Abstract
Engagement of the T cell antigen receptor (TCR) induces the transphosphorylation of the ζ chain–associated protein of 70,000 Mr (ZAP-70) protein tyrosine kinase (PTK) by the CD4/8 coreceptor associated Lck PTK. Phosphorylation of Tyr 493 within ZAP-70's activation loop results in the enzymatic activation of ZAP-70. Additional tyrosines (Tyrs) within ZAP-70 are phosphorylated that play both positive and negative regulatory roles in TCR function. Phosphorylation of Tyr residues (Tyrs 315 and 319) within the Interdomain B region of the ZAP-70 PTK plays important roles in the generation of second messengers after TCR engagement. Here, we demonstrate that phosphorylation of these two Tyr residues also play important roles in mediating the positive and negative selection of T cells in the thymus.
Keywords: protein tyrosine kinases, signal transduction, T cell activation, lymphocyte development, T cell selection
Introduction
The antigen receptor repertoire expressed on T cells is dependent on appropriate positive and negative selection of CD4+CD8+ cells in the thymus (for a review, see reference 1). These selection processes require the sequential activation of the antigen receptor–activated protein tyrosine kinases (PTKs)-Lck, ζ chain–associated protein of 70,000 Mr (ZAP-70), and IL-2–regulated tyrosine (Tyr) kinase (for a review, see reference 2). We and others have previously demonstrated that the TCR-associated ZAP-70 PTK plays essential roles for both positive and negative selection of αβ TCR-bearing thymocytes 3 4 5 6. In addition, ZAP-70 is also required for the generation of multiple signaling intermediates that include calcium and activation of small G proteins after TCR engagement 7 8 9 10 11. Hence, the efficient generation of these second messengers is required for normal thymic selection.
The NH2-terminal tandem src homology (SH)2 domains of ZAP-70 associate with the dually phosphorylated immunoreceptor Tyr-based activation motifs encoded within each of the TCR signaling polypeptides (see Fig. 1 A; references 12 13 14 15). The COOH-terminal domain of ZAP-70 encodes its Tyr kinase activity and contains positive and negative regulatory Tyr residues, Tyrs 493 and 492, respectively, that regulate ZAP-70's enzymatic activity 16 17. Interdomain B bridges the COOH-terminal SH2 and kinase domains and contains three Tyr residues (Tyrs 292, 315, and 319) that are phosphorylated after TCR engagement. Tyr 292 is a binding site for the SH2-like domain of the cbl protooncogene and serves as a negative regulator of T cell function 18 19 20 21 22 23. Phosphorylated Tyr 292 interacts with cbl, a component of the ubiquitination machinery 24 25, and hence may target phospho-Tyr292 ZAP-70 molecules for ubiquitination and degradation. Cells expressing ZAP-70(Y292F) exhibit a hyperactive phenotype in response to TCR cross-linking with enhanced transcriptional activation of nuclear factor of activated T cell (NF-AT) and IL-2–regulated promoter elements 19 20 23.
Figure 1.
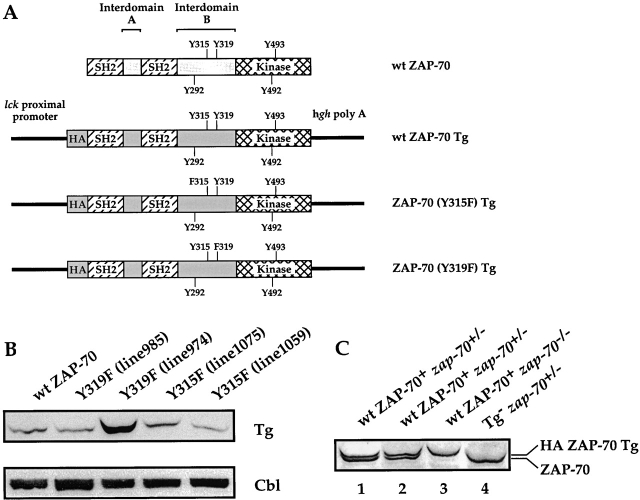
Generation and analysis of transgenic mice. (A) Schematic representation of Tgs used. The schematic structure of ZAP-70 is shown at the top. The Tyr residues that are phosphorylated after TCR engagement are depicted. The positive regulatory Tyr residues are listed above the ZAP-70 structure while the negative regulatory Tyr residues are listed below the structure. The three Tgs (wt ZAP-70, ZAP-70[Y315F], and ZAP-70[Y319F]) regulated by the lck proximal promoter and stabilized by the human growth hormone polyA tail are depicted below the ZAP-70 schematic structure. Each of the Tgs was appended with a HA epitope tag at their NH2 terminus. (B) Expression of the ZAP-70 Tgs. Cell lysates isolated from 107 thymocytes were analyzed by immunoblotting with an anti-HA mAb (top) or an anti-Cbl antiserum (bottom). Two independent lines were derived for each of the mutant ZAP-70 molecules. (C) Expression of wt ZAP-70 Tg is comparable to zap-70 +/− thymocytes. Cell lysates isolated from two representative wt ZAP-70+ zap-70 +/− (lanes 1 and 2), wt ZAP-70+ zap-70 −/ − (lane 3), and zap-70 +/− (lane 4) thymocytes were analyzed by immunoblotting with an anti–ZAP-70 antiserum.
In contrast to the negative regulatory Tyr 292, two additional Tyr residues, Tyrs 315 and 319, play positive regulatory roles in TCR signal transduction. Phosphorylated Tyr 315 has been suggested to provide a binding site for the SH2 domain of the Vav guanine nucleotide exchange factor, an activator of Rho-GTPases 26 27. Expression of a mutant ZAP-70(Y315F) in spleen Tyr kinase (Syk)-deficient DT40 cells results in attenuated B cell antigen receptor–induced NF-AT–regulated responses 27. However, expression of ZAP-70(Y315F) in a ZAP-70–deficient Jurkat T cell line (P116) has no significant effects on TCR-activated NF-AT promoter responses 28. Conversely, expression of ZAP-70(Y319F) in P116 cells results in significant attenuation of TCR-induced calcium responses, Ras activation and NF-AT transcriptional activation 28 29 30. In addition, expression of a mutant ZAP-70 in which the majority of Interdomain B has been deleted, including Tyrs 292, 315, and 319, results in enhanced B cell antigen receptor–induced NF-AT responses using the Syk-deficient DT40 cell system, as compared with wild-type (wt) ZAP-70 31. Hence, the requirement for these putative positive regulatory Tyr residues in T cell function is unclear. Since ZAP-70 is required for both the positive and negative selection processes during T cell development and as generation of second messengers are similarly important for T cell development, we addressed the roles of these two Tyr residues in TCR function during T cell development.
Materials and Methods
Generation of Transgenic Mice.
The human ZAP-70 and mutant ZAP-70 cDNAs were appended with a hemagglutinin (HA) epitope at its NH2 terminus to facilitate protein analysis. The HA-hZAP-70 cDNA was expressed under the lck proximal promoter using the p1017 vector 32. Injection of hZAP-70 transgenes (Tg) into C57BL/6 oocytes and implantation into pseudopregnant recipient mice were performed as described previously 33. Founder mice were screened for Tg expression using a probe encoding the 3′-human growth hormone gene 33. Tg+ mice were crossed with zap-70 − /− mice to generate Tg+ zap-70 +/− mice, which in turn were backcrossed with zap-70 − /− mice to generate Tg+ zap-70 − /− and Tg− zap-70 + /− mice. All mice examined were hemizygous for the ZAP-70 Tg. Genotyping for zap-70 was performed using PCR primers as described previously 3. The zap-70 − /− mice have been maintained on a C57BL/6 background for >20 generations and the Tg+ mice were maintained on a C57BL/6 background.
