Abstract
To study homeostasis of peripheral B lymphocytes in the absence of B cell influx from the bone marrow, we generated a mouse mutant in which the recombination-activating gene (RAG)-2 can be inducibly deleted. When RAG-2 was deleted at the age of 8–10 wk, splenic naive follicular B cells were gradually lost over a year of observation, with a half-life of ∼4.5 mo. By contrast, the pool of marginal zone B cells in the spleen and of B-1 cells in the peritoneal cavity were kept at normal level. In lymph nodes, ∼90% of the B cells were lost within 4 mo, and B cell numbers remained constant thereafter. Mice in which RAG-2 was deleted at birth maintained a small population of activated B cells with an increased proportion of marginal zone B cells. Additionally, an increase of the pool of IgM secreting cells and B-1a cells was observed.
Keywords: RAG-2, mouse, marginal zone B cells, follicular B cells, half-life
Introduction
In mouse and man, B cells are continuously generated from B cell progenitors over lifetime, with the fetal liver and, from around birth on, the bone marrow (BM) as the main sites where this process takes place. The immature B cells generated in the BM emigrate into the peripheral immune system and give rise to a heterogeneous population of peripheral B cells including naive, memory and plasma cells 1. In terms of their anatomical location and surface markers, the B cell population in the periphery consists of recirculating IgMlowIgDhighCD21intCD23high cells located in follicles in spleen and lymph nodes, and IgMhigh IgDlowCD21highCD23low non-recirculating cells enriched mainly in the marginal zone (MZ) of the spleen 2 3. Follicular (FO) B cells in the spleen account for 80–90% and MZ B cells for 5–10% of the splenic B cells in an adult mouse 4. Another B cell subset, consisting of the so-called B-1 cells, has self-renewing capacity and predominates in the peritoneal and pleural cavities 5. Recent data on the function of MZ B cells 6 7 8 and B-1 cells 9 10 suggest that these cells provide a first line of defense against antigens in the blood, and in the peritoneal and pleural cavities, respectively. FO B cells are involved in T cell–dependent antibody responses, in which memory and plasma cells are generated 7.
About 1–2 × 107 immature B cells are generated daily in the adult murine BM 11. Of these, only 3% enter the pool of mature B cells of the various subsets 12. Peripheral B cell pools represent dynamic, yet stable compartments where the input contributed by BM immigrants and antigen driven cell proliferation is balanced by apoptosis and terminal differentiation 13.
For an understanding of this homeostatic control it is of critical importance to determine the life spans of B cells of various subsets under physiological conditions. It is therefore not surprising that B cell life spans have been the subject of many investigations in the past. However, while it is generally agreed that newly generated immature B cells in adult mice have life spans of ∼3-4 d 12 14, the life spans of mature B cells are still controversial. Experiments based on (a) adoptive transfer of LPS-reactive B cells into LPS-nonreactive recipient mice 15; (b) the ablation of cycling B cell precursors with cytotoxic drugs which selectively kill cycling cells 16 17; and (c) induction of BM aplasia using a radioactive strontium isotope, 89S 18 support the hypothesis that most mature B cells have a life span of just a few days 13. In contrast, experiments in which B cell development was blocked by anti–IL-7 19, or anti–IL-7 receptor antibodies 20 or cells were labeled by [3H]thymidine 21 22 23 or bromo deoxyuridine (BrdU) in vivo 24 25 26 lead to the conclusion that the majority of splenic B cells can persist for several weeks or months.
To further clarify this matter and to extend the analysis to the various subsets of mature B cells, we have now generated a transgenic mouse strain in which B cell development can be blocked permanently at any stage of development under quasi-physiological conditions. To achieve this goal we made use of the fact that both B and T cell development critically depend on the somatic assembly of Ig or T cell receptor variable region gene segments, called V, D, and J, by a process known as V(D)J recombination 27. V(D)J recombination is mediated by the products of the recombination-activating genes RAG-1 and RAG-2 28. Consequently, in RAG-1– or RAG-2–deficient mice B and T cell development are blocked at an early progenitor stage 29 30. Induced inactivation of RAG-1 or RAG-2 in B and T cell progenitors in the BM can thus be expected to lead to a block in B and T cell generation. Using conditional gene targeting 31, we established a transgenic mouse strain in which RAG-2 can be inducibly and efficiently inactivated whenever desired. In the present paper, we describe this experimental system and use it to study the maintenance and differentiation of the various peripheral B cell subsets in intact, healthy animals, in the absence of B cell influx from the BM, i.e., a situation in which B cell life spans should be maximized.
Materials and Methods
Construction of the RAG-2 Targeting Vector.
A RAG-2 containing genomic clone from the 129-mouse strain was isolated by screening a 129Sv genomic library by PCR (Genome Systems). The coding sequence for the RAG-2 protein lies within exon 3 of the RAG-2 gene 28. Therefore, the targeting strategy was to flank this exon by loxP sites. The 6-kb XbaI fragment containing the entire RAG-2 coding exon and the 4.8-kb XbaI fragment at the 3′end of RAG-2 gene were cloned by standard cloning techniques into pGEM loxP and pBluscript-tk, respectively (see Fig. 1 A). As a selection marker, the neomycin resistance gene flanked by two loxP sites was cloned into the unique SalI site in intron II. The thymidine kinase (tk) gene was added to the 3′ ends of the vectors (see Fig. 1 A).
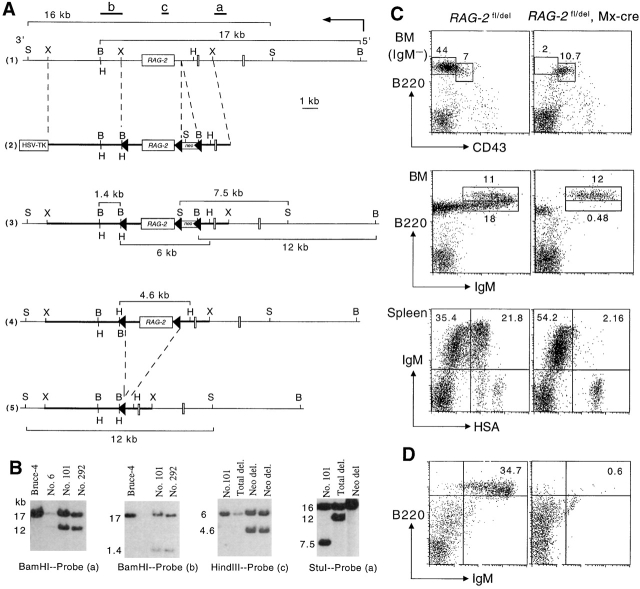
Figure 1.
Generation of RAG-2 fl/+ mice (A) targeting scheme. 1 Restriction map of the RAG-2 locus. Probes used to verify targeting events are indicated as a, b, and c together with the expected sizes of the restriction fragments. The restriction sites of XbaI (X), BamHI (B), HindIII (H), and StuI (S) are indicated. 2 Targeting vector construction. The loxP flanked neomycin resistance gene was inserted into a unique SalI site. A third loxP site was introduced downstream of exon 3. In this way, the entire coding sequence for RAG-2 protein was flanked by loxP sites (triangles). The structure of the targeted RAG-2 locus 3, the targeted locus after removal of the neomycin resistance gene 4, and the locus following deletion of the loxP-flanked exon 3 5 are shown, including sizes of diagnostic restriction fragments. (B) Southern blot analysis of ES cell clones. Genomic DNA from wild-type Bruce-4 cells and clones 6, 101, and 292 were digested with BamHI and hybridized with probe (a) to verify the targeting event or with probe (b) to detect cointegration of the third loxP site. Probe (c) together with HindIII digestion and probe (a) together with StuI digestion were used to distinguish subclones that had deleted only the neomycin resistance gene or the neomycin resistance gene and the entire loxP flanked fragment after transient transfection of the recombinants with a Cre-expressing plasmid. Numbers on the left side of the blots indicate fragment sizes in kb. (C) Block of B cell development upon the induction of RAG-2 deletion. Adult mice carrying or not carrying the Mx-cre transgene were injected with Poly(I)·Poly(C) and BM and spleen cells analyzed by FACS® 2 wk later. Numbers indicate the percentage of total lymphocytes. (D) BM cells of Poly(I)·Poly(C) treated RAG-2 fl/del, Mx-cre mice have no B cell reconstitution potential as shown by transfer into sublethally irradiated RAG-1 −/− mice. A flow cytometric analysis of spleen cells isolated from recipients that had received control (left) or RAG-2–deficient (right) BM cells 4 wk earlier is depicted.
