Abstract
The stroma of solid tumors is a complex network of different cell types. We analyzed stroma cell interactions in two tumor models during cyclophosphamide (Cy)-induced tumor rejection. In growing tumors, tumor infiltrating macrophages (TIMs) produced interleukin (IL)-10. Beginning 6 h after Cy-treatment T cells in the tumor were inactivated and TIMs switched to interferon (IFN)-γ production. Both, IL-10 production before and IFN-γ production after Cy-treatment by TIMs required T cells. With the same kinetics as TIMs started to produce IFN-γ the tumor vasculature was destroyed which required IFN-γ receptor expression on host but not tumor cells. These events preceded hemorrhagic necrosis and residual tumor cell elimination by T cells. Together, T cells regulate the function of TIMs and tumor rejection can be induced by disturbing the stroma network.
Keywords: tumor stroma, tumor infiltrating macrophages, IFN-γ, antiangiogenesis, cyclophosphamid
Introduction
Tumor cells are usually embedded within a stroma which sometimes constitutes a great part of the tumor tissue (1). The tumor stroma is composed of a variety of normal cell types (2) which at least in part appear to be actively recruited by the tumor, e.g., to provide the blood supply (3). Often, substantial numbers of T cells and macrophages are part of the tumor stroma (4). Nevertheless, the tumor grows progressively. The effectiveness of T cells to mediate tumor rejection appears (at least in part) to depend on the time of their activation. In previously immunized mice, T cells can mediate rejection of a challenge tumor (5). In contrast, when naive mice are inoculated with live tumor cells or T cells are transfused to tumor-bearing animals, T cells are activated but subsequently downregulated by the growing tumor and become suppressive or anergic (6, 7). North and Bursuker (8) showed that Ly-1−2+ effector cells (CD8+ cytotoxic T lymphocytes) became activated shortly after inoculation of tumor cells into naive recipients (between days 6 and 9), but then were downregulated by the progressively growing tumor correlating with the appearance of Ly-1+2− (CD4+) suppressor T cells. That T cells can suppress tumor rejection has been demonstrated, since in some tumor models depletion of CD4+ T cells resulted in rejection of established tumors (8–10). Nevertheless, the nature of ‘suppressor’ T cells remains controversial, in particular because the mechanism by which they inhibited tumor rejection is unresolved.
The role of tumor-infiltrating macrophages (TIMs)* is enigmatic. It has been proposed that, depending on the activation state and the environment, they can regulate tumor growth in a positive or negative fashion (4). For example, tumors frequently express macrophage chemotactic factors (11), macrophages can secrete angiogenic factors useful for a growing tumor (12) and inhibition of macrophage infiltration correlated with the inability of the tumor to grow in vivo (13). Conversely, artificial expression of macrophage activating cytokines by tumor cells resulted in macrophage infiltration and diminished tumor growth (14, 15). However, evidence of macrophage function in tumors is largely indirect and a functional dynamics of TIMs during tumor regression has not been shown.
Little is known of interactions between different stroma cell components that either support tumor growth or are involved in tumor rejection. We analyzed such interactions and their alterations during rejection of established tumors. For this purpose, we used cyclophosphamide (Cy) which under certain experimental conditions can induce tumor rejection by host cell modulation rather than direct tumoricidal activity (16–18). For example, a single injection of a certain amount of Cy induced tumor rejection in immunocompetent mice but had no effect on tumor growth in immunodeficient mice (19).
We used IFN-γ as a read-out for tumor stroma cell interactions. A role of IFN-γ for tumor rejection has been demonstrated (15, 20–23). Both IFN-γ−/− and IFN-γ receptor (IFN-γR)−/− mice are severely impaired to develop systemic tumor immunity (21, 23). Additionally, hematopoietic and nonhematopoietic cells within the tumor stroma appear to communicate via IFN-γ during tumor rejection. By immunization/challenge experiments we showed that CD4+ T cell–mediated tumor immunity required IFN-γR expression only on nonhematopoietic cells in the effector phase and involved inhibition of angiogenesis (23). IFN-γ is produced mainly by activated T cells and NK cells. Recently, it was shown that macrophages are able to secrete large amounts of IFN-γ upon appropriate stimulation (24). The IFN-γR is expressed on almost all cell types (25, 26). Here we analyzed the dynamics of stroma cell interactions in a growing tumor and during its rejection. We show that during the phase of tumor establishment, T cells condition TIMs to produce IL-10. Upon Cy-induced T cell inactivation TIMs immediately start to produce IFN-γ and the tumor vasculature is destroyed in an IFN-γR–dependent fashion.
Materials and Methods
Mice.
BALB/c mice were purchased from Charles River Laboratories. Nude mice (BALB/c nu/nu) were obtained from The Jackson Laboratory. IFN-γR α-chain deficient (IFN-γR−/−) mice generated as inbred 129/Sv/Ev line and congenic control 129/Sv/Ev mice were provided by M. Aguet, Epalinges, Switzerland (27). Heterozygous offsprings generated by crossing IFN-γR−/− and 129/Sv/Ev mice were intercrossed to establish IFN-γR+/− and IFN-γR−/− mice in the animal facility of the Max-Delbrück-Centrum, Berlin, Germany. The deficiency of the IFN-γR gene was confirmed by PCR of tail DNA as described previously (27). All mice used in the experiments were sex- and age-matched.
Tumor Growth and In Vivo Treatment.
The BALB/c plasmacytoma J558L and the 129/Sv/Ev fibrosarcoma Mc51.9, derived from IFN-γR−/− mice by treatment with 3-methylcholanthrene (23) were cultured in RPMI 1640 medium supplemented with 10% FCS. For tumor establishment, 106 J558L cells, 2 × 105 or 106 Mc51.9 cells were washed twice with PBS and subcutaneously injected in a volume of 0.2 ml into the flank of mice as indicated. On days 11 or 15 when J558L or Mc51.9 tumors were established, a single dose of 15 mg/kg (BALB/c) or 50 mg/kg (129/Sv/Ev) Cy in Dulbecco's PBS (Sigma-Aldrich) was administered intraperitoneally. Tumor size was measured by a caliper and determined as the mean of the largest diameter and the diameter at right angle. J558L tumors had an average size of 1 cm in diameter 11 d after injection. To estimate the tumor volume J558L tumors of 1 cm in diameter were isolated and the weight was determined as 630 ± 10 mg. Tumor rejection was defined as complete regression after treatment and the absence of recurrent tumor for the entire follow-up period (at least 60 d). For in vivo depletion of T cell subsets, tumor-bearing BALB/c mice were depleted of T cell subsets by intraperitoneal injection of 100 μg rat mAb GK1.5 (anti-CD4) or 2.43 (anti-CD8) in a volume of 0.5 ml, 2 d before tumor cell inoculation and/or 2 d before Cy injection. Depletion of the respective T cell subpopulation was controlled by flow cytometric analysis of peripheral blood cells using PE-labeled anti-CD4 (RM4–5) and anti-CD8 mAbs (53–6.7) (BD PharMingen) and lasted for at least 4 wk.
