Abstract
Bacterial pneumonia is an increasing complication of HIV infection and inversely correlates with the CD4+ lymphocyte count. Interleukin (IL)-17 is a cytokine produced principally by CD4+ T cells, which induces granulopoiesis via granulocyte colony-stimulating factor (G-CSF) production and induces CXC chemokines. We hypothesized that IL-17 receptor (IL-17R) signaling is critical for G-CSF and CXC chemokine production and lung host defenses. To test this, we used a model of Klebsiella pneumoniae lung infection in mice genetically deficient in IL-17R or in mice overexpressing a soluble IL-17R. IL-17R–deficient mice were exquisitely sensitive to intranasal K. pneumoniae with 100% mortality after 48 h compared with only 40% mortality in controls. IL-17R knockout (KO) mice displayed a significant delay in neutrophil recruitment into the alveolar space, and had greater dissemination of K. pneumoniae compared with control mice. This defect was associated with a significant reduction in steady-state levels of G-CSF and macrophage inflammatory protein (MIP)-2 mRNA and protein in the lung in response to the K. pneumoniae challenge in IL-17R KO mice. Thus, IL-17R signaling is critical for optimal production of G-CSF and MIP-2 and local control of pulmonary K. pneumoniae infection. These data support impaired IL-17R signaling as a potential mechanism by which deficiency of CD4 lymphocytes predisposes to bacterial pneumonia.
Keywords: IL-17, T lymphocyte, granulocyte-colony stimulating factor, Klebsiella pneumoniae, chemokine
Introduction
The development of bacterial pneumonia depends on a complex interplay between virulence factors of the organism and lung host defenses. Host resistance requires both innate and adaptive immune responses, which are largely initiated by growth factors termed cytokines. Bacterial pneumonias are an increasingly recognized complication of HIV infection and the incidence of these infections inversely correlates with the CD4+ lymphocyte count 1 2. Some investigators have hypothesized that the increased susceptibility to bacterial pneumonia is due to lack of T cell help and generation of opsonic antibody by B cells 3. Recently a novel cytokine, IL-17, has been found to be produced largely by activated CD4+ lymphocytes 4 5. IL-17 has been shown to stimulate granulopoiesis both in vitro 6 and in vivo 7. Our laboratory has demonstrated that induction of in vivo granulopoiesis by IL-17 is partly due to stimulation of G-CSF and the transmembrane form of stem cell factor 8. Moreover, IL-17 has been shown to induce the production of TNF-α 9 and CXC chemokines 10 in the lung, and our lab has demonstrated its release into the lung in experimental Klebsiella pneumoniae infection 11. In contrast to the relative restriction of IL-17, the IL-17R is ubiquitously expressed in a variety of tissues 12. Based on these data we hypothesized that IL-17R signaling is critical for lung CXC chemokine generation, induction of G-CSF, a cytokine required for ongoing neutrophil production in response to infection, and lung host defense 13. We tested this hypothesis in mice deficient in IL-17R signaling using IL-17R knockout (KO) mice or mice overexpressing a soluble IL-17R:Fc fusion protein to exclude a development defect associated with the IL-17R KO mice. Using a well-described model of K. pneumoniae infection we found that IL-17R signaling is critical for early neutrophil recruitment and local induction of G-CSF and macrophage inflammatory protein (MIP)-2 at both the mRNA and protein level. Moreover, IL-17R–deficient mice had a significantly greater incidence of bacteremia and mortality in response to K. pneumoniae infection.
Materials and Methods
Generation of IL-17R–deficient Mice by Homologous Recombination.
Genomic clones encoding murine IL-17R were isolated from a 129 derived lambda library using a murine IL-17R cDNA probe 4 and mapped by a combination of PCR, restriction digest, and sequence analyses using deposited genomic sequences corresponding to IL-17R locus on mouse chromosome 6 (GenBank/EMBL/DDBJ accession no. AC018559). A gene targeting vector was constructed by replacing 5.7 kb of genomic sequence containing exons 4–11 (corresponding to nucleotides 445–1,172 of the murine IL-17R cDNA) with a PGKneo cassette. A thymidine kinase cassette (MC-TK) was inserted into the 5′ end of the vector. 129 derived embryonic stem (ES) cells were electroporated with the targeting vector and selected in the presence of G418 and ganciclovir as described 14. ES clones carrying a targeted mutation in IL-17R were identified by a combination of PCR and genomic Southern blot analyses and were injected into C57BL/6 blastocysts. The resulting male chimeras were crossed to C57BL/6 females to generate mice heterozygous for the IL-17R mutation (IL-17R+/−), which were subsequently intercrossed to generate IL-17R–deficient mice (IL-17R KO). These mice were moved to a C57BL/6 background by five successive backcrosses to C57BL/6 mice.
Animals.
Specific pathogen-free C57BL/6 mice (6–8-wk-old, male, average weight 25 g; National Cancer Institute, Frederick, MD) were used in all experiments as the control mice. IL-17R KO mice were obtained from Immunex Corporation and bred in LSUHSC Animal Care Facilities. The IL-17R KO mice were confirmed genetically by PCR. Male 6–8-wk-old IL-17R KO and C57BL/6 mice were used in this study. IL-17R+/− mice were generated by crossing IL-17R KO mice with C57BL/6 mice. All mice were housed in specific pathogen-free room within the Animal Care Facility of Louisiana State University Medical Center Vivarium under an IACUC-approved protocol, provided with water ad libitum and food, and housed under 12–h light/dark cycles until date of experiment.
IL-17 Binding and Flow Cytometry.
