Abstract
Dendritic cells (DCs) play a crucial role in the immune responses against infections by sensing microbial invasion through toll-like receptors (TLRs). In humans, two distinct DC subsets, CD11c− plasmacytoid DCs (PDCs) and CD11c+ myeloid DCs (MDCs), have been identified and can respond to different TLR ligands, depending on the differential expression of cognate TLRs. In this study, we have examined the effect of TLR-7 ligands on human DC subsets. Both subsets expressed TLR-7 and could respond to TLR-7 ligands, which enhanced the survival of the subsets and upregulated the surface expression of costimulatory molecules such as CD40, CD80, and CD86. However, the cytokine induction pattern was distinct in that PDCs and MDCs produced interferon (IFN)-α and interleukin (IL)-12, respectively. In response to TLR-7 ligands, the Th1 cell supporting ability of both DC subsets was enhanced, depending on the cytokines the respective subsets produced. This study demonstrates that TLR-7 exerts its biological effect in a DC subset-specific manner.
Keywords: immunity, cytokines, imidazoquinolines, pathogen-associated molecular patterns, Th cell responses
Introduction
Dendritic cells (DCs) sense the presence of invading pathogens, engulf the pathogens, and degrade them intracellularly. The function of DCs is not primarily to destroy pathogens, but to prime naive T cells as professional APCs, thereby linking innate and adaptive immunity (1, 2). However, it remains unknown how DCs regulate the quality of T cell responses. Th cell development is mainly regulated by DC-derived cytokines, such as IL-12 or IFN-α/β (2, 3). Therefore, it is important to clarify how cytokine production is regulated in the DCs. Recent progress in DC biology has suggested that cytokine production depends either on DC subsets (lineage model) or on stimuli that DCs receive (instruction model) (4).
Toll-like receptors (TLRs) are type I transmembrane proteins that are expressed on APCs including macrophages and DCs, and respond to pathogen-associated molecular patterns (PAMPs) (5–9). After pathogen recognition, TLR signaling can activate APCs to induce inflammatory cyto-kines and upregulation of costimulatory molecules (7–14). Expression of TLR family members (TLR-1–10) was investigated on two human blood DC subsets (12–14), CD11c− plasmacytoid DCs (PDCs) and CD11c+ myeloid DCs (MDCs). Each subset expresses a different repertoire of TLRs. For example, MDCs and PDCs express TLR-4 and TLR-9, respectively (13, 14). In accordance with their TLR expression, they can respond to the respective TLR ligands, LPS, or CpG DNA (12–14). These studies suggest that cytokine production is determined by TLR expression on DCs.
It has recently been demonstrated that imidazoquinoline compounds, imiquimod, and its derivative, R-848, are specific ligands for TLR-7 (15). TLR-7 ligands, imidazoquinoline compounds, have potent antiviral and antitumor properties in animals (16–19). In fact, imiquimod has been clinically approved for the treatment of genital warts caused by human papillomavirus (20). In this study, we investigate the biological effects of TLR-7 ligands on human blood DC subsets, and show that (i) TLR-7 ligands are capable of affecting both PDCs and MDCs to enhance their survival and to upregulate costimulatory molecules, and that (ii) TLR-7 signaling selectively facilitates IFN-α production from PDCs and IL-12 production from MDCs, resulting in induction of Th1 balance. These results suggest that the cytokine induction pattern in response to TLR-7 ligands is determined not only by TLR-7 expression but also by cell lineage.
Materials and Methods
Media and Reagents.
