Abstract
CD8+ T cell tolerance to self-proteins prevents autoimmunity but represents an obstacle to generating T cell responses to tumor-associated antigens. We have made a T cell receptor (TCR) transgenic mouse specific for a tumor antigen and crossed TCR-TG mice to transgenic mice expressing the tumor antigen in hepatocytes (gag-TG). TCRxgag mice showed no signs of autoimmunity despite persistence of high avidity transgenic CD8+ T cells in the periphery. Peripheral CD8+ T cells expressed phenotypic markers consistent with antigen encounter in vivo and had upregulated the antiapoptotic molecule Bcl-2. TCRxgag cells failed to proliferate in response to antigen but demonstrated cytolytic activity and the ability to produce interferon γ. This split tolerance was accompanied by inhibition of Ca2+ flux, ERK1/2, and Jun kinasephosphorylation, and a block in both interleukin 2 production and response to exogenous interleukin 2. The data suggest that proliferation and expression of specific effector functions characteristic of reactive cells are not necessarily linked in CD8+ T cell tolerance.
Keywords: CTL, hepatocyte, tumor immunology, autoimmunity, signaling
Introduction
The immune system has evolved to maximize the ability of a host to respond to foreign antigens while preventing reactivity to self-proteins. Negative selection during T cell development in the thymus is not capable of deleting the entire self-reactive repertoire, and potentially self-reactive T cells can still be found in the periphery. As such T cells represent a risk for autoimmune injury, multiple mechanisms operate in the periphery to prevent effective triggering by self-antigen. By contrast, T cell–based immunotherapy of cancer is often dependent on activation of these incompletely deleted self-reactive T cells. Most of the described human tumor antigens are nonmutated proteins also expressed in normal tissues, and targeting such proteins typically overexpressed in tumors provides a platform for treating more than the rare subsets of tumors expressing true tumor-specific antigens such as mutated oncogenes or viral antigens in virally associated malignancies. Thus, tumor immunity in many respects can be viewed as the flip-side of autoimmunity, but in which the T cells reactive with self-proteins mediate a beneficial antitumor effect rather than induce disease. The development of effective T cell therapies for cancer as well as improved methods to prevent and treat autoimmune diseases will require better understanding of how self-reactive T cells are maintained in a tolerant state in the periphery and can be rescued.
Insights into both central (thymic) and peripheral T cell tolerance have been obtained with the use of TCR transgenic mouse strains, which can provide large numbers of T cells with specificity for a self-antigen. Studies in TCR transgenic mice have demonstrated that a large proportion of T cells reactive with self-antigens expressed in the thymus are deleted, although some T cells escape and emigrate to the periphery, in part by downregulation of receptor molecules (1, 2). For antigen expressed in tissues other than the thymus, reactive T cells may be deleted, or rendered nonresponsive in the periphery (3–5). Peripheral anergy can result from downregulation of the TCR (6) or accessory molecules such as CD8 (1), or from molecular disruption of signaling pathways (7, 8). A more passive tolerance in the form of ignorance has also been described previously, in which naive T cells potentially reactive to a self-antigen are not triggered by encounter with the normal tissue, but can be activated to effector cells that do recognize the normal tissue if stimulated by antigen in a more immunogenic context (9).
Signaling and effector functions have been compared in tolerant and nontolerant peripheral CD4+ T cells and, to a more limited extent, CD8+ T cells. Anergic CD4+ or CD8+ T cells produce none or only low levels of IL-2 compared with functional cells (10, 11, for a review, see reference 12). Anergic CD4+ T cells also exhibit a block in both ERK and Jun kinase (JNK)* activation (8, 13), and show a reduced Ca2+ flux after antigen stimulation after induction of anergy with either staphylococcal enterotoxin B (14) or systemic injection of antigenic peptide (15). The signaling defects in tolerant CD8+ T cells are less defined, although Ca2+ flux in tolerant CD8+ cells appears to be diminished (10, 16).
We have previously reported on the generation and initial characterization of a transgenic mouse strain expressing FMuLVgag, the protein targeted by the dominant CTL response to the FMuLV-transformed FBL tumor (17). The strain was developed as a model for a potentially immunogenic tumor antigen expressed in normal tissue. The transgene is under control of the albumin promoter and expressed, as demonstrated by RT-PCR, primarily in hepatic tissue, with much lower levels in kidney and spleen, and marginally detectable levels of mRNA in thymus. The mice are tolerant to FMuLVgag with no detectable CD8+ response after multiple in vivo immunizations, but functional CD8+ T cells can be recovered from immunized gag-TG mice after four weekly cycles of stimulation in vitro with irradiated FBL and supplemental IL-2. These rescued CTL, which had obviously escaped deletion, exhibited similar avidity for FMuLVgag as CTL derived from normal B6 mice. Primed FMuLVgag-specific CTL derived from normal B6 mice retained function after transfer into these transgenic mice and were capable of eradicating FBL tumor in the transgenic host. However, these CTL caused no autoimmune injury, despite sufficient expression of FMuLVgag to tolerize the endogenous FBL-specific CTL repertoire in vivo. These results, which suggest that strategies to rescue such tolerant T cells could yield cells effective and safe in tumor therapy, prompted the current study.
To study the defects in FMuLVgag tolerant T cells, we have now generated a TCR transgenic mouse strain with a receptor from a FMuLVgag-specific CD8+ CTL clone (TCR-TG). Crossing these mice with the gag-TG strain yields mice with a large population of T cells tolerant to a potential tumor antigen. Our results demonstrate that peripheral tolerance is largely maintained by a block in the ability of these self-reactive CTL to expand in response to antigen stimulation, but does not interfere with induction of effector functions such as cytolytic activity and expression of some effector cytokines.
Materials and Methods
Identification and Cloning of Full-Length TCR-α and -β Genes Specific for FBL and FMuLVgag, and Generation of Transgenic Mice.
CD8+ cytotoxic clones were generated by limiting dilution of spleen cells from a B6 mouse primed in vivo with 107 irradiated FBL tumor cells. The TCR-α and -β genes of a selected clone specific for the CCLCLTVFL-epitope (18) were cloned as follows: to determine TCR-α and -β gene usage, total RNA was isolated using the signal transducer and activator of transcription 60 reagent (TEL-TEST “B” Inc.), and cDNA was synthesized from 5 μg of total RNA using oligo(dT) primers and the Superscript system (GIBCO BRL). To allow amplification and identification of TCR sequences in the absence of 5′ sequence information, linker ligation, and subsequent PCR was performed as described in reference 19. PCR amplification products were subcloned and sequenced to determine TCR-α and -β chain usage. Primers that spanned the corresponding full-length TCR-α and -β sequences were then generated and used in PCR reactions to amplify full-length TCR-α and -β chains. Full-length chains were cloned, sequenced, and subcloned using standard molecular methodologies into the hCD2 transgenic expression vector that directs transgene expression exclusively in T cells under control of the human CD2 promoter and LCR (20, 21). For generation of transgenic mice, the FMuLVgag-specific TCR-α and -β constructs were digested with KpnI and NotI to release the human CD2 promoter/LCR/TCR-α or -β genes. The restriction digests were purified, quantified, combined, and injected into B6 embryos using standard techniques. After initial PCR-screening on tail DNA, potential founder mice were screened by flow cytometry for expression of the transgenes, and a founder mouse with both genes integrated on the same chromosome was selected and bred in our facility.
The generation and initial characterization of the gag-TG mice have been described previously (17). C57BL/6 (B6), B6 Thy1.1 congenic and Fas negative (lpr) mice were obtained from The Jackson Laboratory. All mice were maintained under SPF conditions in our animal facility, and our animal protocol was reviewed and approved by the Department of Comparative Medicine's Animal Care Committee.
Thymectomy.
5-wk-old mice were anaesthetized with a mixture of Ketamine (0.13 mg/g body weight) and Xylazine (0.01 mg/g body weight). Thymi were removed by vacuum suction through a 4–5-mm midline sternum incision (22).
