Abstract
Induction of transplantation tolerance with certain therapeutic nondepleting monoclonal antibodies can lead to a robust state of peripheral “dominant” tolerance. Regulatory CD4+ T cells, which mediate this form of “dominant” tolerance, can be isolated from spleens of tolerant animals. To determine whether there were any extra-lymphoid sites that might harbor regulatory T cells we sought their presence in tolerated skin allografts and in normal skin. When tolerated skin grafts are retransplanted onto T cell–depleted hosts, graft-infiltrating T cells exit the graft and recolonize the new host. These colonizing T cells can be shown to contain members with regulatory function, as they can prevent nontolerant lymphocytes from rejecting fresh skin allografts, without hindrance of rejection of third party skin. Our results suggest that T cell suppression of graft rejection is an active process that operates beyond secondary lymphoid tissue, and involves the persistent presence of regulatory T cells at the site of the tolerated transplant.
Keywords: adoptive transfer, monoclonal antibodies, skin transplantation, tolerance, anergy
Introduction
In recent years significant advances have been made in enabling the therapeutic induction of transplantation tolerance (1–6). In rodents it is possible to induce a robust form of peripheral tolerance by treatment with nondepleting mAbs, such as the combination of anti-CD4 and anti-CD8, at the time of transplantation (7–12). Tolerance so achieved is dependent on regulatory T cells that disarm nontolerant naive cells (dominant tolerance) and facilitate the emergence of novel regulatory cells from the naive lymphocyte population (infectious tolerance; references 8, 10, and 13). The regulatory cells which fulfill this role are known to be CD4+ (8), and contained in both the CD4+CD25+ and CD4+CD25− populations (14).
It has been repeatedly demonstrated that such regulatory T cells can be isolated from spleens of tolerant mice (8, 12, 15). Recent work has suggested that in tolerant rats T cells infiltrating tolerated kidneys are enriched for regulatory cells when compared with the splenic T cells (16). We show here that tolerated skin grafts possess regulatory T cells with the capacity to mediate dominant transplantation tolerance. The presence of regulatory T cells in tolerated grafts may indicate that they have a protective role within that tissue.
Materials and Methods
Mice.
CBA/Ca (H-2k), human-CD52 transgenic CP1-CBA/Ca (H-2k; reference 17), recombination activating gene (RAG)1−/−-CBA/Ca (H-2k), and B10.BR (H-2k) mice were bred and maintained in the SPF facilities of the Sir William Dunn School of Pathology (Oxford, UK). All groups were age and sex matched. All procedures were conducted in accordance with the Home Office Animals (Scientific Procedures) Act of 1986.
Thymectomy and Skin Grafting.
Mice were anesthetized with a mixture of 10 mg/ml Hypnodil and 2 μg/ml Sublimaze (Janssen). 0.12 ml per 20 g of body weight was injected intraperitoneally. Thymectomy was conducted as described by Monaco et al. (18). In short, a longitudinal incision was made on the anterior surface of the neck, and the thymus was removed as two intact lobes by the application of negative pressure through a glass tip inserted in the anterior mediastinum. Skin grafting was conducted according to a modified technique of Billingham et al. (19). Briefly, full thickness tailskin (1 × 1 cm) was grafted on the lateral flank. Grafts were observed on alternate days after the removal of the bandage at day 8 and considered rejected when no viable donor skin was present. Tolerated skin grafts were removed from the flank of tolerant mice, washed in PBS and grafted on the lateral flank of the new hosts. Statistical analysis of graft survival was made by the log rank method (20).
Adoptive Cell Transfer.
Cells were obtained from spleens of adult CBA/Ca mice. A single cell suspension was obtained by passing the splenocytes through a 70-μm cell strainer (Becton Dickinson) and the erythrocytes were depleted by water lysis. Cells were counted, diluted in PBS, and injected intravenously into the tail vein.
Cell Depletion, Tolerance Induction, and mAbs.
For depletion of CP1-CBA T cells, 0.25 mg of CAMPATH-1H (21) was injected intraperitoneally. Tolerance was induced in CBA/Ca and CP1-CBA mice by treatment with 1 mg nondepleting CD4 mAb (YTS177.9; reference 7) and 1 mg nondepleting CD8 mAb (YTS105.18; reference 7) at day 0, 2, and 4 after B10.BR skin transplantation. These mAbs were produced in our laboratory by culture in hollow fiber bioreactors, purified from culture supernatants by 50% ammonium sulfate precipitation, dialyzed against PBS, and the purity checked by native and SDS gel electrophoresis (PhastGel; Amersham Pharmacia Biotech).
