Abstract
Continuous lymphocyte recirculation between blood and lymphoid tissues forms a basis for the function of the immune system. Lymphocyte entrance from the blood into the tissues has been thoroughly characterized, but mechanisms controlling lymphocyte exit from the lymphoid tissues via efferent lymphatics have remained virtually unknown. In this work we have identified mannose receptor (MR) on human lymphatic endothelium and demonstrate its involvement in binding of lymphocytes to lymphatic vessels. We also show that the binding requires L-selectin, and L-selectin and MR form a receptor–ligand pair. On the other hand, L-selectin binds to peripheral lymph node addressins (PNAds) on high endothelial venules (HEVs) that are sites where lymphocytes enter the lymphatic organs. Interestingly, MR is absent from HEVs and PNAds from lymphatic endothelium. Thus, lymphocyte L-selectin uses distinct ligand molecules to mediate binding at sites of lymphocyte entrance and exit within lymph nodes. Taken together, interaction between L-selectin and MR is the first molecularly defined mechanism mediating lymphocyte binding to lymphatic endothelium.
Keywords: lymphatics, lymphocyte recirculation, cancer metastasis, endothelium, lymph node
Introduction
The majority of lymphocytes extravasate into the lymph nodes via specialized vessels called high endothelial venules (HEVs). The molecular mechanisms mediating lymphocyte entrance into the lymph nodes via HEVs are rather well known and the multistep interaction between lymphocyte surface molecules and their counter-receptors on endothelium are thoroughly characterized 1 2 3. For example, L-selectin is crucial for lymphocytes to recognize and roll on the luminal surface of peripheral lymph node HEVs. There it uses peripheral lymph node addressins (PNAds) as its endothelial cell counterpart 4. Rolling is followed by an activation step regulated by the function of certain chemokines and their receptors. Thereafter, firm adhesion is mediated by integrins and their Ig–superfamily ligands on endothelium. However, integrins can also function without a preceding rolling step, for example, in smaller capillaries, where the shear is low 1 2 3.
A small part of incoming lymphocytes enter the nodes via afferent lymphatics together with antigens and other types of hematopoietic cells such as dendritic cells, macrophages, and granulocytes. However, only lymphocytes are able to leave the nodes via efferent lymphatic system by first traversing the sinusoidal endothelium and then entering the efferent lymphatic vessel 5 6 7. To maintain the homeostasis in the lymph node the numbers of entering and exiting lymphocytes need to be well in balance. In addition to being of fundamental importance in normal lymphocyte recirculation, the lymphatics also regulate seeding of metastasizing cells in those ∼50% of cancers, which use this type of vessels for spreading.
At the moment, a few molecules present on lymphatic endothelium have been characterized. Those include D6, a β-chemokine receptor 8, and vascular endothelial growth factor receptor (VEGFR)-3 that seems to be critical for normal lymphangiogenesis 9. Moreover, an endocytic receptor for hyaluronan (LYVE-1) is expressed rather dominantly on lymphatic endothelium 10 and podoplanin, first identified as a glomerular podocyte membrane mucoprotein in kidney, has been successfully used as a lymphatic endothelial marker 8 11. However, nothing is known about the molecules mediating lymphocyte exit from the tissues via lymphatics. Therefore, our aim in this work was to characterize such molecules in human lymphatic vessels.
Materials and Methods
Production of Monoclonal Antibodies.
Balb/c mice were immunized to footpads four times at 1 wk intervals with incomplete Freund's adjuvant containing suspension made from efferent lymphatic vessels excised from human lymph nodes. The popliteal lymph node lymphocytes of the immunized mice were fused with Sp2/0 myeloma cells. Hybridoma supernatants were primarily tested on frozen sections of human lymph nodes using immunoperoxidase staining and 3-155 antibody (IgG) was selected for further studies based on its staining of lymphatic endothelium.
Immunostainings.
Immunoperoxidase stainings were performed as described previously 12. In brief, acetone fixed 6-μm frozen sections from different human tissues (lymph nodes, appendix, bronchus, cerebellum, epididymis, oesophagus, heart, small and large intestine, kidney, liver, lung, normal and psoriatic skin, synovium, testis, and tonsil; procedures for tissue collection were approved by the Local and National Boards of Medicolegal Affairs in Finland) were stained with hybridoma supernatants of 3-155 or negative class matched control antibody (3G6 against chicken T cells; reference 12) and lymph nodes also with MECA-79 (rat IgM) against PNAds (a gift from E. Butcher, Stanford University, Stanford, CA; reference 13) followed by peroxidase-conjugated rabbit anti–mouse Ig (1:40; Dako) or peroxidase-conjugated rabbit anti–rat Ig (1:100; Dako), respectively. 3,3-diaminobenzidine in PBS containing 0.03% hydrogen peroxide was used as a chromogen. Finally, the sections were counterstained in hematoxylin (Sigma-Aldrich), dehydrated, cleared in xylene, and permanently mounted in DePex (BDH Limited). Results were analyzed independently by two individuals. To test similarity between our new antibody and earlier known mannose receptor (MR) antibody we used monoclonal anti–human MR antibody (anti–hMR-Ab; Research Diagnostics, Inc.) at 1 μg/ml and stained peripheral lymph nodes, tonsil, and liver samples in parallel with both antibodies using the same protocol as described above.
