The generation of memory T cells in immune responses has been extensively studied over recent years, yet the requirements for production and persistence of a functional memory pool are still unclear. For example, while there is compelling evidence that survival of memory cells does not require TCR–MHC engagements (1, 2), recent evidence indicates that such interactions may be needed to maintain functional activity of these cells (3, 4). Furthermore, there is growing evidence that cytokines play a major role in deciding the fate of CD8 memory cells, although the precise mechanisms by which these effects are mediated remain unknown.
Several new reports, one in the Journal of Immunology (5) and four (6–9) in this issue, now provide further insight into the key cytokine players which induce and maintain the CD8 T cell memory pool. In support of previous studies, IL-15 is implicated as a key regulator of CD8 memory T cell homeostasis. Interestingly, the current data show that in at least one case (5), IL-15 deficiency diminishes the magnitude of CD8 T cell expansion in the primary response, resulting in the production of fewer memory cells. Moreover, the results reveal that once memory cells are generated, IL-15 is critical for their basal proliferation. Surprisingly, several of these studies indicate that, in the absence of IL-15, CD8 homeostasis in immunodeficient hosts can also be maintained by IL-7 as long as the latter is not made limiting by competition with other cells. These data lead to a model where cytokine competition between T cell subsets can influence the fate of the memory pool (Fig. 1) .
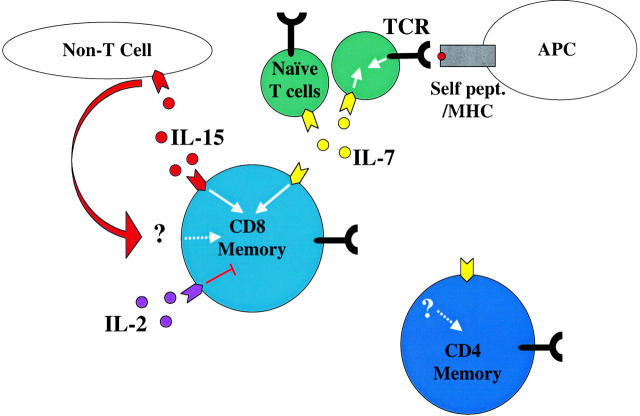
Figure 1.
A model for regulating CD8 memory T cell homeostasis. Naive T cells (green) require signals through their TCR (black) and IL-7R (yellow) for survival and homeostatic expansion. Memory CD8+ T cells (light blue) can use either IL-7R or IL-15R (red) to drive homeostatic proliferation in lymphopenic hosts, but IL-15 is critical for homeostasis in normal animals, where IL-7 may be limiting due to competition with naive T cells. IL-15 might affect memory CD8 T cells directly or via the product/interaction with a “non-T cell.” Some reports suggest IL-2 (purple) might inhibit memory CD8 survival (reference 19), although the mechanism is unclear. None of these cytokines appear to be required for homeostasis of CD4+ memory T cells (dark blue). Neither memory subset requires TCR engagement for survival or homeostatic proliferation. See text for further discussion.
A specialized role for IL-15 in the regulation of T cell memory was suggested by the ability of IL-15 (or IL-15–inducing stimuli) to stimulate proliferation of memory CD8 T cells but not memory CD4 T cells or naive T cells in vivo (10). Furthermore, overexpression of IL-15 leads to persistence of increased numbers of antigen-specific CD8+ memory cells after Listeria infection (11). These findings were amplified by analysis of IL-15 and IL-15Rα knockout mice which display reduced numbers of CD8 T cells in the periphery and virtually lack CD44hi (memory phenotype) CD8 T cells (12, 13). However, these latter reports were focused on analysis of memory phenotype CD8 T cells resident in unimmunized mice, the derivation and specificity of which is unclear. One of these studies (13) reported that IL-15−/− mice cannot mount a protective response to vaccinia, however it was not clear whether this was due to a defect specifically in the CD8 T cell response. Thus, the impact on antigen-specific CD8 memory cells was not addressed. Furthermore, these studies did not determine whether IL-15 was critical for generation and/or persistence of CD8 memory cells.
