specific since there was no effect on the secretion of other Th2 cytokines such as IL-4, IL-5, and IL-10 or on the secretion of the Th1 cytokine IFN-γ. The addition of rIL-12 to the cultures had no such effect (data not shown). Importantly, this downregulatory effect of IL-18 on IL-13 was also observed when MLN cells from IFN-γ KO mice were used demonstrating that this effect is IFN-γ independent. This demonstrates for the first time that IL-18 can directly inhibit IL-13 secretion in an Ag-specific system even when Th2 cells are already polarized in vivo.
To confirm the direct effects of IL-18 on the development of Th2 responses in vivo we treated C57Bl/6 mice with rIL-18 during the course of a high dose T. muris infection. This mouse strain is resistant to high dose infection and will under normal conditions expel the worms within 35 days p.i. 5. However, the animals that received rIL-18 treatment (200 ng/day) from days 4–17 during infection did not expel the worms and developed chronic infections unlike the PBS-treated controls which all became resistant and expelled the parasites. Importantly, cytokine analysis showed that in vivo treatment with rIL-18 did not augment IFN-γ or IL-12 secretion from restimulated MLN cells but significantly reduced both IL-4 and IL-13 secretion. When the same experiment was performed in IFN-γ KO mice a similar delay in worm expulsion and decrease in IL-13 secretion could be seen proving that the effects of IL-18 on worm expulsion and IL-13 secretion is independent of IFN-γ in vivo. These results clearly confirm that the proinflammatory effects of IL-18 during an intestinal nematode infection such as T. muris is not mediated by augmentation of Th1 responses but as a negative regulator of Th2 cytokine secretion.
Previously published work has shown that IL-18 promotes IFN-γ responses in Th1 cells but does not affect IL-4 production from Th2 cells 8 9 35. Some interesting recent observations indicate a role for IL-18 in promoting Th2 responses both in vivo or in vitro by increasing levels of IgE, IL-4, and IL-13 20 21. However, it is noteworthy that very high doses of IL-18 are needed to induce these Th2 type responses 20 21 and a possible explanation may be that the inhibitory or stimulatory effects of IL-18 on Th2 cells are dose dependent in a similar fashion to that seen with other cytokines such as TGF-β 36 37. However, in our experiments, both in vivo and in vitro, we were unable to find any correlation between IL-18 and increased Th2 responses to T. muris infection. There may be other factors that influence these opposing effects of IL-18 including the type of Ag and the dose as well as the site of the immune response. Further experiments are required to clarify the roles of IL-18 in the negative and positive regulation of Th2 cytokine production.
The data presented in this paper provides new information on the cytokine-mediated initiation of chronic T. muris infection. Importantly, the results demonstrate that IL-18, instead of functioning as an IFN-γ inducer in chronic T. muris infection, act as a direct regulator of Th2 cytokines such as IL-13 and/or IL-4. In summary, our studies provide conclusive evidence that IL-18 plays a key pathogenic role in chronic gastrointestinal nematode infections and that IL-18 may have a key regulatory role that is independent of IL-12 and/or IFN-γ. This is the first report showing the importance of IL-18 during parasitic helminth infection and these results extend our knowledge on the cytokine-mediated regulation of intestinal inflammation and may provide important information for the design of rational therapies against helminth infection, allergic reactions, and inflammation of the gut and other mucosal sites.
Acknowledgments
We would like to thank Neil Humphreys for excellent technical assistance, Dr. David Artis for helpful discussions, Dr. Rachel Lawrence for critical reading of the manuscript, and Prof. Nancy Rothwell for providing animals.
H. Helmby is supported by a grant from the Swedish Foundation for International Cooperation in Research and Higher Education (STINT). This work was carried out with support from The Wellcome Trust and The Biotechnology and Biological Sciences Research Council.
Footnotes
Abbreviations used in this paper: ES, excretory/secretory; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; KO, knockout; MLN, mes-enteric lymph node; p.i., post infection; r, recombinant; RPA, RNase protection assay; WT, wild-type.