In double stainings of lymph node sections FITC-conjugated 3-155 (50 μg/ml) together with MECA-79 (10 μg/ml, anti-PNAd) and goat polyclonal anti-VEGFR-3 (10 μg/ml VEGFR-3; R&D Systems) were used. Tetramethyl rhodamine isothiocyanate–conjugated rabbit anti–goat IgG (Zymed Laboratories) with 5% AB-serum and 5% normal mouse serum was the secondary Ab for anti-VEGFR-3 and R-phycoerythrin–conjugated goat anti–rat IgM (Southern Biotechnology Associates, Inc.) with 5% AB-serum was the secondary Ab for anti-PNAds. After staining, the samples were mounted with ProLong® Antifade kit (Molecular Probes). Negative controls in this staining were FITC-conjugated anti–mouse Vβ4, TIB 146 (rat anti–mouse CD45, IgM; American Type Culture Collection), and normal goat serum (Vector Laboratories). In skin samples anti-VEGFR-3 (50 μg/ml) was detected with FITC-conjugated anti–goat IgG (1:100; Sigma-Aldrich) with 5% of AB-serum and 5% of normal mouse serum and staining was enhanced with Alexa Fluor™ antifluorescein (goat IgG; Molecular Probes). Biotinylated 3-155 (50 μg/ml) was detected with PE-conjugated streptavidin (Becton Dickinson). Normal goat serum and biotinylated 3G6 were used as negative controls.
To detect the ligands for L-selectin, supernatant containing human L-selectin-IgM chimera (extracellular part of human L-selectin fused to Fc part of human IgM) was incubated on frozen sections of human lymph nodes. The nonspecific binding sites and reactivity towards IgM positive cells in the section were preblocked (before adding L-selectin-IgM chimera) using normal goat serum and goat anti–human IgM (Caltag). The bound L-selectin-IgM was detected using biotinylated goat anti–human IgM (Caltag) followed by streptavidin-horseradish peroxidase. The reaction was visualized using NovaRed (Vector Laboratories). The control sections were treated otherwise similarly but without addition of L-selectin IgM chimera.
For flow cytometric analyses, peripheral blood mononuclear cells, thoracic duct lymphocytes, and peripheral blood-derived monocytes were used. Peripheral blood mononuclear cells from healthy volunteers were isolated by Ficoll-Hypaque gradient centrifugation. Monocytes were isolated from buffy coats (Finnish Red Cross) first by using Ficoll-Hypaque gradient centrifugation, and then by selecting the adhesive cells after 2-h incubation at +37°C in complete medium which is RPMI 1640 containing 10 mM Hepes, 10% FCS, 4 mM l-glutamine, 100 μg/ml streptomycin, and 100 U/ml penicillin. Monocytes were activated for 24 h in complete medium supplemented with 50 ng/ml GM-CSF. Thoracic duct lymphocytes were obtained from drainages used to treat thoracic duct fistulae.
For staining, the cells were first incubated with 3-155 supernatant or anti-hMR-Ab (2 μg/ml) and then with FITC-conjugated anti–mouse IgG (Sigma-Aldrich). Thereafter, the cells were fixed in PBS containing 1% formaldehyde. 3G6 was used as a negative control antibody. Samples were analyzed using flow cytometer (FACScan™; Becton Dickinson).
In Vitro Adhesion Assay.
Two types of adhesion assays were used. A “static” assay was developed to mimic the conditions of lymphocyte binding to lymphatic endothelium within lymph nodes in the absence of shear. The classic Stamper-Woodruff binding assays were used to mimic the binding of bloodborne cells to vascular endothelium in vessels under physiological shear.
In the static assays lymph node sections were first incubated with 3-155, anti–L-selectin (Dreg-56), or control antibodies, 3G6 or anti-HLA ABC (HB-95; American Type Culture Collection [50 μg/ml of all antibodies were used, if not indicated otherwise]) and then overlaid with Ficoll-Gradient purified peripheral blood mononuclear cells (3 × 106 cells per section). To test whether there is subtype preference in lymphocyte binding to lymphatic endothelium, lymphocytes were stained before the assay with PE-conjugated anti-CD8 or anti-CD4 (both antibodies were from Becton Dickinson). Alternatively, L-selectin negative or positive lymphocytes selected using nonblocking antibody Leu-8 (Becton Dickinson) against L-selectin and goat anti–mouse IgG-coupled supermagnetic beads (MACS®; Miltenyi Biotec) were used. These staining and isolation procedures do not affect endothelial binding capacity of cells 17. Thereafter, the sections were let to stay in static conditions for 15 min, followed by 5 min of rotation at 60 rpm, and then again 15 min without rotation at 7°C. Certain assays were performed under constant rotation. However, static conditions were needed for optimal binding to lymphatic endothelium. The adherent cells were fixed in 1% glutaraldehyde.
The classical Stamper-Woodruff assays were done using the same cell concentrations and antibody concentrations. The only difference was that the cells were applied onto the section under constant rotation (60 rpm) and let adhere for 30 min under these nonstatic conditions.
The number of lymphocytes bound to sinusoidal (lymphatic) endothelium (and to HEVs in certain assays) was counted single blind under dark-field illumination in which setting the sinusoidal vessels are easily recognizable. Epi-illumination was used to detect bound CD4 and CD8 positive cells (their percentage in the input population was assayed using flow cytometer). The results of the inhibition assays are presented as percentage of control binding (the number of adherent cells/vessel in the presence of control mAb defines 100% adherence).
Peptide Analyses.
The molecule recognized by 3-155 antibody was purified from human lymph node lysate (lysis buffer: 150 mM NaCl, 10 mM Tris-base, 1.5 mM MgCl2, 1% NP-40, 1 mM PMSF, and 1% aprotinin) using CnBr-Sepharose 4B (Amersham Pharmacia Biotech) columns coupled with 3-155 antibody (5 mg/ml beads) and a previously described protocol 14. The columns were washed extensively with the lysis buffer and the material bound to the 3-155 column was eluted with 50 mM triethylamine and lyophilized. The eluted material was then subjected to SDS-PAGE analysis and silver staining. The specific band was excised, reduced, alkylated, and digested with trypsin (Promega) overnight at +37°C as described previously 15. The peptides were analyzed using PerSeptive Biosystems Voyager DE-PRO mass spectrometer operated in the reflectron delayed-extraction mode. Calibration of the spectrum was performed internally by using autolysis products of trypsin or with added calibration mixture 2 (PerSeptive BioSystems). Data base search was performed by MS-Fit algorithm (prospector.ucsf. edu/ucsfhtml3.2/msfit.htm) of the UCSF mass spectrometry facility.
