Abstract
Langerhans cells (LCs) represent a subset of immature dendritic cells (DCs) specifically localized in the epidermis and other mucosal epithelia. As surrounding keratinocytes can produce interleukin (IL)-15, a cytokine that utilizes IL-2Rγ chain, we analyzed whether IL-15 could skew monocyte differentiation into LCs. Monocytes cultured for 6 d with granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-15 differentiate into CD1a+HLA-DR+CD14−DCs (IL15-DCs). Agents such as lipopolysaccharide (LPS), tumor necrosis factor (TNF)α, and CD40L induce maturation of IL15-DCs to CD83+, DC-LAMP+ cells. IL15-DCs are potent antigen-presenting cells able to induce the primary (mixed lymphocyte reaction [MLR]) and secondary (recall responses to flu-matrix peptide) immune responses. As opposed to cultures made with GM-CSF/IL-4 (IL4-DCs), a proportion of IL15-DCs expresses LC markers: E-Cadherin, Langerin, and CC chemokine receptor (CCR)6. Accordingly, IL15-DCs, but not IL4-DCs, migrate in response to macrophage inflammatory protein (MIP)-3α/CCL20. However, IL15-DCs cannot be qualified as “genuine” Langerhans cells because, despite the presence of the 43-kD Langerin, they do not express bona fide Birbeck granules. Thus, our results demonstrate a novel pathway in monocyte differentiation into dendritic cells.
Keywords: monocytes, dendritic cells, Langerhans cells, interleukin 15, gamma chain
Introduction
Skin, an efficient barrier against microbes, is composed of two distinct layers: the dermis and the epidermis 1. The dermis is composed of fibroblasts, dermal (interstitial) dendritic cells (DCs), and mast cells. The epidermis includes keratinocytes, melanocytes, Merkel, and Langerhans cells (LCs). LCs are immature DCs that are unique to Malpighian epithelia. LCs are characterized by their Birbeck granules (BGs; reference 1), their expression of Langerhans-associated granule (LAG)/Langerin 2, E-Cadherin 3, and CC chemokine receptor (CCR)6 4. As other immature DCs, they are poised to capture antigens/pathogens, process them, and present their peptides to lymphocytes after their migration into draining lymph nodes 5 6. The lack of LCs in TGF-β1−/− mice 7 suggests an important role for this cytokine in LC development, a conclusion further strengthened by in vitro studies 8 9 10 11.
Keratinocytes (KCs) form the epidermal sheet where the LCs are embedded and produce various cytokines including: (a) GM-CSF, which plays a key role in DC differentiation 12 and (b) IL-15 13, a cytokine that binds to the receptor component IL-2Rγ common to several cytokine receptors including IL-2, IL-4, IL-7, and IL-9 14 15. IL-15 is expressed by nonhematopoietic cells such as fibroblasts, endothelial cells, KCs, and DCs 16 17. This cytokine has the ability to induce the proliferation of activated T cells. In vivo studies have demonstrated that IL-15 plays an important role in the development of memory CD8+ T cells and NK cells 18 19. In particular, IL-15−/− 20 and IL-15Rα−/− 21 mice display reduced numbers of T cells and specifically lack NK and NK T cells.
Inasmuch as monocytes differentiate into immature DCs in response to GM-CSF and IL-4, cytokines produced by the dermal mast cells, we have explored whether the KC-derived cytokines, GM-CSF, and IL-15 may control the differentiation of LCs. Herein, we show that highly purified monocytes cultured in the presence of GM-CSF and IL-15 yield functional DCs (IL15-DCs). Unlike IL-4, IL-15 skews monocyte differentiation into cells that express E-Cadherin, Langerin, and CCR6, as well as migrate in response to macrophage inflammatory protein (MIP)-3α, a phenotype of LCs.
Materials and Methods
Reagents.
mAbs: CD2, CD11b, CD14, HLA-DR (Becton Dickinson); CD86 (BD PharMingen); CD9 (Ancell), CD32, CD36 (Caltag), CD40, HLA-ABC, CCR6 (R&D Systems), CD1a (Dako), CD80, CD83, E-Cadherin, and Langerin (Coulter/Immunotech), IL-2Rγ chain, MIP-3α, TGF-β1 (R&D Systems), Cy5-coupled donkey anti–mouse Ab (Jackson ImmunoResearch Laboratories). Recombinant human cytokines: rhGM-CSF (Leukine; Immunex), rhIL-4 (R&D Systems or Schering-Plough), rhIL-15 (R&D Systems), TGF-β1, and TNFα (R&D Systems). Complete RPMI medium consisted of RPMI 1640, 1% L-glutamine, 1% penicillin/streptomycin, 50 mM 2-ME, 1% sodium-pyruvate, 1% essential amino acids, and heat inactivated 10% FCS (all from GIBCO BRL). LPS was from Sigma-Aldrich.
Cell Culture, Endocytic Activity, and Flow Cytometry.
