Abstract
We present evidence for continuous generation of neurons, oligodendrocytes, and astrocytes in the hippocampal dentate gyrus of adult macaque monkeys, using immunohistochemical double labeling for bromodeoxyuridine and cell-type-specific markers. We estimate that the relative rate of neurogenesis is approximately 10 times less than that reported in the adult rodent dentate gyrus. Nevertheless, the generation of these three cell types in a discreet brain region suggests that a multipotent neural stem cell may be retained in the adult primate hippocampus. This demonstration of adult neurogenesis in nonhuman Old World primates—with their phylogenetic proximity to humans, long life spans, and elaborate cognitive abilities—establishes the macaque as an unexcelled animal model to experimentally investigate issues of neurogenesis in humans and offers new insights into its significance in the adult brain.
Unlike cells in most tissues, which undergo generation and replacement throughout life (1), most neurons of the mammalian brain are entirely generated during early development—either before birth or shortly thereafter—and are not replaced if lost (2, 3). One exception, which was first suspected in rodents 30 years ago (4) and later substantiated (5), is the granule neurons of the dentate gyrus of the hippocampus. These neurons continue to be generated well into adulthood, and their production and survival depend on both genetic (6) and environmental factors (7–11). Evidence has accumulated for neurogenesis in the adult dentate gyrus in other mammals, including a New World monkey, the marmoset (12, 13). Most recently, newly generated cells with neuronal features have been detected in the dentate gyrus of humans, in autopsy material of patients exposed to bromodeoxyuridine (BrdU), a DNA marker, at advanced age (14).
The finding of neurogenesis in adult humans suggests new therapeutic strategies for replacing neurons that have been lost to brain trauma or neurodegenerative disease and may represent a mechanism of adult “neuroplasticity” previously unrecognized in humans (14). However, it also raises important questions that, for practical reasons, cannot be experimentally addressed in the human brain: What is the origin, number, migratory pathway, and terminal fate of these new neurons? What is their survival rate, connectivity, and functional relevance, particularly in a large brain that retains its elaborate cognitive and social abilities over a relatively long life span? Addressing these issues would require a nonhuman primate model as phylogenetically close to humans as possible. We have begun to address some of these questions in macaque monkeys, which, like humans, are also Old World primates, with a hippocampal formation (15, 16) and life-history pattern (17) similar to our own.
The issue of adult neurogenesis in an Old World primate was examined previously in macaque monkeys (Macaca mulatta), by using the method of [3H]thymidine autoradiography and morphologic criteria to detect and identify newly generated cells (2, 18, 19). Although continued gliogenesis and other nonneuronal cell division were detected in examined structures, which included the spinal cord, thalamus, neocortex, and hippocampus, no convincing evidence for newly generated neurons was found in adult monkeys. In the dentate gyrus, neurogenesis continued postnatally for several months but appeared to cease well before sexual maturity (at 3–4 yr) (18, 19), after which only newly generated astrocytes could be positively identified. It was noted, however, that some small [3H]thymidine-labeled cells in the dentate gyrus of adult monkeys were difficult to classify positively, leaving open the possibility that a low level of neurogenesis might persist into adulthood, which went undetected by the methods then available.
We have reexamined the possibility of neurogenesis in the adult macaque monkey, using more recently developed methods to detect and identify newly generated cells and to examine their possible origin and migratory pathways. We now report evidence for the production of new neurons in the hippocampal dentate gyrus of adult macaque monkeys.
MATERIALS AND METHODS
BrdU Injections.
All animal care and experimentation were conducted in accordance with institutional guidelines. Ten adult (5.5 to 16.5 years of age) macaque monkeys, both rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis), received intravenous injections of BrdU (Sigma) dissolved in 0.9% NaCl with 0.007 N NaOH, 50 mg/kg body weight. Either a single injection was administered or one daily injection for 5 consecutive days. Injections were given between 9:00 a.m. and noon. Animals were sacrificed at 2 hr or after 4, 12, 27, 31, 32, 38, or 72 days.
Immunohistochemistry.
