Abstract
Integrin-associated protein (CD47) is a broadly expressed protein that costimulates T cells, facilitates leukocyte migration, and inhibits macrophage scavenger function. To determine the role of CD47 in regulating alloresponses, CD47+/+ or CD47−/− T cells were infused into irradiated or nonconditioned major histocompatibility complex disparate recipients. Graft-versus-host disease lethality was markedly reduced with CD47−/− T cells. Donor CD47−/− T cells failed to engraft in immunodeficient allogeneic recipients. CD47−/− marrow was unable to reconstitute heavily irradiated allogeneic or congenic immune–deficient CD47+/+ recipients. These data suggested that CD47−/− T cells and marrow cells were cleared by the innate immune system. To address this hypothesis, dye-labeled CD47−/− and CD47+/+ lymphocytes or marrow cells were infused in vivo and clearance was followed. Dye-labeled CD47−/− cells were engulfed by splenic dendritic cells and macrophages resulting in the clearance of virtually all CD47−/− lymphohematopoietic cells within 1 day after infusion. Host phagocyte-depleted CD47+/+ recipients partially accepted allogeneic CD47−/− T cells. Thus, dendritic cells and macrophages clear lymphohematopoietic cells that have downregulated CD47 density. CD47 expression may be a critical indicator for determining whether lymphohematopoietic cells will survive or be cleared.
Keywords: transplantation, in vivo animal models, T cells, stem cells, immune tolerance
Introduction
Integrin-associated protein (CD47) is a broadly distributed glycoprotein expressed on all hematopoietic cells 1. CD47 regulates leukocyte activation, chemotaxis, and migration 2 3 4. CD47 ligation can costimulate activated T cells 3 4 5 6. Conversely, CD47 ligation can function as an immune inhibitory signal which limits T cell responses 6 7. Although CD47 ligation has been shown to deliver T cell costimulatory signals in in vitro assays, there are no reports of the potential role of CD47 costimulation in vivo. The counterreceptor for CD47 is the signal regulatory protein (SIRP)α, expressed on neutrophils and monocyte-derived cells including dendritic cells (DCs; references 8 and 9). The binding of SIRPα to CD47 on T cells is associated with T cell activation and induction of antigen-specific CTL responses by DCs 9. Studies in CD47+/+ recipients infused with nonopsonized or opsonized RBCs obtained from CD47+/+ or CD47−/− 10 donors showed that RBC CD47 must deliver an inhibitory signal via macrophage SIRPα to prevent clearance and phagocytosis by splenic macrophages 8 11. The effects of CD47 expression on the clearance of other cell types has not been reported.
To determine whether CD47 ligation would facilitate T cell activation and expansion in vivo, we used an adoptive transfer system in which donor T cells expand and cause target tissue injury known as GVHD in allogeneic recipients 12. Allogeneic CD47−/− T cells had a markedly reduced capacity to cause GVHD and virtually no CD47−/− T cells were found in lymphoid tissues of CD47+/+ recipients. Donor CD47−/− bone marrow (BM) was unable to rescue irradiated congenic immunodeficient CD47+/+ recipients suggesting that the innate immune system might be eliminating CD47−/− cells. Splenic macrophages, CD11b+, and especially CD11b− DCs engulfed dye-labeled CD47−/− LN cells and CD47−/− BM cells clearing these cells within 24 h after infusion in marked contrast to CD47+/+ cells. The elimination of splenic macrophages partially restored the capacity of donor CD47−/− T cells to engraft in allogeneic recipients, indicating that donor CD47−/− T cells can expand in vivo. Thus, host DCs (and macrophages) can receive negative signals delivered via CD47 antigen expressed on donor BM cells or donor T cells. The downregulation of CD47 antigen expression on lymphohematopoietic cells during disease states may signal these cells for clearance by host DCs and macrophages.
Materials and Methods
Mice.
C57BL/6 severe combined immune deficient (B6 SCID), B6.129S7 recombination activating gene (Rag)1tm1Mom recombinase 1–deficient (B6-Rag1−/−), C.H2bm1 (bm1), C.H2bm12 (bm12), and B6.PL-Thy1a/Cy (termed B6-Thy1.1 congenic) mice were purchased from The Jackson Laboratory. C57BL/6 (termed B6:H2b), B6-CD45.1 congenic, Balb/c SCID (H2d), and Balb/c wild-type (CD47+/+) mice were purchased from the National Institutes of Health. B6-CD47 deletional mutant (B6-CD47−/−) and Balb/c CD47−/− mice were generated and backcrossed ≥10 generations 8 10.
GVHD Generation and Quantification of T Cell Expansion In Vivo.
To determine the effects of CD47 expression on the separate contribution of CD4+ or CD8+ T cells to the GVHD response, bm12 or bm1 recipients were irradiated with 6.0 Gray (137Cesium) total body irradiation (TBI) and given CD4+ T cells (0.03–0.3 × 106 cells per recipient) or CD8+ T cells (0.6 × 106 cells per recipient) from CD47−/− or CD47+/+ donors 10. Hematocrit (Hct) values were obtained to assess the degree of GVHD manifested by BM destruction 13. Survival and weight curves were monitored 13. To determine the effects of CD47 expression on GVHD mediated by T cells administered under noninflammatory conditions, nonconditioned Balb/c SCID mice were depleted of NK cells by anti-asialo–GM1 antisera and given B6-CD47+/+ or CD47−/− T cells (0.5 or 2 × 106 cells per recipient). To quantify the number of donor T cells present in the spleen of allogeneic recipients, splenocytes from Balb/c SCID recipients of B6-CD47−/− or CD47+/+ T cells were analyzed on days 6–7 after transfer. Cohorts were treated intravenously with 0.2 ml of liposomes loaded with dichloromethylene diphosphonate (liposomal DMDP; clodronate) on days 2 and 1 before allogeneic T cell transfer to deplete splenic phagocytes 8 14. As an additional indicator of donor T cell expansion, cannulae were inserted in the thoracic duct of other cohorts of recipients at the time of peak proliferation (day 6) after BM transplantation (BMT) and lymphocytes were collected over 18 h 13.
