Abstract
We have analyzed a panel of T cell hybridomas specific for the chemically dominant epitope of hen egg-white lysozyme 48–61 which has asparagine 59 as an important T cell receptor contact residue. A number of T cells recognize 48–61 with asparagine at position 59, but not the aspartic acid or isoaspartic acid derivatives. Conversely, we find T cells that specifically recognize 48–61 bearing an isoaspartic acid at residue 59, but not asparagine. For other T cells, asparagine, aspartic acid, or isoaspartic acid at residue 59 is irrelevant. We present evidence that our previous distinction between type A and type B T cells is not explained by asparagine deamidation at residue 59.
Keywords: class II major histocompatibility molecules, posttranslational modification of peptides, lysozyme, antigen presentation, isoaspartate
Introduction
Some autoimmune diseases can be considered a result of incomplete thymic negative selection or peripheral tolerance, resulting in the activation of T cells specific for autologous proteins. In evaluating these issues, we have been examining the chemistry of peptides selected by class II MHC molecules during antigen processing (summarized in reference 1). We identified two sets of T cells that differed in their recognition of the same peptide segment 48–61 of hen-egg white lysozyme (HEL) 2 3 4 5. A “type A” T cell is the conventional T cell that recognizes the MHC-bound peptide resulting from the processing of the protein in the processing vesicles. It also recognizes the peptide when offered to APCs as an exogenous peptide, i.e., a form that does not require intracellular processing. In contrast, a “type B” T cell recognizes only the exogenous peptide but not that derived from the processed protein. Using HEL as a protein antigen, we have shown that type B T cells escaped thymic negative selection in HEL transgenic mice, thus raising the issue that these T cells could be involved in autoimmunity if an autologous protein is partially proteolyzed in tissues 4.
One explanation for the features of type B T cells is that they are recognizing changes in the exogenous peptide not found in the naturally processed peptide. These changes could occur during the handling of the peptide by the APC or from its synthesis or postsynthetic modifications. Indeed, peptide modifications are important in the context of T cell recognition (for examples, see references 6 and 7), and one such change involves the deamidation of asparagines in proteins or peptides to yield aspartic acid and isoaspartic acid. Mamula et al. reported the important finding that autologous peptides containing an isoAsp modification were immunogenic: immunization of mice with murine cytochrome c peptide, residues 90–104, did not trigger T cell proliferation, but immunization with the isoAsp containing peptide was effective 8. Furthermore, immunization with a peptide derived from a nuclear ribonucleoprotein having the isoAsp also led to antibody production that cross-reacted to the unmodified protein. These findings led Mamula et al. to postulate the possible involvement of such modifications in the autoimmunity of the lupus type.
A recent report claims that the type B T cells described in our previous papers may react with a modification of the exogenous peptide brought about by the spontaneous conversion of Asn to Asp or isoAsp 9. Asn in the peptide segment 52–60 of HEL is an important TCR contact amino acid. Deamidation of Asn in proteins and peptides yields isoAsp and Asp derivatives in the ratios of ∼4:1 10 11 12 13. We demonstrate that an isoAsp residue can be specifically recognized by T cells, confirming the Mamula et al. report at the clonal level. However, we refute the claim of McAdam et al., as the evaluation of a number of T cell clones indicates that the recognition of Asp or isoAsp is not the basis for the difference in the type A and type B reactivity.
Materials and Methods
Antigen and Peptides.
HEL was purchased from Sigma-Aldrich. HEL 48–61, (DGSTDYGILQINSR), referred to as 48–61 N59, the segment in bold binds to I-Ak 14, was synthesized using F-moc chemistry on a Synergy 430A peptide synthesizer (Applied Biosystems). Peptide sequence and purity was confirmed by mass spectrometry. HEL 48–61 with isoAsp or Asp at 59 referred to as 48–61 βD59 and HEL 48–61 D59, respectively, were provided by Dr. Garland Marshall (Washington University). The binding of each peptide to purified I-Ak molecules was done as reported 15 using baculovirus produced molecules in a competitive binding assay.
Mice and Hybridomas.
