Abstract
Coreceptors CD28 and cytotoxic T lymphocyte antigen (CTLA)-4 have opposing effects on TcR/CD3 activation of T cells. While CD28 enhances and CTLA-4 inhibits activation, the underlying molecular basis of these effects has yet to be established. In this context, ganglioside and cholesterol enriched membrane microdomains (rafts, GEMs) serve as centers of signaling in T cells. Although CD28 can promote TcR/raft colocalization, evidence is lacking on whether the surface expression of membrane rafts can be targeted by CTLA-4 in its modulation of T cell responses. In this study, we demonstrate that both CD28 and CTLA-4 profoundly alter the surface expression of membrane rafts during T cell activation. While CD28 increased expression and the number of peripheral T cells induced to express surface rafts in response to TcR ligation, CTLA-4 potently inhibited both TcR and TcR × CD28 induced raft expression on the surface of T cells. Consistent with this, CD28 increased the presence of the linker of activated T cells (LAT) in purified membrane rafts, while CTLA-4 coligation effectively blocked this increase. Further, the reversal of the CTLA-4 block with CD3/CD28 ligation was accompanied by an increase in surface raft expression and associated LAT. Our observations demonstrate for the first time that CTLA-4 targets the release of rafts to the surface of T cells, and provides a mechanism for the opposing effects of CD28 and CTLA-4 on costimulation.
Keywords: T cell function, CD28, CTLA-4, lipid rafts, LAT
Introduction
T cell activation is mediated by the antigen-receptor complex (TcRζ/CD3) and coreceptors CD28, inducible costimulator (ICOS), and cytotoxic T lymphocyte antigen (CTLA)-4 (CD152) (1, 2). CD28 and CTLA-4 have opposing effects with the coreceptors providing positive and negative signals, respectively (3, 4). Despite their importance, the underlying mechanisms responsible for these opposing effects are unclear (5). CD28 has been reported to bind to phosphatidylinositol 3-kinase (PI3-K), adaptors Grb-2/GADS, and the phosphatase PP2A (6–11), while CTLA-4 binds to PI3-K and phosphatases PP2A and SHP-2 (11–14). Debate concerns the relative roles of these elements in CD28 and CTLA-4 function (8, 11, 15, 16).
Glycosphingolipid/cholesterol enriched microdomains (GEMs, Rafts, detergent-insoluble glycolipid-enriched domains [DIGs], detergent-resistant membranes [DRMs]) on the surface of cells act as platforms that compartmentalize key components involved in signaling (17, 18). Rafts are enriched with GPI-linked proteins, palmitoylated transmembrane adaptors such as LAT (linker for activation of T cells), and myristoylated kinases such as p56lck and p59fyn (19–22). While the integrity of rafts is required for efficient T cell activation (22), the engagement of the TcR can promote its movement into rafts (23). In this context, CD28 coengagement can induce raft expression under conditions where TcR ligation failed to achieve this event, and has been reported to promote the colocalization of rafts with TcR complexes (24). In resting T cells, rafts as identified by GM1 staining have been found localized in intracellular compartments and are released to the cell surface with receptor ligation (25). In this context, memory and effector T cells have been reported to express higher levels of rafts than resting T cells (25). In addition to kinases, rafts are enriched with other signaling proteins. In this context, a family of immune cell specific adaptor proteins have been identified in recent years (26–28). Of particular note is the transmembrane adaptor protein LAT which is preferentially associated with rafts due to its palmitoylation (29, 30).
Although CD28 promotes raft expression, there has been a lack of evidence on whether alterations in the formation of surface rafts can explain the opposing effects of CD28 and CTLA-4 on the costimulation of T cells. In this study, we report that while CD28 greatly increased the number of GM1 negative peripheral T cells that become positive as a result of TcR ligation, CTLA-4 profoundly inhibited the expression of surface rafts. This was confirmed biochemically where CD28 augmented the detection of the adaptor LAT in purified rafts, while CTLA-4 coligation blocked this increase at the level of resting T cells. Further, the reversal of the CTLA-4 block by CD3/CD28 ligation was accompanied by an increase in surface raft expression and associated LAT. Our observations demonstrate for the first time that CTLA-4 targets raft expression on the surface of T cells, a finding that provides an explanation for the opposing effects of CD28 and CTLA-4 on the costimulation of T cells.
Materials and Methods
Cells, Reagents, and Antibodies.
Peripheral blood cells were isolated from the buffy coat by lymphocyte separation medium (Ficoll-Paque) density gradient centrifugation. The nonadherent cells were cultured in RPMI 1640 supplemented with 10% (vol/vol) FBS, 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mM L-glutamine. Anti–CTLA-4 mAb was purchased from Immunotech. Avidin-Texas Red, mouse IgG2a (specific for 2,4,6-trinitropenol; TNP), biotinylated anti–CTLA-4 and FITC-labeled CD25 were bought from BD PharMingen. Anti-OKT3 and anti-CD28 (9.3) were obtained from American Type Culture Collection and Bristol-Myers Squibb, respectively. Saponin and Cholera toxin (Ctx, B-subunit) were obtained from Sigma-Aldrich. Polyurethane-coated tosyl-activated Dynabeads were purchased from Dynal. Fluoromount-G was bought from Southern Biotechnology Associates, Inc. Anti-LAT and anti-lck antibodies were purchased from Upstate Biotechnology. Anti-CD3, anti-CD28, anti–CTLA-4, and anti-TNP mAbs were attached to Dynabeads following the manufacturer's instructions. The bead to cell ratio was 1:1. The ratio of anti-CD28 to anti–CTLA-4 was 1:5. Anti-TNP (IgG2a) was used as an isotype specific control for CTLA-4.
Ctx and CTLA-4 Staining of Naive and Activated Peripheral T Cells.