References
- Bundy D.A.P. This wormy world - then and now. Parasitology Today. 1997;13:407–408. [Google Scholar]
- Else K.J., Hultner L., Grencis R.K. Modulation of cytokine production and response phenotypes in murine trichuriasis. Parasite Immunol. 1992;14:441–449. doi: 10.1111/j.1365-3024.1992.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Else K.J., Finkelmann F.D., Maliszewski C.R., Grencis R.K. Cytokine-mediated regulation of intestinal helminth infection. J. Exp. Med. 1994;179:347–351. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft A.J., Else K.J., Sypek J.P., Grencis R.K. Interleukin-12 promotes a chronic intestinal nematode infection. Eur. J. Immunol. 1997;27:866–870. doi: 10.1002/eji.1830270410. [DOI] [PubMed] [Google Scholar]
- Bancroft A.J., McKenzie A.N.J., Grencis R.K. A critical role for IL-13 in resistance to intestinal nematode infection. J. Immunol. 1998;160:3453–3461. [PubMed] [Google Scholar]
- Okamura H., Tsutsui H., Komatsu T., Yutsudo M., Hakura A., Tanimoto T., Torigoe K., Okura T., Nukada Y., Hattori K. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- Ahn H.-Y., Maruo S., Tomura M., Mu J., Hamakoa T., Nakanishi K., Clark S., Kurimoto M., Okamura H., Fujiwara H. A mechanism underlying synergy between IL-12 and IFN-γ-inducing factor in enhanced production of IFN-γ. J. Immunol. 1997;159:2125–2131. [PubMed] [Google Scholar]
- Robinson D., Shibuya K., Mui A., Zonin F., Murphy E., Sana T., Hartley S.B., Menon S., Kastelein R., Bazan F., O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-γ production and activates IRAK and NFκB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- Kohno K., Kataoka J., Ohtsuki T., Suemoto Y., Okamoto I., Usui M., Ikeda M., Kurimoto M. IFN-γ-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J. Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- Yoshimoto T., Okamura H., Tagawa Y., Iwakura Y., Nakanishi K. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-γ production from activated B cells. Proc. Natl. Acad. Sci. USA. 1997;94:3948–3953. doi: 10.1073/pnas.94.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Takeda K., Tanaka T., Ohkusu K., Kashiwamura S., Okamura H., Akira S., Nakanishi K. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cellssynergism with IL-18 for IFN-γ production. J. Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- Munder M., Mallo M., Eichmann K., Modolell M. Murine macrophages secrete interferon-γ upon combined stimulation with interleukin (IL)-12 and IL-18a novel pathway of autocrine macrophage activation. J. Exp. Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa N., Horwood N.J., Elliott J., Mackay A., Owens J., Okamura H., Kurimoto M., Chambers T.J., Martin T.J., Gillespie M.T. Interleukin-18 (interferon-γ–inducing factor) is produced by osteoblasts and acts via granulocyte/macrophage colony-stimulating factor and not via interferon-γ to inhibit osteoclast formation. J. Exp. Med. 1997;185:1005–1012. doi: 10.1084/jem.185.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B., Jahng J.W., Tinti C., Son J.H., Joh T.H. Induction of interferon-γ-inducing factor in the adrenal cortex. J. Biol. Chem. 1997;272:2035–2037. doi: 10.1074/jbc.272.4.2035. [DOI] [PubMed] [Google Scholar]
- Ghayur T., Banerjee S., Hugunin M., Butler D., Herzog L., Carter A., Quintal L., Sekut L., Talanian R., Paskind M. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN γ production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- Gu Y., Kuida K., Tsutsui H., Ku G., Hsiao K., Fleming M.A., Hayshi N., Higashino K., Okamura H., Nakanishi K. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- Takeda K., Tsutsui H., Yoshimoto T., Adachi O., Yoshida N., Kishimoto T., Okamura H., Nakanishi K., Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- Monteleone G., Trapasso F., Parrello T., Biancone L., Stella A., Iuliano R., Luzza F., Fusco A., Pallone F. Bioactive IL-18 expression is up-regulated in Crohn's disease. J. Immunol. 1999;163:143–147. [PubMed] [Google Scholar]
- Pizarro T.T., Michie M.H., Bentz M., Woraratanadharm J., Smith M.S., Jr., Foley E., Moskaluk C.A., Bickston S.J., Cominelli F. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's diseaseexpression and localization in intestinal mucosal cells. J. Immunol. 1999;162:6829–6835. [PubMed] [Google Scholar]