The following hybridomas were generated as described previously: 3A9 16, ALV 11, ALV48, and DAV21 3. The CP series of hybridomas was generated by immunization of B10.BR mice with 10 nmoles of HEL or 48–61 in CFA. Hybridomas MLA11.2 and MLC10.22 were generated by immunization of ML-5 transgenic mice 4 5, expressing a secreted form of HEL with 10 nmoles of the tryptic fragment of HEL, 46–61 in CFA. Hybridoma B10A6.5 was generated by immunization of B10.BR mice with 10 nmoles of the same tryptic fragment. The Iso series of hybridomas were generated by immunization of B10.BR mice with 10 nmoles of HEL48–61βD59. We have used both male and female B10.BR mice which were raised in the small animal facility of Washington University in St. Louis, MO.
T Cell Assays.
T cell reactivity was determined by IL-2 secretion. M12.C3.F6, a B lymphoma expressing I-Ak molecules (5 × 104) was incubated with decreasing doses of peptide in the presence of T cell hybridomas (5 × 104) in a total volume of 200 μl in MEM with 10% fetal calf serum. Incubation was for 18 h, followed by transfer of supernatants to wells containing CTLL-2, an IL-2–dependent cell line. CTLL-2 were cultured for 40 h, with [3H]thymidine added for the last 24 h. [3H]thymidine incorporation was determined using a Wallac β counter. Most of the T cell hybridomas were tested against 48–61 peptide (batches 1255, 1387, or Abbt, with comparable results).
Quantitation of Isoaspartyl Content.
The isoaspartate content of peptides was determined by a procedure based on the incorporation of radioactive methyl groups into isoaspartate residues, using the enzyme protein isoaspartyl methyltransferase and S-adenosyl L-(methyl)-3H methionine (obtained from Amersham Pharmacia Biotech). We used and followed exactly the instructions in the ISOQUANT kit from Promega (lot number 116482, catalog number MA1010). We tested several batches of peptide that were used for several years and kept stored in water at pH 3.
Results and Discussion
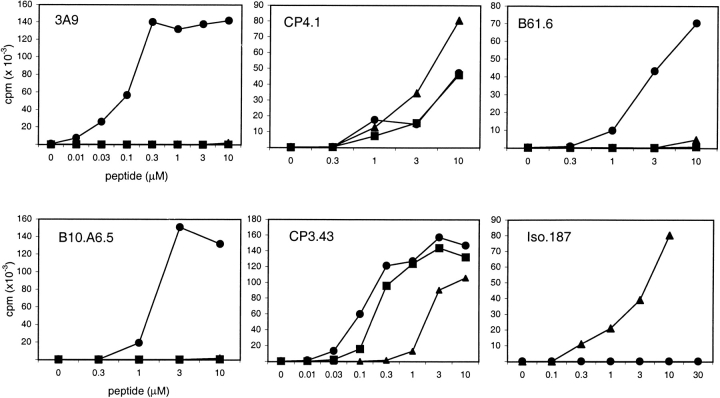
Previous structural and immunological studies indicated that in the core segment of the 52–60 peptide of HEL, Asn 59 is a solvent exposed residue that contacts the TCR 14 17 18. Table indicates that immunization with 48–61 βD59 generated T cells specific for 48–61 βD59 peptide that did not recognize the wild-type 48–61 N59 peptide. These T cells reacted with as little as 0.1 to 0.3 μM of peptide, and yet failed to be triggered by as much as 30 μM of 48–61 N59 (see iso.187 of Fig. 1). We also isolated T cells that recognized specifically the 48–61 βD59 and the 48–61 D59 peptides (Table ).
Table 1.