Cell Lines, Media, Antibodies, and Peptides.
FBL-3, a Friend virus-induced erythroleukemia of B6 (H-2b) origin, expresses FMuLV-env- and -gag-encoded products and MHC class I molecules, but does not express MHC class II molecules (22). E10 is a FMuLV negative subline of the B6 EL-4 thymoma. The mapping and the sequence (CCLCLTVFL) of the dominant H-2Db–restricted FMuLVgag epitope in FBL in B6 mice has been described previously (18), and the peptide was synthesized at the University of Washington peptide facility. Unless otherwise stated, all cell culture was performed in RPMI 1640 supplemented with antibiotics, 2-mercapthoethanol, and 10% FCS. Fluorochrome-conjugated and biotinylated antibodies against CD4, CD8 Vα3, Vβ12, Thy1.1, Thy1.2, CD44, TNF-α, IFN-γ, Bcl-2, and Ly6C were purchased from BD PharMingen, and streptavidin TexasRed from Caltag Laboratories.
Analysis of Autoimmune Injury.
Livers from groups of 4–6-wk-old TCR-TG and TCRxgag mice were fixed in formaldehyde, paraffin embedded, sectioned, and stained with H&E. Coded specimens were analyzed for inflammation and lymphocyte infiltration by a reference pathologist (Phoenix Central Laboratories).
Stimulation of Transgenic T Cells, Cytoxicity, and Proliferation Assays.
For testing cytotoxic potential, 1.5 × 107 splenocytes from TCR-TG or TCRxgag mice were stimulated with 5 × 106 irradiated (10,000R) FBL cells in T25 tissue culture flasks supplemented by 5 U/ml IL-2. After 3 d, the cultures were depleted of CD4+ cells using Dynal magnetic beads (#114.05, Dynal Inc.) according to the manufacturer's protocol, and tested in standard 4 h 51[Cr]-release assays against labeled FBL, E10, or ConA blasts alone, or E10 or ConA blasts pulsed with FMuLVgag peptide at the indicated concentrations. ConA blast targets were generated by first lysing erythrocytes from B6 and lpr spleen cells by osmotic shock, and then incubating 5 × 106 cells in a 24-well plate for 48 h in the presence of 20 μg/ml concanavalin A (model no. C0412; Sigma-Aldrich) before labeling with 51[Cr]. CTL lines were generated in vitro by repetitive stimulation of 2 × 106 cultured cells with 2 × 106 irradiated FBL in T25 flasks containing 5 × 106 irradiated (2,000R) B6 splenocytes as feeders and 20 U/ml IL-2 in a total volume of 10 ml. Lines were tested for cytolytic activity 5 d after an in vitro stimulation.
For proliferative assays, spleen cells were compensated to equilibrate for the number and percentage of potential responders present. Splenocytes from TCR-TG mice contained ∼2.5 to 4.5 times more CD8+ cells compared with splenocytes from TCRxgag mice, as well as ∼2.5 to 4.5 times more TCR-αβhicells in the CD8+ population (see Fig. 1) . Therefore, spleen cells were depleted of CD4 cells, and 106 cells from TCRxgag spleens or 105 cells from TCR mice together with 9 × 105 B6 splenocytes were added to individual wells (a 10-fold compensation) and stimulated with 2 × 104 irradiated FBL for 3 d followed by an 8 h pulse with 3[H]-thymidine.
Figure 1.

FACS® analysis of thymic and splenic T cells from B6, TCR-TG, and TCRxgag. (A) Thymocytes were stained with CD4, CD8, Vα3, and Vβ12 and analyzed for CD4/CD8 expression (top) or Vα3/Vβ12 expression (bottom). Vα3/Vβ12 plots are gated on CD4−/CD8+ thymocytes. (B) Splenocytes were stained with CD4 and CD8 (top) or Vα3; Vβ12 and CD8 (bottom). Vα3/Vβ12 plots are gated on CD8+ splenocytes. (C) For analysis of memory/activation phenotype of splenocytes, cells were stained with CD8 and either Ly-6C (top) or CD44 (bottom). All histograms are gated on CD8+ splenocytes.
Cytokine ELISA.
105 CD4-depleted splenocytes from TCR-TG plus 9 × 105 B6 splenocytes or 106 CD4-depleted TCRxgag were stimulated with 2 × 104 irradiated FBL in a total volume of 200 μl in a 96-well round bottomed plate. After 72 h, 50 μl of culture supernatant was harvested and murine IL-2 measured by ELISA according to the manufacturer's protocols (R&D Systems).
Intracellular Staining for Cytokine and Bcl-2 Expression.
107 splenocytes from TCR-TG and TCRxgag mice were stimulated in a 12-well plate with 3 × 106 irradiated FBL for 48 h. Golgi-Plug (BD PharMingen) was added and 6 h later the cells were washed, permeabilized using Cytofix/Cytoperm (BD PharMingen), and stained for intracellular proteins according to the manufacturer's protocol.
Analysis of p44/42 MAP Kinase and SAPK/JNK Phosphorylation.
Western blot analysis of p44/42 and SAPK/JNK was done with kits (#9100 for P44/42 and #9250 for SAPK/JNK) from New England Biolabs. Responder splenocytes were isolated from TCR-TG and TCRxgag mice and depleted of CD4+ cells using Dynabeads. B6 splenocytes were pulsed with 10 μg/ml FMuLVgag-peptide for 1 h, irradiated and washed for use as APC. The responder cells were mixed in a 1:1 ratio with peptide-pulsed APC, and then lysed in SDS-PAGE sample buffer at indicated time-points. The samples were electrophoresed in SDS-PAGE, and blotted using a semidry transfer system. After blocking for 3 h in TBS, 0.1% Tween-20, 5% BSA at 25°C, the membranes were incubated with primary Abs according to the manufacturer's protocols (New England Biolabs). Specific binding was detected by incubation with secondary HRP-conjugated Ab and ECL Western blotting detection reagents (Amersham Pharmacia Biotech).
Measurement of Ca2+ Flux by Flow Cytometry.
Intracellular Ca2+ was assessed as described previously (23). TCR-TG and TCRxgag splenocytes expressing the Thy1.1 allomarker were depleted of CD4 cells by Dynabeads, and labeled for 30 min with (2 μg/ml) Indo-1 a.m. (model no. I1203; Molecular Probes). During the labeling, cells were stained with Abs to Thy.1.1 and CD4. After washing, the cells were analyzed by flow cytometry. A base line was established and control or peptide treated B6 Thy1.2 APC were added. Intracellular Ca2+ was measured in cells gated as Thy1.1+ and CD4− cells, and Ca2+ was followed over time. As a positive control, responder cells were treated with 2 μg/ml ionomycin (Sigma-Aldrich).
Results
FACS® and Histological Analysis of TCR-TG and TCRxgag T Cells.
Heterozygous TCR-TG mice expressing the Vα3/Vβ12 TCR specific for FMuLVgag in CD8+ T cells were crossed with homo- or heterozygous gag-TG mice (17), and double-transgenic TCRxgag mice identified by flow cytometry for TCR expression and by PCR for expression of FMuLVgag. Both TCR-TG and TCRxgag mice appeared healthy. Histologic analysis of livers from TCRxgag showed no evidence of inflammation, lymphocytic infiltration, or other hepatic abnormalities (data not shown).
Thymocytes from 6-wk-old B6, TCR-TG, and TCRxgag mice were stained with Abs for CD4, CD8, Vα3, and Vβ12, and analyzed by flow cytometry (Fig. 1 A). More thymocytes from TCR-TG mice were CD8 single positive than from B6, consistent with forced expression of a MHC class I–restricted T cell receptor. However, in thymocytes from TCRxgag mice, as compared with TCR-TG mice, the proportion of single positive CD8 cells was reduced from 20 to 6%, suggesting that the presence of FMuLVgag in TCRxgag mice led to negative selection of CD8+ T cells during thymic development. In TCR-TG mice, >90% of single CD8+ cells expressed Vα3 and Vβ12, whereas in TCRxgag mice this proportion was reduced to 52%, These data suggest that, despite expression of FMuLVgag from the liver-specific albumin promoter, some maturing CD8 + T cells in TCRxgag thymocytes were encountering antigen in the thymus, leading to negative selection of a proportion of the gag-specific CTL repertoire.