Purified naive T cells were stimulated with antibody-coated beads (anti-CD3/TNP; anti-CD3/CTLA-4; anti-CD3/CD28/TNP, and anti-CD3/CD28/CTLA-4) for various periods of time at 37°C. Cells were stained with FITC-labeled Ctx B-subunit, fixed with 1% paraformaldehyde, and analyzed by flow cytometry (Cytometer XL; Beckman Coulter). For immunofluorescence microscopy studies, purified naive T cells were activated with plate-bound anti-CD3 and anti-CD28 antibodies (5 μg/ml of each) for 48 h to induce CTLA-4 expression. Staining with FITC-labeled Ctx was performed under permeabilizing and nonpermeabilizing conditions. In the first case, cells were fixed with 2% paraformaldehyde for 20 min, washed, and permeabilized with PBS containing 0.03% saponin. After 30 min incubation with FITC-labeled Ctx and biotinylated CTLA-4 mAb at 4°C in PBS containing 0.3% saponin and 2% BSA, cells were washed and incubated with Avidin-Texas Red (1 μg/ml) for further 30 min at 4°C. Before mounting with Fluoromount G, cells were incubated for 5 min with Hoechst (1 μg/ml) to stain nuclei.
Under nonpermeabilizing conditions, cells were first stained with FITC-labeled Ctx, then washed, fixed, and permeabilized as described above. Cells were then incubated with biotinylated CTLA-4 mAb and Avidin-Texas Red, mounted, and visualized by microscopy.
Proliferation Assays.
Purified naive T cells were cultured in 96-well plates at a density of 2 × 105 cells and activated with anti-CD3/CD28/IgG2a and anti-CD3/CD28/CTLA-4 coated beads for 48 h. After this period of time, cells were washed, and beads were removed by using a magnet. Cells were then restimulated with anti-CD3/CD28 coated beads for further 48 h. To assay proliferation, cells were pulsed with 1 μCi of [3H]thymidine for the last 18 h of the indicated periods of time. In parallel, cells were stained for surface GM1 expression as described above.
GEM Isolation.
The GEM fractions were purified as a Triton X-100 insoluble fraction at the 5/35% interface as described (31). Anti-CD3/CD28 and anti-CD3/CD28/CTLA-4 stimulated T cells (19 h) were washed and resuspended with 1 ml of ice-cold MBS (25 mM MES, 150 mM NaCl, pH 6.5), 0.5% Triton X-100, 1 mM Na3VO4, 2 mM EDTA, 1 mM PMSF, and 1 μg/ml aprotinin. After a 30-min incubation on ice, the lysates were homogenized with 10 strokes of a loose-fitting Dounce homogenizer, gently mixed with an equal volume of 85% sucrose(wt/vol) in MBS, and overlaid with 6.5 ml of 35% sucrose and 3.5 ml of 5% sucrose in MBS with 1 mM Na3VO4 and spun for 16–19 h at 200,000 g at 4°C in a Beckman SW40Ti. 1 ml fractions were harvested from the top of the gradient. The GEM and TSF (triton soluble fraction) fractions were obtained in fractions 3–5 and 10–12, respectively.
Western Blotting.
Equal amounts of cell lysates (40 × 106 cells) from the GEM and TSF fractions was separated on a 12% SDS-PAGE and transferred to nitrocellulose for immunoblotting. Ponceau S staining of the transferred proteins served as a control for loading. The membranes were then blocked with 5% milk in TBS (10 mM Tris-HCL, pH 7.6, 150 mM NaCl) and incubated with the indicated antibodies. Bound antibody was revealed with HRP-conjugated rabbit anti–mouse or donkey anti–rabbit antibodies using enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech).
Results and Discussion
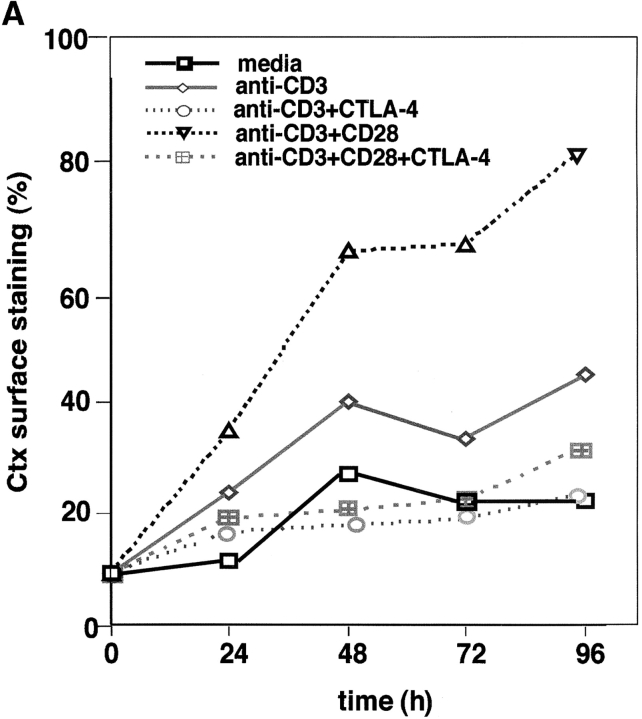
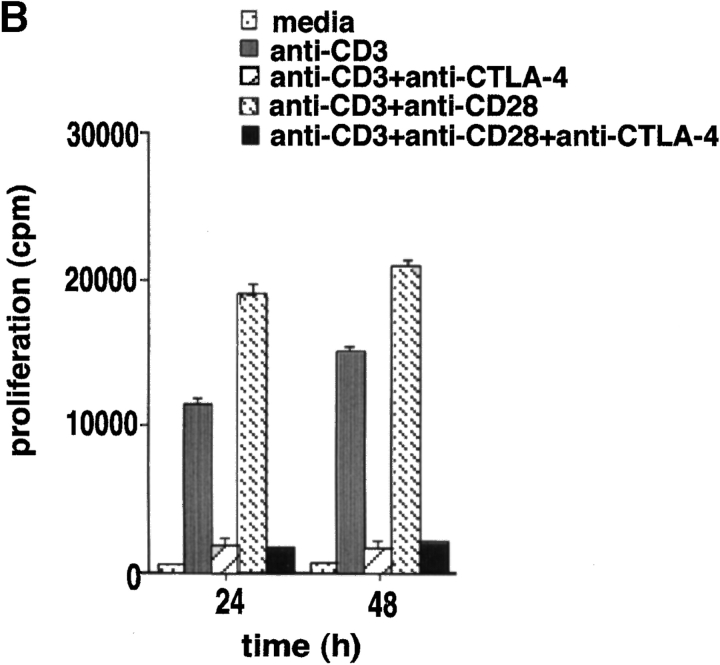