Flow Cytometric Analysis.
Peripheral blood samples were depleted of erythrocytes by water lysis, washed and resuspended in PBS, 1% wt/vol BSA, 5% vol/vol heat-inactivated normal rabbit serum, and 0.1% wt/vol sodium azide. Cells were incubated for 45 min at 4°C with directly conjugated CD3(KT3)-FITC, CD25(PC61)-PE (BD PharMingen), and CD4(H129.19)-CyChrome (BD PharMingen). The cells were washed, resuspended in PBS, 1% wt/vol BSA, 0.1% wt/vol sodium azide, and fixed in 2% vol/vol formaldehyde solution. Three-color FACSCalibur™ analysis was performed using CELLQuest™ software (Becton Dickinson).
Results and Discussion
Tolerated Skin Grafts Can Transfer Dominant Tolerance When Regrafted Onto New Recipients.
We investigated whether tolerated skin from animals exhibiting dominant tolerance play host to regulatory T cells. First, we established that the regrafting of tolerated B10.BR skin transplants into T cell–depleted hosts leads to a dominant tolerant state, such that adoptively transferred splenocytes from naive donors are prevented from rejecting fresh allografts (Fig. 1) .
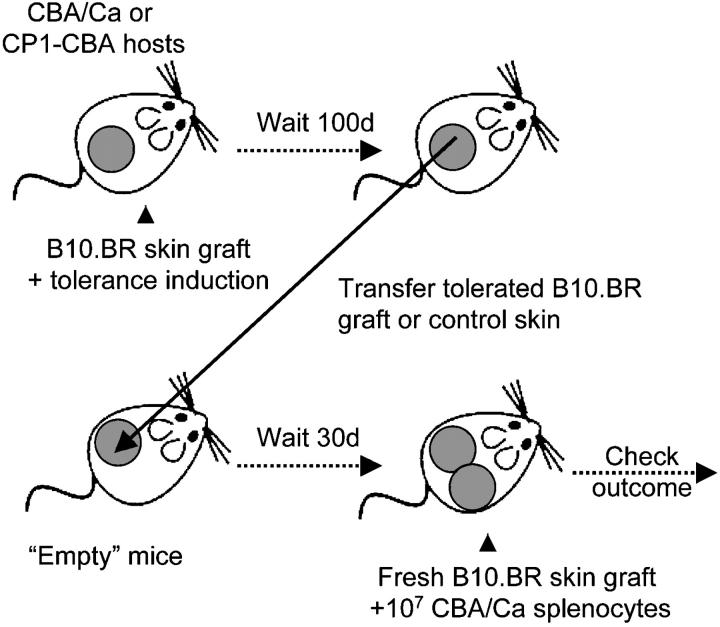
Figure 1.
The experimental system. CBA/Ca or CP1-CBA were made tolerant to B10.BR skin grafts by treatment with nondepleting CD4 and CD8 mAbs. 100 d after tolerance induction the tolerated skin grafts, or autologous control skin, were removed and transplanted onto “empty” mice (either adult thymectomized and T cell–depleted CBA-CP1 mice, or RAG1−/−-CBA mice). After 30 d the mice were transfused with 107 splenocytes from naive CBA/Ca mice, together with a fresh B10.BR skin graft. The possible outcomes are: rejection, when a nontolerant preexisting state permits the transfused cells to mediate graft rejection; or acceptance of the skin grafts, when tolerated grafts lead to a tolerance state that is nonpermissive for graft rejection by the transfused splenocytes.