Splenocytes from TCR-TG mice had an expanded fraction of CD8+ T cells compared with normal B6 mice with an inverted CD4:CD8 ratio, but the CD4:CD8 ratio in TCRxgag mice was more similar to normal B6 mice (Fig. 1 B). More than 90% of the CD8+ population in TCR-TG mice expressed both receptors compared with <1% of the population in B6, indicating good peripheral expression of the transgenes. However, the population of CD8+ T cells expressing both chains in TCRxgag mice decreased to 22%, accompanied by a more heterogeneous pattern of expression with proportionally fewer cells expressing high levels of both chains. This suggested that expression of FMuLVgag in TCRxgag mice caused both a partial deletion of TCR-positive CD8+ cells, as well as downregulation of individual TCR chains on a fraction of CD8+ peripheral cells. Some variation of TCR expression was observed in individual TCRxgag mice, containing between 7- to 16-fold fewer CD8+/TCR-αβhi splenocytes than TCR-TG mice (n = 10). The observed reduction in the proportion of CD8+ cells expressing both receptor chains in the spleen as compared with the thymus of TCRxgag mice suggested that some deletion of these cells is also occurring in the periphery. Similar phenotypes were detected in peripheral blood and lymph nodes (data not shown).
Further evidence that T cells in TCRxgag mice had been influenced by encountering antigen in the periphery was provided by analysis of expression of Ly-6C and CD44, which are both associated with an activation/memory phenotype (Fig. 1 C). More than 70% of CD8+ TCRxgag T cells were Ly-6C+ and CD44Hi, whereas 4% of CD8+ TCR-TG cells were Ly-6C+ and 29% were CD44Hi.
TCR-TG and TCRxgag T Cells Can Both Be Induced to Lyse Targets.
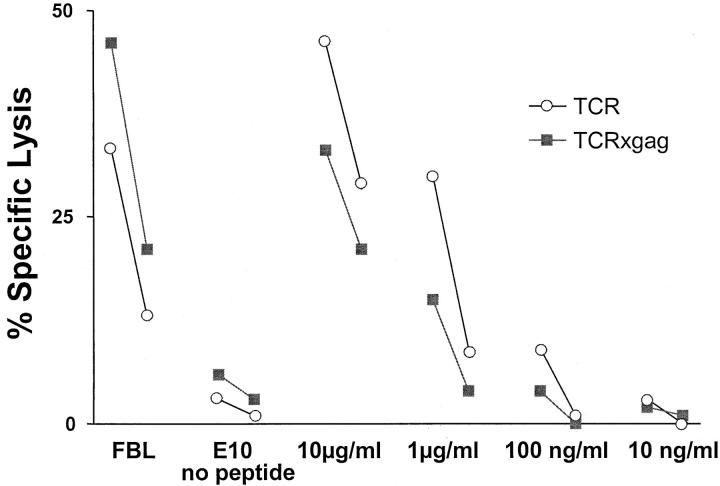
The activated phenotype of CD8+ TCRxgag cells suggested that the T cells had reacted to antigen in vivo. Analysis of spleen cells from TCR-TG and TCRxgag mice revealed that neither population could directly lyse gag-expressing targets ex vivo, suggesting that these were not primed effector cells. The potential for these cells to acquire lytic function was assessed by stimulating for 3 d in vitro with irradiated FBL cells and 15 U/ml IL-2, depleting CD4+ cells, and testing for lysis of FBL and peptide-pulsed E10 targets (Fig. 2) . After culture, effector cells from both TCR-TG and TCRxgag spleens were capable of lysing the FMuLVgag+ FBL target. There was only a small difference observed in the ability of TCR-TG and TCRxgag T cells to lyse targets pulsed with titrating amounts of peptide, with TCR-TG cells appearing slightly more lytic. However, this difference likely reflected the higher number of CD8+/TCR-αβhi cells contained in the initial responder population from TCR-TG mice, as a two to fourfold compensation for the derived E:T ratios would render the cytolytic activities similar.
Figure 2.
CTLs from TCR-TG and TCRxgag lyse gag-positive targets. 1.5 × 107 TCR-TG (white symbols) and TCRxgag (black symbols) splenocytes were stimulated for 3 d with 5 × 106 irradiated FBL cells in the presence of 5 U/ml IL-2. After 3 d, the cells were CD4 depleted and tested in a standard 4 h 51[Cr]-release assay against FBL, E10, or E10 together with FMuLVgag peptide at indicated concentrations. E:T ratio of 50:1 and 10:1 are shown, However, the final CD8+/TCR-αβhi ratio for TCRxgag compared with TCR is overestimated ∼5–15 times based on profiles in Fig. 1.
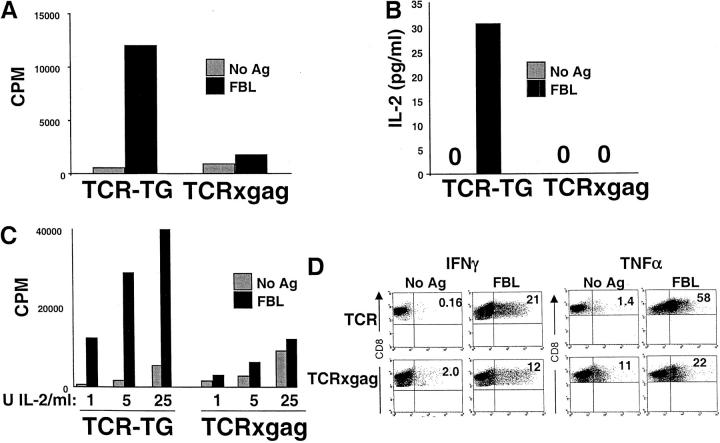
Splenocytes from TCRxgag Mice Do Not Proliferate or Secrete IL-2 but Do Produce IFN-γ and TNF-α Effector Cytokines after Antigen Stimulation.
Because the TCR complex in the TCRxgag mice was capable of activating T cells to deliver a lytic signal upon encounter with gag+ target cells, we next analyzed if these cells also proliferated in response to antigen. To compensate for the lower number of CD8+/TCR-αβhi cells in spleens of TCRxgag mice, (Fig. 1), proliferation and IL-2 production was assessed in stimulating cultures containing 10-fold excess of TCRxgag responder cells compared with TCR-TG cells (Materials and Methods). CD4-depleted TCR-TG and TCRxgag splenocytes were stimulated for 3 d in vitro with FBL, and then pulsed with 3[H]-thymidine. Although TCR-TG T cells proliferated in response to antigen, TCRxgag cells failed to respond (Fig. 3 A). To determine if the lack of proliferation resulted from an inability to produce IL-2, IL-2 production after antigen stimulation was measured. CD4-depleted splenocytes from TCR-TG and TCRxgag mice, compensated 10-fold to equalize the number of potentially responding CD8+ cells, were stimulated with FBL tumor cells for 72 h in vitro, and culture supernatants collected and analyzed by ELISA for the presence of IL-2 (Fig. 3 B). TCR-TG T cells produced IL-2 in response to antigen stimulation, whereas TCRxgag T cells did not. To determine if the failure to proliferate could be entirely explained by this inability to produce IL-2, compensated numbers of CD8+ T cells were stimulated with antigen in the presence of exogenous IL-2. The addition of 1–25 U/ml IL-2 increased background proliferation, but did not result in detectable antigen-specific proliferation by TCRxgag CD8+ T cells (Fig. 3 C), suggesting that the defect in proliferation in response to antigen is more profound than only a deficiency in endogenous IL-2 production. Similar proliferative assays were performed in the presence of 500–50 ng/ml IL-4, 500– 50 ng/ml IL-7, or 500–50 ng/ml IL–15, alternative cytokines with the potential to induce T cell proliferation. Supplementation with these cytokines also failed to augment the proliferative response of TCRxgag T cells to antigen stimulation, with the stimulation index in all conditions tested never equaling or exceeding 2.