Monocytes were isolated from blood mononuclear cells, after Ficoll-Paque™ gradient, by depletion of T-, B-, NK cells, and CD1a+ DCs using purified anti-CD3, anti-CD19, anti-CD56, anti-glycophorin A, and anti-CD1a Abs followed by immunomagnetic depletion (Dynabeads). Enriched CD14+ monocytes were cultured in 6-well plates (106/well) for 6 d in the presence of GM-CSF (100 ng/ml)/IL-15 (200 ng/ml) or GM-CSF (100 ng/ml)/IL-4 (20 ng/ml). In some experiments DCs were activated with LPS (100 ng/ml). When necessary, 500 ng neutralizing anti–TGF-β1 Ab was added every other day to the monocyte cultures. The endocytotic activity was determined by incubating the DCs with 100 μg/ml FITC-Dextran for 30 min at 37°C. As a control, a portion of DCs were incubated with FITC-Dextran on ice. The cells were washed with cold PBS/FCS and analyzed by flow cytometry. Membrane staining: 105 cells were incubated for 30 min at 4°C with fluorochrome-conjugated mAb or purified mAb (1:100 dilution). For indirect staining, FITC-F(ab′)2 sheep anti–mouse Ig or Cy5-donkey anti–mouse Ig were used. Cells are analyzed with the FACSCalibur™ (Becton Dickinson)
Confocal Microscopy.
Cells deposited on poly-L-lysin-coated coverslips were fixed for 15 min with 4% paraformaldehyde in PBS, washed twice in 10 mM glycine in PBS and twice in PBS, and permeabilized with 0.5% saponin-1% BSA-PBS for 30 min. Coverslips were incubated for 30 min at room temperature with 5 μg/ml anti–DC-LAMP or anti-Langerin (Coulter/Immunotech as well as gifts from Drs. Lebecque and Saeland, Schering-Plough, Dardilly, France). Cells were incubated for 30 min with secondary-labeled donkey anti–mouse Abs coupled to Texas red (Jackson ImmunoResearch Laboratories), washed, incubated with mouse serum for 30 min, washed again, and incubated for 30 min with a second primary Ab anti–HLA-DR directly coupled to fluorescein (Becton Dickinson). Confocal microscopy was performed using a TCS SP microscope equipped with argon and krypton ion lasers (Leica).
Electron Transmission Microscopy.
Langerin+DR+ IL15-DCs were first sorted using FACSVantage™ or positively selected by anti-Langerin coupled with goat anti–mouse beads-IgG and afterwards fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) and postfixed with 1% OsO4. After dehydration with graded ethanol, they were embedded in Spurr's plastic. Ultra-thin sections were stained with lead citrate and uranyl acetate, and studied using a LEO 906E electron microscope (Germany).
T Cell Proliferation.
DCs were cultured at graded doses with 105 freshly isolated CD4+ or CD8+ allogeneic T cells for 5 d in cRPMI plus 10% human AB serum. To assay autologous T cell proliferation, IL15-DCs or IL4-DCs were pulsed with tetanus toxoid (TT; 4 LFU/ml) for 48 h and cultured at graded doses with 105 autologous T cells. Cells were pulsed for the last 16 h with 0.5 μCi [3H]thymidine per well (New England Nuclear).
Generation and Assessment of Antigen-specific CTLs.
On day 5, IL15-DCs or IL4-DCs were induced to maturation using macrophage cytokines: GM-CSF, IL-1α (5 ng/ml), IL-6 (25 ng/ml), and TNFα (10 ng/ml) for 16 h and pulsed with 0.1 μg/ml of Flu-MP58–66 for further 18 h CD8+ effector T cells (106) were isolated by magnetic beads separation and cocultured with either unpulsed or Flu-MP pulsed DCs in 1 ml complete RPMI plus 10% human AB serum. All cultures received IL-7 (10 ng/ml) at the onset and IL-2 (10 UI/ml) on day 7 of culture. T cells were stimulated twice with DCs and grown for 14 d as described earlier 22. For CTL assay, effector cells (30 × 103/well) were plated in 96-well round-bottom plates with T2 (103/well) target cells pulsed with Flu-MP58–66 and labeled with 51Cr (Amersham Pharmacia Biotech). After 4 h, supernatants were harvested and chromium release was measured using a γ-counter (Packard Instrument Co.). Percentage of specific lysis was then determined. For tetramer binding assay after two stimulation cycles, T cells were labeled with anti-CD3 FITC (Becton Dickinson) and anti-CD8 PE Abs (BD PharMingen) for 45 min at 4°C, washed and stained with APC-conjugated Flu-MP/HLA-A*0201 class I tetramer (provided by National Institute of Allergy and Infectious Diseases) for 10 min at room temperature. After fixation in 1% PFA, samples were analyzed on FACSCalibur™ (Becton Dickinson).
Immunoblotting.
Cell lysis and immunoblotting were performed as described previously 12. Gels were transferred onto Immun-Blot® PVDF membranes. The transferred proteins were detected using anti-Langerin or IgG1 as control revealed with ECL Western blotting detection system (Amersham Pharmacia Biotech).
Chemotaxis Assay.