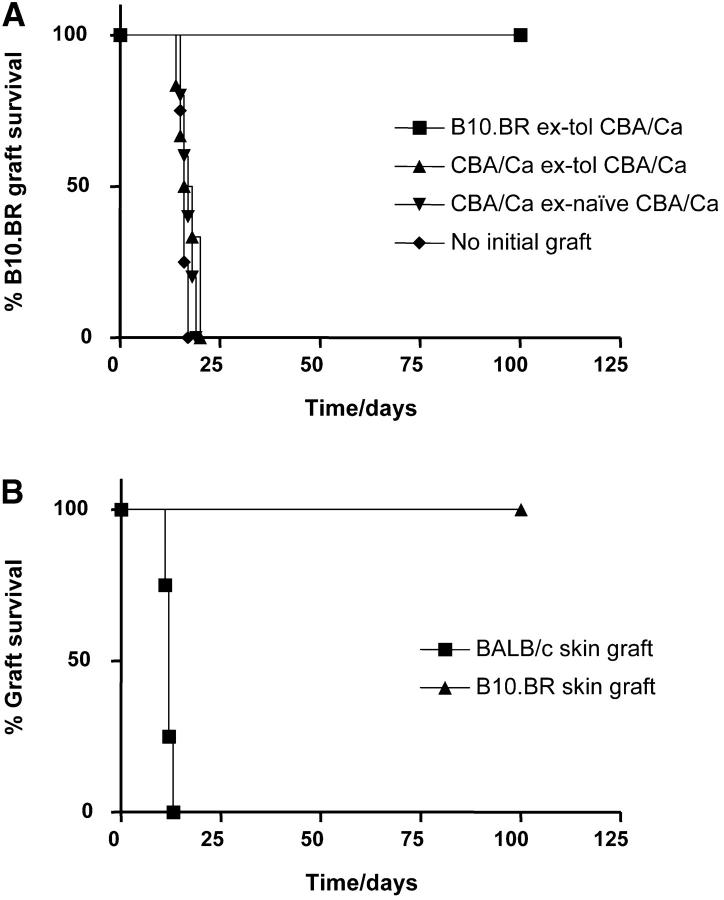
Tolerated B10.BR skin grafts, as well as control CBA/Ca skin, were obtained from CBA/Ca mice 100 to 120 d after skin grafting, such tolerance having been induced initially with 3 doses of 1 mg nondepleting CD4 and CD8 mAbs given over 1 wk. T cell–depleted CP1-CBA mice were used as recipients for the regrafted skin. The CP1-CBA strain is histocompatible with CBA/Ca and expresses human-CD52 under the control of the CD2 promoter in all T cells, allowing selective T cell depletion with the hCD52 mAb CAMPATH-1H (13). “Recipient” CP1-CBA mice were thymectomized at 4 wk of age, and depleted of T cells with CAMPATH-1H 1 wk before skin grafting (designated as “empty” mice). 30 d after the grafting of these empty CP1-CBA mice with tolerated B10.BR or control CBA/Ca skin grafts, all mice were transfused with 107 splenocytes from naive CBA/Ca donors and challenged with a fresh B10.BR skin graft. Fig. 2 A shows that the group of mice, that had been transplanted with tolerated B10.BR skin grafts, were able to resist the rejection by naive cells. However, groups transplanted with CBA/Ca skin from the same tolerant donors were permissive for rejection, with a rate similar to the animals grafted with CBA/Ca skin from naive donors, and to the control recipients that had not received any preparatory skin graft.
Figure 2.
Tolerated skin grafts can transfer the tolerant state upon regraft. CP1-CBA mice were thymectomized at 4 wk of age, and depleted of T cells with 0.25 mg CAMPATH-1H. (A) At day −30, these mice were transplanted with tolerated B10.BR skin grafts from tolerant CBA/Ca (▪), CBA/Ca skin from the CBA/Ca tolerant to B10.BR skin grafts (▴), or CBA/Ca skin from naive donors (▾). A control group of mice did not receive any initial skin graft (♦). All mice were transfused with 107 spleen cells from naive CBA/Ca at day −1, and transplanted with a fresh B10.BR skin on the following day. Only mice with tolerated skin grafts resisted the challenge transfusion of nontolerant splenocytes and accepted the B10.BR skin grafts indefinitely (▪, n = 5, MST > 100 d, P < 0.002 to other groups). In all other groups the B10.BR skin grafts were rejected at a similar rate. (B) Tolerant mice were grafted with both BALB/c (▪) and B10.BR (▴) skin grafts in the same graft bed, 60 d after challenge with naive CBA/Ca splenocytes and a fresh B10.BR skin. Only BALB/c skin grafts were rejected (P < 0.007).
To confirm that tolerant animals were not immunosuppressed, we showed that the same recipient test animals remained permissive for rejection of third-party skin. BALB/c and fresh B10.BR skin were transplanted in the same graft bed of mice of the nonpermissive group. Fig. 2 B shows that the third-party BALB/c skin grafts were promptly rejected while the B10.BR skin grafts were accepted indefinitely.
Taken together, these results confirm that only the tolerated skin grafts, but not autologous skin from tolerant animals, have the capacity to transfer dominant tolerance.
Tolerance Is Not Due to Microchimerism.