Figure 3.
TCRxgag mice do not proliferate or produce IL-2 but retain IFN-γ and TNF-α production after antigen stimulation. (A) To measure antigen-specific proliferation, 0.1 × 106 CD4-depleted splenocytes from TCR-TG plus 0.9 × 106 B6 splenocytes and 106 splenocytes from TCRxgag mice were stimulated with 2 × 104 irradiated FBL for 3 d, and then pulsed with 3[H]-thymidine for 8 h. For analysis of IL-2 production (B), 0.1 × 106 CD4-depleted splenocytes from TCR-TG plus 0.9 × 106 B6 and 106 CD4-depleted TCRxgag were stimulated with 2 × 104 irradiated FBL in a total volume of 200 μl in a 96-well round bottomed plate. After 72 h, 50 μl of culture supernatant was harvested and murine IL-2 measured by ELISA. (C) The antigen-specific response of TCRxgag in the presence of IL-2 was measured as in A, but with indicated amounts of IL-2 added to the wells. Intracellular staining for IFN-γ and TNF-α production (D) was performed after stimulation of TCR-TG or TCRxgag splenocytes with irradiated FBL. After 48 h, secretion of protein was prevented by addition of Golgi-Block reagent for 6 h and cells permeabilized by Cytofix/Cytoperm. The cells were stained with anti-CD8 and anti–IFN-γ or anti–TNF-α. Plots were gated on CD8+ cells.
Despite the block in proliferation in response to antigen even in the presence of supplemental cytokines, the presence of lytic function after stimulation of TCRxgag CD8+ cells suggested that some effector functions were intact. Therefore, we analyzed if TCRxgag T cells could produce effector cytokines. IFN-γ and TNF-α production was assessed by intracellular staining for these cytokines after 6 h of antigen stimulation. Both TCR-TG and TCRxgag T cells produced IFN-γ and TNF-α (Fig. 3 C). The lower percentage of IFN-γ– and TNF-α–producing CD8+ cells from TCRxgag mice compared with TCR-TG CD8+ splenocytes is consistent with the 2.5- to 4.5-fold lower percentage of TCR-αβhi cells in TCRxgag mice (Fig. 1). Moreover, the amount of IFN-γ and TNF-α being produced in responding cells appears to be relatively similar. Thus, the only difference observed in IFN-γ and TNF-α production is a higher background in TCRxgag CD8+ cells, which may reflect consequences of a previous or recent encounter with antigen in vivo.
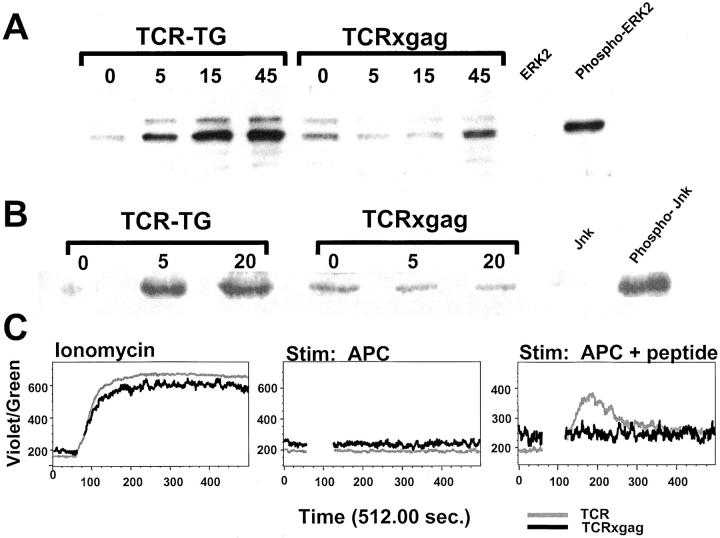
TCRxgag CD8+ T Cells Are Deficient in the Ras/MAPK and SAPK/JNK Signaling Pathways and in Flux of Ca2+ after Antigen Stimulation.
The ability of CD8+ T cells from TCRxgag mice to lyse targets and produce some IFN-γ after antigen stimulation, but not proliferate or produce IL-2, suggested interference with but not disruption of signals through the TCR. Therefore, TCR-TG and TCRxgag splenocytes were depleted of CD4+ cells, stimulated in vitro with peptide-pulsed APC, and phosphorylation of ERK1/ERK2 and JNK as reflections of the Ras/MAPK and SAPK/JNK pathways, respectively, was determined by Western blot analysis. Nonstimulated CD8+ T cells from TCRxgag appeared to express slightly higher basal levels of phosphorylated ERK and JNK than resting CD8+ T cells from TCR-TG mice, which would again be consistent with a recent antigen encounter in vivo. T cells from TCRxgag showed delayed kinetics and decreased total phosphorylation of ERK1 and ERK2 after 45 min stimulation (Fig. 4 A), and no evidence of Jun kinase phosphorylation (Fig. 4 B). Similarly decreased ERK and JNK phosphorylation was seen in TCRxgag CD8+ T cells after stimulation in vitro for 72 h (data not shown). Thus, the inhibition of activation of these pathways could not be overcome by increasing the duration of stimulation to the time when lytic activity was detected, suggesting these signals need not be fully intact for transmission of a lytic signal in CD8+ effector cells.
Figure 4.
TCRxgag T cells exhibit deficiencies in Ras/MAPK and SAPK/JNK as well as in flux of Ca2+ after antigen stimulation. CD4-depleted splenocytes from TCR-TG and TCRxgag mice were stimulated with irradiated B6 splenocytes pulsed with 10 μg/ml gag-peptide. After stimulation, the cells were lysed in SDS-PAGE sample buffer at indicated time points. The samples were run on a SDS-PAGE gel and blotted. Blots were probed using primary Abs against phosphorylated forms of ERK1/2 (A) or JNK (B). Specific binding was detected by incubation with a secondary HRP-conjugated Ab and ECL Western blotting detection reagents. For analysis of Ca2+ flux (C), TCR-TG (black line) and TCRxgag (gray line) splenocytes expressing the Thy1.1 allomarker were depleted of CD4 cells and labeled with Indo-1. During the loading, cells were stained with Abs against Thy.1.1 and CD4. After washes, the cells were analyzed by flow cytometry in order to establish a base line. Control or peptide treated B6 Thy1.2 APC were added and after gating on Thy1.1+/CD4− cells, the Ca2+ was followed over time. As a positive control, responder cells were treated with 1 μg/ml ionomycin.
A more proximal signaling event, flux of Ca2+ after stimulation, was then examined: TCR-TG and TCRxgag splenocytes congenic for the Thy1.1 allomarker were loaded with Indo-1, labeled with antibodies against Thy1.1 and CD4, and analyzed by flow cytometry. After establishing an Indo-1 baseline for the unstimulated gated Thy1.1+ CD4− cells, peptide-pulsed Thy1.2+ B6 splenocytes were added. TCR-TG T cells responded with a clear peak of increased Ca2+ to peptide-pulsed APC, but TCRxgag T cells displayed only low oscillating levels of Ca2+. By contrast, T cells from both TCR-TG and TCRxgag mice fluxed Ca2+ equivalently after exposure to ionomycin, suggesting that the failure to flux Ca2+ after antigen stimulation in TCRxgag T cells resulted from a defect in the quality of the signal from the TCR.