Surface IL-17R expression was detected by flow cytometry using murine IL-17.Fc on gated populations of either splenocytes, or peritoneal exudate cells obtained 18 h after intraperitoneal injection of 3 ml 3% thioglycollate broth (Sigma-Aldrich). Briefly, after incubation in 50 μl blocking buffer containing 2% normal mouse serum, 2% normal goat serum, and 10 μg Fc Block (BD PharMingen), 106 cells were incubated with either 20 μg/ml murine IL-17.Fc or the negative control, human IL-4R.Fc, and then stained with biotinylated mouse anti–human IgG1 Fc (Jackson ImmunoResearch Laboratories) and streptavidin-allophycocyanin (APC; BD PharMingen). PE- and FITC-conjugated antibodies against CD4, CD8, Gr-1, and CD19 were purchased from BD PharMingen.
K. pneumoniae Infection.
K. pneumoniae strain 43816, serotype 2 (American Type Culture Collection) was grown in tryptic soy broth (Difco) for 18 h at 37°C. 1 ml of the culture was added into 200 ml of fresh tryptic soy broth, and grown for another 2 h until the organism reached log phage. Bacteria was pelleted by centrifugation at 5,000 rpm for 15 min, washed twice in PBS, and resuspended at desired concentration. The concentration of K. pneumoniae was determined by measuring the absorbency at 600 nm. A standard of absorbencies based on known CFUs was used to calculate inoculum concentration. For inoculation, mice were briefly anesthetized with ether. The bacterial inoculum (50 μl) was applied to the nose tip of mouse with a pipet tip and involuntarily inhaled. Mice were held vertically for 2 min after the inoculation.
Bronchoalveolar Lavage.
At serial time points, mice were anesthetized with Ketamine (Schering-Plough Animal Health Corp). A tracheal cannula was inserted into the upper cervical trachea through a tracheotomy. The lungs were lavaged with 0.5-ml aliquots of warmed PBS + 0.5 mM EDTA up to 11 ml. The first ml of recovered bronchoalveolar lavage (BAL) fluid was centrifuged at 500 g and the supernatant was stored at –80°C for subsequent cytokine ELISAs. The remaining cell pellet was combined with the other lavage fluid and analyzed for total cell count followed by differential cell counts performed in cytospins stained with Wright-Giemsa (Baxter McGaw Park).
Lung Histological Examination.
At serial time points, mice were anesthetized followed by euthanasia by transection of the abdominal aorta. The lungs were removed en bloc and inflated with 10% formalin up to 20 cm H2O. The lungs were placed in 10% formalin and subsequently embedded in paraffin. Sections were stained with hematoxylin and eosin for histological examination.
K. pneumoniae CFU in Lung Homogenates.
At serial time points after K. pneumoniae challenge, mice were killed and the lungs were then removed aseptically. The lung was placed in sterile PBS (volume ml = 0.9 × the lung tissue weight g) and homogenized with a tissue homogenizer. The lung homogenates were placed on ice, and serial 1:10 dilutions were made. 10 μl of each dilution were plated on soy base blood agar plates (Difco) and incubated for 18 h at 37°C, then colonies were counted. Bacterial concentrations were expressed in terms of CFU/lung.
Total RNA Isolation and Ribonuclease Protection Assays.
At serial time points after K. pneumoniae infection, mice were euthanized and the lungs were perfused blood free by injection of PBS through the right ventricle. Lungs were homogenized in Trizol solution (Life Technologies). 20 μg of total RNA from each sample was used for ribonuclease protection assay (RPA) with mCK-4 and mCK-5 templates, purchased from BD PharMingen, following the manufacturer's protocol.
ELISA for MIP-2, G-CSF, and IL-17.
MIP-2 and IL-17 was measured by ELISA kits (R&D Systems) following the manufacturer's protocol. G-CSF was measured using a sandwich ELISA using matched antibody pairs from R&D Systems.
Statistical Analysis.
Comparison between means was performed with analysis of variance (ANOVA) followed by Fisher's follow-up testing. Survival comparisons were performed using Kaplan-Meier analysis (StatView; Abacus Concepts). P < 0.05 was considered to represent a significant difference.
Results
Generation of IL-17R KO Mice.
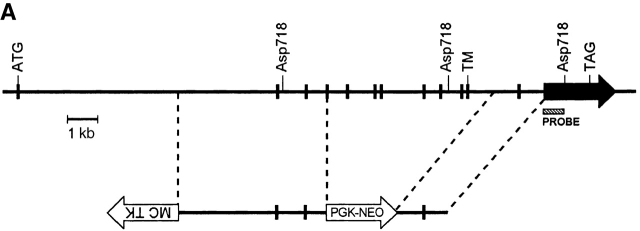
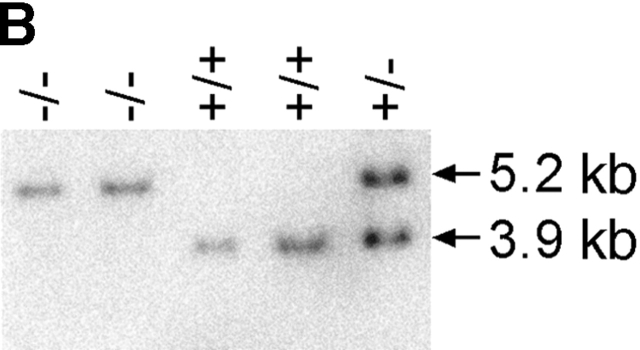
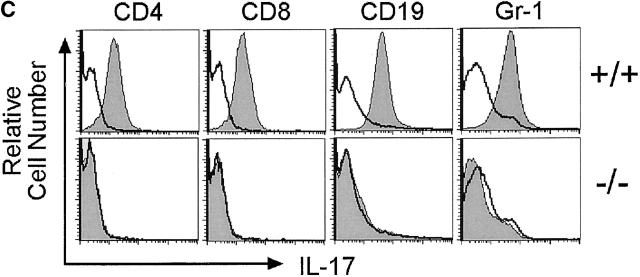
Mice genetically deficient in IL-17R (IL-17R KO) were generated by homologous recombination in ES cells using a targeting vector that replaces exons 4–11, encoding nucleotides 445–1,172 of the IL-17R cDNA 4, with a PGK-neo cassette (Fig. 1 A). Southern blot analyses of genomic DNAs derived from wild-type, IL-17R+/−, and IL-17R KO genomic confirmed that the IL-17R gene was disrupted by specific gene targeting (Fig. 1 B). IL-17R KO mice were born at the expected Mendelian frequency from IL-17R+/− intercrosses (data not shown) and displayed no gross external phenotype. To confirm that the targeted mutation abrogates IL-17 binding, flow cytometric analyses using IL-17:Fc 4 were performed on subpopulations of wild-type and IL-17R KO spleen and peritoneal exudate cells. As shown in Fig. 1 C, IL-17R KO T cells, B cells, and myeloid cells fail to bind IL-17, whereas binding is clearly observed to wild-type cells.