Generation of RAG-2 fl/fl Mice.
The experimental procedures used for the culture, transfection, and selection of embryonic stem (ES) cells were as described previously 32. Bruce-4 ES cells (1.5 × 107) 33 derived from C57BL/6 mice were transfected by electroporation with a PvuI-linearized targeting vector (40 μg). After double selection with G418 (350 μg/ml) and Gancyclovir (2 μM) for around 10 d, colonies were picked and screened for homologous recombination of the RAG-2 floxed allele by Southern blotting analysis as shown in Fig. 1 B. BamHI-digested genomic DNA from double resistant colonies were hybridized with external probe (a) to yield bands of 17 kb against 12 kb for wild-type and targeted loci, respectively. To screen for clones that had cointegrated the third loxP site, BamHI-digested genomic DNA from homologous recombinants were hybridized with internal probe (b). The presence of a 1.4 kb band indicates the third loxP cointegration event.
To delete the neomycin resistance gene in vitro, targeted ES cell clones were transiently transfected with a Cre-encoding plasmid. DNA from neomycin-sensitive clones were digested with HindIII and hybridized with probe (c). Bands of 6 kb only and 6 kb against 4.6 kb indicate targeted loci with total deletion and the neomycin resistance gene deletion, respectively (Fig. 1 B). To confirm this, DNA from neomycin-sensitive clones were digested with StuI and hybridized with probe (a). The size of the bands was 16 kb against 12 kb for the neomycin resistance gene deletion and 16 kb only for total deletion. Two targeted ES cell clones were injected into 3.5-d blastocysts harvested from CB.20 or BALB/c mice, and the blastocysts transferred to the uteri of pseudopregnant (C57BL/6 × BALB/c) F1 foster mothers. Male chimeric mice were mated with C57BL/6 females to generate mutant offspring on the C57BL/6 genetic background. Germline transmission was scored by coat color and Southern blotting analysis of tail DNA.
To establish a system of inducible RAG-2 deletion, mice with the RAG-2 floxed allele (RAG-2 fl/fl) were bred with Mx-cre transgenic mice in which the cre transgene is under the control of the type I IFN-inducible Mx promoter 34. The Mx-cre transgenic mice used in our experiments had been backcrossed to C57BL/6 for at least five generations. All mice used in this study were bred and maintained in a conventional animal facility in the Institute for Genetics, Cologne. All animal studies were approved by the institutional review board.
Induced Deletion of RAG-2.
To induce Cre expression and subsequent deletion of RAG-2, mice at 8–10 weeks of age with the genotype of RAG-2 fl/del, Mx-cre were given three doses of Poly(I)·Poly(C) (400 μg/dose; Amersham Pharmacia Biotech) by intraperitoneal injection on days 0, 3, and 6 and analyzed at various time points thereafter. The animals used in the BrdU labeling experiments had been treated with four doses of Poly(I)·Poly(C) (100 μg/dose) at 1 d intervals. In newborn or 2-wk-old mice, RAG-2 deletion was induced by three doses of type I IFN injection (106 U/dose for neonates and 2 × 106 U/dose for 2-wk-old mice) given at 2-d intervals. To measure the degree of RAG-2 gene deletion, cells were isolated from different organs to prepare genomic DNA for Southern blotting.
BM Transfer.
For reconstitution assays, single cell suspensions were prepared from the BM and B and T cells were depleted by MACS (Miltenyi Biotec), using anti-IgM and anti-Thy1.2 mAb-coupled magnetic beads. Purified BM cells (5 × 106) were injected intravenously into 9–16-wk-old C57BL/6, RAG-1 –/– mice after irradiation (400 rad).
Flow Cytometry.
Single-cell suspensions were prepared from spleen, superficial inguinal lymph nodes, BM (one femur), and peritoneal cavity of RAG-2 fl/del, Mx-cre, or littermate controls. 106 cells were stained with fluorochrome- (FITC, PE, or Cychrome) or biotin-conjugated monoclonal antibodies for flow cytometric analysis on a FACSCalibur™ (Becton Dickinson). Streptavidin-Cychrome or streptavidin-Alexa were used to reveal biotinylated antibodies. The following homemade mAbs were used: R33–24–12 (anti-IgM), F30–1 (anti-heat shock antigen [HSA]), 7G6 (anti-CD21), 1.3–5 (anti-IgD), RA3–6B2 (anti-B220), M5–114 (anti-MHC class II). mAbs against CD3, CD4, CD5, CD8, CD19, CD23, CD25, CD69, MHCII, CD62L, and CD86 were purchased from BD PharMingen.
Immunohistology.
Spleens were embedded in OCT and frozen on dry ice. Frozen sections were cut, air-dried, and fixed with cold (−20°C) acetone. For double immunostaining, sections were rehydrated in PBS and stained. We used biotin-conjugated anti-MadCAM (Southern Biotechnology Associates, Inc.) plus peroxidase-labeled anti-IgM (goat anti–mouse; Sigma-Aldrich) and MOMA-1 (rat, IgG2a, specific for mouse splenic metallophilic macrophages; Dianova) plus biotin-conjugated rabbit anti-IgG plus peroxidase-coupled anti-IgM to locate the MZ and FO B cells. After washing, bound peroxidase was revealed by 3-amino-9-ethyl carbazole (Sigma-Aldrich), and biotin by alkaline-phosphatase substrate kit III/blue (Vector Laboratories).
BrdU Labeling and Cell Cycle Analysis.
Mice were fed with BrdU (Sigma-Aldrich) in the drinking water (1 mg/ml) for 3 d. Single cell suspensions from spleens were stained with IgM-allophycocyanin (APC), CD23-PE, and CD21-bio and sorted into MZ and FO B cells by FACS®. The sorted cells were fixed in 70% cold ethanol; their DNA denatured with 2 M HCl and stained with FITC conjugated ant-BrdU antibody (Becton Dickinson). The cells were then washed twice with PBS and resuspended in 500 μl of PBS containing 5 μg/ml of propidium iodide (Sigma-Aldrich). The fraction of BrdU-labeled cells in each sorted cell population and DNA content of single cells, excluding doublets, was analyzed on FACS®.
ELISA and Enzyme-linked Immunospot. Serum Igs levels were measured by ELISA as described 35. The enumeration of IgM and IgG3 secreting cells was done by enzyme-linked immunospot (ELISPOT) according to Czerkinsky 36.
Results
Generation and Characterization of a Mouse Strain Allowing Conditional Inactivation of RAG-2.
The gene targeting strategy and screening of recombinant ES cells by Southern blotting are shown in Fig. 1A and Fig. B, respectively. The conditional RAG-2 allele (RAG-2fl) was transmitted into the C57BL/6 germline and the mutant mice were analyzed. B cell lymphopoiesis in the BM of RAG-2 fl/fl mice was normal, and the size of the peripheral B cell pool was comparable to that of wild-type mice, indicating no interference with RAG-2 expression by the insertion of the two loxP sites (data not shown). To generate mice homozygous for RAG-2 deletion (RAG-2 del/del), RAG-2 fl/fl mice were crossed to the deleter strain, which allows ubiquitous deletion of loxP-flanked gene segments including germ cells 37. B and T cell development in RAG-2 del/del mice was blocked at the pro-B and the pro-T cell stages, producing the phenotype of classical RAG-1 and RAG-2 knockout mice (29 30; data not shown).