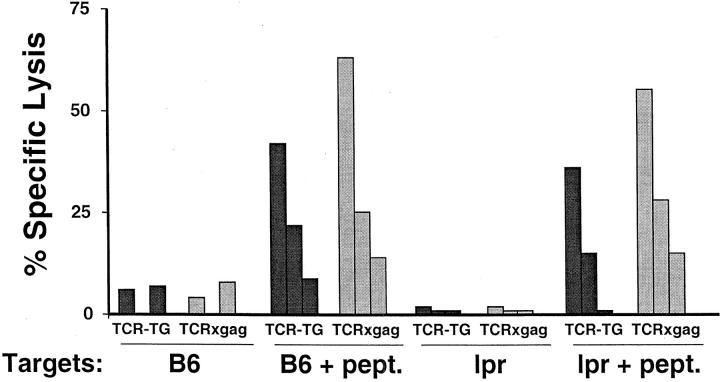
TCRxgag CD8+ CTL-mediated Lysis Is Independent on Fas Expression on Target Cells.
TCRxgag CD8+ T cells efficiently killed target cells in 4 h cytotoxicity assays despite defects in signaling following target recognition. Such acute cytotoxicity in short-term in vitro assays has been shown to be mediated by either the Fas–FasL or perforin pathway (24). To determine if TCRxgag T cells are mediating target lysis via Fas–FasL interactions, CD4-depleted splenocytes from TCR-TG and TCRxgag mice were stimulated for 3 d in vitro with irradiated FBL in the presence of IL-2, and tested for lysis of peptide pulsed ConA-induced blast targets from B6 or Fas-negative lpr mice (Fig. 5) . Both TCR-TG and TCRxgag T cells efficiently lysed targets derived from lpr-mice, demonstrating that the lytic activity was not dependent on Fas–FasL interactions. An alternative mechanism for CTL-mediated lysis other than Fas or perforin could result from the activity of TNF-α or TNF-β, although this generally is not detectable in short-term cytolytic assays. To determine if the targets used in our lytic assays were sensitive to TNF-mediated lysis, FBL and E10 cells were cocultured with 5 ng/ml TNF-α or 100 pg/ml TNF-β. Neither tumor was sensitive to these concentrations of TNF in either 4 or 24 h assays, whereas L-cells which were not killed after 4 h were lysed at 24 h (data not shown).
Figure 5.
CTL from TCRxgag lyse gag-positive targets independently of Fas-expression. 1.5 × 107 TCR-TG (black symbols) and TCRxgag (white symbols) splenocytes were stimulated for 3 d with 5 × 106 irradiated FBL cells in the presence of 5 U/ml IL-2. After 3 d, the cells were CD4 depleted and tested in a standard 51[Cr]-release assay using ConA-induced blasts from B6 or Fas-negative lpr mice as targets. Target cells were labeled with 51[Cr] and used either untreated or after pulse with 5 μg/ml gag-peptide. E:T ratios were 50:1, 10:1, and 2:1.
These results suggest that the observed lysis is mediated via the perforin pathway. Analysis of freshly obtained TCR as well as TCRxgag CD8+ T cells revealed only low levels of perforin expression by Western blot analysis. However, after 3 d of in vitro stimulation with FBL, when cytolytic activity is detectable, both TCR and TCRxgag CD8+ cells expressed high levels of perforin (data not shown), which would be consistent with involvement of the perforin pathway in target lysis.
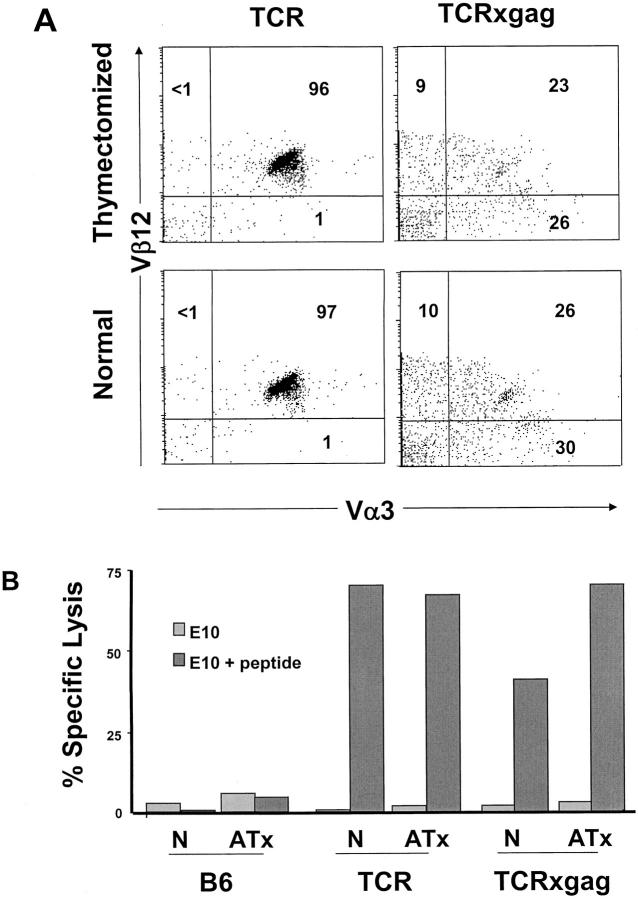
Tolerant T Cells Persist in TCRxgag Mice after Thymectomy.
It was possible that the T cells with cytolytic activity detected in TCRxgag mice were cells that had not yet undergone deletion or anergy. Thus, although such cells may be destined for tolerization, the high output of naive T cells from the thymus might permit recovery of very recent emigrants. Therefore, the outflux of new T cells to the periphery was eliminated by thymectomy of TCR-TG, TCRxgag, and control B6 mice at 5–6 wk of age. 5 wk after surgery, peripheral T cells were analyzed by flow cytometry for changes in the Vα3 and Vβ12 positive population (Fig. 6 A). The percent of CD8+/TCR-αβhi cells and TCR staining intensity was unchanged in both TCR-TG and TCRxgag splenocytes 5 wk after thymectomy. After stimulation of splenocytes for 3 d in vitro with irradiated FBL, splenocytes from thymectomized mice exhibited similar lytic activity as T cells from their euthymic littermates (Fig. 6 B). Thus, the functional and morphological phenotype observed in the periphery of TCRxgag mice represents a stable population that is not being rapidly deleted and replaced.
Figure 6.
The tolerant cells in TCRxgag are a stable population and not deleted in thymectomized animals. 5-wk-old TCR-TG and TCRxgag mice were anaesthetized and their thymi removed. After 5 wk, splenocytes from control and thymectomized mice were (A) stained with CD8; Vα3 and Vβ12, and after gating on CD8+ cells, T cell receptor expression was analyzed. For analysis of lysis by thymectomized mice (B), 1.5 × 107 TCR-TG and TCRxgag splenocytes from normal control (N) or adult-thymectomized (ATx) mice were stimulated with 5 × 106 irradiated FBL cells in the presence of 5 U/ml IL-2. After 3 d, the cells were CD4 depleted and tested in a standard 51[Cr]-release assay against E10 or E10 pulsed with FMuLVgag. E:T ratio was 50:1.
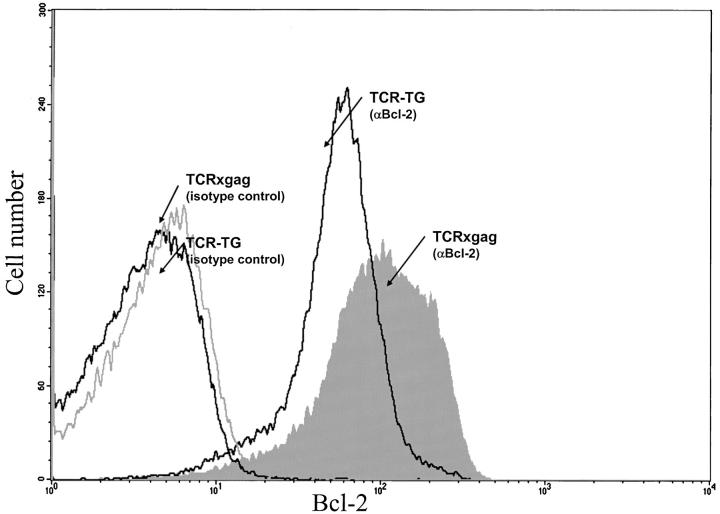
Tolerant TCRxgag T Cells Express Elevated Levels of Bcl-2.