Specificity of HEL48–61 Reactive T Cells
| HEL48–61 | ||||
|---|---|---|---|---|
| N59 | D59 | βD59 | ||
| Type A | CP4.1 | ++ | ++ | ++ |
| CP4.22 | +++ | +/− | ++ | |
| 3A9 | +++ | − | − | |
| ALV11 | ++ | − | − | |
| Type B | ALV48 | ++ | +++ | + |
| DAV21 | ++ | +++ | +/− | |
| CP3.43 | +++ | +++ | ++ | |
| CP3.42 | +++ | +++ | ++ | |
| CP3.12 | ++ | ++ | + | |
| B10A8.4 | +++ | +/− | +++ | |
| CP1.9 | +++ | + | + | |
| CP1.7 | +++ | +++ | + | |
| CP1.17 | +++ | +++ | ++ | |
| CP3.11 | +++ | ++ | − | |
| CP4.17 | ++ | + | + | |
| MLA11.2 | +++ | − | +/− | |
| B61.6 | +++ | − | − | |
| MLC10.22 | ++ | − | − | |
| CP2.11 | ++ | − | − | |
| B10A6.5 | +++ | − | − | |
| Isoaspartyl | Iso.187 | − | − | ++ |
| Iso.210 | − | − | ++ | |
| Iso.198 | − | + | ++ | |
| Iso.203 | +++ | + | +++ | |
| Iso.156 | +++ | − | +++ | |
Patterns of reactivities to peptides among type A and B T cells. Type A T cell hybridomas recognize native HEL and HEL48–61 N59 with similar sensitivity, while type B hybridomas are at least 100-fold more sensitive to peptide than to native HEL. Shown here is a list of T cell hybridomas that follow the criteria for A and B T cells. References 3 and 4 have reported on the reactivity of many of these. The “Iso” series of hybridomas were generated by immunization with HEL48–61βD59. All hybridomas were tested against the following synthetic peptides: HEL48–61 N59 (indicated as N59), HEL48–61D59 (D59), or HEL48–61βD59 (βD59). For easier comparison, the sensitivity of the hybridomas to the given peptides are represented as: +++ for 0.1 μM or less, ++ for 1–10 μM, + for greater than 10 μM, +/− for trace response, − for no response at the highest concentration tested.
Figure 1.
Type A and type B hybridomas were tested for responses to HEL48–61 N59 (•), HEL48–61D59 (▪), and HEL48–61βD59 (▴). T cell hybridomas were incubated with M12.C3.F6, a B lymphoma expressing I-Ak, and the designated amount of peptide overnight. T cell recognition was measured by IL-2 secretion, as determined by incubation of assay supernatant with the IL-2–dependent cell line, CTLL-2, and incorporation of [3H]thymidine. Iso.187, and Iso.210 (in Table ) did not respond to native HEL, denatured HEL, and 48–61N59 at concentrations up to 30 μM.
A type A T cell is defined as reacting with both HEL as well as to 48–61 3 4. A type B T cell will preferentially recognize the peptide and will react poorly, if at all, to HEL. A small response can be obtained at the high levels of HEL in culture, of 30 to 100 μM. In Table , all type A T cells were triggered by ∼0.1 μM HEL, while none of the type B were triggered up to 10 μM. When a number of type A and type B T cells were examined for their response to the three peptides, we found two patterns of reactivity: the first set responded to the three peptides with slight differences in the sensitivities for each (CP4.1 and CP3.43; Fig. 1). The second set only recognized the 48–61 N59 peptide, but not the isoAsp or Asp derivatives (3A9, B61.6, and B10.A6.5; Fig. 1). These two sets were found in both type A and B subsets. Two of four type A T cells only recognized the 48–61 N59, while the other two did not discriminate among the Asn, Asp, and isoAsp 59 residues. Likewise, 5 of 16 type B T cells were completely specific for the N59 TCR contact residue, while the remaining 11 had variable degrees of responses among the three peptides. Studies are now being done to examine the use of the various TCR contact residues among these different T cells.
The finding that the wild-type peptide, 48–61 N59, was recognized uniquely by either A and B types while, conversely, the 48–61 βD59 specific T cells failed to recognize it, argues strongly that spontaneous conversion of the Asn 59 TCR contact residue in the 48–61 N59 peptide is not the explanation for the type B specificity. We also tested for the degree of isoAsp conversion in each of the peptides used in the past 6 yr. These peptides were repeatedly used and kept in the laboratory under various temperature conditions. Using the incorporation of radioactive methyl groups, we failed to find significant levels of isoaspartyl conversion in 6/7 preparations that were tested (Table ). Whether the Asn in these peptides is converted during the assay becomes irrelevant in light of the results with T cells. Indeed, based on the sensitivities of the T cells indicated above, the amount of spontaneous isoAsp conversion of the 48–61 N59 peptide must be limited to <1% in the 18 h of the assay. Finally, we found only slight differences in binding of the three peptides to purified I-Ak molecules, with IC 50% of 0.12, 0.24, and 0.82 μM for 48–61 N59,48–61 D59, and 48–61 βD59, respectively.
Table 2.