Given the profound opposing effects of CD28 and CTLA-4 on T cell activation (1–3) and the importance of ganglioside/cholesterol enriched rafts in signaling (17, 18), we investigated whether raft formation on the cell surface is targeted by the coreceptors. GM1 serves as a marker for the presence of membrane rafts on the surface of mammalian cells (18). The B-subunit of Ctx conjugated to FITC binds to GM1 and as such can detect surface expression of the ganglioside (32). Peripheral T cells were cross-linked with anti-CD3 with or without anti-CD28 or anti–CTLA-4, and assessed for GM1 expression by Ctx-FITC staining (Fig. 1 A). Anti-CD3 ligation induced raft surface expression where the percentage of GM1-positive cells increased from 7–10 on resting cells to 35–40% of the population over a period of 96 h on activated cells. Significantly, coligation with CD28 enhanced the percentage of GM1 positive cells to 70–80% of the population (i.e., an increase of two- to threefold that was observed in six experiments; Fig. 1 A). CD28 coligation also increased the mean intensity of fluorescence (MIF) on positively gated cells (i.e., 48 h: anti-CD3: 12.1, anti-CD3/CD28: 13.7; 72 h: anti-CD3: 15.6, anti-CD3/CD28: 17.4). However, the CD28 effect was more pronounced on the increase in numbers of naive peripheral T cells to express surface rafts as a result of TcR ligation (i.e., GM1-positive cells at 48 h: anti-CD3 versus anti-CD3/CD28: 40 to 60%; MIF: anti-CD3 versus anti-CD3/CD28: 12.1 [61%] to 13.7 [69%]). Anti-CD28 alone had not obvious effect on raft formation. As a control, upregulation in the presence of membrane rafts was accompanied by enhanced T cell proliferation (Fig. 1 B). These data show that CD28 can cooperate with the TcR in the potentiation of raft expression, especially by enhancing the percentage of peripheral T cells that are induced to express rafts in response to TcR ligation.
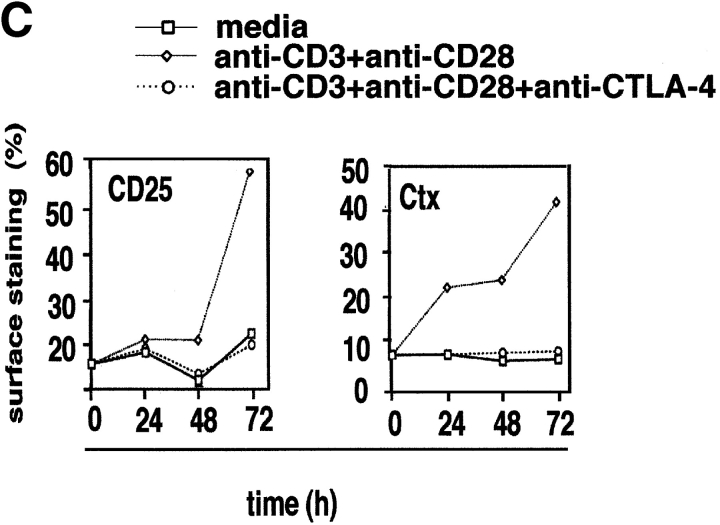
Figure 1.
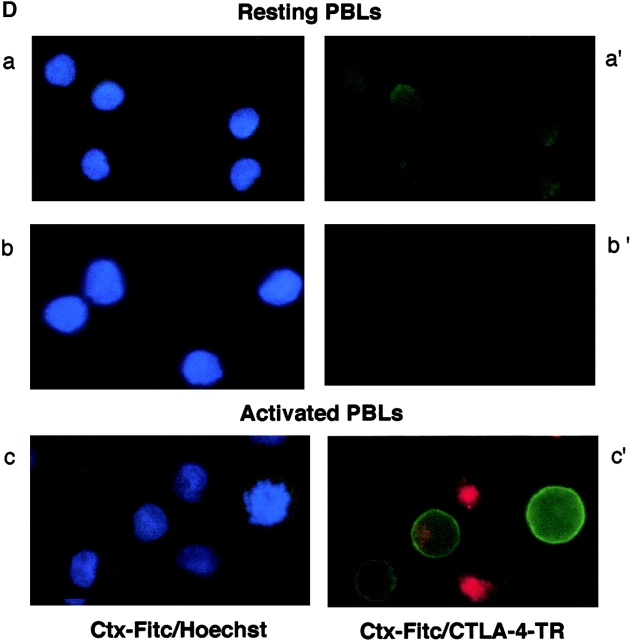
CD28 and CTLA-4 regulate the formation of membrane rafts on the surface of T cells. (A) Resting human peripheral T cells were (2 × 105) were stimulated with the following mAbs coated to Dynabeads: anti-CD3 (rectangle), anti-CD3/CTLA-4 (circle), anti-CD3/CD28 (triangle), and anti-CD3/CD28/CTLA-4 (crossed square) for 0, 24, 48, 72, and 96 h. Nonstimulated cells (square) served as a negative control. After the indicated time, cells were washed, stained with Ctx-FITC, fixed, and analyzed for GM1-positive cells (percentage). (B) Resting human cells stimulated with anti-CD3 (gray bars), anti-CD3/CTLA-4 (striped bars), anti-CD3/CD28 (hatched bars), and anti-CD3/CD28/CTLA-4 (black bars) coated beads for 24 and 48 h were pulsed with [3H]thymidine for the last 18 h at the indicated periods of time. Cells cultured in media alone served as a negative control (dotted bars). (C) Resting human peripheral T cells were stimulated with anti-CD3/CD28 (triangle) and anti-CD3/CD28/CTLA-4 (circle) coated beads for 0, 24, 48, and 72 h. Nonstimulated cells served as a negative control (square). After the indicated time, cells were washed and stained with anti CD25-FITC and Ctx-FITC, fixed, and analyzed for CD25 and Ctx surface expression by FACS®. (D) Immunofluorescence visualization of GM1 and CTLA-4 expression in resting and activated peripheral T cells. Resting lymphocytes were first permeabilized and then stained with Hoechst and FITC-labeled Ctx (a) or FITC-labeled Ctx and biotinylated CTLA-4/anti–IgG2a- Avidin-Texas Red (a′). Panel b: nonpermeabilized resting lymphocytes were stained with Hoechst and FITC-labeled Ctx (b), or stained only with FITC-labeled Ctx followed by fixation, permeabilization, and staining with biotinylated CTLA-4/anti–IgG2a-Avidin-Texas Red (b′). Panel c: to activate peripheral blood lymphocytes, cells were incubated with plate-bound anti-CD3 plus anti-CD28 mAbs (5 μg/ml of each). After 48 h, cells were first stained with Hoechst and FITC-labeled Ctx (c), and then additionally fixed, permeabilized, and stained with biotinylated CTLA-4/anti-IgG2a-Avidin-Texas Red (c′).