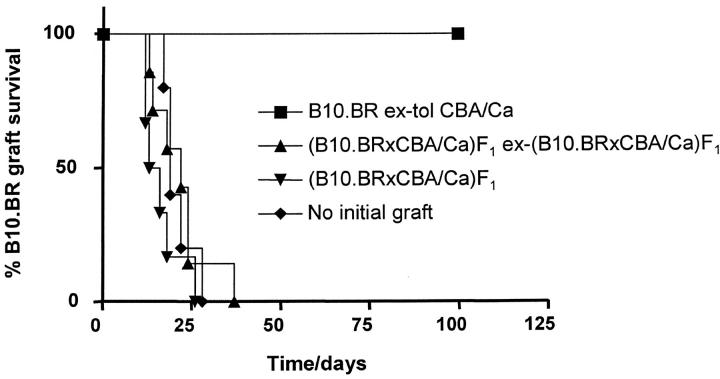
There is evidence implicating donor microchimerism as a mechanism capable of enhancing graft acceptance (22, 23). To investigate whether microchimerism was the explanation for tolerance induced by transfer of tolerated skin grafts, we repeated the experiment but this time grafting CBA/Ca mice with skin from (B10.BR × CBA/Ca)F1. Such skin grafts could contribute to the generation of donor-type microchimerism with cells simultaneously carrying CBA/Ca and B10.BR antigens and being naturally tolerant, by deletion, to both sets of antigens (without B10.BR-specific regulatory T cells). “Empty” CP1-CBA mice were transplanted with tolerated B10.BR skin grafts from tolerant CBA/Ca, another group with (B10.BR × CBA/Ca)F1 skin grafts transplanted previously in syngeneic F1 mice, and yet another group with fresh (B10.BR × CBA/Ca)F1 skin. In one control group, the empty CP1-CBA received no grafts. A challenge intravenous injection with 107 spleen cells from naive CBA/Ca was administered to all CP1-CBA mice 30 d after grafting. All mice received a new B10.BR skin graft on the following day. Fig. 3 shows that only the animals grafted with tolerated B10.BR skin from tolerant CBA/Ca were nonpermissive for naive cells to reject the B10.BR skin grafts. The empty mice, which had been grafted with (B10.BR × CBA/Ca)F1 skin, remained permissive and skin was rejected at rate similar to controls.
Figure 3.
Tolerance is not due to microchimerism. Empty CP1-CBA mice were transplanted at day −30 with tolerated B10.BR skin grafts from tolerant CBA/Ca (▪), (B10.BR × CBA/Ca)F1 skin grafts (▾), or (B10.BR × CBA/Ca)F1 skin grafts transplanted 30 d before into syngeneic hosts (▴). A control group of mice did not receive any initial skin graft (♦). All mice were transfused with 107 spleen cells from naive CBA/Ca at day −1, and transplanted with a fresh B10.BR skin on the following day. Only recipients of tolerated skin grafts resisted the transfusion of nontolerant splenocytes and accepted the B10.BR skin grafts indefinitely (▪, n = 5, MST > 100 d, P < 0.002). In all other groups the B10.BR skin grafts were rejected at a similar rate.
These results exclude microchimerism as being sufficient to drive tolerance achieved by transferring tolerated skin grafts. In addition, we can exclude any requirement for the thymus in the maintenance of the tolerant state, as all recipient mice had been adult thymectomized.
Tolerance Is Due to Regulatory T Cells Present in the Skin Graft.
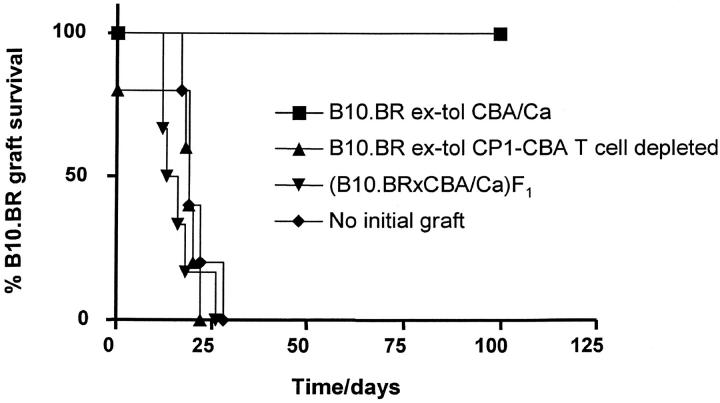
To establish the role of putative regulatory T cells infiltrating the skin graft we used CP1-CBA mice, tolerized to B10.BR skin grafts, as donors of tolerated B10.BR skin. This enabled us to use CAMPATH-1H mAb to deplete donor T cells present in the tolerated skin, once it had been retransplanted. Fig. 4 shows that when tolerated skin was obtained from tolerant CBA/Ca donors, hosts became nonpermissive for the rejection of fresh B10.BR skin graft after transfusion of 107 nontolerant spleen cells. However, when the tolerated B10.BR skin was derived from tolerant CP1-CBA donors, and the hosts depleted of all donor-derived and recipient T cells with 0.25 mg CAMPATH-1H at the time of regraft, grafts were rejected after the transfusion of naive CBA/Ca splenocytes.
