Abstract
We studied complement 1 inhibitor (C1-INH) as an inhibitor of the alternative complement pathway. C1-INH prevented lysis, induced by the alternative complement pathway, of paroxysmal nocturnal hemoglobinuria (PNH) erythrocytes in human serum. It inhibited the binding of both factors B and C3 to PNH and rabbit erythrocytes and blocked the ability of factor B to restore alternative-pathway function in factor B–depleted serum. C1-INH did not bind to factors B or D but did bind to immobilized C3b and cobra venom factor (CVF), a C3b analogue. C1-INH prevented factor B from binding to CVF-coated beads and dissociated bound factor B from such beads. Factor B and C1-INH showed cross competition in binding to CVF-coated beads. Factor D cleaved factor B into Bb and Ba in the presence of C3b. Cleavage was markedly inhibited when C3b was preincubated with C1-INH. C1-INH inhibited the formation of CVFBb and decreased the C3 cleavage. Removal of C1-INH from serum, in the presence of Mg-EGTA with an anti–C1-INH immunoabsorbant, markedly increased alternative-pathway lysis. C1-INH interacts with C3b to inhibit binding of factor B to C3b. At physiologic concentrations, it is a downregulator of the alternative pathway convertase.
Keywords: complement, serpins, hematological diseases, regulation, immunity
Introduction
Complement 1 inhibitor (C1-INH)* is a critically important protein that controls activation of multiple plasma mediator pathways (1). This protein, a member of the serine protease inhibitor (serpin) group, originally was described as an inhibitor of C1 (2). It binds stoichiometrically to the active sites on both C1r and C1s to form a complex C1-INH-C1r-C1s-C1-INH and thus inhibits activated C1 (3). In addition, C1-INH has been reported to remove the intact C1qrs complex from an activating surface (4) and to inhibit autoactivation of C1 (5). C1-INH is a known inhibitor of kinin generating (kallikrein), fibrinolytic (plasmin), and contact activation (intrinsic) pathway of the coagulation cascade (factor XIIa, XIIf, and factor XIa) (1, 6–9). Recently, it has been shown to be an inhibitor of the mannan-binding lectin pathway of complement activation, inhibiting mannan-binding lectin-associated serine proteases (MASPs) in that pathway (10).
Because the alternative complement pathway has many features in common with the classical complement pathway, and because many proteins of that pathway function in a manner analogous to proteins of the classical pathway, we studied the role of the C1-INH in inhibition of the alternative complement pathway. In the alternative complement pathway, C3b serves a function analogous to that of C4b; factor B serves a function analogous to C2 and is a very similar protein; and factor D serves a function analogous to that of C1 of the classical pathway. We began these experiments with the hypothesis that C1-INH regulates factor D activity.
Materials and Methods
Buffers and Reagents.
We prepared Veronal-buffered saline (VBS), pH 7.4; VBS containing 0.1% (wt/vol) gelatin (GVBS); GVBS containing 0.15 mM CaCl2 and 1 mM MgCl2 (GVBS++); GVBS containing 5 mM MgCl2 and 8 mM EGTA (Mg-EGTA buffer); and GVBS containing 10 mM EDTA (EDTA buffer) as described (11). All chemicals were purchased from Sigma-Aldrich, and 125I was obtained from Amersham Pharmacia Biotech. The Mg-EGTA buffer was at pH 7.4 unless otherwise indicated, when the pH was reduced with HCl to pH 6.5.
Complement Components and Antibodies.
Human factor B, factor D, C3, cobra venom factor (CVF), and C1-INH and factor B-depleted, C3-depleted, and C8-depleted human sera were purchased from Advanced Research Technologies, Inc. The IgG fractions of polyclonal anti-factor B, antifactor D, antifactor H, anti–C1-INH, anti–human albumin, and anti-C3 were obtained from Binding Site, Inc.
Preparation of Cells and Sera.
Peripheral blood from normal human donors, patients with paroxysmal nocturnal hemoglobinuria (PNH), and rabbits was collected into tubes containing EDTA. The erythrocytes (normal donors, Ehu; PNH donors, Epnh; and rabbits, Erb) were washed with EDTA buffer and then stored at 4°C in GVBS++. To obtain serum, blood was allowed to clot at room temperature for 30 min, chilled, and then centrifuged. The patients with PNH all were followed in the hematology-oncology clinic at Duke University Medical Center. In each case, the diagnosis was confirmed by flow cytometry. Analysis of blood cells showed the absence of the phosphatidylinositol-linked membrane protein CD59. For most of the studies, the erythrocytes were from a patient who had >90% type III PNH erythrocytes, which lyse extensively in acidified serum (12). Fresh serum was obtained from the same donor, who was Coombs-negative, to exclude the presence of autoantibodies. For studies of protein binding or lysis of rabbit erythrocytes, human serum was absorbed extensively with washed rabbit red cells at 0°C before use.
Purification of C1-INH.
The C1-INH was further purified from C1-INH prepared by Immuno-AG (Baxter) using a modification of the method of Pilatte et al. (13). Briefly, commercial C1-INH in 175 mM, pH 7.4 Tris buffer was applied to a 50-ml jacalin-agarose column and eluted with the same buffer containing 125 mM Melibiose. The pooled and concentrated C1-INH fraction was dialyzed and then mixed with Sepharose beads coupled with anti-factor B to remove trace amounts of factor B. The absorbed C1-INH was applied to a fast protein liquid chromatography (FPLC) Superose 12-gel filtration column in VBS to remove trace amounts of IgA and anti-factor B IgG. It was further purified by a FPLC Mono Q column in 20 mM Tris, pH 7.3, and eluted with the same buffer containing 1 M NaCl. The final C1-INH preparation contained a major band at 105 kD with a trace band of degraded C1-INH at 96 kD. It was free of factors H, D, and B by Western blot analysis.