Figure 1.
Generation of IL-17R KO mice. (A) A gene targeting vector was constructed that replaces exons 4–11 with a PGK-neo cassette. A thymidine kinase cassette (MC-TK) was inserted into the 5′ end of the vector in the opposite orientation of the IL-17R gene. Exons are depicted as filled boxes. The initiation (ATG) and termination (TAG) codons as well as the transmembrane domain (TM) are indicated. The Asp718 restriction sites and probe used for genomic Southern blot analyses are indicated. (B) Genomic DNAs from wild-type (+/+), IL-17R+/−, and IL-17R KO mice were digested with Asp718 and subject to Southern blot analysis using the depicted probe. The sizes of the mutant and wild-type alleles are indicated. (C) Analysis of IL-17 binding to wild-type and IL-17R–deficient cells. The binding of IL-17:Fc to wild-type (+/+) and IL-17R–deficient (−/−) spleen cells gated on expression of the T cell markers CD4 or CD8 or the B cell marker CD19 as well as thioglycollate elicited peritoneal exudate cells gated on expression of the granulocytic marker Gr-1 was determined by flow cytometry and shown in the shaded histograms. Background binding to an irrelevant Fc protein is shown in the open histograms.
IL-17R Signaling Is Required for Survival Against K. pneumoniae Infection.
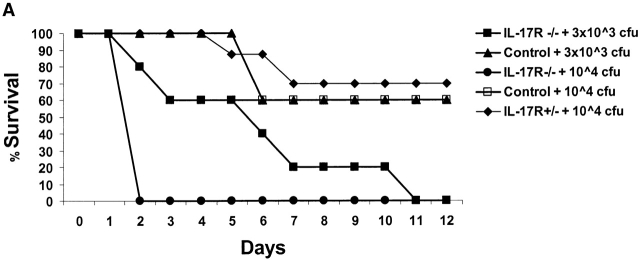
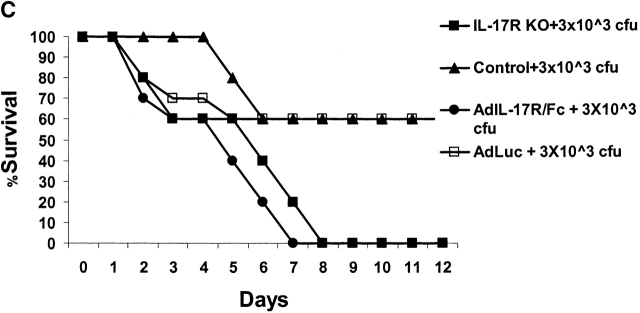
Pilot studies were performed with varying does (3 × 103–106 CFU) of K. pneumoniae given intranasally to 6–8-wk-old male C57BL/6 mice (n = 56). Based on these experiments, the LD50 (at 12 d) of K. pneumoniae was determined to be 3 × 104 CFU. We have previously shown that this inoculum can induce IL-17 within 12 h of infection in the lungs of mice 11. Based on these studies we chose 3 × 103 and 104 CFU for subsequent intranasal challenges in control and IL-17R–deficient mice. IL-17R KO mice showed a significantly reduced survival rate after K. pneumoniae inoculation. At the 104 CFU dose, 100% of IL-17R KO mice perished by 48 h, compared with only 20% of the controls (Fig. 2 A). At 3 × 103 CFU dose, 80% of IL-17R KO mice were dead by day 7, compared with only 40% of the controls (Fig. 2 A). Although IL-17R KO mice have normal peripheral blood counts and normal numbers of CD4+ and CD8+ lymphocytes in the blood and spleen 12, we needed to exclude any potential developmental defects in these mice, which could, in part, explain a reduced survival to K. pneumoniae challenge. To accomplish this, we constructed a fusion protein consisting of the IL-17R extracellular domain with the murine IgG1 CH2 and CH3 domains using recombinant PCR as described previously by our group with the TNF p55 extracellular domain 15. The IL-17R:Fc fusion protein migrated as a 140-kD dimer on nonreduced SDS-PAGE (Fig. 2 B). Moreover, this protein is biologically active, as it significantly inhibited recombinant IL-17–induced production of IL-6 by 3T3 fibroblasts (data not shown). To express this construct in vivo we generated an E1-deleted recombinant adenovirus (AdIL17R/Fc) as described previously 15. C57BL/6 mice were administered 109 PFU of AdIL17R/Fc or AdLuc (which encodes firefly luciferase) as a control. 72 h later (the time of maximal expression of the IL-17R:Fc fusion protein), mice were given an intranasal challenge with 3 × 103 CFU K. pneumoniae. Parallel studies were performed in IL-17R KO mice or littermate controls. Both AdIL-17R/Fc–treated mice and IL-17R KO mice had a similar phenotype in that their survival was significantly worse than control mice (Fig. 2 C).