H-Y TCR+/H-Y TCR+ zap-70 − /− mice were generated by crossing H-Y TCR+/H-Y TCR+ mice with zap-70 − /− mice and intercrossing H-Y TCR+ zap-70 +/− mice to generate H-Y TCR+/H-Y TCR+ zap-70 − /− mice 3 34. These mice were then bred with Tg+ zap-70 +/− mice to generate H-Y TCR+ Tg+ zap-70 − /− and H-Y TCR+Tg− zap-70 +/− for analysis. All mice were analyzed within an age range of 4–6 wk.
Antibodies, Cell Analysis, and Protein Analysis.
Antibodies used for protein analysis in this study include: 12CA5, an anti-HA epitope mAb (Babco), anti-phospholipase C γ (PLCγ)1 raised against a fusion protein encoding both SH2 and the SH3 domains, anti-linker of activated T cell (LAT) antisera (Upstate Biotechnology), 4G10, an antiphophotyrosine mAb (Upstate Biotechnology), anti-pErk and Erk antisera (New England Biolabs, Inc. and Santa Cruz Biotechnology, Inc., respectively), anti–ZAP-70 antiserum 19, and an anti-Syk mAb (Upstate Biotechnology). Antibodies used for FACS® analysis were purchased from BD PharMingen. The anti-HY mAb was a gift of Dr. H.S. Teh (University of British Columbia, Vancouver, Canada).
For protein analysis, cells were lysed at 2 × 108 cells per milliliter in 10 mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40 and protease and phosphatase inhibitors as described previously (lysis buffer) 19. Lysates were clarified by centrifugation at 14,000× g for 10 min at 4°C. Cell lysates were incubated with the appropriate antiserum for 2 h at 4°C, immunoprecipitated with protein A-Sepharose (Pierce Chemical Co.) for 1 h at 4°C, washed three times with lysis buffer, and proteins resolved by SDS-PAGE. Western blot analysis was performed using enhanced chemiluminescence (Amersham Pharmacia Biotech) according to the manufacturer's recommendations. Quantitation of band intensity was performed using UN-SCAN-IT software.
Analysis of cell surface markers was performed using FACSCalibur™ (Becton Dickinson) and analyzed using CELLQuest™ software (Becton Dickinson).
T Cell Activation.
For analysis of free cytoplasmic calcium ([Ca2+]i) mobilization after TCR cross-linking, cells were incubated with the calcium-sensitive dye, Fura-2 (Molecular Probes), and analyzed by spectrofluorimetry using a FT2000 fluorimeter. In brief, cells were incubated at 37°C with a biotinylated anti-CD3ε mAb (2C11, 10 μg/ml) and cross-linked with 2.5 μg/ml avidin. Loading of cells was monitored by the ability of cells to increase [Ca2+]i in response to ionomycin. The maxima and minima to determine absolute calcium concentrations were obtained by lysing cells with Triton X-100 and quenching with EDTA, respectively.
For biochemical studies, thymocytes were suspended in complete media (2 × 108 cells per milliliter) and rested for 15 min at 37°C. Anti-CD3ε mAb (2C11, 10 μg/ml) was added to cells and incubated for the appropriate stimulation time before cell lysis as described above.
Results
Generation of ZAP-70(Y315F) and ZAP-70(Y319F) Tg Mouse Lines.
Tg encoding either ZAP-70(Y315F) or ZAP-70(Y319F) were expressed in the thymus under the control of the lck proximal promoter (Fig. 1 A; reference 32). Four independent transgenic lines were established for each mutant molecule. Based on the levels of protein expression, two lines for each mutant ZAP-70 molecule were selected for further study. To ensure that expression of ZAP-70 under a heterologous promoter did not alter T cell development, Tg mice expressing wt ZAP-70 were also generated under the control of the lck proximal promoter and bred with zap-70 − /− mice for two generations. The Tg+ zap-70 − /− mice were then compared with zap-70 +/+ and zap-70 +/− mice. All exogenous ZAP-70 molecules were linked to an epitope tag at their NH2 termini to facilitate analysis of protein expression levels. Studies in Jurkat T cells have demonstrated that this epitope tag does not interfere with ZAP-70 function (data not shown).
Immunoblot analysis of total thymocytes demonstrated comparable levels of exogenous ZAP-70 were expressed amongst the transgenic lines with the exception of line 974 which expressed the mutant ZAP-70(Y319F) at approximately fourfold higher levels of ZAP-70 as compared with the other Tgs (Fig. 1 B). In the remaining three transgenic lines, the exogenous ZAP-70 molecules were expressed at levels comparable to zap-70 +/− thymocytes (Fig. 1 C and data not shown). Consistent with the decreased transcriptional activity of the lck proximal promoter in peripheral as compared with immature T cells 32, the expression of the Tg ZAP-70 proteins was decreased in the periphery. This prohibited a valid comparison of peripheral T cell function in Tg+ zap-70 − /− mice with zap-70 +/− mice (data not shown).
T Cell Development of zap-70−/− Mice with Enforced Expression of wt ZAP-70 under the Control of the Lck Proximal Promoter Is Comparable to zap-70+/− Mice.
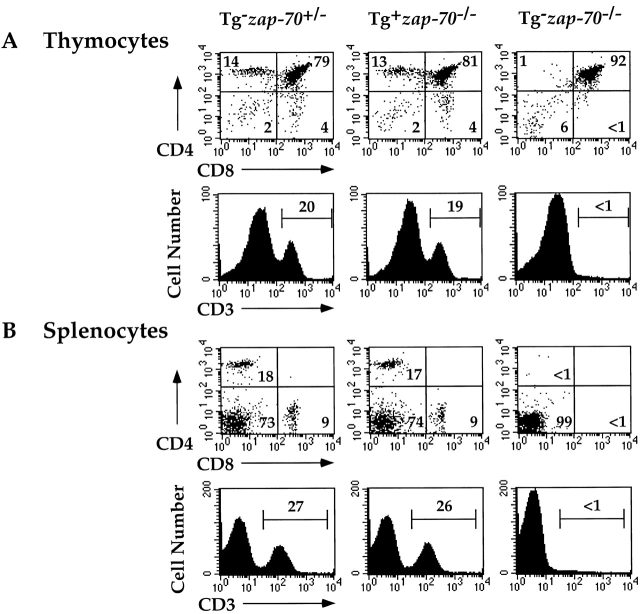
To ensure that expression of ZAP-70 under the control of the lck proximal promoter did not affect the function of ZAP-70 in T cell development, we first compared the phenotype of Tg(wt ZAP-70)+ zap-70 − /− mice with zap-70 +/− mice. While zap-70 − /− mice accumulate CD4+CD8+ thymocytes 3, expression of either the wt ZAP-70 Tg or endogenous ZAP-70 restored the development of CD4+ and CD8+ thymocytes (Fig. 2 A). Total thymocyte number and subsets (CD4−CD8−, CD4+CD8+, CD4+ and CD8+ stages) were comparable in Tg(wt ZAP-70)+ zap-70 − /− and zap-70 +/− mice (Fig. 2 A and Table ). The percentage of CD3+ T cells and the mean fluorescence intensity (MFI) for CD3 were comparable between the two groups. Similar profiles of CD4+ and CD8+ T cells were also observed in the spleen and lymph node (Fig. 2 B and data not shown).
Figure 2.
Expression of wt ZAP-70 under the control of the lck proximal promoter restores T cell development in zap-70 −/ − mice. Thymocytes (A) and splenocytes (B) from zap-70 +/−, wt ZAP-70+zap-70− / −, or zap-70 −/ − mice were analyzed by FACS® for the expression of CD4 and CD8 (top) or CD3 (bottom). The percentage of cells detected in each quadrant is listed within each quadrant or above each gate. Total cell number isolated from each genotype is represented in Table .