For immunoperoxidase staining, animals were anaesthetized and perfused with 70% ethanol, and brains were blocked and postfixed overnight at 4°C. Blocks were dehydrated in graded alcohol solutions, cleared in xylene, embedded in paraffin, and serially sectioned at either 8 or 10 μm in the coronal plane. Sections were mounted on glass slides and processed as follows. For proliferating-cell nuclear antigen (PCNA) immunoperoxidase staining, rehydrated sections were blocked then incubated overnight at 4°C with a mouse anti-PCNA antibody (Boehringer Mannheim; 1:500). BrdU immunoperoxidase staining was performed as described previously (20). Briefly, rehydrated sections were placed in 2 N HCl for 1 hr, rinsed, and incubated with a mouse anti-BrdU antibody (Becton Dickinson; 1:100) for 30 min at room temperature. Either antibody was visualized by using a biotinylated horse anti-mouse IgG (Vector Laboratories; 1:200), the Vector ABC Elite kit, and H2O2/diaminobenzidine (DAB) with 0.02% cobalt chloride and 0.02% nickel ammonium sulfate to yield a black reaction product. Sections were counterstained by using 0.1% basic fuchsin.
For peroxidase double-immunostaining for oligodendrocyte markers, sections were first immunoreacted for BrdU as described above. After PBS rinsing and blocking, sections were incubated overnight at 4°C with either mouse anti-O4 (Boehringer Mannheim; 1:10) or mouse anti-2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP) (Boehringer Mannheim; 1:100). O4 immunoreactivity was visualized by using a peroxidase-conjugated goat anti-mouse IgM (Jackson ImmunoResearch; 1:100), and H2O2/DAB, which produces a brown precipitate. CNP immunoreactivity was visualized by using a biotinylated horse anti-mouse IgG (Vector Laboratories; 1:200), the Vector ABC Elite kit, and H2O2/DAB. Immunopositivity for BrdU could be distinguished from that for the glial markers on the basis of color (black vs. brown) and distinct subcellular localization (nuclear vs. cytoplasmic).
For immunofluorescence double-labeling for BrdU and neuronal markers, we perfused 32 days after the final of five BrdU injections with 0.9% saline, followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB), pH 7.4. Blocks of brain tissue were postfixed in PFA–PB for 6 hr at 4°C, then sunk in graded sucrose solutions to 30%. Blocks were frozen, and coronal cryostat sections (40-μm) were placed in PBS and immediately processed. To detect BrdU, the free-floating sections were pretreated to denature DNA by the following steps: 2-hr incubation in 50% formamide/2 × SSC at 65°C; 5-min rinse in 2 × SSC; 30-min incubation in 2 N HCl at 37°C; 10-min rinse in 0.1 M boric acid, pH 8.5. Sections were then blocked and incubated for 48 hr at 4°C with a pooled solution of rat anti-BrdU antibody (Accurate Scientific, Westbury, NY; 1:100) and either mouse anti-NeuN antibody (Chemicon; 1:100) or mouse anti-class III β-tubulin antibody (TuJ1; a gift from Anthony Frankfurter, University of Virginia; 1:400). To visualize BrdU with green fluorescence and neuronal markers with red fluorescence, nonadjacent sections were incubated for 2 hr in a pooled solution of Alexa 488-conjugated goat anti-rat IgG and Alexa 546-conjugated goat anti-mouse IgG (both from Molecular Probes; 1:200). To ensure that our labeling patterns were not the consequence of a dye artifact, we “switched” immunofluorescent markers: other sections adjacent to the former were incubated in a pooled solution of biotinylated goat anti-rat IgG (Jackson ImmunoResearch; 1:200) and Alexa 488-conjugated goat anti-mouse IgG, followed by a 2-hr incubation in Alexa 546-conjugated streptavidin (both from Molecular Probes; 1:200). Omitting primary antibodies from the immunohistochemistry processing steps eliminated fluorescence or peroxidase labeling of cells. Sections were mounted and coverslipped with mounting medium. To determine the phenotype of BrdU-labeled cells, we examined 20 nonadjacent tissue sections double-immunolabeled with BrdU and TuJ1 and 20 nonadjacent sections double-labeled with BrdU and NeuN.
Brightfield images were obtained by using a charge-coupled device camera mounted on a Zeiss microscope. Fluorescent signals were imaged by using a confocal laser scanning microscope (Zeiss LSM 510). Same-field fluorescent images from separate channels were combined by using Adobe Photoshop (Adobe Systems, Mountain View, CA).
RESULTS
Neural Progenitor Cells in the Macaque Dentate Gyrus.
To detect the existence and location of dividing cells and their progeny in the adult macaque hippocampus, we injected ten postpubertal (at least 5 years old) macaque monkeys of both sexes with the DNA marker, BrdU. BrdU, a thymidine analog, is incorporated into DNA during S phase of the cell cycle and thus labels dividing cells and their subsequent progeny for detection by using immunohistochemistry (20, 21). Each monkey received either a single injection or a series of one daily injection over 5 consecutive days to cumulatively label a proliferative cell population. After postinjection survival periods ranging from 2 hr to 75 days, we harvested the brains and examined the hippocampal region for the presence of BrdU-labeled cells.