RPMI 1640 supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 ng/ml streptomycin, and heat-inactivated 10% FCS (Irvine Scientific) was used for the cell culture throughout the experiments. Imiquimod (R-837) and R-848 were synthesized in Pharmaceuticals and Biotechnology Laboratory, Japan Energy Corporation (Saitama, Japan). CpG-oligodeoxynucleotides (ODNs), phosphorothioate form: 2006 (TCGTCGTTTTGTCGTTTTGTCGTT) and phosphodiester form: AAC-30 (ACCGATAACGTTGCCGGTGACGGCACCACG) were purchased from Hokkaido System Science. ODN 2006 and AAC-30 were used at concentrations of 10−6 M and 5 × 10−6 M, respectively. LPS (Salmonella typhimurium) (1 μg/ml) was purchased from Sigma-Aldrich. UV-irradiated Sendai virus (SV) (HVJ: Cantell strain, provided by Sumitomo Pharmaceuticals was used at 5 hemagglutinating U/ml. Recombinant human cytokines, GM-CSF (used at a concentration of 100 ng/ml) and IL-3 (at 10 ng/ml) were purchased from PeproTech EC.
Isolation of Blood DC Subsets.
Peripheral blood DC subsets (MDCs and PDCs) were isolated according to the modified protocol, as described previously (21, 22). Briefly, the DC-enriched population (CD4+/CD3−/CD14− cells) was obtained from PBMCs by negative and subsequent positive immunoselections. The CD11c+/lineage−/DR+ cells (MDCs) and CD11c−/lineage−/DR+ cells (PDCs) were sorted by an EPICS ALTRA® flow cytometer (Beckman Coulter) by using PE-labeled anti-CD11c (Leu-M5; Becton Dickinson), mixture of FITC-labeled mAbs against lineage markers, CD3 (M2AB; Exalpha), CD14 (FWKW-1; Exalpha), CD15 (Leu-M1; Becton Dickinson), CD16 (J5511; Exalpha), CD19 (SJ25C1; Becton Dickinson), and CD56 (NCAM16.2; Becton Dickinson), and allophycocyanin (APC)-labeled HLA-DR (Tü36; Becton Dickinson). Blood CD14+ CD16− monocytes were purified from PBMCs stained with PE-labeled anti-CD16 (J5511) and FITC-labeled CD14 (FWKW-1) by cell sorting. The purity of each group of cells was >98%.
Analyses of Cultured DCs.
The sorted blood DC subsets or PBMCs were cultured in 96-well, round-bottomed tissue culture plates at 3×104 cells in 200 μl of medium per well for 24 h. In the viability assay, viable cells were evaluated as annexin V-negative fractions using annexin V-FITC Apoptosis Detection kit (Genzyme) after cell debris was excluded by an appropriate forward scatter threshold. To analyze the expression of costimulatory molecules, the culture cells were stained with FITC-labeled anti-CD40 (5C3; BD PharMingen), CD86 (2331; BD PharMingen), or CD80 (BB-1; Ancell) and analyzed by a FACScan™ (Becton Dickinson). The production of cytokines in the culture supernatants was determined by ELISA 24 h later (kits for IL-12 p40+p70 and IFN-α were purchased from Endogen).
DC–T Cell Coculture.
CD4+/CD45RA+ naive T cells were obtained from allogeneic healthy volunteers using CD4+ T cell Isolation kit (GmbH; Miltenyi Biotec) followed by positive selection with CD45RA-conjugated microbeads (Miltenyi Biotec). The purity of the cells was 95% or greater by reanalysis. MDC and PDC preincubated with GM-CSF, IL-3, or R-848 (10−6 M) for 24 h were washed and then cocultured with allogeneic CD4+/CD45RA+ naive T cells (105 cells per well, DC–T ratio, 1:5) for 7 d in 96-well, round-bottomed tissue culture plates. In some experiments, neutralizing goat polyclonal anti–human IL-12 Ab (AB-219-NA: 20 μg/ml) (R&D Systems) or a mixture of neutralizing rabbit polyclonal anti–human IFN-α Ab (2,000 neutralization U/ml), neutralizing rabbit polyclonal anti–human IFN-β Ab (1,000 neutralization U/ml), and mouse anti–human IFN-α/β receptor mAb (20 μg/ml) (PBL Biomedical Laboratories) was added into the coculture.
Intracellular Cytokine Staining.