Hemolytic Assays.
The erythrocytes (107 Epnh or Erb) were incubated with 1:5 diluted human serum in Mg-EGTA-GVBS in a final volume of 200 μl at 37°C for 30 min. To stop the reaction, 1 ml of EDTA buffer was added. After centrifugation, the supernatant optical density was measured at 412 nm. Before adding the cells, C1-INH or control protein was added to diluted serum to study the inhibitory activity of C1-INH.
Factor B Hemolytic Activity.
Rabbit red cells (1.5 × 108, 100 μl in Mg-EGTA buffer, pH 6.5) were incubated with 100 μl of a 1:10 dilution of factor B-depleted human serum in the same buffer, with 5 μl of factor B (0.25 μg) added. To this mixture, 10 μl of buffer containing human serum albumin (HSA) or C1-INH was added. The mixture was then incubated at 37°C for 30 min. To stop the reaction, 1 ml of EDTA buffer was added. After centrifugation, the optical density of the supernatant was measured at 412 nm.
Isotope Labeling.
Factor B (1.4 μCi/μg), factor D (0.04 μCi/μg), C3 (1.43 μCi/μg), C1-INH (0.32 μCi/μg), and anti-B (0.113 μCi/μg), anti-D (0.06 μCi/μg), and anti-C3 (0.19 μCi/μg) were radiolabeled with 125I using Iodobeads according to the manufacturer's instructions (Pierce Chemical Co.). Free iodine was removed by small-scale gel filtration on a Bio-Spin column (Bio-Rad Laboratories). The labeled factor B and C3 were functional in a hemolytic assay.
Dot-Blot Analysis.
C3b, Factor D, Factor B, or BSA, all at 50 μg/ml in 200 μl of PBS was dotted onto nitrocellulose paper using Bio-Dot apparatus (Bio-Rad Laboratories). The paper with dotted proteins was blocked with 3% nonfat drymilk for 1 h at room temperature, washed, and incubated with 125I–C1-INH (2.5 μg/ml) for 1 h at room temperature. Radioactivity was determined after washing four times in PBS-Tween.
Coupling of Proteins to Cyanogen Bromide–activated Sepharose 4B.
The CVF, anti-C1-INH, and anti-HSA were coupled to CNBr-activated Sepharose 4B beads according to the manufacturer's instructions (Sigma-Aldrich). Briefly, CNBr-activated Sepharose 4B powder was allowed to swell in cold 1 mM HCl (200 ml/g dry powder). The beads were washed five times with 0.1 M NaHCO3, 0.5 M NaCl, pH 8.4 (coupling buffer), and immediately the various proteins in coupling buffer were added. Beads with added proteins at 1 mg/ml/gram dry power were incubated overnight at 4°C, using an end-over-end mixer. The beads were spun down, and unbound protein was washed away with coupling buffer. Unbound sites were blocked with 0.2 M glycine, pH 8.0 overnight at 4°C, and the beads were then washed multiple times to remove the blocking solution. The first wash solution contained coupling buffer; the second acetate buffer (0.1 M, pH 4.0, 0.5 M NaCl). This wash cycle was repeated three times. After washing in VBS the beads were stored at 4°C with 0.05% azide. The protein concentration in the coupling buffer was measured at 280 nm before and after coupling; the coupling efficiency was higher than 90%.
Effect of C1-INH on Binding of Serum Factor B and C3 to Erythrocytes.
The erythrocytes (5 × 107 Ehu, Epnh, or Erb) were incubated with C8-depleted serum (1:10 diluted in Mg-EGTA buffer) at pH 6.5 or 7.4 at 37°C for 30 min. After washing twice with cold Mg-EGTA buffer, 125I-labeled antibodies were added as indicated. The mixture was incubated at 4°C for 1 h. After washing twice with Mg-EGTA buffer, the radioactivity of the cell pellet was determined. Bovine serum albumin or human serum albumin was used as the control protein.
Effect of C1-INH on Binding of Factor B to CVF Sepharose Beads.
Sepharose beads with bound CVF, 50 μl containing ∼50% beads in pH 6.5 Mg-EGTA buffer, were incubated with 125I-labeled factor B (5–25 ng), with or without C1-INH or control protein (300 μg/ml) added at 37°C for 15 min. After washing twice with 2 ml of cold Mg-EGTA buffer, the bound factor B was measured in a gamma scintillation counter. In factor B dissociation studies, CVF beads with bound factor B were further incubated with C1-INH or control protein (300 μg/ml) at 37°C for 15 min. We added 1 ml of cold Mg-EGTA buffer and, after centrifugation, measured the pellet and supernatant radioactivity.
Factor B Cleavage.
Factor B was cleaved by incubating 250 ng (5 μl) 125I-labeled factor B, 5–100 ng of C3b (1 μl) and 0.1–100 ng of factor D (1 μl) at 37°C for 60 min. The reaction was stopped by adding 10 μl of twice-concentrated SDS-PAGE sample buffer. In some experiments, a limiting amount of C3b (10 ng) or factor D (0.5 ng) was preincubated with C1-INH (2 μg) or control HSA (2 μg) at 37°C for 10 min before adding other components. We loaded 10 μl into wells of a 7.5% SDS-PAGE in a mini electrophoresis system (Bio-Rad Laboratories).
C3 Cleavage.
CVF Sepharose beads 20 μl in GVBS were incubated with factor B (1 μg) and D (0.1 μg) at 37°C for 10 min to generate Sepharose CVFBb. After washing in GVBS++ to remove free B and D, the solid phase CVFBb beads were incubated with 125I-labeled human C3 (250 ng) at 37°C for 60 min. To stop the reaction, 20 μl of twice-concentrated SDS-PAGE sample buffer was then added. In the C1-INH inhibition experiments, CVF beads were preincubated with C1-INH (2 μg) or control HSA (2 μg) at 37°C for 10 min before adding factor B and D. After washing, 125I-C3 was added and the procedure above was followed.