Table 1.
Cell Number and Subset Distribution of Thymocytes
| Percentage of cells within T cell subset | ||||||
|---|---|---|---|---|---|---|
| Genotype | Number of miceanalyzed | Cell number | CD4−CD8− | CD4+CD8+ | CD4+CD8− | CD4−CD8+ |
| n | × 10−6 | |||||
| Tg− zap-70 +/− | 35 | 330 ± 19 | 4.3 ± 0.3 | 81.2 ± 2.2 | 8.6 ± 0.4 | 3.6 ± 0.2 |
| wt ZAP-70+ zap-70 −/− | 10 | 311 ± 56 | 4.4 ± 0.5 | 80.8 ± 1.1 | 10.5 ± 0.5 | 4.2 ± 0.5 |
| ZAP-70(Y319F)+ zap-70 −/− (line 985) | 10 | 263 ± 16 | 6.0 ± 0.5 | 87.6 ±0.5 | 3.1 ± 0.2 | 3.0 ± 0.2 |
| ZAP-70(Y319F)+ zap-70 −/− (line 974) | 8 | 266 ± 40 | 5.2 ± 0.5 | 85.9 ± 0.8 | 5.7 ± 0.8 | 3.2 ± 0.2 |
| ZAP-70(Y315F)+ zap-70 −/− (line 1059) | 17 | 265 ± 34 | 3.7 ± 0.7 | 83.0 ± 0.9 | 8.6 ± 0.4 | 4.4 ± 0.3 |
| ZAP-70(Y315F)+ zap-70 −/− (line 1075) | 9 | 348 ± 45 | 3.7 ± 0.3 | 86.0 ±1.3 | 7.4 ± 0.8 | 3.1 ± 0.4 |
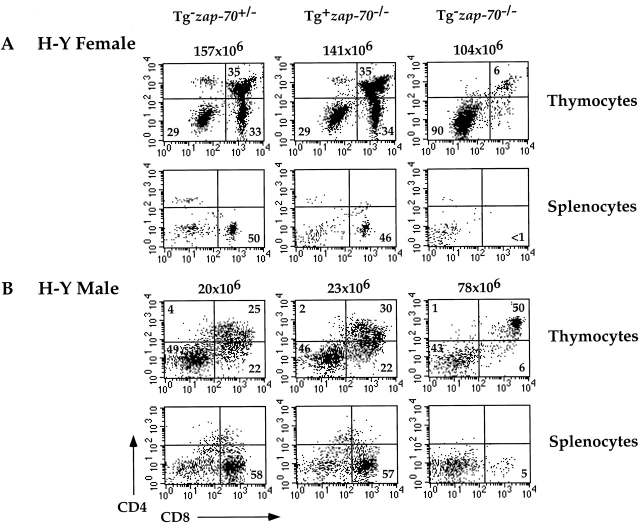
To permit analysis of mutant ZAP-70 molecules during T cell development, we generated Tg(wt ZAP-70)+ zap-70 − /− or zap-70 +/− mice expressing an H-Y transgenic TCR specific for H-Y male antigen under the H-2b selecting background 35. Positive selection of H-Y TCR+ T cells, as determined by the numbers of H-Y TCR+CD4+CD8+ and CD8+ T cells in female mice was comparable between Tg(wt ZAP-70)+ zap-70 − /− and zap-70 +/− mice (Fig. 3 A and Table ). The MFI of CD8 and CD3 was also similar between the two groups (Fig. 3 A and data not shown). Hence, positive selection of T cells was not affected by the enforced expression of wt ZAP-70 as compared with endogenous ZAP-70.
Figure 3.
Expression of wt ZAP-70 regulated by the lck proximal promoter reconstitutes both positive and negative selection of H-Y TCR+ thymocytes. (A) H-Y TCR+ thymocytes (top) and splenocytes (bottom) from zap-70 +/−, wt ZAP-70+ zap-70−/−, or zap-70 −/ − H-Y TCR+ female mice were analyzed for CD4 and CD8 expression by FACS® analysis. The number of thymocytes isolated from each mouse is depicted above each analysis. Thymocytes shown represent the H-Y TCR+ population as defined by T3.70 staining. Average thymocyte number isolated for each genotype is represented in Table . (B) H-Y TCR+ thymocytes (top) and splenocytes (bottom) from zap-70 +/−, wt ZAP-70+ zap-70−/−, or zap-70 −/ − H-Y TCR+ male mice were analyzed by FACS® analysis as described above. Thymocytes shown represent the H-Y TCR+ population as defined by T3.70 staining. Average thymocyte number isolated for each genotype is represented in Table .
Table 2.
Cell Number from H-Y TCR+ Female and Male Mice
| H-Y TCR+ Female | H-Y TCR+ Male | |||
|---|---|---|---|---|
| Genotype | Cell number | Number of mice analyzed | Cell number | Number of mice analyzed |
| × 10−6 | n | × 10−6 | n | |
| Tg− zap-70 +/+ | 185 ± 27 | 8 | 27.6 ± 3.5 | 3 |
| Tg− zap-70 +/− | 167 ± 11 | 13 | 28.2 ± 2.6 | 14 |
| Tg− zap-70 −/− | 88 ± 10 | 7 | 104 ± 16 | 5 |
| wt ZAP-70+ zap-70 −/− | 137 ± 6 | 3 | 27.7 ± 6.2 | 3 |
| ZAP-70(Y319F)+ zap-70 −/− | 213 ± 22 | 11 | 202 ±37 | 6 |
| ZAP-70(Y315F)+ zap-70 −/− | 121 ± 11 | 7 | 116 ± 17 | 3 |
Surprisingly, while zap-70 − /− mice without a Tg TCR accumulate CD4+CD8+ T cells 3, the expression of the H-Y TCR in zap-70 − /− thymocytes induced an earlier block at the CD4−CD8− stage and decreased development of H-Y TCR+CD4+CD8+ thymocytes (Fig. 3 A). This earlier block was not unique to the H-Y TCR since zap-70 − /− mice expressing the DO11.10 TCR also accumulated a greater percentage of CD4−CD8− thymocytes (data not shown). As expression of Syk was not altered in the Tg(wt ZAP-70)+ zap-70 − /− mice as compared with zap-70 +/− mice (data not shown), these differences likely reflect the earlier enforced expression of the TCR Tg and hence, signaling differences in pre- and αβ TCRs (see Discussion).
Finally, we examined the ability of the Tg(wt ZAP-70) to mediate the deletion of H-Y TCR+ T cells in male mice. Consistent with the requirement for ZAP-70 in negative selection, H-Y TCR+ zap-70 − /− male mice demonstrated increased thymocyte number and accumulated H-Y TCR+CD4+CD8+ thymocytes (Table and Fig. 3 B). However, no H-Y TCR+CD8+ T cells were observed in the thymus or periphery. Reconstitution of zap-70 − /− mice with the Tg(wt ZAP-70) restored the ability of the H-Y TCR+ T cells to undergo negative selection. H-Y TCR+ T cells in zap-70 +/− male mice were deleted to a comparable extent as that observed in Tg(wt ZAP-70)+ zap-70 − /− male mice (Fig. 3 B and Table ). Hence, transgenic expression of wt ZAP-70 in zap-70 − /− mice does not compromise either positive or negative selection processes.
Analysis of Thymocyte Development in Mice Expressing ZAP-70(Y315F) or ZAP-70(Y319F).
