Abstract
Lymphocytes residing in the intestinal epithelium are exclusively T cells and account for one of the largest collection of T cells in the organism. However, their function remains obscure. We and others have shown that the development of intestinal intraepithelial T cells is compromised in mutant mice prone to chronic intestinal inflammation. These results led us to directly assess their role in regulating the development of colitis secondary to transfer of primary splenic TCRαβ+CD4+CD45RBhi T cells into severe combined immunodeficiency (SCID) mice. Here we demonstrate that prior reconstitution of SCID recipients with intraintestinal TCRαβ+CD4−CD8α+β− T cells prevents disease, and does so in an interleukin (IL)-10–dependent fashion. In contrast, reconstitution with either TCRγδ+ or TCRαβ+CD4− CD8α+β+ intestinal T cells did not prevent colitis. TCRαβ+CD4−8α+β− T cells are unique to the intestinal epithelium of both rodents and humans. Previous repertoire analyses of TCRαβ+CD4−CD8α+β− T cells revealed a high proportion of cells expressing high affinity, self-specific TCR within this subset. We demonstrate that monoclonal, self specific TCRαβ+CD4−CD8α+β− cells derived from TCR transgenic mice also prevent the onset of colitis. Thus, intestinal TCRαβ+CD4−CD8α+β− T cells, selected based on their self-reactivity, maintain gut integrity in a IL-10–dependent fashion.
Keywords: regulatory, autoreactive, TCRαβ+CD4−CD8α+β− IEL, IL-10, IBD
Introduction
There is general consensus that inflammation of the intestinal mucosa results from a dysregulated response of CD4+TCRαβ+ T cells, resident within the intestinal lamina propria, to environmental antigen(s), in genetically susceptible individuals (1). Further, it has been proposed that onset of disease is due to an imbalance between regulatory and inflammatory subsets of CD4+ T cells (1).
Evidence has accumulated over the last 10 yr which established the T lymphopoietic capacity of the murine intestinal mucosa (2–5). Indirect evidence supports this conclusion in humans as well (6). Analyses of intraintestinal mononuclear cells (MNCs) developing in athymic radiation chimeras has enabled the phenotypic characterization of the various subsets of gut-derived T cells.
The first important observation is that donor-derived T cells are found on both sides of the basement membrane of the intestinal epithelium in numbers comparable to those observed in unmanipulated animals (2, 4, 7). Among intraepithelial T lymphocytes (iIELs), 30–50% express TCRγδ. The remaining CD3+ iIELs express a high level of TCRαβ. 5–10% express CD4 only, while 5–30%, in an age- and strain-dependent fashion, coexpress high levels of CD4 and the α chain of CD8. The remaining 60–80% of TCRαβ+ iIELs are divided into two subsets of roughly equal size, those expressing only the α chain of CD8, and those coexpressing CD8α and CD8β. In humans, the latter two TCRαβ+ subsets account for virtually all iIELs (8).
Murine TCRαβ+ iIELs are also distinguished based on their differential expression of functional antigen receptor complexes. Specifically, the CD4+CD8− and CD4− CD8α+β+ subsets express functional TCR/CD3 complexes (4, 7, 9), while CD4+CD8α+β− and CD4−CD8α+β− TCRαβ+ iIEL subsets are refractory to antigen, superantigen, anti-TCRαβ stimulation (4, 7, 9). However, these latter subsets do respond to lectin (4), and mAb specific for CD3 (4), in vitro.
There is evidence that the selection of CD4− CD8α+β−TCRαβ+ iIELs is unique (4, 10, 11). Specifically, this subset is comprised of a mixture of cells, some specific for antigen presented by classical MHC class I molecules (12), and others recognizing antigen presented by molecules encoded by the Qa-2 locus (13). What is remarkable is that the development of CD4−CD8α+ β−TCRαβ+ iIELs restricted by classical MHC class I is predicated by the expression of high affinity, self-specific TCR (10).
The first indication that iIELs play a role in the maintenance of mucosal integrity was derived from the analysis of IL-2/IL-2R–deficient mice, which are prone to IBD (14). The analysis of euthymic and athymic hemopoietic radiation chimeras demonstrated the lack of intraintestinal T lymphopoiesis in IL-2−/−, IL-2Rα−/−, and IL-2Rβ−/− mice. The absence of IBD in athymic radiation chimeras, and the presence of intestinal inflammation in euthymic recipients of hemopoietic precursors derived from the above three strains demonstrated the central role of thymus-derived T cells resident in the intestinal mucosa as mediators of disease, and the importance of a normal complement of gut-derived T cells in the maintenance of intestinal integrity. The above results are consistent with previous results demonstrating that normal thymus-derived T cells can induce gut inflammation upon adoptive transfer to T cell–deficient animals (15, 16). Specifically, when low numbers of CD4+CD45RBhi T cells of thymus origin cells are passively transferred into SCID mice, lacking both gut- and thymus-derived T cells, donor T cells migrate to the intestinal mucosa, expand, secrete proinflammatory cytokines, and induce an ulcerative colitis-like syndrome in the recipients (16). Furthermore, and in the context of this model system, it was demonstrated that TCRαβ+CD4+ CD45RBlow T cells of thymus origin prevented the development of this syndrome (16), and did so in an IL-10–dependent fashion (17).