Electrophoresis and Autoradiography.
PAGE was performed according to the procedure of Laemmli (14) using 7.5% ready gel (Bio-Rad Laboratories). The 125I-labeled protein was loaded into wells (10 μl/well). After electrophoresis, the gel was dried, and Eastman Kodak XRP X-ray film was exposed to the dried slab gel wrapped in cellophane for 24 h at −20°C before developing.
Data Presentation.
Data represent the mean ± SEM of single experiments and is representative of at least three experiments all of which gave similar results. Means were compared using a two-tailed t test and in each case the difference was significant at the 0.01 level or below.
Results
C1-INH Inhibits PNH Cell Lysis by the Alternative Complement Pathway.
The PNH erythrocytes were incubated with acidified (pH 6.5) normal human serum in Mg-EGTA buffer (Fig. 1) or the patient's own acidified serum (data not shown) with purified C1-INH or control protein added. Highly purified C1-INH, but not control protein, prevented lysis of PNH erythrocytes in Mg-EGTA buffer in a dose-dependent manner. In experiments not shown, C1-INH similarly inhibited lysis of rabbit erythrocytes in Mg-EGTA buffer by the alternative pathway.
Figure 1.
C1-INH inhibits PNH cell lysis in acidified serum. Epnh in pH 6.5 Mg-EGTA buffer (50 μl) was incubated with 100 μl diluted (1:5) acidified normal human serum. To this mixture, 50 μl of HSA (○) or C1-INH (•) was added. The mixture was then incubated at 37°C for 30 min. To stop the reaction, 1 ml of EDTA buffer was added. After centrifugation, the optical density of the supernatant was measured at 412 nm and percent lysis was calculated.
C1-INH Inhibits Factor B and C3 Binding to Rabbit and PNH Erythrocytes.
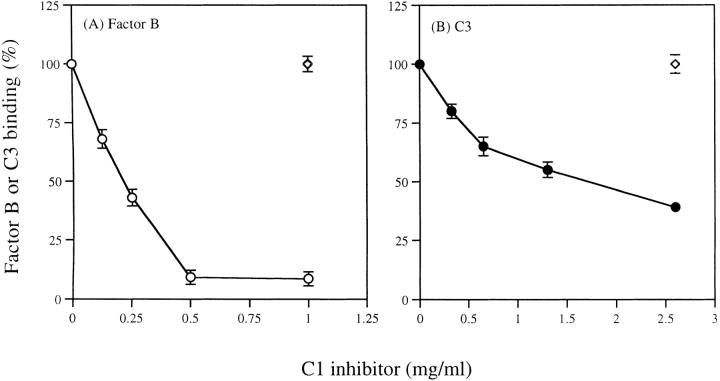
The Erb or Epnh were incubated with diluted (1:10) C8-depleted serum brought to pH 6.5 in Mg-EGTA buffer with or without added purified C1-INH or control protein (BSA). After incubation and washing, labeled anti-factor B or anti-C3 was added to detect factor B or C3 binding. The C1-INH inhibited factor B and C3 binding to rabbit erythrocytes in a dose-dependent manner (Fig. 2). Control protein (BSA) had no effect. Similarly, C1-INH inhibited factor B and C3 binding to PNH erythrocytes (data not shown).
Figure 2.
C1-INH inhibits factor B and C3 binding to rabbit erythrocytes. The Erb was incubated with C8-depleted serum (1:10 diluted in Mg-EGTA buffer) at pH 6.5 with added C1-INH or BSA (⋄) for 30 min at 37°C. After washing, 125I-labeled antibodies were added to detect factor B (A: ○) or C3 (B: •) binding.
C1-INH Does Not Interact with Factor B or Factor D, but Does Interact with C3b.
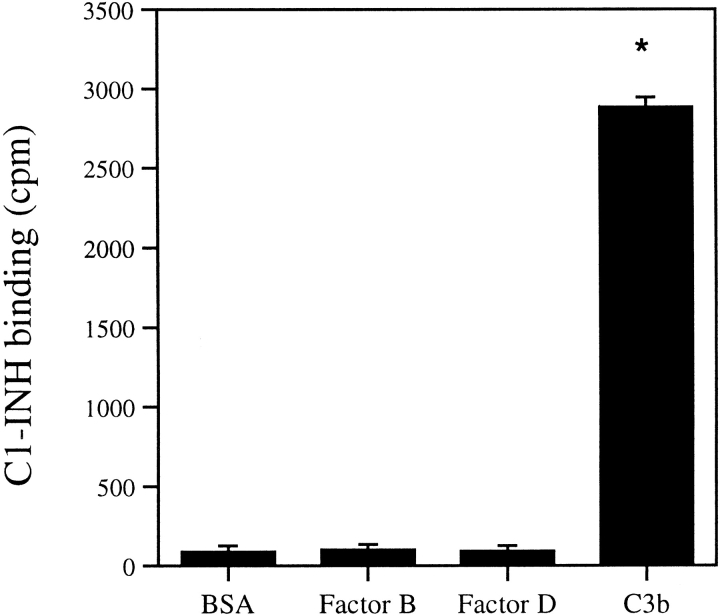
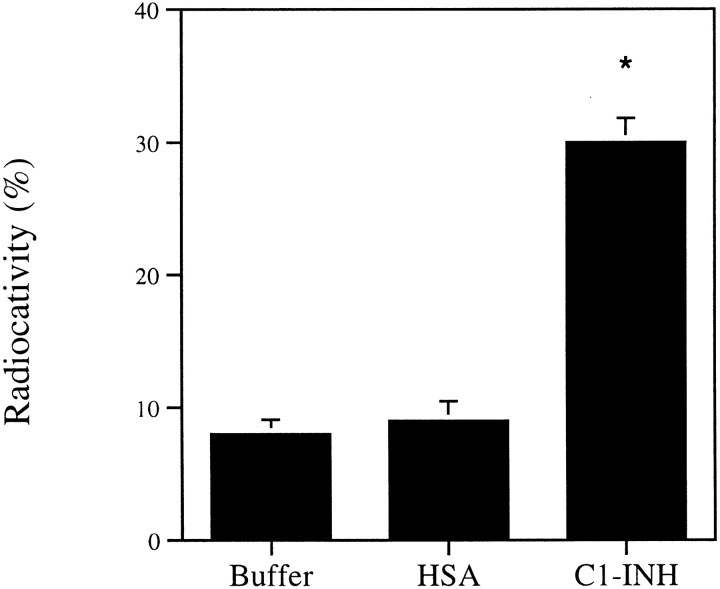