Cytokine Detection.
Mc51.9 tumors were established by subcutaneous injection of 2 × 105 cells into 129/Sv/Ev (IFN-γR+/−) and nude mice. 11 d later, mice were treated with 50 mg/kg Cy. Before, 6 h, 24 h, and 72 h after treatment spleen and tumors were excised. Splenocytes (three mice per group) were cultured at 106 cells per milliliter in a 24-well plate without stimulant, with 10 μg/ml anti-CD3 mAb (37.51), and 5 μg/ml anti-CD28 mAb (145–2C11; BD PharMingen) or with 1 μg/ml ConA (Sigma-Aldrich) for 24–48h. Single cell suspensions of tumors (3–5 mice per group) were prepared by mechanical dissociation and collagenase/DNase I treatment for 45–60 min at 37°C (1 mg/ml collagenase, 1× trypsin/EDTA; GIBCO BRL; 1 mg/ml Dnase I; Boehringer). The cells were then plated in medium at 4 × 105 cells per milliliter in a 24-well plate and incubated at 37°C for 2 h. Nonadherent tumor infiltrating cells were collected and cultured at 106 cells per milliliter with or without stimulation as described for spleen cells. T cell numbers in tumors did not differ significantly before and within the first 3 d after Cy-treatment. For separation of macrophages, cell suspensions were incubated with microbeads coupled anti–Mac-1 (CD11b) (Miltenyi Biotec) and passed over a MACS® column according to the manufacturer's recommendation. The purity of macrophage preparations was between 71 and 93% as determined by cell morphology, plastic adherence, and flow cytometric analysis (data not shown). Mac-1+ cells were plated in medium at 106 cells per milliliter in a 24-well plate and cultured with or without 1 μg/ml LPS for 24 h. IFN-γ or IL-10 in the culture supernatant was determined by ELISA (BD PharMingen). The detection limit was between 31 and 62 pg/ml for both cytokines. Mc51.9 cells did not secrete IL-10 or IFN-γ, either spontaneously or after LPS stimulation (data not shown). The T cell–regulated IFN-γ production by TIMs was also analyzed in a second tumor model. J558L tumors were established by subcutaneous injection of 106 cells into BALB/c mice. 11 d later, mice were left untreated or treated with 15 mg/kg Cy. The mice (3–5/group) were additionally depleted of CD4+/CD8+ T cells starting either before tumor cell inoculation (day –2 and day 9) or before Cy application (day 9) by use of mAb GK1.5 (anti-CD4) and 2.43 (anti-CD8) as described previously. One group of mice was not depleted of T cells. TIMs were isolated on day 12 from not Cy-treated mice or 24 h after Cy-treatment and cultured as above without stimulation or stimulated with IL-12 (10 ng/ml) and IL-18 (10 ng/ml). Mac-1+ cells isolated via MACS® had a purity of ∼90%. After 48-h incubation, culture supernatant was collected and IFN-γ was determined as described previously.
Flow Cytometric Analysis.
Tumors were established by subcutaneous injection of 2 × 105 Mc51.9 cells into IFN-γR+/− and nude mice. 11 d later, single cell suspension of tumors (3–5 mice per group) was prepared as described previously. Cells were directly stained using PE-labeled anti–Mac-1 mAb (M1/70; BD PharMingen) and analyzed with an Epics-XL flow cytometer (Coulter Electronics). Mc51.9 cells did not express detectable Mac-1 (data not shown).
Immunohistochemical Analysis.
Isolation of tumor tissues, preparation of cryostat sections, and alkaline phosphatase immunostaining were done as described previously (14). 2 × 105 Mc51.9 cells were injected subcutaneously into IFN-γR+/− and IFN-γR−/− or nude mice. 11 d later, mice were treated with 50 mg/kg Cy. Tumors were excised before, 6 h, 24 h, and 72 h after treatment. For detection of TIMs consecutive sections were stained with anti-F4/80 (cl: A3–1; Serotec). Endothelial cells were identified with Meca 32 (28) or anti-CD31 mAb (MEC 13.3; BD PharMingen). The alkaline phosphatase-conjugated goat anti–rat IgG was purchased from Jackson ImmunoResearch Laboratories. All sections were then counterstained with Mayer's hematoxylin (Chroma Gesellschaft GmbH, Münster, Germany). Tissue sections of 3–4 mice per group were evaluated.
Results
T Cell-dependent and -independent Phases during Cy-induced Tumor Rejection.
We employed a model in which rejection of established solid tumors was induced by a treatment which did not act directly on the tumor cells. Nude and BALB/c mice bearing J558L tumors of a size of ∼1 cm in diameter were treated with a single injection of Cy (15 mg/kg). Whereas tumors in nude mice progressively grew without any retardation, tumors in BALB/c mice became severely necrotic after ∼3 d and finally were rejected within 10–20 d (Fig. 1). This experiment showed that Cy, at the applied dose, had no significant direct effect on the tumor cells and that tumor necrosis and subsequent rejection was T cell dependent. The involvement of T cells is further indicated by the observation that BALB/c mice which rejected the J558L tumor upon Cy-treatment rejected a second challenge of J558L but not unrelated tumor cells (data not shown).
Figure 1.