In this study, we directly assessed the capacity of specific iIEL subsets to protect the intestinal mucosa from chronic inflammation induced by TCRαβ+CD4+CD45RBhi T cells of thymic origin.
Materials and Methods
Animals.
Ly5.2 C57Bl/6, SCID.C57Bl/6, and IL-10−/− C57Bl/6 mice were purchased from The Jackson Laboratory. Ly5.1 C57Bl/6 mice and Ly5.2, recombination activating gene (RAG)-2−/−, C57Bl/6 mice transgenic for HY/H2Db-specific TCRαβ (10) were bred in our animal facility. Prior to transfer, both donors and recipients (7–10 wk old) were bred and maintained under specific pathogen-free conditions. Bimonthly assessment of sentinel mice failed to detect viruses, parasites, or pathogenic bacteria (including Helicobacter sp.) throughout the course of the study. After adoptive transfer of MNCs, recipients were fed non-sterile food and water, and the filter tops of their cages were removed. Three times a week, recipients were weighed and examined for the presence of diarrhea, bloody stools, and rectal prolapse. Animals were killed when body weight loss was ≥20%, and/or any combination of the three other intestinal symptoms developed, or at 150 d.
Monoclonal Antibodies, Three-Color Immunofluorescence, FACS® Analysis, Cell Preparation, Cell Sorting, and Cell Transfer.
The preparation of iIELs and lamina propria (LP) lymphocytes, and the monoclonal antibodies used in this study have been described previously (4, 7, 14, 16). Cells were then analyzed by flow cytometry on a Becton Dickinson FACSCalibur™ or sorted using a Moflo (Cytomation, Inc.). At least 104 cells/sample were acquired for analysis. The absolute number of cells within an MNC subset was calculated by multiplying the total number of MNCs isolated by the proportion of cells accounted for by this subset. The total number of MNCs from the intestinal epithelium and lamina propria was determined before their purification by discontinuous Percoll gradient centrifugation.
For sorting, iIELs and splenocyte suspensions were prepared and stained in sterile conditions with the appropriate combination of fluorochrome-conjugated antibodies (4). The purity of sorted iIEL and splenocyte subsets was analyzed before their injection into SCID recipients and was always ≥99%.
For iIEL reconstitution, a total number of 5 × 106 cells from each of the studied iIEL subsets was administered intravenously to the SCID recipients. Adoptive transfer of TCRαβCD4+ CD45RBhi splenic T cells (0.5 × 106/recipient) was performed intravenously 1 wk after the iIEL reconstitution.
Histology.
The gut of experimental animals was differentially processed for histological examination depending on whether suspensions of MNCs from the intestinal epithelium and lamina propria were also prepared for flow cytometry analysis. Prior to the preparation of suspensions of gut-derived MNCs, 3 proximal, median, and distal fragments (5–10 mm long) of both small and large bowel were collected for histological examination. For the other experimental animals, the entire length of small and large bowel from SCID recipients was wrapped to form a roll before fixation in 10% neutral buffered formaldehyde for 24 h. Specimens were then embedded in paraffin and three 6-μm sections were taken 250 μm apart through the entire thickness of the bowel loops. Each section was stained with hematoxylin and eosin. Each section was examined and scored in a blind fashion by one of us (D. Banerjee) as described (17). Briefly, grade 0 corresponded to the absence of histological abnormalities; grade 1 to the presence of minimal and scattered inflammatory cell infiltrates, with or without minimal epithelial hyperplasia; grade 2 to mild scattered to diffuse inflammatory cell infiltrates, sometimes invading the submucosa and associated with erosions, with minimal to mild epithelial hyperplasia, and minimal to mild loss of mucin from goblet cells; grade 3 to mild to moderate inflammatory cell infiltrates, sometimes transmural, often associated with ulceration, with moderate epithelial hyperplasia, and loss of mucin; grade 4 to severe inflammatory cell infiltrates, often transmural and associated with ulceration, with marked epithelial hyperplasia, and loss of mucin; grade 5 to severe, transmural inflammatory cell infiltrates, with severe ulceration and loss of intestinal crypts.
Statistical Analysis.
The Mann-Whitney test and log-rank analysis were applied to compare histological scores and Kaplan-Meier survival curves, respectively. Student's t test was applied to weight variations. Differences were considered as statistically significant with P < 0.05.
Results and Discussion
CD4−8α+β−TCRαβ+ iIELs Protect Against Colitis.
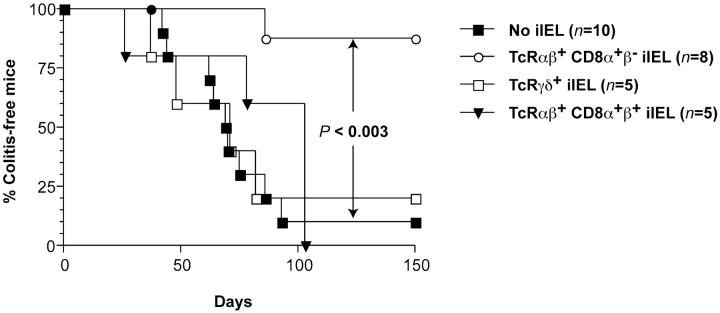
We used the murine model which led to the original characterization of CD4+CD45RBhiTCRαβ+ thymus-derived T cells expressing a Th1 cytokine pattern, as potent inducers of colitis when injected into histocompatible SCID recipients (16, 18). As illustrated in Figs. 1, 2 A, and 2 C, mild to severe colitis developed in 22/26 Ly 5.2 SCID.B6 recipients of 0.5 × 106 splenic TCRαβ+CD4+CD45RBhi T cells derived from Ly 5.1 C57Bl/6 donors in 150 d. All animals that developed clinical symptoms had severe lesions restricted to the colon (Fig. 3 a). Animals were considered as colitis free only when clinically normal and devoid of histological abnormalities.