MLR Response.
B6-CD47+/+ or CD47−/− T cells were mixed with irradiated (30 Gray) T cell depleted Balb/c splenocyte stimulators, plated in replicates of six into 96-well round-bottomed plates containing 105 responders and 105 irradiated stimulators, and incubated for 2–8 d 13. Microtiter wells were pulsed with tritiated thymidine (1 μCi) for 18 h before harvesting and counted in the absence of scintillation fluid on a β-plate reader.
Long-Term Engraftment Studies.
To determine whether CD47−/− recipients could reject donor BM grafts, B6-CD47−/− or B6-CD47+/+ recipients were irradiated (5.5 Gray TBI) and infused with Balb/c BM cells (107). To determine whether CD47−/− or CD47+/+ BM would engraft and provide long-term hematopoietic reconstitution, B6 recipients were irradiated (5.5–6.5 Gray TBI) on day 1 and were reconstituted with Balb/c CD47+/+ or CD47−/− T cell depleted BM (107 cells) on day 0 15. In other studies, B6-Thy1.1 recipients were irradiated (9.0 Gray TBI) and reconstituted with B6-CD45.2 CD47−/− and B6-CD45.1 CD47+/+ BM cells (5 × 106 each). A cohort was splenectomized 10 d pre-BMT. For chimerism assessment, peripheral blood mononuclear cells were stained with anti-H2b–PE and anti-H2d–FITC (BD PharMingen) and analyzed using a FACScalibur™ (Becton Dickinson). To preclude a host antidonor T cell response against donor CD47−/− BM cells, CD47+/+ B6-Rag1−/−, or B6-CD47−/− recipients were irradiated (9.5 Gray TBI) on day 0 and given 5 × 106 congenic B6-CD47−/− or CD47+/+ BM cells.
Short-Term In Vivo BM Proliferation Assay.
To determine the effect of CD47 expression on the short-term reconstituting capacity of CD47−/− BM, B6 recipients were irradiated (9.5 Gray TBI) to eliminate host hematopoiesis and given 3 × 106 CD47−/− or CD47+/+ BM cells on day 0 16. To preclude NK cell–mediated graft rejection, cohorts were injected with anti-NK1.1 (PK136) mAb (200 μg) on day 2 to deplete NK cells. Anti-NK1.1 mAb (100 μg) results in depletion by day 1 and lasts for >14 d. On day 5, 3 μCi of [125I]deoxyuridine and 10−11 molar fluorodeoxyuridine (Amersham Pharmacia Biotech) was given. Spleens were harvested on day 6, rinsed, and incorporated radioactivity determined using a γ counter.
Kinetic Assessment of CD47−/− Lymphohematopoietic Cell Clearance in the Spleen of CD47+/+ Recipients.
Single cell suspensions of LN cells were prepared from B6-CD47+/+ and CD47−/− mice and labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) according to the manufacturer's protocol. Cells were injected intravenously into unirradiated B6 recipients. Single cell splenocyte suspensions were prepared using 10 mM EDTA. Cells were incubated on ice with cychrome-labeled anti-B220, cychrome-labeled anti-CD3, PE-labeled anti-CD11c, and either allophycocyanin-labeled anti-CD11b or biotin-labeled anti-F4/80 followed by allophycocyanin-labeled streptavidin. Data were analyzed on a FACScan™ flow cytometer (Becton Dickinson). A portion of the spleens was frozen in precooled isopentane, sectioned (10 μ), dehydrated in acetone, rehydrated in PBS, and sequentially incubated with anti-FcR mAb (2.4G2), avidin/biotin solutions (Vector Laboratories), biotin-labeled anti-CD11c mAb (N418), streptavidin-labeled horseradish peroxidase (NEN Life Science Products), and Cy5-labeled tyramide (NEN Life Science Products). Confocal microscopy and image analysis were performed as described previously 17.
Statistical Analyses.
Group comparisons of continuous data were made by Student's t test. Survival data were analyzed by lifetable methods using the Mantel-Peto-Cox summary of chi-squared. P values < 0.05 were considered significant.
Results
CD47− /− T Cells Have a Reduced Capacity to Mediate GVH-induced Lethality and Fail to Expand In Vivo.