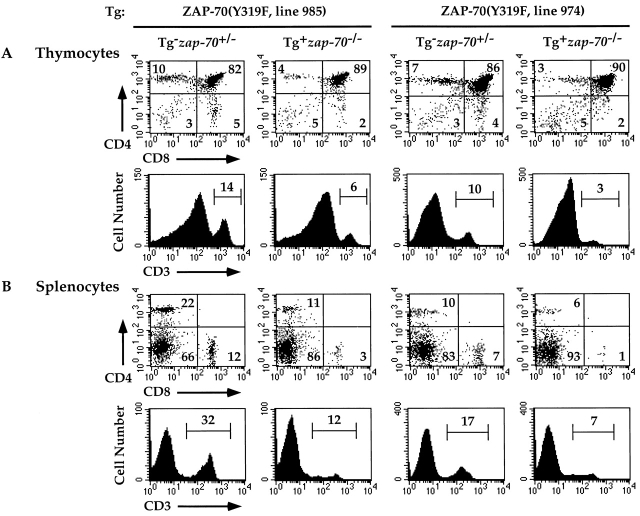
Since zap-70 − /− mice expressing Tg(wt ZAP-70) under the lck proximal promoter reconstituted the ability of cells to undergo positive and negative selection, we next analyzed the ability of mutant ZAP-70 molecules that cannot be phosphorylated on Tyrs 315 or 319 to reconstitute T cell development in zap-70 − /− mice. Using a similar strategy as that employed for Tg(wt ZAP-70), we analyzed two independent lines for mice expressing ZAP-70(Y319F) (lines 985 and 974) or ZAP-70(Y315F) (lines 1059 and 1075). Thymocytes harvested from zap-70 − /− mice expressing the ZAP-70(Y319F) Tg demonstrated an ∼50% decrease in CD4+ T cells (Fig. 4 A and Table ). A small decrease in CD8+ T cells was observed, though the difference was not statistically significant. However, a decrease in both CD4+ and CD8+ T cells was observed in the periphery of mice expressing ZAP-70(Y319F) (Fig. 4 B). Hence, mice expressing ZAP-70(Y319F) demonstrate compromised development of mature single positive T cells as compared with zap-70 +/− or Tg(wt ZAP-70)+ zap-70 − /− mice. The differences in the level of ZAP-70(Y319F) expression amongst the two transgenic lines did not affect the extent of the T cell developmental defect. Hence, the fourfold overexpression of ZAP-70(Y319F) observed in line 974 did not rescue this developmental defect.
Figure 4.
Attenuated development of T cells in zap-70 −/ − mice expressing ZAP-70(Y319F). Thymocytes (A) and splenocytes (B) isolated from ZAP-70(Y319F)+ zap-70 −/ − mice were analyzed for CD4 and CD8 (top) and CD3 (bottom) expression. Representative profiles of two independent transgenic lines (985 and 974) are shown. Average thymocyte number isolated for each genotype is represented in Table .
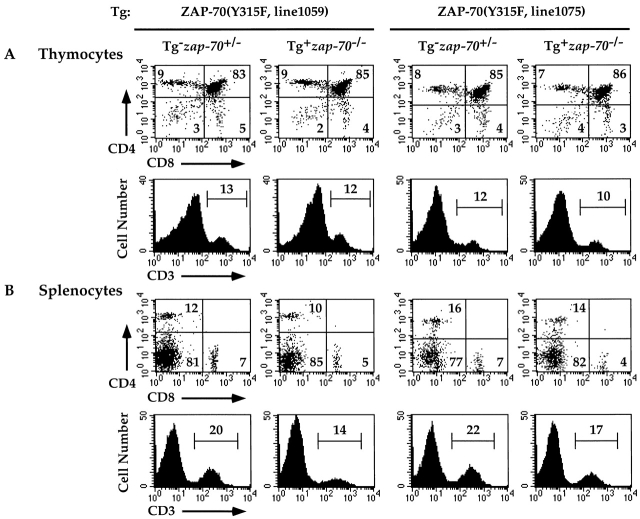
In contrast to the developmental abnormalities observed in mice expressing the ZAP-70(Y319F) Tg, mice expressing the ZAP-70(Y315F) Tg demonstrated comparable T cell numbers and T cell subsets as zap-70 +/− mice (Fig. 5 A and Table ). The percentage of CD3+ T cells and the MFI for CD3 expression were comparable between ZAP-70(Y315F)+ zap-70 − /−, wt ZAP-70+ zap-70 − /−, and zap-70 +/− mice. These cells were similarly able to populate peripheral lymphoid organs as comparable numbers of CD4+ and CD8+ T cells were detected in the spleen and lymph node (Fig. 5 B and data not shown).
Figure 5.
Normal development of T cells in zap-70 −/ − mice expressing ZAP-70(Y315F). Thymocytes (A) and splenocytes (B) isolated from ZAP-70(Y315F)+ zap-70 −/ − mice were analyzed for CD4 and CD8 (top) and CD3 (bottom) expression. Representative profiles of two independent transgenic lines (1059 and 1075) are shown. Average thymocyte number isolated for each genotype is represented in Table .
Analysis of Mice Expressing Transgenic TCRs and Mutant ZAP-70 Molecules.
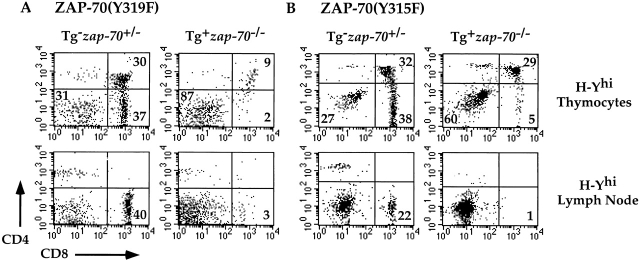
We then analyzed the effects of the two Tyr residues in mice expressing the H-Y transgenic TCR. While ZAP-70(Y319F)+ zap-70 − /− mice demonstrated a moderate compromise in the development of CD4+ T cells as compared with zap-70 +/− mice (Fig. 4), mice expressing ZAP-70(Y319F) when bred upon the H-Y TCR background demonstrated a more substantial block. The development of CD8+ H-Y TCR+ thymocytes was compromised in ZAP-70(Y319F)+ zap-70 − /− female mice bearing the H-Y TCR (Fig. 6 A). This decrease did not reflect increased emigration of cells from the thymus into the periphery since the peripheral T cell compartment was also devoid of these H-Y TCR+ T cells (Fig. 6 A, bottom, and data not shown). Similar data were obtained for both transgenic lines expressing ZAP-70(Y319F) (data not shown). Hence, Tyr 319 is critical in the positive selection of the H-Y TCR+–bearing thymocytes.
Figure 6.
Attenuated positive selection of H-Y TCR+ T cells in zap-70 −/ − mice expressing ZAP-70(Y319F) or ZAP-70(Y315F). H-Y TCR+ thymi (top) or lymph nodes (bottom) from ZAP-70(Y319F)+ zap-70 −/ − (A) or ZAP-70(Y315F)+ zap-70 −/ − (B) female mice were analyzed for CD4 and CD8 expression. Cells shown represent the H-Y TCR+ population as defined by T3.70 staining. Average thymocyte number isolated for each genotype is represented in Table .
While zap-70 − /− mice expressing the ZAP-70(Y315F) Tg did not demonstrate any significant developmental defects on a non-TCR transgenic background, these mice exhibited a significant block in development when bred upon the H-Y TCR background. Development of CD8+ H-Y TCR+ thymocytes was compromised in ZAP-70(Y315F)+ zap-70 − /− female mice bearing the H-Y TCR (Fig. 6 B). The decrease in CD8+ H-Y TCR+ thymocytes was not due to increased emigration to the periphery as there was a paucity of CD8+ H-Y TCR+ T cells found in the spleen or lymph node (Fig. 6 B and data not shown). Similar data were obtained for both transgenic lines expressing ZAP-70(Y315F) (data not shown). Hence, Tyr 315 also appears critical for the positive selection of H-Y TCR+ thymocytes, though the degree of compromise appeared less severe than the H-Y TCR+ ZAP-70(Y319F)+ zap-70 − /− female mice. This lesser developmental block may reflect the more mild signaling defects observed in the ZAP-70(Y315F)+ zap-70 − /− mice (see below).