Direct binding of C1-INH to various complement proteins was studied using 125I-labeled factor B or factor D. Labeled factor B or D was incubated with C1-INH at room temperature for 1 h and anti–C1-INH was added and incubated for 30 min. Saturated ammonium sulfate was added at 4°C for 1 h to precipitate complexes. After washing twice with 50% saturated ammonium sulfate, we measured the radioactivity of the pellet. In a second type of experiment, labeled C1-INH was added to a 96-well microtiter plate, to which was bound factor B, factor D, or BSA control. After incubation and washing, radioactivity in the well was determined. In a third type of experiment, a PAGE migration-retardation method was used. Labeled factor B or factor D was incubated with C1-INH or BSA. After electrophoresis, the proteins were transferred to nitrocellulose membrane and exposed to films for 24 h before developing. The protein-migration pattern with or without addition of C1-INH was compared. All the above attempts failed to detect interaction between labeled proteins and C1-INH or control proteins. However, C1-INH interacted with immobilized C3b in the dot-blot analysis (Fig. 3), but not with Factor B, Factor D, and BSA.
Figure 3.
C1-INH binds to immobilized C3b. C3b, Factor B, Factor D, BSA, or buffer control on nitrocellulose paper was incubated with 125I-labeled C1-INH for 1 h at room temperature. After washing, radioactivity was determined. Nonspecific binding from the buffer control was subtracted from C3b, factor B, factor D or BSA binding. *P < 0.01 versus BSA control.
C1-INH Blocks the Ability of Factor B to Restore the Hemolytic Activity of Factor B–depleted Serum.
Factor B–depleted serum (1:10 diluted) in Mg-EGTA buffer had no ability to lyse rabbit red cells in alternative-pathway assays. Its activity was restored when purified human factor B (5 μg) was added. Addition of C1-INH prevented factor B from restoring the activity of factor B-depleted serum in a dose-dependent manner (Fig. 4). Control HSA had no effect. The C1-INH purchased from Advanced Research Technologies as well as C1-INH prepared by a rigorous purification procedure (see Materials and Methods) showed similar activity.
Figure 4.
C1-INH blocks factor B hemolytic activity. The Erb in pH 6.5 Mg-EGTA buffer was incubated with factor B-depleted human serum. To this mixture, 15 μl of factor B and HSA (⋄), factor B, and purified C1-INH from two sources (prepared as in Materials and Methods, solid circles and from Advanced Research Technologies; ○) was added. The mixture was then incubated at 37°C for 30 min. To stop the reaction, 1 ml of EDTA buffer was added. After centrifugation, the optical density of the supernatant was measured at 412 nm, and percent lysis was calculated.
Binding of Factor B and C1-INH to CVF-coated Sepharose Beads and Binding Inhibition by C1-INH or Factor B.
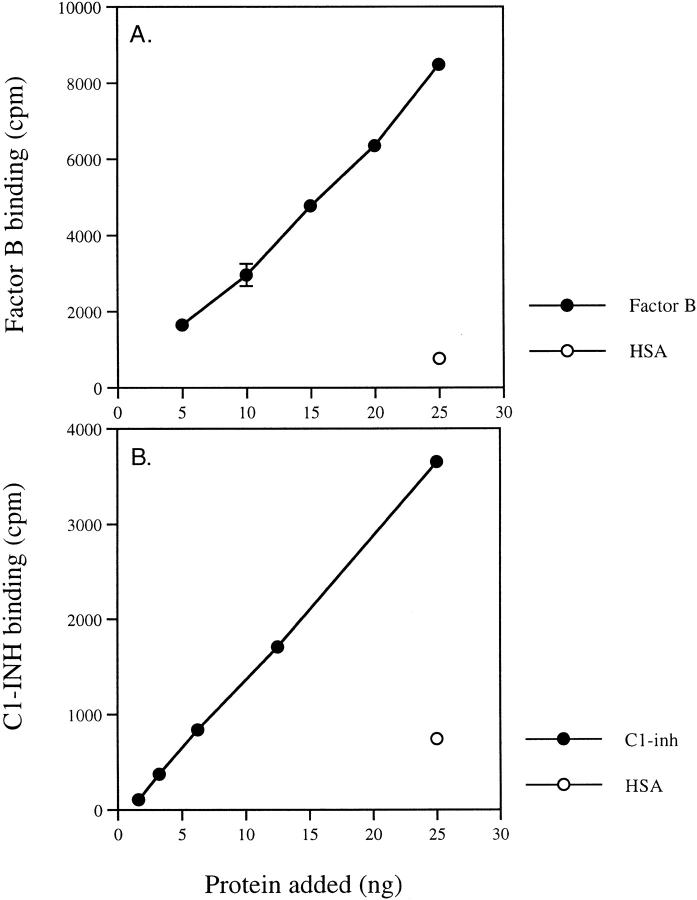
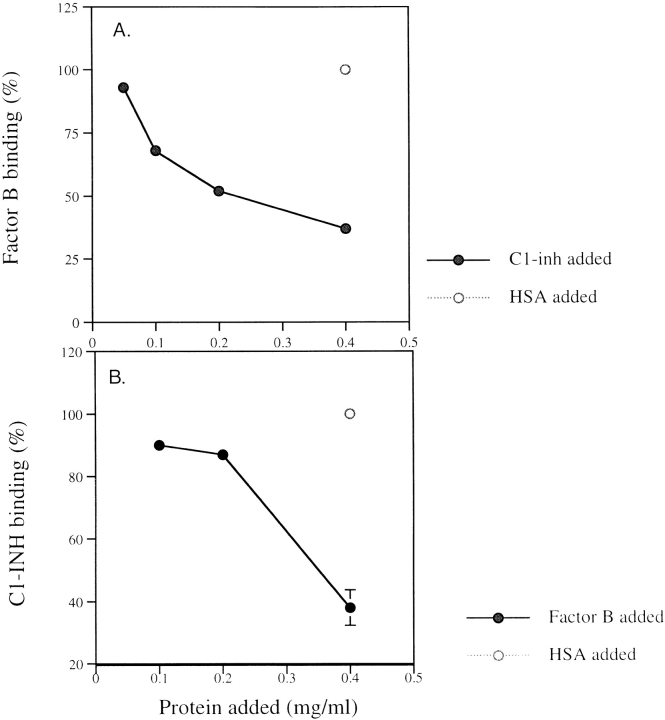