T cell involvement during Cy-mediated tumor rejection. (a) Tumors were established by subcutaneous injection of 106 J558L cells into BALB/c and nude mice. 11 d later, when tumor reached a size of ∼1 cm in diameter, BALB/c (▪, n = 10) and nude mice (•, n = 10) were treated intraperitoneally with 15 mg/kg Cy. BALB/c (□, n = 10) and nude mice (○, n = 10) which had not received Cy treatment served as controls. All Cy-treated BALB/c mice rejected the tumor, whereas all mice from the other groups did not. SD did not exceed 10% in all cases. Data are representative for three independent experiments. (b–d) An example of a tumor before (b), three (c), and 10 d after Cy-treatment (d). (e) Tumor 10 d after Cy treatment in mice depleted of CD8+ T cells (see Table I). The arrow indicates the ring-like structure of the growing tumor around the necrotic area.
To elucidate the contribution of T cells for tumor necrosis or rejection and the time point of their requirement, CD4+, CD8+, or both T cell subsets were depleted in tumor-bearing mice before Cy-injection. Cy administration induced tumor necrosis in CD4+, CD8+, and surprisingly also in CD4+/CD8+ T cell–depleted animals (Table I) . Similar as observed in nude mice, Cy did not induce tumor necrosis in BALB/c mice depleted of CD4+/CD8+ T cells before tumor inoculation (Table I), indicating that T cells were necessary during tumor establishment but not during Cy-treatment for tumor necrosis. However, complete tumor rejection was found only in CD4+ but not in CD8+ or CD4+/CD8+ T cell–depleted mice. After a short period of time tumors in CD8+ T cell–depleted mice started to grow again by forming a ring-like structure around the necrotic area (Fig. 1 e). Of note, some mice (2/10) rejected the tumor after CD4+ T cell depletion without Cy-treatment, indicating partially overlapping effects induced by Cy and CD4+ T cell depletion (see below).
Table I.
T Cell-dependent and -independent Phases during Cy-induced Tumor Necrosis/Rejection
| T cell depletion | Cy treatment | Tumor necrosisa | Rejection (No./Total)b | |
|---|---|---|---|---|
| Subset | Time | |||
| None | − | 0/5 | 0/5 | |
| + | 10/10 | 10/10 | ||
| αCD4 | day 9 | − | 2/10 | 2/10 |
| + | 5/5 | 5/5 | ||
| αCD8 | day 9 | − | 0/5 | 0/5 |
| + | 10/10 | 0/10 | ||
| αCD4, αCD8 | day 9 | − | 0/3 | 0/3 |
| + | 7/7 | 0/7 | ||
| αCD4, αCD8 | days −2 and 9 | − | 0/3 | 0/3 |
| + | 0/3 | 0/3 | ||
BALB/c mice were inoculated subcutaneously with 106 J558L cells. 11 d later, when tumors reached a size of ∼1 cm in diameter, mice were treated with 15 mg/kg Cy. 2 d prior to Cy-administration or tumor cell injection mice were depleted of CD4+ and/or CD8+ T cells. Untreated mice served as control.
Tumor necrosis was observed on days 3–5 after treatment.
Mice were observed for 60 d.
Cy-mediated Tumor Rejection Requires IFN-γR Expression by Host but not Tumor Cells.
IFN-γ has been associated in several studies with tumor rejection (15, 20–23). We asked whether it was necessary for Cy-mediated tumor rejection. To confirm the Cy-effect in a second tumor model and to distinguish whether a possible contribution of IFN-γ involved its activity on tumor or host cells, we used the fibrosarcoma Mc51.9, derived from a 129/Sv/Ev IFN-γR−/− mouse. Mc51.9 cells were injected into IFN-γR+/− and IFN-γR−/− mice. After 11 d when tumors were established, mice were treated with Cy. All IFN-γR+/− mice rejected the tumors, whereas they continued to grow without Cy-treatment (Fig. 2 a). In contrast, tumors in IFN-γR−/− mice were not rejected after treatment. Because Mc51.9 tumors grew slightly faster in IFN-γR−/− compared with IFN-γR+/− mice, IFN-γR+/− and IFN-γR−/− mice were also treated with Cy when Mc51.9 tumors had grown to a similar size (Fig. 2 b). Again, Cy-treatment of IFN-γR+/− mice resulted in complete tumor rejection, whereas tumors in IFN-γR−/− mice continued to grow with a short retardation. These data show that Cy-induced tumor rejection depends on IFN-γR expression, yet only by host and not tumor cells.
Figure 2.
Cy-mediated tumor rejection requires IFN-γR expression on host but not tumor cells. (a) IFN-γR+/− (▪, n = 10) and IFN-γR−/− 129/Sv/Ev mice (•, n = 10) were injected subcutaneously with 2 × 105 Mc51.9 cells (derived from an IFN-γR−/− mouse) and 11 d later treated intraperitoneally with 50 mg/kg Cy. IFN-γR+/− (□, n = 5) and IFN-γR−/− mice (○, n = 5) which did not receive Cy treatment served as controls. Data are representative for two independent experiments. (b) IFN-γR−/− mice (•, n = 5) were injected subcutaneously with 2 × 105 and IFN-γR+/− mice (▪, n = 5) with 106 Mc51.9 cells. 11 (for IFN-γR−/− mice) and 15 d (for IFN-γR+/− mice) later, respectively, when tumors reached a size of ∼1 cm in diameter, mice were treated intraperitoneally with 50 mg/kg Cy. Untreated tumor bearing IFN-γR+/− (□, n = 5) and IFN-γR−/− (○, n = 5) mice served as controls. All Cy-treated IFN-γR+/− mice rejected the tumor, whereas all mice from the other groups did not. SD did not exceed 10% in all cases.
Cy-Treatment Rapidly Inactivates Tumor-infiltrating T Cells. Because of the requirement of IFN-γR for Cy-mediated tumor rejection we analyzed the ability of tumor-infiltrating T cells (TILs) to produce IFN-γ after Cy-treatment. 11-d-old Mc51.9 tumors contained CD4+ and CD8+ T cells as determined by flow cytometry and immunohistochemistry (data not shown). Nonadherent infiltrating cells of tumors grown in 129/Sv/Ev mice were isolated before, 6, 24, and 72 h after Cy-treatment of the mice, stimulated in vitro with αCD3/αCD28 mAb and IFN-γ production was measured. Without stimulation the cells produced either very little or no IFN-γ (Fig. 3). αCD3/αCD28 mAb-stimulation of tumor-infiltrating cells isolated before Cy-treatment led to the production of large amounts of IFN-γ. Starting 6 h after Cy-treatment IFN-γ production was dramatically reduced and after 72 h the cells had completely lost the ability to produce IFN-γ. Spleen cells isolated from tumor-bearing mice at the different time points after Cy-treatment showed little changes in IFN-γ production upon αCD3/αCD28 mAb-stimulation (Fig. 3). Similar results were obtained after ConA stimulation (data not shown). Therefore, we conclude that TILs are functionally inhibited rapidly after Cy-treatment and are unlikely the source of IFN-γ necessary for Cy-mediated tumor rejection. This is compatible with the observation that T cells are not necessary early after Cy-treatment for tumor necrosis. Additionally, in the early phase of tumor rejection the most pronounced effect of Cy was locally on the TILs even though systemic effects cannot be excluded.