Intracellular cytokine staining in MDCs and PDCs was performed after 5 h R-848 (10−6 M) stimulation. 10 μg/ml brefeldin A (Sigma-Aldrich) was added during the last 1 h. After the stimulation, MDCs and PDCs were stained with Cy-Chrome-labeled CD40 (5C3; BD PharMingen), and then fixed, permeabilized (FIX and PERM kit; Caltag Laboratories), and stained with FITC-labeled anti–IL-12 p40+p70 mAb (B-P24; DIACLONE Research) or unconjugated mouse anti–human IFN-α mAb (MC-16; Genzyme). FITC-labeled goat anti–mouse IgG F(ab′)2 (Becton Dickinson) was used as the secondary antibody for anti–IFN-α mAb after repermeabilization. For detection of intracellular T cell cytokines, T cells after the 7 d coculture were restimulated with PMA (50 ng/ml) and ionomycin (2 μg/ml) for 6 h, and brefeldin A (10 μg/ml) was added during the last 2 h (all from Sigma-Aldrich). The cells were stained with phycoerythrin-cyanin 5.1 (PC5)-labeled anti-CD4 (ImmunoTech), and then with PE-labeled anti–IL-4 (Becton Dickinson) plus FITC-labeled anti–IFN-γ mAb (Becton Dickinson) using FIX and PERM kit.
RT-PCR for TLRs.
Total RNA was prepared from sorted MDCs, PDCs, and CD14+CD16− blood monocytes using TRIsol reagent (GIBCO BRL). RT reactions were performed on 2 μg of total RNA using an oligo dT primer with SuperScript II (GIBCO BRL). 1 μl aliquots of the DNA resulting from each RT reaction were then subjected to PCR. The temperature profiles of PCR were as follows: an initial denaturation step of 94°C for 5 min, followed by 35 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 1 min, and a final elongation step of 72°C for 7 min. For detection of TLR mRNA, a primer set each of TLR4 and TLR9 and three primer sets of TLR-7, as described previously (12–14), were used. The sequences of primers were as follows: TLR-4, forward: 5′-TTTCTGCAATGGATCAAGGACCA-3′, reverse: 5′-GGACACCACAACAATCACCTTTC-3′; TLR-9, forward: 5′-TTATGGACTTCCTGCTGGAGGTGC-3′, reverse: 5′-CTGCGTTTTGTCGAAGACCA-3′; the first set of TLR-7 (13), forward: 5′-TCTACCTGGGCCAAAACTGTT-3′, reverse: 5′-GGCACATGCTGAAGAGAGTTA-3′; the second set of TLR-7 (12), forward: 5′-AGTGTCTAAAGAACCTGG-3′, reverse: 5′-CTTGGCCTTACAGAAATG-3′; the third set of TLR-7 (14), forward: 5′-CGAACCTCACCCTCACCATTA-3′, reverse: 5′-GGGACGGCTGTGACATTGTTA-3′; and β-actin, forward: 5′-GACCCAGATCATGTTTGAGACCTTCAACAC-3′, reverse: 5′-TACTTGCGCTCAGGAGGAGCAATGATCTTG-3′. A ΦX174 DNA-HaeIII Digest (New England BioLabs) was used as a DNA size marker.
Results
Expression of TLR-7 in DCs.