Factor B or C1-INH bound to CVF beads in Mg-EGTA buffer in a dose-dependent manner (Fig. 5). The control protein (HSA) showed little binding. When CVF-coated Sepharose beads were preincubated with C1-INH or factor B, the binding of labeled factor B was inhibited by C1-INH and C1-INH binding was inhibited by factor B in a dose-dependent manner (Fig. 6). The control protein (HSA) had no inhibition activity.
Figure 5.
Factor B and C1-INH binding to CVF beads. CVF-Sepharose beads were incubated with: (A) 125I-labeled factor B (•) or control protein HSA (○); (B) 125I-labeled C1-INH (•) or control protein HSA (○) at 37°C for 15 min. After washing twice with 2 ml of cold Mg-EGTA buffer, the bound proteins were measured in a gamma scintillation counter.
Figure 6.
Inhibition of factor B or C1-INH binding by C1-INH or factor B. The CVF-Sepharose beads were incubated with unlabeled C1-INH (•) or HSA (○) before adding 125I-labeled factor B. Inhibition of binding of factor B by C1-INH but not control protein is shown (A). Then, CVF Sepharose beads were incubated with unlabeled factor B (•) or HSA (○), to which 125I-labeled C1-INH was added. After washing twice with two ml cold Mg-EGTA buffer, the bound proteins were measured in a gamma counter. Inhibition of binding of C1-INH by factor B is shown (B).
Dissociation of Factor B from CVF-coated Beads Complex by C1-INH.
When complex of CVF-bound beads with bound 125I-factor B was incubated with C1-INH for 15 min at 37°C, a portion of the radioactivity in the pellet was released into the supernatant (30% compared with 8% for control; Fig. 7), indicating that C1-INH dissociated bound factor B from the CVF beads.
Figure 7.
C1-INH dissociates bound factor B from CVF. The CVF beads with bound factor B were washed and incubated with C1-INH or control protein HSA at 37°C for 15 min. We added 1 ml of cold Mg-EGTA buffer, and, after centrifugation, measured the supernatant radioactivity. *P < 0.001 versus HSA control.
C1-INH Inhibits Factor B and C3 Cleavage.
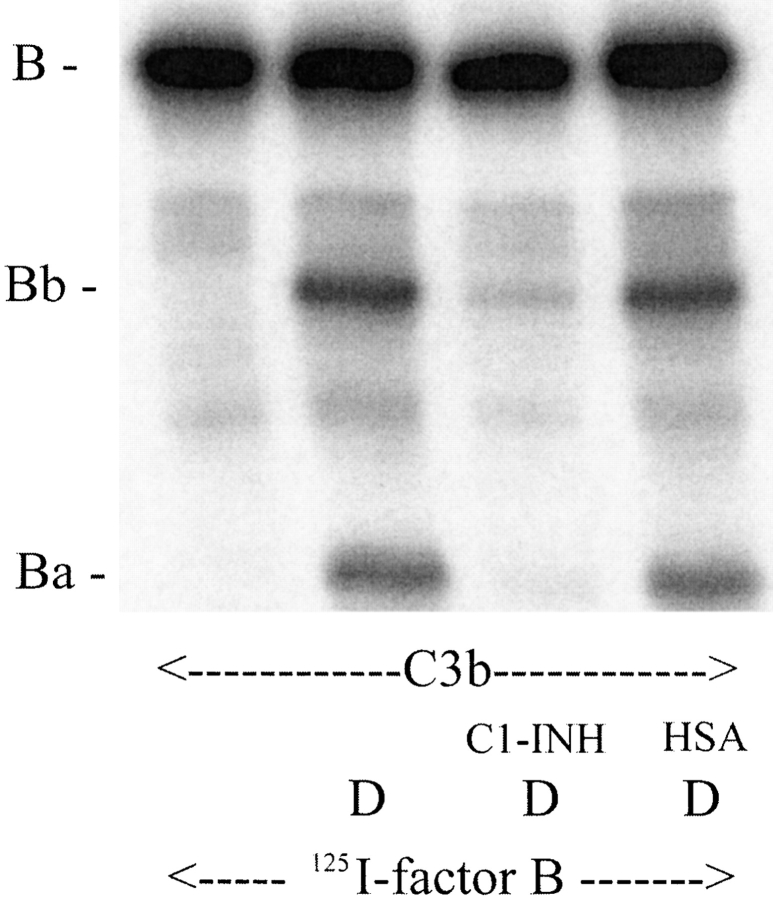
Factor B bound to C3b, and, once bound, was cleaved by factor D into Bb and Ba. When C3b was incubated with C1-INH before adding 125I-labeled factor B and factor D, factor B cleavage was inhibited. Less Bb and Ba were formed, as shown in Fig. 8. The control HSA had no effect. To determine whether reduced B cleavage, in turn, results in less C3 cleavage, the solid phase CVFBb (C3 convertase) was generated by incubation of CVF Sepharose beads, factor B, and factor D in the presence or absence of C1-INH and then washed to remove fluid-phase Bb and unbound proteins. In the absence of C1-INH, the formed CVFBb was able to cleave C3 to form C3b (C3 α chain into α′chain) (Fig. 9, lane 2). When C1-INH was present during generation of CVFBb, the 125I-C3 α chain remained intact (Fig. 9, lane 4), indicating that less CVFBb was formed. The control HSA had no effect (Fig. 9, lane 3). We also measured the activity of solid-phase CVFBb in consuming hemolytic C3. The C1-INH inhibited the generation of CVFBb, and thus more hemolytic C3 activity remained in the supernatant (data not shown).