Glycosidase Treatments.
NP-40 (1%) containing lysates from GM-CSF activated monocytes and lymph nodes were treated with 40 mU Vibrio cholerae neuraminidase (4 h; Dade Behring, Inc.), 40 mU neuraminidase (4 h) plus 2 mU O-glycanase (overnight; Glyko, Inc.), and 10 mU N-glycanase (overnight; Glyko [reference16]). All incubations were done at 37°C and control incubations were performed under similar conditions without enzymes. Thereafter, the samples were run on SDS-PAGE, blotted to nitrocellulose sheets, and probed with 3-155 antibody or a negative control antibody (3G6); both antibodies were used at concentration of 2 μg/ml. Peroxidase-conjugated rabbit anti–mouse Ig (1:1,000; Dako) was used as the second stage reagent. Detection was performed using enhanced chemiluminescence system (Hybond-ECL; Amersham Pharmacia Biotech).
Crossprecipitation Studies and Ligand Analyses.
Antibodies 3-155, anti-hMR (HyCult Biotechnology), and 3G6 (neg co) were loaded to protein A-Sepharose beads coupled to rabbit anti–mouse Igs (Dako). These beads were used to immunoprecipitate antigens from human lymph node lysates. The original and depleted lysates were analyzed by SDS-PAGE and immunoblotting using enhanced chemiluminescence (Amersham Pharmacia Biotech) with anti-hMR as a primary Ab.
L-selectin-IgM chimera was loaded on protein A-Sepharose beads coupled to goat anti–human IgM (Caltag). After washings, these beads and the control beads without L-selectin chimera were used to immunoprecipitate antigens from human lymph node lysates. The resultant depleted lysates were analyzed by SDS-PAGE and immunoblotting using enhanced chemiluminescence (Amersham Pharmacia Biotech). The intensity of the 3-155 bands was quantified using Microcomputer Imaging Device (MCID; Imaging Research, Inc.).
Results
A New Antibody Detects a Molecule Mediating Lymphocyte Binding to Lymphatics.
The molecular mechanisms involved in lymphocyte exit are unknown. To identify migration-associated structures we produced monoclonal antibodies against isolated efferent lymphatic vessels of human lymph nodes. One of the antibodies (3-155) clearly stained lymphatic endothelium both in afferent and efferent lymphatic systems (Fig. 1). This was confirmed by using a double staining technique with an antibody against a known lymphatic endothelial marker VEGFR-3. Remarkably, 3-155 did not stain HEVs that were brightly PNAd positive (Fig. 1).
Figure 1.
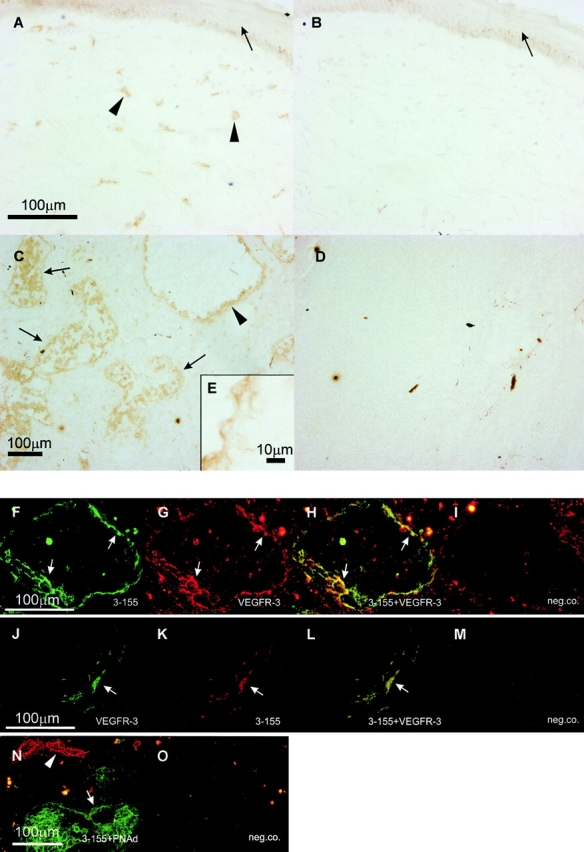
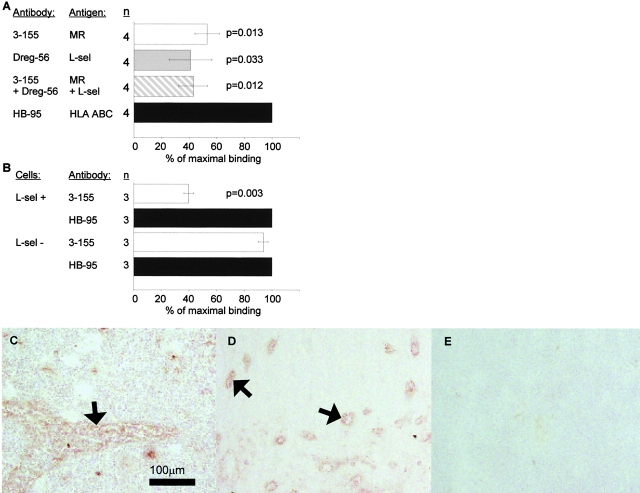
The monoclonal antibody 3-155 recognizes lymphatic endothelium both in afferent and efferent lymphatic systems. In indirect immunoperoxidase staining the afferent lymphatic sacs (arrowheads) in the skin stain positively (epidermis pointed out by an arrow). Some of the positive structures are cells belonging to macrophage lineage. (A) The staining with a negative control antibody does not show any specific reactivity (B). 3-155 reacts with small lymphatic vessels (cross-sectional profile, arrows), and with larger sinusoids (a single endothelial cell layer is seen, arrowhead) in the lymph node (C). The same area lacks any reactivity when stained with a negative control antibody (D). (E) The inset demonstrates expression of 3-155 antigen in sinusoidal endothelium with a high power magnification. In other organs studied sinusoidal endothelium of liver, occasional tissue macrophages, and lymphatic vessels stained positively with 3-155 (data not shown). Double stainings of lymphatic vessels in a lymph node (F–I) and skin (J–M) with 3-155, anti–VEGFR-3, and negative control antibodies (I and M). Two-color staining of lymph node sections with 3-155 (green), MECA-79 (against PNAd; red) (N) and negative control antibodies (O). The opposite phenotypes of lymphatic endothelium and HEVs regarding expression of MR and PNAds are obvious. Arrows point to the lymphatic endothelium (F, G, H, J, K, L, and N) and an arrowhead to HEVs (N).