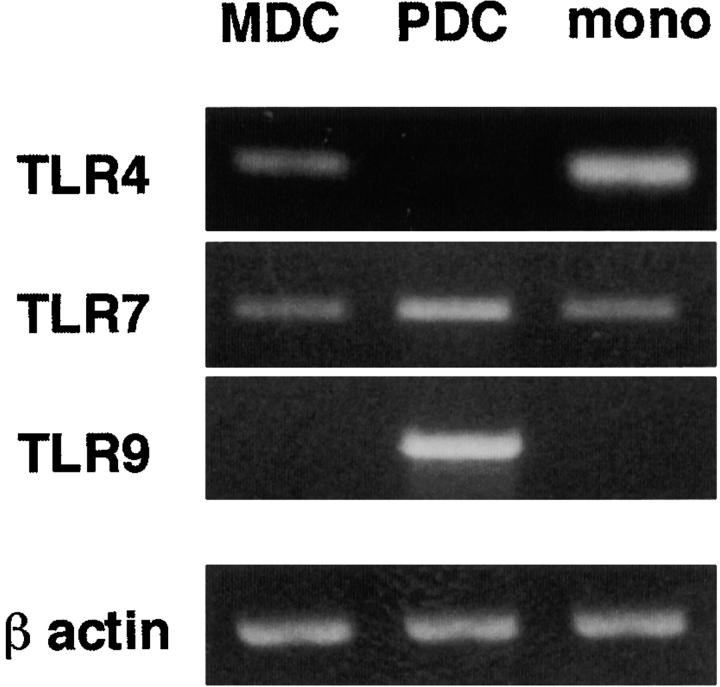
First, we analyzed the expression of TLR-7 in MDCs and PDCs using the RT-PCR method (Fig. 1) . Krug et al. (13), but not Jorossay et al.(14), observed TLR-7 expression in MDCs. Kadowaki et al. detected weak, but significant expression of TLR-7 in MDCs through real-time PCR (12). Taking this discrepancy into consideration, we investigated TLR-7 expression with the three different sets of PCR primers employed in these studies. TLR-7 mRNA was shown to be unequivocally expressed in both MDCs and PDCs (Fig. 1 and data not shown). On the other hand, MDCs and PDCs reciprocally expressed TLR-4 and TLR-9; MDCs expressed TLR-4 but not TLR-9, while PDCs expressed TLR-9 but not TLR-4.
Figure 1.
mRNA expression of TLRs in MDCs and PDCs. mRNA expression of TLR-4, -7, and -9 was examined in purified blood MDCs, PDCs, and CD14+CD16− monocytes by RT-PCR. For the detection of TLR-7 mRNA, three sets of primers were used, and similar results were obtained when using each set. The results using the first set of TLR-7 primers (as described in Materials and Methods) are shown in the figure. The data shown are representative of two independent experiments.
Effects of TLR-7 Ligands on Survival and Expression of Costimulatory Molecules on DCs.
We next examined the biological effects of TLR-7 ligands, imiquimod and its derivative, R-848 (15), on MDCs and PDCs. Isolated MDCs and PDCs were incubated with various agents for 24 h and were then analyzed for cell viability and the expression of costimulatory molecules. As shown in Fig. 2 , both DC subsets, especially PDCs, mostly died within 24 h in the absence of stimuli. GM-CSF prevented MDCs from dying, while IL-3 or CpG-ODNs (phosphorothioate form [2006] and phosphodiester form [AAC30]) efficiently blocked the cell death of PDCs, a result that was consistent with the previous studies (11, 13, 22). TLR-7 ligands were shown to enhance the survival of both MDCs and PDCs. The survival-promoting effect of R-848 was ∼100-fold more potent than that of imiquimod, which is consistent with their effects on murine cells (15).
Figure 2.
TLR-7 ligands enhance the survival of both MDCs and PDCs. MDCs and PDCs were incubated for 24 h with various stimuli. The viable cells in each DC subset were evaluated as annexin V-negative fractions by flow cytometry. The results are representative of three independent experiments.
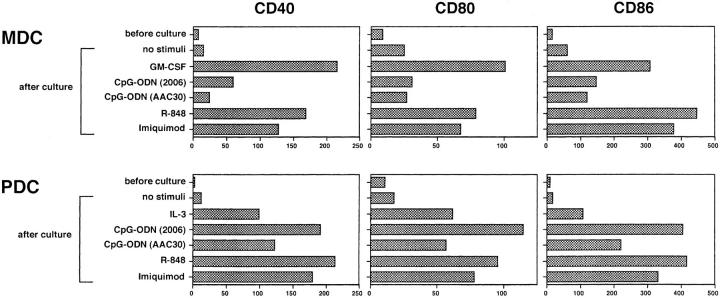
Furthermore, TLR-7 ligands could upregulate the surface expression of the costimulatory molecules, CD40, CD80 and CD86 on both MDCs and PDCs (Fig. 3) . Their expression levels were comparable between the two DC subsets. As previously demonstrated, CpG-ODNs (2006 or AAC30) could enhance surface expression of these costimulatory molecules on PDCs but not on MDCs (11, 13). The effects of TLR-7 ligands on PDCs were equivalent to those of CpG-ODN (2006). Collectively, these results suggest that TLR-7 signaling can enhance survival rates and costimulatory molecule expression on PDCs and MDCs at comparable levels.