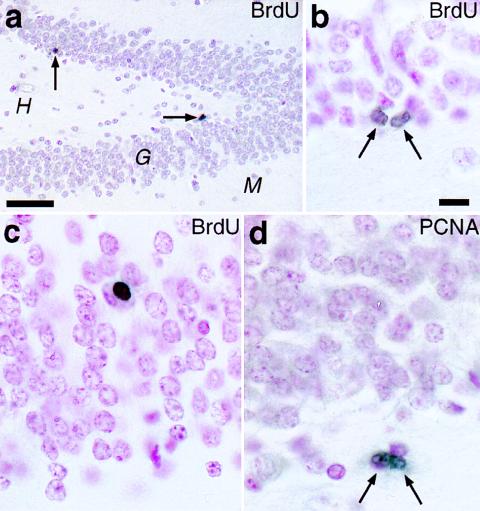
In all BrdU-injected monkeys, cells with BrdU-labeled nuclei were observed in the dentate gyrus. Specifically, at 2 hr after either a single or five daily injections, BrdU-labeled cells were present in the hilus, the molecular layer, and the border between the hilus and granule cell layer (GCL), the subgranular zone (SGZ) (Fig. 1 a and b). At longer postinjection survival times (12 or more days), BrdU-labeled cells were additionally found in the GCL (Fig. 1c). BrdU immunoreactivity usually filled the cell nucleus. Occasionally, a pair of closely apposed BrdU-labeled cells were observed in the hilus or SGZ; such “doublets” likely represent the daughter cells of a progenitor that had incorporated BrdU (Fig. 1b). These observations strongly suggest that the BrdU label in cell nuclei signified DNA synthesis during cell division rather than other processes that could potentially result in BrdU labeling, such as DNA repair or apoptosis (22, 23). To verify the presence of cell-proliferative activity in the dentate gyrus, we examined tissue sections from the same brains immunolabeled with antibodies against PCNA, an endogenous marker expressed in nuclei of cells engaged in the cell cycle (24). Cells with PCNA-immunopositive nuclei, including “doublets,” were observed in the same aforementioned hippocampal regions, except in the GCL proper. This result provided independent confirmation of the presence of cell proliferative activity in the dentate gyrus (Fig. 1d).
Figure 1.
The presence of newly generated cells in the dentate gyrus of adult macaque monkeys, as revealed by independent immunohistochemical markers. (a and b) The hippocampal dentate gyrus 2 hr after the last of five daily injections of BrdU shows BrdU-labeled nuclei (arrows) in the subgranular zone of the granule cell layer (G). H, hilus; M, molecular layer. (b) A BrdU-labeled “doublet” in the SGZ, possibly the daughter cell progeny of a recent mitotic event. (c) A BrdU-labeled nucleus deep in the GCL, 27 days after a single BrdU injection. Note the rounded appearance, similar to that of neighboring granule neurons and oligodendrocytes. (d) An example of a PCNA-immunopositive doublet in the SGZ. [Bars = (a) 50 μm; (b–d) 10 μm.]
The presence of BrdU-labeled cells in the dentate gyrus at 75 days postinjection (the longest survival period examined) indicated that at least some newly generated cells survive more than 2 months after incorporating BrdU. Such an extended survival argues against both the possibility of a rapid turnover of new cells and of BrdU cytotoxicity (25) at the doses used in this study. In sum, these results indicate that, as in humans and other mammals, the dentate gyrus of macaque monkeys retains progenitor cells into adulthood.
New Neurons.