Figure 3.
Rapid functional inactivation of TILs but not spleen cells after Cy-treatment. 129/Sv/Ev (IFN-γR+/−) mice were inoculated subcutaneously with 2 × 105 Mc51.9 cells and 11 d later intraperitoneally injected with 50 mg/kg Cy. Before, 6 h, 24 h, and 72 h after Cy treatment tumors (3–5 mice per group) and spleens (three mice per group) were excised. Single cell suspensions of tumors were prepared and nonadherent cells were cultured at 106 cells per milliliter without or with stimulation by α-CD3/α-CD28 mAb's for 24 h. Spleen cells (106 cells per milliliter) were cultured without or with α-CD3/α-CD28 mAb stimulation for 48 h. IFN-γ in the culture supernatants was determined by ELISA. Data are representative for two independent experiments.
Cy-treatment Switches Cytokine Production of TIMs from IL-10 to IFN-γ.
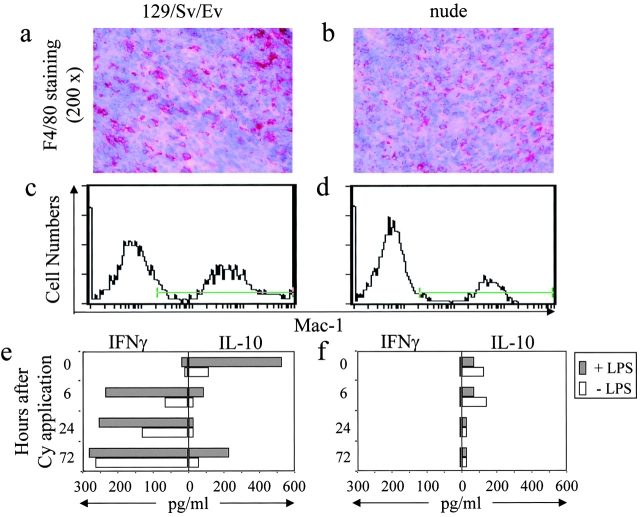
To search for an alternative source of IFN-γ after Cy-treatment we analyzed cytokine production of TIMs before and after Cy-treatment of Mc51.9 tumor-bearing mice. At the time of Cy-treatment (11 d after tumor cell injection) tumors were infiltrated by substantial numbers of macrophages as judged by immunohistochemical and flow cytometric analysis with F4/80 and α-Mac1 (CD11b) mAbs, respectively (Fig. 4 a and c), and by morphology and plastic adherence upon isolation. Macrophages isolated from tumors before Cy-treatment spontaneously produced IL-10 that increased by stimulation with LPS (Fig. 4 e). TIMs isolated 6 and 24 h after Cy-treatment did not produce IL-10 and little IL-10 was detected 72 h after Cy-treatment. Conversely, TIMs isolated before Cy-treatment did not produce IFN-γ but within 6 h after treatment started to produce IFN-γ that gradually increased after 24 and 72 h (Fig. 4 e). Similar results were obtained when macrophages were stimulated with LPS. These results show rapid and profound effects of Cy-application on TIMs.
Figure 4.
Cy treatment switches T cell–dependent cytokine production of TIMs from IL-10 to IFN-γ. (a–d) IFN-γR+/− 129/Sv/Ev (left) and nude mice (right) were inoculated subcutaneously with 2 × 105 Mc51.9 cells. 11 d later, tumors were obtained and macrophages were identified by immunohistology with α-F4/80 mAb (a and b) and flow cytometry with α–Mac-1 (CD11b) mAb (c and d). (e) IFN-γR+/− 129/Sv/Ev and (f) nude mice were inoculated with tumor cells as above and 11 d later injected intraperitoneally with 50 mg/kg Cy. Before, 6 h, 24 h, and 72 h after Cy treatment single cell suspension of tumors (3–5 mice per group) were prepared, Mac-1+ cells were isolated and cultured at 106 cells per milliliter without or with stimulation by 1 μg/ml LPS for 24 h. IFN-γ and IL-10 in the culture supernatants were determined by ELISA. One of two experiments with similar results is shown.
Cy-induced IFN-γ Production by TIMs Is T Cell Dependent.
Since the induction of tumor necrosis required the presence of T cells before Cy-treatment, we asked whether IFN-γ production by macrophages was T cell dependent. Macrophages infiltrated Mc51.9 tumors in nude mice (Fig. 4 b and d) to a similar extent as those in T cell–competent mice (Fig. 4 a and c) indicating that they infiltrated the tumor largely in a T cell–independent fashion. Macrophages isolated from tumors grown in nude mice produced very little or no IL-10 before Cy-application and LPS was unable to enhance the IL-10 production (Fig. 4 f). Remarkably, macrophages isolated from tumors in nude mice did not produce IFN-γ either before or at any time point after Cy-treatment (Fig. 4 f). Thus, Cy-induced IFN-γ production by macrophages required the presence of T cells.