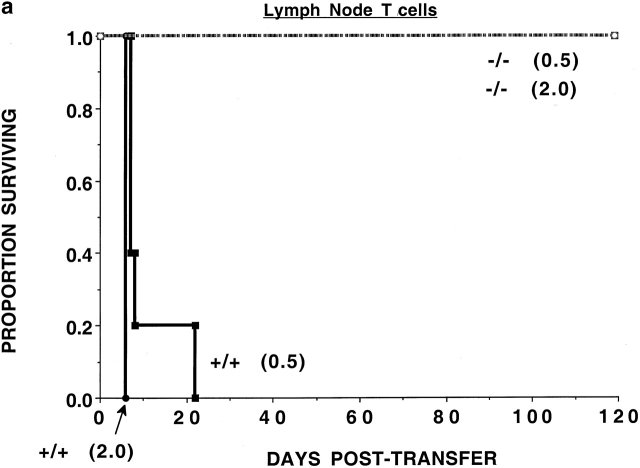
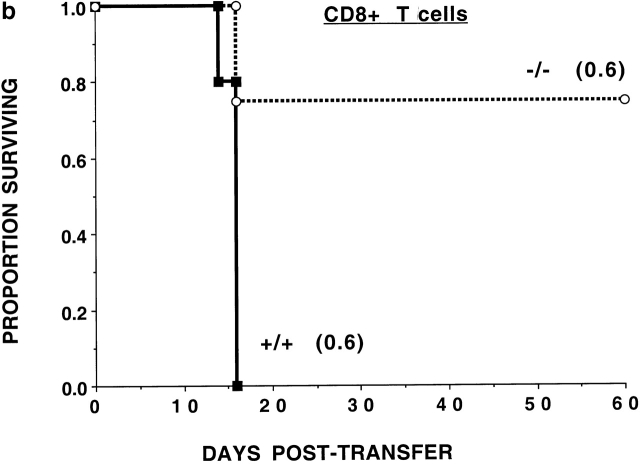
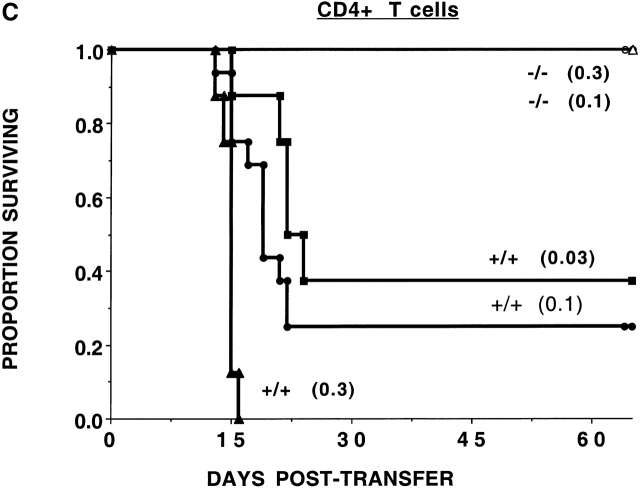
To determine whether the CD47 expression on donor T cells was required for optimal alloresponses in vivo, nonconditioned NK-depleted Balb/c SCID mice were given 0.5 or 2.0 × 106 purified LN T cells from B6-CD47+/+ or CD47−/− donors (Fig. 1 a). All recipients of CD47−/− T cells survived long-term, while recipients of CD47+/+ T cells died by 3 wk after transfer.
Figure 1.
CD47−/− T cells have a reduced GVHD lethality capacity. (a) Nonirradiated, NK cell–depleted Balb/c SCID recipients (n = 5 per group) were given B6-CD47−/− or CD47+/+ T cells. Cell doses × 10−6 are indicated in parentheses. Recipients of 2 × 106 CD47−/− T cells had a significantly (P = 0.0015) higher survival rate versus those given 0.5 × 106 CD47+/+ T cells. (b) Irradiated (6.0 Gray TBI) bm1 recipients (n = 8 per group) were given B6-CD47−/− or B6-CD47+/+ CD8+ T cells. Recipients of CD47−/− T cells had a significantly higher survival versus B6-CD47+/+ T cells. (c) Irradiated (6.0 Gray TBI) bm12 recipients (n = 8 or 16 per group) were given B6-CD47+/+ CD4+ T cells. Recipients of B6-CD47−/− CD4+ T cells at a dose of 0.3 × 106 had a significantly higher survival rate than recipients of 10-fold fewer B6-CD47+/+ cells.
To assess the potential effects of irradiation in supporting GVHD lethality and to determine whether differences existed in the effects of CD47 expression on CD8+ versus CD4+ T cell–mediated GVHD lethality, bm1 or bm12 recipients were sublethally irradiated (6 Gray TBI) and given purified CD8+ or CD4+ T cells, respectively, from B6-CD47−/− or CD47+/+ donors. CD47+/+ CD8+ T cells were rapidly lethal (Fig. 1 b). In contrast, 75% of recipients of CD47−/− CD8+ T cells survived long-term. Day 14 mean Hct values were higher in recipients of CD47−/− versus CD47+/+ T cells (25 vs. 9%, respectively; P = 0.066), reflective of less GVHD-induced tissue destruction from CD47−/− T cells. Recipients of CD4+ T cells from CD47+/+ donors had 25% survival versus 100% survival in recipients of CD47−/− cells (Fig. 1 c). Day 14 mean Hct values were significantly higher in recipients of CD47−/− versus CD47+/+ T cells (40 vs. 16%, respectively). Titration studies revealed a >10-fold reduction in GVH lethality by CD47−/− versus CD47+/+ T cells. Thus, GVHD lethality mediated by either CD4+ or CD8+ CD47−/− T cells was markedly reduced even in the setting of irradiation-induced tissue injury.
To investigate the mechanism(s) responsible for impaired GVHD lethality observed with CD47−/− T cells, MLR responses were analyzed. As compared with CD47+/+ T cells, B6-CD47−/− T cells generated a comparable peak proliferative response to Balb/c stimulator cells (mean ± SD 6,409 ± 968 vs. 7,709 ± 1,309 cpm [day 3]; 5,175 ± 827 vs. 5,124 ± 512 cpm [day 4]; P values > 0.1]. These data indicate that CD47 expression was not critical for in vitro alloproliferation.
It is possible that reduced GVHD by CD47−/− T cells was due to a defect in the response of CD47−/− T cells to alloantigen-bearing cells in vivo which could not be uncovered by in vitro studies. As another indicator of in vivo alloresponses, we examined the capacity of host CD47−/− recipients to reject allogeneic donor BM grafts, a process which is predominantly T cell dependent. Sublethally irradiated (5.5 Gray TBI) B6-CD47+/+ or CD47−/− recipients were given Balb/c T cell–depleted BM grafts. Donor chimerism levels at 6 wk after BMT revealed no significant differences (P = 0.29) between CD47−/− versus CD47+/+ recipients (39 ± 8% vs. 27 ± 7%) (n = 18–20 per group). Therefore, CD47−/− host T cells do not have a major defect in allogeneic BM graft rejection.