Mice of the genotype RAG-2 fl/del, Mx-cre, in which RAG-2 deletion can be efficiently induced by IFN or its inducer, Poly(I)·Poly(C) 34, were used in our further experiments. 2 wk after intraperitoneal injection of Poly(I)·Poly(C) into adult RAG-2 fl/del, Mx-cre mice, slightly fewer BM cells were recovered from these animals than from similarly treated littermate controls. Gating on the IgM-negative population, the B220lowCD43+ pro-B cell compartment in the experimental animals was comparable to that of the controls, whereas B220low CD43− pre-B cells were essentially absent, indicating a block of B cell development at the pro-B cell stage (Fig. 1 C). B220lowIgM+ immature B cells were at background levels, while B220highIgM+ mature B cells were not affected. Similar to B cell development in the BM, T cell development in the thymus was blocked at the pro-T cell stage upon induction of RAG-2 deletion (data not shown). In agreement with the short life span of immature B cells 12 14, the IgM+HSAhigh immature B cell compartment in the spleen of the Poly(I)·Poly(C) treated mutants was reduced to background levels (see Fig. 1 C; the few IgM+HSAhigh cells in the spleens of the mutants were mostly CD21+ and therefore presumably MZ B cells). As in adult mice, B cell development was also blocked at the pro-B cell stage in mice in which RAG-2 was deleted at birth or at the age of 2 wk (data not shown). In these mice as well as in those treated with the inducer at adult age, the block of B cell development as depicted in Fig. 1 C was maintained over the entire period of observation (see below; data not shown). The deletion efficiency of the RAG-2 fl allele in the BM as quantified by Southern blotting analysis was close to 100% both in adult and neonates, whereas it was ∼90% in the spleen and cells in the peritoneal cavity (data not shown).
To verify that the induced block of B cell development was indeed complete, we reconstituted sublethally irradiated syngeneic RAG-1 −/− mice (that lack B and T cells) with BM cells of 13-wk-old RAG-2 fl/del, Mx-cre mice which had been treated with Poly(I)·Poly(C) at the age of 8 wk. BM cells from similarly treated RAG-2 fl/del mice served as a control. The total number of splenic cells recovered 4 wk after transfer was 2.8 × 106 in recipients reconstituted with BM from Poly(I)·Poly(C) treated RAG-2 fl/del, Mx-cre mice and 2.2 × 107 in the controls. As shown in Fig. 1 D, mice that had received BM cells from the induced mutants exhibited no detectable reconstitution for B cells, whereas the controls were fully reconstituted. Similar results were obtained using BM cells from 19-wk-old donor mice in which RAG-2 had been inactivated at birth (data not shown). In both reconstitution experiments, BM cells from two donor mice were individually tested. In all cases, the donor-derived RAG-2 deletion could be detected by PCR in the BM and spleen of the recipients, indicating that the donor cells had been accepted by the latter (data not shown).
Slow Decline and Subsequent Persistence of Peripheral B Cells in Adult Mice upon Induced Blockade of B Cell Influx from the BM.
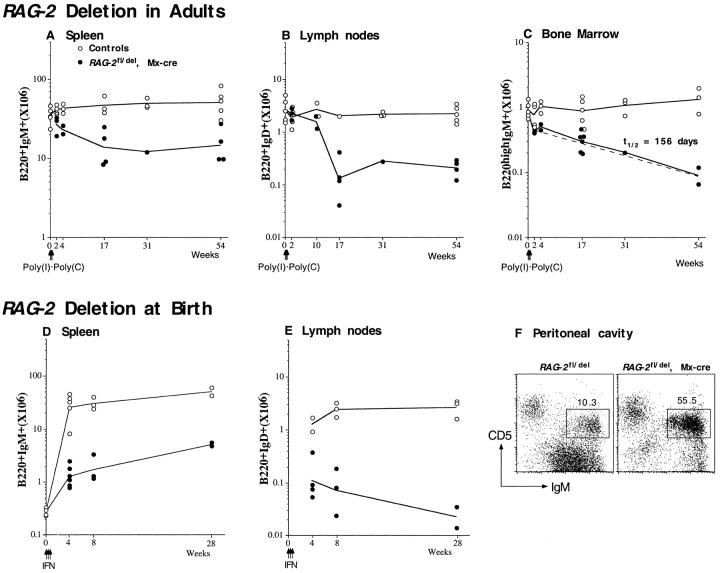
The survival kinetics of B cells in the spleen, lymph nodes, and BM in the absence of new immigrants from the BM was followed in mice in which RAG-2 was deleted at the age of 8–10 wk. Compared with the controls, the size of the B cell pool in the spleen of RAG-2 fl/del, Mx-cre mice was reduced from 67% at week 2 (at which time the immature cells had disappeared [Fig. 1 C]) to 32% at week 17 after Poly(I)·Poly(C) injection and subsequently was kept stable up to 54 wk (Fig. 2 A). In the lymph nodes, the numbers of B220+IgD+ B cells were not reduced during the first 2 wk, and declined subsequently, with a rapid drop of cell numbers (from 58 to 11% of the controls) between week 10 and week 17 after induced RAG-2 deletion (Fig. 2 B). There was no further reduction in B cell numbers in the lymph nodes between 17 and 54 wk. As shown in Fig. 2 C, the size of the B220highIgM+ mature B cell pool in the BM was reduced by half in comparison to the controls 2 wk after Poly(I)·Poly(C) injection, and subsequently declined slowly over the period of observation, with a half-life of 156 d. As in the spleen, the reduction of B cell numbers during the first 2 wk was mainly due to the loss of HSAhigh B cells. Taken together, the overall numbers of B cells in the secondary lymphoid organs of adult mice in the absence of B cell influx from the BM declined over the first 17 wk and were subsequently maintained for up to 54 wk. In contrast, the numbers of IgM+CD5+ B-1a cells in the peritoneal cavity of the mutants were comparable to those of the controls (data not shown).
Figure 2.
Survival kinetics of B cells in the spleen, lymph nodes, and BM of adult mice after induction of RAG-2 deletion (A–C) or of mice in which RAG-2 was deleted at birth (D–F). Mice of the genotype of RAG-2 fl/del, Mx-cre, and littermate controls were either injected with Poly(I)·Poly(C) at the age of 8–10 wk or with type I IFN at birth. Cells from spleen, BM, lymph nodes, and peritoneal cavity were isolated at various periods after induced RAG-2 deletion and analyzed by FACS®. Numbers of B220+IgM+ cells in the spleen (A and D); B220+IgD+ cells in the lymph nodes (B and E) and B220highIgM+ cells in the BM (C) are plotted against time (weeks) after induction of RAG-2 deletion. The half-life (t1/2) of B220highIgM+ cells in the BM was calculated from a linear regression line (dashed), using Sigma Plot software. Circles represent data from individual mice, open circles representing controls, and solid circles RAG-2 fl/del, Mx-cre mutants. Solid lines connect mean values of each group. A representative flow cytometric analysis of cells in the peritoneal cavity at the age of 8 wk is shown in F. Numbers are percentages of CD5+IgM+ cells in the lymphocyte population.
Decline of FO B Cells and Maintenance of MZ B Cells after RAG-2 Deletion in Adults.
MZ B cells are distinguished from recirculating FO B cells by anatomical location and phenotypic characteristics. According to the expression levels of CD21 and CD23, we divided the splenic B cells into MZ (CD21highCD23low) and FO (CD21intCD23high) B cells and followed their numbers after induction of RAG-2 deletion in adult mice. As shown in Fig. 3 A, the proportion of MZ B cells within the population of surface IgM-positive cells increased from less than 10% to 17% at week 2 and ∼40% at week 31 after RAG-2 deletion, whereas it was constant in the controls over the same period of time. In terms of cell numbers, the MZ B cell pool in the spleen of the experimental animals was comparable to that of the controls and stable over 54 wk (Fig. 3 B). In contrast, the number of FO B cells declined slowly, with a calculated half-life of 134 d (Fig. 3 B) and thus in reasonable accord with the decline of recirculating B cells in the BM (Fig. 2 C).