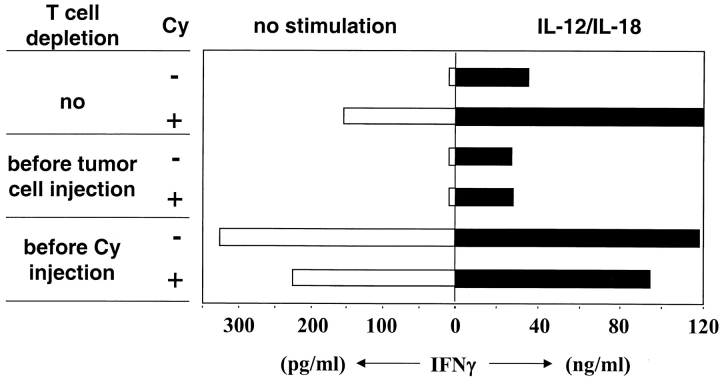
T Cells Prevent IFN-γ Production by TIMs in Growing Tumors. The correlation between functional inhibition of TILs and IFN-γ production of TIMs raised the question whether T cells, although necessary for IFN-γ production by TIMs upon Cy-treatment, were also responsible for the failure of TIMs to do so in growing tumors. TIMs were isolated from J558L tumors grown in BALB/c mice before or 24 h after Cy-treatment. Three groups of mice were analyzed: mice that were not depleted, depleted of CD4+/CD8+ T cells starting before tumor cell injection, or before Cy-treatment (Fig. 5). TIMs isolated from nonT cell–depleted mice did not produce IFN-γ without Cy-treatment, however in treated mice they produced IFN-γ similar as observed in the MC51.9 tumor model. TIMs isolated from tumors of mice depleted of T cells throughout the experiment did not produce IFN-γ regardless of Cy-treatment. This confirms that Cy-induced IFN-γ production by TIMs requires the presence of T cells during the period between tumor cell injection and Cy-treatment.
Figure 5.
T cells prevent IFN-γ production by TIMs in growing tumors. BALB/c mice were subcutaneously inoculated with 106 J558L cells and 11 d later intraperitoneally injected with 15 mg/kg Cy. Additionally, mice were depleted of CD4+/CD8+ T cells starting before tumor cell injection, before Cy-treatment, or as control not depleted of T cells. Before and 24 h after Cy-treatment single cell suspensions of tumors (3–5 mice per group) were prepared, Mac-1+ cells were isolated and cultured at 106 cells per milliliter without or with stimulation by IL-12 and IL-18 for 24 h. IFN-γ in the culture supernatant was determined.
Remarkably, T cell depletion in tumor-bearing mice induced IFN-γ production by TIMs even without Cy-treatment. In parallel, TIMs of all experimental groups were in vitro stimulated with IL-12 and IL-18, a strong stimulus for macrophages to produce IFN-γ (24). TIMs of all groups had the ability to produce large amounts of IFN-γ. The amount was proportional to that seen without in vitro stimulation (Fig. 5). Collectively, these data demonstrate that in the early phase TIMs require T cells to acquire the ability to produce IFN-γ, while in the later phase T cells inhibited IFN-γ production by TIMs.
Cy Induces IFN-γR–mediated Destruction of the Tumor Vasculature.
Finally, we searched for the target of IFN-γ during Cy-induced tumor rejection. Because we had previously shown that CD4+ T cell–mediated tumor immunity involved inhibition of angiogenesis which required IFN-γR expression on nonhematopoetic cells, most likely in the tumor stroma, and because of the rapid hemorrhagic necrosis after Cy treatment, we analyzed the tumor vasculature after Cy treatment. IFN-γR+/− and IFN-γR−/− mice bearing established Mc51.9 tumors were treated with Cy. Tumors were isolated before and after treatment and cryosections were stained with mAb α-Meca 32, which recognizes an epitope constitutively expressed on vascular endothelia (28). A dense net of blood vessels was visible in tumors of IFN-γR+/− and IFN-γR−/− mice before treatment (Fig. 6 a and f). Cy treatment of IFN-γR+/− mice resulted in a significant decrease in the number of blood vessels, as early as 6 h after treatment. After 24 h a further reduction of vessel density was observed and after 72 h blood vessels in the tumor were almost undetectable (Fig. 6 b–d). Importantly, blood vessel destruction preceded significant necrosis in the tumor tissue. Cy treatment of IFN-γR−/− mice did not induce any decrease in tumor blood vessel density compared with untreated tumors (Fig. 6 g–i). Instead, a continuous increase in blood vessel density could be observed in tumors of treated and control IFN-γR−/− mice. These results were confirmed by using a second mAb, α-CD31 (platelet/endothelial cell adhesion molecule-1), directed against endothelial intracellular junctions (Fig. 6 e and j). Thus, Cy rapidly induces the destruction of the tumor vasculature in an IFN-γR–dependent fashion.
Figure 6.

Cy induces IFN-γR–dependent destruction of the tumor vasculature. Tumors were established in IFN-γR+/− and IFN-γR−/− 129/Sv/Ev mice by subcutaneous injection of 2 × 105 Mc51.9 cells. 11 d later, mice were injected intraperitoneally with 50 mg/kg Cy and tumors were excised before, 6 h, 24 h, and 72 h after Cy treatment. Immunohistochemical analysis of tissue sections from IFN-γR+/− mice (a–e) and IFN-γR−/− mice (f–j) was performed with mAb Meca 32 (a-d, f–i) or with mAb anti-CD31 (e and j). Original magnification: 100×. A representative staining of tumors from 3–4 mice per group is shown.
Discussion
We analyzed the time-course of tumor stroma cell interactions during rejection of established tumors. It is known that a single injection of a certain amount of Cy can induce rejection of some established solid tumors (17, 18). Several findings already indicated that tumor rejection by Cy can be the result of host cell modulation rather than direct tumoricidal activity: (i) Cy-induced tumor rejection was T cell dependent (19); (ii) low dose was more effective than high dose treatment (29); (iii) the time interval between tumor inoculation and Cy administration rather than the tumor size was critical for tumor rejection (30); and (iv) Cy-mediated tumor rejection was inhibited, if mice had been treated earlier with Cy (29). An indirect mode of action was confirmed here, since Cy, at the given doses, had no obvious effect on tumors growing in nude mice or normal mice which were depleted of T cells from the time of tumor cell inoculation. Based on the results presented here we distinguish three phases during Cy-induced tumor rejection: (i) the time between tumor cell inoculation and Cy-application; (ii) the early phase after Cy-treatment defined by hemorrhagic necrosis and; (iii) the late phase defined by elimination of residual tumor cells which survived hemorrhagic necrosis at the rim of the tumor.