Isoaspartyl Content of Peptides
| Peptide | Batch (date) | cpm/pmol | pmol (percentage of isoaspartyl) |
|---|---|---|---|
| 48–61 | 388 (1/96) | 0 | 0 (0%) |
| 48–61 | 924 (11/97) | 971 | 0.6 (0.2%) |
| 48–61 | 1255 (4/99) | 75 | 0.4 (0.2%) |
| 48–61 | 1317 (7/99) | 31,083 | 17.7 (7.1%) |
| 48–63 | 1387 (8/99) | 508 | 0.3 (0.1%) |
| 48–63 | Abbt (6/98) | 0 | 0 (0%) |
| 46–61 | RD (7/00) | 3,716 | 2.1 (0.9%) |
Isoaspartyl content of peptides. Each preparation was tested at 250 pmol. With the standard provided by the manufacturer, the cpm per pmol was estimated at 1,754. Peptides had been made at the indicated dates and stored frozen or at 4°C. The various peptides had been used to generate and evaluate the cells shown in Table . The above data has been selected from a series of experiments performed during April and May, 2000. Peptide 1317 was made in another laboratory and never used in these experiments. 46–61 refers to a tryptic peptide from HEL. The peptide labeled “Abbt” was produced by a commercial source. The preparation of 48–61 βD59 was tested at 125 pmol and found to bind 98% of the radioactive material.
In summary, our results extend the report by Mamula et al., calling attention to the fact, using cloned cells, that T cells can make an important discrimination between Asn, Asp, and isoAsp residues in a peptide. This discrimination is important in the context of changes that may take place in autologous proteins in tissues, as they correctly speculated. It is also important in the context of peptides made in the laboratory 8: as noted, one of our peptides (1317 in Table ) had 17% of conversion and could have induced specific T cells. In our case, the importance of this issue is highlighted because Asn 59 has been documented as a solvent exposed residue available for T cell recognition of the 52–60 core segment of the HEL peptide.
From a thorough analysis of a large panel of T cell hybridomas, it is obvious that the recognition of Asp or isoAsp at the TCR contact residue 59, which is P8 in the peptide, is independent of the type A and type B states and is not the feature that distinguishes them. Concerning the claim made in the McAdam et al. paper we contend that conclusions are difficult to make on one or two T cells, particularly when the issue of sensitivity in the various manipulations of the assays are not taken into consideration. Also, to note that other HEL peptides in which we found the type A and type B T cells did not have the sequence of Asn followed by the Ser or Gly, the destabilizing residues that favor the deamidation. These include the 84–96 peptide 2.
We made the distinction between type A and B T cells based on our identification of the natural processed peptides of HEL, from which we identified and quantitated their amounts displayed after processing. The surprise was that offering the identical peptide to APCs induced a novel reactivity—type B—which was not displayed by the endogenous processing of HEL. As the linear sequence was identical (and not explained by postsynthetic modifications), we have sought alternative explanations. The best explanation for the type B state of a peptide-MHC complex is that it results from a unique conformation 3 4 formed when exogenous peptides bind to class II molecules in early endosomal compartments or on plasma membrane, as described in reference 19. This conformation is distinct from the type A that results when peptides bind from a processed protein to a nascent class II molecule in the processing compartments. As mentioned, the importance of type B T cells is that many escape thymic negative selection and peripheralize, representing a set of T cells that can potentially react with peptides formed at sites of inflammation 4 5.
Acknowledgments
We thank Dr. Garland Marshall and Lori Quick for their help in peptide synthesis and for their advice. We also thank Shirley Petzold for technical assistance, and Richard DiPaolo and Brian T. Edelson for their useful comments.
This work was supported by grants from the National Institutes of Health. Thomas Cirrito was supported in part by the Cancer Research Institute.
