Abstract
ARO4 and HIS7 are two tandemly orientated genes of Saccharomyces cerevisiae that are transcribed into the same direction. The ARO4 terminator and the HIS7 promoter regions are sensitive to Micrococcus nuclease (Mnase) and separated by a positioned nucleosome. The HIS7 promoter is target for the transcription factors Gcn4p and Bas1p/Bas2p that activate its transcription upon amino acid starvation and purine limitation, respectively. Activation of the HIS7 gene by Gcn4p overexpression but not by Bas1p/Bas2p releases an ordered nucleosome distribution to yield increased Mnase sensitivity throughout the intergenic region. This remodeling is SNF2 dependent but mostly GCN5 independent. Accordingly, SNF2 is necessary for the Gcn4p-mediated transcriptional activation of the HIS7 gene. GCN5 is required for activation upon adenine limitation by Bas1p/Bas2p. Our data suggest that activation of HIS7 transcription by Gcn4p and Bas1p/Bas2p is supported by a nucleosome position-dependent and -independent mechanism, respectively. Whereas Gcn4p activation causes Swi/Snf-mediated remodeling of the nucleosomal architecture at the HIS7 promoter, the Bas1p/Bas2p complex presumably activates in combination with Gcn5p-dependent histone acetylation.
Regulation of transcription involves specific transcription factors and multiple complexes that act directly or indirectly by changing the nucleosomal structure of the chromatin (2). In general, 147 bp of DNA coil around a histone octamer that together form the nucleosome, the smallest unit of chromatin structure. Tight interactions between promoter DNA and/or upstream activation sequences with the histone octamer can repress gene transcription and result in silent or basally transcribed genes (26, 40). The process of destabilizing nucleosomes in order to facilitate access for the general transcription machinery to promoters requires not only sequence specific transcription factors but also cooperation with histones and cofactors that help to remodel or displace nucleosomes (31). Genetic studies and subsequent biochemical analyses have identified a number of factors required for transcriptional regulation in the context of chromatin structure. The multitude of these proteins functions as part of large complexes, such as the Swi/Snf complex; the RSC (for remodeling the structure of chromatin), Ada, and SAGA complexes; and the Srb/mediator/holoenzyme complex (32, 28).
Swi/Snf was purified as a 2-MDa protein complex that is composed of the Swi1, Swi2/Snf2, Swi3, Snf5, and Snf6 proteins plus five additional polypeptides (7). Swi/Snf can bind to nucleosomes and DNA, thereby creating loops in nucleosomal arrays or naked DNA, respectively, bringing distant sites into close proximity (4). In an ATP-dependent fashion it can reposition nucleosomes in cis on the same DNA molecule (47), with the SWI2/SNF2 gene encoding the DNA-dependent ATPase activity (33). Transcriptome analyses with a swi2/snf2 mutant strain have revealed that Swi/Snf controls transcription of only 6% of all Saccharomyces cerevisiae genes and that the control is exerted at the level of individual promoters rather than over chromosomal domains (39). Swi/Snf thereby both activates and represses transcription of different target genes. Recruitment of Swi/Snf to specific promoters by DNA-binding regulatory proteins, as well as targeting of the complex by the general transcription machinery, has been suggested (48).
Two high-molecular-mass Ada-Gcn5 complexes (0.8 and 1.8 MDa) have been biochemically isolated from S. cerevisiae and shown to be able to acetylate nucleosomes both in vitro and in vivo at specific lysine residues of histones H3 and H4 (14). Both complexes share Gcn5p, Ada2p, and Ada3p, whereas the larger one additionally contains Spt proteins (Spt20p, Spt3p, Spt8p, and Spt7p) and is called SAGA (for Spt/ADa/Gcn5 acetyltransferase). Gcn5p comprises the histone acetyltransferase (HAT) activity to acetylate histones in promoter regions in a manner that is correlated with Gcn5p-dependent transcriptional activation and HAT activity in vitro (23). SAGA interacts with both TATA-binding protein and acidic transcriptional activators such as the herpesvirus VP16 activation domain and yeast Gcn4p, suggesting that the complex might also have a transcriptional adaptor function for some promoters (15).
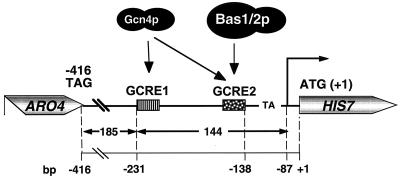
HIS7 is a typical housekeeping gene of S. cerevisiae, and its gene product is necessary for the biosynthesis of the amino acid histidine as well as purines (21). Its expression has previously been shown to be activated by two major stimuli: the lack of amino acid availability and limitation of external purines (37). Starvation for amino acids triggers increased expression of Gcn4 protein that in turn activates transcription of amino acid biosynthetic genes (general control of amino acid biosynthesis) (6, 18). Upon starvation, two Gcn4p-recognition elements (GCREs) within the HIS7 promoter are targeted by the transcription factor, thereby mediating a sixfold increase in HIS7 expression (Fig. 1) (37). The absence of purines causes a twofold increase in HIS7 expression and is mediated by the heterodimeric transcription factor Bas1/Bas2p that shares a common binding site with Gcn4p, the TATA-proximal GCRE2 (Fig. 1). This activation requires both factors Bas1p and Bas2p (37). Nevertheless, both activation pathways act independently of each other and, moreover, are additive upon simultaneous amino acid and purine limitation (37). Located 416 bp upstream of the HIS7 gene is the ARO4 gene, encoding an enzyme that is required for the biosynthesis of aromatic amino acids (22). Both transcriptional units are separated by a positioned nucleosome in between them (45).
FIG. 1.
Diagram of the yeast HIS7 promoter. Two binding sites for Gcn4p, GCRE1 and GCRE2, are functional parts of the promoter. GCRE2 additionally functions as recognition element for the heterodimeric transcription factor Bas1p/Bas2p. TA at position −120 reflects the putative TATA element. The arrow at −87 indicates the initiator element of the major transcriptional start site. The positions are relative to the translational start codon AUG, which is shown as +1.
A recent study on the field of chromatin elucidated how different chromatin-related coactivators act together on the regulation of genes (28). However, up to now there has been little information about how chromatin-modifying complexes such as Swi/Snf or HATs contribute to the regulation of a single gene under different activation systems. Whether different transcriptional activators require different, possibly independently acting, chromatin-modifying activities or whether they compete in these activities is poorly understood. We show here that two different transcription factors, although sharing a common binding site, use different chromatin-modifying activities to achieve their goal of transcriptional gene activation. Transcriptional HIS7 activation by Gcn4p requires the Swi/Snf-dependent rearrangement of nucleosomes, whereas Bas1/Bas2p-mediated HIS7 transcription requires the presence of Gcn5p HAT activity.