Because MLR results were similar with CD47−/− versus CD47+/+ T cells, we sought to determine whether the impaired GVHD response was a result of poor T cell expansion in vivo. NK-depleted Balb/c SCID mice were given B6-CD47−/− or CD47+/+ T cells (106 cells per recipient). On day 6, we observed a mean of ≥2.6 × 106 donor CD47+/+ T cells as compared with <0.05 × 106 donor CD47−/− T cells in recipient spleens (n = three per group individually analyzed; P < 0.001). We found <0.05 × 106 CD47−/− T cells in the thoracic duct lymphatics of a separate cohort of mice that received CD47−/− T cells versus 0.6 × 106 T cells in mice given CD47+/+ T cells (n = 6 per group individually analyzed; P < 0.001). Thus, CD47−/− T cells do not appear to expand efficiently in or are eliminated by CD47+/+ allogeneic recipients.
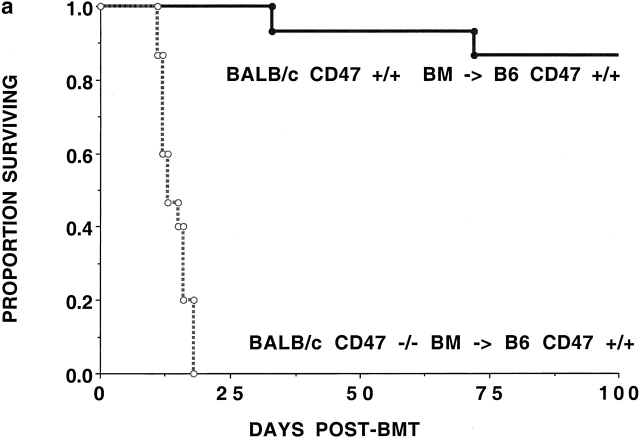
CD47− /− BM Cannot Reconstitute Lethally Irradiated CD47+/+ Allogeneic or Congenic Recipients.
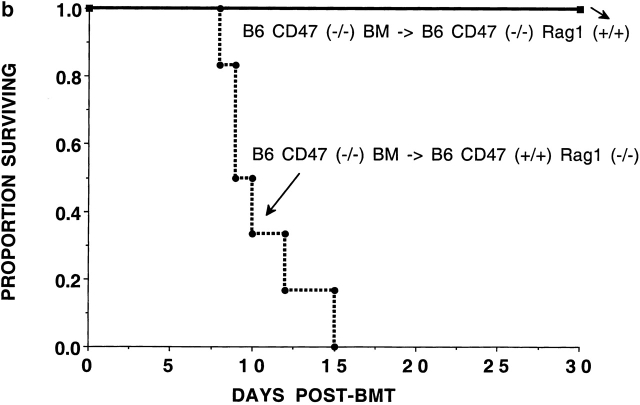
The data suggested that the reduction in GVHD by CD47−/− cells in CD47+/+ allogeneic recipients may not be due to a profound defect in generating an alloresponse. The reduced GVH response in irradiated recipients could be due to the clearance of CD47−/− T cells by CD47+/+ recipients. To determine whether CD47−/− BM cells would be eliminated by CD47+/+ recipients, irradiated (6.5 Gray TBI) B6 recipients were given Balb/c T cell–depleted BM grafts from CD47−/− or CD47+/+ donors. CD47+/+ BM infusion resulted in 87% survival and a mean of 79% donor cells in the peripheral blood at 2 mo after BMT. In marked contrast, recipients of CD47−/− BM cells died between days 12–19 after BMT (Fig. 2 a). Consistent with the presumed hematopoietic failure, recipients of CD47−/− BM cells experienced a progressive severe (28%) body weight loss. To determine whether the absence of CD47 expression was responsible for impaired hematopoietic reconstitution in the absence of an alloresponse, we infused congenic CD47−/− BM into CD47+/+ recipients. To preclude a T cell or B cell immune response to the donor graft, we used lethally irradiated (9.5 Gray TBI) CD47+/+Rag1−/− or Rag1+/+ mice as recipients of either CD47−/− or CD47+/+ BM. CD47−/− BM was able to rescue CD47−/− recipients from hematopoietic failure (Fig. 2 b). In contrast, CD47−/− BM was not able to rescue lethally irradiated CD47+/+ Rag1−/− recipients. Day 14 Hct values indicated that the Rag1−/− mice given CD47−/− but not CD47+/+ BM were dying of hematopoietic failure (13 ± 2% vs. 41 ± 1%; P < 0.001). Because congenic CD47−/− BM is able to rescue CD47−/− but not CD47+/+ recipients, these data are most consistent with a non-T cell, non-B cell immune–mediated resistance of CD47+/+ recipients to CD47−/− BM.
Figure 2.
CD47+/+ but not CD47−/− BM rescues lethally irradiated recipients. (a) B6 recipients were given 6.5 Gray TBI and infused with Balb/c-CD47+/+ or CD47−/− BM (n = 15 per group). Recipients of CD47−/− BM had a significantly lower survival versus those given CD47+/+ BM. (b) Lethally irradiated (9.5 Gray TBI) B6-CD47−/− or B6-CD47+/+ Rag1−/− recipients (n = 15 per group) were infused with B6-CD47−/− BM. B6-CD47−/− recipients of B6-CD47−/− BM had a significantly higher survival than B6-CD47+/+Rag1−/− recipients given B6-CD47−/− BM.
Further evidence as to whether CD47−/− BM cells would be preferentially eliminated in CD47+/+ recipients, lethally irradiated (9.0 Gray TBI) B6-Thy1.1 were given equal numbers of B6-CD47−/− (CD45.2+, Thy1.2+) and congenic B6-CD45.1 CD47+/+(Thy1.2+) BM cells. Long-term peripheral blood chimerism indicated that only B6-CD45.1 CD47+/+ cells repopulated the recipient (n = 8 per group). Recipient splenectomy did not alter long-term chimerism results, indicating that the spleen was not essential for the clearance of CD47−/− BM cells. Together, these data indicate that CD47−/− BM cells are preferentially eliminated by CD47+/+ recipients.