Figure 1.
Differential susceptibility to colitis in SCID.C57Bl/6 recipients of various iIEL subsets and splenic CD4+CD45RBhiTCRαβ+ T cells. Significant (P < 0.01) protection from colitis was afforded by CD4−CD8α+β−TCRαβ+ iIELs. Results taken from two of the 2–8 experiments performed.
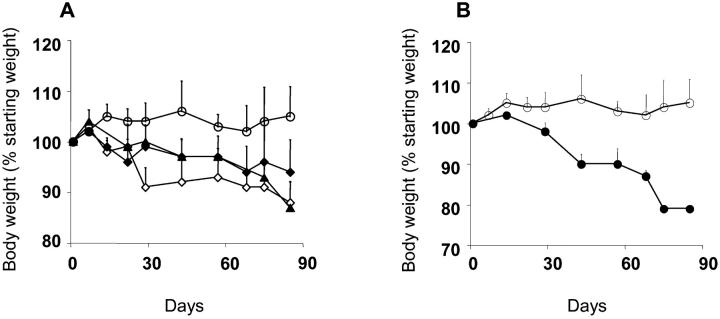
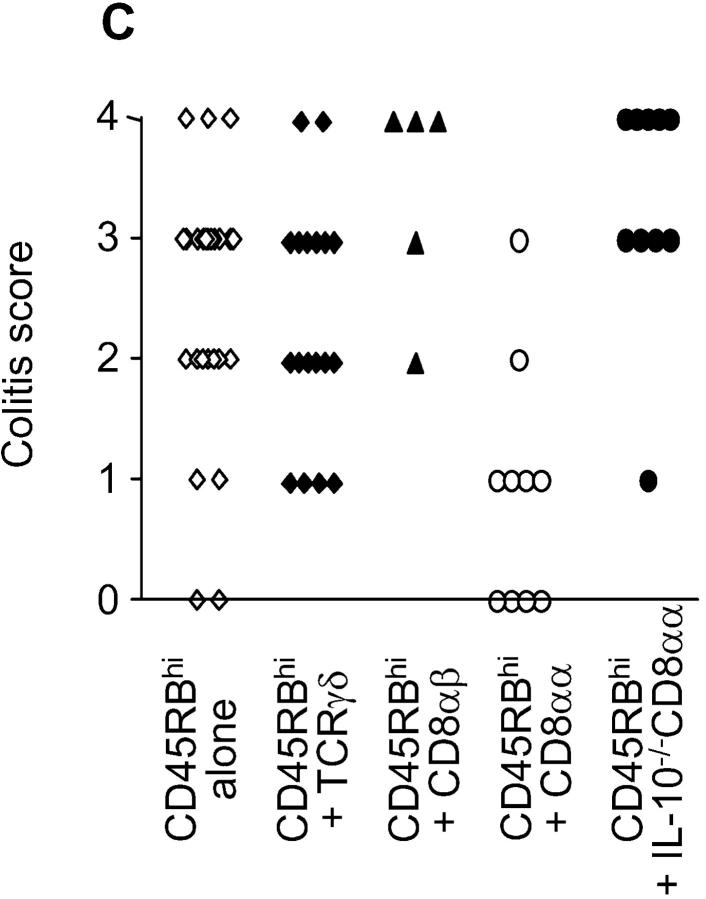
Figure 2.
Differential susceptibility to colitis in SCID.C57Bl/6 recipients of various iIEL subsets and splenic CD4+45RBhiTCRαβ+ T cells. (A and B) Weight variations over time of experimental groups of recipients. Data represent the mean ± SEM. (A) Statistical significance (Student's t test): no reconstitution (⋄) versus reconstitution with CD4−CD8α+β−TCRαβ+ iIELs (○), P < 0.02; no reconstitution (⋄) versus reconstitution with TCRγδ+ iIELs (♦) or CD4−CD8α+β+TCRαβ+ iIELs (▴), not significant. (B) Reconstitution with CD4−CD8α+β−TCRαβ+ iIELs (○) versus IL-10−/−CD4−CD8α+β−TCRαβ+ iIELs (•), P < 0.05. (C) Pathology scores of individual animals from experimental groups of recipients. Statistical significance (Mann-Whitney test): no reconstitution versus reconstitution with CD4−CD8α+β−TCRαβ+ iIELs, P < 0.002; no reconstitution versus reconstitution with TCRγδ+ iIELs or CD4−CD8α+β+TCRαβ+ iIELs, not significant; reconstitution with CD4−CD8α+β−TCRαβ+ iIELs versus IL-10−/−CD4−CD8α+β−TCRαβ+ iIELs, P < 0.0005; no reconstitution versus reconstitution with IL-10−/−CD4−CD8α+β−TCRαβ+ iIELs, P < 0.05.