Cell migration was determined by using a chemotaxis microchamber. MIP-3α (1 μg/ml) was diluted in RPMI plus 2% human serum, and was added to the lower wells of the chemotaxis chamber. IL-15- or IL4-DCs (105/80 μl) were applied to the upper wells of the chamber, with a standard 5-μm pore polyvinylyrrolidone-free polycarbonate separating the lower wells. The chamber was incubated at 37°C for 3 h. Afterwards, cells that had migrated to the lower side of the filter were collected and counted using a light microscope. Each assay was performed in triplicate and results were expressed as the mean ± SD of migrated cells per field.
Results and Discussion
Monocytes Cultured with GM-CSF and IL-15 Yield DCs (IL15-DCs).
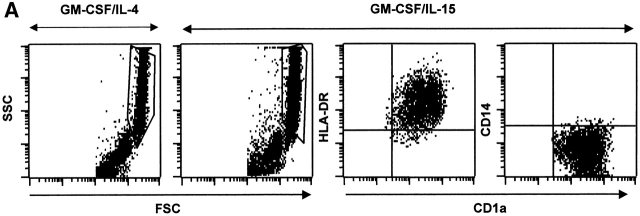
As IL-4 and IL-15 receptors share the IL-2Rγ chain, we analyzed whether IL-15, just like IL-4, may be involved in the differentiation of monocytes into DCs. Highly purified monocytes (>98% of CD14+ cells) were cultured with IL-15 or IL-4 combined with GM-CSF. Cells aggregated within 24 h, and within 6 d aggregates revealed protruding veils suggestive of DCs (not shown). Giemsa staining, after 6 d of culture, revealed cells with a typical DC morphology (not shown). The cells harvested from cultures of monocytes with GM-CSF/IL-15 show the same forward/side scatter properties as those from GM-CSF/IL-4 cultures. Furthermore, large cells acquire CD1a, express high levels of HLA-DR and have lost CD14 (Fig. 1 A), similarly to GM-CSF/IL-4 cultures. DC differentiation requires 4–6 d of culture (not shown). Thus, monocytes cultured with GM-CSF/IL-15 give rise to cells with a phenotype of DCs. For the sake of simplicity, we will subsequently call these cells IL15-DCs by contrast to IL4-DCs.
Figure 1.
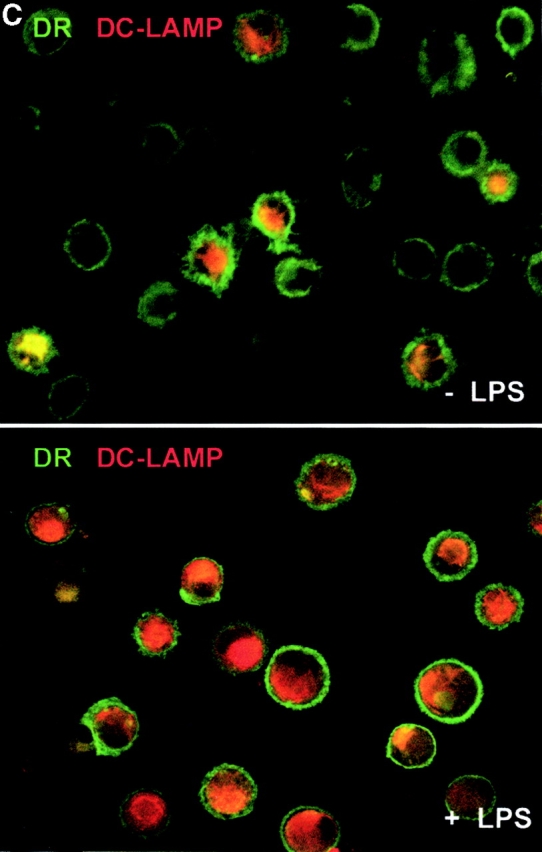
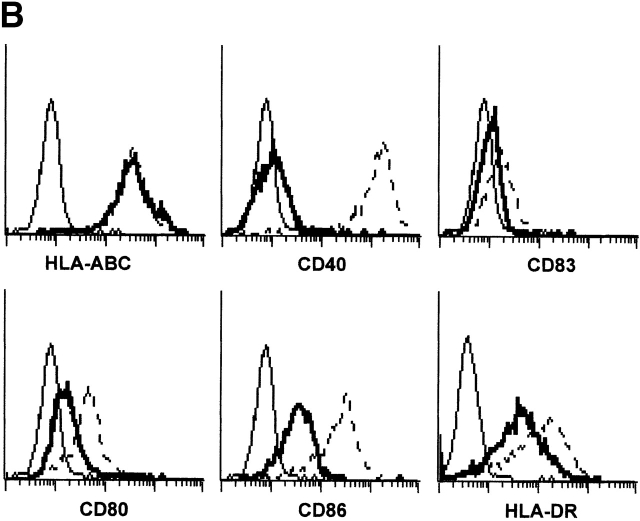
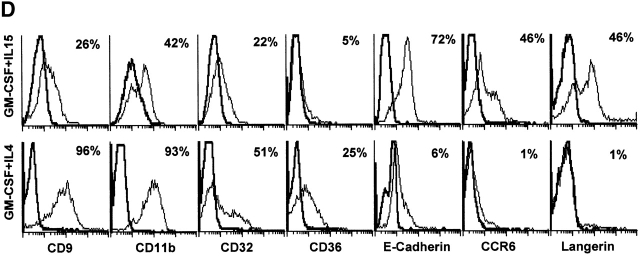