Figure 4.
Tolerance is due to regulatory T cells present in the skin graft. Empty CP1-CBA mice were transplanted at day −30 with tolerated B10.BR skin grafts from tolerant CBA/Ca (▪), tolerated B10.BR skin grafts from tolerant CP1-CBA (▴), or (B10.BR × CBA/Ca)F1 skin (▾). A control group of mice did not receive any initial skin graft (♦). All mice were transfused with 107 spleen cells from naive CBA/Ca at day −1, and transplanted with a fresh B10.BR skin on the following day. Mice transplanted with tolerated B10.BR skin grafts from tolerant CP1-CBA (▴) were depleted of infiltrating T cells by treatment with 0.25 mg CAMPATH-1H at days −30 and −1. Recipients of tolerated B10.BR skin grafts from tolerant CBA/Ca were also treated with CAMPATH-1H as described. Only recipients of tolerated skin grafts whose T cells had not been ablated resisted the transfusion of nontolerant splenocytes and accepted B10.BR skin grafts indefinitely (▪, n = 5, MST > 100 d, P < 0.002). In all other groups the B10.BR skin grafts were rejected at a similar rate. Note that one animal in the tolerated skin, T cell–depleted group (▴) rejected the initial B10.BR graft before transfusion with CBA/Ca splenocytes.
From these results we can conclude that when any T cells carried over with tolerated skin grafts are depleted, then tolerance is not imposed on the recipient. As a corollary, when we observe nonpermissiveness, it must be due to regulatory T cells which had infiltrated the tolerated skin grafts, and not to other pro-tolerogenic properties of the tolerated skin, as in the example of neonatal tolerance (24, 25).
T Cells Can Expand from the Tolerated B10.BR Skin Grafts.
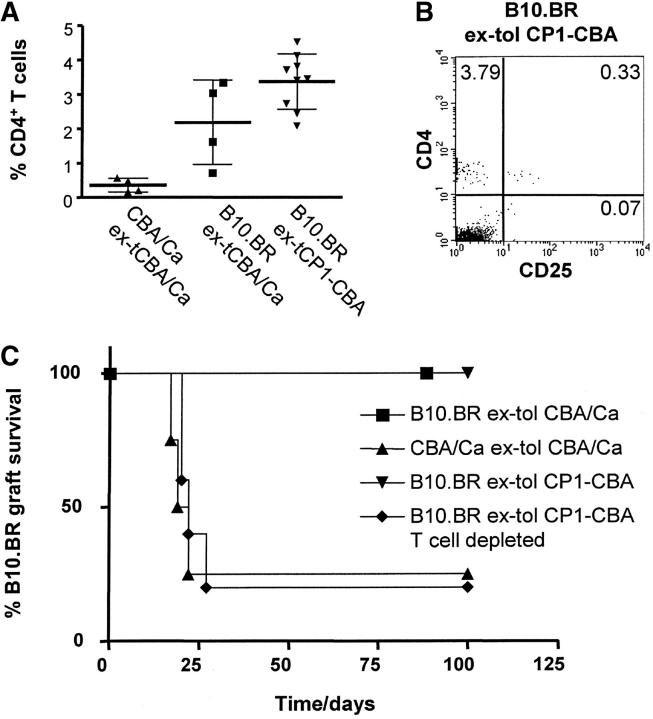
We used RAG1−/−-CBA mice as T cell–deficient hosts to determine whether T cells infiltrating tolerated grafts can expand from the skin. These mice were grafted with tolerated B10.BR skin from either tolerant CBA/Ca or tolerant CP1-CBA, or autologous CBA/Ca skin from CBA/Ca mice tolerant to B10.BR skin grafts. A sample of peripheral blood was collected 30 d after transplantation, stained, and analyzed by flow cytometry. Fig. 5 A shows that CD4+ T cells can be detected in the peripheral blood of transplanted RAG1−/−-CBA mice 30 d after tolerated skin transplantation. Remarkably, the CD4+ T cell frequency was significantly increased in recipients of tolerated skin grafts when compared with recipients of autologous skin from tolerant mice. In all mice the majority of CD4+ cells that had expanded from the graft were CD4+CD25−, but a minority of CD4+CD25+ cells could also be detected (Fig. 5 B). The frequency of CD4+CD25+ T cells within the CD4+ T cell population derived from tolerated skins was not significantly different from the usual frequency in naive CBA/Ca mice. 1 wk after the blood sampling, all animals were transfused with 107 spleen cells from naive CBA/Ca mice, and challenged with a fresh B10.BR skin graft on the following day. In one group of mice transplanted with tolerated B10.BR skin from tolerant CP1-CBA donors, donor T cells were depleted with 0.25 mg CAMPATH-1H at the time of CBA/Ca cell transfusion. These mice became permissive for rejection by naive CBA/Ca cells, with a rejection rate comparable to the group initially grafted with CBA/Ca skin from tolerant CBA/Ca mice (Fig. 5 C). In contrast, when the RAG1−/− -CBA mice were initially transplanted with tolerated B10.BR skin grafts, in the absence of T cell depletion, all mice became nonpermissive for rejection, and consequently all B10.BR grafts were held indefinitely.