Figure 8.
C1-INH inhibits factor B cleavage. A limiting amount of C3b (10 ng) was preincubated with C1-INH (2 μg) or control HSA (2 μg) at 37°C for 10 min before adding factor D (10 ng) and 125I-labeled factor B (250 ng). The mixture was incubated at 37°C for 60 min and stopped by adding 10 μl of twice concentrated SDS-PAGE sample buffer. 10 μl was loaded into wells of a 7.5% SDS-PAGE. Autoradiography indicates that C1-INH inhibits factor B cleavage.
Figure 9.
C1-INH inhibits C3 convertase. CVF beads were preincubated with C1-INH (2 μg) or control HSA (2 μg) at 37°C for 10 min before adding factors B (1 μg) and D (0.1 μg). The mixture was incubated at 37°C for 10 min to generate Sepharose CVFBb. After washing in GVBS++ to remove free factors B and D, the solid-phase CVFBb beads were incubated with 125I-labeled human C3 (250 ng) at 37°C for 60 min. To stop the reaction, 20 μl of twice-concentrated SDS-PAGE sample buffer was added. Autoradiography after SDS-PAGE indicated that C1-INH inhibits C3 cleavage.
Absorption of C1-INH from Normal Serum.
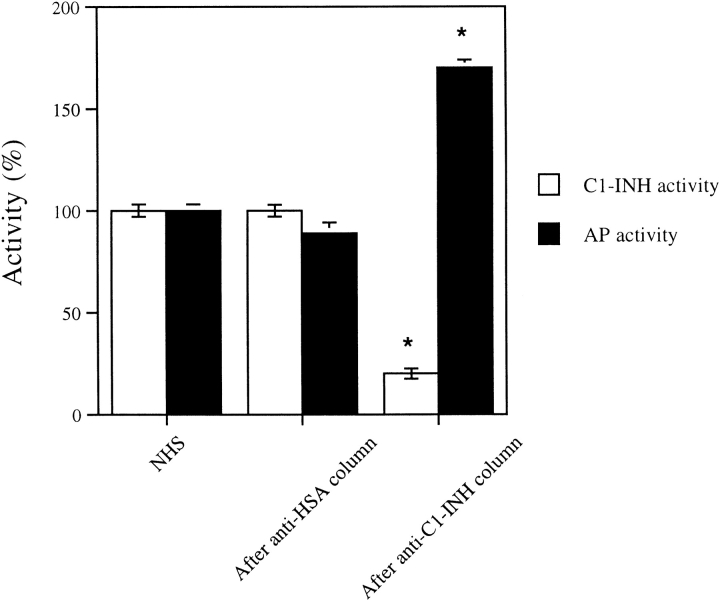
It is important to know whether levels of C1-INH in normal plasma regulate alternative-pathway activity. To examine this question, serum in Mg-EGTA buffer (to prevent activation of the classical pathway) was exposed in the cold to an anti–C1-INH or anti-HSA immunoabsorbant. The anti–C1-INH column, removed ∼80% of the C1-INH activity but not the anti-HSA column (Fig. 10). Lytic activity of the absorbed serum by the alternative pathway increased whereas that of the control was unchanged, suggesting that physiologic concentrations of C1-INH in normal serum downregulate activity of the alternative pathway.
Figure 10.
C1-INH and alternative-complement activities in C1-INH depleted serum. C1-INH functional activity (white bars) and alternative-complement pathway activity (black bars) in normal human serum, serum after passage over Sepharose-bound anti-HSA columns, and serum after passage over anti-C1-INH columns at 0°C. *P < 0.001 versus NHS or anti-HSA control.
Discussion
C1-INH is a heavily glycosylated, single chain, plasma glycoprotein with an apparent molecular weight of 105 kd on sodium dodecyl sulfate-PAGE. It consists of 478 amino acids comprising a backbone molecular weight of 52,880 (1). The protein acts as a serine protease inhibitor (serpin) binding to and forming covalent bonds with a variety of plasma proteases and thus inhibiting their activity (2, 3). The protein is known to inhibit C1s and C1r, two subcomponents of the complement protein C1. It is for these properties that it received its name. However, it is a known inhibitor of factor FXIIa and FXIIf, kallikrein, FXIa, plasmin, MASP1, and MASP2. Thus it inhibits proteins of the intrinsic coagulation, kinin generating, and fibrinolytic pathways, as well as the mannan binding lectin pathway of complement activation (5–9). As such, it is a potent down regulator of inflammation. C1-INH has been administered to animals in a variety of animal models of disease and shown to have profound inhibitory activities in situations ranging from ischemia-reperfusion injury as occurs in myocardial infarct to transplant rejection (15–17).