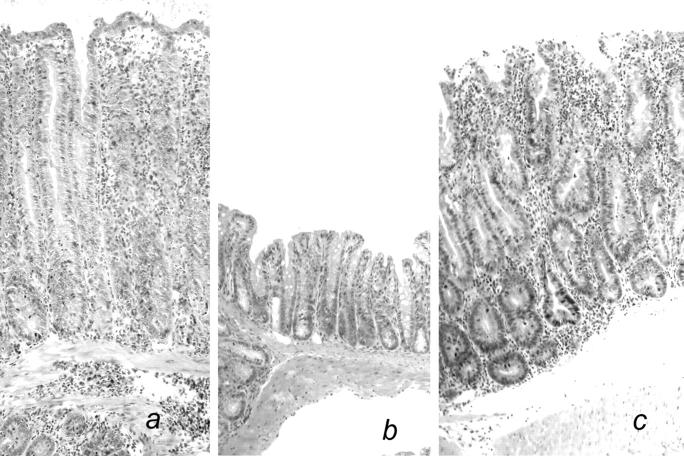
Figure 3.
Histological analysis of the colonic mucosa from representative SCID.C57Bl/6 recipients of CD4+CD45RBhiTCRαβ+ T cells and different iIEL subsets. (a) Severe colitis in a SCID.C57Bl/6 recipient of CD4+CD45RBhi TCRαβ+ T cells. (b) Lack of colitis in a SCID.C57Bl/6 recipient of CD4−CD8α+ β−TCRαβ+ iIELs and CD4+CD45RBhi TCRαβ+ T cells. (c) Severe colitis in a SCID.C57Bl/6 recipient of IL-10−/− CD4− CD8α+β−TCRαβ+ iIELs and CD4+CD45RBhi TCRαβ+ T cells. Hematoxylin and eosin stain; original magnification: ×100.
The capacity of three iIEL subsets to regulate the course of colitis was assessed. Specifically, 1 wk before the adoptive transfer of splenic TCRαβ+CD4+CD45RBhi T cells, recipients were reconstituted with 5 × 106 cells of one of the three following iIEL subsets: CD4−CD8α+β− TCRαβ+, CD4−CD8α+β+TCRαβ+, or TCRγδ+ isolated from Ly 5.2 C57Bl/6 donors. As illustrated in Figs. 1, 2 A, 2 C, and 3 b, 8/10 recipients of CD4−CD8α+β−TCRαβ+ iIELs remained disease free for up to 150 d. In contrast, 5/5 recipients of CD4−CD8α+β+TCRαβ+ iIEL and 14/18 recipients of TCRγδ+ iIELs developed mild to severe colitis (Figs. 1, 2 A, and 2 C).
The results presented herein demonstrate a heretofore unrecognized function of iIELs. Specifically, of the two major subsets of iIELs in rodents and humans (8, 11, 19), those expressing CD4−CD8α+β−TCRαβ+ and those expressing CD4−CD8α+β+TCRαβ+, we demonstrate that the former but not the latter can function to protect gut integrity in the presence of thymus-derived inflammatory T cells. In this regard, it is of note that there is precedent for the role of iIELs in modulating systemic immune responses. Specifically, TCRγδ+, iIELs have been shown to modulate low dose oral tolerance induction in an IL-10–dependent fashion (20).
Protective iIELs Regulate Numbers of CD4+ T Cells in the Colonic Lamina Propria.
The use of CD45 congenic C57Bl/6 lines enabled us to discriminate amongst recipient, donor-derived splenic T cells and iIELs, and hence to assess the fate of donor-derived T cell subsets.
Tracking of donor T cell subsets using this strain combination demonstrated that the inability of an iIEL subset to afford protection was not related to its capacity to reconstitute the iIEL compartment of the recipient. As illustrated in Table I, >95% of donor (Ly 5.2) iIELs were rescued from the iIEL compartment of recipient small bowel while <2% of these donor cells were rescued from secondary lymphoid organs. Further, the total number of donor-derived CD4−CD8α+β+TCRαβ+, CD4−CD8α+β−TCRαβ+, and TCRγδ+ iIELs recovered was not significantly different, and hence differential reconstitution did not correlate with protection. Of note, the number of rescued cells approximated the number of cells injected, and their membrane CD4/CD8 phenotype remained unchanged.
Table I.