The first phase (tumor establishment) is characterized by the accumulation of different host-derived stroma cells, e.g., inflammatory cells such as T cells and macrophages or cells involved in the establishment of the tumor vasculature. The inflammatory response to the tumor is slow in comparison to that observed in previously immunized mice (23) and, in contrast to preimmunized mice, the tumor continues to grow. Critical for a tumor permissive environment appeared to be the interaction between macrophages and T cells. Macrophages infiltrated the tumor to a large extent in a T cell–independent manner, yet their IL-10 production before and IFN-γ production after Cy-treatment required the presence of T cells. Therefore, it is likely that macrophages interacted with T cells within the tumor. The role of T cells during the tumor establishment phase is complex. On the one hand macrophages need T cells to obtain the ability to produce IFN-γ, on the other hand T cells prevent macrophages to not produce IFN-γ. It has to be shown whether the T cells themselves are regulated by the tumor similar as demonstrated in other tumor models which showed activation and subsequent conversion of T cells by the growing tumor (7, 8).
A suppressive nature of T cells in the established tumor is indirectly indicated by T cell–dependent IL-10 production of TIMs. It has been shown that IL-10 acts immunsuppressive, e.g., downmodulates T cell function (31), inhibits the accumulation of dendritic cells in the tumor (32) or downregulates MHC class I expression on tumor cells (33, 34). However, direct evidence for the suppressive activity of T cells and the mode of suppression was demonstrated by their inactivation and the immediate changes within the tumor environment. Similar as shown before (9, 10), CD4+ T cell depletion induced rejection of J558L tumors in some of the mice (2/10). Furthermore, Cy-treatment rapidly inactivated TILs, as shown by their inability to produce IFN-γ. Thus, TILs are unlikely the source of IFN-γ necessary for Cy-mediated tumor necrosis. This is consistent with the observation that CD4+/CD8+ T cell depletion at the time of Cy-treatment still led to tumor necrosis. However, we cannot exclude that IFN-γ produced by T cells contributes to tumor vasculature destruction. The fact that the TILs could be induced in vitro to produce IFN-γ when isolated before Cy-treatment does not mean that they in fact produced IFN-γ in situ. Rather, their inability to produce IFN-γ after Cy-treatment could reflect the inability to produce other unknown factors that regulate macrophage function. With the same kinetics as TILs were inactivated by Cy, TIMs stopped to produce IL-10 and started to produce IFN-γ. Sica et al. recently showed that macrophages from mouse and human tumors produced IL-10 that inhibited IL-12 production in an autocrine manner (35). Because macrophages can be induced to produce IFN-γ by IL-12 and IL-18 (24), it is possible that these cytokines produced by macrophages themselves or other cells within the tumor stroma contributed to Cy-mediated tumor rejection. Recently, it has been shown that IL-12 can support Cy-mediated tumor rejection (36). In any case, IFN-γ most likely produced by macrophages was of pivotal importance, since Cy did not induce tumor rejection in IFN-γR−/− mice. Cy caused a short delay of tumor growth in IFN-γR−/− mice that we cannot explain at the present time but could be due to other factors induced by Cy. To exclude that Cy directly induced the functional switch of TIMs, we showed that T cell depletion led to IFN-γ production by TIMs similar as Cy-treatment. This shows that the TIMs were directly suppressed by T cells and that Cy-treatment and T cell depletion induced at least partially overlapping effects. Since (CD4+) T cell depletion did not or only rarely induce tumor rejection compared with Cy-treatment, yet both treatments let to IFN-γ production by TIMs, IFN-γ may be a necessary but not sufficient factor. Collectively, the results suggest that one factor determining a permissive tumor environment is ‘holding in check’ the TIMs by T cells whose suppressive/regulatory phenotype may be caused by their too late arrival in the tumor.
The tumor cells did not need to express IFN-γR for Cy-induced tumor rejection. Which host cells have to express the IFN-γR is not entirely clear. Previously we showed that tumor immunity mediated by CD4+ T cells against the MHC class II− Mc51.9 tumor involves inhibition of angiogenesis which required IFN-γR expression by nonhematopoietic cells, most likely within the tumor stroma (23). Therefore, we assume that IFN-γ has a similar cellular target during Cy-induced tumor rejection. The most striking effect of Cy was the destruction of the tumor vasculature in IFN-γR+/− but not IFN-γR−/− mice which preceded tumor necrosis and occurred with similar kinetics as TILs were inactivated and TIMs started to produce IFN-γ. A possible target of IFN-γ are endothelial cells themselves, supported by the demonstration that IFN-γ (together with TNF) impairs survival of endothelial cells in vitro (37). Alternatively, other cells of the tumor stroma such as fibroblasts could be the target of IFN-γ and be involved in blood vessel destruction by release of other factors (38, 39). It should be noted that TILs from IFN-γR−/− mice could be induced to produce IFN-γ before and lost the ability after Cy-treatment similar to those isolated from IFN-γR+/− mice. Also, TIMs from IFN-γR−/− mice switched from IL-10 to IFN-γ production during Cy-treatment (data not shown). Compatible with a xenograft model of arteriosclerosis (40), IFN-γ seems to have an effector rather than regulatory role during Cy-induced tumor rejection. Different from our results it was recently suggested that Cy directly affected tumor angiogenesis (41). In this study Cy was repeatedly applied in high amounts (170 mg/kg). We can exclude direct effect of Cy in our model, since the tumor vasculature appeared to be unaffected in IFN-γR−/− mice.
After destruction of the tumor vasculature the tumor becomes centrally necrotic. In the J558L tumor model few cells at the periphery of the tumor appear to survive and they are eventually eliminated by CD8+ T cells. We did not analyze T cell subset requirement in the Mc51.9 tumor model where CD4+ T cells are essential effector cells in immunization/challenge experiments (23). The CD8+ T cells necessary for elimination of residual J558L cells could either be liberated from a suppressive activity of CD4+ T cells abolished by Cy (9) or, alternatively, induced as a result of high amounts of antigens available for APCs (e.g., the activated macrophages in the tumor) during tumor necrosis. Further Cy-treatment during this period might impair residual tumor cell elimination by T cells which upon activation can be assumed to proliferate, and thus should be more susceptible to Cy-toxicity.
Several approaches have been developed to induce inhibition of tumor angiogenesis (3, 42) or tumor infarction (43). Cy is particularly interesting because for no currently used antiangiogenic compound more clinical data exist. Whether some of the therapeutic or toxic effects in the clinic can be explained by Cy-induced vascular damage and whether they are immune-mediated is currently not known. The rapid kinetics of Cy-induced alterations within the tumor stroma, however, would be difficult to reconcile with the supposed effect of Cy to act on rapidly dividing cells. In summary, the importance of stroma for protection of solid tumors, e.g., as barrier for infiltrating immune cells has been recognized (44, 45). We have shown here that the rejection of some transplanted tumors can be induced by altering the balance between individual components of the tumor stroma.