Figure 3.
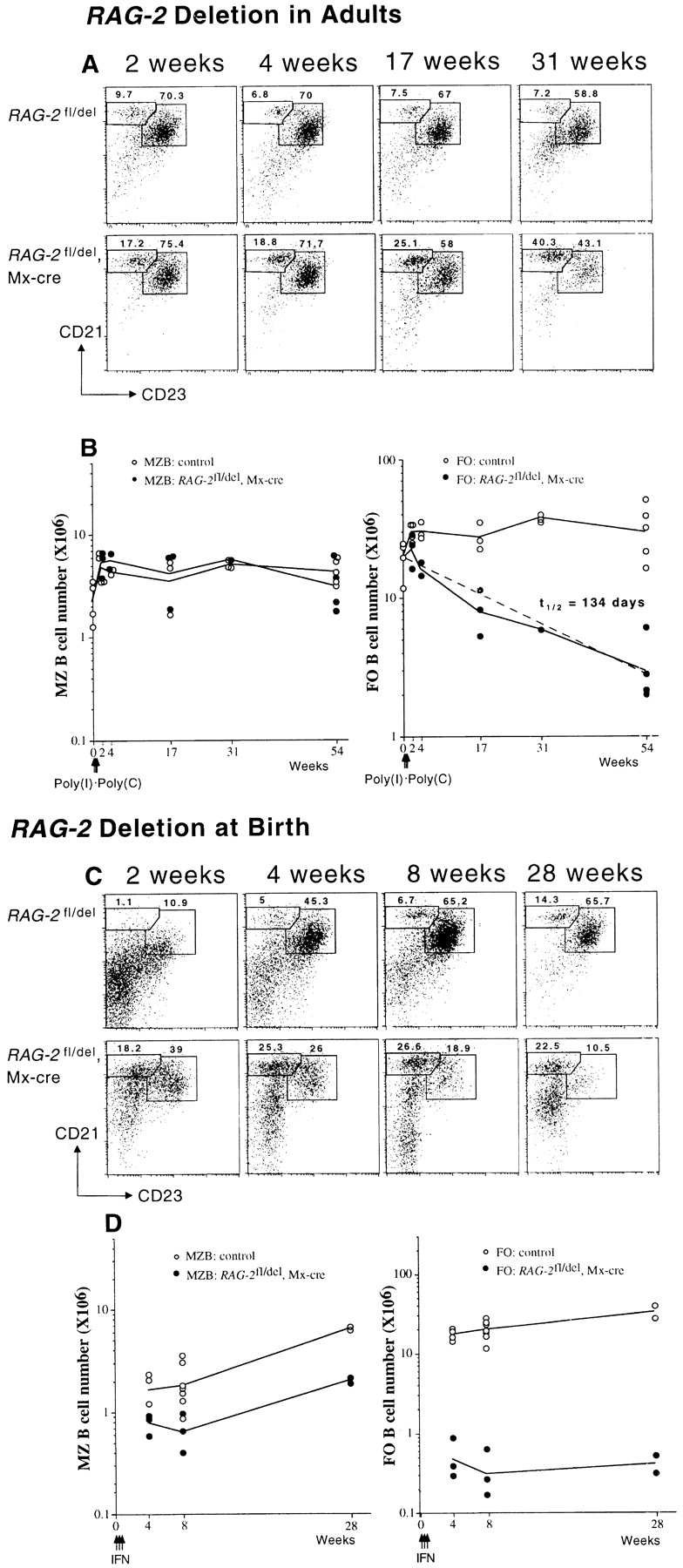
Inactivation of RAG-2 leads to a relative enlargement of MZ B cell compartment. Analysis of MZ and FO B cells in the spleen of RAG-2 fl/del, Mx-cre, and RAG-2 fl/del control mice at various times after RAG-2 deletion at the age of 8–10 wk (A and B) or at birth (C and D). FACS® profiles were obtained by gating on IgM-positive cells, and numbers indicate percentages of MZ (CD21highCD23low) and FO B cells (CD21intCD23high) (A and C). In C, in the induced mutants, cells falling outside the corresponding windows represent mainly B-1a cells (bottom left panel; see text). In B and D, the numbers of MZ and FO B cells are plotted against time (weeks). The half-life (t1/2) of FO B cells in the spleen was calculated from a linear regression line (dashed) as indicated in the legend to Fig. 2. Solid lines connect mean values.
When B Cell Development Is Blocked at Birth, Small Numbers of B Cells Are Maintained, with an Expanded Population of B-1 Cells in the Peritoneal Cavity and MZ B Cells in the Spleen.
To what extent can the immature B cells present in newborn mice build a peripheral B cell compartment in the absence of B cell influx from the BM? When RAG-2 was deleted in newborns by injection of IFN, the animals maintained a small population of B cells in spleen and lymph nodes over long periods of time, but B cell numbers were far below those developing in the controls (Fig. 2D and Fig. E). In contrast, the development of B-1a cells (IgM+CD5+) in the peritoneal cavity was undisturbed, and from week 8 after IFN injection on, 2 to 3 times more such cells were recovered from the animals than from controls (Fig. 2 F). Thus, the block of B cell generation at birth did not perturb the development and expansion of the B-1a cells, a result that is in line with the earlier findings showing that B1 cells are self-renewing cells 5.
The MZ in mice usually does not develop until 3 wk of age 38. All splenic B cells in the newborn mice express high levels of HSA and have an immature phenotype (data not shown). We induced RAG-2 deletion in newborn mice by IFN injection and asked whether B cells in the spleens of these animals would differentiate into MZ B cells in the absence of B cell influx from the BM. Surprisingly, some B cells acquired a MZ B cell phenotype and some a FO B cell phenotype already 2 wk after RAG-2 deletion, which was less evident in the age-matched controls (Fig. 3 C). The percentage of MZ B cells within the population of surface IgM-positive cells was ∼25% from week 4 up to week 28 after RAG-2 deletion (Fig. 3 C). In terms of cell numbers, MZ B cells in the induced RAG-2–deficient mutants increased slightly over time, from ∼8 × 105 at week 4 to 2 × 106 at week 28 after RAG-2 deletion (Fig. 3 D). The FO B cells developing in the animals (Fig. 3 C) remained constant in numbers over time (Fig. 3 D). Apart from MZ and FO B cells, CD5+ B cells could be detected in the spleen of the animals, amounting to 30–50% of the IgM+ or CD19+ cells. In absolute numbers, this matches the compartment of CD5+ B cells (B-1a cells) in the spleen of the controls (data not shown; but see Fig. 3 C, bottom panel).
The relative increase in the MZ B cell pool seen by flow cytometry was also evident at the level of histology. At the age of 8–10 wk, an increase in MZ B cells and a decrease in FO B cells could be observed in histological sections of the spleen in the mutants, in comparison to controls (Fig. 4).
Figure 4.
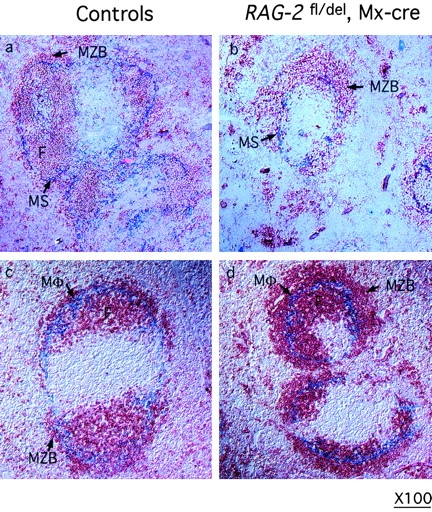
Enlarged MZs in spleens of mice with induced RAG-2 deficiency from birth. Spleen sections from 8 to 10-wk-old control (a and c) and RAG-2 fl/del, Mx-cre mice (b and d) treated with type I IFN at birth, stained with antibodies against IgM (red, a to d) for B cells, MadCAM-1 for cells lining the marginal sinus (blue, a and b) and MOMA-1 (blue, c and d) for metallophilic macrophages. MZB, marginal zone B cells; MS, marginal sinus; M, macrophages; F, follicular B cells. Original magnification: ×100.