As described above, tolerant CD8+ T cells were capable of persisting in the periphery, and therefore must be capable of avoiding deletion despite expression of the tolerizing antigens. Upregulation of antiapoptotic molecules in CD8+ T cells receiving the tolerizing signals might provide a mechanism of avoiding deletion. Therefore, splenocytes from TCR and TCRxgag were permeabilized and stained with anti–Bcl-2 antibodies, and intracellular expression of the protein analyzed by flow cytometry (Fig. 7) . CD8+ TCRxgag cells expressed more Bcl-2 protein than CD8+ cells from TCR mice.
Figure 7.
Tolerant TCRxgag cells CD8+ express high levels of Bcl-2. Fresh splenocytes from TCR and TCRxgag were stained for CD8, and then permeabilized and stained with antibodies for Bcl-2 or with isotype control antibodies diluted in permeabilization buffer. After three washes, cells were analyzed by flow cytometry for CD8 and Bcl-2 expression.
Recovery of Proliferating CD8+ T Cells after Repeated In Vitro Stimulation.
The failure to detect a proliferative response in CD8+ T cells from TCRxgag mice could result from an irreversible defect in signaling, or from an actively maintained tolerizing process. The latter possibility would suggest that it might be possible to recover functional T cells with high avidity. Therefore, we attempted to overcome the tolerant state and expand FMuLVgag reactive CD8+ T cells by removing the cells from the tolerogenic in vivo environment and repetitively stimulating the cells in vitro in the presence of supplemental IL-2. Splenocytes from naive TCR-TG and TCRxgag mice were stimulated weekly in vitro with irradiated FBL and IL-2. Cell cultures from both TCR-TG and TCRxgag splenocytes exhibited an initial and similar contraction due to ∼80% cell death of the CD8+ population during the first weekly cycle. During the second week and subsequent stimulation cycles, TCR-TG CD8+ cells responded with proliferation leading to accumulation of CD8+ T cells. TCRxgag T cells, however, did not begin expanding the second week, and showed no change in total cell number (data not shown). After 4 wk of culture, TCRxgag cultures began expanding after stimulation in a similar manner to cultures of TCR-TG T cells, and at this time the CD8+ splenocytes from cultured TCRxgag mice showed no difference in proliferative response to titrating amounts of peptide compared with CD8+ cells from TCR-TG mice (Fig. 8) The results suggest that the defect(s) leading to proliferative inhibition were reversible, and that high avidity clones were neither deleted nor permanently anergized during tolerance induction.
Figure 8.
TCRxgag T cells that have been expanded in vitro by repetitive in vitro stimulations have similar avidity as T cells from TCR-TG mice. 1.5 × 107 splenocytes from TCR-TG or TCRxgag mice were stimulated with 5 × 106 irradiated FBL in T25 flasks in a total volume of 10 ml supplemented with 20 U/ml IL-2. The cultures were restimulated every 7 d for 11 wk with 2 × 106 irradiated FBL, 5 × 106 irradiated B6 splenocytes as feeder cells and 20 U/ml IL-2. 8 d after last stimulation cultures were tested in proliferation assay. 5 × 104 CD4-depleted cells from TCR-TG (white symbols) and TCRxgag (black symbols) cultures were stimulated with 5 × 105 irradiated B6 splenocytes pulsed with indicated concentrations of peptide for 4 d in the presence of 5 U/ml IL-2, and then pulsed with 3[H]-thymidine for 8 h.
Discussion
We have developed a transgenic model for the study of tolerance to peripherally expressed candidate tumor-associated antigens (TAAs) based on a TCR transgenic mouse expressing a FMuLVgag-specific, H-2Db-restricted TCR and a transgenic mouse expressing FMuLVgag from the albumin promoter. In double transgenic mice, the CD8+ T cells specific for the TAAs which is expressed mainly in the liver, are maintained in a tolerant state. This tolerance reflects not only partial deletion during T cell development in the thymus, but a peripheral population of antigen experienced cells that is functionally compromised.
The tolerant CD8+ CTL retained the capacity to lyse FMuLVgag+ targets but had lost the ability to proliferate and produce IL-2 in response to antigen stimulation. This phenotype of “split tolerance” is similar to one described in an in vitro model in which an initially IL-2 producing CD8+ CTL clone was tolerized by stimulation with fixed APC, and became unable to proliferate in response to antigen while maintaining lytic ability (11). In both our in vivo and this in vitro model, production of the effector cytokine IFN-γ was less impaired than IL-2 production. However, in another transgenic model of in vivo–induced tolerance in CD8+ cells in which the self-antigen is a ubiquitously expressed male antigen, more global tolerance was observed with not only a block in proliferation and cytokine production, but also the absence of cytotoxicity (10). The differences in these in vivo models may reflect the magnitude as well as the ubiquitous nature of expression of the tolerizing antigen HY, with the more restricted expression of FMuLVgag resulting in tolerance but a less severe functional inhibition.
We did not detect a defect in lytic function after antigen stimulation of CD8+ T cells from TCR-TG and TCRxgag mice, particularly after compensating for the proportionally lower number of CD8+/TCR-αβhi cells in TCRxgag mice compared with TCR-TG mice. Despite demonstrable cytolytic activity, signaling through the Ras/MAPK and SAP/JNK pathways was abnormal. Cytolytic function has been shown to be in part dependent on ERK phosphorylation, but a subpopulation of CTL can lyse target cells via the perforin pathway independent of activation of ERK (25). The role of JNK in CTL lysis has not been extensively analyzed, but our results suggest that even a severe defect in the ability to phosphorylate JNK is not detrimental for lytic function by CD8+ T cells. This data is consistent with a report showing that spontaneous and ADCC-mediated lysis by NK cells is also independent of JNK activation (26).
CTL can kill target cells in short term in vitro assays via perforin release and by FasL-mediated ligation of Fas on target cells (for a review, see reference 24). The effective lysis of Fas-negative lpr targets by TCRxgag CTL show that the lytic activity is not dependent on the Fas/FasL pathway. Our results suggested that killing was mediated via the perforin pathway. However, perforin-mediated lysis has been reported to be Ca2+ dependent (27, 28) and our results showed freshly obtained TCRxgag T cells did not efficiently flux Ca2+. Therefore, either this defect was sufficiently restored after 3 d of in vitro stimulation to permit activation of the cytolytic pathway, or the known sensitivity for granular exocytosis to small changes in Ca2+ is below the level of detection in our assay (29). Other alternative mechanisms of killing, such as mediated by TNF-release, are possible but in our view less likely because the targets analyzed in this study are resistant to TNF, and also because such mechanisms of lysis are generally not detectable in a 4 h cytolytic assay (24).
Several studies have suggested that maintenance of the anergic state in self-reactive T cells requires continuous exposure of the T cells to the tolerogen, and that the nonresponsive state can be reversed after withdrawal of the antigen (30–32). However, it is very difficult to mimic this rescue condition in vitro and to keep T cells alive for prolonged periods of time in vitro in the absence of antigen stimulation. Our results suggest an alternative strategy for reversing anergy, in which the T cells are removed from the tolerizing environment and repetitively stimulated in vitro with the antigen presented by a functionally competent APC in the presence of IL-2. The recovery of reactive CD8+ cells is encouraging for developing T cell therapy against TAAs in which tolerance may be an obstacle.
Other transgenic in vivo models analyzing tolerance induction have demonstrated that expression of an antigen in peripheral tissues may lead to deletion of high avidity CD8+ cells from the host, with only low avidity T cells persisting (33–35). In our model, CD8+ cells with potentially high avidity but a tolerant phenotype were shown to persist in the host, suggesting a mechanism may be operating that provides protection from peripheral deletion. Increased expression of Bcl-2 has been shown to promote survival and protect lymphocytes from apoptosis (36–38) (for a review, see reference 39), and the tolerant CD8+ cells from TCRxgag mice were demonstrated to have upregulated Bcl-2. Thus, the encounter of CD8+ T cells with high affinity TCR with a tolerizing antigen in vivo can lead to upregulation of antiapoptotic molecules despite the development of anergy.