Figure 5.
T cells expand from the tolerated B10.BR skin grafts. RAG1−/−-CBA mice were grafted with tolerated B10.BR skin from tolerant CBA/Ca mice (▪), CBA/Ca skin from CBA/Ca mice tolerant to B10.BR skin grafts (▴), and tolerated B10.BR skin grafts from tolerant CP1-CBA mice (▾ and ♦). Tolerated B10.BR skin grafts in group ♦ were depleted of putative infiltrating T cells with 0.25 mg CAMPATH-1H at day −1. (A) Blood samples were collected 30 d after skin grafting and analyzed by FACS®. The graph represents the percentage of CD4+ T cells within blood mononuclear cells. The percentage of CD4+ T cells that expanded from tolerated skin grafts is significantly higher than in the animals grafted with CBA/Ca skin from tolerant syngeneic donors (P < 0.05, unpaired t test). (B) FACS® staining from a mouse of the tolerated skin group (▾), showing that expanded T cells are mainly CD4+CD25−. (C) All mice were transfused with 107 spleen cells from naive CBA/Ca 1 wk after blood tests, and transplanted with a fresh B10.BR skin on the following day (day 0). Recipients of tolerated B10.BR skin grafts whose putative regulatory T cells had not been depleted resisted the challenge with transfused CBA/Ca splenocytes and accepted the B10.BR skin grafts indefinitely (▪ and ▾, MST > 100 d, P < 0.05). Mice that were recipients of tolerated B10.BR skin grafts depleted of T cells rejected the grafts shortly after transfusion of CBA/Ca splenocytes (▴, MST = 22 d). Recipients of CBA/Ca skin from CBA/Ca mice tolerated to B10.BR skin grafts also rejected B10.BR skin grafts (♦, MST = 20.5 d).
We needed to exclude the possibility that the process of transplanting donor skin to RAG1−/−-CBA recipients was not itself conducive to the development of dominant tolerance. We first transplanted CBA/Ca mice with B10.BR skin grafts in the absence of any further treatment. At day 8 after transplantation, when the skin grafts still appeared healthy (rejection usually occurs at days 11–15), the grafts were removed and retransplanted onto RAG1−/−-CBA mice. These grafts were all rejected (n = 6, median survival time [MST] = 9 d from the time of regraft) and contributed to CD4+ T cell expansion as assessed 30 d after transplantation (4.92% ± 0.37 CD4+ T cells in peripheral blood). After transfusion of 107 splenocytes from naive CBA/Ca and transplantation of a second skin graft we confirmed that the cell expansion did not alter the rejection permissive state, as all skin grafts were readily rejected (n = 6, MST = 17 d). This reinforces our conclusion that the regulatory T cells preexisted in tolerated skin before retransplantation onto RAG1−/−-CBA recipients.
We have recently shown that B10.BR skin graft rejection mediated by 107 splenocytes transfused from naive CBA/Ca into empty CP1-CBA mice, can be prevented by cotransfer of regulatory T cells (14). By titrating the number of transfused regulatory cells we concluded that abrogation of rejection requires cotransfer of 106 CD4+CD25+ cells or 107 CD4+CD25− cells from CBA/Ca tolerized to B10.BR skin grafts (14). Such observations, taken together with our present results, suggest that at the time 107 splenocytes from naive CBA/Ca mice are transfused, regulatory cells from tolerated allografts have expanded to evoke regulatory function equivalent to 106 CD4+CD25+ cells from a tolerized spleen.
These results confirm that tolerance achieved by retransplantation of tolerated skin grafts is due to regulatory T cells that infiltrate the transplant, and are not present (at least at comparable levels) within the autologous skin of such tolerized mice. This observation may in part explain the phenomenon of linked suppression, that could operate at the level of the graft, therefore yielding graft acceptance when the tolerated and “third-party” antigens coexist in close proximity, usually on the same cells. In contrast to the situation where the two sets of antigens are present in two different grafts in the same graft-bed, when the third party graft is rejected (9, 10, 26, 27).