Figure 3.
TLR-7 ligands upregulate the expression of costimulatory molecules on both MDCs and PDCs. After 24-h culture with various stimuli, the expression levels of CD40, CD80, and CD86 on each DC subset were analyzed by flow cytometry. Imiquimod and R-848 were used at concentrations of 10−5 M and 10−7 M, respectively. The results are shown as ΔMFI, which is calculated by subtraction of MFI with the isotype-matched control from that with each mAb. The results shown here are representative of three independent experiments.
Effects of TLR-7 Ligands on Cytokine Production of DCs.
Serum levels of IL-12 and IFN-α were elevated when mice were intraperitoneally injected with the TLR-7 ligands (15). Therefore, we next examined the effect of TLR-7 ligands on cytokine production from DCs, especially focusing on IL-12 and IFN-α. LPS and Sendai virus (SV) differentially activated MDCs and PDCs to produce large amounts of IL-12 p40+p70 (total IL-12) (Fig. 4 A) and IFN-α (Fig. 4 B), respectively.
Figure 4.
TLR-7 ligands induce the production of IL-12 and IFN-α from MDCs and PDCs, respectively. (A and B) After 24-h culture with various stimuli, concentration of IL-12 p40+p70 (A) and IFN-α (B) in the culture supernatants of MDCs, PDCs, and PBMCs were measured by ELISA. The data are shown by means ± SEM of five independent experiments. (C) After 5-h culture with R-848 (10−6 M), intracellular staining of IL-12 and IFN-α in MDCs and PDCs, together with the staining of surface expression of CD40, was performed. Percentages of the respective cytokine producing DCs are indicated. This figure represents the results from one of three experiments.
Both imiquimod and R-848 definitely induced total IL-12 production from MDCs in a dose–dependent manner (Fig. 4 A). However, these compounds as well as the other stimuli tested were much less effective or had no effect on IL-12 production from PDCs. By contrast, TLR-7 ligands were demonstrated to be potent stimulators of IFN-α production of PDCs (Fig. 4 B). The effects were manifested in a dose–dependent manner and were even more potent than those of the CpG-ODNs tested. However, the TLR-7 ligands had no capacity to induce IFN-α production from MDCs. Titration analysis again demonstrated that the effect of the imidazoquinolines on cytokine induction (IL-12 from MDCs and IFN-α from PDCs) was ∼100-fold stronger in R-848 than in imiquimod.
Intracellular cytokine staining also showed that R-848 induced the synthesis of IL-12 and IFN-α in MDCs and PDCs, respectively (Fig. 4 C). Both IL-12–producing MDCs and IFN-α–producing PDCs exclusively expressed CD40, indicating that DCs maturated by TLR-7 ligands produce cytokines.
Effects of TLR-7 Ligands on DC-mediated Th Polarization.