The new reports address these questions using two main approaches. The first approach was to determine the role of IL-15 in regulating antigen-specific memory T cell generation and survival. Two groups studied CD8 antiviral responses to VSV (5) or LCMV (6) infections in IL-15 and IL-15Rα–deficient animals. The new papers reveal that IL-15 deficiency partially reduces the magnitude (or duration) of the primary CD8 response to VSV, while this effect was less prominent in the LCMV primary response. An intriguing finding was that the primary anti-VSV response in IL-15−/− mice was diminished while the response was normal in IL-15Rα mice, suggesting that an alternative form of the IL-15 receptor might be used in the absence of IL-15Rα. Nevertheless, the overall findings were that CD8 memory T cells were generated in both IL-15−/− and IL-15Rα−/− mice and these cells appeared capable of responding normally, at least in terms of recall cytokine responses (6).
What then accounts for the CD8 memory deficit in IL-15 and IL-15Rα knockout animals? The answer lies in the control of memory cell turnover. Both groups studied the fate of antigen-specific CD8 memory cells transferred into IL-15−/− hosts, an approach also used by Goldrath et al. (8). All three groups show that in the absence of IL-15, CD8 memory cells gradually decline in numbers. This is chiefly due to a dramatic loss in memory CD8 proliferation, since it appears that a subset of nondividing, or extremely slowly dividing, CD8 memory cells survive quite well in the absence of IL-15. This highlights the role of memory cell turnover in the “basal homeostasis” (as Goldrath et al. put it) of this subset; it has been estimated that ∼80% of memory phenotype CD8 cells proliferate in a 5-wk period (14) leading to the idea that memory T cells are not strictly long lived but rather are fecund. IL-15 appears critical for this mechanism of homeostasis. On the other hand, a role for IL-15 in mediating CD8 memory survival is not ruled out by these studies and recent reports indicate IL-15 signaling is important for expression of the antiapoptotic protein Bcl-2 in CD8 T cells (15). Survival and proliferation could be intimately linked in the CD8 memory subset, making resolution of these mechanisms difficult, although the present data might imply that proliferation is not an absolute requirement for survival.
The second experimental approach used in the current reports was to analyze the cytokine requirements for CD8 memory proliferation in lymphopenic hosts (8, 9). Numerous groups have reported that naive T cells (both CD4 and CD8) will proliferate in T cell–deficient hosts (e.g., recombination activation gene−/− or sublethally irradiated mice). This response, typically called homeostatic proliferation or homeostatic expansion, requires both TCR engagement with self-MHC ligands and IL-7R engagement with IL-7 (16–18). In the studies described above the animals have a partial deficiency in total CD8 T cell numbers, which is insufficient to induce homeostatic proliferation (note that Becker et al. [reference 6] use the phrase “homeostatic proliferation” to describe memory turnover in a normal, T cell–replete host, equivalent to the “basal homeostasis” described by Goldrath et al. [reference 8]. As we will see below, the requirements for memory CD8 T cell homeostatic proliferation differ in normal and lymphopenic hosts, so this distinction is important).
Given the studies above, it might be expected that CD8 memory homeostatic proliferation in lymphopenic hosts would be spectacularly diminished in the absence of IL-15. However, this was not the case: all the groups report barely any defect of CD8 memory proliferation in irradiated IL-15−/− hosts compared with irradiated normal hosts. How does CD8 memory proliferation become IL-15 independent in lymphopenic hosts? The solution, it transpires, is IL-7. As discussed above, IL-7 has been shown to be a prerequisite for naive T cell survival and homeostatic proliferation. That IL-7 might also be involved in regulating memory was suggested by the finding that CD8 memory T cells express IL-7R and that IL-7R−/− cells respond to activation normally but are poor producers of CD8 memory cells (18). On the other hand, blocking IL-7 in vivo did not reveal a clear role for IL-7 in memory homeostasis (19).