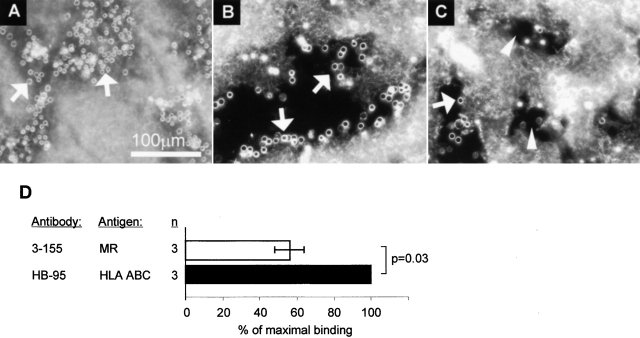
To analyze whether the molecule recognized by 3-155 is involved in mediating lymphocyte binding to lymphatic endothelium, a new adhesion assay was developed. This was necessary, because lymphocytes under constant rotation (normally used condition in a Stamper-Woodruff type of assay) do not bind to lymphatic endothelium. If static conditions are used lymphocytes effectively bind to lymphatic endothelium and binding to HEVs is 71.8 ± 11.3% (n = 4) of the adhesion in nonstatic conditions. Static conditions obviously seem to bypass the need of selectin-mediated interaction between lymphocytes and HEVs, because L-selectin positive and negative lymphocytes bind equally wellx to HEV in static conditions (L-selectin negative cells bound 1.04 ± 0.04 times better than L-selectin positive lymphocytes, n = 2). In contrast, under rotatory conditions L-selectin negative cells bind approximately three times less efficiently to peripheral lymph node HEVs than unseparated peripheral blood lymphocytes 17.
Using the static assay lymphocyte binding to endothelium in lymphatic sinuses on frozen sections of lymph nodes could be measured (Fig. 2 A–C). Lymphatic sinuses are the sites where lymphocytes exit from the organized lymphatic areas of the lymph node and thus belong to the efferent lymphatic system. Although the isolated peripheral blood mononuclear cell population used in the assays contains a small fraction of monocytes, (easily recognizable by their ruffle appearance under dark field microscopy) they do not seem to bind to lymphatic endothelium. To test the efficiency of binding of T cell subtypes to lymphatic endothelium we stained the lymphocytes with PE-conjugated anti-CD4 and CD8 antibodies before the assays. CD4 and CD8 T cells had almost equal capacity to bind to lymphatic endothelium, because the percentages of bound CD8 and CD4 positive cells were almost the same as in the input population (CD4 cells bound 1.1 ± 0.1 times better than CD8 cells, n = 4).
Figure 2.
The molecule recognized by 3-155 is involved in lymphocyte binding to lymphatic endothelium. An adhesion assay was performed to measure lymphocyte binding to lymphatic endothelium. In this assay the lymphocytes (some pointed out by arrows) specifically bind to endothelium of smaller lymphatic vessels that appear as bunches (A) or to endothelial cells that are lining the larger sinusoids (B) (see staining in Fig. 1 C for comparison of these two types of lymphatic vessels). After incubating the sections with 3-155 antibody the number of bound cells is decreased. Two sinuses with markedly decreased number of lymphocytes are indicated by arrowheads (C). In D, the results of three independent inhibition experiments with 3-155 hybridoma supernatant are shown as mean percentage of maximal binding ± SEM. (E) Concentration dependent-inhibition of 3-155.
When the lymph node sections were pre-treated with 3-155 antibody lymphocyte binding to lymphatic endothelium was reduced by 45% (Fig. 2 D). Moreover, inhibition of the binding was dependent on the concentration of the antibody used (Fig. 2 E). These data indicate that the molecule recognized by 3-155 on lymphatic vessels indeed mediates lymphocyte binding.
The Molecule Detected by 3-155 mAb Is MR or its Close Homologue.
We purified 3-155 protein using affinity chromatography. After cleavage with trypsin, mass spectrometric analyses yielded 16 peptides that had identical sequences with MR. These sequences covered altogether 182 amino acids (12% of the 1,456 amino acids of MR) and spanned practically the entire length of the molecule (the amino acids between 24 and 1,285). These results suggested that either the molecule recognized by 3-155 is MR or its close homologue. No identical sequences were found in the other known members of the MR family (phospholipase A2 receptor, reference 18; DEC-205, reference 19; and a novel lectin, reference 20).