Numbers (Mean ± SEM) of Donor-derived Lymphocytes in the Intestinal Mucosa and Secondary Lymphoid Organs (Spleen and Lymph Nodes) of SCID.C57Bl/6 Recipients (n = 3–5/Group Derived from 2–3 Experiments Killed at the Onset of Clinical Colitis or at 150 d).a
| iIEL subsets transferred prior to administration of CD4+CD8−CD45RBhighTCRαβ splenocytes
|
||||||
|---|---|---|---|---|---|---|
| Donor lymphocytes | No iIEL | CD8α1β1TCRαβ+ | CD8α1β2TCRαβ+ | IL-10−/−CD8α1β2TCRαβ1 | TCRγδ1 | |
| CD4+CD45RBhiTCRαβ1 | ||||||
| S.B. | iIEL | 1.4 ± 0.8 × 107 | 8.3 ± 1.4 × 106 | 5.1 ± 1.4 × 106 | 9.2 ± 0.9 × 106 | 2.0 ± 0.7 × 107 |
| LP | 1.7 ± 0.9 × 107 | 8.7 ± 1.9 × 106 | 8.8 ± 1.9 × 106 | 2.0 ± 2 × 107 | 6.2 ± 1 × 106 | |
| iIEL | 0.3 ± 0.1 × 106 | 0.5 ± 0.1 × 105 | 0.4 ± 0.2 × 105 | 1.1 ± 0.2 × 105 | 1.2 ± 0.3 × 105 | |
| L.B. | LP | 8.3 ± 0.3 × 106 | 8.6 ± 0.4 × 106 | 1.4 ± 0.3 × 106a | 9.9 ± 0.6 × 106 | 6.9 ± 0.4 × 106 |
| Spleen plus LN | 5.0 ± 1.7 × 106 | 0.8 ± 0.6 × 106 | 1.8 ± 1.0 × 106 | 3.2 ± 1.1 × 106 | 1.2 ± 0.6 × 106 | |
| iIEL | ||||||
| S.B. | iIEL | NA | 7 ± 0.4 × 106 | 5.8 ± 0.7 × 106 | 5.2 ± 0.4 × 106 | 1 ± 0.4 × 107 |
| LP | NA | 0.5 ± 0.3 × 106 | 0.3 ± 0.3 × 106 | 0.3 ± 0.3 × 106 | 0.8 ± 0.3 × 106 | |
| L.B. | iIEL | NA | 0.2 ± 0.2 × 105 | 0.3 ± 0.1 × 105 | 0.4 ± 0.3 × 105 | 0.4 ± 0.3 × 106 |
| LP | NA | 0.4 ± 0.2 × 106 | 0.2 ± 0.2 × 105 | 0.4 ± 0.2 × 105 | 0.4 ± 0.3 × 105 | |
| Spleen plus LN | NA | 1.3 ± 0.6 × 105 | 1.0 ± 0.8 × 105 | 6.4 ± 1.4 × 105 | 2.5 ± 1.6 × 104 | |
Statistically significant (P < 0.05, Mann-Whitney test) compared to other groups of recipients. L.B., large bowel; S.B., small bowel.
While the majority of the cells derived from the injected Ly 5.1 TCRαβ+CD4+CD45RBhi were rescued in the small bowel of recipients (Table I), these animals showed no small bowel pathology. The cells homed to both the iIEL and LP compartments of the small bowel with comparable efficiency, and the number of donor cells rescued was 16–60-fold higher than the number of injected cells. Homing of the progeny of CD4+45RBhi cells to the recipient spleen and lymph nodes only amounted to 3–13% of the number of cells of the same origin recovered from the recipient gut (Table I).
The lamina propria of the large bowel was the exclusive site where the number of cells derived from Ly 5.1 TCRαβ+CD4+CD45RBhi T cells was differentially and significantly affected by the subset of reconstituting iIELs. Specifically, 8–9-fold more CD4+ T cells were rescued from this site in animals that developed colitis compared with those healthy recipients of protective CD4−CD8α+ β−TCRαβ+ iIELs (Table I).
It is striking that protective iIELs were rescued almost exclusively in the small bowel. While consistent with the location of this subset in conventional animals, this study reveals two remarkable facts. The cells derived from CD4+CD45RBhi TCRαβ+ T cells expanded 50–100-fold in all animals, independent of iIEL reconstitution. However, the majority of the progeny were found in the intestinal mucosa of the small bowel, which was free of pathology. The influence of the reconstituting iIEL subsets was only observed in the lamina propria of the large bowel. We conclude that progeny of CD4+CD45RBhiTCRαβ+ T cells resident in the small bowel must not be pathogenic, and that the protective function of CD4−CD8α+ β−TCRαβ+ iIELs does not involve direct interaction with inflammatory effector cells.
Protection Against Colitis Afforded by CD4−CD8α+β− TCRαβ+ iIELs Is IL-10–dependent.