Figure 2.
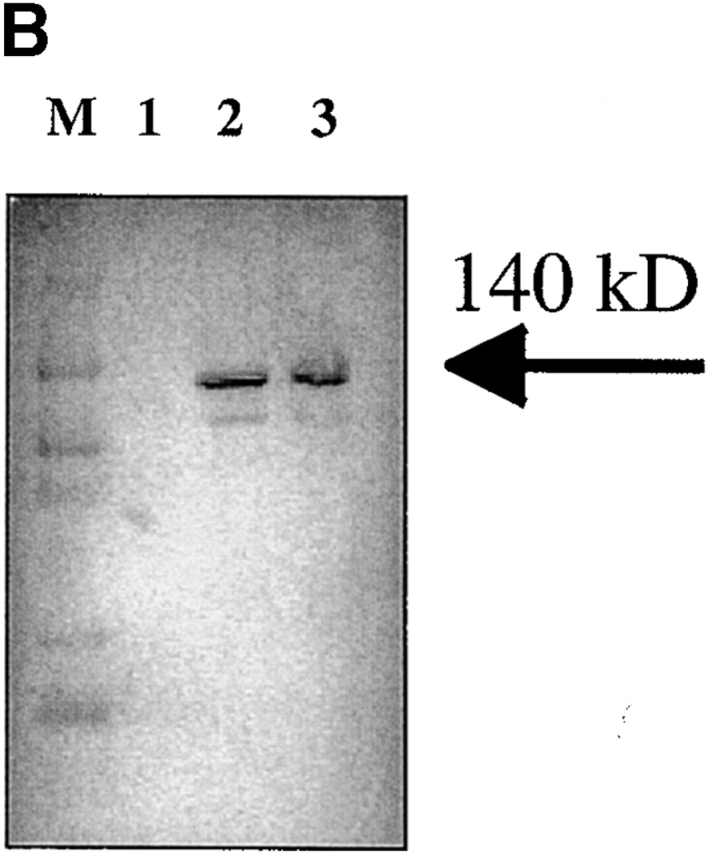
Reduced survival of mice deficient in IL-17R signaling. (A) IL-17R KO, IL-17R+/−, or control mice (n = 10 each group) were challenged with intranasal K. pneumoniae and survival was recorded every 12 h. To confirm that the reduced survival phenotype was due to a lack of IL-17R signaling and not due to a developmental defect, 6–8-wk-old IL-17R KO mice or C57BL/6 mice infused with AdIL-17R Fc (Western blot of IL-17R/Fc fusion protein depicted in B) or a control virus were challenged with intranasal K. pneumoniae and survival was recorded every 12 h (C; n = 10 each group).
Lung Bacterial Burden after K. pneumoniae Intranasal Challenge.
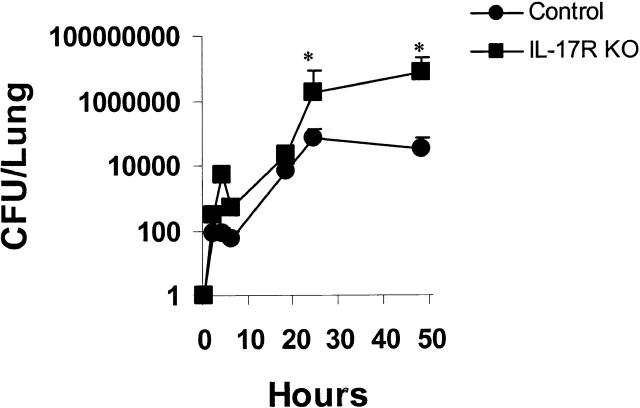
The bacterial burden of K. pneumoniae was determined by quantitative culture of lung homogenates. Intrapulmonary growth of K. pneumoniae was similar in control and IL-17R KO mice for the first 18 h of infection (Fig. 3). However, by 24 h there were significantly greater organisms in the lungs of IL-17R KO mice compared with control mice. This accentuated growth was observed up to 48 h. Thereafter, there was significant mortality in the IL-17R KO mice and thus comparisons of bacterial burdens at late time points between the IL-17R KO mice and control mice were not made due to selection bias for surviving mice. However, surviving control mice showed a decline in lung burden by 56 h (data not shown). Moreover, IL-17R KO mice all had positive spleen culture at 18 h after K. pneumoniae challenge (n = 10) compared with only 2 of 10 control mice.
Figure 3.
K. pneumoniae growth curves in control and IL-17R KO mice (n = 4–6, *P < 0.05).
Failure of IL-17R KO Mice to Mobilize and Recruit Neutrophils to the Lung.