Peripheral B Cells in Mice in which B Cell Development Was Blocked at Birth Acquire an Activated Phenotype and Have Proliferative Activity.
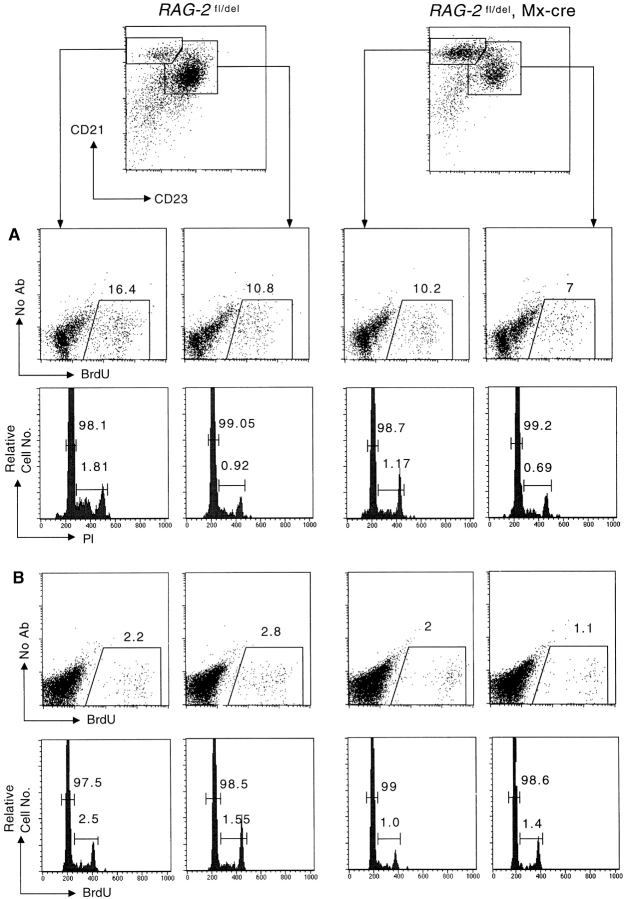
The small B cell population maintained in the spleen of animals in which B cell development was blocked at birth are not only unusual with respect to their tendency to develop into MZ B cells. These cells also had increased size and showed an upregulation of activation markers like CD25, CD69, CD86, and MHC class II when analyzed at the age of 8 wk (Fig. 5). The activated cells, irrespective whether of MZ or FO phenotype, exhibited substantial proliferative activity, with 10% of MZ B cells and 7% of FO B cells incorporating BrdU after a 3-d labeling period, compared with 16 and 11% in the controls (in which there is influx of newly generated B cells from the BM; Fig. 6 A). An analysis of the cells for DNA content showed that up to 1% of the cells were in the S or G2/M phase of the cell cycle (Fig. 6 A). These data suggest that a substantial fraction of the B cells maintained in these animals in spleen and lymph nodes undergo self-renewal. However, not all cells may participate in this self-renewal activity, as BrdU administration for a period of 3 wk resulted in at most one quarter of the cells being labeled (data not shown).
Figure 5.
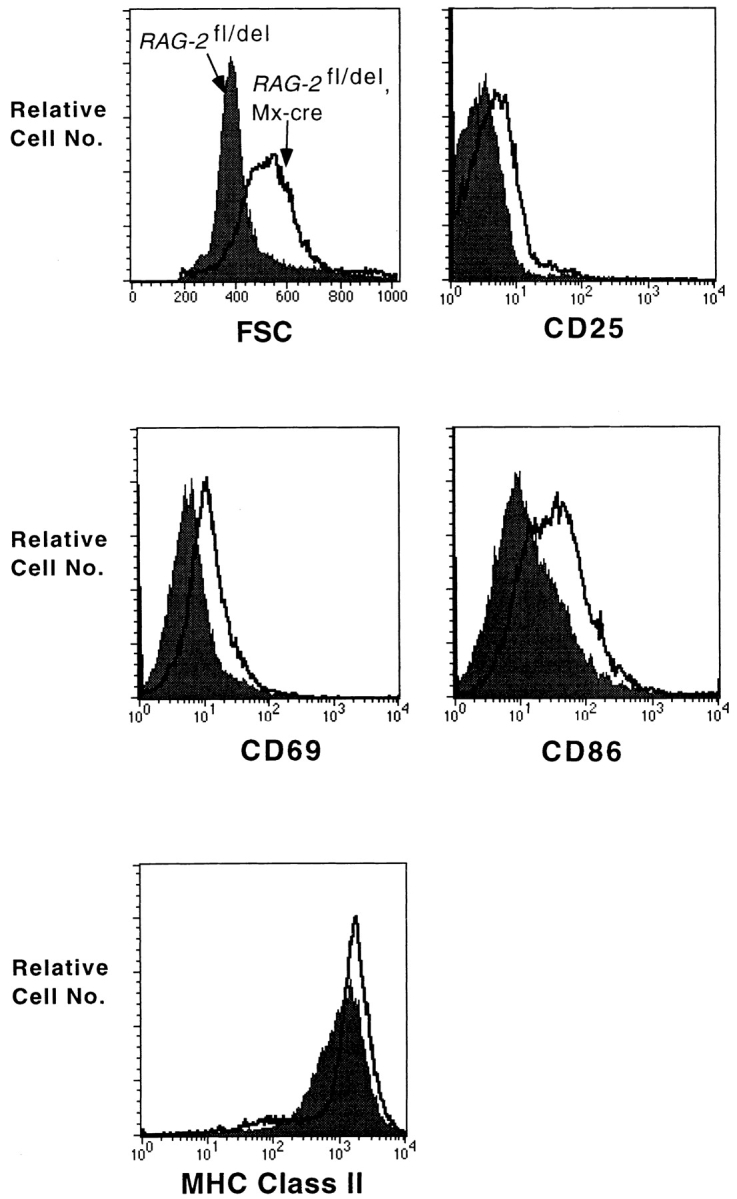
Splenic B cells in mice with induced RAG-2 deficiency from birth are activated. RAG-2 fl/del, Mx-cre mice, and littermate controls were injected with type I IFN at birth. At the age of 8 wk, splenocytes were analyzed by FACS® for the expression of activation markers as indicated. Shaded histograms represent controls and unshaded histograms the induced mutants. FSC, forward scatter.
Figure 6.
Proliferative activity of peripheral splenic B cells revealed by BrdU-labeling and cell cycle analysis. RAG-2 fl/del, Mx-cre mice, and littermate were treated with Poly(I)·Poly(C) at the age of 8 wk (A) or with type I IFN at the age of 2 wk (B). 10 and 6 wk after induction, respectively, the mice were fed with BrdU in the drinking water for 3 d. At the end of the third day, MZ and FO B cells were sorted, fixed, and stained with anti-BrdU antibody and propidium iodide. One representative experiment of two to four is shown. Numbers indicate the percentage of BrdU-positive cells or cells in G1/G0, and S and G2/M phase, respectively.