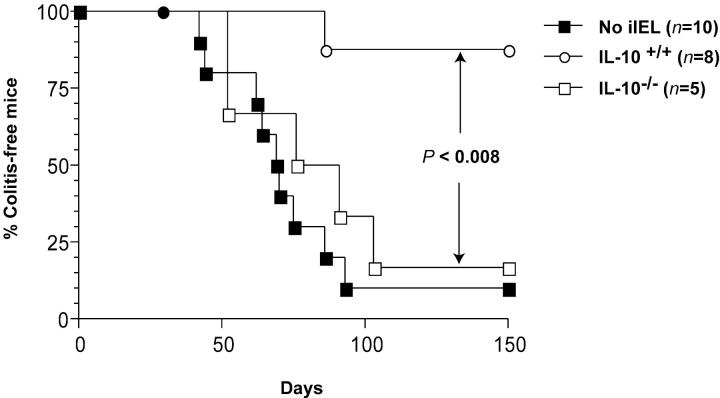
When adoptive transfer of Ly 5.1 TCRαβ+CD4+CD45RBhi splenic T cells was preceded by reconstitution of the recipients with 5 × 106 CD4−CD8α+β−TCRαβ+ iIELs derived from IL-10−/− donors, mild to severe colitis developed in 9/10 animals, compared with 1/10 animals reconstituted with IL-10+/+ CD4−CD8α+β−TCRαβ+ iIELs (Figs. 2 C, 3, and 4, and Table I). Importantly, this lack of protection was not related to an impaired iIEL reconstitution, as the number of donor-derived CD4−CD8α+β−TCRαβ+ iIELs rescued approximated the number of injected cells independent of their IL-10 genotype (Table I). Notwithstanding, the number of cells derived from donor TCRαβ+CD4+CD45RBhi T cells within the lamina propria of the large bowel was significantly higher in recipients of IL-10−/− CD4−CD8α+β− TCRαβ+ iIELs (Table I), and comparable to that observed in both unreconstituted animals and recipients of nonprotective, IL-10+/+ iIELs (Table I). Of note, reconstitution of SCID.B6 mice with CD4−CD8α+β−TCRαβ+ iIELs derived from IL-10−/− donors did not result in the development of colitis in the absence of subsequent injection of TCRαβ+CD4+CD45RBhi splenic T cells (data not shown).
The IL-10–dependent protection afforded by CD4− CD8α+β−TCRαβ+ iIELs is consistent with a previous report demonstrating that administration of exogenous IL-10 to recipients of CD4+CD45RBhiTCRαβ+ T cells prevents colitis (18). However, we have been unable to obtain evidence for the capacity of CD4−CD8α+β− TCRαβ+ iIELs to secrete IL-10. Specifically, we have assessed the secretion of IL-10 by FACS®-purified (>99%) CD4−CD8α+β−TCRαβ+ iIELs in vitro, without stimulation, as well as in response to a combination of plate bound anti-CD3 and anti-CD28 monoclonal antibodies. In both circumstances, CD4−CD8α+β−TCRαβ+ iIELs failed to secrete IL-10 (data not shown). Further, we did not detect surface expression of IL-10R on CD4−CD8α+β−TCRαβ+ iIELs flow cytometrically (data not shown). These results, which are consistent with those of a recent study showing very low levels of transcripts for both IL-10 and IL-10R in iIELs expressing TCRαβ (21), strongly suggest that the IL-10 dependency of protection conferred by this subset is indirect. Importantly, murine enterocytes which are in physical contact with iIELs, constitutively express IL-10R (22). It is not implausible that the differentiation of CD4− CD8α+β−TCRαβ+ iIELs into effector regulatory T cells depends on signals generated by surrounding enterocytes induced by IL-10.
Self-specific CD4−CD8α+β−TCRαβ+ iIELs Protect Against Colitis.
One of the paradoxes of intraintestinal T cell development is the presence of CD4−CD8α+β− TCRαβ+ iIELs expressing potentially self-specific TCR. In conventional mice this subset, while oligoclonal, contains high proportions of cells expressing Vβ specific for endogenous retroviral superantigens (4, 11, 23). Analysis of RAG-deficient animals transgenic for MHC class I–restricted TCR has demonstrated that CD4−CD8α+β− TCRαβ+ iIELs expressing the transgenic TCR develop in animals that express both the restricting MHC and the specific antigen, exclusively (7, 10, 12). Thus, 100% of the CD4− CD8α+β− iIEL subset in RAG−/− H-2Db male mice transgenic for a H-Y/H-2Db-specific TCRαβ, express high levels of the transgenic TCR. This enabled us to directly assess the protective capacity of self-specific CD4− CD8α+β− iIEL against intestinal inflammation.
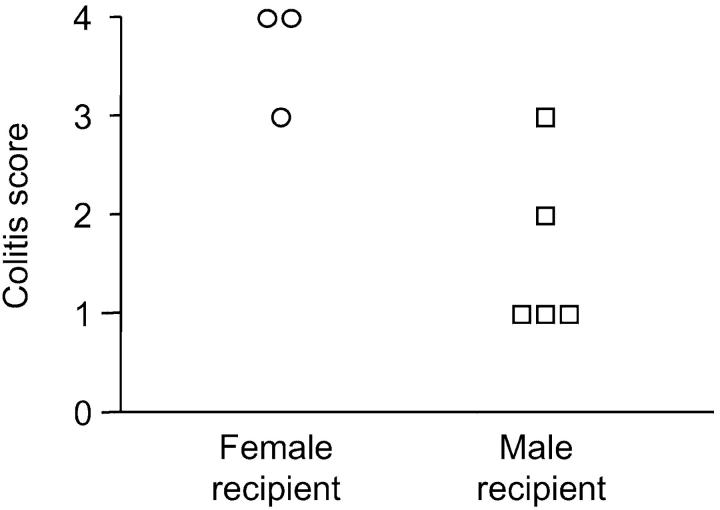
Toward this end, 5 × 106 CD4−CD8α+β− iIELs derived from RAG−/− H-2Db male mice transgenic for a H-Y/H-2Db-specific TCRαβ were used to reconstitute Ly 5.2 SCID.B6 male (n = 5) and female (n = 3) recipients. As illustrated in Fig. 5 , of the male recipients only one developed severe colitis within 150 d after the transfer of 0.5 × 106 splenic CD4+CD45RBhi T cells derived from male Ly 5.1 C57Bl/6 donors (Fig. 5). The female recipients, which provided a specificity control in this system, all developed severe colitis within the same time frame (Fig. 5).