CD8+ T cell tolerance can potentially result from nonresponsiveness either at the afferent or at the efferent phase of the immune response. Control of autoreactive T cells only at the effector phase would be inefficient, as it would permit in vivo expansion and accumulation of unusable tolerant T cells. Similarly, control of self-reactive T cells only by prevention of proliferation seems undesirable, as retention of lytic function could result in autoimmune injury. However, as long as tolerance keeps the frequency of autoreactive T cells low by blockade of expansion, a small amount of self-reactivity may be permissible to the host. Normally, the frequency of CD8+ CTL reactive with a particular self-antigen in the absence of expansion is low due to clonal diversity during T cell development. In our transgenic model, however, the frequency of potentially self-reactive CTL is very high, suggesting that there must be an alternative explanation for the lack of detectable autoimmune injury. The TCRxgag T cells may not be directly lytic in vivo, and only retain the capacity to become cytolytic if exposed to antigen via a functionally competent APC rather than normal tissue. This would be consistent with the absence of spontaneous killing when TCRxgag splenocytes were directly tested for cytolysis before 3 d of in vitro stimulation with irradiated FBL. However, other protective mechanisms may also be operative in vivo. In a previous report describing the gag-TG mice, we reported that gag-TG mice showed a remarkable resistance to CTL induced liver injury after infusion of large numbers of FMuLVgag-reactive effector CTL (17). The resistance of hepatocytes to autoimmune damage may be a general phenomenon for this tissue. In a transgenic model in which tolerance was induced by expression of the alloantigen H-2Kb from the albumin promoter, hepatocytes were also resistant to H-2Kb–specific CTL-mediated autoimmune injury (40). Additionally, potentially cytolytic CD8+ T cells are able to control viral replication in a hepatitis model, presumably by release of inflammatory cytokines such as IFN-γ, without a cytopathic effect on hepatocytes (41).
The means by which hepatocytes may be protected from cytolysis in the above examples are unknown, but similar mechanisms could be operative in our model as well as in other normal tissues. In the induction phase of a T cell response, inhibitory signals can be triggered through cross-linking of CTLA-4 by B7.1 or B7.2 (for reviews, see references 42 and 43). Recently two new molecules with homology to the B7 family, PD-L1 (44, 45), and PD-L2 (46), were described and shown to inhibit T cell proliferation and cytokine production upon binding to PD-1 on T cells. In addition to being expressed in lymphoid tissues, PD-L1 is constitutively expressed in distinct parenchymal organs such as murine heart and lung (45), while PD-L2 is expressed in mouse liver and lung (46). Both molecules might thus be capable of inducing tissue specific tolerance in vivo (44–46), and it is possible that these or some still unidentified molecules could be mediating inhibitory signals to TCRxgag T cells in our model.
There are probably multiple mechanisms that can contribute to the establishment and maintenance of T cell tolerance to self-proteins, and the difference in data derived from different transgenic models may reflect this complexity. Depending on the nature of the tissue and level of protein expression, some or all of these tolerance mechanisms may be induced, resulting in either deletion or in functionally tolerant T cells with a range of potential phenotypes. Thus, it may not be possible to design a universal strategy for breaking tolerance to self-antigens for the generation of tumor-reactive T cells, just as there are presumably multiple distinct events that might lead to autoimmunity. Further analysis of this and other models should continue to illuminate and ultimately resolve these issues.
Acknowledgments
We thank Dimitris Kioussis, National Institute for Medical Research, Mill Hill, UK, for providing the CD2 constructs; Benjamin Jacobsen and Christopher Wilson, University of Washington, Seattle, WA, for microinjection and generation of the TCR-TG strain; Xaioxia Tan and Jennifer Young for expert technical assistance; and Joanne Factor for her assistance in preparation of this manuscript.
This work was supported by grant number CA33084 from the National Institutes of Health/National Cancer Institute, and M. Kalos was supported by a grant from the Cancer Research Institute.
M. Kalos's present address is Corixa Corporation, 1124 Columbia St., Seattle, WA 98104.
E.S. Huseby's present address is HHMI, National Jewish Medical and Research Center, 1400 Jackson St., Denver, CO 80206.
Footnotes
Abbreviations used in this paper: JNK, Jun kinase; TAA, tumor-associated antigen.
References
- 1.Kisielow, P., H. Bluthmann, U.D. Staerz, M. Steinmetz, and H. von Boehmer. 1988. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 333:742–746. [DOI] [PubMed] [Google Scholar]
- 2.Blackman, M., J. Kappler, and P. Marrack. 1990. The role of the T cell receptor in positive and negative selection of developing T cells. Science. 248:1335–1341. [DOI] [PubMed] [Google Scholar]
- 3.Lo, D., L.C. Burkly, G. Widera, C. Cowing, R.A. Flavell, R.D. Palmiter, and R.L. Brinster. 1988. Diabetes and tolerance in transgenic mice expressing class II MHC molecules in pancreatic β cells. Cell. 53:159–168. [DOI] [PubMed] [Google Scholar]
- 4.Morahan, G., J. Allison, and J.F. Miller. 1989. Tolerance of class I histocompatibility antigens expressed extrathymically. Nature. 339:622–624. [DOI] [PubMed] [Google Scholar]
- 5.Schonrich, G., F. Momburg, M. Malissen, A.M. Schmitt-Verhulst, B. Malissen, G.J. Hammerling, and B. Arnold. 1992. Distinct mechanisms of extrathymic T cell tolerance due to differential expression of self antigen. Int. Immunol. 4:581–590. [DOI] [PubMed] [Google Scholar]
- 6.Schonrich, G., U. Kalinke, F. Momburg, M. Malissen, A.M. Schmitt-Verhulst, B. Malissen, G.J. Hammerling, and B. Arnold. 1991. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 65:293–304. [DOI] [PubMed] [Google Scholar]
- 7.DeSilva, D.R., W.S. Feeser, E.J. Tancula, and P.A. Scherle. 1996. Anergic T cells are defective in both jun NH2-terminal kinase and mitogen-activated protein kinase signaling pathways. J. Exp. Med. 183:2017–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields, P.E., T.F. Gajewski, and F.W. Fitch. 1996. Blocked Ras activation in anergic CD4+ T cells. Science. 271:1276–1278. [DOI] [PubMed] [Google Scholar]
- 9.Ohashi, P.S., S. Oehen, K. Buerki, H. Pircher, C.T. Ohashi, B. Odermatt, B. Malissen, R.M. Zinkernagel, and H. Hengartner. 1991. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 65:305–317. [DOI] [PubMed] [Google Scholar]
- 10.Tanchot, C., S. Guillaume, J. Delon, C. Bourgeois, A. Franzke, A. Sarukhan, A. Trautmann, and B. Rocha. 1998. Modifications of CD8+ T cell function during in vivo memory or tolerance induction. Immunity. 8:581–590. [DOI] [PubMed] [Google Scholar]
- 11.Otten, G.R., and R.N. Germain. 1991. Split anergy in a CD8+ T cell: receptor-dependent cytolysis in the absence of interleukin-2 production. Science. 251:1228–1231. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz, R.H. 1997. T cell clonal anergy. Curr. Opin. Immunol. 9:351–357. [DOI] [PubMed] [Google Scholar]