Because long-term reconstitution could not be achieved with CD47−/− BM, we used a short-term in vivo assay to assess the proliferation of CD47−/− BM cells in CD47+/+ recipients early after infusion. We considered the possibility that NK cells were involved in graft resistance as has been shown for congenic MHC class I−/− BM cells. When CD47−/− BM is given to lethally irradiated (9.5 Gray TBI) CD47−/− recipients, we observed high proliferation by day 5 after BMT (Table ). In marked contrast, CD47−/− BM was unable to proliferate in CD47+/+ recipients regardless as to whether host NK cells were depleted. These data indicate that CD47−/− BM is able to home to the spleen and proliferate in CD47−/− but not in CD47+/+ recipients early after BMT.
Table 1.
CD47−/− BM Cells Will Proliferate in CD47−/− but not CD47+/+ Recipients
| Experiment No. | No. Analyzed | Donor | Recipient | NK depletion | Mean ± SEM |
|---|---|---|---|---|---|
| 1 | 6 | −/− | −/− | no | 142,645 ± 24,908 |
| 1 | 6 | −/− | +/+ | no | 484 ± 114 |
| 1 | 6 | −/− | +/+ | yes | 4,225 ± 9,347 |
| 2 | 5 | −/− | −/− | no | 125,453 ± 22,089 |
| 2 | 5 | −/− | +/+ | no | 331 ± 47 |
| 2 | 5 | −/− | +/+ | yes | 407 ± 99 |
| 3 | 4 | −/− | −/− | no | 146,057 ± 17,621 |
| 3 | 5 | −/− | +/+ | no | 532 ± 248 |
| 3 | 5 | −/− | +/+ | yes | 518 ± 95 |
Host Macrophages Can Resist the Engraftment of CD47− /− Donor Cells Transferred into Allogeneic CD47+/+ Recipients.
To determine whether host CD47+/+ macrophages would clear CD47−/− donor T cells, NK-depleted Balb/c SCID recipients were given B6-CD47−/− or B6-CD47+/+ spleen cells and analyzed 6 d after transfer (Table ). In marked contrast to the fivefold expansion of CD47+/+ T cells, CD47−/− T cells failed to reconstitute the spleen of allogeneic SCID recipients. Host macrophage and partial DC depletion by clodronate reduced the expansion of allogeneic T cells in controls, suggesting that host APCs supported the expansion of CD47+/+ T cells in CD47+/+ recipients. In contrast, APC depletion increased the absolute number of donor CD4+ and CD8+ T cells in the spleen of SCID recipients of CD47−/− T cells, consistent with a partial restoration of donor CD47−/− T cell engraftment.
Table 2.
CD47−/− T Cells Have Impaired In Vivo Engraftment As Compared to CD47+/+ T Cells when Infused into NonconditionedAllogeneic Recipients
| Group | DMDP | Spleen No. | CD4+ | Total CD4+ | CD8+ | Total CD8+ |
|---|---|---|---|---|---|---|
| % | % | |||||
| +/+→Balb/c SCID | no | 25 ± 5 | 10 ± 1 | 2.4 ± 0.5 | 20 ± 5 | 5.0 ± 1.6 |
| +/+→Balb/c SCID | yes | 3 ± 2 | 13 ± 4 | 0.5 ± 0.4 | 34 ± 7 | 1.0 ± 0.4 |
| −/−→Balb/c SCID | no | 6 ± 2 | 0 ± 0 | 0.0 ± 0.0 | 0 ± 0 | 0.0 ± 0.0 |
| −/−→Balb/c SCID | yes | 2 ± 1 | 10 ± 3 | 0.2 ± 0.2 | 28 ± 18 | 0.4 ± 0.3, |
Nonconditioned, NK cell–depleted Balb/c SCID were given T cells (106 cells per recipient) from B6-CD47+/+ or B6-CD47−/−donors as indicated. Mice received either saline or liposomal DMDP injections on days 2 and 1. Four mice per group were individually studied on day 6 after transfer for evidence of engraftment of donor T cells in the spleen of recipients. Data for lymphoid cell subsets are shown. All cell numbers are ×10−6. DMDP, dichloromethylene diphosphanate (clodronate). No., number.
Next, we sought to determine whether macrophage-poor organs had a higher number of donor CD47−/− T cells as compared with macrophage-rich organs. As compared with nonconditioned or sublethal irradiated recipients, lethally irradiated recipients may have an impairment in the removal of donor CD47−/− T cells by CD47+/+ host cells. Therefore, we chose a lethal irradiation system in which B10.BR recipients were prepared with lethal irradiation (8.0 Gray TBI) to increase the likelihood of donor T cell infiltration into GVHD target organs. Cohorts of mice were reconstituted with BM and either B6 CD47+/+ or CD47−/− T cells. Recipients of allogeneic CD47−/− T cells uniformly succumbed to GVHD lethality, albeit significantly delayed as compared with recipients receiving CD47+/+ T cells (data not shown). Immunohistochemistry was performed on tissue sections obtained from known GVHD target organs (liver, colon, and skin) in this model system. On day 14 after BMT, there were at least 15-fold fewer donor T cells present in the liver of recipients of CD47−/− versus CD47+/+ T cells (mean values: CD4+ T cells, 9 ± 7 vs. 133 ± 11/mm2; CD8+ T cells: 7 ± 2 vs. 122 ± 32/mm2, respectively). The liver is a rich source of Kupffer cells, macrophages which clear cellular and particulate material. In lethally irradiated recipients of MHC disparate donor T cells, 25% of Kupffer cells are of host origin as late as 2 wk after BMT 18. Thus, the liver could function in part as a clearinghouse for CD47−/− T cells. In the skin, also a rich source of APCs, T cells were present at fivefold lower frequency in recipients of CD47−/− as compared with CD47+/+ T cells (mean values: CD4+ T cells, 19 ± 6 vs. 11 ± 1/mm2; CD8+ T cells: 36 ± 18 vs. 0 ± 0/mm2, respectively). In contrast, in other organs not as rich in macrophages, such as the colon, donor T cell infiltration in recipients of CD47−/− T cells was reduced by only 63% as compared with recipients of CD47+/+ T cells (mean values: CD4+ T cells, 91 ± 41 vs. 322 ± 89/mm2; CD8+ T cells: 150 ± 17 vs. 350 ± 35/mm2, respectively). These data are consistent with a greater degree of clearance of allogeneic CD47−/− T cells in macrophage-rich as compared with macrophage-poor organs in CD47+/+ recipients.