MATERIALS AND METHODS
Yeast strains and growth conditions.
The yeast strains and their genotypes used here are listed in Table 1. For high GCN4 expression, strains were transformed with plasmid p238 harboring a constitutively highly expressed GCN4 allele (27). To prepare strains FY1553 and FY1360 starving for histidine by using its analog 3-aminotriazole (3-AT), histidine prototrophy was initially regained by transforming these strains with plasmid pRS303 (HIS3) (35), yielding strains RH2561 and RH2563. Strains RH2569 and RH2570 with deletions of GCN5 and translational Phis7-lacZ fusions were obtained by transforming RH1615 and RH1616 with the deletion cassette of plasmid pME1236. The inserted KANR marker gene was then removed by using the Cre-loxP recombination system as described earlier (17).
TABLE 1.
Yeast strains and plasmids used in this study
| Strain or plasmid | Genotype or descriptiona | Reference or source |
|---|---|---|
| Strains | ||
| FY1353 | MATα his3Δ200 ura3-52 leu2Δ1 lys2-173R2 | 39 |
| RH2561 | Same as FY1353 but with plasmid pRS303 (HIS3) | This work |
| FY1354 | MATα his3Δ200 ura3-52 leu2Δ1 lys2-173R2 gcn5Δ::HIS3 | 39 |
| FY1360 | MATa his3Δ200 ura3-52 leu2Δ1 lys2-173R2 snf2Δ::LEU2 | 39 |
| RH2563 | Same as FY1360 but with plasmid pRS303 (HIS3) | This work |
| FY1352 | MATa his3Δ200 ura3-52 leu2Δ1 lys2-173R2 gcn5Δ::HIS3 snf2Δ::LEU2 | 39 |
| RH1381 | MATa ura3-52 aro3-2 gcn4-101 | 37 |
| RH1616 | MATa ura3-52 aro3-2 his7-lacZ gcn4-101 | 37 |
| RH2029 | MATa ura3-52, gcn4-101 bas1-2 bas2-2 | 45 |
| RH2569 | MATa ura3-52 aro3-2 his7-lacZ gcn5Δ::loxP | This work |
| RH2570 | MATa ura3-52 aro3-2 his7-lacZ gcn4-101 gcn5Δ::loxP | This work |
| RH1619 | MATa ura3-52 aro3-2 his7(mut GCRE1)-lacZ gcn4-101 | 37 |
| RH1622 | MATa ura3-52 aro3-2 his7(mut GCRE2)-lacZ gcn4-101 | 37 |
| RH1830 | MATa ura3-52 aro3-2 his7(mut ABS)-lacZ gcn4-101 | 36 |
| Plasmids | ||
| pBluescript II SK(+) | Commercial cloning vector with polylinker; lacZ | Stratagene |
| pUG6 | loxP-kanMX-loxP module with Kanr marker flanked by TEF2 promoter and terminator, Ampr | 17 |
| pME1768 | loxP-kanMX-loxP module from pUG6 in pBluescript II SK(+) | |
| pME2034 | 774-bp GCN5 3′ fragment in pBluescript II SK(+) | This work |
| pME2035 | 862-bp GCN5 5′ fragment in pBluescript II SK(+) | This work |
| pME2036 | 1.6-kb kanMX fragment from pME1768 in between GCN5 5′ and 3′ fragment | This work |
| pSH47 | cre recombinase expression vector | 17 |
| pRS303 | Yeast integrative vector; lacZ HIS3 | 35 |
| pCB159 | pGAL-BAS1 (SpeI-MluI 3.6-kb fragment) in B656 backbone | 44 |
| p238 | YCp50 carrying a GCN4 allele with mutated uORFsb | 27 |
Kanr, kanamycin resistance; Ampr, ampicillin resistance.
uORFs, upstream open reading frames.
Strains were cultivated in minimal-vitamin medium (25). Adenine repression was achieved by supplementation with 0.3 mM adenine (37).
Plasmids.
The plasmids used in the present study are listed in Table 1. Plasmid pME1236, carrying the gcn5::KANR deletion cassette, was created by replacing the GCN5 coding sequence with the kanamycin resistance cassette by a PCR-based three-step cloning strategy, with plasmids pME1234 and pME1235 as intermediates. The other plasmids used here have been described previously.
Genomic chromatin preparation and nuclease digestions.
These methods have been described previously (42). Biodyne B nylon membranes (Pall, Dreieich, Germany) were used for Southern transfer. Probes were labeled by the random primer method (11).
Indirect end labeling.
Chromosomal DNA from the nuclease digestion was digested with XbaI and MluI and fractionated on 1.2% agarose gels. The fractionated DNA was blotted on a Hybond-N nylon membrane by the alkaline blotting method and then hybridized with a radioactively labeled 250-bp PCR amplicon, generated with oligonucleotides HIS7-CHR1 (5′-GAGATTAAAGAAATTGTCAGA-3′) and HIS7-CHR2 (5′-CAAGTATTGAGGAGAAATGGTA-3′), with annealing just downstream of the XbaI site. A DNA ladder consisting of multiples of 256 bp was used for calibration (43).
RNA analysis.
Total RNA from S. cerevisiae was isolated according to the method of Cross and Tinkelenberg (9). For Northern hybridization analysis, 20 μg of total RNAs were separated on a formaldehyde-agarose gel and transferred to a positively charged nylon membrane (Biodyne B; Pall) by capillary blotting. Hybridization with specific DNA probes was performed after 32P labeling of the respective DNA fragments with the Prime-It II DNA labeling kit from Stratagene. The HIS7 probe was generated with oligonucleotides HIS-OL1 (5′-GTGGTAACCTACAGTCACTAACC-3′) and HIS-OL2 (5′-CCGATCGATACTTTATCAGCACC-3′), and the ACT probe was generated with oligonucleotides ACT-OL1 (5′-GCTGCTTTGGTTATTGATAACGG-3′) and ACT-OL2 (5′-CACTTGTGGTGAACGATAGATGG-3′). Signal intensities were visualized by autoradiography and quantified by using a BAS-1500 Phospho-Imaging scanner (Fuji).
β-Galactosidase assay.