Requirement for Tyrs 315 and 319 in Negative Selection.
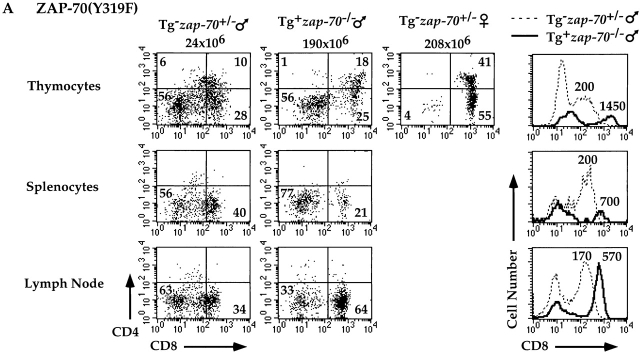
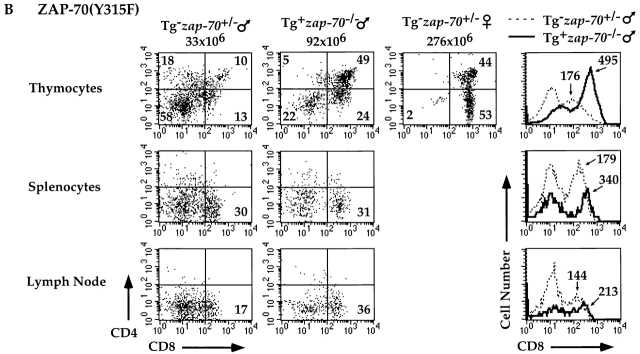
Analysis of H-Y TCR+ male mice expressing either Y315F or Y319F mutants permitted us to further assess the requirements of these two Tyr residues in the elimination of T cells engaging self-antigen. While H-Y TCR+ zap-70 +/− and wt ZAP-70+ zap-70 − /− male mice demonstrated deletion of H-Y TCR+ thymocytes (Fig. 3 and Table ), H-Y TCR+ male mice expressing ZAP-70(Y319F) accumulated approximately sixfold greater numbers of thymocytes (Table ). These mutant male mice had a greater percentage and number of H-Y TCR+ CD4+CD8+ thymocytes as well as H-Y TCR+ CD8+ peripheral T cells as compared with zap-70 +/− male mice (Fig. 7 A). In addition, the CD8 MFI in the ZAP-70(Y319F)+ zap-70 − /− male mice was higher than the zap-70 +/− male controls. Analysis of the H-Y TCR+ ZAP-70(Y315F)+ zap-70 − /− male mice also demonstrated a requirement for Tyr 315 in negative selection. H-Y TCR+ male mice expressing ZAP-70(Y315F) accumulated approximately fourfold greater numbers of thymocytes as compared with H-Y TCR+ zap-70 +/− male mice (Table ). These mutant mice also expressed a greater percentage and number of both H-Y TCR+ CD4+CD8+ T cells as compared with zap-70 +/− mice (Fig. 7 B). Interestingly, there was significantly less development of H-Y TCR+CD4−CD8+ thymocytes in male mice expressing ZAP-70(Y315F)+ as compared with ZAP-70(Y319F)+ zap-70 − /− mice. Hence, the degree of compromise observed for ZAP-70(Y319F) was again more profound than for ZAP-70(Y315F). The CD8 MFI of these ZAP-70(Y315F)+ zap-70 − /− cells was also higher than the H-Y TCR+ CD8+ zap-70 +/− male mice though the difference was less marked as those found in mice expressing ZAP-70(Y319F). Together, these data indicate that both Tyrs 315 and 319 are important for both positive and negative selection of H-Y TCR+ thymocytes.
Figure 7.
Attenuated negative selection of H-Y TCR+ T cells in zap-70 −/ − mice expressing ZAP-70(Y319F) or ZAP-70(Y315F). (A) H-Y TCR+ thymi (top), splenocytes (center), or lymph nodes (bottom) were analyzed for CD4 and CD8 expression. A representative FACS® profile from an H-Y TCR+ zap-70 +/ − male mouse (left) and ZAP-70(Y319F)+ zap-70 −/ − male mouse (second from left) is shown. A representative profile of an H-Y TCR+ zap-70 +/− female mouse (third from left) is also depicted for comparison. Cells shown represent the H-Y TCR+ population as defined by T3.70 staining. A histogram for CD8 expression of H-Y TCR+ thymocytes is shown on the right. The dotted line denotes the H-Y TCR+ zap-70 +/− male mouse while the solid line denotes the H-Y TCR+ZAP-70(Y319F)+ zap-70 −/ − mouse. Quantitation of the MFI of the right peak for each mouse is depicted above the peak. (B) H-Y TCR+ thymi (top), splenocytes (center), or lymph nodes (bottom) were analyzed for CD4 and CD8 expression as described above. The mode of presentation is the same as in A except the Tg represents the ZAP-70(Y315F) mutant molecule.
Defects in [Ca2+]i Signaling in ZAP-70(Y315F) and ZAP-70(Y319F) Thymocytes.
Since ZAP-70 is required for the increases in [Ca2+]i after TCR cross-linking, we analyzed the TCR induced increase in [Ca2+] i in thymocytes isolated from zap-70 +/−, Tg(wt ZAP-70)+ zap-70 − /−, ZAP-70(Y319F)+ zap-70 − /−, or ZAP-70(Y315F)+ zap-70 − /− mice. While thymocytes isolated from zap-70 +/− or Tg (wt ZAP-70)+ zap-70 − /− mice demonstrated comparable increases in [Ca2+]i, thymocytes isolated from ZAP-70(Y319F)+ zap-70 − /− mice demonstrated a significant attenuation in TCR-induced increase in [Ca2+]i (Fig. 8 A). The attenuation in [Ca2+]i mobilization was observed not only in the total thymocyte population, but also in purified CD4+CD8+ and CD4+ thymocytes (Fig. 8B and Fig. C). In contrast, thymocytes isolated from mice expressing ZAP-70(Y315F)+ zap-70 − /− demonstrated a small, but reproducible, attenuation in TCR-induced increase in [Ca2+] i (Fig. 8 A). This difference was not observed in the less responsive CD4+CD8+ thymocytes, but was more accentuated in the CD4+ thymocyte population (Fig. 8B and Fig. C). Consistent with the requirement for PLCγ activation in Erk activation 36 37, Erk activation was slightly attenuated (25% reduction) in both zap-70 − /− thymocytes expressing either ZAP-70(Y315F) or ZAP-70(Y319F) (Fig. 8 D). Hence, phosphorylation of Tyrs 315 and 319 contribute to the generation of second messengers after TCR cross-linking.
Figure 8.