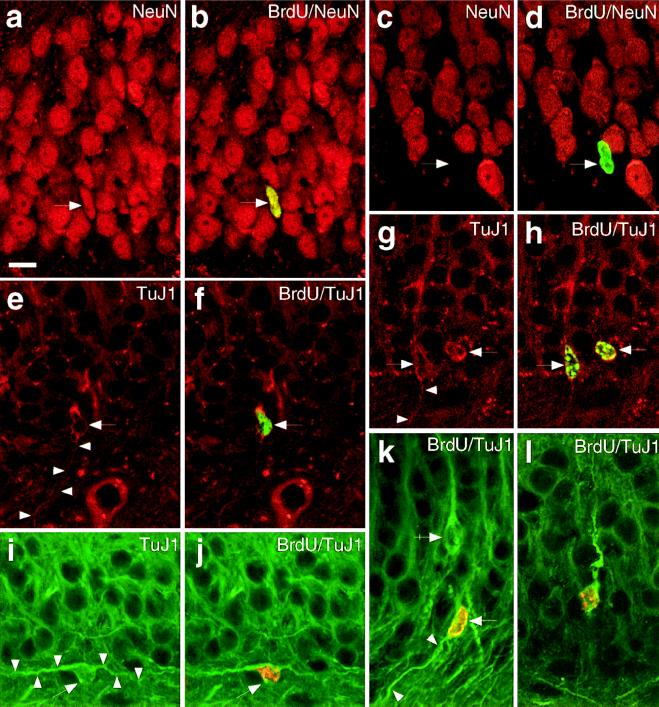
To determine whether BrdU-labeled cells in the hippocampus expressed a neuronal phenotype, we used double-label immunofluorescence for BrdU and either of two neuronal markers, NeuN (26, 27) or TuJ1 (28). The phenotype of BrdU-labeled cells at 32 days after the final of five daily injections was examined by using confocal microscopy to detect double-labeled cells. All cells that were double-labeled for BrdU and either NeuN or TuJ1 were located in the GCL and SGZ (Fig. 2), indicating the presence of newly generated neurons in the adult macaque monkey dentate gyrus. None of the BrdU-labeled cells in the hilus, molecular layer, or any other regions of the hippocampal formation also expressed immunoreactivity to the neuronal markers. Double-labeled cells had nuclei that varied in shape from round to oval to elongated. The elongated nuclei were similar in size and shape to nuclei of young migrating neurons in the GCL of neonatal monkeys (29) (Fig. 2).
Figure 2.
Newly generated cells in the adult macaque dentate gyrus express neuronal phenotypic markers 32 days after five BrdU injections, as detected by immunofluorescence double-label and confocal microscopy. (a–d) Neurons in the dentate gyrus express NeuN (red). The same cell in the GCL that is labeled with BrdU (arrow, green in b) also expresses NeuN (arrow, a). (c and d) An example of a BrdU-labeled nucleus (d, arrow, green) that did not emit a red fluorescence signal (c, arrow), demonstrating that the BrdU fluorescent signal did not “bleed” into the red channel; this might be a progenitor or new glial cell. (e and f) A TuJ1-positive cell in the SGZ (arrow, red) colabels with BrdU in its nucleus (f, arrow, green). Note the slender process (arrowheads) emanating from the cell body, resembling the trailing process of a newly generated migrating neuron. The BrdU in its nucleus confirms its recent generation. (g and h) Two cells in the SGZ expressing TuJ1 in the cytoplasm surrounding their nuclei (red), which are immunopositive for BrdU (h, green). Their close proximity suggests that these two cells might be newly generated “siblings.” The long thin process (arrowheads), consistent with migratory behavior, is clearly seen in one of the cells. (i and j) A bipolar cell in the SGZ coexpressing TuJ1 (green) and nuclear BrdU (j, orange). Although most double-labeled cells were oriented radially in the GCL, occasionally a cell was oriented parallel to the GCL. This example shows such a BrdU-labeled cell with an extended process on either side of the nucleus. (k) A TuJ1-positive cell (green, arrow) with a BrdU-positive nucleus (orange) has an immature migratory appearance. Note the thin trailing process (arrowheads) and a nearby BrdU-negative neuron, with a mature, apical process (arrow–cross). (l) A cell deep in the GCL colabels with TuJ1 (green) and BrdU (orange) with an apical process that is thick and tortuous, similar to the dynamic, exploratory leading process of a migrating neuron (its trailing process is out of the optical plane). Compare this with the straighter apical process of the more mature BrdU-immunonegative granule neuron in k (arrow–cross). [Bar (a–l) = 10 μm.]
NeuN immunofluorescence stained predominantly cell nuclei and only partially the surrounding cytoplasm (Fig. 2 a–d). In BrdU/TuJ1 double-labeled cells, TuJ1 immunofluorescence was confined to the cytoplasm, often revealing a bipolar morphology that resembled an immature migrating neuron (29, 30), having a thick leading process directed into the GCL and a slender trailing process at the “hilar” pole of the cell (Fig. 2 e–l). Although most double-labeled cells were oriented radially in the granule cell layer, occasionally BrdU/TuJ1-labeled cells were oriented obliquely or parallel to the GCL, with their nuclei at or near the SGZ (Fig. 2 i–k). To ensure that our double-label results did not reflect a fluorescent label artifact, we “switched” immunofluorescent markers and obtained similar results (e.g., Fig. 2 e–l).