Figure 5.
Self-specific CD4− CD8α+β−TCRαβ+ iIELs protect SCID.C57Bl/6 recipients of splenic CD4+CD45RBhiTCRαβ+ T cells from colitis. Pathology scores of individual male (□) and female (○) SCID.C57Bl/6 recipients of CD4−CD8α+β− iIELs expressing an HY/Db-specific transgenic TCRαβ, and splenic CD4+CD45RBhiTCRαβ+ T cells derived from male donors. Significant (P < 0.05) protection of male recipients was observed.
These results formally validate the capacity of self-specific T cells within the CD4−CD8α+β− iIEL subset to protect the intestinal mucosa against chronic inflammation. Thus, positive selection of self-specific CD4−CD8α+β− TCRαβ+ T cells, which is restricted to the intestinal epithelium, is advantageous to the integrity of the intestinal mucosa. Of note, the positive selection of self-specific T cells is not unique to the murine intestinal epithelium. Specifically, dendritic epidermal T cells are monoclonal, and protect the cutaneous epithelium in response to self antigen subsequent to injury (24).
It is tempting to speculate that CD4−CD8α+β−TCRαβ+ iIEL, present in other species, including humans (8) fulfill a similar role in the maintenance of intestinal integrity. The observation that exogenous IL-10 is inefficient in ameliorating symptoms of inflammatory bowel disease in humans (25), would suggest that, if protective, human CD4−CD8α+ β−TCRαβ+ iIELs, may not function in an IL-10–dependent manner. However, results obtained in murine models of colitis underscore the importance of the methodology used to assess the role of IL-10 in regulating IBD. Specifically, the first attempt to demonstrate its role, involved the administration of neutralizing antibodies, and was unsuccessful. In contrast, subsequent studies established that either systemic administration of IL-10, or the forced expression of IL-10 in CD4+CD45RBhi TCRαβ+ T cells, prevented colitis (18, 26). Hence, resolution of the apparent discrepancy regarding the role of IL-10 in humans may require the assessment of various regimens of IL-10 administration, minimally the assessment of outcome in circumstances in which it is certain that IL-10 reaches the target organ. Furthermore, murine models of inflammatory bowel diseases have not established the capacity of IL-10 to reverse the course of ongoing colitis, rather, its effectiveness in preventing the development of chronic inflammation. It is therefore inappropriate to compare the preventive role of IL-10 in murine colitis with its curative role in humans with established inflammatory bowel disease.
Figure 4.
Protection of SCID.C57Bl/6 recipients of splenic CD4+CD45RBhiTCRαβ+ T cells from colitis by CD4−CD8α+β− TCRαβ+ iIELs is IL-10 dependent. Reconstitution with CD4−CD8α+ β−TCRαβ+ iIEL (○) versus IL-10−/− CD4−CD8α+β−TCRαβ+ iIELs (□), P < 0.008; no reconstitution (▪) versus reconstitution with IL-10−/−CD4−CD8α+β−TCRαβ+ iIELs (□), not significant. Results taken from two of the 3–8 experiments performed.
Acknowledgments
We thank C. Cantin and Gisele Knowles for expert assistance with flow cytometry.
This work was supported by the Canadian Institutes of Health Research.
References
- 1.Powrie, F. 1995. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 3:171–174. [DOI] [PubMed] [Google Scholar]
- 2.Mosley, R.L., D. Styre, and J.R. Klein. 1990. Differentiation and functional maturation of bone marrow-derived intestinal epithelial T cells expressing membrane T cell receptor in athymic radiation chimeras. J. Immunol. 145:1369–1375. [PubMed] [Google Scholar]
- 3.Rocha, B., P. Vassalli, and D. Guy-Grand. 1994. Thymic and extrathymic origins of gut intraepithelial lymphocyte populations in mice. J. Exp. Med. 180:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poussier, P., P. Edouard, C. Lee, M. Binnie, and M. Julius. 1992. Thymus-independent development and negative selection of T cells expressing T cell receptor alpha/beta in the intestinal epithelium: evidence for distinct circulation patterns of gut- and thymus-derived T lymphocytes. J. Exp. Med. 176:187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandeira, A., S. Itohara, M. Bonneville, O. Burlen-Defranoux, T. Mota-Santos, A. Coutinho, and S. Tonegawa. 1991. Extrathymic origin of intestinal intraepithelial lymphocytes bearing T- cell antigen receptor gamma delta. Proc. Natl. Acad. Sci. USA. 88:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch, S., D. Kelleher, R. McManus, and C. O'Farrelly. 1995. RAG1 and RAG2 expression in human intestinal epithelium: evidence of extrathymic T cell differentiation. Eur. J. Immunol. 25:1143–1147. [DOI] [PubMed] [Google Scholar]