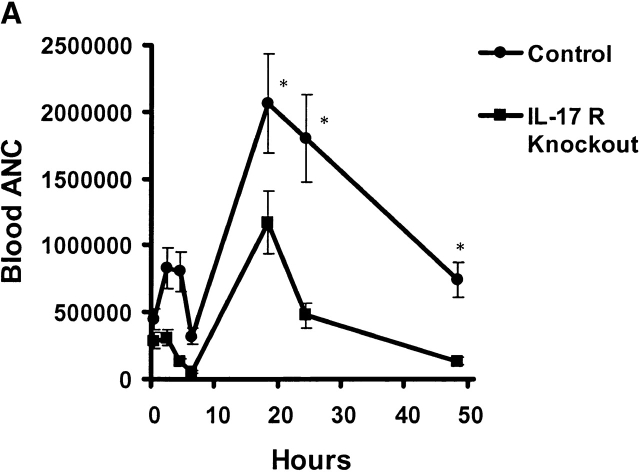
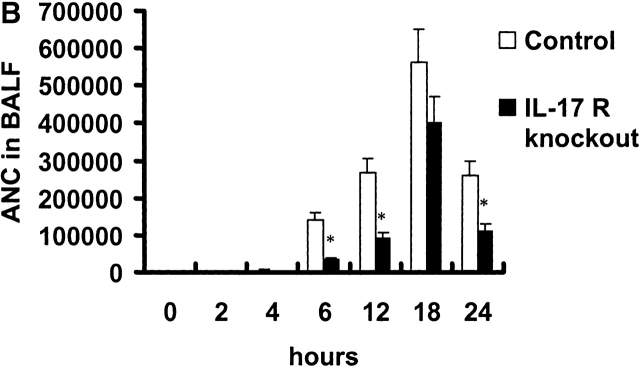
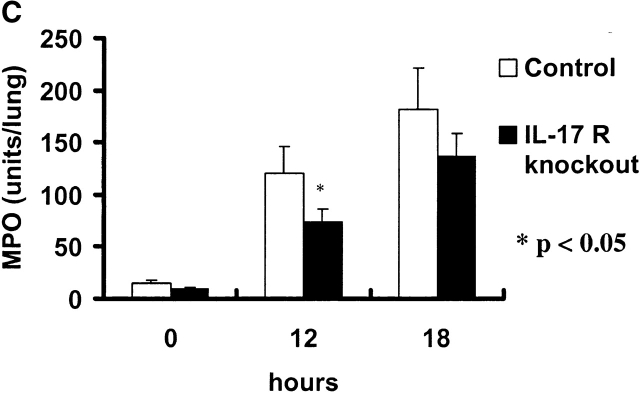
Absolute neutrophil counts (ANCs) were measured in blood and BAL fluid over time in control and IL-17R KO mice after K. pneumoniae challenge. Baseline blood ANC was similar in both groups of mice (Fig. 4 A). After K. pneumoniae challenge, control mice demonstrated a mild increase in blood ANCs up to 6 h, which was not observed in IL-17R KO mice (Fig. 4 A). Both groups of mice showed a transient fall in blood ANCs at 6 h. This was followed by a significant increase in blood ANCs in control mice, which peaked at 18 h, and slowly declined as the infection was controlled (Fig. 4 A). In contrast, IL-17R KO mice showed a significant attenuation in blood ANCs at 18, 24, and 48 h and these mice were significantly neutropenic by 48 h, consistent with overwhelming infection. Control mice demonstrated the ability to recruit significant numbers of neutrophils into the lung, as measured by the ANCs in the BAL fluid (Fig. 4 B). In contrast, IL-17R KO mice displayed decreased neutrophil recruitment, which was statistically lower than control mice up to 18 h into the infection (Fig. 4 B). This decreased neutrophil recruitment was also supported by a reduced level of lung myeloperoxidase activity measured at 12 h into the infection (Fig. 4 C).
Figure 4.
Reduced ANCs in the blood and lungs of IL-17R KO mice in response to K. pneumoniae infection. (A) Attenuation of increase in blood ANC in IL-17 R KO mice (n = 5–8, * denotes P < 0.05). (B) Reduction in lung neutrophil recruitment as measured as ANCs in BAL fluid (BALF) in IL-17R KO mice (n = 5–8, * denotes P < 0.05). (C) The reduced lung neutrophil recruitment was associated with a significant decrease in lung MPO activity in IL-17 R KO mice (n = 5–8, * denotes P < 0.05).
Lungs from both control and IL-17R KO mice were morphologically normal before K. pneumoniae challenge (Fig. 5). 18 h after 104 CFU, both groups of animals showed focal peribronchiolar inflammation (Fig. 5). However, by 24 h, IL-17R KO mice developed significant areas of perivascular edema and associated with large number of organisms in the perivenous space (Fig. 5). The combination of delayed neutrophil recruitment and the early dissemination of bacteria as measured by splenic culture and histologically by perivascular organisms led us to investigate known factors involved in early host defense against K. pneumoniae, specifically the CXC chemokine, MIP-2 16. Moreover, a failure to increase peripheral blood ANCs suggested that IL-17R signaling was required for infection-induced granulopoiesis, which is partly dependent on G-CSF and stem cell factor (SCF; reference 8). This led us to investigate whether IL-17R signaling was involved in lung MIP-2, G-CSF, and SCF production.
Figure 5.