Of an average of 11 BrdU-labeled cells in the SGZ/GCL per coronal section, an average of two of these cells per section were also TuJ1-labeled. Given that the macaque dentate gyrus extends approximately 19 mm rostrocaudally (from a M. mulatta brain atlas, by M. Schwarcbart and H.E. Rosvold, National Institutes of Health, and from MRI images of four M. mulatta brains in our collection), and the coronal sections were 40-μm thick, we thus estimate that the total number of BrdU/TuJ1 double-labeled cells present at 32 days after five daily BrdU injections is approximately 1,000 per hippocampus. This number represents 0.02% of the total neuronal population [4.8 million neurons (31)] in the macaque monkey GCL. Thus we approximate that one-fifth of this fraction, or 0.004% of the total GCL population, were generated per day (see Discussion).
New Glia.
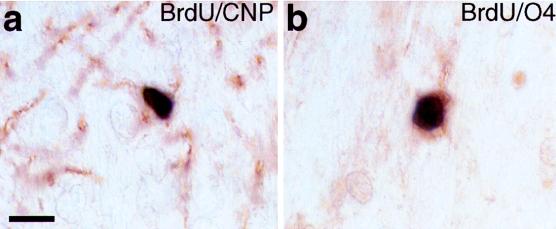
The BrdU-labeled cells in the dentate gyrus that were immunonegative for the neuronal markers could represent undifferentiated progenitors, new neurons that did not stain, or new nonneuronal cells. At least some of the newly generated nonneuronal cells in the dentate gyrus become astrocytes in adult macaque monkeys (19) as well as in humans (14). To determine whether oligodendroglia are also generated in the adult monkey dentate gyrus, we used immunoperoxidase double-labeling for BrdU and either of two independent markers expressed by oligodendrocytes, O4 (32) or CNP (33). In the hilus and GCL, some of the BrdU-labeled nuclei were surrounded by cytoplasm immunopositive for O4 or CNP (Fig. 3), indicating that, in addition to astrocytes and neurons, oligodendrocytes also comprise part of the population of newly generated cells in the adult monkey dentate gyrus.
Figure 3.
Newly generated oligodendrocytes in the adult macaque dentate gyrus. These two examples show cells that are immunoperoxidase double-labeled; the black reaction product in the nucleus indicates BrdU, and the brown stain in the surrounding cytoplasm indicates the expression of oligodendrocyte-specific markers, CNP (a) or O4 (b). The cell in (a) is in the GCL and the cell in (b) is in the hilus of an adult monkey, 27 days after a single BrdU injection. (Bar = 10 μm.)
DISCUSSION
Our results indicate that, in addition to new astrocytes (19), new cells with oligodendroglial and neuronal features are generated in the dentate gyrus of adult macaque monkeys. We infer that the new neurons are generated from progenitor cells in the SGZ, based on their immature appearance and their localization at or near the SGZ, the only region of the hippocampus known to produce neurons during adulthood in other species (5, 10).
It is unknown whether the new neurons, oligodendrocytes, and astrocytes in the macaque monkey GCL are each generated from a distinct committed progenitor, or whether all are derived from a multipotent progenitor. The existence of such multipotent neural stem cells in the adult dentate gyrus in vivo has been inferred from in vitro and transplantation experiments (34, 35). Our finding that, in macaques, all three neural cell lineages are generated in or near a discrete region—the SGZ—provides further support for the in vivo existence of a multipotent neural stem cell in the adult primate brain.
Previous studies in adult rodents (5) and perinatal monkeys (36) have shown that new neurons originate from progenitors in the SGZ, then migrate into the GCL where they differentiate. Our results suggest that this sequence of events transpires in adult monkeys as well. The immature, bipolar shape of double-labeled cells we observed in the SGZ/GCL resembles that of migrating young neurons during hippocampal development in this species (29, 30). Migration during development is supported and guided by radial glial fibers that penetrate through the GCL; these fibers are also present in adulthood in monkeys (29, 30) and thus may provide a substrate for the migration of new neurons in the adult dentate gyrus. Although the BrdU-labeled neurons described in adult human dentate gyrus had a mature morphology, and the presence of immature neurons was not noted (14), our findings in macaque monkeys suggest that these cells in humans may also undergo a similar series of developmental stages and do not originate de novo from preexisting mature neurons.