References
- Latek R.R., Unanue E.R. Mechanisms and consequences of peptide selection by the I-Ak class II molecule. Immunol. Rev. 1999;172:209–228. doi: 10.1111/j.1600-065x.1999.tb01367.x. [DOI] [PubMed] [Google Scholar]
- Viner N.J., Nelson C.A., Unanue E.R. Identification of a major I-Ek-restricted determinant of hen egg lysozymelimitations of lymph node proliferation studies in defining immunodominance and crypticity. Proc. Natl. Acad. Sci. USA. 1995;92:2214–2218. doi: 10.1073/pnas.92.6.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner N.J., Nelson C.A., Deck B., Unanue E.R. Complexes generated by the binding of free peptides to class II MHC molecules are antigenically diverse compared with those generated by intracellular processing. J. Immunol. 1996;156:2365–2368. [PubMed] [Google Scholar]
- Peterson D.A., DiPaolo R.J., Kanagawa O., Unanue E.R. Quantitative analysis of the T cell repertoire that escapes negative selection. Immunity. 1999;11:453–462. doi: 10.1016/s1074-7613(00)80120-x. [DOI] [PubMed] [Google Scholar]
- Peterson D.A., DiPaolo R.J., Kanagawa O., Unanue E.R. Cutting edgea single MHC anchor residue alters the conformation of a peptide-MHC complex inducing T cells that survive negative selection. J. Immunol. 2001;166:5874–5877. doi: 10.4049/jimmunol.166.10.5874. [DOI] [PubMed] [Google Scholar]
- Skipper J.C., Hendrickson R.C., Gulden P.H., Brichard V., Van Pel A., Chen Y., Shabanowitz J., Wolfel T., Slingluff C.L., Jr., Boon T. An HLA-A2-restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J. Exp. Med. 1996;183:527–534. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C.V., Kihlberg J., Elofsson M., Magnusson G., Unanue E.R. Glycopeptides bind MHC molecules and elicit specific T cell responses. J. Immunol. 1993;151:2419–2425. [PubMed] [Google Scholar]
- Mamula M.J., Gee R.J., Elliott J.I., Sette A., Southwood S., Jones P.J., Blier P.R. Isoaspartyl post-translational modification triggers autoimmune responses to self-proteins. J. Biol. Chem. 1999;274:22321–22327. doi: 10.1074/jbc.274.32.22321. [DOI] [PubMed] [Google Scholar]
- McAdam S.N., Fleckenstein B., Rasmussen I.B., Schmid D.G., Sandlie I., Bogen B., Viner N.J., Sollid L.M. T cell recognition of the dominant I-Ak-restricted hen egg lysozyme epitope. Critical role for asparagine deamidation. J. Exp. Med. 2001;193:1239–1246. doi: 10.1084/jem.193.11.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson A.D., Riggin R.M. Development of improved high-performance liquid chromatography conditions for nonisotopic detection of isoaspartic acid to determine the extent of protein deamidation. Anal. Biochem. 2000;278:150–155. doi: 10.1006/abio.1999.4421. [DOI] [PubMed] [Google Scholar]
- Johnson B.A., Murray E.D., Jr., Clarke S., Glass D.B., Aswad D.W. Protein carboxyl methyltransferase facilitates conversion of atypical L-isoaspartyl peptides to normal L-aspartyl peptides. J. Biol. Chem. 1987;262:5622–5629. [PubMed] [Google Scholar]
- Geiger T., Clarke S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J. Biol. Chem. 1987;262:785–794. [PubMed] [Google Scholar]
- Brennan T.V., Clarke S. Deamidation and isoaspartate formation in model synthetic peptidesThe effects of sequence and solution environment. In: Aswad D.W., editor. Deamidation and Isoaspartate Formation in Peptides and Proteins. Chapter 5. CRS Press, Inc.; 1995. pp. 66–113. [Google Scholar]
- Fremont D.H., Monnaie D., Nelson C.A., Hendrickson W.A., Unanue E.R. Crystal structure of I-Ak in complex with a dominant epitope of lysozyme. Immunity. 1998;8:305–317. doi: 10.1016/s1074-7613(00)80536-1. [DOI] [PubMed] [Google Scholar]
- Latek R.R., Suri A., Petzold S.J., Nelson C.A., Kanagawa O., Unanue E.R. Structural basis of peptide binding and presentation by the type 1 diabetes-associated MHC class II molecule of NOD mice. Immunity. 2000;12:699–710. doi: 10.1016/s1074-7613(00)80220-4. [DOI] [PubMed] [Google Scholar]
- Allen P.M., Unanue E.R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J. Immunol. 1984;132:1077–1079. [PubMed] [Google Scholar]
- Allen P.M., Matsueda G.R., Evans R.J., Dunbar J.B., Jr., Marshall G.R., Unanue E.R. Identification of the T-cell and Ia contact residues of a T-cell antigenic epitope. Nature. 1987;327:713–715. doi: 10.1038/327713a0. [DOI] [PubMed] [Google Scholar]
- Nelson C.A., Viner N.J., Young S.P., Petzold S.J., Unanue E.R. A negatively charged anchor residue promotes high affinity binding to the MHC class II molecule I-Ak . J. Immunol. 1996;157:755–762. [PubMed] [Google Scholar]
- Lindner R., Unanue E.R. Distinct antigen MHC class II complexes generated by separate processing pathways. EMBO J. 1996;15:6910–6920. [PMC free article] [PubMed] [Google Scholar]