The situation was quite different in mice in which B cell development had been blocked at the age of 8 wk. When these animals was fed with BrdU in the drinking water for 3 d, 10 wk after application of Poly(I)·Poly(C), only 1% of the FO B cells and 2% of the MZ B cells were labeled, compared with 2 and 3%, respectively, in the controls (Fig. 6 B). According to DNA content, again around 1% of the cells was in S or G2/M phase of the cell cycle (Fig. 6 B). It is possible that in these animals the remaining B cells acquire full self-renewing activity only later, when cell numbers have dropped to a level comparable to that seen in animals in which B cell development was blocked at an early stage. In accord with this possibility, when RAG-2 deletion was induced in adult mice, the B cell acquired an activated phenotype like the one depicted in Fig. 5 only around 4 mo later (data not shown).
RAG-2 Deletion Induced at Birth Leads to an Increase of Serum IgM Levels and the Numbers of IgM Secreting Cells.
The number of B cells in animals in which RAG-2 was deleted at birth is less than 10% of the controls (Fig. 2D and Fig. E). Still, at the age of 8 and 17 wk, serum immunoglobulin levels in the these mice were close to, and occasionally even above, those in the controls (Fig. 7A and Fig. B). To understand this phenomenon, we employed ELISPOT to determine the number of IgM and IgG3 secreting cells in the spleen and BM of the mice at the age of 8 weeks. As shown in Fig. 7 C, the total number of IgM secreting cells was three- to fourfold larger in the induced RAG-2 mutants than in the controls, and the animals also harbored substantial numbers of IgG3 secreting cells. The antibody secreting cells may well largely derive from B-1 cells which are present in these animals in normal numbers and known to contribute significantly to the production of IgM 39.
Figure 7.
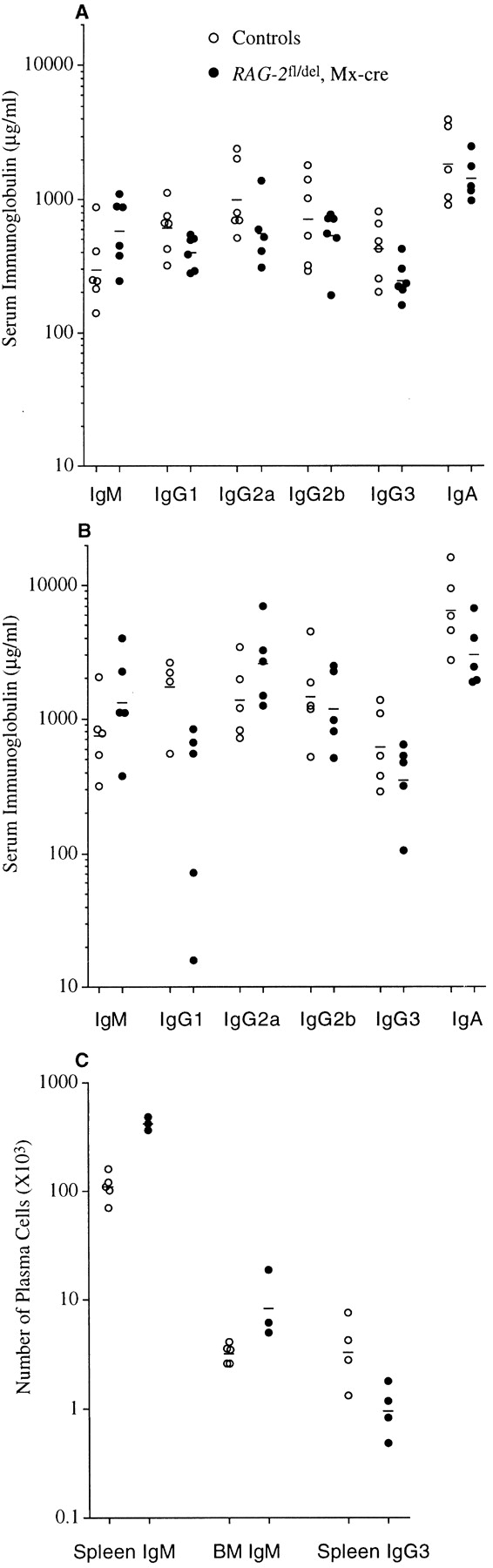
Serum immunoglobulin levels and numbers of IgM and IgG3 secreting cells in mice having undergone RAG-2 deletion at birth. Immunoglobulin levels in the sera of mice in which RAG-2 had been deleted at birth were measured by ELISA at the age of 8 wk (A) and 17 wk (B). Numbers of IgM and IgG3 secreting cells in the spleen and BM (one femur) of individual 8-wk-old mutant mice and littermate controls were determined by ELISPOT (C). Dashes indicate geometric means.
Discussion
Approaches to Study the Stability and Self-renewing Potential of the Peripheral B Cell Pool.
A recent review of the literature on B cell life spans in the mouse 40 documents strikingly to which extent the methods used to approach this problem determine the experimental outcome. Depending on whether B cell progenitors were ablated in situ, B cells were transferred into immunocompetent or immunodeficient, irradiated, or nonirradiated hosts, or the incorporation of nucleotide analogs or radioactively labeled nucleotides into the DNA of B cells was measured, life span determinations ranged from a few days to several weeks or months for mature B cells. This complexity is not surprising given the requirement for the organism to keep the size of the peripheral B cell pool constant in a first approximation, although there is continuous but variable loss or expansion of cells in immune reactions and competition of the various B cell subsets for space. Therefore, the entry of newly generated B cells into the peripheral B cell pool must be carefully controlled and B cell life spans likely depend heavily on the extent to which this homeostatic control is disturbed by the experimental approach 13.
In previous work we had come to the conclusion that in intact mice the life span of most mature B cells is in the order of weeks or months, based on BrdU incorporation measurements using both direct labeling of the cells and a pulse-chase protocol 24. We have now attempted to establish an experimental system in which B cell life spans can be measured in vivo under conditions under which they should be maximized by homeostatic control, namely in the absence of influx of new B cells from the BM. Such a system would also allow the study of the self-renewing capacity of the peripheral B cell pool, and it would be of practical relevance as well, by mimicking situations in which B cell generation is suppressed, e.g. by cytotoxic drugs as they are used in the chemotherapy of tumors.
Earlier attempts in this direction have used chemicals like Strontium-89 18, hydroxyurea 15, Gancyclovir in herpes simplex thymidine kinase-transgenic mice 17, or antibodies to IL-7 19 or IL-7R 20, which all block B cell generation in the BM to various extents. However, these experiments suffered from the severe side effects of the chemical drugs and the necessity of continuous treatment of the animals in case of the anti–IL7 and anti–IL-7R antibodies, such that the latter experiments were performed over only short time periods. Another approach consisted of the transfer of mature B cells into immunodeficient, syngeneic hosts 41 42. These experiments are limited by the amounts of cells that can be transferred, and, as an additional potential problem besides the cell transfer as such, in part involved X-irradiation of the hosts 42. The results obtained are still relevant for the present work as further discussed below.
The experimental system described in the present paper was developed to allow the induced ablation of B and T cell generation under quasi-physiological conditions in intact mice. The transgenic mice carry a deleted and a loxP-flanked allele of RAG-2, in combination with the Mx-Cre transgene 34 from which Cre-recombinase can be expressed upon stimulation by IFN or its inducer Poly (I)·Poly (C). In the absence of induction, B and T cell development proceed normally in these mice; upon Cre induction and RAG-2 deletion, development is blocked, and we show that in the case of B cell development in the BM, the developmental block is indeed complete. While it is difficult to formally demonstrate that the system is absolutely tight in all parts of the body, its efficiency is further supported by the finding that in 62-wk-old animals in which RAG-2 had been deleted at birth, only B-1 and virtually no B-2 cells could be detected in the spleen (unpublished data). Thus, in this system B cell development can be ablated at any point in time, and the composition of the peripheral B cell pool monitored thereafter.