Host CD47+/+ Splenic DCs and Macrophages Rapidly Engulf Donor CD47− /− Lymphohematopoietic Cells In Vivo.
In contrast to the critical role of host macrophages in RBC clearance 8 11, these cells appeared to play only a partial role in lymphohematopoietic cell clearance. To directly examine which host cell populations would engulf CD47−/− lymphohematopoietic cells, B6-CD47+/+ recipients were given CFSE-labeled LN cells from B6-CD47−/− or CD47+/+ donors. Splenocytes were analyzed at various time periods after infusion to enumerate CD47−/− versus CD47+/+ cells and determine whether there was evidence that CD47−/− T cells were phagocytosed to a greater extent than CD47+/+ T cells. Flow cytometric analysis indicated that a higher proportion of splenic CD11b−CD11c+ DCs had phagocytosed CFSE-labeled CD47−/− than CD47+/+ congenic T cells by 1 h after infusion (5.5 vs. 1.0%, respectively; Fig. 3 a). Analysis of time kinetics revealed that CFSE-labeled CD47−/− versus CD47+/+ T cells had been engulfed by a higher proportion of CD11b−CD11c+ DCs (1, 2, and 4 h after infusion), CD11b+CD11c+ DCs (1 h), and F4/80+CD11c− macrophage populations (1 h; Fig. 3 b). Splenic sections obtained 1 h after infusion showed that CFSE-labeled T cells of both genotypes migrated to the periarteriolar sheath areas but approximately one-half of CFSE-labeled CD47−/− and virtually no CD47+/+ T cells could be found inside host CD11c+ DCs (Fig. 3 c). By 1 d after infusion, there were no detectable CFSE-labeled CD47−/− T cells present in the spleen as determined by either flow cytometry or splenic sections, in contrast to readily detectable CFSE-labeled CD47+/+ T cells which were still present (data not shown). Thus, CD11b− DCs and to a lesser extent CD11b+ DCs and macrophages preferentially engulfed CD47−/− versus CD47+/+ T cells.
Figure 3.
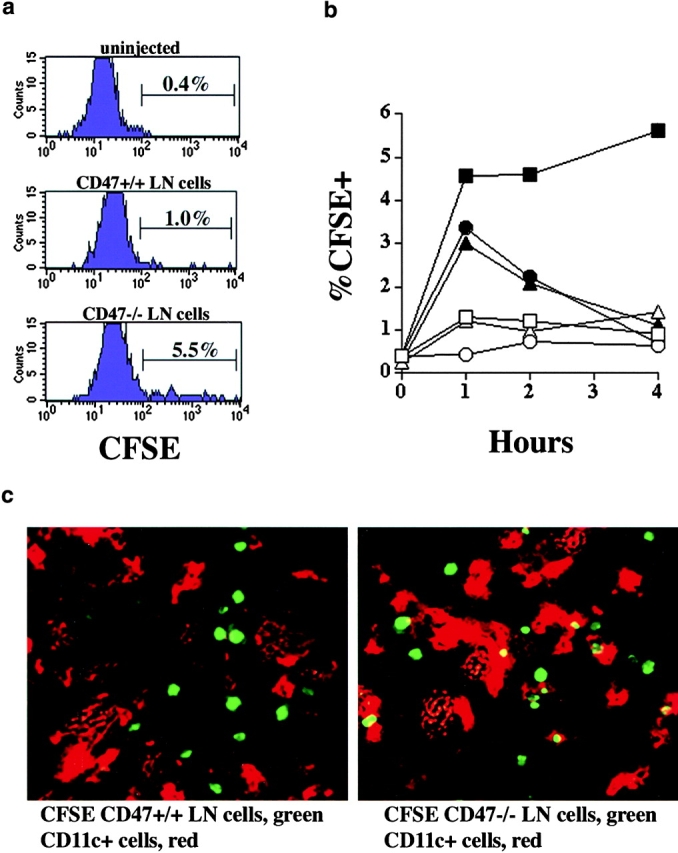
CD47−/− lymph node cells are cleared by APC in vivo. LN cells were isolated from B6-CD47+/+ or CD47−/− mice, CFSE-labeled, and injected (5 × 106 per mouse) intravenously into B6 recipients. Some mice were left uninjected (time 0 h). Spleens from mice were harvested 1, 2, and 4 h after injection (n = 3 per time point) and stained with mAb (anti-B220, anti-CD3, anti-CD11c, anti-CD11b, or anti-F4/80) to identify CD11b− DCs (B220−, CD3−, CD11c+), CD11b+ DCs (B220−, CD3−, CD11c+), and F4/80+ macrophages (B220−, CD3−, CD11c−). A portion of the spleens examined using confocal microscopic analysis. (a) Representative histograms depicting CD11b− DCs 1 h after injection of CD47+/+ cells (center), CD47−/− cells (bottom) or not injected (top) are shown. (b) Kinetics of the appearance of CFSE+CD11b−DCs (squares) CD11b+ DCs (circles), and F4/80+ macrophages (triangles) after injection of CD47+/+ (open symbols) or CD47−/− (closed symbols) LN cells. The percentage of CFSE+ cells contained within the indicated cell population is listed on the y axis. Values for standard error of the mean were all ≤26% except for CD11b+ DCs engulfing CD47+/+ T cells. At the indicated time points, there was a significantly (P ≥ 0.03) higher proportion of CD11b− CD11c+ (1, 2, 4 h) DCs, CD11b+ DCs (1 h), and F4/80+ macrophages (1 h) which had engulfed CFSE-labeled CD47−/− versus CD47+/+ cells. (c) Photomicrographs of spleen sections from mice at 1 h after injection with CFSE-labeled CD47+/+ (left) or CD47−/− (right) LN cells (green). CD11c+ DCs are red.