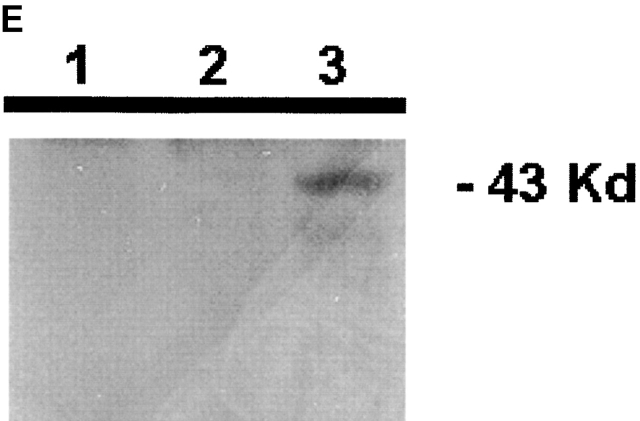
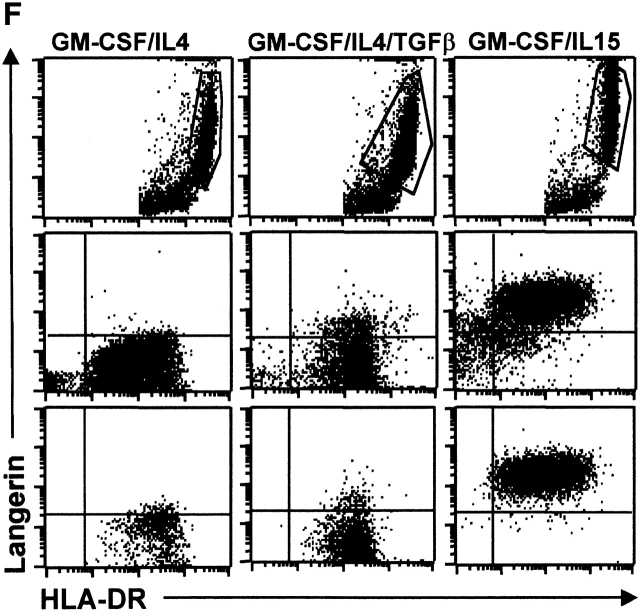
IL-15 and GM-CSF skew monocyte differentiation into DCs with features of LCs. (A) Purified monocytes are cultured with GM-CSF/IL-4 (IL4-DCs) or with GM-CSF/IL-15 (IL15-DCs) for 6 d. Both cultures display similar SSC/FSC properties (left panels). IL15-DCs acquire CD1a, lose CD14, and are HLA-DR+ (right panels), similarly to IL4-DCs (not shown). (B) IL15-DCs (bold line) acquire a mature DC phenotype (CD40high, CD83+, CD80high, CD86high, and HLA-DRhigh), when cultured with LPS (100 ng/ml) (dotted line). Thin solid line shows isotype control. (C) Some IL15-DCs express intracellular DC-LAMP, the expression of which is considerably upregulated by LPS-activation (confocal microscopy, field 130 × 100 μm). (D) IL15-DCs, but not IL4-DCs, express the surface phenotype of LCs expressing E-Cadherin, CCR6, and Langerin. Conversely, IL4-DCs, but not IL15-DCs, express high levels of CD9, CD11b, CD32, and CD36. Purified monocytes are cultured for 6 d, stained with indicated Abs and analyzed by flow cytometry. (E) Immunoblot-detection of Langerin in IL15-DCs. Proteins of SDS-gels were transferred onto Immun-Blot® PVDF membranes and these were stained using anti-Langerin and ECL Western blotting detection system. Lane 1: T cells; lane 2: IL4-DCs; lane 3: IL15-DCs. (F) Monocytes cultured with GM-CSF/IL-15 display high Langerin expression when compared with monocytes cultured with GM-CSF/IL-4/TGF-β1 (representative of four experiments).
After 6 d, 106 monocytes yield 0.23 × 106 IL15-DCs (mean of 12 experiments, SD = 0.41). The generation of IL15-DCs is independent of endogenous IL-4 as daily addition of neutralizing anti–IL-4 Ab (400 ng/ml) does not prevent DC generation (not shown). Conversely, adding anti–IL-15 or anti–IL-2Rγ chain Abs blocks significantly the generation of CD1a+ DCs in response to GM-CSF/IL15 (not shown).