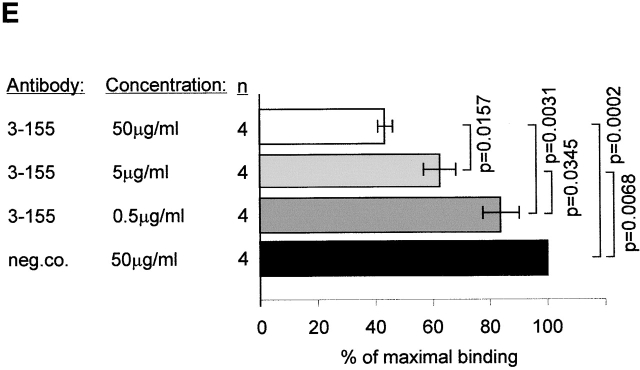
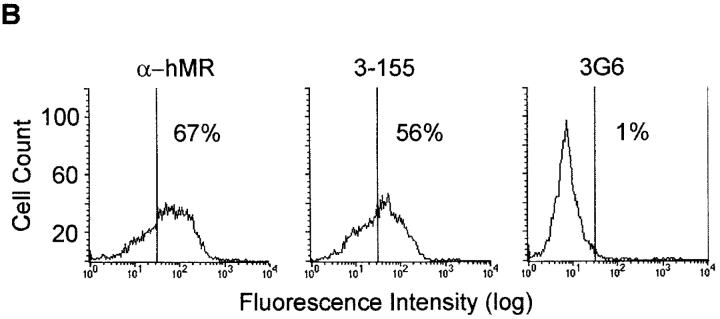
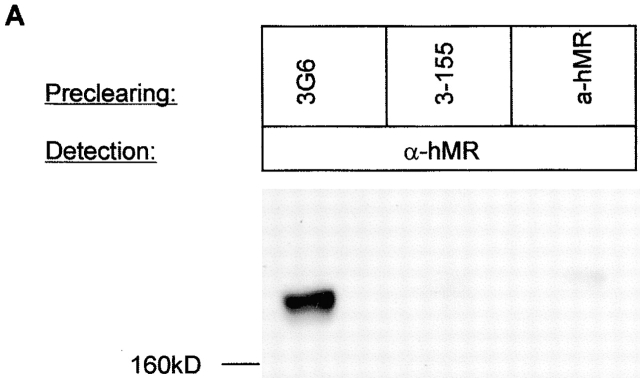
To further study the identity of the antigen recognized by 3-155 with MR, crossprecipitations were performed. Beads coupled to 3-155 and commercial anti-MR antibody were both able to deplete MR from the lysate although the beads coupled to a negative control antibody were not able to do so (Fig. 3 A). The characteristic feature of MR is its inducibility on activated monocytes. As can be seen in Fig. 3 B, 3-155 reacted positively with activated blood monocytes and gave practically an identical staining pattern with a known anti-MR antibody. Lymphocytes from blood and thoracic duct were negative with both antibodies (data not shown). These results strongly suggest that 3-155 antigen is indeed a MR.
Figure 3.
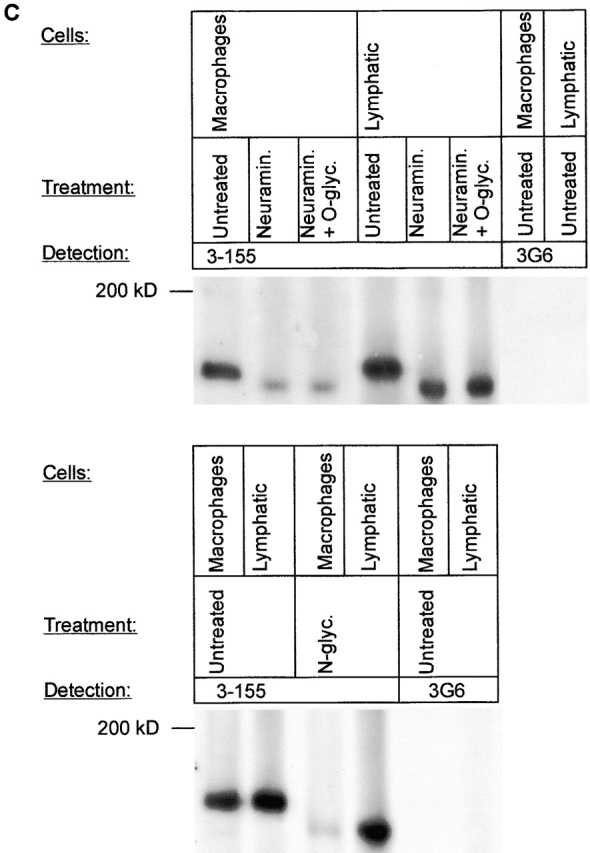
3-155 antigen is an MR and macrophage and lymphatic MR have identical cell distribution and indistinguishable glycosylation profiles. (A) 3-155 antibody coupled to Sepharose 4B beads via rabbit anti–mouse IgG as well as the positive control precipitation with the commercial anti-MR antibody (same as the detecting antibody) were able to remove MR from lymphocyte lysate when tested by immunoblotting using a commercial anti-MR antibody. After the negative control precipitation with beads coupled to an irrelevant antibody (3G6) the MR band is clearly visible. (B) Histograms from flow cytometric analyses demonstrate that GM-CSF–activated monocytes show positive and identical staining both with a known anti–human MR antibody (α-hMR) and 3-155. 3G6 is a negative control antibody. (C) After various glycosidase treatments of activated monocyte (macrophages) and lymph node lysates (lymphatic), molecules were separated by SDS-PAGE and analyzed by immunoblotting with 3-155 and a negative control antibody (3G6).
Lymphatic MR Has Indistinguishable Molecular Mass and Glycosylation with Macrophage MR.
Because the cDNA encoding MR can produce molecules with different binding specificities even in the same cell type 21, we next analyzed whether MR on macrophages and lymphatic endothelium are similar regarding their molecular mass and glycosylation. We treated lysates from activated monocytes and lymph nodes with different glycosidases. The molecular masses of macrophage and lymphatic MRs were practically identical after neuraminidase (removes sialic acids), O-glycanase (cleaves off O-linked carbohydrates), and N-glycanase (cuts off N-linked carbohydrates) digestions (Fig. 3 C). This indicates that lymph node MR that almost entirely originates from lymphoid endothelium (Fig. 1 C) is structurally very similar if not identical to macrophage MR.
L-selectin Is a Lymphocyte Counter-Receptor of Lymphatic MR.