Discussion
Our data show that CD47 expression is necessary to prevent host CD47+/+ splenic DCs and macrophages from clearing CD47+/+ lymphohematopoietic cells. In the absence of CD47 expression, donor BM reconstituting cells are rapidly cleared in CD47+/+ recipients indicating that this antigen is expressed on short-term reconstituting BM cells. Congenic or allogeneic donor T cells which do not express CD47 are rapidly cleared by CD47+/+ DCs and macrophages. Thus, CD47 antigen on BM cells and T cells can function as a negative regulator of the scavenger effects of host DCs and macrophages in eliminating lymphohematopoietic cells which fail to express this antigen.
CD47−/− BM is able to proliferate and reconstitute lethally irradiated CD47−/− but not CD47+/+ recipients. These data are consistent with a clearing of CD47−/− hematopoietic progenitor cells by the host as further evidenced by an almost complete absence of BM proliferation early after transfer. Similarly, despite the pressure to generate effective alloresponses and for homeostatic expansion in vivo, B6-CD47−/− T cells could not be detected in allogeneic Balb/c SCID CD47+/+ recipients. Recently, we have reported that CD47−/− RBCs are cleared in CD47+/+ mice by splenic macrophages 8 11. The macrophage receptor engaged by CD47 is SIRPα, a signal-regulatory protein which inhibits tyrosine-kinase signaling pathways 8 11 19. NK inhibitory receptors also recruit and activate the same tyrosine-kinase signaling pathways so that NK cells won't eliminate lymphohematopoietic cells that express self-MHC class I antigens 20 21. Conversely, tumor cells that have lost or downregulated MHC class I antigens could be removed by NK cells 19. In an analogous fashion, splenic macrophages clear RBCs which lack MHC antigens. Although an ovarian cancer cell line has been shown to be CD47 deficient, it is possible that CD47 expression is downregulated on other types of tumors or damaged lymphohematopoietic cells 22. In those instances, a low level of CD47 expression may cause the host to eliminate these cells.
Regulation of CD47 expression may be an important mechanism for host macrophages to remove damaged RBCs since the clearance of opsonized RBCs is controlled by CD47-SIRPα. For other lymphohematopoietic cells such as T cells and BM cells, DCs appear to be more critical than macrophages in eliminating CD47−/− cells in a CD47+/+ microenvironment. We provide direct evidence that both CFSE-labeled CD47−/− and CD47+/+ T cells initially localized to the splenic periarteriolar sheath but that CD47−/− T cells were engulfed by CD11b− DCs and to a lesser extent by CD11b+ DCs and macrophages by 1 h after infusion, resulting in complete clearance of CD47−/− but not CD47+/+ T cells within 1 d after infusion. In contrast, RBCs normally stay within the macrophage-rich red pulp area of the spleen, which is why macrophages are the primary populations to eliminate CD47−/− RBCs. Consistent with CFSE-labeled T cell data, the elimination of host splenic CD11b+CD11c+ DCs and macrophages by clodronate only partially inhibited CD47−/− T cell clearance by CD47+/+ recipients. Because DCs are widely distributed throughout the body especially including DC-rich tissues such as the liver, lung, LN, and skin as well as the spleen, we hypothesize that the spleen may not be the major site of clearance for CD47−/− T cells or BM. In support of this hypothesis, irradiated, splenectomized recipients given equal numbers of congenic CD47−/− and CD47+/+ BM cells had exclusively congenic CD47+/+ BM-derived cells present in the peripheral blood when analyzed 1 mo later. Thus, CD47+/+ DCs and macrophages engulf and clear CD47−/− lymphohematopoietic cells, a process which can occur both within or outside the spleen. For lymphohematopoietic cells which express MHC antigens, CD47 expression may represent a safeguard for eliminating abnormal cells that have not downregulated MHC class I antigens.
We were unable to demonstrate a critical role for CD47 in regulating alloresponses. In vitro, MLR responses with CD47−/− T cells were similar to CD47+/+ T cells. In vivo, CD47−/− recipients could reject allogeneic donor BM grafts indicating that host antidonor responses by CD47−/− T cells were not markedly impaired. Several investigators have shown that CD47 can costimulate T cells that have received TCR signals 3 4 5 6 7 19. In nonirradiated or sublethally irradiated recipients, the donor T cells are rapidly cleared and therefore not available to mediate lethality. Because under lethal irradiation conditions, recipients of allogeneic CD47−/− T cells eventually succumbed to GVHD lethality, it is possible that heavy irradiation either eliminated SIRPα-expressing host cells or inhibited their function. Regardless, CD47−/− T cells can cause lethality, providing evidence that CD47−/− T cell alloresponses are not markedly impaired in vivo.
In summary, we have found that CD47 expression is required to prevent clearance of lymphohematopoietic cells by DCs and macrophages. This rapid response mechanism may protect the host from damaged or defective cells that downregulate CD47 antigen in disease states. Approaches to reduce CD47 antigen expression on lymphohematopoietic cells may provide a means of eliminating abnormal or harmful cells in various disease settings.
Acknowledgments
This work was supported in part by National Institutes of Health grants R01AI-34495, R01HL55209, R01HL63452, R01GM-57573, R01AI-33903, R01-AI-34285, R37HL-56067, P01AI-35225, and K08AI 01503. P.-A. Oldenborg was supported by a postdoctoral fellowship from the Umea University-Washington University exchange program and by the Swedish Medical Research Council.
Footnotes
B.R. Blazar and F.P. Lindberg contributed equally to this work.