Here we report that C1-INH is also active in downregulating function of the alternative complement pathway. C1-INH downregulates lysis of complement sensitive erythrocytes from patients with paroxysmal nocturnal hemoglobinuria in acidified, Mg-EGTA serum, lysis known to be caused by activation of the alternative pathway. It also is a potent inhibitor of lysis in a second, widely used, assay for alternative pathway function, the lysis of rabbit erythrocytes in Mg-EGTA serum. In both of these cases, we find that acidification of serum contributes to increased binding of factor B and C3 to the erythrocytes when examined directly and this increase in binding is inhibited by C1-INH.
We have explored the basis of this unexpected observation. Our initial hypothesis was that C1-INH would bind and inhibit factor D, the C1 like enzyme of the alternative pathway. If such were the case, it might be expected to inhibit the protease by forming a covalent bond, as with all of the aforementioned proteases. Initial experiments demonstrated binding of C1-INH to C3b, but not factor B or D. In a variety of experimental models we also found that it binds to CVF, a C3b analogue found in the venom of cobras. Further studies demonstrated that C1-INH blocked the ability of factor B to restore the hemolytic activity of factor B-depleted serum. In binding to CVF it appears to interact with a site close to or identical with the factor B binding site on CVF. There is dose-dependent cross-inhibition of C1-INH and factor B binding. Moreover factor B is known to form a firm complex with CVF-coated beads and it is of interest that C1-INH can partially displace factor B from the complex.
It is known that when CVF coated beads are mixed with purified factor B and factor D, C3 cleavage, via the alternative pathway, is initiated. We find that C1-INH on interacting with CVF prevents such C3 cleavage.
To asses the point further, purified C3b was incubated with purified factor B and factor D in the presence or absence of C1-INH. In this reaction, factor B binds to C3b and in the presence of factor D, factor B is cleaved into Ba and Bb. This cleavage leads to the activation of the alternative pathway convertase and formation of a C3-cleaving enzyme. In the presence of C1-INH, but not in the presence of control protein, B cleavage was inhibited and the generation of Ba and Bb was markedly reduced.
These experiments suggest that one function of C1-INH is to regulate activation of the alternative pathway, presumably by binding to C3b and inhibiting the formation of or destabilizing the complex with factor B. We have no evidence thus far that the function of C1-INH in these studies is to act as a serpin. It does not appear to inhibit enzymatic activity, as it has in other functional studies.
In these studies, C1-INH is in marked molar excess and it was important to assess whether C1-INH has a regulatory function in normal serum. To normal serum was added Mg-EGTA to prevent activation of the classic pathway. C1-INH was removed by an immunoabsorption method. Activity of the alternative pathway increased markedly, suggesting that even at physiological concentrations C1-INH acts to control activation of the alternative pathway.
It is of interest that this is not the first observation of inhibition of protein function by C1-INH without formation of a covalent bond. It is known that C1-INH downregulates activation of C1 in normal plasma via a noncovalent interaction (5).
The plasma concentration of C1-INH is 200–300 μg/ml. It is an acute-phase protein and rises strikingly during inflammatory states. In alternative pathway activation small amounts of C3 with a cleaved thioester bond are generated spontaneously. If they bind to a surface on which the C3 is protected from inactivation by factors H and I, a C3 convertase is formed after the binding of factors B and D and marked alternative pathway amplification occurs. It is likely that C1-INH acts at these early steps in alternative pathway activation when limited amounts of C3 are activated and can be inhibited by C1-INH.
Interestingly, individuals who are heterozygous for a C1-INH gene defect and who functionally have one third to one half normal C1-INH function have HAE associated with attacks of mediator activation and tissue swelling. This disease can be life threatening. Current evidence suggests that these attacks are caused by the inability of heterozygous levels of C1-INH protein to regulate the generation of bradykinin by the kinin generating pathway (18). Patients with HAE have low levels of C4, reflecting uncontrolled activation of C1. The level of high-molecular-weight kininogen however, is normal between attacks, but falls during attacks, suggesting that C1-INH is not needed for regulation of the kinin-generating pathway between attacks of edema (18–20). Our data suggest that C1-INH also regulates the alternative pathway under physiologic conditions. Presumably, because there are many other regulators of C3 in plasma, including factors H and I, DAF (CD55), CR1 (CD35), and MCP (CD46), the C3 level does not fall during attacks of HAE. Nevertheless, C3 turnover has been shown to be increased and C3 degradation products do rise during these attacks (20).
In conclusion, these data present evidence that C1-INH, thought to inhibit classical pathway and mannan-binding lectin binding pathway, also functions as a regulatory protein of the third pathway of complement activation, the alternative pathway. At sites of inflammation, local concentration of C1-INH may be very important in controlling alternative pathway activity. There are many papers that suggest that C1-INH infusions can control a wide variety of inflammatory process (17). At present, therapeutic preparations of C1-INH are used to raise C1-INH protein levels, at times to above physiologic values. The ability of C1-INH to control all pathways of complement activation may be therapeutically important in the future.
Acknowledgments
The authors wish to acknowledge T. Waytes, M.D., and Baxter Healthcare Corporation, Glendale, CA, for the gift of partially purified C1 inhibitor.
This work was supported by a National Institutes of Health grant, 1R01 HL63937-01A1 (PI: Page Anderson, M.D. and the Marine/Freshwater Biomedical Center Project. The Role of Nurse Shark C4 Inhibitor in Inhibiting Mammalian C4).
This work was presented in part at the Eighteenth International Complement Workshop, Salt Lake City, UT. Immunopharmacology 49:61, 2000.