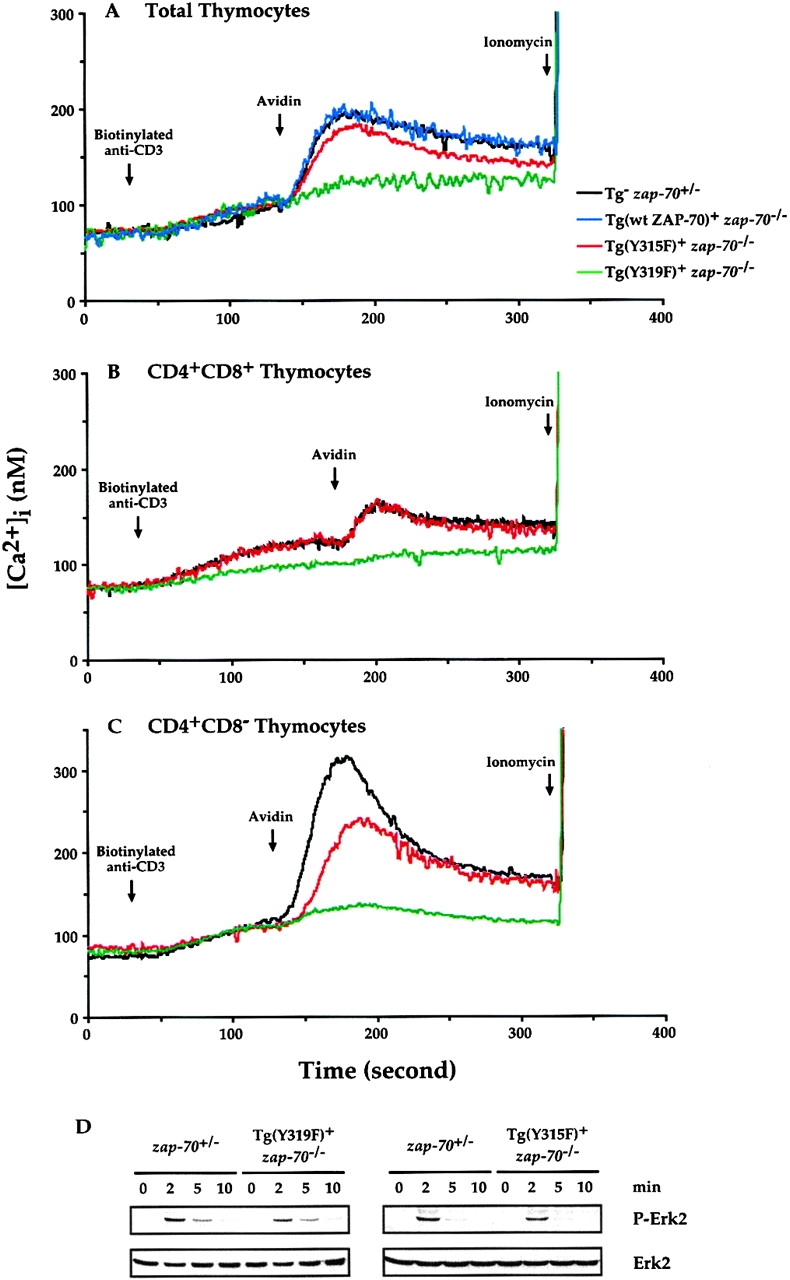
Signaling defects in ZAP-70(Y319F)+ zap-70 −/ − and ZAP-70(Y315F)+ zap-70 −/ − thymocytes. Increases in [Ca2+]i after TCR engagement from zap-70 +/−, (wt ZAP-70)+ zap-70 −/ −, ZAP-70(Y315F)+ zap-70 −/ −, or ZAP-70(Y319F)+ zap-70 −/ − T cells were measured as described in Materials and Methods. The arrows denote the additions of biotinylated anti-CD3ε mAb followed by cross-linking with avidin. Increases in [Ca2+]i were measured for total thymocytes (A), purified CD4+CD8+ thymocytes (B) as well as for CD4+ thymocytes (C). (D) Erk activation was measured by immunoblot analysis of cell lysates using an anti-pErk antibody (top). Equal loading of cell lysates was monitored by immunoblotting with an anti-total Erk antibody (bottom). These measurements were representative of a minimum of three independent experiments. Similar results were obtained independent of the method for isolation of the thymocyte subsets.
Discussion
The ZAP-70 PTK has been demonstrated to be required for the positive and negative selection of thymocytes as well as for the efficient activation of multiple signaling pathways in T cells 3 4 6. In this study, we generated zap-70 − /− mice expressing mutant ZAP-70(Y315F) or ZAP-70(Y319F) Tg that permitted us to examine the roles of two Interdomain B containing–Tyr residues in mediating T cell development. ZAP-70(Y319F)+ zap-70 − /− mice demonstrated compromised development of CD4+ thymocytes and CD4+ and CD8+ T cells in peripheral lymphoid organs (Fig. 4 and Table ). In contrast, no significant defects were observed in zap-70 − /− mice expressing the ZAP-70(Y315F) Tg (Fig. 5 and Table ). While the levels of protein expression achieved in the transgenic lines were selected to closely resemble the endogenous level, we cannot exclude potential positional effects of the Tgs. However, two independent lines of each mutant ZAP-70 molecule provided similar results and the analysis of the ZAP-70(Y315F)+ zap-70 − /− mice is similar to the phenotype generated using a knockin approach 38. Moreover, the developmental defect observed in ZAP-70(Y319F)+ zap-70 − /− mice was not restored even with fourfold overexpression of the ZAP-70(Y319F) mutant protein as achieved in the transgenic line 974. Together, these data indicate a hierarchy in the requirements for these Tyr residues in mediating T cell development.
While T cells developed in the absence of either Tyr 315 or 319, the affinities of the receptors in T cells of mice expressing ZAP-70(Y315F) or ZAP-70(Y319F) may be altered to compensate for potential signaling deficiencies activated by these mutant ZAP-70 molecules. To address this possibility, we analyzed further the roles of these two Tyr residues with a fixed affinity transgenic TCR. Using the H-Y TCR transgenic system, we analyzed H-Y TCR+ zap-70 − /− mice and H-Y TCR+ zap-70 − /− mice encoding the two mutant ZAP-70 molecules.
Surprisingly, while zap-70 − /− mice develop and accumulate TCRloCD4+CD8+ thymocytes, the enforced expression of the H-Y TCR substantially compromised the development of the H-Y TCR+ CD4+CD8+ population in female mice (Fig. 3 A). This earlier developmental block was not unique to the H-Y TCR transgenic system as a similar block was also observed in zap-70 − /− mice bearing the DO11.10 TCR specific for OVA (data not shown). While CD4−CD8− thymocytes can utilize either ZAP-70 or Syk in mediating the function of the αβ TCR 6 39, the earlier expression of an H-Y αβ TCR Tg likely results in an earlier downregulation of the Syk PTK and hence a greater dependence on ZAP-70 40. As expression of the H-Y αβ TCR results in accelerated development of CD25−CD44−CD4−CD8− thymocytes (DN4 stage) with a paucity of CD25+CD44−CD4−CD8− thymocytes (DN3 stage), we were unable to determine whether the H-Y TCR induces an earlier decrease in the level of Syk expression at the DN3 to DN4 transition (data not shown). However, consistent with the idea that the accelerated expression of the H-Y TCR is more dependent on ZAP-70, H-Y TCR–bearing thymocytes expressing a Syk Tg are unable to mature into CD4+CD8+ thymocytes (unpublished data). These data implicate potential signaling differences in the ZAP-70 and Syk kinases in shaping the antigen receptor repertoire, and may account for the earlier block in thymocyte development observed in H-Y TCR+ zap-70 − /− female mice.
Consistent with the requirement for ZAP-70 in the in vitro deletion of DO11.10 TCR+ zap-70 − /− thymocytes 3, H-Y TCR+ zap-70 − /− male mice demonstrate compromised negative selection of H-Y TCR+ thymocytes (Fig. 3 B and Table ). H-Y TCR+ zap-70 − /− male mice also demonstrate a threefold increase in total thymocyte number and accumulate H-Y TCR+CD4+CD8+ thymocytes. However, these cells do not mature further to CD8+ thymocytes or peripheral T cells.