- 7.Poussier, P., H.S. Teh, and M. Julius. 1993. Thymus-independent positive and negative selection of T cells expressing a major histocompatibility complex class I–restricted transgenic T cell receptor alpha/beta in the intestinal epithelium. J. Exp. Med. 178:1947–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latthe, M., L. Terry, and T.T. MacDonald. 1994. High frequency of CD8αα homodimer-bearing T cells in human fetal intestine. Eur. J. Immunol. 24:1703–1705. [DOI] [PubMed] [Google Scholar]
- 9.Ohteki, T., and H.R. MacDonald. 1993. Expression of the CD28 costimulatory molecule on subsets of murine intestinal intraepithelial lymphocytes correlates with lineage and responsiveness. Eur. J. Immunol. 23:1251–1255. [DOI] [PubMed] [Google Scholar]
- 10.Cruz, D., B.C. Sydora, K. Hetzel, G. Yakoub, M. Kronenberg, and H. Cheroutre. 1998. An opposite pattern of selection of a single T cell antigen receptor in the thymus and among intraepithelial lymphocytes. J. Exp. Med. 188:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guy-Grand, D., N. Cerf-Bensussan, B. Malissen, M. Malassis-Seris, C. Briottet, and P. Vassalli. 1991. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J. Exp. Med. 173:471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocha, B., H. von Boehmer, and D. Guy-Grand. 1992. Selection of intraepithelial lymphocytes with CD8 alpha/alpha co- receptors by self-antigen in the murine gut. Proc. Natl. Acad. Sci. USA. 89:5336–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das, G., D.S. Gould, M.M. Augustine, G. Fragoso, E. Sciutto, I. Stroynowski, L. Van Kaer, D.J. Schust, H. Ploegh, C.A. Janeway, Jr., and E. Scitto. 2000. Qa-2-dependent selection of CD8alpha/alpha T cell receptor alpha/beta(+) cells in murine intestinal intraepithelial lymphocytes. J. Exp. Med. 192:1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poussier, P., T. Ning, J. Chen, D. Banerjee, and M. Julius. 2000. Intestinal inflammation observed in IL-2R/IL-2 mutant mice is associated with impaired intestinal T lymphopoiesis. Gastroenterology. 118:880–891. [DOI] [PubMed] [Google Scholar]
- 15.Claesson, M.H., A. Rudolphi, S. Kofoed, S.S. Poulsen, and J. Reimann. 1996. CD4+ T lymphocytes injected into severe combined immunodeficient (SCID) mice lead to an inflammatory and lethal bowel disease. Clin. Exp. Immunol. 104:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powrie, F., M.W. Leach, S. Mauze, L.B. Caddle, and R.L. Coffman. 1993. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 5:1461–1471. [DOI] [PubMed] [Google Scholar]
- 17.Asseman, C., S. Mauze, M.W. Leach, R.L. Coffman, and F. Powrie. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powrie, F., M.W. Leach, S. Mauze, S. Menon, L.B. Caddle, and R.L. Coffman. 1994. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1:553–562. [DOI] [PubMed] [Google Scholar]
- 19.Torres-Nagel, N., E. Kraus, M.H. Brown, G. Tiefenthaler, R. Mitnacht, A.F. Williams, and T. Hunig. 1992. Differential thymus dependence of rat CD8 isoform expression. Eur. J. Immunol. 22:2841–2848. [DOI] [PubMed] [Google Scholar]
- 20.Fujihashi, K., T. Dohi, M.N. Kweon, J.R. McGhee, T. Koga, M.D. Cooper, S. Tonegawa, and H. Kiyono. 1999. gammadelta T cells regulate mucosally induced tolerance in a dose-dependent fashion. Int. Immunol. 11:1907–1916. [DOI] [PubMed] [Google Scholar]
- 21.Shires, J., E. Theodoridis, and A.C. Hayday. 2001. Biological insights into TCRgammadelta+ and TCRalphabeta+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE). Immunity. 15:419–434. [DOI] [PubMed] [Google Scholar]
- 22.Denning, T.L., N.A. Campbell, F. Song, R.P. Garofalo, G.R. Klimpel, V.E. Reyes, and P.B. Ernst. 2000. Expression of IL-10 receptors on epithelial cells from the murine small and large intestine. Int. Immunol. 12:133–139. [DOI] [PubMed] [Google Scholar]
- 23.Rocha, B., P. Vassalli, and D. Guy-Grand. 1991. The V beta repertoire of mouse gut homodimeric alpha CD8+ intraepithelial T cell receptor alpha/beta + lymphocytes reveals a major extrathymic pathway of T cell differentiation. J. Exp. Med. 173:483–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boismenu, R., and W.L. Havran. 1998. Gammadelta T cells in host defense and epithelial cell biology. Clin. Immunol. Immunopathol. 86:121–133. [DOI] [PubMed] [Google Scholar]
- 25.Fiocchi, C. 1999. From immune activation to gut tissue injury: the pieces of the puzzle are coming together. Gastroenterology. 117:1238–1241. [DOI] [PubMed] [Google Scholar]
- 26.Hagenbaugh, A., S. Sharma, S.M. Dubinett, S.H. Wei, R. Aranda, H. Cheroutre, D.J. Fowell, S. Binder, B. Tsao, R.M. Locksley, et al. 1997. Altered immune responses in interleukin 10 transgenic mice. J. Exp. Med. 185:2101–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]