Given the opposing negative effect of CTLA-4 on T cell activation (4, 5), we next assessed whether CTLA-4 coligation could alter the surface expression of rafts. Coligation of CTLA-4 with either TcR, or the combination of TcR and CD28 potently inhibited GM1 expression (Fig. 1 A). On average, CTLA-4 inhibited the percentage of GM1-positive cells by 70–90% so that the level of expression at 48–96 h exceeded that of the resting population by only 5–10%. CTLA-4 also inhibited the expression level on cells that had been induced to express GM1 as a result of anti-CD3 or anti-CD3/CD28 ligation (i.e., MIF of positively gated cells), albeit to a much lesser extent (i.e., MFI: 48 h: anti-CD3 versus anti-CD3/CTLA-4: 12.1 [61%] to 11.0 [55%]; anti-CD3/CD28 versus anti-CD3/CD28/CTLA-4: 13.6 [69%] to 11.9 [60%]). Inhibition of GM1 expression correlated with the anti–CTLA-4 blockage of proliferation (Fig. 1 B), and CD25 expression (Fig. 1 C). Our findings therefore show that CD28 and CTLA-4 have striking opposite effects on the surface expression of rafts in a manner that mimic their effects on T cell proliferation. To our knowledge, this is the first report to document a regulatory effect of CTLA-4 on the expression of membrane rafts on the surface of T cells.
Membrane rafts are released from intracellular compartments to the plasma membrane due to receptor ligation (18, 25, 33). Immunofluorescence microscopy confirmed that anti-CD3/CD28 induced the release of GM1 from the cytoplasm to surface of resting human T cells (Fig. 1 D). Peripheral T cells were either permeabilized with saponin to visualize both intracellular/surface staining (Fig. 1 D, a and c), or were left in a nonpermeabilized state to visualize surface staining (Fig. 1 D, b). Hoechst staining was used to visualize the nucleus of permeabilized and nonpermeabilized cells (Fig. 1 D, a–c). Ctx staining of permeabilized resting T cells showed extensive intracellular staining for GM1 (Fig. 1 D, a′), while the majority of nonpermeabilized resting cells showed no immunofluorescence staining (Fig. 1 D, b′). By contrast, anti-CD3/CD28 activation showed extensive surface expression of GM1 (Fig. 1 D, c and c′). As expected from the detection of staining by FACS®, CTLA-4 coligation reduced the number of cells that stained positively for surface GM1 (data not shown; same as in Fig. 1 D, b′). An additional observation was that amongst the anti-CD3/CD28 activated T cells, individual cells that stained strongly for CTLA-4 failed to express GM1, while other cells that expressed high levels of CTLA-4 tended to be negative for GM1 (Fig. 1 D, c′).
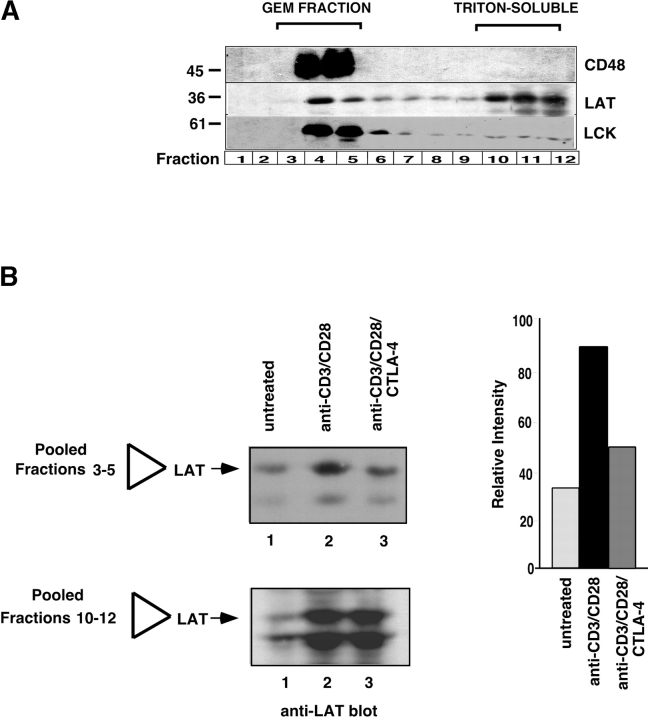
A key signaling protein in membrane rafts is the palmitoylated transmembrane adaptor protein LAT (29, 30). To obtain a biochemical correlate to raft formation, we next examined the ability of anti–CTLA-4 to inhibit raft formation by examining the presence of LAT in purified membrane rafts or GEMs. A Triton X-100 insoluble fraction (i.e., raft fractions) was isolated at the 5/35% interface of discontinuous sucrose gradient (31, 34). Of the 12 collected fractions, fractions 3–5 correspond to the GEM fraction, while fractions 10–12 correspond to the Triton-soluble fraction (Fig. 2 A). Subsequent blotting with GPI-linked CD48 showed an exclusive localization to the GEM fraction (Fig. 2 A, top panel). Blotting with anti-LAT showed its presence in both the GEM and non-GEM fractions (Fig. 2 A, middle panel), as reported previously (21). Blotting with anti-p56lck showed that Lck segregated primarily to the GEM fraction (Fig. 2 A, bottom panel). Our first observation was that anti-CD28 co-cross-linking resulted in the presence of a larger band at the sucrose interface (i.e., two- to threefold larger than in anti-CD3 activated samples), while anti–CTLA-4 blocked this increase (data not shown). Consistent with this, anti-CD3/CD28 stimulation increased the amount of LAT protein from purified GEMs when compared with untreated membranes (i.e., pooled samples from fractions 3–5; Fig. 2 B, lanes 2 versus 1). Further, coligation of CTLA-4 with TcR × CD28 inhibited the increase in the presence of LAT associated with the raft fraction (Fig. 2 B, lanes 3 versus 2). Significantly, anti-CTLA-4 blocked the TcR/CD28-mediated increase in LAT expression by 50–70% as measured by densitometric scanning (Fig. 2 B, right panel). An alteration in the presence of LAT in the non-raft fraction was not as easily observed, presumably due to the relatively small amount of LAT transferred to the raft fraction relative to the total amount of protein (Fig. 2 B, bottom panel). Despite this, the finding that the coreceptors altered the level of LAT in the raft fraction was a consistent observation.