Interestingly, a reverse transcription (RT)-PCR analysis of genes expressed in tolerated and rejecting tissues, showed that genes associated with regulatory T cells were found to be differential. This was not, however, the case, when draining lymph nodes or spleens from the same animals were compared, suggested that regulatory activity is concentrated in the graft (28). It is intriguing that on a functional basis, regulatory cells with the capacity to prevent graft rejection can be demonstrated in both the spleen and tolerated skin grafts. It is not clear at this time, given the RT-PCR data, whether graft infiltrating regulatory cells constitute a special resident population different from splenic regulatory cells. The observation that T cells expand from graft infiltrating regulatory cells may imply that regulatory T cells in grafts result from a steady-state recirculation. Perhaps, regulatory cells recirculate through the body and accumulate preferentially at the sites where their target antigens are present. As a consequence it is possible they exert their regulatory activity on peripheral tissues by default, until inflammatory signals or other as yet unknown ligands turn off their suppressive function, so permitting a “normal” protective immune response to occur. In any case, our observations strongly support the view that at least some of the suppressive activity of regulatory T cells occurs beyond secondary lymphoid tissues at the sites where their target antigens are present.
Acknowledgments
The authors are grateful to S. Humm for preparing many of the mAbs used in vivo and to the PSB staff, especially M. Coates and S. Laynes, for excellent animal care. This work was funded by a program grant from the Medical Research Council, UK.
L. Graca was supported by the Calouste Gulbenkian Foundation's PhD Program in Biology and Medicine and the Portuguese Foundation for Science and Technology/Praxis XXI.
References
- 1.Waldmann, H. 1999. Transplantation tolerance-where do we stand? Nat. Med. 5:1245–1248. [DOI] [PubMed] [Google Scholar]
- 2.Wekerle, T., and M. Sykes. 2001. Mixed chimerism and transplantation tolerance. Annu. Rev. Med. 52:353–370. [DOI] [PubMed] [Google Scholar]
- 3.Knechtle, S.J. 2000. Knowledge about transplantation tolerance gained in primates. Curr. Opin. Immunol. 12:552–556. [DOI] [PubMed] [Google Scholar]
- 4.Kirk, A.D., and D.M. Harlan. 2000. Challenges for the clinical application of transplant tolerance strategies. Curr. Opin. Organ Transplant. 5:108–113. [Google Scholar]
- 5.Li, X.C., T.B. Strom, L.A. Turka, and A.D. Wells. 2001. T cell death and transplantation tolerance. Immunity. 14:407–416. [DOI] [PubMed] [Google Scholar]
- 6.Waldmann, H. 2001. Therapeutic approaches for transplantation. Curr. Opin. Immunol. 13:606–610. [DOI] [PubMed] [Google Scholar]
- 7.Qin, S.X., M. Wise, S.P. Cobbold, L. Leong, Y.C. Kong, J.R. Parnes, and H. Waldmann. 1990. Induction of tolerance in peripheral T cells with monoclonal antibodies. Eur. J. Immunol. 20:2737–2745. [DOI] [PubMed] [Google Scholar]
- 8.Qin, S., S.P. Cobbold, H. Pope, J. Elliott, D. Kioussis, J. Davies, and H. Waldmann. 1993. Infectious transplantation tolerance. Science. 259:974–977. [DOI] [PubMed] [Google Scholar]
- 9.Davies, J.D., L.Y. Leong, A. Mellor, S.P. Cobbold, and H. Waldmann. 1996. T cell suppression in transplantation tolerance through linked recognition. J. Immunol. 156:3602–3607. [PubMed] [Google Scholar]
- 10.Chen, Z.K., S.P. Cobbold, H. Waldmann, and S. Metcalfe. 1996. Amplification of natural regulatory immune mechanisms for transplantation tolerance. Transplantation. 62:1200–1206. [DOI] [PubMed] [Google Scholar]
- 11.Waldmann, H., and S. Cobbold. 1998. How do monoclonal antibodies induce tolerance? A role for infectious tolerance? Annu. Rev. Immunol. 16:619–644. [DOI] [PubMed] [Google Scholar]