Acknowledgments
We thank M. Rösch and C. Westen for excellent technical assistance and A. Pezzutto for critically reading this manuscript. We thank M. Aguet for IFN-γR−/− mice.
This work was supported by grants from the Bundesministerium für Bildung und Forschung (01KV9911), the Deutsche Krebshilfe, Mildred-Scheel-Stiftung E.V. (10-1535-B1 2), and the Deutsche Forschungsgemeinschaft (SFB 506).
S. Ibe and Z. Qin contributed equally to this paper.
Footnotes
Abbreviations used in this paper: Cy, cyclophosphamide; IFN-γR, IFN-γ receptor; TIL, tumor-infiltrating T cell; TIM, tumor-infiltrating macrophage.
References
- 1.Dvorak, H.F. 1986. Similarities between tumor stroma generation and wound healing. New Engl. J. Med. 315:1650–1659. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber, H., and D.A. Rowley. 1999. Inflammation and Cancer. In Inflammation: Basic Principles and Clinical Correlates. 3rd ed. Lippincott Williams & Wilkins, Philadelphia. pp 1117–1129.
- 3.O'Reilly, M.S., L. Holmgren, Y. Shing, C. Chen, R.A. Rosenthal, M. Moses, W.S. Lane, Y. Cao, E.H. Sage, and J. Folkman. 1994. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 79:315–328. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani, A., B. Bottazzi, F. Colotta, S. Sozzani, and L. Ruco. 1992. The origin and function of tumor associated macrophages. Immunol. Today. 13:265–270. [DOI] [PubMed] [Google Scholar]
- 5.Blankenstein, T., S. Cayeux, and Z. Qin. 1996. Genetic approaches to cancer immunotherapy. Rev. Physiol. Biochem. Pharmacol. 129:1–49. [DOI] [PubMed] [Google Scholar]
- 6.North, R.J. 1984. The murine antitumor immune response and its therapeutic manipulation. Adv. Immunol. 35:89–155. [DOI] [PubMed] [Google Scholar]
- 7.Staveley-O'Carroll, K., E. Sotomayor, J. Montgomery, I. Borrello, L. Hwang, S. Fein, D. Pardoll, and H. Levitsky. 1998. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc. Natl. Acad. Sci. USA. 95:1178–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.North, R.J., and I. Bursuker. 1984. Generation and decay of the immune response to a progressive fibrosarcoma. Lyt-1+2− suppressor T cells down-regulate of Lyt-1−2+ effector T cells. J. Exp. Med. 59:1295–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.North, R.J. 1982. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J. Exp. Med. 155:1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koeppen, H.K., S. Singh, H.J. Stauss, B.H. Park, D.A. Rowley, and H. Schreiber. 1993. CD4-positive and B lymphocytes in transplantation immunity. I. Promotion of tumor allograft rejection through elimination of CD4-positive lymphocytes. Transplantation. 55:1349–1355. [DOI] [PubMed] [Google Scholar]
- 11.Bottazzi, B., N. Polentarutti, R. Acero, A. Balsari, D. Boraschi, P. Ghezzi, M. Salmona, and A. Mantovani. 1983. Regulation of the macrophage content of neoplasms by chemoattractans. Science. 220:210–212. [DOI] [PubMed] [Google Scholar]
- 12.Polverini, P., and S.J. Leibovich. 1984. Induction of neovascularization in vivo and endothelial proliferation in vitro by tumor-associated macrophages. Lab. Invest. 51:635–642. [PubMed] [Google Scholar]
- 13.Richter, G., S. Kruger-Krasagakes, G. Hein, C. Huls, E. Schmitt, T. Diamantstein, and T. Blankenstein. 1993. Interleukin 10 gene transfected into Chinese hamster ovary cells prevents tumor growth and macrophage infiltration. Cancer Res. 53:4134–4137. [PubMed] [Google Scholar]
- 14.Blankenstein, T., Z. Qin, K. Überla, W. Müller, H. Rosen, H.D. Volk, and T. Diamantstein. 1991. Tumor suppression after tumor cell-targeted tumor necrosis factor α gene transfer. J. Exp. Med. 173:1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hock, H., M. Dorsch, U. Kunzendorf, Z. Qin, T. Diamantstein, and T. Blankenstein. 1993. Mechanisms of rejection induced by tumor cell-targeted gene transfer of interleukin-2, interleukin-4, interleukin-7, tumor-necrosis factor, or interferon-γ. Proc. Natl. Acad. Sci. USA. 90:2774–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awwad, M., and R.J. North. 1988. Cyclophosphamide (Cy)-facilitated adoptive immunotherapy of a Cy-resistant tumor. Evidence that Cy permits the expression of adoptive T-cell mediated immunity by removing suppressor T cells rather than by reducing tumor burden. Immunology. 65:87–92. [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrke, M.J., E. Mihich, and D. Berd. 1989. Effects of anticancer drugs on the immune system in man. Semin. Oncol. 16:230–253. [PubMed] [Google Scholar]
- 18.Bass, K.K., and M.J. Mastrangelo. 1998. Immunopotentiation with low-dose cyclophosphamide in the active specific immunotherapy of cancer. Cancer Immunol. Immunother. 47:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hengst, J.C., M.B. Mokyr, and S. Dray. 1981. Cooperation between cyclophosphamide tumoricidal activity and host antitumor immunity in the cure of mice bearing large MOPC-315 tumors. Cancer Res. 41:2163–2167. [PubMed] [Google Scholar]
- 20.Dighe, A.S., E. Richards, L.J. Old, and R.D. Schreiber. 1994. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN γ receptors. Immunity. 1:447–456. [DOI] [PubMed] [Google Scholar]
- 21.Hung, K., R. Hayashi, A. Lafond-Walker, C. Lowenstein, D. Pardoll, and H. Levitsky. 1998. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 188:2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mumberg, D., P.A. Monach, S. Wanderling, M. Philip, A.Y. Toledano, R.D. Schreiber, and H. Schreiber. 1999. CD4+ T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-γ. Proc. Natl. Acad. Sci. USA. 96:8633–8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin, Z., and T. Blankenstein. 2000. CD4+ T cell-mediated tumor rejection involves inhibition of angiogenesis which is dependent on IFNγ receptor expression by non-hematopoietic cells. Immunity. 12:677–686. [DOI] [PubMed] [Google Scholar]