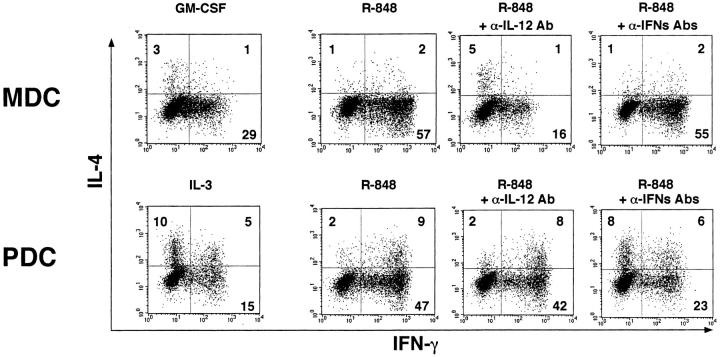
To elucidate how TLR-7 ligands affect the DC-mediated T cell responses, MDCs and PDCs were preincubated with R-848 for 24 h, and then cocultured with allogeneic naive CD4+ T cells. As shown in Fig. 5 , GM-CSF–treated MDCs preferentially induced Th1 development, while IL-3–treated PDCs facilitated Th2 skewing, in agreement with the previous reports (22–24). TLR-7 ligand-stimulated MDCs more strongly induced Th1 development than GM-CSF–treated MDCs, and this effect was markedly blocked by the addition of anti–IL-12 Ab (IL-12α Ab), but not by the addition of a mixture of anti–IFN-α Ab, anti–IFN-β Ab, and anti–IFN-α/β R Ab (IFN-αs Abs). TLR-7 ligand-stimulated PDCs also developed IFN-γ–producing cells. This Th1 induction was substantially inhibited by the addition of IFN-αs Abs, but not by the addition of IL-12α Ab. Thus, both PDCs and MDCs can augment their Th1 cell supporting ability in response to TLR-7 ligands through distinct cytokines.
Figure 5.
MDCs and PDCs stimulated by TLR-7 ligands induce Th1 development depending on each DC-derived cytokine. MDCs and PDCs were preincubated with the cytokines or R-848 (10−6 M) for 24 h, washed, and subsequently cocultured with allogeneic CD4+ naive T cells for 7 d in the presence or absence with neutralizing anti-IL-12 Ab (IL-12α Ab) or a mixture of neutralizing anti–IFN-α Ab, neutralizing anti–IFN-β Ab, and anti-IFN-α/β R mAb (IFN-αs Abs). Intracellular cytokine profiles in the T cells were analyzed by flow cytometry. Percentages of the respective cytokine-producing T cells are indicated. This figure represents the results from one of three experiments.
Discussion
It remains a key issue whether DC response is determined by cell lineage or by stimuli. TLRs on DCs are major receptors for various pathogenic stimuli. Therefore, it is quite important to evaluate how DCs are activated by TLR signaling. Of the TLRs, TLR-2, -4, and -9 are expressed in either MDCs or PDCs (12–14). In accordance with the expression of TLR-2, -4, and -9, each DC subset differentially responds to respective TLR ligands. MDCs can secrete IL-12 through TLR-2 or TLR-4 signaling (12–14), whereas PDCs can produce IFN-α/β through TLR-9 signaling (11–14, 25, 26). Thus, human DCs can produce subset-specific cytokines in response to PAMPs. These findings favor the idea that the DC lineage is critical for establishing DC response. However, TLRs for these PAMPs are differentially expressed in the DC subsets (12–14, and Fig. 1). Therefore, it cannot formally be concluded whether the microbial response is determined by TLR expression or DC lineage.
Unlike TLR-4 and -9, TLR-7 was constitutively expressed on both PDCs and MDCs (Fig. 1). In this context, TLR-7 ligands should be useful tools to clarify how the same pattern recognition stimuli lead to activation of distinct DC subsets. Consistent with the TLR-7 expression, the ligands, imiquimod and R-848, enhanced the survival of both PDCs and MDCs (Fig. 2) and induced their phenotypical maturation (Fig. 3). In contrast, TLR-7 ligands showed differential cytokine-inducing effects on different DC subsets; IFN-α from PDCs (Fig. 4 B) versus IL-12 from MDCs (Fig. 4 A). These results indicate that the TLR-7–induced cytokine induction pattern is determined not only by TLR expression but also by DC lineage. In other words, DC lineage as well as instructive stimuli are decisive factors affecting DC response, at least in the TLR-7 system.