Figure 2.
Raft associated LAT is reduced by CTLA-4 coligation. (A) Separation of GEMs and Triton X-100 soluble material by discontinuous sucrose gradient. The GEM fraction were purified as a Triton X-100 insoluble fraction at the 5/35% interface as described (reference 31). Fractions 3–5 correspond to the GEM fraction, while fractions 10–12 correspond to the Triton-soluble fraction. Equal amounts of cell lysates of fractions 1–12 were loaded and immunoblotting was performed with anti-CD48 (top panel), anti-LAT (middle panel), and anti-lck (bottom panel) antibodies. (B) CTLA-4 coligation with anti-CD28/CD3 reduces the presence of LAT in the GEM fraction. Left panel: equal amounts of cell lysates from GEM fractions (3–5) and triton-soluble fractions (10–12) obtained from untreated T cells (lane 1), or stimulated for 19 h with anti-CD3/CD28 (lane 2) and anti-CD3/CD8/CTLA-4 (lane 3) were separated on a 12% SDS-PAGE and immunoblotted with anti-LAT antibody. Right panel: histogram depiction of the levels of LAT protein as detected by densitometric reading.
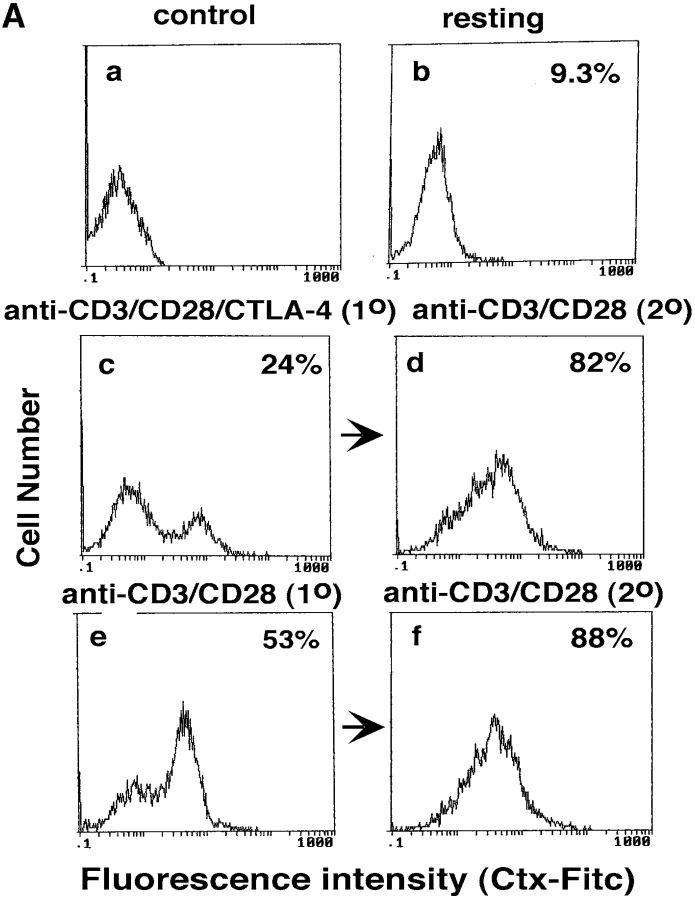
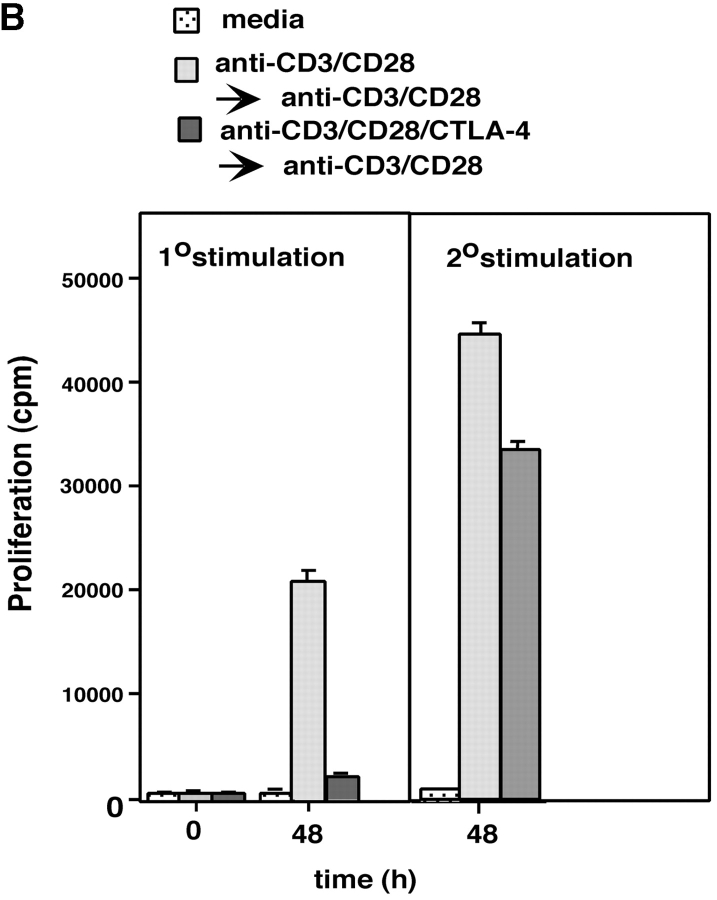
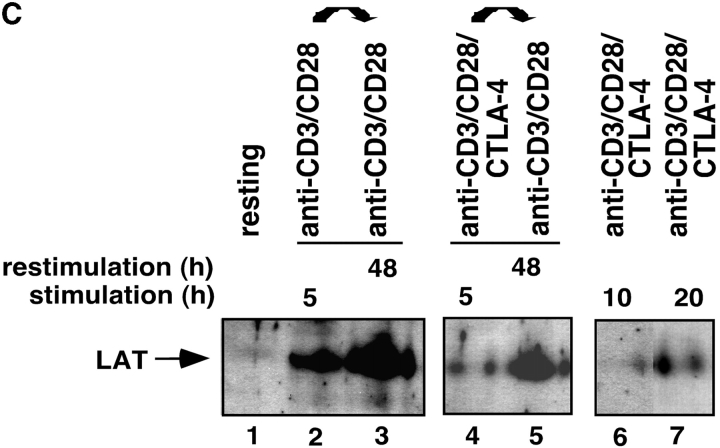
Given the above data, we next assessed whether the reversal of the CTLA-4 blockade by secondary stimulation with anti-CD3/CD28 resulted in the restoration of raft formation on the surface of cells (Fig. 3 A). Anti–CTLA-4 inhibited CD3/CD28-induced appearance of GM1 positive cells when assayed at 48 h (i.e., Fig. 3 A, b, c, and e). Restimulation of these cells with anti-CD3/CD28 reversed this inhibition with an increase in GM1 expression where 80–85% of the population stained positive for GM1 48 h later (Fig. 3 A, d). The level of this increase in expression was similar to that observed in primary anti-CD3/CD28 activated cells that had been restimulated with anti-CD3/CD28 (i.e., 88% expression; Fig. 3 A, f). Restimulation of cells with anti-CD3/CD28 caused a concordant recovery of proliferation (Fig. 3 B). Further, as shown in Fig. 3 C, the recovery of raft formation was also evident with an increase in the detection of raft-associated LAT. Cells stimulated with anti-CD3/CD28 (i.e., for 5 and 48 h) showed increased amounts of LAT in the raft fraction when compared with untreated cells (Fig. 3 C, lanes 1 versus 2 and 3). Coligation with anti–CTLA-4 suppressed this increase in LAT expression (Fig. 3 C, lane 4 versus 2). However, restimulation with anti-CD3/CD28 reversed the CTLA-4–mediated blockage by increasing the amount of LAT in the rafts (Fig. 3 C, lane 5). As mentioned, this reversal of LAT expression was mimicked by the ability of anti-CD3/CD28 to reverse the blockage in proliferation (Fig. 3 B). These findings further underlie the close relationship between CD28 and CTLA-4 modulation of proliferation and the expression of membrane rafts on the surface of T cells.