The new reports show that CD8 memory cell homeostatic proliferation occurred normally (or was only modestly reduced) in animals lacking IL-7 or treated with anti–IL-7Rα antibodies (8, 9), in keeping with previous reports (18). However, when both IL-15 and IL-7 are removed from the system, CD8 memory homeostatic proliferation was completely halted (8, 9). Why then does IL-7 not substitute for IL-15 in the intact host? Since naive T cells “consume” IL-7 in order to survive, and this population easily outnumbers the typical CD8 memory subset, IL-7 might be limiting in normal animals (Fig. 1). Lack of naive T cells (e.g., lymphopenia) would effectively raise available IL-7 levels. Indeed, it has been suggested that such increased IL-7 levels might be one cause for the induction of homeostatic proliferation among naive T cells transferred into T cell–deficient hosts (16).
Support for this idea comes from analysis of mice which overexpress IL-7, as shown by Kieper et al. (7) in this issue. These IL-7 transgenic animals have 25× to 50× elevated IL-7 levels and show a marked increased frequency and absolute number of CD8 memory phenotype cells (as well as less extreme increases in other T cell subsets and in B cells). Most strikingly, the size and characteristics of the CD8 memory subset is similar in IL-7 transgenic mice on either the wild-type or IL-15−/− backgrounds, indicating that IL-7 overexpression functionally compensates for IL-15 deficiency. The IL-7 transgenic IL-15−/− animals are not lymphopenic (quite the opposite, having T cell numbers similar to plain IL-7 transgenics), but the data fit in nicely with the IL-7 competition model described above.
Hence, neither IL-7 nor IL-15 seem to be unique in their effect on promoting memory CD8 T cell proliferation in immunodeficient hosts. It remains to be seen whether IL-7 can affect antigen-specific memory cells in normal hosts. In addition, it has yet to be determined whether CD8 memory cells arising from IL-7–mediated proliferation in an IL-15–deficient environment are functionally normal. Furthermore, the way in which IL-7 substitutes for IL-15 is unclear. Increased signaling through IL-7R could potentially deliver proliferative or survival signals to expand or sustain the memory pool. Goldrath et al. raise the possibility of different functions for IL-15 and IL-7 in memory CD8 survival, the former being involved in proliferation and the latter in survival (8). At face value, the data from Kieper et al. (7) also suggest survival as a more likely role for IL-7, since the percentage of memory phenotype cells proliferating is unchanged in IL-7 transgenic versus normal mice. However, these authors did find an increase in absolute numbers of proliferating memory cells in the IL-7 transgenics, which might suggest a role in driving proliferation, and clearly IL-7 can support proliferative responses during homeostatic proliferation in lymphopenic hosts. Overexpression of IL-7 might also oppose the negative influence of IL-2 which, despite being the canonical T cell growth factor, was shown to be a counterregulator of memory T cell homeostasis by the Marrack laboratory (19).
There are some unexpected findings in the current reports, which suggest further subtleties. Tan et al. (9) describe an experiment to test the model that naive T cell competition for IL-7 restrains CD8 memory expansion in the absence of IL-15. They studied the impact of cotransferring large numbers of naive CD8 T cells on homeostatic proliferation of either naive or memory CD8 T cell populations in irradiated hosts. Since the memory cells might be expected to respond to IL-15, these experiments were performed in irradiated IL15−/− hosts. Such cotransfers halt efficiently homeostatic proliferation of naive T cells, presumably in part through competition for IL-7. Surprisingly, however, the cotransferred naive cells did not slow down memory CD8 T cell expansion in the IL-15−/− hosts. Tan et al. (9) propose that this is due to segregation of naive and memory subsets to different physical locations in the body (while naive cells are confined to T cell zones of the secondary lymphoid organs, memory T cells are much more widely distributed, being found in nonlymphoid tissues; references 20 and 21). Such an argument brings into question the basis for the initial hypothesis however, since it implies that naive and memory subsets do not compete for the same local pool of IL-7. While these results may relate more to technical limitations in the approach than a flaw in the underlying logic, future studies will be needed to tease out the situations in which naive and memory T cells compete for limiting resources.