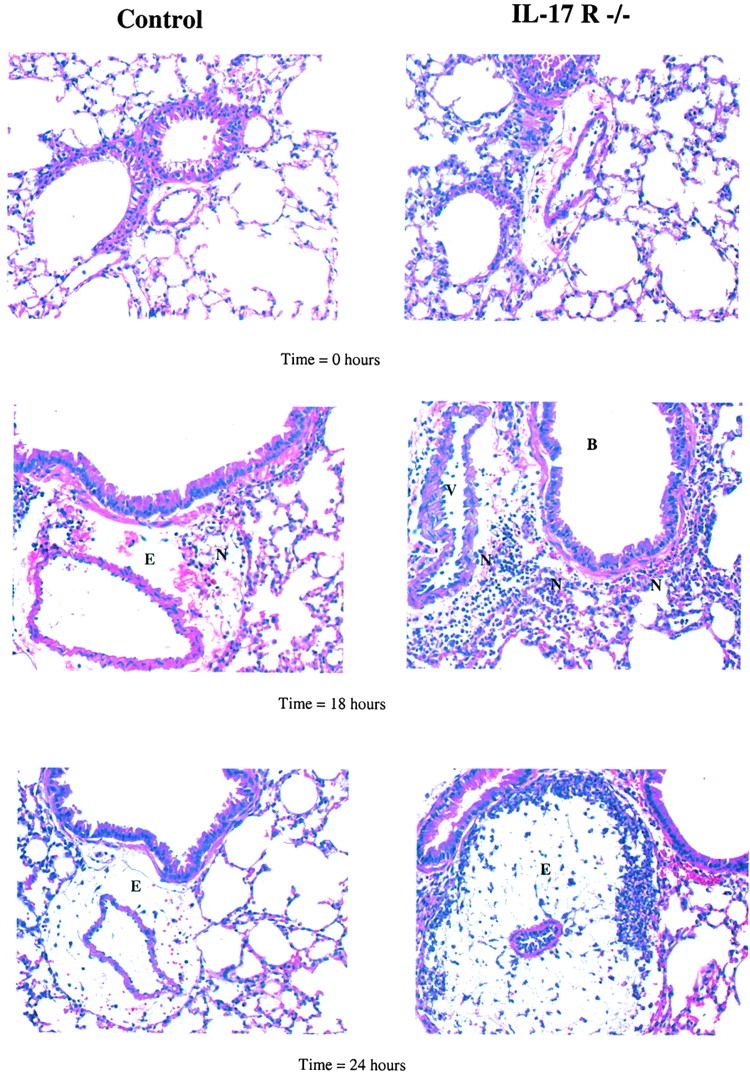
Representative lung histology of control and IL-17R KO mice after K. pneumoniae challenge. At 0 h, the lungs of control wild-type mice and IL-17R KO mice were essentially normal with no inflammatory infiltrate and mild edema. 18 h after infection there is minimal perivascular infiltration of neutrophils and moderate edema in the lungs of wild-type mice at 18 h after infection. In contrast, IL-17R KO mice had perivascular, peribronchiolar, and intraalveolar neutrophils at 18 h after infection associated with moderate diffuse perivascular edema. At 24 h after infection, wild-type mice had minimal perivascular infiltration of neutrophils and moderate edema in the lungs. In contrast, IL17R−/− mice had severe perivascular edema that partially compressed and distorted adjacent alveoli. Within, and marginating the edema were large numbers of degenerate neutrophils and large numbers of bacterial rods compared with few visible bacteria in control mice (all images 40×).
Failure of IL-17R KO Mice to Induce expression of MIP-2, G-CSF, and SCF.
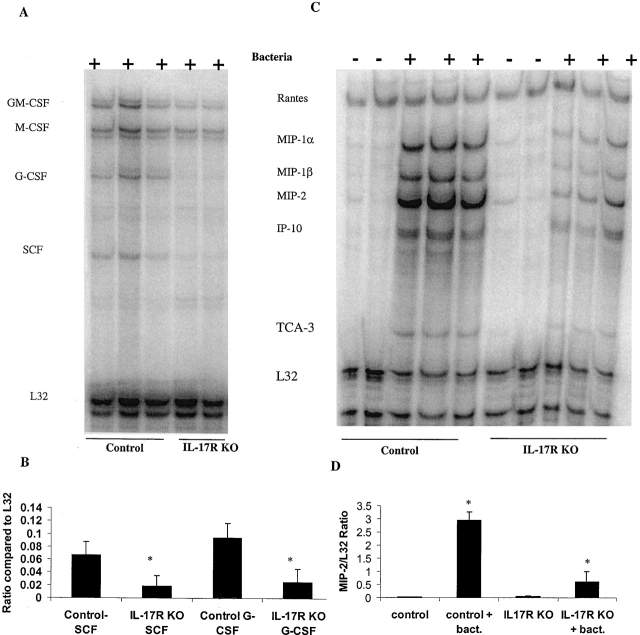
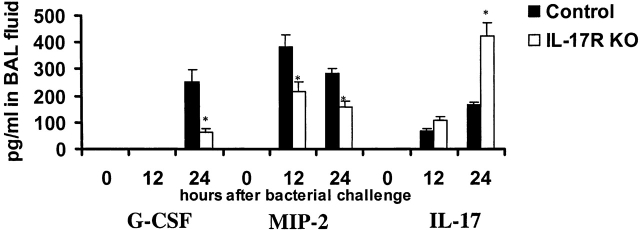
For these experiments both IL-17R KO and control mice were challenged intranasally with 104 CFU of K. pneumoniae. 12 h later when bacterial burdens were similar, but myeloperoxidase (MPO) and neutrophil recruitment were significantly different between both groups of animals, mice were killed and total RNA was isolated from whole lung. Transcripts for G-CSF, SCF, and MIP-2 were measured by RPA. Transcripts were normalized to the L32 transcript. The results indicated that after the K. pneumoniae challenge, IL-17R KO mice failed to significantly induce either G-CSF or SCF (Fig. 6). No G-CSF or SCF transcripts were detected in unchallenged IL-17 R KO or control mice (data not shown). Moreover, IL-17R KO mice also demonstrated significantly lower transcripts for MIP-1α, MIP-1β, and MIP-2 (Fig. 6). Attenuation of lung MIP-2 in IL-17R KO mice was also confirmed at the protein level at both 12 and 24 h after K. pneumoniae infection. No G-CSF protein was detected in the BAL fluid 12 h after K. pneumoniae infection. However, G-CSF was readily detectable at 24 h in BAL fluid of control mice but was reduced by over 60% in IL-17R KO mice (Fig. 7). IL-17 was also readily detectable in BAL fluid at 12 and 18 h after K. pneumoniae infection in both control and IL-17R KO mice (Fig. 7) and thus, local release of IL-17 is part of the normal host response to this infection.