At present, it remains unknown why TLR-7 exerts differential outcomes in distinct DC subsets. In response to TLR-4 ligand, cytokine induction depends on MyD88, whereas DC maturation can proceed without MyD88, indicating that MyD88 is a bifurcation point in TLR-4 signaling (27). However, all effects of TLR-7 signaling depend on MyD88 (15). Therefore, it is likely there is another bifurcation point leading to the expression of IFN-α or IL-12.
In determining Th responses, the DC system shows not only lineage heterogeneity, but also functional plasticity depending on the signals or environmental instruction (2, 4, 22–24, 28). In the microbial environment, MDCs acquire Th1-inducing activity in response to TLR-2 or TLR-4 ligands (12–14). Meanwhile, PDCs can also support Th1 cell development in response to a TLR-9 ligand, CpG DNA (25, 26). These immunogenic Th1 responses are mediated by IL-12 and IFN-α/β from MDCs and PDCs, respectively (22, 29). In line with this paradigm of Th1 responses, this study shows that TLR-7 signaling affects both DC subsets so that they support the differentiation of naive Th cells also toward Th1 cells, depending on the cytokines each DC subset secretes (Fig. 5). Thus, DC subsets are programmed to make different innate responses in terms of cytokine production, but can both eventually cause the same Th1 induction.
In conclusion, TLR-7 ligands, imiquimod and its derivative, had discernible immunostimulatory effects on both human PDCs and MDCs. In addition, our results uncovered a possible new member of TLRs that has the potential to evoke IFN-α production from PDCs. Remarkably, the TLR-7–dependent cytokine production pattern is determined by the lineage commitment of the DC subsets.
Acknowledgments
This work was supported by a grant from Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
References
- 1.Mellman, I., and R.M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell. 106:255–258. [DOI] [PubMed] [Google Scholar]
- 2.Liu, Y.-J. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 106:259–262. [DOI] [PubMed] [Google Scholar]
- 3.Lanzavecchia, A., and F. Sallusto. 2001. Regulation of T cell immunity by dendritic cells. Cell. 106:263–266. [DOI] [PubMed] [Google Scholar]
- 4.Liu, Y.-J., H. Kanzler, V. Soumelis, and M. Gilliet. 2001. Dendritic cell lineage, plasticity and cross-regulation. Nat. Immunol. 2:585–589. [DOI] [PubMed] [Google Scholar]
- 5.Medzhihtov, R., and C. Janeway, Jr. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 91:295–298. [DOI] [PubMed] [Google Scholar]
- 6.Medzhihtov, R., and C. Janeway, Jr. 2000. Innate immune recognition: mechanism and pathways. Immunol. Rev. 173:89–97. [DOI] [PubMed] [Google Scholar]
- 7.Aderem, A., and R.J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature. 406:782–787. [DOI] [PubMed] [Google Scholar]
- 8.Akira, S., K. Takada, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680. [DOI] [PubMed] [Google Scholar]
- 9.Kaisho, T., and S. Akira. 2001. Dendritic-cell function in toll-like receptor- and MyD88-knockout mice. Trends Immunol. 22:78–83. [DOI] [PubMed] [Google Scholar]
- 10.Thoma-Uszynski, S., S.M. Kiertscher, M.T. Ochoa, D.A. Bouis, M.V. Norgard, K. Miyake, P.J. Godowski, M.D. Roth, and R.L. Modlin. 2000. Activation of toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J. Immunol. 165:3804–3810. [DOI] [PubMed] [Google Scholar]
- 11.Kadowaki, N., S. Antonenko, and Y.-J. Liu. 2001. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c− type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN. J. Immunol. 166:2291–2295. [DOI] [PubMed] [Google Scholar]