β-Galactosidase activities were determined by using permeabilized yeast cells and the fluorogenic substrate 4-methylumbelliferyl-β-d-galactoside (MUF) as described previously (21).
RESULTS
Positioned nucleosomes flank an open HIS7 promoter.
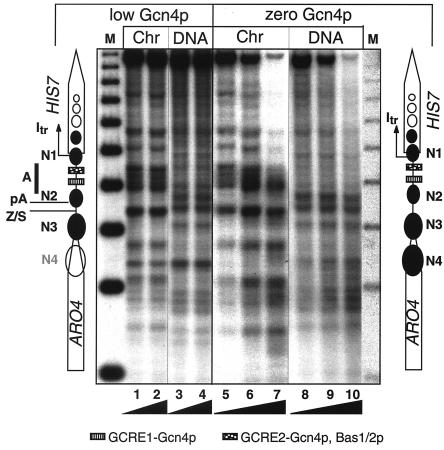
The nucleosomes within the yeast HIS7 promoter were mapped by Micrococcus nuclease (Mnase) treatment of crude nuclei extracts. In a first approach, the HIS7 promoter was investigated for cells grown without amino acid starvation and in the absence of adenine. Under these conditions the cells express only low amounts of Gcn4p, and the lack of adenine results in Bas1/Bas2p-mediated HIS7 expression. Since this expression level of HIS7 corresponds to the expression in minimal medium, it is here referred to as “basal” expression. We have used this nomenclature in the discussion below. Crude nuclear extracts from overnight cultures were partially digested with Mnase and treated further as previously described (42). The comparison of the Mnase-treated chromatin with that of naked DNA revealed that under these conditions the HIS7 promoter with its binding sites for Gcn4p and Bas1p/Bas2p lays in a region sensitive to the nuclease (Fig. 2, region A of lanes 1 to 4). This extended sensitive region A is flanked on both sites by protected DNA stretches, here referred to as positioned nucleosomes N1 and N2. Thus, N1 covers the transcriptional initiation site of the HIS7 promoter. The ARO4 gene is located 417 bp upstream of HIS7 and is transcribed in the same direction (21). The site that defines where the poly(A) tail is added during ARO4 mRNA 3′-end processing (pA) lies within nucleosome N2 that separates the ARO4 gene from the HIS7 promoter. The sensitive region further upstream encodes a Zaret/Sherman element that is required for efficient ARO4 3′-end processing (36). A further nucleosome N3 is positioned at the very 3′-end of the ARO4 open reading frame.
FIG. 2.
Chromatin structure at the ARO4-HIS7 intergenic region. Mnase protection experiments are shown with chromatin from strains FY1353 (wild-type GCN4) or RH1381 (gcn4Δ). Cells were cultivated in the absence of exogenous adenine. Control Mnase digests of naked DNA are shown in lanes 3, 4, 8, 9, and 10. Lanes M on the far left and right show a 256-bp DNA-ladder (42). Black ramps at the bottom indicate increasing amounts of Mnase and exactly represent 20 and 40 U for lanes 1 and 2; 20, 40, and 60 U for lanes 5, 6, and 7; 4 and 8 U for lanes 3 and 4; and 4, 8, and 12 U for lanes 8, 9, and 10, respectively. The diagrams on the left and right show the location of the open reading frames of ARO4 and HIS7. Protected regions are shown as black ovals N1-N3 (here referred to as positioned nucleosomes). The binding sites for Gcn4p and Bas1p/Bas2p are indicated at the bottom. The site of HIS7 transcriptional initiation is indicated by Itr. pA, poly(A) addition site of the ARO4 mRNA; Z/S, Zaret/Sherman element required for efficient ARO4 mRNA 3′-end processing.
Although Gcn4p expression is rather low during growth with sufficient supply of amino acids, binding to the GCREs in the HIS7 promoter could be responsible for the accessible chromatin detected. We therefore tested the nucleosome positions of cells lacking any Gcn4p with a gcn4Δ strain. However, they display the same Mnase-sensitive promoter region flanked by nucleosomes on both sides, as shown for cells with low Gcn4p levels (Fig. 2, lanes 5 to 10). A positioned nucleosome N4 in the 3′ end of the ARO4 open reading frame is absent in cells with low Gcn4p levels but present in Gcn4p-lacking cells.
Gcn4p overexpression releases the ordered chromatin structure at the intergenic region.
Cells starving for amino acids increase expression of Gcn4p, the master regulator of the general control system of amino acid biosynthesis. For experimental purposes, amino acid starvation is often induced by adding 3-AT or 5-methyltryptophan to the growth culture. 3-AT and 5-methyltryptophan are amino acid analogs acting as false feedback inhibitors of the histidine or tryptophan biosynthetic pathways, respectively. The general control network can also be activated by using a constitutively highly expressed GCN4 allele (27). For Gcn4p-dependent HIS7 activation both induction methods were shown to increase its transcription (21, 37).
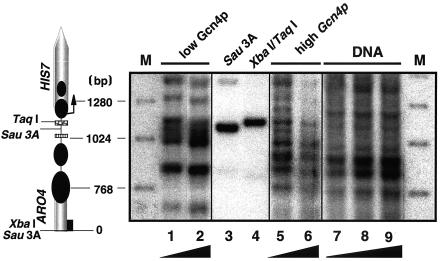
Basing our studies on the HIS7 promoter chromatin structure of the basally expressed gene as described above (for low Gcn4p, Bas1/Bas2p activation caused by absence of adenine), we now addressed the question of whether activation of HIS7 expression mediated by Gcn4p overexpression affects the HIS7 promoter chromatin structure.
To answer this question, we investigated the chromatin structure with a strain that harbors a highly expressed GCN4 allele and therefore constitutively expressing the general control system (27). Cells were cultivated in the presence of adenine so that the Gcn4p activation of HIS7 is not affected by Bas1/Bas2p. In striking contrast to the ordered HIS7 promoter chromatin structure described for the basal expression (Fig. 2, lanes 1 and 2), which is also observed for growth with 0.3 mM adenine (Fig. 3, lanes 1 and 2), several Mnase-sensitive bands in close proximity appear throughout the entire ARO4-HIS7 intergenic region as a result of Gcn4p overexpression (Fig. 3, lanes 5 and 6). Unambiguous localization of positioned nucleosomes that protect the DNA from the nuclease activity in that region is no longer feasible. We conclude that Gcn4p overexpression alters the chromatin structure at the ARO4-HIS7 intergenic region specifically and at the same time causes increased HIS7 and ARO4 transcription. We conclude that the HIS7 activation by high Gcn4p levels is associated with severe changes of the local ARO4-HIS7 chromatin structure, whereas the regulation of HIS7 transcription mediated by Bas1p/Bas2p seems not to be associated with any remodeling of nucleosomes.