Figure 6.
Analysis of lung gene expression by RPA. (A) Representative gel of lung G-CSF and SCF gene expression after K. pneumoniae challenge. (B) Densitometric analysis of G-CSF and SCF gene expression (n = 3–4, *P < 0.05). (C) Representative gel of lung chemokine gene expression after K. pneumoniae challenge. (D) Densitometric analysis of MIP-2 gene expression (n = 3–4, * P < 0.05). bact., bacteria.
Figure 7.
BAL fluid levels of G-CSF, MIP-2, and IL-17 after K. pneumoniae challenge.
Our laboratory has previously shown that G-CSF is not compartmentalized in the lung such as other growth factors such as TNF 17 18. Thus, the lung may be a significant source of G-CSF during infection, which results in an appropriate granulopoietic response 17. To investigate whether granulopoiesis was altered in response to infection, splenic CFU-GM and high proliferative potential (HPP) colonies were measured at 0 and 18 h after K. pneumoniae infection, as described previously 7. Control mice demonstrated a 98 ± 16% increase in CFU-GM and a 189 ± 31% increase in HPP, whereas IL-17R KO mice showed a 7 ± 5% and a 15 ± 8% decrease (P < 0.01, ANOVA). These data are consistent with the fact that IL-17R KO could not appropriately increase their ANCs in response to K. pneumoniae infection (Fig. 4).
Discussion
IL-17, a 32-kD dimer, is a cytokine produced principally by activated CD4+ T cells. It signals through a unique type I transmembrane receptor, which is ubiquitously distributed. Interestingly, the cytoplasmic domain has no known homology with existing known signaling pathways 12. IL-17 clearly supports hematopoiesis both in vitro and in vivo 6 7. IL-17–induced hematopoiesis results in maturation of cells along the granulocytic pathway 6 7. Moreover, IL-17 has been shown to induce translocation of nuclear factor (NF)-κB and production of IL-1 and TNF by monocyte/macrophages 9. The proinflammatory nature of this cytokine has also been implicated in inflammatory arthritis 9 19 20, lung inflammation 10 21, organ allograft rejection 22, and infection with K. pneumoniae 11. To further define the role of this ligand/receptor in regulating inflammatory response, we analyzed the response of mice deficient in IL-17 signaling to K. pneumoniae infection. We chose this model because host defense is dependent on CXC chemokine expression 16 and data from our laboratory and others has demonstrated that IL-17 is a potent inducer of lung chemokine expression 10. Moreover, microbial lipopeptides have been shown to be potent inducers of IL-17 in T cells 23.
Our data demonstrate that IL-17R signaling is critical for lung chemokine gene expression, early lung neutrophil recruitment, and survival against K. pneumoniae challenge. Moreover, IL-17 was readily detectable in BAL fluid as early as 12 h after K. pneumoniae infection and thus is a normal response to infection with this pathogen. In fact, IL-17R KO mice had higher levels of IL-17, suggesting that this receptor is important in clearance of IL-17 from the lung. Although we did not directly investigate the source of IL-17 in the lung in these studies, studies ongoing in our laboratory support the fact that both CD4+ and CD8+ T cells are detectable in the lung as early as 6 h after K. pneumoniae infection, and represent a significant source of IL-17 in this infection model, as described previously 4 5. These data further suggest that lung lymphocytes can influence antibacterial host defenses within the first 12–24 h of infection, which is largely an innate response. In further support of this concept, our laboratory has previously shown that depletion of CD4+ T cells can attenuate lung macrophage production of TNF after intratracheal endotoxin administration 24.
In summary, our study shows that the lack of signaling via IL-17Rs severely impairs host defense against K. pneumoniae infection in a mouse model. We found that the ANC both in blood and lungs in IL-17R KO mice was significantly lower compared with control mice after infection. Moreover, this failure to increase the ANCs in blood was associated with failure to increase neutrophil progenitors in splenic CFU assays. IL-17R KO mice failed to normally induce MIP-2, a chemokine gene critical for neutrophils diapedesis and chemotaxis into the lung 16, and G-CSF, a growth factor that is produced in the lung in response to infection, which secondarily increases granulopoiesis 13. The excessive growth of bacteria in the lungs of IL-17R KO mice was associated with significantly more perivascular edema and bacterial dissemination compared with control mice. These data demonstrate that signaling through the IL-17R is critical for lung PMN recruitment and host defenses against K. pneumoniae. We speculate that relative IL-17 deficiency may in part explain the pulmonary host defense defect associated with either HIV infection or congenital immunodeficiency of CD4+ lymphocytes.
Acknowledgments
The authors would like to thank Ms. Sharon Lee in editorial assistance in manuscript preparation.
This work was supported by Public Health Service Grants AA10384 (J.K. Kolls), HL62052 (J.K. Kolls), and HL61721 (J.K. Kolls).
Footnotes
Abbreviations used in this paper: ANC, absolute neutrophil count; BAL, bronchoalveolar lavage; ES, embryonic stem; KO, knockout; MIP, macrophage inflammatory protein; RPA, ribonuclease protection assay; SCF, stem cell factor.