The primary goal of the present study was to test the existence of neurogenesis in the adult macaque monkey. Nonetheless, some provisional estimates regarding cell number and its potential significance can be ventured. In the adult macaque monkey, we estimated that at least 0.004% of the neuronal population in the GCL are new neurons generated per day, i.e., one new neuron per 24,000 existing GCL neurons per day. In a similar study performed in adult mice that received BrdU injections (the same dose as that used in the present study) and also survived 1 mo postinjection, it was estimated that more than one new neuron in 2,000 existing granule neurons was generated per day (6). This fraction in rodents is an order of magnitude larger than our estimate in the macaque monkey. Considering that the duration of S phase of neural progenitors in monkeys is less than 1 day and is similar to that in rodents (20), it thus appears that in macaque monkeys, as in humans (14), there is considerably less adult hippocampal neurogenesis than in rodents. It remains to be determined whether the reduced neurogenesis in these Old World primates reflects lower rates of neuronal production, survival, or both.
The reasons for the apparent reduction of neurogenesis in adult macaque monkeys and humans are unclear. Nonetheless, this reduction is in harmony with the general decline of adult neurogenesis during vertebrate evolution, which could be an adaptive strategy to maintain stable neuronal populations throughout life (2, 37). This hypothesis is consistent with the restriction of adult neurogenesis in the mammalian brain to phylogenetically older structures, i.e., the olfactory bulbs (38, 39) and hippocampal formation and its absence in the more recently evolved neocortex (2, 37, 40). Moreover, the reduced neurogenesis in the adult dentate gyrus of Old World primates may also be related to their prolonged period of adolescence and longer life span. Many studies of adult neurogenesis in rodents are based on animals that were 2–3 months of age (5, 6, 10, 41–43), whereas studies in Old World macaques, including the present one, tested animals older than 5 years of age (2, 18, 19, 37). When neurogenesis in the rodent is examined later in life, i.e., after 1 yr of age, which is considered senescence in these species, levels of neurogenesis in the dentate gyrus are drastically reduced (44, 45). If the macaque brain is examined at 2 postnatal months, the same age as “adult” rodents, there is a significant amount of neurogenesis in the dentate gyrus that even continues for several months (18, 19). By 4 years of age, the time at which macaques have attained sexual maturity, neurogenesis has declined. These observations suggest that levels of neurogenesis in the mammalian dentate gyrus may be more closely associated with absolute rather than relative age, i.e., 2-mo-old rodents and 2-mo-old macaques both show substantial neurogenesis. This hypothesis would predict that “adult” neurogenesis should be greater in short-lived early-maturing mammals than in longer-lived late-maturing Old World primates. This hypothesis is consistent with the findings of substantial neurogenesis in the dentate gyrus of “fast-maturing” mammals and New World monkeys examined at early ages, i.e., 7-mo- to 2.5-yr-old tree shrews (sexual maturity between 4 and 5 months) (13) and 3-yr-old marmosets (sexual maturity at 14 months) (12). Presumably, if these species were examined after 5 yr of age (i.e., the age of the monkeys in the present study), they would also show reduced levels of neurogenesis. Because of differences in the methods and experimental designs used in previous studies (e.g., survival times, dose and type of proliferation marker, environmental conditions), it is difficult to compare the amount of neurogenesis across species until identical experimental manipulations can be performed in several species in a single study.
Newly generated neurons in the adult primate dentate gyrus could either cumulatively add to the population of older cells, resulting in continuous growth of the GCL, or alternatively they could replace older neurons, without a net increase in cell number. In rodents, new neurons are added to the neuronal population, resulting in a gradual increase in granule cell number during adulthood (45–47). At least some of these new neurons extend axons (43, 48) and receive synaptic input (5, 49). Moreover, environmental enrichment can cause a further increase in the number of neurons in the GCL, suggesting a functional role for new neurons in adult brain plasticity (10). In contrast, such neuronal accumulation may not occur in Old World primates. Cell-counting studies indicate that the number of neurons in the normal human GCL remains relatively stable throughout most of postnatal life, despite a lifetime of enriched experiences (16, 50, 51). For example, the number of neurons in the GCL of an 11-mo-old child was similar to that of adults (16). In macaques, other neuronal elements in the dentate gyrus (e.g., the total number of synapses) also remain stable from 4 to 35 years of age (52), suggesting that there is no increase in neuron number. That there seems to be no significant net accumulation of neurons in the primate GCL implies that the rate of neuronal production in adulthood is balanced by an equal rate of apoptosis and cell removal. Whether such turnover would ultimately replace all neurons in the primate GCL, like successive rows of shark teeth, or alternatively, would be restricted to a subpopulation in the GCL, consisting of young, recently generated cells, is an issue for future investigations. Thus in addition to differences between adult Old World primates and rodents in levels of adult neurogenesis, there may also be differences in the regulation of the ultimate fate of new neurons and their functional role.