- 12.Waldmann, H., and S. Cobbold. 2001. Regulating the immune response to transplants. A role for CD4+ regulatory cells? Immunity. 14:399–406. [DOI] [PubMed] [Google Scholar]
- 13.Graca, L., K. Honey, E. Adams, S.P. Cobbold, and H. Waldmann. 2000. Cutting edge: anti-CD154 therapeutic antibodies induce infectious transplantation tolerance. J. Immunol. 165:4783–4786. [DOI] [PubMed] [Google Scholar]
- 14.Graca, L., and S. Thompson. C.-Y. Lin, E. Adams, S.P. Cobbold, and H. Waldmann. 2002. Both CD4+CD25+ and CD4+CD25− regulatory cells mediate dominant transplantation tolerance. J. Immunol. 168:5558–5567. [DOI] [PubMed] [Google Scholar]
- 15.Zhai, Y., and J.W. Kupiec-Weglinski. 1999. What is the role of regulatory T cells in transplantation tolerance? Curr. Opin. Immunol. 11:497–503. [DOI] [PubMed] [Google Scholar]
- 16.Sawitzki, B., M. Lehmann, T. Ritter, E. Graser, J.W. Kupiec-Weglinski, and H.D. Volk. 2001. Regulatory tolerance-mediating T cells in transplantation tolerance. Transplant. Proc. 33:2092–2093. [DOI] [PubMed] [Google Scholar]
- 17.Gilliland, L.K., L.A. Walsh, M.R. Frewin, M.P. Wise, M. Tone, G. Hale, D. Kioussis, and H. Waldmann. 1999. Elimination of the immunogenicity of therapeutic antibodies. J. Immunol. 162:3663–3671. [PubMed] [Google Scholar]
- 18.Monaco, A.P., M.L. Wood, J.G. Gray, and P.S. Russell. 1966. Studies on heterologous anti-lymphocyte serum in mice. II. Effect on the immune response. J. Immunol. 96:229–238. [PubMed] [Google Scholar]
- 19.Billingham, R.E., L. Brent, and P.B. Medawar. 1953. Actively acquired tolerance to foreign cells. Nature. 172:603–606. [DOI] [PubMed] [Google Scholar]
- 20.Peto, R., M.C. Pike, P. Armitage, N.E. Breslow, D.R. Cox, S.V. Howard, N. Mantel, K. McPherson, J. Peto, and P.G. Smith. 1977. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. Br. J. Cancer. 35:1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riechmann, L., M. Clark, H. Waldmann, and G. Winter. 1988. Reshaping human antibodies for therapy. Nature. 332:323–327. [DOI] [PubMed] [Google Scholar]
- 22.Ko, S., A. Deiwick, M.D. Jager, A. Dinkel, F. Rohde, R. Fischer, T.Y. Tsui, K.L. Rittmann, K. Wonigeit, and H.J. Schlitt. 1999. The functional relevance of passenger leukocytes and microchimerism for heart allograft acceptance in the rat. Nat. Med. 5:1292–1297. [DOI] [PubMed] [Google Scholar]
- 23.Anderson, C.C., and P. Matzinger. 2001. Immunity or tolerance: opposite outcomes of microchimerism from skin grafts. Nat. Med. 7:80–87. [DOI] [PubMed] [Google Scholar]
- 24.Alferink, J., A. Tafuri, D. Vestweber, R. Hallmann, G.J. Hammerling, and B. Arnold. 1998. Control of neonatal tolerance to tissue antigens by peripheral T cell trafficking. Science. 282:1338–1341. [DOI] [PubMed] [Google Scholar]
- 25.Alferink, J., S. Aigner, R. Reibke, G.J. Hammerling, and B. Arnold. 1999. Peripheral T-cell tolerance: the contribution of permissive T-cell migration into parenchymal tissues of the neonate. Immunol. Rev. 169:255–261. [DOI] [PubMed] [Google Scholar]
- 26.Wong, W., P.J. Morris, and K.J. Wood. 1997. Pretransplant administration of a single donor class I major histocompatibility complex molecule is sufficient for the indefinite survival of fully allogeneic cardiac allografts: evidence for linked epitope suppression. Transplantation. 63:1490–1494. [DOI] [PubMed] [Google Scholar]
- 27.Honey, K., S.P. Cobbold, and H. Waldmann. 1999. CD40 ligand blockade induces CD4+ T cell tolerance and linked suppression. J. Immunol. 163:4805–4810. [PubMed] [Google Scholar]
- 28.Zelenika, D., E. Adams, S. Humm, L. Graca, S. Thompson, S.P. Cobbold, and H. Waldmann. 2002. Regulatory T cells over-express a subset of Th2 gene transcripts. J. Immunol. 168:1069–1079. [DOI] [PubMed] [Google Scholar]