- Hoshino T., Yagita H., Ortaldo J.R., Wiltrout R.H., Young H.A. In vivo administration of IL-18 can induce IgE production through Th2 cytokine induction and up-regulation of CD40 ligand (CD154) expression on CD4+ T cells. Eur. J. Immunol. 2000;30:1998–2006. doi: 10.1002/1521-4141(200007)30:7<1998::AID-IMMU1998>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Mitzutani H., Tsuitui H., Noben-Trauth N., Yamanaka K.-I., Tanaka M., Izumi S., Okamura H., Paul W.E., Nakanishi K. IL-18 induction of IgEdependence on CD4+ T cells, IL-4 and STAT 6. Nat. Immunol. 2000;1:132–137. doi: 10.1038/77811. [DOI] [PubMed] [Google Scholar]
- Magram J., Connaughton S.E., Warrier R.R., Carvajal D.M., Wu C.-Y., Ferrante J., Stewart C., Sarmiento U., Faherty D.A., Gately M.K. IL-12-deficient mice are defective in IFN-γ production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- Bancroft A.J., Else K.J., Grencis R.K. Low-level infection with Trichuris muris significantly affects the polarization of the CD4 response. Eur. J. Immunol. 1994;24:3113–3118. doi: 10.1002/eji.1830241230. [DOI] [PubMed] [Google Scholar]
- Snijders A., Hilkens C.M.U., van der Pouw Kraan T.C.T.M., Engel M., Aarden L.A., Kapsenberg M.L. Regulation of bioactive IL-12 production in lipopolysaccharide-stimulated human monocytes is determined by the expression of the p35 subunit. J. Immunol. 1996;156:1207–1212. [PubMed] [Google Scholar]
- Verhasselt V., Buelens C., Willems F., De Groote D., Haeffner-Cavaillon N., Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cellsevidence for a soluble CD14-dependent pathway. J. Immunol. 1997;158:2919–2925. [PubMed] [Google Scholar]
- Reis e Sousa C., Hieny S., Scharton-Kersten T., Jankovic D., Charest H., Germain R.N., Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of IL-12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puren A.J., Fantuzzi G., Dinarello C.A. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1β are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc. Natl. Acad. Sci. USA. 1999;96:2256–2261. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Chan W., Leung B.P., Hunter D., Schultz K., Carter R.W., McInnes I.B., Robinson J.H., Liew F.Y. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J. Exp. Med. 1998;188:1485–1492. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelrich H., Parra-Lopez C., Tacchini-Cottier F., Louis J.A., Launois P. The IL-4 rapidly produced in BALB/c mice after infection with Leishmania major down-regulates IL-12 receptor β2-chain expression on CD4+ T cells resulting in a state of unresponsiveness to IL-12. J. Immunol. 1998;161:6156–6163. [PubMed] [Google Scholar]
- Jones D., Elloso M.M., Showe L., Williams D., Trinchieri G., Scott P. Differential regulation of the interleukin-12 receptor during the innate immune response to Leishmania major . Infect. Immun. 1998;66:3818–3824. doi: 10.1128/iai.66.8.3818-3824.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Shen C., Brunham R.C. Chlamydia trachomatis infection of epithelial cells induces the activation of caspase-1 and release of mature IL-18. J. Immunol. 2000;165:1463–1469. doi: 10.4049/jimmunol.165.3.1463. [DOI] [PubMed] [Google Scholar]
- Wei X., Leung B.P., Niedbala W., Piedrafita D., Feng G., Sweet M., Dobbie L., Smith A.J.H., Liew F.Y. Altered immune responses and susceptibility to Leishmania major and Staphylococcus aureus infection in IL-18-deficient mice. J. Immunol. 1999;163:2821–2828. [PubMed] [Google Scholar]
- Monteforte G.M., Takeda K., Rodriguez-Sosa M., Akira S., David J.R., Satoskar A.R. Genetically resistant mice lacking IL-18 gene develop Th1 response and control cutaneous Leishmania major infection. J. Immunol. 2000;164:5890–5893. doi: 10.4049/jimmunol.164.11.5890. [DOI] [PubMed] [Google Scholar]
- Ohkusu K., Yoshimoto T., Takeda K., Ogura T., Kashiwamura S.-I., Iwakura Y., Akira S., Okamura H., Nakanishi K. Potentiality of interleukin-18 as a useful reagent for treatment and prevention of Leishmania major infection. Infect. Immun. 2000;68:2449–2456. doi: 10.1128/iai.68.5.2449-2456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio S., Namba M., Okura T., Hattori K., Nukada Y., Akita K., Tanabe F., Konishi K., Micallef M., Fujii M. Cloning of the cDNA for human IFN-γ-inducing factor, expression in Escherichia coli, and studies on the biological activities of the protein. J. Immunol. 1996;156:4274–4279. [PubMed] [Google Scholar]
- Wahl S.M. Transforming growth factor βthe good, the bad, and the ugly. J. Exp. Med. 1994;180:1587–1590. doi: 10.1084/jem.180.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorham J.D., Guler M.L., Fenoglio D., Guebler U., Murphy K.M. Low dose TGF-β attenuates IL-12 responsiveness in murine Th cells. J. Immunol. 1998;161:1664–1670. [PubMed] [Google Scholar]