- 12.Kadowaki, N., S. Ho, S. Antonenko, R.W. Malefyt, R.A. Kastelein, F. Bazan, and Y.-J. Liu. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krug, A., A. Towarowski, S. Britsch, S. Rothenfusser, V. Hornung, R. Bals, T. Giese, H. Engelmann, S. Endres, A.M. Krieg, et al. 2001. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 31:3026–3037. [DOI] [PubMed] [Google Scholar]
- 14.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388–3393. [DOI] [PubMed] [Google Scholar]
- 15.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via TLR7-MyD88-dependent signaling pathway. Nat. Immunol. 3:196–200. [DOI] [PubMed] [Google Scholar]
- 16.Miller, R.L., J.F. Gerster, M.L. Owens, H.B. Slade, and M.A. Tomai. 1999. Imiquimod applied topically: a novel immune response modifier and new class of drug. Int. J. Immunopharmacol. 21:1–14. [DOI] [PubMed] [Google Scholar]
- 17.Harrison, C.J., R.L. Miller, and D.I. Bernstein. 1994. Posttherapy suppression of genital herpes simplex virus (HSV) recurrences and enhancement of HSV-specific T-cell memory by imiquimod in guinea pigs. Antimicrob. Agents Chemother. 38:2059–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen, M., B.P. Griffith, H.L. Lucia, and G.D. Hsiung. 1988. Efficacy of S26308 against guinea pig cytomegalovirus infection. Antimicrob. Agents Chemother. 32:678–683. [DOI] [PMC free article] [PubMed]
- 19.Bernstein, D.I., C.J. Harrison, M.A. Tomai, and R.L. Miller. 2001. Daily or weekly therapy with resiquimod (R-848) reduces genital recurrences in herpes simplex virus-infected guinea pigs during and after treatment. J. Infect. Dis. 183:844–849. [DOI] [PubMed] [Google Scholar]
- 20.von Krogh, G., C.J. Lacey, G. Gross, R. Barrasso, and A. Schneider. 2000. European course on HPV associated pathology: guideline for primary case physicians for the diagnosis and management of anogenital warts. Sex. Transm. Infect. 76:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito, T., M. Inaba, K. Inaba, J. Toki, S. Sogo, T. Iguchi, Y. Adachi, K. Yamaguchi, R. Amakawa, J. Valladeau, et al. 1999. A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J. Immunol. 163:1409–1419. [PubMed] [Google Scholar]
- 22.Ito, T., R. Amakawa, M. Inaba, S. Ikehara, K. Inaba, and S. Fukuhara. 2001. Differential regulation of human blood dendritic cell subsets by IFNs. J. Immunol. 166:2961–2969. [DOI] [PubMed] [Google Scholar]
- 23.Rissoan, M.-C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, R.W. Malefyt, and Y.-J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 283:1183–1186. [DOI] [PubMed] [Google Scholar]
- 24.Kadowaki, N., S. Antonenko, J.Y.-N. Lau, and Y.-J. Liu. 2000. Natural interferon α/β-producing cells link innate and adaptive immunity. J. Exp. Med. 192:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krug, A., S. Rothenfusser, V. Hornung, B. Jahrsdörfer, S. Blackwell, Z.K. Ballas, S. Endres, A.M. Krieg, and G. Hartmann. 2001. Identification of CpG oligonucleotide sequences with high induction of IFN-α/β in plasmacytoid dendritic cells. Eur. J. Immunol. 31:2154–2163. [DOI] [PubMed] [Google Scholar]
- 26.Bauer, M., V. Redecke, J.W. Ellwart, B. Scherer, J.-P. Kremer, H. Wagner, and G.B. Lipford. 2001. Bacterial CpG-DNA triggers activation and maturation of human CD11c−, CD123+ dendritic cells. J. Immunol. 166:5000–5007. [DOI] [PubMed] [Google Scholar]
- 27.Kaisho, T., O. Takeuchi, T. Kawai, K. Hoshino, and S. Akira. 2001. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol. 166:5688–5694. [DOI] [PubMed] [Google Scholar]
- 28.Vieira, P.L., E.C. de Jong, E.A. Wierenga, M.L. Kapsenberg, and P. Kaliñski. 2000. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J. Immunol. 164:4507–4512. [DOI] [PubMed] [Google Scholar]
- 29.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1:305–310. [DOI] [PubMed] [Google Scholar]