Cre induction in the mutant mice also leads to an ablation of T cell development. The consequence of this for the peripheral T cell pool will be described in detail elsewhere. However, we mention a few points here which are relevant in this context. Thus, in adult mice, induced RAG-2 inactivation also results in a decline of peripheral T cell numbers, but the decline is less pronounced than in the case of B cells, and the cells do not acquire an activated phenotype. When T cell development is blocked at birth, peripheral T cells are generated in normal numbers for the first 3 weeks, probably because RAG-2 deletion is not fully efficient in the thymus. Thereafter, T cell numbers slowly decrease, but again we do not observe the generalized cellular activation, as it is seen in the B cell compartment of these animals (unpublished data). We thus see no reason to suspect that the blockade of T cell development leads to a situation in which the remaining T cells affect B cell homeostasis in some unusual way. However, we are still interested to assess B cell homeostasis in the present system in the total absence of T cells, an experiment that will require the introduction of further targeted mutations and thus extensive mouse crossing. The persistence of T cells in the experimental animals is likely responsible for the presence of T cell–dependent antibody isotypes in their blood.
While the present experimental system approaches what it was intended for, namely a block of B and T cell generation under quasi-physiological conditions, it should still be kept in mind that it involves short-term treatment of the animals with type I IFN or Poly (I)·Poly (C), compounds which are potent activators of the immune system. However, we have not observed any long-term effects of these inducers on the homeostasis of the B and T cell compartments in control animals (see also the present results).
Gradual Loss of FO, but Maintenance of MZ and B-1 B Cells in Adult Mice upon RAG-2 Deletion.
When B cell generation was blocked in adult mice, the immature B cells were rapidly lost from BM and spleen, as expected. Mature B cells also declined slowly over time, but the decline differed for the various B cell subsets and anatomical locations: there was a severe loss of B cells (∼90%) from the inguinal lymph nodes over a 17-wk period, and the cells then persisted in this environment for at least 54 wk, perhaps on the basis of a homeostatic mechanism. In the spleen, FO B cells declined to a similar extent over the 54-wk period, but the decline was constant over time with a calculated half-life of 4.5 mo. This half-life is close to that determined for the population of recirculating B cells in the BM (∼5 mo), supporting the view that these cells are in equilibrium with the splenic FO cells. Given that the FO B cells represent the largest B cell subset in the spleen, it is likely that the half-life of 4.5 mo truly reflects the average life span of those cells, although it cannot be excluded that some FO B cells convert to MZ B cells whose persistence over time could be due to cellular influx from the FO B cell compartment. However, the maintenance of MZ B cells as well as of the B1 cells in spleen and peritoneal cavity over the 54 wk of observation more likely indicates that MZ B cells, like B1 cells 5, represent a self-renewing population, a possibility that is supported by the analysis of mice in which RAG-2 had been deleted at birth (see below). Overall, the results attribute long life spans to most peripheral mature B cells in the absence of B cell influx from the BM, but clearly indicates that the subsets of MZ and B-1 B cells are more stable under these conditions than the majority of FO B cells (which seems to decline in spleen and lymph nodes with different kinetics, for reasons that remain to be explored) and can presumably persist without decline for lifetime, at the population level. Influx of B cells from the BM should result in shorter life spans of (some) peripheral mature cells, as is indeed suggested by earlier BrdU labeling experiments 24 25 26.
When B Cell Generation Is Blocked at Birth, Activated B Cells Persist in the Animals, and a Large Compartment of Plasma Cells Is Generated.
Only small numbers of B cells are present in newborn mice. Many of these cells seem to belong to the B-1 subset 43, and they express a restricted antibody repertoire due to the paucity of N nucleotide insertions and other special features of V(D)J recombination predominating at this stage of development 1. There is also evidence that the antibody repertoire of B cells in the newborn is biased toward the production of “natural” polyreactive antibodies prone to autoreactivity 44. How do these B cells develop over time in the absence of subsequent influx of cells expressing a more diverse antibody repertoire as it is typical for the adult mouse?
Upon RAG-2 deletion at birth, the animals maintain a small, only slightly increasing B cell population for the 28-wk period of observation and thus presumably for lifetime. These cells assume an activated phenotype and resemble in their majority B-1 and MZ B cells. Similar to B-1 cells 5, the latter exhibit self-renewing capacity as indicated by BrdU incorporation in vivo (∼10% of the cells incorporating label over a 3-d period). With respect to cellular activation, these results are strikingly similar to those of a recent study of B cell development in IL-7–deficient mice 45. In these animals B cells are generated only in the first 7 wk of life and are maintained with a similar phenotype as in the induced RAG-2 knockouts described above. In both systems large compartments of peritoneal B-1 cells and antibody secreting cells are generated, similar or greater in size compared with those of wild-type animals.
These results are in good accord with observations made in immunodeficient and/or irradiated mice reconstituted with small numbers of mature, peripheral B cells. In such animals B cells persisted at levels comparable to those seen in the present study 41 42. These cells also displayed an activated phenotype and gave rise to a large compartment of plasma cells from which normal levels of serum antibodies are generated 42. A quick and efficient reconstitution of the B-1 compartment including B-1–derived plasma cells is also seen in such experimental models 46.
Whether the cells we see self-propagating in the animals in which B cell development was blocked at birth belong to a separate “fetal” lineage as has been postulated 39 43 47 is not addressed by the present experiments, although the results are compatible with such a notion. Be this as it may, the results directly demonstrate in the intact animal a principle that has recently emerged mainly from cell transfer experiments 42, namely that when B cell numbers are limiting, the system preferentially propagates and maintains activated and/or highly responsive cells (as shown for MZ B cells in reference 6), from which a normal size or even increased compartment of antibody secreting cells is generated. It will be of interest to elucidate the mechanisms by which these cells and their antibody repertoire are selected, and in particular to what extent (self-) antigens are involved in this process as positive regulators. Recent experiments 48 have indeed directly demonstrated positive selection of B-1 cells by an autoantigen.
Acknowledgments
We are grateful to S. Casola for advice and help in targeting vector construction; to S. Casola, A. Weisman, and M. Alimzhanov for critical reading of the manuscript; to M. Pasparakis and C. Schmedt for helpful discussion; to A. Egert, C. Göttlinger, B. Hample, A. Roth, and C. Uthoff-Hachenberg for technical help; to F. Alt and R.J. Monroe for the RAG-2 plasmid; to W. Ma for help in calculation of half-life. We thank P. Vieira for communicating to us unpublished results.
This work was supported by the Deutsche Forschungsgemeinschaft through SFB 243, the Land Nordrhein-Westfalen, and the Körber Foundation.
Footnotes
Abbreviations used in this paper: BM, bone marrow; BrdU, bromo deoxyuridine; ELISPOT, enzyme-linked immunospot; ES, embryonic stem; FO, follicular; HSA, heat shock antigen; MZ, marginal zone; RAG, recombination-activating gene.