FIG. 3.
Chromatin structure of the ARO4-HIS7 intergenic region at low and high intracellular Gcn4p levels. Yeast cells were grown in the presence of 0.3 mM adenine to prevent Bas1p/Bas2p-mediated HIS7 transcription. The Mnase protection pattern as described in Fig. 2 for cells with basal HIS7 expression levels of strain FY1353 can be seen in lanes 1 and 2. Strain FY1353[p238] expresses constitutively high amounts of Gcn4p and yields a protection pattern as shown in lanes 5 and 6. In lanes 3 and 4, restriction digests with Sau3A and XbaI/TaqI show DNA fragments precisely located within the HIS7 promoter. The diagram on the left and the black ramps at the bottom are as described for Fig. 2.
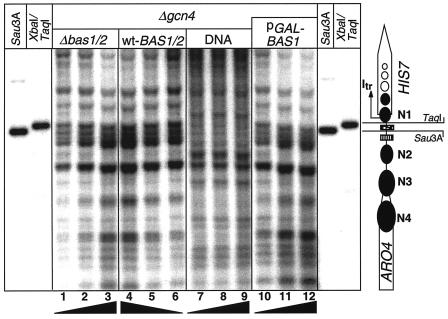
Different Bas1/2p levels do not affect the nucleosome positioning at the HIS7 promoter.
We investigated further the impact of Bas1/2p on HIS7 chromatin by varying its cellular levels. To eliminate conflicting data due to the Gcn4p response, the experiments were performed with yeast of a gcn4Δ genetic background. Cells were cultivated in the absence of adenine in the growth medium to induce Bas1/2p-dependent HIS7 transcription. The chromatin mappings of strain RH2029 (bas1Δ and bas2Δ), which lacks any Bas1/Bas2 protein, revealed a distribution of Mnase-sensitive and Mnase-protected sites identical to the BAS1/2 wild-type strain RH1381 (Fig. 4, lanes 1 to 3 and 4 to 6). The absence of Bas1/2p does not cause changes at the HIS7 promoter chromatin structure. We conclude that the heterodimeric complex Bas1/2p is not required to keep the HIS7 promoter and its cis-acting elements accessible. Overexpression of BAS1 is toxic for cell viability. We analyzed HIS7 transcription and its promoter chromatin structure after a 2-h burst of BAS1 expression to test whether toxicity correlates with a changed nucleosome structure. High BAS1 expression was achieved with a high-copy-number plasmid, pCB159 (Table 1), carrying BAS1 under the control of the GAL1 promoter, induced by shifting cells to medium with galactose as single carbon source. Surprisingly, HIS7 transcription is not only not affected by high Bas1p levels but in fact nearly prevented under these circumstances (data not shown). The HIS7 chromatin structure during BAS1 overexpression remains unaffected and is identical to that of zero or low Bas1p levels, shown in lanes 10 to 12 of Fig. 4. Thus, Bas1p, in conjunction with Bas2p, neither requires nor triggers chromatin remodeling to fulfill its function at the HIS7 promoter.
FIG. 4.
Chromatin structure of the ARO4-HIS7 intergenic region at various intracellular Bas protein levels. Yeast cells were grown in the absence of adenine. Lanes: 1 to 3, chromatin of strain RH2029; 4 to 6, chromatin of strain RH1381; 10 to 12, chromatin of strain RH1381 with the BAS1 overexpression plasmid pCB159 (44). On this high-copy-number plasmid the BAS1 open reading frame is fused to the GAL1 promoter. After shifting from glucose to galactose medium, BAS1 expression is strongly induced (44). The location of the promoter region is labeled by Southern hybridizations of chromosomal DNA digested with either XbaI/TaqI or Sau3A.
Remodeling of the HIS7 promoter chromatin structure upon Gcn4p overexpression is SNF2 dependent.
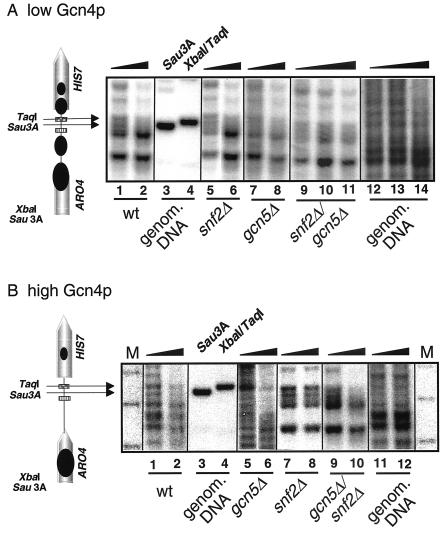
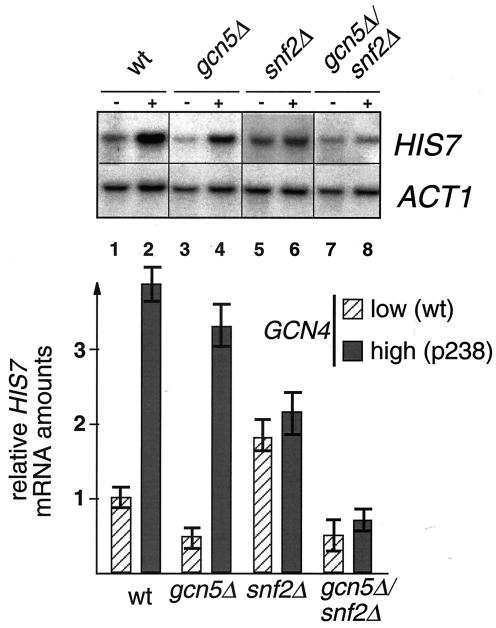
Chromatin remodeling upon transcriptional repression or activation of a variety of genes requires a functional Swi/Snf complex. As described above, the chromatin structure of the HIS7 promoter changes upon high Gcn4p expression. An influence of Swi/Snf on HIS7 chromatin is therefore conceivable for the described Gcn4p-mediated chromatin change. By using Mnase protection experiments we investigated the HIS7 promoter chromatin of cells with a snf2Δ background. We analyzed whether the ordered chromatin structure of the basal expressed HIS7 promoter or the Gcn4p-dependent change in chromatin structure is Swi/Snf dependent. Experiments with the snf2Δ mutant strain grown in the presence of low Gcn4p levels show a HIS7 promoter chromatin structure similar to the wild-type strain grown under basal gene expression conditions (Fig. 5 A, compare lanes 1 and 2 with lanes 5 and 6). The positions of sensitive, as well as protected, regions within the intergenic region are similar for the snf2 mutant and the wild-type SNF2 strains. This suggests that the Swi/Snf complex is not involved in the establishment and maintenance of the defined HIS7 promoter chromatin structure at low Gcn4p-levels (low Gcn4p, adenine limitation). However, the basal transcription of HIS7 is affected by the Swi/Snf complex, since in comparison to wild-type cells the snf2Δ strain showed a twofold increase in HIS7 mRNA levels (Fig. 6, lanes 1 and 5). The mechanism of Swi/Snf action remains unsolved here. One possibility is that it actually binds as a repressor to the promoter or, alternatively, this might be the result of an indirect effect. However, the transition in chromatin structure during high Gcn4p expression as described above is prevented in the snf2Δ background. In the absence of SNF2, the chromatin of the HIS7 promoter remains in its ordered structure of the basal transcribed promoter (Fig. 5 B, lanes 1, 2, 7, and 8). Therefore, the Swi/Snf complex is essential for the nucleosomal change upon Gcn4p-overexpression. Consistent with this influence on chromatin, Gcn4p-dependent activation of HIS7 transcription is almost absent in the snf2Δ background (Fig. 6, lanes 5 and 6).