Figure 3.
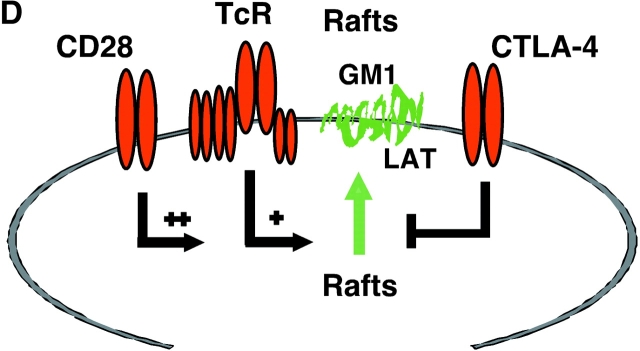
CD28 reverses the CTLA-4 block in the formation of rafts as assessed by GM1 expression and levels of raft-associated LAT. (A) Purified naive T cells were activated with anti-CD3/CD28/CTLA-4 or anti-CD3/CD28/TNP coated beads for 48 h (primary stimulation). After this period of time, anti-CD3/CD28/CTLA-4 or anti-CD3/CD28/TNP activated cells were washed and stained with Ctx-FITC for surface GM1 expression, respectively (c and e). Cells stained with goat anti–mouse FITC served as a negative control (a). Restimulation (secondary stimulation) was then performed by incubating the cells with anti-CD3/CD28 coated beads for further 48 h, respectively (d and f). After this time, cells were washed, stained with Ctx-FITC and analyzed for GM1 surface expression by FACS®. GM1 surface expression in resting PBLs is shown in panel b. (B) Proliferation was measured by pulsing the cells, stimulated as described in panel A, with [3H]thymidine for the last 18 h. Control (dotted bars), anti-CD3/CD28 (1°) followed by anti-CD3/CD28 (2°) (light gray bars), and anti-CD3/CD28/CTLA-4 (1°) followed by anti-CD3/CD28 (2°) (dark gray bars). (C) Equal amounts of cell lysates from GEM fractions (3–5) obtained from untreated and stimulated cells were separated on a 12% PAGE-Gel and immunoblotted with anti-LAT antibody. Lane 1: untreated cells; lanes 2 and 3: cells stimulated with anti-CD3/CD28 mAbs for 5 h (lane 2) and restimulated with anti-CD3/CD28 mAbs for 48 h (lane 3); lanes 4 and 5: cells stimulated with anti-CD3/CD28/CTLA-4 mAb for 5 h (lane 4) and restimulated with anti-CD3/CD28 mAbs for 48 h (lane 5); lanes 6 and 7: cells stimulated with anti-CD3/CD28/CTLA-4 mAbs for 10 h (lane 6) and 20 h (lane 7). (D) Model of CTLA-4 blockage of raft expression on the surface of T cells. TcR can induce the appearance of rafts on the surface of T cells. CD28 enhances raft expression induced by TcR ligation, and especially can increase the recruitment of cells to express membrane rafts that would not normally do so with TcR ligation alone. By contrast, CTLA-4 reduced the expression on TcR-induced GM1-positive cells, and dramatically on the numbers of cells to express rafts as a result of CD28 costimulation. Both the level of GM1 expression and the presence of the adaptor protein LAT in rafts was altered in a manner consistent with the opposing effects on the coreceptors on T cell function.