P.-A. Oldenborg's present address is Department of Integrative Medical Biology, Section for Histology and Cell Biology, Umeå University SE-901 87, Umeå, Sweden.
References
- Brown E., Hooper L., Ho T., Gresham H. Integrin-associated proteina 50-kD plasma membrane antigen physically and functionally associated with integrins. J. Cell Biol. 1990;111:2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof B.A., Weerasinghe D., Brown E.J., Lindberg F.P., Hammel P., Piali L., Dessing M., Gisler R. Cross talk between α(v)β3 and α4β1 integrins regulates lymphocyte migration on vascular cell adhesion molecule 1. Eur. J. Immunol. 1997;27:3242–3252. doi: 10.1002/eji.1830271223. [DOI] [PubMed] [Google Scholar]
- Waclavicek M., Majdic O., Stulnig T., Berger M., Baumruker T., Knapp W., Pickl W.F. T cell stimulation via CD47agonistic and antagonistic effects of CD47 monoclonal antibody 1/1A4. J. Immunol. 1997;159:5345–5354. [PubMed] [Google Scholar]
- Reinhold M.I., Lindberg F.P., Kersh G.J., Allen P.M., Brown E.J. Costimulation of T cell activation by integrin-associated protein (CD47) is an adhesion-dependent, CD28-independent signaling pathway. J. Exp. Med. 1997;185:1–11. doi: 10.1084/jem.185.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticchioni M., Deckert M., Mary F., Bernard G., Brown E.J., Bernard A. Integrin-associated protein (CD47) is a comitogenic molecule on CD3-activated human T cells. J. Immunol. 1997;158:677–684. [PubMed] [Google Scholar]
- Reinhold M.I., Green J.M., Lindberg F.P., Ticchioni M., Brown E.J. Cell spreading distinguishes the mechanism of augmentation of T cell activation by integrin-associated protein/CD47 and CD28. Int. Immunol. 1999;11:707–718. doi: 10.1093/intimm/11.5.707. [DOI] [PubMed] [Google Scholar]
- Pettersen R.D., Hestdal K., Olafsen M.K., Lie S.O., Lindberg F.P. CD47 signals T cell death. J. Immunol. 1999;162:7031–7040. [PubMed] [Google Scholar]
- Oldenborg P.A., Zheleznyak A., Fang Y.F., Lagenaur C.F., Gresham H.D., Lindberg F.P. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- Seiffert M., Brossart P., Cant C., Cella M., Colonna M., Brugger W., Kanz L., Ullrich A., Buhring H.J. Signal-regulatory protein α (SIRPα) but not SIRPβ is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34+CD38− hematopoietic cells. Blood. 2001;97:2741–2749. doi: 10.1182/blood.v97.9.2741. [DOI] [PubMed] [Google Scholar]
- Lindberg F.P., Bullard D.C., Caver T.E., Gresham H.D., Beaudet A.L., Brown E.J. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- Oldenborg P.A., Gresham H.D., Lindberg F.P. CD47-signal regulatory protein α (SIRPα) regulates Fcγ and complement receptor-mediated phagocytosis. J. Exp. Med. 2001;193:855–862. doi: 10.1084/jem.193.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W.J., Blazar B.R. New strategies for preventing graft-versus-host disease. Curr. Opin. Immunol. 1999;11:509–515. doi: 10.1016/s0952-7915(99)00002-3. [DOI] [PubMed] [Google Scholar]
- Blazar B.R., Taylor P.A., Panoskaltsis-Mortari A., Sharpe A.H., Vallera D.A. Opposing roles of CD28:B7 and CTLA-4:B7 pathways in regulating in vivo alloresponses in murine recipients of MHC disparate T cells. J. Immunol. 1999;162:6368–6377. [PubMed] [Google Scholar]
- Van Rooijen N., Sanders A. Liposome mediated depletion of macrophagesmechanism of action, preparation of liposomes and applications. J. Immunol. Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Blazar B.R., Taylor P.A., Panoskaltsis-Mortari A., Yagita H., Bromberg J.S., Vallera D.A. A critical role for CD48 antigen in regulating alloengraftment and lymphohematopoietic recovery after bone marrow transplantation. Blood. 1998;92:4453–4463. [PubMed] [Google Scholar]
- Iizuka K., Chaplin D.D., Wang Y., Wu Q., Pegg L.E., Yokoyama W.M., Fu Y.X. Requirement for membrane lymphotoxin in natural killer cell development. Proc. Natl. Acad. Sci. USA. 1999;96:6336–6340. doi: 10.1073/pnas.96.11.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingulli E., Mondino A., Khoruts A., Jenkins M.K. In vivo detection of dendritic cell antigen presentation to CD4+ T cells. J. Exp. Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis K., Sharp H.L., Vallera D.A., Blazar B.R. Kupffer cell engraftment across the major histocompatibility barrier in micebone marrow origin, class II antigen expression, and antigen-presenting capacity. J. Pediatr. Gastroenterol. Nutr. 1990;11:525–533. doi: 10.1097/00005176-199011000-00014. [DOI] [PubMed] [Google Scholar]
- Vernon-Wilson E.F., Kee W.J., Willis A.C., Barclay A.N., Simmons D.L., Brown M.H. CD47 is a ligand for rat macrophage membrane signal regulatory protein SIRP (OX41) and human SIRPα 1. Eur. J. Immunol. 2000;30:2130–2137. doi: 10.1002/1521-4141(2000)30:8<2130::AID-IMMU2130>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Yokoyama W.M. Natural killer cells. Right-side-up and up-side-down NK–cell receptors. Curr. Biol. 1995;5:982–985. doi: 10.1016/s0960-9822(95)00194-1. [DOI] [PubMed] [Google Scholar]
- Ravetch J.V., Lanier L.L. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- Gao A.G., Lindberg F.P., Finn M.B., Blystone S.D., Brown E.J., Frazier W.A. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J. Biol. Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]