FIG. 5.
Chromatin structure of the ARO4/HIS7 intergenic region of strains deficient in nucleosome modifying activity. Yeast strains FY1360 (snf2Δ), FY1354 (gcn5Δ), or FY1352 (snf2Δ/gcn5Δ) were cultivated without exogenous adenine. Experiments were performed with strains expressing either low (A) or high (B) amounts of Gcn4p from wild-type GCN4 (wt) or the additional CGCN4 (constitutively expressed GCN4) allele of p238, respectively.
FIG. 6.
HIS7 mRNA levels in yeast cells expressing either a low or a high amount of Gcn4 protein after cultivation without exogenous adenine. Deletions of components required for chromatin modification are as indicated. Low Gcn4p amounts are expressed from the wild-type GCN4 gene (−), whereas high Gcn4p amounts are expressed from plasmid p238 (+) for strains FY1353 (wt), FY1360 (snf2D), FY1354 (gcn5D), and FY1352 (snf2Δ/gcn5D). Four independent RNA isolations were hybridized twice in Northern experiments and then equalized to ACT1 mRNA levels, resulting in the mean values given in the graph.
GCN5 is only required for the Gcn4p-independent HIS7 expression.
Besides the remodeling of nucleosomes, histones are also targets for various modifications, including acetylation and/or deacetylation. Reversible acetylation of histones H3 and H4 has been shown to be associated with transcriptional gene activation. GCN5 encodes a respective HAT activity and is essential for the function of the yeast HAT complexes SAGA and Ada.
We then tested whether GCN5 is involved in the Gcn4p-mediated HIS7 transcription. Northern hybridization with total RNA of gcn5Δ mutant cells revealed that Gcn4p activation of HIS7 transcription is similar to that of the GCN5 wild-type strain (Fig. 6, lanes 3 and 4). Therefore, Gcn4p-dependent HIS7 activation seems to be independent of Gcn5p-containing HAT complexes. However, since the double mutation gcn5Δ snf2Δ led to a much stronger reduction of HIS7 transcription than Δsnf2 by itself does, a synergistic contribution of both activities seems obvious. Chromatin mappings Furthermore, showed that the remodeling of the nucleosomes upon Gcn4p overexpression as described above also takes place in gcn5Δ mutant cells (Fig. 5B, lanes 5 and 6). Gcn4p-dependent HIS7 gene expression and its associated nucleosome remodeling do not require functional Gcn5p-containing HAT complex. In contrast, the Bas1p/Bas2p-dependent basal HIS7 transcription is halved in a gcn5Δ background (Fig. 6, lanes 1 and 3). The nucleosome mappings, depicted in Fig. 5A (lanes 7 and 8), show no changes in the absence of Gcn5p.
Gcn5p is required for the Gcn4p-independent activation of the HIS7 promoter by adenine limitation.
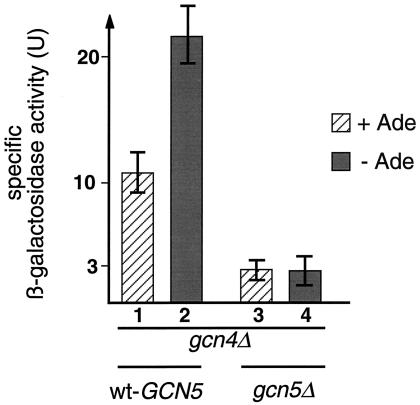
Low amounts of Gcn4p are expressed in cells even during the absence of amino acid starvation. It is known that these low amounts are responsible for various promoters to maintain certain expression levels. To confirm that the Gcn5p-dependency of the basal HIS7 expression is mediated through Bas1p/Bas2p and not through low Gcn4p, we tested the role of GCN5 in the gcn4Δ background. Specific β-galactosidase activities of cells harboring a translational his7-lacZ fusion instead of the wild-type HIS7 gene were measured to determine HIS7 expression. The activity of the reporter gene is strongly reduced in cells harboring the gcn5Δ background. In the presence of adenine when his7-lacZ expression is low anyway, deletion of GCN5 further reduces it to ca. 30% of that value (Fig. 7, lanes 1 and 3). Furthermore, any activation of his7-lacZ expression upon adenine limitation is prevented in a gcn5Δ background (Fig. 7, lane 4). This means that the Bas1p/Bas2p-dependent activation of his7-lacZ requires the HAT activity encoded from GCN5 to enable increased expression upon adenine limitation.
FIG. 7.
HIS7 promoter driving a lacZ reporter in cells defective in GCN4, as well as with defects in chromatin modification. The effects of external adenine on his7-lacZ expression for strains RH1616 (GCN5) and RH2570 (gcn5D) were measured as specific β-galactosidase activity. The graphs give average values of three individual enzyme assays performed with crude extracts from four independent cultures. Strains were cultivated either in adenine-deficient medium (−Ade) or in medium containing 0.3 mM adenine (+Ade). Specific β-galactosidase units correspond to nanomoles/(hour · milliliter · optical density at 546 nm)−1.