At day 6 of culture, IL15-DCs are immature with low or no expression of CD40, CD80, and CD83 (Fig. 1 B). However, unlike IL4-DCs, IL15-DCs contain some cells (5–10%) that express DC-LAMP. Upon activation with LPS (100 ng/ml), IL15-DCs show increased surface expression of HLA-DR, CD40, CD80, CD86, and CD83 (Fig. 1 B), as well as intracellular expression of DC-LAMP (23; Fig. 1 C) consistent with DC maturation. Maturation of IL15-DCs can be induced equally well with LPS or CD40L (not shown). As expected, immature IL15-DCs efficiently capture soluble FITC-Dextran and particulate antigen (e.g., killed tumor cells) and lose this capacity upon maturation (not shown).
IL15-DCs Display the Surface Phenotype of LCs.
CD34+ hematopoietic progenitor cells cultured with GM-CSF/TNFα yield both interstitial DCs and LCs 24. A few markers distinguish LCs from other DCs: (a) Langerin, a C-type lectin uniquely expressed in LCs and possibly controlling the formation of BGs 2, (b) CCR6, the receptor for MIP-3α/CCL20 and β-defensin, and (c) E-Cadherin, an adhesion molecule allowing binding to KCs 3. IL15-DCs express Langerin on their surface while IL4-DCs do not (Fig. 1 D, and Table ). Western blot analysis reveals an expected 43-kD band in IL15-DC lysate (Fig. 1 E, lane 3) but not in lysates of IL4-DC and CD4 T cells (lanes 2 and 1, respectively). Moreover, a much larger proportion of cells express Langerin in IL15-DCs when compared with monocytes cultured with GM-CSF/IL-4/TGF-β1 (Fig. 1 F, and Table ), a combination of cytokines earlier suggested to induce monocytes to become LCs 9. Daily addition of a neutralizing anti–TGF-β1 mAb inhibits Langerin expression on GM-CSF/IL4/TGF-β1 cells, while it does not affect the IL-15–induced expression of Langerin (not shown). This suggests that the induction of DCs with LC phenotype in response to GM-CSF and IL-15 is TGF-β1 independent.
Table 1.
IL-15 Skews Monocyte Differentiation toward Langerin-positive Cells
| Culture conditions | GM-CSF/ IL-4 | GM-CSF/ IL-4/TGFβ | GM-CSF/ IL-15 |
|---|---|---|---|
| Mean ± SEM percentage of Langerin-positive cells | 1.7 ± 0.4 | 9.6 ± 4.3 | 40 ± 8.7 |
| Median | 1 | 7.5 | 42 |
| No. experiments with >5% positive cells | 0/11 | 6/8 | 11/11 |
| Range | 0–5 | 3–37 | 5–78 |
Percentages of Langerin-expressing cells in monocyte cultures with either GM-CSF/IL-4, or GM-CSF/IL-4/TGFβ, or GM-CSF/IL-15 (day 6) (average ± SEM as well as median from indicated number of experiments). Percentages of Langerin positive cells in total (nongated) population.
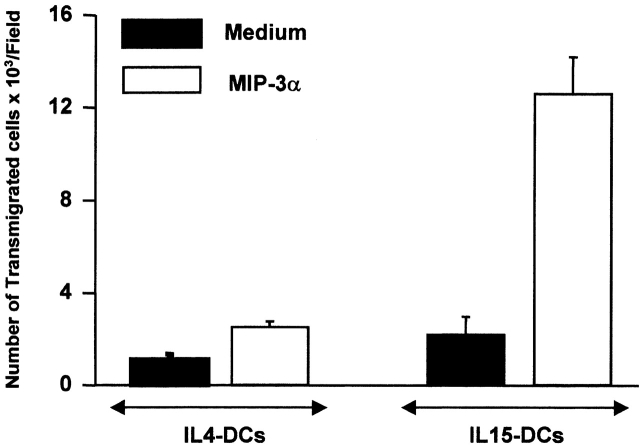
As shown in Fig. 1 D, IL4-DCs, but not IL15-DCs, express high levels of CD9, CD11b, CD32, and CD36. Conversely, IL15-DCs express E-Cadherin and CCR6 molecules while IL4-DCs express neither. To determine whether this chemokine receptor is functional, cells were tested for their ability to migrate toward MIP-3α in a chemotaxis microchamber. As shown in Fig. 2, IL15-DCs but not IL4-DCs migrate in response to MIP-3α/CCL20. Thus, the phenotype of IL-4 and IL-15 monocyte-derived DCs is respectively comparable to that of DCs derived from the CD14+ and CD1a+ subsets isolated from CD34+ HPCs grown with GM-CSF and TNFα 24.
Figure 2.
IL15-DCs migrate in response to MIP-3α/CCL20. MIP-3α was added to the lower wells of the chemotaxis chamber. IL15- or IL4-DCs (105/80 μl) were applied to the upper wells of the chamber, with a standard 5-μm pore polyvinylyrrolidone-free polycarbonate separating the lower wells. The chamber was incubated at 37°C for 3 h. Cells that had migrated to the lower well were collected and counted. Each assay was performed in triplicate and results are expressed as the mean ± SD number of migrated cells (representative of two experiments).