References
- Tumbarello M., Tacconelli E., de Gaetano K., Ardito F., Pirronti T., Cauda R., Ortona L. Bacterial pneumonia in HIV-infected patientsanalysis of risk factors and prognostic indicators. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 1998;18:39–45. doi: 10.1097/00042560-199805010-00006. [DOI] [PubMed] [Google Scholar]
- Wallace J.M., Hansen N.I., Lavange L., Glassroth J., Browdy B.L., Rosen M.J., Kvale P.A., Mangura B.T., Reichman L.B., Hopewell P.C. Respiratory disease trends in the pulmonary complications of HIV infection study cohort. Pulmonary complications of HIV infection study group. Am. J. Respir. Crit. Care Med. 1997;155:72–80. doi: 10.1164/ajrccm.155.1.9001292. [DOI] [PubMed] [Google Scholar]
- Twigg H.L., III, Spain B.A., Soliman D.M., Bowen L.K., Heidler K.M., Wilkes D.S. Impaired IgG production in the lungs of HIV-infected individuals. Cell. Immunol. 1996;170:127–133. doi: 10.1006/cimm.1996.0142. [DOI] [PubMed] [Google Scholar]
- Yao Z., Fanslow W.C., Seldin M.F., Rousseau A.-M., Painter S.L., Comeau M.R., Cohen J.I., Spriggs M.K. Herpesvirus saimiri encodes a new cytokine, IL-17, which binds to novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Yao Z., Painter S.L., Fanslow W.C., Ulrich D., Macduff B.M., Spriggs M.K., Armitage R.J. Human IL-17a novel cytokine derived from T cells. J. Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- Fossiez F., Djossou O., Chomarat P., Flores-Romo L., Ait-Yahia S., Maat C., Pin J.J., Garrone P., Garcia E., Saeland S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenberger P., La Russa V., Miller A., Ye P., Huang W., Zieske A., Nelson S., Bagby G.J., Stoltz D., Mynatt R.L., Spriggs M., Kolls J.K. IL-17 stimulates granulopoiesis in miceuse of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J. Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- Schwarzenberger P., Huang W., Ye P., Oliver P., Manuel M., Zhang Z., Bagby G., Nelson S., Kolls J.K. Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J. Immunol. 2000;164:4783–4789. doi: 10.4049/jimmunol.164.9.4783. [DOI] [PubMed] [Google Scholar]
- Jovanovic D.V., Di Battista J.A., Martel-Pelletier J., Jolicoeur F.C., He Y., Zhang M., Mineau F., Pelletier J.P. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J. Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- Laan M., Cui Z.H., Hoshino H., Lotvall J., Sjostrand M., Gruenert D.C., Skoogh B.E., Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- Ye P., Garvey P.B., Zhang P., Nelson S., Bagby G., Summer W.R., Schwarzenberger P., Shellito J.E., Kolls J.K. IL-17 and lung host defense against K. pneumoniae infection. Am. J. Respir. Cell. Mol. Biol. 2001;In press doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- Spriggs M.K. Interleukin-17 and its receptor. J. Clin. Immunol. 1997;17:366–369. doi: 10.1023/a:1027360106635. [DOI] [PubMed] [Google Scholar]
- Dale D.C., Liles W.C., Summer W.R., Nelson S. Reviewgranulocyte colony-stimulating factor—role and relationships in infectious diseases. J. Infect. Dis. 1995;172:1061–1075. doi: 10.1093/infdis/172.4.1061. [DOI] [PubMed] [Google Scholar]
- Peschon J.J., Torrance D.S., Stocking K.L., Glaccum M.B., Otten C., Willis C.R., Charrier K., Morrissey P.J., Ware C.B., Mohler K.M. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- Kolls J., Peppel K., Silva M., Beutler B. Prolonged and effective blockade of tumor necrosis factor activity through adenovirus-mediated gene transfer. Proc. Natl. Acad. Sci. USA. 1994;91:215–219. doi: 10.1073/pnas.91.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger M.J., Strieter R.M., Kunkel S.L., Danforth J.M., Laichalk L.L., McGillicuddy D.C., Standiford T.J. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J. Infect. Dis. 1996;173:159–165. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- Nelson S. Novel nonantibiotic therapies for pneumoniacytokines and host defense. Chest. 2001;119:419S–425S. doi: 10.1378/chest.119.2_suppl.419s. [DOI] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophagedefender against bacterial infection of the lung. J. Clin. Invest. 1974;54:519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabaud M., Fossiez F., Taupin J.L., Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J. Immunol. 1998;161:409–414. [PubMed] [Google Scholar]
- Jovanovic D.V., Martel-Pelletier J., Di Battista J.A., Mineau F., Jolicoeur F.C., Benderdour M., Pelletier J.P. Stimulation of 92-kd gelatinase (matrix metalloproteinase 9) production by interleukin-17 in human monocyte/macrophagesa possible role in rheumatoid arthritis. Arthritis Rheum. 2000;43:1134–1144. doi: 10.1002/1529-0131(200005)43:5<1134::AID-ANR24>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Hoshino H., Lotvall J., Skoogh B.E., Linden A. Neutrophil recruitment by interleukin-17 into rat airways in vivo. Role of tachykinins. Am. J. Respir. Crit. Care Med. 1999;159:1423–1428. doi: 10.1164/ajrccm.159.5.9806008. [DOI] [PubMed] [Google Scholar]
- Antonysamy M.A., Fanslow W.C., Fu F., Li W., Qian S., Troutt A.B., Thomson A.W. Evidence for a role of IL-17 in organ allograft rejectionIL-17 promotes the functional differentiation of dendritic cell progenitors. J. Immunol. 1999;162:577–584. [PubMed] [Google Scholar]
- Infante-Duarte C., Horton H.F., Byrne M.C., Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- D'Souza N.B., Mandujano F.J., Nelson S., Summer W.R., Shellito J.E. CD4+ T lymphocyte depletion attenuates lipopolysaccharide-induced tumor necrosis factor secretion by alveolar macrophages in the mouse. Lymphokine Cytokine Res. 1994;13:359–366. [PubMed] [Google Scholar]