In summary, the HIS7 transcription is highly activated upon Gcn4p overexpression by mechanisms that require a functional Swi/Snf complex. This activation is accompanied by changes of the promoter chromatin structure. This response of disruption or randomizing nucleosomes does not depend on GCN5 but on SWI2/SNF2. In contrast, the Bas1p/Bas2p-mediated HIS7 expression during purine limitation conditions takes place with the aid of Gcn5p. Nucleosome remodeling as in the case of Gcn4p overexpression was not detected.
DISCUSSION
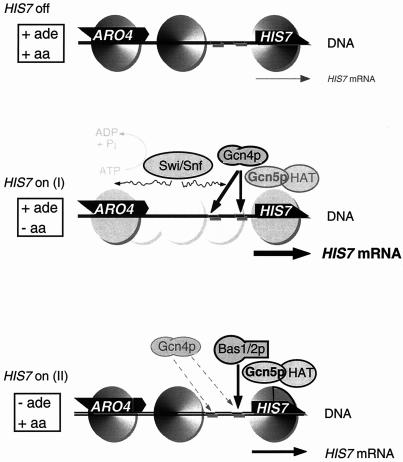
We have shown here that two eukaryotic transcriptional activators that share a common cis element in the promoter of their common target gene HIS7 require the activities of two different nucleosome-associated protein complexes. One of them, the jun-like transcription factor Gcn4p depends on the multiprotein complex Swi/Snf, leading to the remodeling of the promoter chromatin structure. The second factor, the heterodimeric complex Bas1p/Bas2p, requires Gcn5p/HAT complexes, whereas nucleosome remodeling does not take place (Fig. 8).
FIG. 8.
Model of the alternative mechanisms of nucleosome-dependent transcriptional activation of yeast HIS7 initiated by two different transcription factors. +ade/+aa, Growth in the presence of adenine and amino acids, that is, without any acid starvation; +ade/−aa, growth in presence of adenine but without amino acids, that is, with amino acid starvation; −ade/+aa, growth without adenine but in the presence of amino acids, that is, with purine starvation. Dark circles represent positioned nucleosomes with strong DNA-histone interactions.
Activation by Gcn4p overexpression but not Bas1p/Bas2p induction alters an ordered chromatin structure at the HIS7 promoter.
The HIS7 gene is an example for a yeast housekeeping gene that is regulated by two different and independent activation pathways. As a result of amino acid starvation, the transcription factor Gcn4p activates the transcription. In the absence of amino acid starvation, when yeast cells are not supplemented with purines and have to synthesize them de novo, the heterodimeric transcription factor Bas1p/Bas2p activates HIS7 expression to a level that has been termed basal expression (3). Supplementation with adenine represses this basal expression to a lower level that corresponds to the HIS7 expression of a bas1Δ or bas2Δ mutant strain (37).
Changes of chromatin structure at promoters during gene activation are a common phenomenon previously reported for numerous genes as, e.g., PHO5, PHO8, SUC2, CHA1, HIS4, GAL10, and CUP1 (1, 8, 10, 12, 16, 26, 34). Activators that specifically increase gene expression comprise transcription factors of different DNA-binding motif classes, including the basic-helix-loop-helix activator Pho4p, the acidic Cys6-Zn cluster activators Gal4p or Cha4p, and the basic-leucine-zipper Gcn4p.
Yeast HIS7 is an example of a gene with a promoter chromatin structure that changes upon the activation by one but not the other physiological operating activator (Fig. 7). High Gcn4p levels change the chromatin structure within the ARO4-HIS7 intergenic region and at the same time increase HIS7 transcription. We assume that the simultaneous binding to GCRE1 and GCRE2 causes the observed nucleosome randomization or disruption at the locus. This would support the previously described synergistic nature of HIS7 transcription by the binding of Gcn4p to two binding sites within the HIS7 promoter (37). Different levels of Bas1/2p influence HIS7 transcription (BAS1 overexpression astonishingly even represses it) but do not affect the chromatin structure.
Gcn4p-dependent HIS7 chromatin remodeling demands for a functional Swi/Snf-complex.
Genome-wide expression analysis revealed that ca. 6% of all yeast genes are affected twofold or more by the inactivation of Swi/Snf. The affected genes are subdivided into two groups: one with reduced amounts of transcript and the other with an increased transcript level (20, 39). Remodeling by the Swi/Snf-complex can in general have two consequences. One is that repressive nucleosomes are removed from the promoter region, thereby enforcing gene activity. The other is that nucleosomes are positioned to promoter elements, leading to the repression of gene activity.
An active role of the Swi/Snf complex in Gcn4p-mediated activation of the yeast HIS3 gene was previously described (29). However, a preferential accessibility for Mnase to the HIS3 promoter was shown to be a general property of the DNA sequence and not mediated by the Gcn4p-binding site (24). Moreover, several studies have described direct interactions between transcription factors and Swi/Snf that support the recruitment of the remodeling activity to the promoter by binding the DNA-bound activator (30, 46, 48).
The chromatin of the noninduced HIS7-promoter is an accessible open one, sustaining weak HIS7 expression (Fig. 7). This open structure is compatible with the Bas1p/Bas2p-mediated HIS7 activation during adenine limitation and does not require further remodeling. However, this open configuration does not seem to be suitable for high HIS7 expression at high Gcn4p levels. High HIS7 expression levels caused by Gcn4p overexpression result in the randomization or disruption of nucleosomes within the ARO4-HIS7 intergenic region. Mechanistically, this remodeling is coupled to the Swi/Snf complex.
The Bas1p/Bas2p-dependent adenine response on HIS7 expression depends on a functional SAGA/Ada (Gcn5p) complex.
The HAT activity of the SAGA or Ada complexes in yeast is encoded by GCN5 and is necessary for the transcriptional activation of several genes (5), including both Gcn4p-regulated and Gcn4p-independent genes. Previous studies reported Gcn5p dependence for the Gcn4p activation of an artificial PHO5 promoter that also harbors a binding site for Gcn4p and was therefore inducible by amino acid starvation. Moreover, the same PHO5 promoter is activated upon phosphate limitation by the transcription factor Pho4p and yet independently of Gcn5p (41). Other genes that require a functional GCN5 gene for the Gcn4p-dependent transcriptional activation are HIS3, TRP3, and ILV1 (13). In contrast, the Gcn4p-dependent activation of the HIS4 and ARG4 genes has been shown to be strictly GCN5 independent (13). In common with these latter genes the Gcn4p response of the HIS7 promoter does not require GCN5. The Gcn4p-binding sites of the HIS4, ARG4, and HIS7 promoters nearly perfectly matches the consensus sequence 5′-TGACTC-3′, whereas the HIS3, TRP3, and ILV1 promoters possess weak Gcn4p-binding sites (19, 38). Possibly, the requirement for Gcn5p increases with the decreasing strength of the respective Gcn4p recognition element.