References
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- Kumararatne D.S., MacLennan I.C. The origin of marginal-zone cells. Adv. Exp. Med. Biol. 1982;149:83–90. doi: 10.1007/978-1-4684-9066-4_12. [DOI] [PubMed] [Google Scholar]
- Kraal G. Cells in the marginal zone of the spleen. Int. Rev. Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- Oliver A.M., Martin F., Gartland G.L., Carter R.H., Kearney J.F. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur. J. Immunol. 1997;27:2366–2374. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- Kantor A.B., Herzenberg L.A. Origin of murine B cell lineages. Annu. Rev. Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- Oliver A.M., Martin F., Kearney J.F. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J. Immunol. 1999;162:7198–7207. [PubMed] [Google Scholar]
- Martin F., Kearney J.F. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory. Immunol. Rev. 2000;175:70–79. [PubMed] [Google Scholar]
- Martin F., Oliver A.M., Kearney J.F. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- Karras J.G., Wang Z., Huo L., Howard R.G., Frank D.A., Rothstein T.L. Signal transducer and activator of transcription-3 (STAT3) is constitutively activated in normal, self-renewing B-1 cells but only inducibly expressed in conventional B lymphocytes. J. Exp. Med. 1997;185:1035–1042. doi: 10.1084/jem.185.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguay D.A., Colarusso T.P., Pavlovic S., Irigoyen M., Howard R.G., Bartek J., Chiles T.C., Rothstein T.L. Early induction of cyclin D2 expression in phorbol ester-responsive B-1 lymphocytes. J. Exp. Med. 1999;189:1685–1690. doi: 10.1084/jem.189.11.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond D.G. Population dynamics of bone marrow B lymphocytes. Immunol. Rev. 1986;93:103–124. doi: 10.1111/j.1600-065x.1986.tb01504.x. [DOI] [PubMed] [Google Scholar]
- Allman D.M., Ferguson S.E., Lentz V.M., Cancro M.P. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J. Immunol. 1993;151:4431–4444. [PubMed] [Google Scholar]
- Freitas A.A., Rocha B., Coutinho A.A. Lymphocyte population kinetics in the mouse. Immunol. Rev. 1986;91:5–37. doi: 10.1111/j.1600-065x.1986.tb01482.x. [DOI] [PubMed] [Google Scholar]
- Allman D.M., Ferguson S.E., Cancro M.P. Peripheral B cell maturation. I. Immature peripheral B cells in adults are heat-stable antigenhi and exhibit unique signaling characteristics. J. Immunol. 1992;149:2533–2540. [PubMed] [Google Scholar]
- de Freitas A.A., Coutinho A. Very rapid decay of mature B lymphocytes in the spleen. J. Exp. Med. 1981;154:994–999. doi: 10.1084/jem.154.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas A.A., Rocha B., Forni L., Coutinho A. Population dynamics of B lymphocytes and their precursorsdemonstration of high turnover in the central and peripheral lymphoid organs. J. Immunol. 1982;128:54–60. [PubMed] [Google Scholar]
- Heyman R.A., Borrelli E., Lesley J., Anderson D., Richman D.D., Baird S.M., Hyman R., Evans R.M. Thymidine kinase obliterationcreation of transgenic mice with controlled immune deficiency. Proc. Natl. Acad. Sci. USA. 1989;86:2698–2702. doi: 10.1073/pnas.86.8.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozing J., Buurman W.A., Benner R. B lymphocyte differentiation in lethally irradiated and reconstituted mice. I. The effect of Strontium-89 induced bone marrow aplasia on the recovery of the B cell compartment in the spleen. Cell. Immunol. 1976;24:79–89. doi: 10.1016/0008-8749(76)90133-7. [DOI] [PubMed] [Google Scholar]
- Grabstein K.H., Waldschmidt T.J., Finkelman F.D., Hess B.W., Alpert A.R., Boiani N.E., Namen A.E., Morrissey P.J. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J. Exp. Med. 1993;178:257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T., Nishikawa S., Ohno N., Akiyama N., Tamakoshi M., Yoshida H. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc. Natl. Acad. Sci. USA. 1993;90:9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Miller J.F. Thoracic duct lymphocytes from nude micemigratory properties and life-span. Eur. J. Immunol. 1972;2:384–387. doi: 10.1002/eji.1830020420. [DOI] [PubMed] [Google Scholar]
- Sprent J., Basten A. Circulating T and B lymphocytes of the mouse. II. Lifespan. Cell. Immunol. 1973;7:40–59. doi: 10.1016/0008-8749(73)90181-0. [DOI] [PubMed] [Google Scholar]
- Ropke C., Hougen H.P., Everett N.B. Long-lived T and B lymphocytes in the bone marrow and thoracic duct lymph of the mouse. Cell. Immunol. 1975;15:82–93. doi: 10.1016/0008-8749(75)90166-5. [DOI] [PubMed] [Google Scholar]
- Forster I., Rajewsky K. The bulk of the peripheral B-cell pool in mice is stable and not rapidly renewed from the bone marrow. Proc. Natl. Acad. Sci. USA. 1990;87:4781–4784. doi: 10.1073/pnas.87.12.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster I., Muller W., Schittek B., Rajewsky K. Generation of long-lived B cells in germ-free mice. Eur. J. Immunol. 1991;21:1779–1782. doi: 10.1002/eji.1830210732. [DOI] [PubMed] [Google Scholar]
- Fulcher D.A., Basten A. Influences on the lifespan of B cell subpopulations defined by different phenotypes. Eur. J. Immunol. 1997;27:1188–1199. doi: 10.1002/eji.1830270521. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Oettinger M.A., Schatz D.G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R.S., Herrup K., Tonegawa S., Papaioannou V.E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K.P., Oltz E.M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A.M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Gu H., Kuhn R., Betz U.A., Muller W., Roes J., Schwenk F. Conditional gene targeting. J. Clin. Invest. 1996;98:600–603. doi: 10.1172/JCI118828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres R., Kühn R. Laboratory Protocols for Conditional Gene Targeting 1997. Oxford University Press, ; Oxford, UK: pp. 167 pp [Google Scholar]
- Kontgen F., Suss G., Stewart C., Steinmetz M., Bluethmann H. Targeted disruption of the MHC class II Aa gene in C57BL/6 mice. Int. Immunol. 1993;5:957–964. doi: 10.1093/intimm/5.8.957. [DOI] [PubMed] [Google Scholar]
- Kuhn R., Schwenk F., Aguet M., Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Roes J., Rajewsky K. Immunoglobulin D (IgD)-deficient mice reveal an auxiliary receptor function for IgD in antigen-mediated recruitment of B cells. J. Exp. Med. 1993;177:45–55. doi: 10.1084/jem.177.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C.C., Nilsson L.A., Nygren H., Ouchterlony O., Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J. Immunol. Methods. 1983;65:109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- Schwenk F., Baron U., Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Kozono Y., Waldschmidt T.J., Berthiaume D., Quigg R.J., Baron A., Holers V.M. Mouse complement receptors type 1 (CR1;CD35) and type 2 (CR2;CD21)expression on normal B cell subpopulations and decreased levels during the development of autoimmunity in MRL/lpr mice. J. Immunol. 1997;159:1557–1569. [PubMed] [Google Scholar]
- Herzenberg L.A. B-1 cellsthe lineage question revisited. Immunol. Rev. 2000;175:9–22. [PubMed] [Google Scholar]
- Fulcher D.A., Basten A. B cell life spana review. Immunol. Cell Biol. 1997;75:446–455. doi: 10.1038/icb.1997.69. [DOI] [PubMed] [Google Scholar]
- Sprent J., Schaefer M., Hurd M., Surh C.D., Ron Y. Mature murine B and T cells transferred to SCID mice can survive indefinitely and many maintain a virgin phenotype. J. Exp. Med. 1991;174:717–728. doi: 10.1084/jem.174.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agenes F., Freitas A.A. Transfer of small resting B cells into immunodeficient hosts results in the selection of a self-renewing activated B cell population. J. Exp. Med. 1999;189:319–330. doi: 10.1084/jem.189.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R. Development and function of B-1 cells. Curr. Opin. Immunol. 2000;12:346–353. doi: 10.1016/s0952-7915(00)00098-4. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Kazatchkine M.D., Avrameas S. Natural autoantibodies. Curr. Opin. Immunol. 1995;7:812–818. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- Carvalho T.L., Mota-Santos T., Cumano A., Demengeot J., Vieira P. Arrested B lymphopoesis and persistence of activated B cells in adult interleukin 7−/− mice. J. Exp. Med. 2001;194:1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroese F.G., Butcher E.C., Stall A.M., Lalor P.A., Adams S., Herzenberg L.A. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int. Immunol. 1989;1:75–84. doi: 10.1093/intimm/1.1.75. [DOI] [PubMed] [Google Scholar]
- Herzenberg L.A. Toward a layered immune system. Cell. 1989;59:953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Asano M., Shinton S.A., Gui M., Allman D., Stewart C.L., Silver J., Hardy R.R. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]