A hallmark of epidermal LCs in situ is the presence of BGs. However, IL15-DCs cannot be qualified as “genuine” LCs because they do not express bona fide BGs, despite the presence of the 43-kD Langerin. While a virally transformed cell line 2 is induced to display BGs upon transduction with Langerin, DCs expressing the Langerin protein do not necessarily display BGs (this study and reference 9). Thus, Langerin expression does not fully correlate with BG formation and additional signals must be necessary to complete LC differentiation.
IL15-DCs Are Efficient Antigen-presenting Cells.
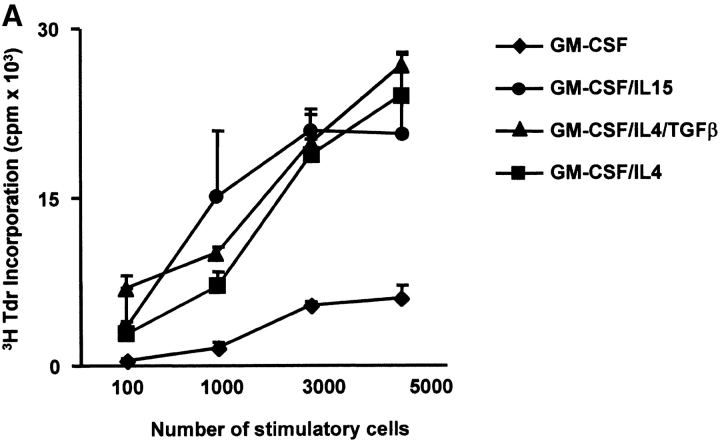
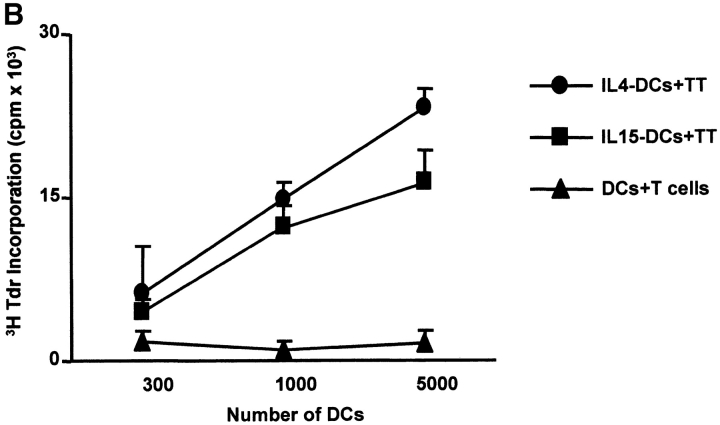
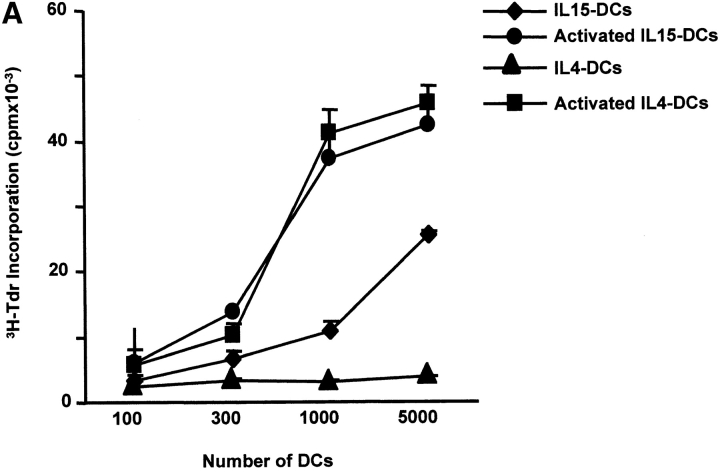
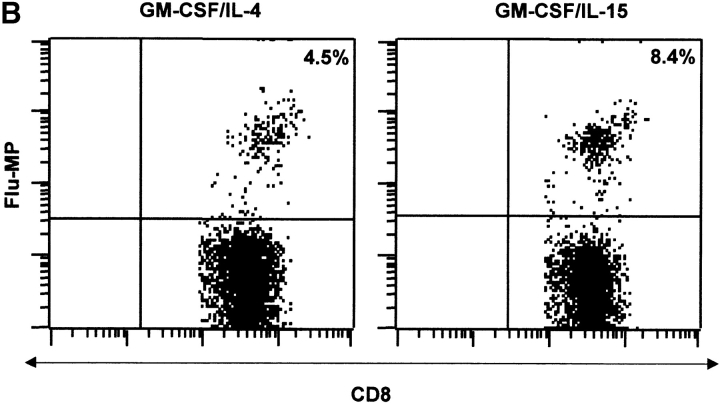
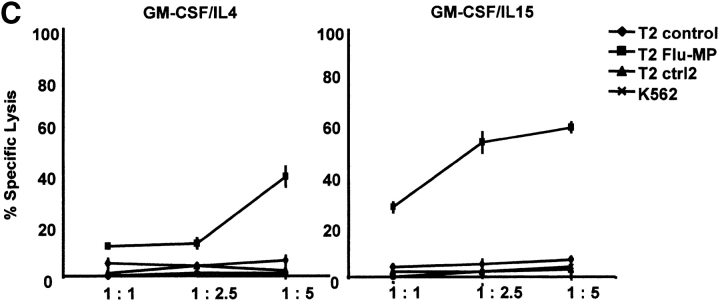
The capacity to induce allogeneic T cell proliferation is a functional in vitro hallmark of DCs. Thus, we compared the allostimulatory capacity of monocytes cultured with GM-CSF alone or in combination with IL-15, IL-4, or IL-4/TGF-β1. As shown in Fig. 3 A, IL15-DCs are as efficient in stimulating CD4+ T cell proliferation as IL4-DCs. The allostimulatory capacity of IL15-DCs is enhanced upon maturation (not shown). Furthermore, IL15-DCs can present antigens to autologous CD4+ T cells and induce T cell proliferation to either soluble antigens such as TT (Fig. 3 B) or particulate antigens such as killed tumor cells (not shown). Interestingly, while immature IL4-DCs require an exogenous maturation factor to induce CD8+ T cell proliferation, immature IL15-DCs can induce purified allogeneic CD8+ T cells to proliferate significantly (Fig. 4 A), a property that is enhanced during their maturation. Finally, IL15-DCs pulsed with Flu-MP peptide induce recall CTL responses as demonstrated by (a) frequency of Flu-MP tetramer positive CD8+ T cells (Fig. 4 B), and (b) CTL activity against peptide-pulsed target (Fig. 4 C). Thus, IL15-DCs are capable antigen-presenting cells that might be more efficient than IL4-DCs in inducing primary CD8+ T cell responses.
Figure 3.
IL15-DCs present antigens to CD4+ T cells. (A) Allostimulatory capacity of monocytes cultured with GM-CSF/IL-15, GM-CSF/IL-4, GM-CSF/IL-4/TGF-β1, or GM-CSF alone. Proliferation of allogeneic CD4+ T cells (105) cultured for 5 d with graded dose of antigen-presenting cells, is determined by thymidine uptake. (B) IL15-DCs pulsed with TT (4 LFU/ml) induce proliferation of autologous CD4+ T cells (105) as determined by thymidine incorporation at day 5. Representative of three experiments.
Figure 4.