However, since GCN5 is required for basal Bas1p/Bas2p-dependent HIS7 transcription, the situation is complex for this promoter, enabling individual responses to different stimuli by using different mechanisms. Altogether, we demonstrated that the transcriptional regulation of the HIS7 gene by two independent activation pathways uses different chromatin-modifying machineries. Gcn4p changes the nucleosomal distribution at the HIS7 promoter upon its overexpression. This process requires a functional Swi/Snf complex but no functional SAGA/Ada (Gcn5p) complex. Bas1p/Bas2p-dependent HIS7 activation, in contrast, requires a functional SAGA/Ada (Gcn5p) complex but is not associated with chromatin remodeling. Astonishingly, this is possible even though both activators share a common cis element of the HIS7 promoter. Chromatin immunoprecipitation studies shall further elucidate the recruitment of the cofactors to the HIS7 promoter chromatin and their relationship to the bound transcription factors at the different stages of HIS7 expression.
Acknowledgments
We thank Meike Andermann for excellent technical assistance during the course of this study. We are grateful to Fred Winston for generously providing yeast strains FY1352, FY1353, FY1354, and FY1360 and to Claudia Weber and Georg Schnappauf for their initial help.
This work was supported by grants from the Deutsche Forschungsgemeinschaft, the Volkswagenstiftung, and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Almer, A., H. Rudolph, A. Hinnen, and W. Hörz. 1986. Removal of positioned nucleosomes from the yeast PHO5 promoter upon PHO5 induction releases additional upstream activating DNA elements. EMBO J. 5:2689-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong, J. A., and B. M. Emerson. 1998. Transcription of chromatin: these are complex times. Curr. Opin. Genet. Dev. 8:165-172. [DOI] [PubMed] [Google Scholar]
- 3.Arndt, K. T., C. Styles, and G. R. Fink. 1987. Multiple global regulators control HIS4 transcription in yeast. Science 237:874-880. [DOI] [PubMed] [Google Scholar]
- 4.Bazett-Jones, D. P., J. Cote, C. C. Landel, C. L. Peterson, and J. L. Workman. 1999. The SWI/SNF complex creates loop domains in DNA and polynucleosome arrays and can disrupt DNA-histone contacts within these domains. Mol. Cell. Biol. 19:1470-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger, S. L. 1999. Gene activation by histone and factor acetyltransferases. Curr. Opin. Cell Biol. 11:336-341. [DOI] [PubMed] [Google Scholar]
- 6.Braus, G. H. 1991. Aromatic amino acid biosynthesis in the yeast Saccharomyces cerevisiae: a model system for the regulation of a eukaryotic biosynthetic pathway. Microbiol. Rev. 55:349-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cairns, B. R., Y. J. Kim, M. H. Sayre, B. C. Laurent, and R. D. Kornberg. 1994. A multisubunit complex containing the SWI1/ADR6, SWI2/SNF2, SWI3, SNF5, and SNF6 gene products isolated from yeast. Proc. Natl. Acad. Sci. USA 91:1950-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalli, G., and F. Thoma. 1993. Chromatin transitions during activation and repression of galactose-regulated genes in yeast. EMBO J. 12:4603-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross, F. R., and A. H. Tinkelenberg. 1991. A potential positive feedback loop controlling CLN1 and CLN2 gene expression at the start of the yeast cell cycle. Cell 65:875-883. [DOI] [PubMed] [Google Scholar]
- 10.Devlin, C., K. Tice-Baldwin, D. Shore, and K. T. Arndt. 1991. RAP1 is required for BAS1/BAS2- and GCN4-dependent transcription of the yeast HIS4 gene. Mol. Cell. Biol. 11:3642-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feinberg, A. P., and B. Vogelstein. 1983. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 132:6-13. [DOI] [PubMed] [Google Scholar]
- 12.Gavin, I. M., and R. T. Simpson. 1997. Interplay of yeast global transcriptional regulators Ssn6p-Tup1p and Swi/Snf and their effect on chromatin structure. EMBO J. 16:6263-6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georgakopoulos, T., and G. Thireos. 1992. Two distinct yeast transcriptional activators require the function of the GCN5 protein to promote normal levels of transcription. EMBO J. 11:4145-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant, P. A., L. Duggan, J. Cote, S. M. Roberts, J. E. Brownell, R. Candau, R. Ohba, T. Owen-Hughes, C. D. Allis, F. Winston, S. L. Berger, and J. L. Workman. 1997. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11:1640-1650. [DOI] [PubMed] [Google Scholar]
- 15.Grant, P. A., D. E. Sterner, L. J. Duggan, J. L. Workman, and S. L. Berger. 1998. The SAGA unfolds: convergence of transcription regulators in chromatin-modifying complexes. Trends Cell. Biol. 8:193-197. [DOI] [PubMed] [Google Scholar]
- 16.Gregory, P. D., A. Schmid, M. Zavari, M. Munsterkotter, and W. Hörz. 1999. Chromatin remodeling at the PHO8 promoter requires SWI/SNF and SAGA at a step subsequent to activator binding. EMBO J. 18:6407-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Güldener, U., S. Heck, T. Fielder, J. Beinhauer, and J. H. Hegemann. 1996. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24:2519-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinnebusch, A. G. 1997. Translational regulation of yeast GCN4. A window on factors that control initiator-tRNA binding to the ribosome. J. Biol. Chem. 272:21661-21664. [DOI] [PubMed] [Google Scholar]
- 19.Holmberg, S., and J. G. Petersen. 1988. Regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. Curr. Genet. 13:207-217. [DOI] [PubMed] [Google Scholar]
- 20.Holstege, F. C., E. G. Jennings, J. J. Wyrick, T. I. Lee, C. J. Hengartner, M. R. Green, T. R. Golub, E. S. Lander, and R. A. Young. 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95:717-728. [DOI] [PubMed] [Google Scholar]