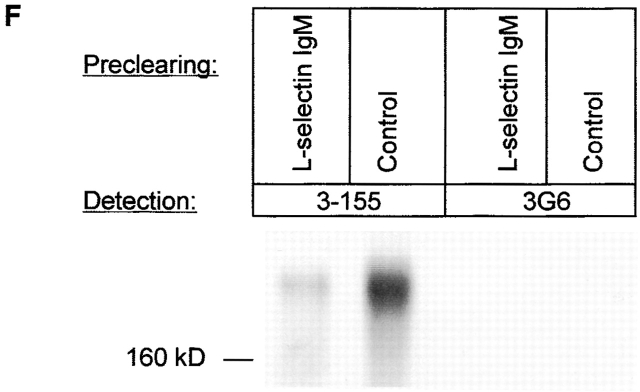
Mouse L-selectin chimera produced in silk worm cells has been reported to have affinity towards MR. However, due to absence of MR on HEVs, this interaction has been considered as an artefact resulting from the baculovirus expression system 22. Because MR on lymphatic endothelium unambiguously was involved in lymphocyte binding we tested whether a function-blocking anti–L-selectin antibody Dreg-56 is able to inhibit lymphocyte binding to lymphatic endothelium. In these studies blocking of L-selectin diminished lymphocyte adhesion by 59%. Simultaneous blocking of L-selectin and lymphatic MR did not show any additive inhibition strongly suggesting that both molecules are mediating the adhesion of the same cell population and perhaps they even act as a receptor ligand pair (Fig. 4 A). We also selected L-selectin positive and negative lymphocytes and tested their capacity to adhere to lymphatic endothelium. Both populations showed comparable binding efficiency to lymphatic vessels (binding of L-selectin negative cells was 98.4 ± 7.6% that of L-selectin positive cells) but only the binding of L-selectin positive population could be significantly inhibited with 3-155 anti-MR antibody (Fig. 4 B). Finally we used an L-selectin-IgM chimera to test whether it can bind to lymphatic endothelium in lymph nodes. In these experiments, L-selectin-IgM chimera strongly bound to lymphatic endothelium as well as to HEVs, demonstrating the presence of its ligand also in lymph vessels (Fig. 4 C and D). Proof for direct binding between L-selectin-IgM and lymphatic MR was obtained from depletion experiments. When lymph node lysate was precleared with beads coupled to L-selectin-IgM, the majority (77%) of MR concomitantly disappeared. In contrast, preclearing with the control beads could not remove the MR (Fig. 4 F). Together, these data strongly indicate that MR is a novel ligand for L-selectin.
Figure 4.
L-selectin is a counter-receptor of MR on lymphatic endothelium. (A) Frozen section adhesion assays were performed by inhibiting lymphocyte binding to sinusoidal endothelium with 3-155, Dreg-56 (anti–L-selectin), both antibodies together, or with a control antibody (anti–human HLA ABC). The results of four independent assays are shown (mean percentage of maximal binding ± SEM). (B) 3-155 significantly inhibits binding of L-selectin positive cells to lymphatic endothelium. In contrast, only minor inhibition is seen when the binding of L-selectin negative cells is tested. Results of three independent assays are presented. L-selectin-IgM chimera was incubated on frozen section of a human lymph node. It binds to sinusoidal endothelial cells (arrow, C) to HEVs (pointed out by arrows, D). (E) Negative control. The L-selectin-IgM chimera coupled beads and control beads were used to deplete lymph node lysate and the nonprecipitated molecules were analyzed using immunoblotting and the monoclonal antibodies indicated (F).
Discussion
MR has a well established role both in endocytotic clearance of certain glycoproteins and in macrophage phagocytosis of microorganisms 23. Presence of MR on sinusoidal endothelial cells has been reported but its role there has remained enigmatic 24 25. Results of this work reveal a novel role for lymphatic MR and represent the first molecular identification of the system controlling lymphocyte exit from lymph nodes. Moreover, discovery of L-selectin as a counter-receptor of lymphatic MR expands the repertoire of L-selectin ligands.
Both MR and L-selectin contain lectin domains and recognize a wide variety of carbohydrates. Furthermore, they both are heavily glycosylated themselves creating theoretical possibilities for different types of recognition events between these two molecules 26 27 28 29 30. However, the antibody Dreg-56 against L-selectin used in the inhibition assays is reported to recognize a lectin domain of L-selectin 31, strongly suggesting involvement of the lectin domain of L-selectin in the binding to a carbohydrate epitope on MR. Theoretically, the possibility remains that a carbohydrate forms a link between the lectin domains of both L-selectin and MR.
As L-selectin interacts with PNAds on HEVs and the present data identifies MR as an L-selectin ligand on lymphatic endothelium, it may implicate that using these alternative ligands L-selectin guides lymphocyte migration at various steps of the recirculation cascade. Thus, first in blood L-selectin directs lymphocyte trafficking to lymph nodes by binding to PNAds on HEVs 4. Notably, this interaction requires shear force over 0.6 dynes/cm2 that is present in blood circulation at the entrance site of lymphocytes 32. Then during the activation step in the adhesion cascade L-selectin is lost by proteolytic cleavage 33 34. However, lymphocytes regain L-selectin expression within the lymph node. Finally, under static conditions L-selectin binds to MR on lymphatic endothelium. Therefore, the recognition domains used by L-selectin on HEVs (PNAd+, MR−) and on lymphatic endothelium (having exactly the opposite phenotype PNAd−, MR+) may be different. Although for technical reasons we were able to test lymphocyte binding only to sinusoidal endothelial cells, the presence of MR also in the afferent lymphatic vessels for example in the skin may indicate that L-selectin plays a role in lymphocyte exit from nonlymphoid tissues as well.