- 13.Li, W., C.D. Whaley, A. Mondino, and D.L. Mueller. 1996. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 271:1272–1276. [DOI] [PubMed] [Google Scholar]
- 14.Kimura, M., M. Yamashita, M. Kubo, M. Iwashima, C. Shimizu, K. Tokoyoda, J. Chiba, M. Taniguchi, M. Katsumata, and T. Nakayama. 2000. Impaired Ca/calcineurin pathway in in vivo anergized CD4 T cells. Int. Immunol. 12:817–824. [DOI] [PubMed] [Google Scholar]
- 15.Bercovici, N., J. Delon, C. Cambouris, N. Escriou, P. Debre, and R.S. Liblau. 1999. Chronic intravenous injections of antigen induce and maintain tolerance in T cell receptor-transgenic mice. Eur. J. Immunol. 29:345–354. [DOI] [PubMed] [Google Scholar]
- 16.Dubois, P.M., M. Pihlgren, M. Tomkowiak, M. Van Mechelen, and J. Marvel. 1998. Tolerant CD8 T cells induced by multiple injections of peptide antigen show impaired TCR signaling and altered proliferative responses in vitro and in vivo. J. Immunol. 161:5260–5267. [PubMed] [Google Scholar]
- 17.Öhlén, C., M. Kalos, D.J. Hong, A.C. Shur, and P.D. Greenberg. 2001. Expression of a tolerizing tumor antigen in peripheral tissue does not preclude recovery of high-affinity CD8+ T cells or CTL immunotherapy of tumors expressing the antigen. J. Immunol. 166:2863–2870. [DOI] [PubMed] [Google Scholar]
- 18.Chen, W., H. Qin, B. Chesebro, and M.A. Cheever. 1996. Identification of a gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by friend, moloney, and rauscher murine leukemia virus-induced tumors. J. Virol. 70:7773–7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao, C., G.E. Osman, S. Adams, and S.K. Datta. 1994. T cell receptor α-chain repertoire of pathogenic autoantibody-inducing T cells in lupus mice. J. Immunol. 152:1462–1470. [PubMed] [Google Scholar]
- 20.Zhumabekov, T., P. Corbella, M. Tolaini, and D. Kioussis. 1995. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J. Immunol. Methods. 185:133–140. [DOI] [PubMed] [Google Scholar]
- 21.Lang, G., D. Wotton, M.J. Owen, W.A. Sewell, M.H. Brown, D.Y. Mason, M.J. Crumpton, and D. Kioussis. 1988. The structure of the human CD2 gene and its expression in transgenic mice. EMBO J. 7:1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg, P.D., D.E. Kern, and M.A. Cheever. 1985. Therapy of disseminated murine leukemia with cyclophosphamide and immune Lyt-1+, 2− T cells. Tumor eradication does not require participation of cytotoxic T cells. J. Exp. Med. 161:1122–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coligan, J., A. Kruisbeck, D. Margulies, E. Shevach, and W. Strober. 1992. Immunofluorescence and cell sorting. Current Protocols in Immunology. John Wiley & Sons, New York. 5.5.1–5.5.3.
- 24.Berke, G. 1995. The CTL's kiss of death. Cell. 81:9–12. [DOI] [PubMed] [Google Scholar]
- 25.Lilic, M., K. Kulig, I. Messaoudi, K. Remus, M. Jankovic, J. Nikolic-Zugic, and S. Vukmanovic. 1999. CD8+ T cell cytolytic activity independent of mitogen-activated protein kinase/extracellular regulatory kinase signaling (MAP kinase/ERK). Eur. J. Immunol. 29:3971–3977. [DOI] [PubMed] [Google Scholar]
- 26.Trotta, R., K. Fettucciari, L. Azzoni, B. Abebe, K.A. Puorro, L.C. Eisenlohr, and B. Perussia. 2000. Differential role of p38 and c-Jun N-terminal kinase 1 mitogen-activated protein kinases in NK cell cytotoxicity. J. Immunol. 165:1782–1789. [DOI] [PubMed] [Google Scholar]
- 27.Gray, L.S., J.R. Gnarra, and V.H. Engelhard. 1987. Demonstration of a calcium influx in cytolytic T lymphocytes in response to target cell binding. J. Immunol. 138:63–69. [PubMed] [Google Scholar]
- 28.Takayama, H., and M.V. Sitkovsky. 1987. Antigen receptor-regulated exocytosis in cytotoxic T lymphocytes. J. Exp. Med. 166:725–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyubchenko, T.A., G.A. Wurth, and A. Zweifach. 2001. Role of calcium influx in cytotoxic T lymphocyte lytic granule exocytosis during target cell killing. Immunity. 15:847–859. [DOI] [PubMed] [Google Scholar]
- 30.Rocha, B., C. Tanchot, and H. Von-Boehmer. 1993. Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen. J. Exp. Med. 177:1517–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Migita, K., and A. Ochi. 1993. The fate of anergic T cells in vivo. J. Immunol. 150:763–770. [PubMed] [Google Scholar]
- 32.Ramsdell, F., and B.J. Fowlkes. 1992. Maintenance of in vivo tolerance by persistence of antigen. Science. 257:1130–1134. [DOI] [PubMed] [Google Scholar]
- 33.Morgan, D.J., H.T. Kreuwel, S. Fleck, H.I. Levitsky, D.M. Pardoll, and L.A. Sherman. 1998. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J. Immunol. 160:643–651. [PubMed] [Google Scholar]
- 34.Theobald, M., J. Biggs, J. Hernandez, J. Lustgarten, C. Labadie, and L.A. Sherman. 1997. Tolerance to p53 by A2.1-restricted cytotoxic T lymphocytes. J. Exp. Med. 185:833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurts, C., H. Kosaka, F.R. Carbone, J.F. Miller, and W.R. Heath. 1997. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 186:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaux, D.L., S. Cory, and J.M. Adams. 1988. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 335:440–442. [DOI] [PubMed] [Google Scholar]
- 37.McDonnell, T.J., N. Deane, F.M. Platt, G. Nunez, U. Jaeger, J.P. McKearn, and S.J. Korsmeyer. 1989. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 57:79–88. [DOI] [PubMed] [Google Scholar]
- 38.Hockenbery, D., G. Nunez, C. Milliman, R.D. Schreiber, and S.J. Korsmeyer. 1990. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 348:334–336. [DOI] [PubMed] [Google Scholar]
- 39.Arch, R.H., and C.B. Thompson. 1999. Lymphocyte survival—the struggle against death. Annu. Rev. Cell Dev. Biol. 15:113–140. [DOI] [PubMed] [Google Scholar]
- 40.Limmer, A., T. Sacher, J. Alferink, M. Kretschmar, G. Schonrich, T. Nichterlein, B. Arnold, and G.J. Hammerling. 1998. Failure to induce organ-specific autoimmunity by breaking of tolerance: importance of the microenvironment. Eur. J. Immunol. 28:2395–2406. [DOI] [PubMed] [Google Scholar]
- 41.Guidotti, L.G., T. Ishikawa, M.V. Hobbs, B. Matzke, R. Schreiber, and F.V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 4:25–36. [DOI] [PubMed] [Google Scholar]
- 42.Sperling, A.I., and J.A. Bluestone. 1996. The complexities of T-cell co-stimulation: CD28 and beyond. Immunol. Rev. 153:155–182. [DOI] [PubMed] [Google Scholar]
- 43.Chambers, C.A., M.S. Kuhns, J.G. Egen, and J.P. Allison. 2001. Ctla-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 19:565–594. [DOI] [PubMed] [Google Scholar]
- 44.Dong, H., G. Zhu, K. Tamada, and L. Chen. 1999. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5:1365–1369. [DOI] [PubMed] [Google Scholar]
- 45.Freeman, G.J., A.J. Long, Y. Iwai, K. Bourque, T. Chernova, H. Nishimura, L.J. Fitz, N. Malenkovich, T. Okazaki, M.C. Byrne, et al. 2000. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Latchman, Y., C.R. Wood, T. Chernova, D. Chaudhary, M. Borde, I. Chernova, Y. Iwai, A.J. Long, J.A. Brown, R. Nunes, et al. 2001. PD-L2 is a second ligand for PD-I and inhibits T cell activation. Nat. Immunol. 2:261–268. [DOI] [PubMed] [Google Scholar]