Using this H-Y TCR+ zap-70 − /− transgenic system, analysis of mice expressing either mutant ZAP-70 molecule revealed a range of developmental defects. While non-TCR transgenic mice demonstrate a twofold decrease in the development of CD4+ thymocytes and in peripheral T cells, H-Y TCR+ZAP-70(Y319F)+ zap-70 − /− female mice exhibit a more substantial block that was qualitatively similar to the developmental block observed in H-Y TCR+ zap-70 − /− mice (Fig. 3 A and 6 A). Conversely, non-TCR Tg ZAP-70(Y315F)+ zap-70 − /− mice demonstrate no significant thymic developmental defects. However, H-Y TCR+ZAP-70(Y315F)+ zap-70 − /− female mice demonstrate compromised development of H-Y TCR+ CD8+ thymocytes and, correspondingly, do not develop H-Y TCR+CD8+ peripheral T cells (Fig. 6).
Similar to the range of defects in the positive selection of H-Y TCR+ T cells observed in H-Y TCR+ female mice, these differences are paralleled in their H-Y TCR+ male counterparts. H-Y TCR+ zap-70 − /− mice expressing either ZAP-70(Y319F) or ZAP-70(Y315F) Tgs accumulate six and fourfold, respectively, greater thymocyte number as compared with H-Y TCR+ zap-70+/- or H-Y TCR+ wt ZAP-70+ zap-70 − /− male mice (Table ). In addition, the MFIs for CD4 and CD8 expression follow a hierarchy that paralleled the efficiency of selection in the male mice: ZAP-70(Y319F)+ zap-70 − /− > ZAP-70(Y315F)+ zap-70 − /− > zap-70 +/− thymocytes. These differences further support the notion that the ZAP-70(Y319F)+ zap-70 − /− and to a lesser degree ZAP-70(Y315F)+ zap-70 − /− thymocytes are unable to undergo efficient deletion.
Finally, the biochemical defects observed in the ZAP-70(Y319F)-expressing thymocytes is consistent with the analysis of Jurkat T cells expressing ZAP-70(Y319F). While TCR activation appears to be unaltered, Tyr phosphorylation of PLCγ1 and LAT is attenuated 28. In turn, these thymocytes demonstrate defects in [Ca2+]i mobilization and Erk activation (Fig. 8). Additionally, the ability of these thymocytes to upregulate CD69 in vitro in response to anti-CD3ε cross-linking was also compromised (data not shown). Hence, phosphorylated 319 through its potential interactions with Lck and PLCγ1 may contribute to the assembly and stability of macromolecular signaling complexes required for efficient and sustained generation of second messengers 29 30 28.
Minimal biochemical defects were observed in ZAP-70(Y315F)+ zap-70 − /− thymocytes. Tyr phosphorylation of cellular proteins, including PLCγ1, LAT, SH2 domain–containing leukocyte protein of 76,000 Mr, and Vav, in ZAP-70(Y315F)+ zap-70 − /− thymocytes was minimally decreased as compared with zap-70 +/− thymocytes (data not shown). One functional defect observed in these thymocytes was a slight, but reproducible, decrease in [Ca2+]i in total thymocytes with a slightly more substantial decrease in CD4+CD8− thymocytes. This defect is reminiscent of the signaling differences observed in fyn − /− mice in which CD4+ thymocytes demonstrated greater [Ca2+]i signaling defects as compared with CD4+CD8+ thymocytes 41. In addition, a slight attenuation in TCR-induced Erk activation was observed in ZAP-70(Y315F)+ zap-70 − /− thymocytes. Differences in the stability of the ZAP-70(Y315F)–TCR complex have been observed (reference 38 and data not shown). This compromised stability of ZAP-70(Y315F)–TCR complexes and, hence, decreased longevity of the activated ZAP-70 kinase may provide a biochemical basis for these functional and developmental abnormalities.
While the biochemical defects observed in ZAP-70(Y315F)+ zap-70 − /− and ZAP-70(Y319F)+ zap-70 − /− thymocytes are mild to moderate and while only a moderate degree of compromise in T cell development was observed in ZAP-70(Y319F)+ zap-70 − /− mice, the expression of a Tg TCR resulted in severe compromise in both the positive and negative selection of H-Y TCR+ thymocytes. While it is possible that these alterations may be unique to the H-Y TCR Tg system, it is more likely that the enforced expression of a low affinity TCR magnifies more subtle and prolonged signaling defects that would otherwise be compromised by alterations receptor affinity or repertoire. As the affinity of the H-Y TCR for H-Y antigen is still higher than for a non-Tg TCR for its cognate ligand, it is likely that mutations in these two Interdomain B Tyr residues may result in attenuated signaling and shift the repertoire of receptors such that self-reactive antigen receptors may not be efficiently eliminated and contribute to autoimmunity.
Acknowledgments
The authors thank Dr. H.S Teh (University of British Columbia) for the gift of the anticlonotypic H-Y TCR mAb, Dr. R. Perlmutter for the p1017 vector, and Drs. Bernard and Marie Malissen for communicating their unpublished data.
A grant from the National Institutes of Health (NIH, CA74881) provided partial support for this work. A.C. Chan is an Associate Investigator of the Howard Hughes Medical Institute. J.B. Wardenburg is supported by the NIH (5T32GM07200).
Footnotes
Abbreviations used in this paper: HA, hemagglutinin; LAT, linker of activated T cell; MFI, mean fluorescence intensity; NF-AT, nuclear factor of activated T cell; PLCγ, phospholipase C γ; PTK, protein Tyr kinase; SH, src homology; Syk, spleen Tyr kinase; Tg, transgene; Tyr, tyrosine; wt, wild-type; ZAP-70, ζ chain–associated protein of 70,000 Mr.
References
- Goldrath A., Bevan M. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- Cheng A.M., Chan A.C. Protein tyrosine kinases in thymocyte development. Curr. Opin. Immunol. 1997;8:394–401. doi: 10.1016/s0952-7915(97)80106-9. [DOI] [PubMed] [Google Scholar]
- Negishi I., Motoyama N., Nakayama K.-I., Nakayama K., Senju S., Hatakeyama S., Zhang Q., Chan A.C., Loh D.Y. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- Wiest D.L., Ashe J.M., Howcraft K., Lee H.-M., Kemper D.M., Negishi I., Singer A., Abe R. A spontaneously arising mutation in the DLAARN motif of murine ZAP-70 abrogates kinase activity and arrests thymocyte development. Immunity. 1997;6:663–671. doi: 10.1016/s1074-7613(00)80442-2. [DOI] [PubMed] [Google Scholar]
- Kadlecek T., van Oers N., Lefrancois L., Olson S., Finlay D., Chu D., Connolly K., Killeen N., Weiss A. Differential requirements for ZAP-70 in TCR signaling and T cell development. J. Immunol. 1998;161:4688–4694. [PubMed] [Google Scholar]
- Sugawara T., DiBartolo V., Miyazaki T., Nakauchi H., Acuto O., Takahama Y. An improved retroviral gene transfer technique demonstrates inhibition of CD4−CD8− thymocyte development by kinase-inactive ZAP-70. J. Immunol. 1998;161:2888–2894. [PubMed] [Google Scholar]
- Arpaia E., Shahar M., Dadi H., Cohen A., Roifman C. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking ZAP-70 kinase. Cell. 1994;76:947–958. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- Chan A., Kadlecek T., Elder M., Filipovich A., Grey J., Iwashima M., Parslow T., Weiss A. ZAP-70 protein tyrosine kinase deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264:1599–1601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- Elder M., Lin D., Clever J., Chan A., Hope T., Weiss A., Parslow T. Human severe combined immunodeficiency due to a defect in ZAP-70, a T-cell receptor-associated tyrosine kinase. Science. 1994;264:1596–1599. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- Gelfand E.W., Weiberg K., Mazer B.D., Kadlecek T.A., Weiss A. Absence of ZAP-70 prevents signaling through the antigen receptor on peripheral blood T cells but not on thymocytes. J. Exp. Med. 1995;182:1057–1065. doi: 10.1084/jem.182.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D., Mollenauer M.N., Weiss A. Dominant-negative ζ-associated protein 70 inhibits T cell antigen receptor signaling. J. Exp. Med. 1996;183:611–620. doi: 10.1084/jem.183.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wange R.L., Malek S.N., Desiderio S., Samelson L.E. The tandem SH2 domains of ZAP-70 bind to TCR ζ and CD3ε from activated Jurkat T cells. J. Biol. Chem. 1993;268:19757–19801. [PubMed] [Google Scholar]