- 21.Künzler, M., T. Balmelli, C. M. Egli, G. Paravicini, and G. H. Braus. 1993. Cloning, primary structure, and regulation of the HIS7 gene encoding a bifunctional glutamine amidotransferase:cyclase from Saccharomyces cerevisiae. J. Bacteriol. 175:5548-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Künzler, M., G. Paravicini, C. M. Egli, S. Irniger, and G. H. Braus. 1992. Cloning, primary structure and regulation of the ARO4 gene, encoding the tyrosine-inhibited 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase from Saccharomyces cerevisiae. Gene 113:67-74. [DOI] [PubMed] [Google Scholar]
- 23.Kuo, M. H., J. Zhou, P. Jambeck, M. E. Churchill, and C. D. Allis. 1998. Histone acetyltransferase activity of yeast Gcn5p is required for the activation of target genes in vivo. Genes Dev. 12:627-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mai, X., S. Chou, and K. Struhl. 2000. Preferential accessibility of the yeast his3 promoter is determined by a general property of the DNA sequence, not by specific elements. Mol. Cell. Biol. 20:6668-6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miozzari, G., P. Niederberger, and R. Hütter. 1978. Tryptophan biosynthesis in Saccharomyces cerevisiae: control of the flux through the pathway. J. Bacteriol. 134:48-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreira, J. M., and S. Holmberg. 1998. Nucleosome structure of the yeast CHA1 promoter: analysis of activation-dependent chromatin remodeling of an RNA-polymerase-II-transcribed gene in TBP and RNA pol II mutants defective in vivo in response to acidic activators. EMBO J. 17:6028-6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller, P. P., and A. G. Hinnebusch. 1986. Multiple upstream AUG codons mediate translational control of GCN4. Cell 45:201-207. [DOI] [PubMed] [Google Scholar]
- 28.Narlikar, G. J., H. Y. Fan, and R. E. Kingston. 2002. Cooperation between complexes that regulate chromatin structure and transcription. Cell 108:475-487. [DOI] [PubMed] [Google Scholar]
- 29.Natarajan, K., B. M. Jackson, H. Zhou, F. Winston, and A. G. Hinnebusch. 1999. Transcriptional activation by Gcn4p involves independent interactions with the SWI/SNF complex and the SRB/mediator. Mol. Cell 4:657-664. [DOI] [PubMed] [Google Scholar]
- 30.Neely, K. E., A. H. Hassan, A. E. Wallberg, D. J. Steger, B. R. Cairns, A. P. Wright, and J. L. Workman. 1999. Activation domain-mediated targeting of the SWI/SNF complex to promoters stimulates transcription from nucleosome arrays. Mol. Cell 4:649-655. [DOI] [PubMed] [Google Scholar]
- 31.Orphanides, G., and D. Reinberg. 2002. A unified theory of gene expression. Cell 108:439-451. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Martin, J. 1999. Chromatin and transcription in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 23:503-523. [DOI] [PubMed] [Google Scholar]
- 33.Richmond, E., and C. L. Peterson. 1996. Functional analysis of the DNA-stimulated ATPase domain of yeast. SWI2/SNF2. Nucleic Acids Res. 24:3685-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen, C. H., B. P. Leblanc, J. A. Alfieri, and D. J. Clark. 2001. Remodeling of yeast CUP1 chromatin involves activator-dependent repositioning of nucleosomes over the entire gene and flanking sequences. Mol. Cell. Biol. 2:534-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Springer, C., S. Krappmann, M. Künzler, C. Zmasek, and G. H. Braus. 1997. Regulation of the yeast HIS7 gene by the global transcription factor Abf1p. Mol. Gen. Genet. 256:136-146. [DOI] [PubMed] [Google Scholar]
- 37.Springer, C., M. Künzler, T. Balmelli, and G. H. Braus. 1996. Amino acid and adenine cross-pathway regulation act through the same 5′-TGACTC-3′ motif in the yeast HIS7 promoter. J. Biol. Chem. 271:29637-29643. [DOI] [PubMed] [Google Scholar]
- 38.Struhl, K. 1982. Regulatory sites for his3 gene expression in yeast. Nature 300:285-286. [DOI] [PubMed] [Google Scholar]
- 39.Sudarsanam, P., V. R. Iyer, P. O. Brown, and F. Winston. 2000. Whole-genome expression analysis of snf/swi mutants of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 97:3364-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Svaren, J., and W. Hörz. 1995. Interplay between nucleosomes and transcription factors at the yeast PHO5 promoter. Semin. Cell. Biol. 6:177-183. [DOI] [PubMed] [Google Scholar]
- 41.Syntichaki, P., I. Topalidou, and G. Thireos. 2000. The Gcn5 bromodomain co-ordinates nucleosome remodeling. Nature 404:414-417. [DOI] [PubMed] [Google Scholar]
- 42.Thoma, F. 1996. Mapping of nucleosome positions. Methods Enzymol. 274:197-214. [DOI] [PubMed] [Google Scholar]
- 43.Thoma, F., L. W. Bergman, and R. T. Simpson. 1984. Nuclease digestion of circular TRP1ARS1 chromatin reveals positioned nucleosomes separated by nuclease-sensitive regions. J. Mol. Biol. 177:715-733. [DOI] [PubMed] [Google Scholar]
- 44.Tice-Baldwin, K., G. R. Fink, and K. T. Arndt. 1989. BAS1 has a Myb motif and activates HIS4 transcription only in combination with. BAS2. Science 246:931-935. [DOI] [PubMed] [Google Scholar]
- 45.Valerius, O., C. Brendel, K. Düvel, and G. H. Braus. 2002. Multiple factors prevent transcriptional interference at the yeast ARO4-HIS7 locus J. Biol. Chem. 277:21440-21445. [DOI] [PubMed] [Google Scholar]
- 46.Wallberg, A. E., K. E. Neely, A. H. Hassan, J. A. Gustafsson, J. L. Workman, and A. P. Wright. 2000. Recruitment of the SWI/SNF chromatin remodeling complex as a mechanism of gene activation by the glucocorticoid receptor tau1 activation domain. Mol. Cell. Biol. 20:2004-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitehouse, I., A. Flaus, B. R. Cairns, M. F. White, J. L. Workman, and T. Owen-Hughes. 1999. Nucleosome mobilization catalyzed by the yeast SWI/SNF complex. Nature 400:784-787. [DOI] [PubMed] [Google Scholar]
- 48.Yudkovsky, N., C. Logie, S. Hahn, and C. L. Peterson. 1999. Recruitment of the SWI/SNF chromatin remodeling complex by transcriptional activators. Genes Dev. 13:2369-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]