We believe that the experimental conditions used in this work closely reflect the situation in vivo. When lymphocytes are gathering into lymphatic sinusoids ready to leave the lymph node, practically no shear is present. Also lymphocyte binding to lymphatic endothelium in vitro requires static conditions, because in the presence of shear stress only occasional lymphocytes (not even enough to get any meaningful numbers to count) were able to adhere to lymphatic endothelium. In contrast, L-selectin–mediated binding to PNAds at HEVs is shear dependent, because in the absence of shear both L-selectin negative and positive lymphocytes bind with equal efficiency to peripheral lymph node HEVs, presumably by integrin-mediated mechanisms not requiring the shear. In nonstatic conditions, L-selectin positive cells bind at least three times better to peripheral lymph node HEV than L-selectin negative lymphocytes 17.
Interestingly, CD4- and CD8-positive T cells seem to have almost equal capacity to bind to lymphatic endothelium. This is in striking contrast to the entrance site, because CD8 positive cells bind 1.5–2.0 times better than CD4+ cells to peripheral lymph node HEVs 35. The better capacity of CD8 to enter the lymph nodes may compensate their smaller number among blood lymphocytes and result in rather equal numbers of entering CD4 and CD8 cells. Comparable binding to lymphatic endothelium may then allow these different subtypes to leave the lymph nodes with equal efficiency. Although the cell populations (Ficoll-purified mononuclear cells from blood) used in our binding assays contains small number of monocytes and they bind very efficiently to HEV-like vessels at sites of inflammation such as for example in inflamed synovium 36 monocytes did not bind to lymphatic endothelium. This is well in line with in vivo situation, because monocytes are not found in the efferent lymph in normal conditions 37.
In conclusion, based on our in vitro findings we hypothesize that L-selectin could be involved in both lymphocyte entrance to and exit from lymph nodes. We suggest that L-selectin-MR interactions may be involved in the exit phase of physiologic lymphocyte recirculation. These may theoretically also be involved in the exit of L-selectin–expressing malignant cells and thus the spread of L-selectin positive tumors. However, in vivo experiments will be required to elucidate the actual role of L-selectin-MR interactions in lymphocyte exit from the lymph nodes.
Acknowledgments
We thank M. Singer and S. Rosen for providing the L-selectin-IgM chimera, E. Butcher for anti-PNAds and anti–L-selectin antibodies, and J. Lowe for donating the IgM cassette containing vector, J. Hellman for mass spectrometric analyses, K. Elima for sequence analyses, M. Kääriäinen for technical help, and A. Sovikoski-Georgieva for secretarial help.
This work was supported by the Finnish Academy, the Sigrid Juselius Foundation, the Finnish Cancer Union, CIMO, the Swedish Institute, the Research and Science Foundation of Farmos, and Turku Graduate School of Biomedical Sciences.
Footnotes
H. Irjala and E.-L. Johansson contributed equally to this paper.
Abbreviations used in this paper: HEV, high endothelial venule; MR, mannose receptor; PNAd, peripheral lymph node addressin; VEGFR, vascular endothelial growth factor receptor.
References
- Butcher E.C. Leukocyte-endothelial cell recognitionthree (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigrationthe multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Salmi M., Jalkanen S. How do lymphocytes know where to gocurrent concepts and enigmas of lymphocyte homing. Adv. Immunol. 1997;64:139–218. doi: 10.1016/s0065-2776(08)60889-5. [DOI] [PubMed] [Google Scholar]
- Rosen S.D. Endothelial ligands for L-selectinfrom lymphocyte recirculation to allograft rejection. Am. J. Pathol. 1999;155:1013–1020. doi: 10.1016/S0002-9440(10)65201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.G., Morris B. The origin of cells in the efferent lymph from a single lymph node. J. Exp. Med. 1965;121:901–910. doi: 10.1084/jem.121.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay C.R., Kimpton W.G., Brandon M.R., Cahill R.N.P. Lymphocyte subsets show marked differences in their distribution between blood and the afferent and efferent lymph of peripheral lymph nodes. J. Exp. Med. 1988;167:1755–1765. doi: 10.1084/jem.167.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E.C., Picker L.J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Nibbs R.J., Kriehuber E., Ponath P.D., Parent D., Qin S., Campbell J.D., Henderson A., Kerjaschki D., Maurer D., Graham G.J., Rot A. The β-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am. J. Pathol. 2001;158:867–877. doi: 10.1016/s0002-9440(10)64035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen T., Jussila L., Veikkola T., Karpanen T., Kettunen M.I., Pulkkanen K.J., Kauppinen R., Jackson D.G., Kubo H., Nishikawa S.-I. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat. Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- Prevo R., Banerji S., Ferguson D.J., Clasper S., Jackson D.G. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J. Biol. Chem. 2001;276:19420–19430. doi: 10.1074/jbc.M011004200. [DOI] [PubMed] [Google Scholar]
- Breiteneder-Geleff S., Soleiman A., Kowalski H., Horvat R., Amann G., Kriehuber E., Diem K., Weninger W., Tschachler E., Alitalo K., Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillariespodoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi M., Jalkanen S. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science. 1992;257:1407–1409. doi: 10.1126/science.1529341. [DOI] [PubMed] [Google Scholar]