IL15-DCs present antigens to CD8+ T cells. (A) Immature IL-15 DCs induce substantial proliferation of allogeneic CD8+ T cells. CD8+ T cells (105) are cultured with graded doses of IL15-DCs and proliferation is determined by thymidine incorporation. (B) IL15-DCs induce higher frequency of Flu-MP tetramer-positive CD8+ T cells. Autologous CD8+ T cells are cultured with HLA-A201 plus activated IL15- or IL4-DCs pulsed with Flu-MP peptide. After two stimulations (14 d of culture, IL-7 in the first and IL-2 in the second week), CD8+ T cells are harvested and stained with anti-CD3 FITC, anti-CD8 PE, and Flu-MP HLA-A201 class I tetramer-APC. Representative of three experiments. (C) CTL activity of CD8+ T cells cultured with HLA-A201 plus IL15- or IL4-DCs pulsed with Flu-MP peptide. T cells are activated as described above and tested in chromium release assay using Flu-MP pulsed T2 cells as targets (percentage specific lysis). K562, unpulsed T2 cells, or T2 pulsed with irrelevant peptide are used as controls.
Concluding Remarks.
This study demonstrates that IL-15, in conjunction with GM-CSF, induces highly purified monocytes to differentiate into DCs. The cells generated under these conditions are in many ways comparable to DCs generated with GM-CSF/IL-4. They share: (a) the basic DC phenotype; (b) the capacity to capture FITC-Dextran; (c) the capacity to mature in response to various stimuli; and (d) the ability to induce vigorous T cell proliferation. However, a major difference between IL-4 and IL-15 is that IL-15 cultures yield DCs with a surface phenotype of LCs.
Our study demonstrates that IL-15 acts directly on monocytes. Although IL-4 and IL-15 mobilize a common receptor component, the IL-2Rγ chain, they also induce differentiation of two distinct DC subsets. Thus, the cytokine specific receptor provides unique signals that control monocyte differentiation.
The ability to generate DCs with LC phenotype from monocytes will permit us to assess their unique biological function in humans compared with interstitial DCs generated by culturing monocytes with GM-CSF and IL-4. Finally, the lack of T cells and NK cells observed in IL-15−/− and IL-15R−/− mice may now be reanalyzed in the context of the possible effect of IL-15 on in vivo DC differentiation in mice.
Acknowledgments
We wish to thank Dr. Sem Saeland for his gift of Langerin Ab and the critical review of the manuscript, Serge Lebecque for the gift of DC-LAMP Ab, Dr. Nikolas Romani for the critical discussions and suggestions to our studies, and Sandra Clayton for help with confocal analysis.