Although neurogenesis in the adult dentate gyrus has been known for 3 decades in the rodent, its functional significance in any mammal remains largely unknown. The demonstration of adult neurogenesis in humans (14) and now in the macaque monkey raises further questions regarding its role in long-lived primates with elaborate cognitive and social capacities. For example, the understanding and treating of human brain disorders that implicate hippocampal dysfunction [e.g., temporal lobe epilepsy (53, 54), schizophrenia (55, 56)] may require consideration of the status and potential involvement of ongoing neurogenesis in the dentate gyrus of affected patients. The present study, together with known similarities between macaque monkeys and humans in hippocampal anatomy and function (15, 16), establishes the macaque monkey as an invaluable, phylogenetically appropriate model for investigating various issues of adult neurogenesis in humans.
Acknowledgments
This research was supported by the United States Public Health Service. We thank Joseph Musco for excellent technical assistance and Dr. Tarik Haydar for his generous assistance with confocal imaging.
ABBREVIATIONS
- BrdU
bromodeoxyuridine
- GCL
granule cell layer
- SGZ
subgranular zone
- PCNA
proliferating-cell nuclear antigen
- CNP
2′,3′-cyclic nucleotide 3′-phosphodiesterase
References
- 1.Leblond C P. Natl Cancer Inst Monogr. 1964;14:119–150. [PubMed] [Google Scholar]
- 2.Rakic P. Science. 1985;227:1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Bayer S A. In: Restorative Neurology, Vol. 6, Neuronal Cell Death and Repair. Cuello A C, editor. Amsterdam: Elsevier; 1993. pp. 203–225. [Google Scholar]
- 4.Altman J, Das G D. J Comp Neurol. 1965;124:319–336. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan M S, Hinds J W. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 6.Kempermann G, Kuhn H G, Gage F H. Proc Natl Acad Sci USA. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McEwen B S. Cell Mol Neurobiol. 1996;16:103–116. doi: 10.1007/BF02088170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould E, Cameron H A. Dev Neurosci (Basel) 1996;18:22–35. doi: 10.1159/000111392. [DOI] [PubMed] [Google Scholar]
- 9.Bengzon J, Kokaia Z, Elmér E, Nanobashvili A, Kokaia M, Lindvall O. Proc Natl Acad Sci USA. 1997;94:10432–10437. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kempermann G, Kuhn H G, Gage F H. Nature (London) 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 11.Parent J M, Yu T W, Leibowitz R T, Geschwind D H, Sloviter R S, Lowenstein D H. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gould E, Tanapat P, McEwen B S, Flugge G, Fuchs E. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gould E, McEwen B S, Tanapat P, Galea L A M, Fuchs E. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriksson P S, Perfilieva E, Björk-Eriksson T, Alborn A, Nordborg C, Peterson D A, Gage F H. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 15.Rosene D L, Van Hoesen G W. In: Cerebral Cortex, Vol. 6, Further Aspects of Cortical Function, Including Hippocampus. Jones E G, Peters A, editors. New York: Plenum; 1987. pp. 345–456. [Google Scholar]
- 16.Seress L. Epilepsy Res (Suppl) 1992;7:3–28. [PubMed] [Google Scholar]
- 17.Harvey P H, Martin R D, Clutton-Brock T H. In: Primate Societies. Smuts B B, Cheney D L, Seyfarth R M, Wrangham R W, Struhsaker T T, editors. Chicago: Chicago University Press; 1987. pp. 181–196. [Google Scholar]
- 18.Rakic P, Nowakowski R S. J Comp Neurol. 1981;196:99–128. doi: 10.1002/cne.901960109. [DOI] [PubMed] [Google Scholar]
- 19.Eckenhoff M F, Rakic P. J Neurosci. 1988;8:2729–2747. doi: 10.1523/JNEUROSCI.08-08-02729.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kornack D R, Rakic P. Proc Natl Acad Sci USA. 1998;95:1242–1246. doi: 10.1073/pnas.95.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowakowski R S, Lewin S B, Miller M W. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- 22.Ninomiya T, Adams R, Morriss-Kay G M, Eto K. J Cell Physiol. 1997;172:25–35. doi: 10.1002/(SICI)1097-4652(199707)172:1<25::AID-JCP3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 23.ElShamy W M, Klevenvall L K, Ernfors P. Neuron. 1998;21:1003–1015. doi: 10.1016/s0896-6273(00)80619-4. [DOI] [PubMed] [Google Scholar]