Footnotes
Abbreviations used in this paper: C1-INH, complement 1 inhibitor; CVF, cobra venom factor; Epnh, PNH erythrocytes; Erb, rabbit erythrocytes; GVBS, gelatin Veronal-buffered saline; MASP, mannan-binding lectin associated serine protease; PNH, paroxysmal nocturnal hemoglobinuria.
References
- 1.Davis, A.E., III. 1998. C1-INH and hereditary angioedema. The Human Complement System in Health and Disease. J.E. Volanakis and M.M. Frank, editors. Marcel Dekker, Inc., New York, NY. 455–480.
- 2.Levy, L., and I. Lepow. 1959. Assay and properties of serum inhibitor of C'1 esterase. Proc. Soc. Exp. Biol. Med. 101:608–611. [DOI] [PubMed] [Google Scholar]
- 3.Harpel, P.C., and N.R. Cooper. 1975. Studies on human plasma C1-inactivator-enzyme interactions. I. Mechanisms of interaction with C1s, plasmin and trypsin. J. Clin. Invest. 55:593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, C.H., and R. Boackle. 1998. A newly discovered function for C1-INH, removal of the entire C1qr2s2 complex from immobilized human IgG subclasses. Clin. Immunol. Immunopathol. 87:68–74. [DOI] [PubMed] [Google Scholar]
- 5.Ziccardi, R.J. 1985. Demonstration of the interaction of native C1 with monomeric immunoglobulins and C1 inhibitor. J. Immunol. 134:2559–2563. [PubMed] [Google Scholar]
- 6.Ratnoff, O., J. Pensky, D. Ogston, and G. Naff. 1969. The inhibition of plasmin, plasma kallikrein, plasma permeability factor, and the C1'r subcomponent of complement by serum C1' esterase inhibitor. J. Exp. Med. 129:315–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gigli, I., J.W. Mason, R.W. Colman, and K.F. Austen. 1970. Interaction of plasma kallikrein with the C1 inhibitor. J. Immunol. 104:574–581. [PubMed] [Google Scholar]
- 8.Wuillemin, W.A., M. Minnema, and J.C. Meijers. 1995. Inactivation of factor XIa in human plasma assessed by measuring factor XIa-protease inhibitor complexes: major role for C1-inhibitor. Blood. 85:1517–1526. [PubMed] [Google Scholar]
- 9.Schreiber, A.D., A.P. Kaplan, and K.F. Austen. 1973. Inhibition by C1-INH of Hagemann factor fragment activation of coagulation, fibrinolysis, and kinin generation. J. Clin. Invest. 52:1402–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsushita, M., S. Thiel, J.C. Jensenius, I. Terai, and T. Fujita. 2000. Proteolytic activities of two types of mannose-binding lectin-associated serine protease. J. Immunol. 165:2637–2642. [DOI] [PubMed] [Google Scholar]
- 11.Wagner, E., H. Jiang, and M.M. Frank. 2001. Complement and kinins: mediators of inflammation. Clinical Diagnosis and Management by Laboratory Methods, 20th ed. J.B. Henry, editor. W.B. Saunders Company, Philadelphia, PA. 892–913.
- 12.Hillmen, P., and S.J. Richard. 2000. Implications of recent insights into the pathophysiology of paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 108:470–479. [DOI] [PubMed] [Google Scholar]
- 13.Pilatte, Y., C.H. Hammer, M.M. Frank, and L.F. Fries. 1989. A new simplified procedure for C1 inhibitor preparation. A novel use for jacalin-agarose. J. Immunol. Methods. 120:37–43. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly o f the head of bacteriophage T4. Nature (London). 227:680–685. [DOI] [PubMed] [Google Scholar]
- 15.Buerke, M., T. Murohara, and A.M. Lefer. 1995. Cardioprotective effects of a C1 esterase inhibitor in myocardial ischemia and reperfusion. Circulation. 91:393–402. [DOI] [PubMed] [Google Scholar]
- 16.Horstick, G., A. Heimann, O. Götze, G. Hafner, O. Berg, P. Böehmer, P. Becker, H. Darius, H.J. Rupprecht, M. Loos, et al. 1997. Intracoronary application of C1 esterase inhibitor improves cardiac function and reduces myocardial necrosis in an experimental model of ischemia and reperfusion. Circulation. 95:701–708. [DOI] [PubMed] [Google Scholar]
- 17.Caliezi, C., W.A. Wuillemin, S. Zeerleder, M. Redondo, B. Eisele, and C.E. Hack. 2000. C1-Esterase inhibitor: an anti-inflammatory agent and its potential use in the treatment of diseases other than hereditary angioedema. Pharmacol. Rev. 52:91–112. [PubMed] [Google Scholar]
- 18.Waage Nielsen, E.W., H.T. Thidemann Johanes, K. Hogasen, W.A. Wuillemin, C.E. Haak, and T.E. Mollnes. 1996. Activation of the complement, coagulation, fibrinolytic, and kallikrein-kinin systems during attacks of hereditary angioedema. Scand. J. Immunol. 44:185–192. [DOI] [PubMed] [Google Scholar]
- 19.Schapiram M., L.D. Silver, C.F. Scott, A.H. Schmaier, L.J. Prograis, Jr., J.G. Curd, and R.W. Colman. 1983. Prekallikrein activation and high-molecular-weight kininogen consumption in hereditary angioedema. N. Engl. J. Med. 308:1050–1053. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen, E.W., H.T. Johansen, O. Gaudesen, B. Osterud, J.O. Olsen, K. Hogasen, C.E. Hack, and T.E. Mollnes. 1995. C3 is activated in hereditary angioedema, and C1/C1-inhibitor complexes rise during physical stress in untreated patients. Scand. J. Immunol. 42:679–685. [DOI] [PubMed] [Google Scholar]