This work is supported by grants from the National Institutes of Health CA-78846-01A1 and from the Baylor Health Care System Foundation. Flu-MP tetramers were provided by National Institute of Allergy and Infectious Diseases Tetramer Facility and the National Institutes of Health AIDS Research and Reference Reagent Program.
Footnotes
M. Mohamadzadeh and F. Berard contributed equally to this work.
References
- Norris D.A. Immune Mechanisms in Cutaneous Disease 1989. Marcel Dekker, Inc; New York and Basel: pp. 826 pp [Google Scholar]
- Valladeau J., Ravel O., Dezutter-Dambuyant C., Moore K., Kleijmeer M., Liu Y., Duvert-Frances V., Vincent C., Schmitt D., Davoust J. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- Tang A., Amagai M., Granger L.G., Stanley J.R., Udey M.C. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature. 1993;361:82–85. doi: 10.1038/361082a0. [DOI] [PubMed] [Google Scholar]
- Greaves D.R., Wang W., Dairaghi D.J., Dieu M.C., Saint-Vis B., Franz-Bacon K., Rossi D., Caux C., McClanahan T., Gordon S. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3alpha and is highly expressed in human dendritic cells. J. Exp. Med. 1997;186:837–868. doi: 10.1084/jem.186.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Borkowski T.A., Letterio J.J., Farr A.G., Udey M.C. A role for endogenous transforming growth factor beta 1 in Langerhans cell biologythe skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J. Exp. Med. 1996;184:2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl H., Riedl E., Scheinecker C., Bello-Fernandez C., Pickl W.F., Rappersberger K., Majdic O., Knapp W. TGF-beta 1 promotes in vitro development of dendritic cells from CD34+ hemopoietic progenitors. J. Immunol. 1996;157:1499–1507. [PubMed] [Google Scholar]
- Ito T., Inaba M., Inaba K., Toki J., Sogo S., Iguchi T., Adachi Y., Yamaguchi K., Amakawa R., Valladeau J. A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J. Immunol. 1999;163:1409–1419. [PubMed] [Google Scholar]
- Geissmann F., Prost C., Monnet J.P., Dy M., Brousse N., Hermine O. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J. Exp. Med. 1998;187:961–966. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Howard O.M., Chen Q., Oppenheim J.J. Cutting edgeimmature dendritic cells generated from monocytes in the presence of TGF-beta 1 express functional C-C chemokine receptor 6. J. Immunol. 1999;163:1737–1741. [PubMed] [Google Scholar]
- Caux C., Dezutter-Dambuyant C., Schmitt D., Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- Mohamadzadeh M., Takashima A., Dougherty I., Knop J., Bergstresser P.R., Cruz P.D., Jr. Ultraviolet B radiation up-regulates the expression of IL-15 in human skin. J. Immunol. 1995;155:4492–4496. [PubMed] [Google Scholar]
- Edelbaum D., Mohamadzadeh M., Bergstresser P.R., Sugamura K., Takashima A. Interleukin (IL)-15 promotes the growth of murine epidermal gamma delta T cells by a mechanism involving the beta- and gamma c-chains of the IL-2 receptor. J. Invest. Dermatol. 1995;105:837–843. doi: 10.1111/1523-1747.ep12326630. [DOI] [PubMed] [Google Scholar]
- Waldmann T.A., Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- Mohamadzadeh M., McGuire M.J., Dougherty I., Cruz P.D., Jr. Interleukin-15 expression by human endothelial cellsup-regulation by ultraviolet B and psoralen plus ultraviolet A treatment. Photodermatol. Photoimmunol. Photomed. 1996;12:17–21. doi: 10.1111/j.1600-0781.1996.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Brezinschek R.I., Mohamadzadeh M., Vita R., Lipsky P.E. Interleukin 15 is produced by endothelial cells and increases the transendothelial migration of T cells In vitro and in the SCID mouse-human rheumatoid arthritis model In vivo. J. Clin. Invest. 1998;101:1261–1272. doi: 10.1172/JCI1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Sun S., Hwang I., Tough D.F., Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- Ku C.C., Murakami M., Sakamoto A., Kappler J., Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- Kennedy M.K., Glaccum M., Brown S.N., Butz E.A., Viney J.L., Embers M., Matsuki N., Charrier K., Sedger L., Willis C.R. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodolce J.P., Boone D.L., Chai S., Swain R.E., Dassopoulos T., Trettin S., Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- Berard F., Blanco P., Davoust J., Neidhart-Berard E.M., Nouri-Shirazi M., Taquet N., Rimoldi D., Cerottini J.C., Banchereau J., Palucka A.K. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J. Exp. Med. 2000;192:1535–1544. doi: 10.1084/jem.192.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Saint-Vis B., Vincent J., Vandenabeele S., Vanbervliet B., Pin J.J., Ait-Yahia S., Patel S., Mattei M.G., Banchereau J., Zurawski S. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–336. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- Caux C., Vanbervliet B., Massacrier C., Dezutter-Dambuyant C., de Saint-Vis B., Jacquet C., Yoneda K., Imamura S., Schmitt D., Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J. Exp. Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]