- 24.Bravo R, Macdonald-Bravo H. J Cell Biol. 1987;105:1549–1554. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris S M. Mutat Res. 1991;258:161–188. doi: 10.1016/0165-1110(91)90007-i. [DOI] [PubMed] [Google Scholar]
- 26.Mullen R J, Buck C R, Smith A M. Development (Cambridge, UK) 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 27.Wolf H K, Buslei R, Schmidt-Kastner R, Schmidt-Kastner P K, Pietsch T, Wiestler O D, Bluhmke I. J Histochem Cytochem. 1996;44:1167–1171. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- 28.Lee M K, Rebhun L I, Frankfurter A. Proc Natl Acad Sci USA. 1990;87:7195–7199. doi: 10.1073/pnas.87.18.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckenhoff M F, Rakic P. J Comp Neurol. 1984;223:1–21. doi: 10.1002/cne.902230102. [DOI] [PubMed] [Google Scholar]
- 30.Nowakowski R S, Rakic P. J Neurocytol. 1979;8:697–718. doi: 10.1007/BF01206671. [DOI] [PubMed] [Google Scholar]
- 31.Seress L. J Hirnforsch. 1988;29:335–340. [PubMed] [Google Scholar]
- 32.Sommer I, Schachner M. Dev Biol. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- 33.Sprinkle T J. CRC Crit Rev Clin Neurobiol. 1989;4:235–301. [PubMed] [Google Scholar]
- 34.Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser R A R, Goldman S A. Cereb Cortex. 1994;6:576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- 35.Gage F G, Kemperman G, Palmer T D, Peterson D A, Ray J. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Nowakowski R S, Rakic P. J Comp Neurol. 1981;196:129–154. doi: 10.1002/cne.901960110. [DOI] [PubMed] [Google Scholar]
- 37.Rakic P, Kornack D R. In: Restorative Neurology, Vol. 6, Neuronal Cell Death and Repair. Cuello A C, editor. Amsterdam: Elsevier; 1993. pp. 257–266. [Google Scholar]
- 38.Lois C, Alvarez-Buylla A. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 39.Goldman S A, Luskin M B. Trends Neurosci. 1998;21:107–114. doi: 10.1016/s0166-2236(97)01191-0. [DOI] [PubMed] [Google Scholar]
- 40.Rakic P. Nat Neurosci. 1998;1:3–5. doi: 10.1038/3643. [DOI] [PubMed] [Google Scholar]
- 41.Gould E, Cameron H A, Daniels D C, Wooley C S, McEwen B S. J Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gould E, Tanapat P. Neuroscience. 1997;80:427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- 43.Markakis E A, Gage F H. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- 44.Kuhn H G, Dickinson-Anson H, Gage F H. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kemperman G, Kuhn H G, Gage F H. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bayer S A, Yackel J W, Puri P S. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- 47.Crespo D, Stanfield B B, Cowan W M. Exp Brain Res. 1986;62:541–548. doi: 10.1007/BF00236032. [DOI] [PubMed] [Google Scholar]
- 48.Stanfield B B, Trice J E. Exp Brain Res. 1988;72:399–406. doi: 10.1007/BF00250261. [DOI] [PubMed] [Google Scholar]
- 49.Kaplan M S, Bell D H. J Neurosci. 1984;4:1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simic G, Kostovic I, Winblad B, Bogdanovic N. J Comp Neurol. 1997;379:482–494. doi: 10.1002/(sici)1096-9861(19970324)379:4<482::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 51.Harding A J, Halliday G M, Kril J J. Cereb Cortex. 1998;8:710–718. doi: 10.1093/cercor/8.8.710. [DOI] [PubMed] [Google Scholar]
- 52.Tiggs J, Herndon J G, Rosene D L. Acta Anat. 1995;153:39–48. doi: 10.1159/000147713. [DOI] [PubMed] [Google Scholar]
- 53.Houser C R. In: The Dentate Gyrus and Its Role in Seizures, Epilepsy Research [Suppl. 7] Ribak C E, Gall C M, Mody I, editors. Amsterdam: Elsevier; 1992. pp. 223–234. [Google Scholar]
- 54.Mathern G W, Leite J P, Pretorius J K, Quinn B, Peacock W J, Babb T L. Dev Brain Res. 1994;78:70–80. doi: 10.1016/0165-3806(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 55.Weinberger D R, Berman K F, Suddath R, Torrey E F. Am J Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- 56.Heckers S, Rauch S L, Goff D, Savage C R, Schacter D L, Fischman A J, Alpert N M. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]