In summary, our data demonstrating that CTLA-4 as well as CD28 can regulate the expression of membrane rafts on the surface of T cells provides a mechanism to account for the opposing effects of these coreceptors on the T cell response. CTLA-4 ligation potently blocked the induction of raft surface expression caused by TcR ligation alone, or in combination with CD28 (Fig. 3 D). Alterations in raft surface expression therefore represent a novel target for CTLA-4, and provide a mechanism by which this coreceptor can attenuate TcR signaling. Of particular note was the potency with which CTLA-4 blocked both TcR and TcR × CD28 induced raft expression, and the degree to which the positive and negative effects of CD28 and CTLA-4 on raft formation was closely paralleled effects on T cell activation. After anti–CTLA-4 coligation, the percentage of cells expressing rafts was only slightly greater than for resting T cells. Although CTLA-4 reduced the expression on TcR-induced GM1-positive cells, a more profound effect was observed on the enhanced numbers of cells to appear as a result of CD28 costimulation. This is in keeping with the fact that the primary function of CD28 was to increase the number of cells that become positive as a result of TcR ligation. By preventing the intracellular release of these crucial signaling platforms, CTLA-4 will prevent TcR-raft interactions needed for efficient signaling. Indeed, the reduction in overall raft expression was accompanied by the reduction in the level of raft-associated LAT, a key integrator of TcR signaling. This may account for the reported reduction in LAT phosphorylation upon CTLA-4 coligation (13). Further, our findings show that this inhibitory effect was not fixed, but rather readily reversed with anti-CD3/CD28 coligation, an event that caused the reexpression of GM1, raft-associated LAT, and proliferation (Fig. 3). Raft microdomains serve as platforms that are enriched with key signaling molecules for efficient TcR signaling. By modulating raft expression and the percent of the surface area of the plasma membrane that constitutes raft microdomains, the two coreceptors would promote or prevent crucial interactions between TcR, LAT, and other components needed for signaling. These opposing effects on raft formation could account for the opposing effects of these coreceptors on the T cell response.
Our findings also provide a different perspective on the enhancing effects of CD28 on raft expression. While Viola and coworkers made the first important observation that CD28 can induce GM1 surface expression on cells, they failed to observe an effect of TcRζ/CD3 itself (24). Our findings show that anti-CD3 ligation can also induce raft expression on the T cell surface, and that this antigen receptor–mediated effect is potentiated by anti-CD28 ligation. CD28 therefore does not provide a unique signal for the release of rafts to the cell surface that cannot also be provided by the TcR. Second, our findings show that while CD28 can enhance GM1 expression on cells induced by TcR ligation, its principal effect was to increase the recruitment of new cells to express membrane rafts that would not normally do so with TcR ligation alone. By increasing the number of cells with the appropriate microenvironment for more efficient TcR signaling, CD28 would be expected to increase the number of cells capable of responding to a given antigen. Lastly, although Tuosto et al. (25) have previously shown increased expression levels of GM1 after anti-CD3/CD28 stimulation, the issue of whether CD28 could potentiate the anti-CD3–mediated induction of GM1 was not assessed. Both of our studies confirm that GM1 is released from intracellular stores in response to signals from TcR (in our study) or TcR × CD28 (both studies). Our findings are also in keeping with the observation that effector T cells with higher levels of raft expression than naive T cells are less dependent on co-stimulation (29, 35). Given that a function of CD28 is to promote raft expression, cells with high levels of expression would be expected to be less dependent on the function of the coreceptor.
Regulation of surface rafts may eventually be found to represent a general mechanism by which costimulation by other coreceptors such as ICOS amplify signaling. Further studies will be needed to unravel the proximal biochemical signals that connect coreceptors with the release of rafts from intracellular stores.
Acknowledgments
C.E. Rudd is a Principal Research Fellow of the Wellcome Trust, London, UK.