- Streeter P.R., Rouse B.T.N., Butcher E.C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J. Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.J., Salmi M., Bono P., Hellman J., Leu T., Jalkanen S. Cloning of vascular adhesion protein-1 reveals a novel multifunctional adhesion molecule. J. Exp. Med. 1998;188:17–27. doi: 10.1084/jem.188.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A., Wilm M., Vorm O., Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Salmi M., Jalkanen S. Human vascular adhesion protein-1 (VAP-1) is a unique sialoglycoprotein that mediates carbohydrate-dependent binding of lymphocytes to endothelial cells. J. Exp. Med. 1996;183:569–579. doi: 10.1084/jem.183.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi M., Tohka S., Berg E.L., Butcher E.C., Jalkanen S. Vascular adhesion protein 1 (VAP-1) mediates lymphocyte subtype-specific, selectin-independent recognition of vascular endothelium in human lymph nodes. J. Exp. Med. 1997;186:589–600. doi: 10.1084/jem.186.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki J., Hanasaki K., Higashino K., Kishino J., Kikuchi N., Ohara O., Arita H. Molecular cloning of pancreatic group I phospholipase A2 receptor. J. Biol. Chem. 1994;269:5897–5904. [PubMed] [Google Scholar]
- Jiang W., Swiggard W.J., Heufler C., Peng M., Mirza A., Steinman R.M., Nussenzweig M.C. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- Wu K., Yuan J., Lasky L.A. Characterization of a novel member of the macrophage mannose receptor type C lectin family. J. Biol. Chem. 1996;271:21323–21330. doi: 10.1074/jbc.271.35.21323. [DOI] [PubMed] [Google Scholar]
- Fiete D., Beranek M.C., Baenziger J.U. The macrophage/endothelial cell mannose receptor cDNA encodes a protein that binds oligosaccharides terminating with SO4-4- GalNAcβ1,4GlcNAcβ or Man at independent sites. Proc. Natl. Acad. Sci. USA. 1997;94:11256–11261. doi: 10.1073/pnas.94.21.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima H., Watanabe N., Li Y.F., Hirose M., Miyasaka M. Characterization of a 180 kDa molecule apparently reactive with recombinant L-selectin. Glycoconj. J. 1997;14:321–330. doi: 10.1023/a:1018518611341. [DOI] [PubMed] [Google Scholar]
- Stahl P.D., Ezekowitz R.A. The mannose receptor is a pattern recognition receptor involved in host defense. Curr. Opin. Immunol. 1998;10:50–55. doi: 10.1016/s0952-7915(98)80031-9. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Donovan M.J., Rogers R.A., Ezekowitz R.A. Distribution of murine mannose receptor expression from early embryogenesis through to adulthood. Cell Tissue Res. 1998;292:311–323. doi: 10.1007/s004410051062. [DOI] [PubMed] [Google Scholar]
- Uccini S., Sirianni M.C., Vincenzi L., Topino S., Stoppacciaro A., Lesnoni La Parola I., Capuano M., Masini C., Cerimele D., Cella M. Kaposi's sarcoma cells express the macrophage-associated antigen mannose receptor and develop in peripheral blood cultures of Kaposi's sarcoma patients. Am. J. Pathol. 1997;150:929–938. [PMC free article] [PubMed] [Google Scholar]
- Leteux C., Chai W., Loveless R.W., Yuen C.-T., Uhlin-Hansen L., Combarnous Y., Jankovic M., Maric S.C., Misulovin Z., Nussenzweig M.C., Feizi T. The cysteine-rich domain of the macrophage mannose receptor is a multispecific lectin that recognizes chondroitin sulfates A and B and sulfated oligosaccharides of blood group Lewisa and Lewisx types in addition to the sulfated N-glycans of lutropin. J. Exp. Med. 2000;191:1117–1126. doi: 10.1084/jem.191.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Chirino A.J., Misulovin Z., Leteux C., Feizi T., Nussenzweig M.C., Bjorkman P.J. Crystal structure of the cysteine-rich domain of mannose receptor complexed with a sulfated carbohydrate ligand. J. Exp. Med. 2000;191:1105–1116. doi: 10.1084/jem.191.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Pomares L., Kosco-Vilbois M., Darley E., Tree P., Herren S., Bonnefoy J.-Y., Gordon S. Fc chimeric protein containing the cysteine-rich domain of the murine mannose receptor binds to macrophages from splenic marginal zone and lymph node subcapsular sinus and to germinal centers. J. Exp. Med. 1996;184:1927–1937. doi: 10.1084/jem.184.5.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder T.F., Steeber D.A., Chen A., Engel P. The selectinsvascular adhesion molecules. FASEB J. 1995;9:866–873. [PubMed] [Google Scholar]
- Weis W.I., Taylor M.E., Drickamer K. The C-type lectin superfamily in the immune system. Immunol. Rev. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto T.K., Jutila M.A., Butcher E.C. Identification of a human peripheral lymph node homing receptora rapidly down-regulated molecule. Proc. Natl. Acad. Sci. USA. 1990;87:2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger E.B., Puri K.D., Alon R., Lawrence M.B., von Andrian U.H., Springer T.A. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 1996;379:266–269. doi: 10.1038/379266a0. [DOI] [PubMed] [Google Scholar]
- Walcheck B., Kahn J., Fisher J.M., Wang B.B., Fisk R.S., Payan D.G., Feehan C., Betageri R., Darlak K., Spatola A.F., Kishimoto T.K. Neutrophil rolling altered by inhibition of L-selectin shedding in vitro. Science. 1996;380:720–723. doi: 10.1038/380720a0. [DOI] [PubMed] [Google Scholar]
- Chen J.R., Gu B.J., Dao L.-P., Bradley C.J., Mulligan S.P., Wiley J.S. Transendothelial migration of lymphocytes in chronic lymphocytic leukaemia is impaired and involved down-regulation of both L-selectin and CD23. Br. J. Haematol. 1999;105:181–189. [PubMed] [Google Scholar]
- Butcher E.C. The regulation of lymphocyte traffic. Curr. Top. Microbiol. Immunol. 1986;128:85–122. doi: 10.1007/978-3-642-71272-2_3. [DOI] [PubMed] [Google Scholar]
- Salmi M., Jalkanen S. Human leukocyte subpopulations from inflamed gut bind to joint vasculature using distinct sets of adhesion molecules. J. Immunol. 2001;166:4650–4657. doi: 10.4049/jimmunol.166.7.4650. [DOI] [PubMed] [Google Scholar]
- Lemaire L.C., van Deventer S.J., van Lanschot J.J., Meenan J., Gouma D.J. Phenotypical characterization of cells in the thoracic duct of patients with and without systemic inflammatory response syndrome and multiple organ failure. Scand. J. Immunol. 1998;47:69–75. doi: 10.1046/j.1365-3083.1998.00265.x. [DOI] [PubMed] [Google Scholar]