- 24.Munder, M., M. Mallo, K. Eichmann, and M. Modolell. 1998. Murine macrophages secret interferon γ upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J. Exp. Med. 187:2103–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valente, G., L. Ozmen, F. Novelli, M. Geuna, G. Palestro, G. Forni, and G. Garotta. 1992. Distribution of interferon-γ receptor in human tissues. Eur. J. Immunol. 22:2403–2412. [DOI] [PubMed] [Google Scholar]
- 26.Farrar, M.A., and R.D. Schreiber. 1993. The molecular cell biology of interferon-γ and its receptor. Annu. Rev. Immunol. 11:571–611. [DOI] [PubMed] [Google Scholar]
- 27.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R.M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-γ receptor. Science. 259:1742–1745. [DOI] [PubMed] [Google Scholar]
- 28.Leppink, D.M., D.K. Bishop, D.D. Sedmak, M.L. Henry, R.M. Ferguson, P.R. Streeter, E.C. Butcher, and C.G. Orosz. 1989. Inducible expression of an endothelial cell antigen on murine myocardial vasculature in association with interstitial cellular infiltration. Transplantation. 48:874–877. [DOI] [PubMed] [Google Scholar]
- 29.Mokyr, M.B., and S. Dray. 1983. Some advantages of curing mice bearing a large subcutaneous MOPC-315 tumor with a low dose rather than a high dose of cyclophosphamide. Cancer Res. 43:3112–3119. [PubMed] [Google Scholar]
- 30.Hengst, J.C., M.B. Mokyr, and S. Dray. 1980. Importance of timing in cyclophosphamide therapy of MOPC-315 tumor-bearing mice. Cancer Res. 40:2135–2141. [PubMed] [Google Scholar]
- 31.de Waal Malefyt, R., J. Haanen, H. Spits, M.G. Roncarolo, A. te Velde, C. Figdor, K. Johnson, R. Kastelein, H. Yssel, and J.E. de Vries. 1991. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 174:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin, Z., G. Noffz, M. Mohaupt, and T. Blankenstein. 1997. Interleukin 10 prevents dendritic cell accumulation and vaccination with granulocyte-macrophage-colony-stimulating-factor gene modified tumor cells. J. Immunol. 159:770–776. [PubMed] [Google Scholar]
- 33.Salazar-Onfray, F., J. Charo, M. Petersson, S. Freland, G. Noffz, Z. Qin, T. Blankenstein, H.G. Ljunggren, and R. Kiessling. 1997. Down-regulation of the expression and function of the transporter associated with antigen processing in murine tumor cell lines expressing interleukin 10. J. Immunol. 159:3195–3202. [PubMed] [Google Scholar]
- 34.Petersson, M., J. Charo, F. Salazar-Onfray, G. Noffz, M. Mohaupt, Z. Qin, G. Klein, T. Blankenstein, and R. Kiessling. 1998. Constitutive IL-10 production accounts for the high NK sensitivity, low MHC class I expression and poor transporter associated with antigen processing (TAP)-1/2 function in the prototype NK target YAC-1. J. Immunol. 161:2099–2105. [PubMed] [Google Scholar]
- 35.Sica, A., A. Saccani, B. Bottazzi, N. Polentarutti, A. Vecchi, J. van Damme, and A. Mantovani. 2000. Autocrine production of IL-10 mediates defective IL-12 poduction and NF-kB activation in tumor-associated macrophages. J. Immunol. 164:762–767. [DOI] [PubMed] [Google Scholar]
- 36.Tsung, K., J.B. Meko, Y.L. Tsung, G.R. Peplinski, and J.A. Norton. 1998. Immune response against large tumors eradicated by treatment with cyclophosphamide and IL-12. J. Immunol. 160:1369–1377. [PubMed] [Google Scholar]
- 37.Ruegg, C., A. Yilmaz, G. Bieler, J. Bamat, P. Chaubert, and F.J. Lejeune. 1998. Evidence for the involvement of endothelial cell integrin αVβ3 in the disruption of the tumor vasculature induced by TNF and IFN-γ. Nat. Med. 4:408–414. [DOI] [PubMed] [Google Scholar]
- 38.Sgadari, C., A.L. Angiolillo, B.W. Cherney, S.E. Pike, J.M. Farber, L.G. Koniaris, P. Vanguri, P.R. Burd, N. Sheikh, G. Gupta, et al. 1996. Interferon-inducible protein-10 identified as a mediator of tumor necrosis in vivo. Proc. Natl. Acad. Sci. USA. 93:13791–13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sgadari, C., J.M. Farber, A.L. Angiolillo, F. Liao, J. Teruya-Feldstein, P.R. Burd, L. Yao, G. Gupta, C. Kanegane, and G. Tosato, G. 1997. Mig, the monokine induced by interferon-γ, promotes tumor necrosis in vivo. Blood. 89:2635–2643. [PubMed] [Google Scholar]
- 40.Tellides, G., D.A. Tereb, N.C. Kirkiles-Smith, R.W. Kim, J.H. Wilson, J.S. Schechner, M.I. Lorber, and J.S. Pober. 2000. Interferon-γ elicits arteriosclerosis in the absence of leukocytes. Nature. 403:207–211. [DOI] [PubMed] [Google Scholar]
- 41.Browder, T., C.E. Butterfield, B.M. Kraling, B. Shi, B. Marshall, M.S. O'Reilly, and J. Folkman. 2000. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 60:1878–1886. [PubMed] [Google Scholar]
- 42.Bergers, G., K. Javaherian, K.M. Lo, J. Folkman, and D. Hanahan. 1999. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 284:808–812. [DOI] [PubMed] [Google Scholar]
- 43.Huang, X., et al. 1997. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science. 275:547–550. [DOI] [PubMed] [Google Scholar]
- 44.Singh, S., S.R. Ross, M. Acena, D.A. Rowley, and H. Schreiber. 1992. Stroma is critical for preventing or permitting immunological destruction of antigenic cancer cells. J. Exp. Med. 175:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willimsky, G., and T. Blankenstein. 2000. Interleukin-7/B7.1-encoding adenoviruses induce rejection of transplanted but not nontransplanted tumors. Cancer Res. 60:685–692. [PubMed] [Google Scholar]