M. Martin and H. Schneider contributed equally to this work.
References
- 1.June, C.H., J.A. Bluestone, L.M. Nadler, and C.B. Thompson. 1994. The B7 and CD28 receptor families. Immunol. Today. 15:321–331. [DOI] [PubMed] [Google Scholar]
- 2.Chambers, C.A., and J.P. Allison. 1999. Co-stimulatory regulation of T cell function. Curr. Opin. Cell. Biol. 11:203–210. [DOI] [PubMed] [Google Scholar]
- 3.Walunas, T.L., D.J. Lenschow, C.Y. Bakker, P.S. Linsley, G.J. Freeman, J.M. Green, C.B. Thompson, and J.A. Bluestone. 1994. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1:405–413. [DOI] [PubMed] [Google Scholar]
- 4.Krummel, M.F., and J.P. Allison. 1995. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 182:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudd, C.E. 1996. Upstream-downstream: CD28 cosignaling pathways and T cell function. Immunity. 4:527–534. [DOI] [PubMed] [Google Scholar]
- 6.Truitt, K.E., C.M. Hicks, and J.B. Imboden. 1994. Stimulation of CD28 triggers an association between CD28 and phosphatidylinositol 3-kinase in Jurkat cells. J. Exp. Med. 179:1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad, K., Y.C. Cai, M. Raab, B. Duckworth, L. Cantley, S.E. Shoelson, and C.E. Rudd. 1994. T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Try(P)-Met-Xaa-Met motif. Proc. Natl. Acad. Sci. USA. 91:2834–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pages, F., M. Ragueneau, R. Rottapel, A. Truneh, J. Nunes, J. Imbert, and D. Olive. 1994. Binding of phosphatidyl-3-OH kinase to CD28 is required for T cell signaling. Nature. 369:327–329. [DOI] [PubMed] [Google Scholar]
- 9.Schneider, H., Y.C. Cai, K. Prasad, and C.E. Rudd. 1995. T cell antigen CD28 binds to the GRB-2/SOS complex, regulators of p21ras. Eur. J. Immunol. 25:1044–1050. [DOI] [PubMed] [Google Scholar]
- 10.Ellis, J.H., C. Ashman, M.N. Burden, K.E. Kilpatrick, M.A. Morse, and P.A. Hamblin. 2000. GRID: a novel Grb-2-related adapter protein that interacts with the activated T cell costimulatory receptor CD28. J. Immunol. 164:5805–5814. [DOI] [PubMed] [Google Scholar]
- 11.Chuang, E., T.S. Fisher, R.W. Morgan, M.D. Robbins, J.M. Duerr, M.G. Vander Heiden, J.P. Gardner, J.E. Hambor, M.J. Nevue, and C.B. Thompson. 2000. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 13:313–322. [DOI] [PubMed] [Google Scholar]
- 12.Schneider, H., K. Prasad, S.E. Shoelson, and C.E. Rudd. 1995. CTLA-4 binding to the lipid kinase phosphatidylinositol 3-kinase in T cells. J. Exp. Med. 181:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, K.M., E. Chuang, M. Griffin, R. Khattri, D.K. Hong, D.K. Zhang, W. Zhang, D. Strauss, L. Samelson, C.B. Thompson, and J.A. Bluestone. 1998. Molecular basis of T cell inactivation by CTLA-4. Science. 282:2263–2266. [DOI] [PubMed] [Google Scholar]
- 14.Schneider, H., and C.E. Rudd. 2000. Tyrosine phosphatase SHP-2 binding to CTLA-4: absence of direct YVKM/YFIP motif recognition. Biochem. Biophys. Res. Comm. 269:279–283. [DOI] [PubMed] [Google Scholar]
- 15.Cai, Y.C., D. Cefai, H. Schneider, M. Raab, N. Nabavi, and C.E. Rudd. 1995. Selective CD28pYMNM mutations implicate phosphatidylinositol 3-kinase in CD86-CD28 mediated costimulation. Immunity. 3:417–426. [DOI] [PubMed] [Google Scholar]
- 16.Lu, Y., C.A. Phillips, and J.M. Trevillyan. 1995. Phosphatidylinositol 3-kinase activity is not essential for CD28 costimulatory activity in Jurkat cells: studies with selective inhibitor, wortmannin. Eur. J. Immunol. 25:533–537. [DOI] [PubMed] [Google Scholar]
- 17.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- 18.Brown, D.A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell. Dev. Biol. 14:111–136. [DOI] [PubMed] [Google Scholar]
- 19.Rogers, W., and J.K. Rose. 1996. Exclusion of CD45 inhibition activity of p56lck associated with glycolipid-enriched membrane domains. J. Cell Biol. 135:1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran, M., and M.C. Miceli. 1998. Engagement of GPI-linked CD48 contributes to TCR signals and cytoskeletal organization: a role for lipid rafts in T-cell activation. Immunity. 9:787–796. [DOI] [PubMed] [Google Scholar]
- 21.Zhang, W., R.P. Trible, and L.E. Samelson. 1998. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 9:239–246. [DOI] [PubMed] [Google Scholar]
- 22.Xavier, R., T. Brennan, Q. Li, C. McCormack, and B. Seed. 1998. Membrane compartmentation is required for efficient T cell activation. Immunity. 8:723–732. [DOI] [PubMed] [Google Scholar]
- 23.Montixi, C., C. Langlet, A.M. Bernard, J. Thimonier, C. Dubois, M.A. Wurbel, J.P. Chauvin, M. Pierres, and H.T. He. 1998. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 17:5334–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viola, A., S. Schroeder, Y. Sakakibara, and A. Lanzavecchia. 1999. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 283:680–682. [DOI] [PubMed] [Google Scholar]
- 25.Tuosto, T., I. Parolini, S. Schroder, M. Sargiacomo, A. Lanzavecchia, and A. Viola. 2001. Organization of plasma membrane functional rafts upon T cell activation. Eur. J. Immunol. 31:345–349. [DOI] [PubMed] [Google Scholar]
- 26.Zhang, W., and L.E. Samelson. 2000. The role of membrane-associated adaptors in T cell receptor signaling. Semin. Immunol. 12:35–41. [DOI] [PubMed] [Google Scholar]
- 27.Myung, P.S., N.J. Boerthe, and G. Koretzky. 2000. Adapter proteins in lymphocyte antigen-receptor signaling. Curr. Opin. Immunol. 12:254–266. [DOI] [PubMed] [Google Scholar]
- 28.Rudd, C.E. 1999. Adaptors and molecular scaffolds in immune cell signaling. Cell. 96:5–8. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, W., J. Sloan-Lancaster, J. Kitchen, R.P. Trible, and L.E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Immunity. 9:83–92. [DOI] [PubMed] [Google Scholar]
- 30.Lin, J., A. Weiss, and T.S. Finco. 1999. Localization of LAT in glycolipid-enriched microdomains is required for T cell activation. J. Biol. Chem. 274:28861–28864. [DOI] [PubMed] [Google Scholar]
- 31.Brown, D.A., and J.K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to apical cell surface. Cell. 68:533–544. [DOI] [PubMed] [Google Scholar]
- 32.Schon, A., and E. Freire. 1989. Thermodynamics of intersubunits interactions in choleratoxin upon binding to the oligosaccharide portion of its cell surface receptor, ganglioside GM1. Biochemistry. 28:5019–5024. [DOI] [PubMed] [Google Scholar]
- 33.Sadeghlar, F., K. Sandhoff, and G. van Echten-Deckert. 2000. Cell type specific localization of sphingomyelin biosynthesis. FEBS Lett. 478:9–12. [DOI] [PubMed] [Google Scholar]
- 34.Rogers, W., and J.K. Rose. 1996. Exclusion of CD45 inhibition activity of p56lck associated with glycolipid-enriched membrane domains. J. Cell Biol. 135:1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iezzi, G., K. Karjalainen, and A. Lanzavecchia. 1998. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 8:89–95. [DOI] [PubMed] [Google Scholar]