- Iwashima M., Irving B., van Oers N., Chan A., Weiss A. The sequential interaction of two cytoplasmic protein tyrosine kinases in T cell antigen receptor signaling. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- Hatada M.H., Lu X., Laird E.R., Green J., Morgenstern J.P., Lous M., Marr C.S., Phillips T.B., Ram M.K., Theriault K. Molecular basis for interaction of the protein tyrosine kinase ZAP-70 with the T-cell receptor. Nature. 1995;377:32–38. doi: 10.1038/377032a0. [DOI] [PubMed] [Google Scholar]
- Isakov N., Wange R.L., Burgess W.H., Watts J.D., Aebersold R., Samelson L.E. ZAP-70 binding specificity to T cell receptor tyrosine-based activation motifsthe tandem SH2 domains of ZAP-70 bind distinct tyrosine-based activation motifs with varying affinity. J. Exp. Med. 1995;181:375–380. doi: 10.1084/jem.181.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A.C., Dalton M., Johnson R., Kong G.-H., Wang T., Thoma R., Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14:2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wange R.L., Guitian R., Isakov N., Watts J.D., Aebersold R., Samelson L.E. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J. Biol. Chem. 1995;270:18730–18733. doi: 10.1074/jbc.270.32.18730. [DOI] [PubMed] [Google Scholar]
- Fournel M., Davidson D., Weil R., Veillette A. Association of tyrosine protein kinase Zap-70 with the protooncogene product p120 c-cbl in T lymphocytes. J. Exp. Med. 1996;183:301–306. doi: 10.1084/jem.183.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G., Dalton M., Wardenburg J., Straus D., Kurosaki T., Chan A. Distinct tyrosine phosphorylation sites within ZAP-70 mediate activation and negative regulation of antigen receptor function. Mol. Cell. Biol. 1996;16:5026–5035. doi: 10.1128/mcb.16.9.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Weiss A. Enhancement of lymphocyte responsiveness by a gain-of-function mutation of ZAP-70. Mol. Cell. Biol. 1996;16:6765–6774. doi: 10.1128/mcb.16.12.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupher M., Songyang Z., Shoelson S., Cantley L., Band H. The cbl phosphotyrosine-binding domain selects a D(N/D)XpY motif and binds to the Tyr 292 negative regulatory phosphorylation site of ZAP-70. J. Biol. Chem. 1997;272:33140–33144. doi: 10.1074/jbc.272.52.33140. [DOI] [PubMed] [Google Scholar]
- Meng W., Sawasdikosol S., Burakoff S.J., Eck M.J. Structure of the amino-terminal domain of Cbl complexed to its binding site on ZAP-70 kinase. Nature. 1999;398:84–90. doi: 10.1038/18050. [DOI] [PubMed] [Google Scholar]
- Rao N., Lupher M.L.J., Ota S., Reedquist K.A., Druker B.J., Band H. The linker phosphorylation site Tyr 292 mediates the negative regulatory effect of Cbl on ZAP-70 in T cells. J. Immunol. 2000;164:4616–4626. doi: 10.4049/jimmunol.164.9.4616. [DOI] [PubMed] [Google Scholar]
- Joazeirro C., Wing S., Huang H., Leverson J., Hunter T., Liu Y. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- Levkowitz G., Waterman H., Etternberg S., Katz M., Tsygankov A., Ahoy I., Lavi S., Iwai K., Reiss Y., Ciechanover A. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- Katzav S., Sutherland M., Packham G., Yi T., Weiss A. The protein tyrosine kinase ZAP-70 can associate with the SH2 domain of proto-Vav. J. Biol. Chem. 1994;269:32579–32585. [PubMed] [Google Scholar]
- Wu J., Zhao Q., Kurosaki T., Weiss A. The Vav binding site (Y315) in ZAP-70 is critical for antigen receptor-mediated signal transduction. J. Exp. Med. 1997;185:1877–1882. doi: 10.1084/jem.185.10.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B., Irvin B., Sutor S., Chini C., Yacyshyn Y., Bubeck Wardenburg J., Dalton M., Chan A., Abraham R. Phosphorylation of Tyr 319 in ZAP-70 is required for T-cell antigen receptor phospholipase C-γ1 and ras activation. EMBO J. 1999;18:1832–1844. doi: 10.1093/emboj/18.7.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bartolo V., Mege D., Germain V., Pelosi M., Dufour E., Michel F., Magistrelli G., Isacchi A., Acuto O. Tyrosine 319, a newly identified phosphorylation site of ZAP-70, plays a critical role in T cell antigen receptor signaling. J. Biol. Chem. 1999;274:6285–6294. doi: 10.1074/jbc.274.10.6285. [DOI] [PubMed] [Google Scholar]
- Pelose M., Di Bartolo V., Mounier V., Mege D., Pascussi J., Dufour E., Blondel A., Acuto O. Tyrosine 319 in the Interdomain B of ZAP-70 is a binding site for the Src homology 2 domain of Lck. J. Biol. Chem. 1999;274:14229–14237. doi: 10.1074/jbc.274.20.14229. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Williams B., Abraham R., Weiss A. Interdomain B in ZAP-70 regulates but is not required for ZAP-70 signaling function in lymphocytes. Mol. Cell. Biol. 1999;19:948–956. doi: 10.1128/mcb.19.1.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J., Forbush K., Perlmutter R. Functional dissection of the lck proximal promoter. Mol. Cell. Biol. 1992;12:2758–2768. doi: 10.1128/mcb.12.6.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q., White L., Johnson R., White M., Negishi I., Thomas M., Chan A.C. Restoration of thymocyte development and function in zap-70−/− mice by the Syk protein tyrosine kinase. Immunity. 1997;7:369–378. doi: 10.1016/s1074-7613(00)80358-1. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Teh H.S., Bluthmann H., von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Bluthmann H., Staerz U.D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Dower N., Stang S., Bottorff D., Ebinu J., Dickie P., Ostergaard H., Stone J. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat. Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- Ebinu J., Stang S., Teixeira C., Bottorff D., Hooton J., Blumberg P., Barry M., Bleakley R., Ostergaard H., Stone J. RasGRP links T-cell receptor signaling to Ras. Blood. 2000;95:3199–3203. [PubMed] [Google Scholar]
- Magnan A., Di Bartolo V., Pichonnat A.-M., Boyer C., Richelme M., Lin Y.-L., Roure A., Gillet A., Arrieumerlou C., Trautmann A. T cell development and T cell responses in mice with mutations affecting tyrosines 292 or 315 of the ZAP-70 protein tyrosine kinase. J. Exp. Med. 2001;194:491–505. doi: 10.1084/jem.194.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A.M., Negishi I., Anderson S.J., Chan A.C., Bolen J., Loh D.Y., Pawson T. Arrested development of double negative thymocytes in mice lacking both the Syk and ZAP-70 tyrosine kinases. Proc. Natl. Acad. Sci. USA. 1997;94:9797–9801. doi: 10.1073/pnas.94.18.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D., van Oers N., Malissen M., Harris J., Elder M., Weiss A. Pre-T cell receptor signals are responsible for the down-regulation of Syk protein tyrosine kinase expression. J. Immunol. 1999;163:2610–2620. [PubMed] [Google Scholar]
- Appleby M.W., Gross J.A., Cooke M.P., Levin S.D., Qian X., Perlmutter R.M. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992;70:751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]