In addition, the basis by which IL-15 exerts its effects on CD8 memory is less obvious than it might appear. Although IL-15 can clearly have an effect on CD8 memory T cells directly, there is also evidence that IL-15 acts on other cells to influence CD8 memory responses (Fig. 1). A recent report from Lodolce et al. (22) showed that normal T cells do not respond to poly I:C treatment in IL-15Rα2/− hosts, but that IL-15Rα2/− T cells can respond to poly I:C induced activation in a wild type host. Such data indicate IL-15/IL-15R interactions affect memory T cells nonautonomously. The identity of this “bystander” population which responds to IL-15 is still unknown. The current reports do not address the question of whether the role of IL-15 studied is autonomous to the T cell population or not, but this will be an important future question. In light of these new studies, it would be interesting to determine whether IL-7 plays a role in mediating the effect of the IL-15 responsive “bystander” population.
Lastly, one of these reports also analyzes requirements for CD4 memory T cell homeostasis (9). Tan et al. show that, similar to the CD8 memory subset, expansion of CD4 memory T cells is independent of MHC ligands for the TCR, confirming previous studies (1, 2). However, they go on to show that CD4 memory cells are quite content to undergo homeostatic proliferation in the absence of both IL-15 and IL-7. These data support the findings of Lantz et al. (23) who proposed that CD4 memory cells can survive in the absence of the common γ chain (γC) a component of many cytokine receptors, including IL-2, IL-4, IL-7, and IL-15. The situation might be different for human T cells; Geginat et al. recently showed that human CD4+ memory cells (especially the “effector memory” pool) proliferate in response to IL-7 and IL-15, while naive human CD4+ T cells do not proliferate in response to either cytokine (24). What exogenous factors, if any, are required for homeostasis of mouse CD4 memory cells remains a mystery to be unraveled in future studies.
Acknowledgments
This work was supported by National Institutes of Health grants AI38903 (to. S.C. Jameson), AI41576 and DK45260 (to L. Lefrancois), and by the American Cancer Society award RPG-99-264 (to S.C. Jameson).
References
- 1.Murali-Krishna, K., L.L. Lau, S. Sambhara, F. Lemonnier, J. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 286:1377–1381. [DOI] [PubMed] [Google Scholar]
- 2.Swain, S.L., H. Hu, and G. Huston. 1999. Class II-independent generation of CD4 memory T cells from effectors. Science. 286:1381–1383. [DOI] [PubMed] [Google Scholar]
- 3.Kassiotis, G., S. Garcia, E. Simpson, and B. Stockinger. 2002. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat. Immunol. 3:244–250. [DOI] [PubMed] [Google Scholar]
- 4.Rocha, B. 2002. Requirements for memory maintenance. Nat. Immunol. 3:209–210. [DOI] [PubMed] [Google Scholar]
- 5.Schluns, K.S., K. Williams, A. Ma, X.X. Zheng, and L. Lefrancois. 2002. Requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 168:4827–4831. [DOI] [PubMed] [Google Scholar]
- 6.Becker, T.C., E.J. Wherry, D.L. Boone, K. Murali-Krishna, R. Antia, A. Ma, and R. Ahmed. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kieper, W.C., J.T. Tan, B. Bondi-Boyd, L. Gapin, J. Sprent, R. Ceredig, and C.D. Surh. 2002. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J. Exp. Med. 195:1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldrath, A.W., P.V. Sivakumar, M. Glaccum, M.K. Kennedy, M.J. Bevan, C. Benoist, D. Mathis, and E.A. Butz. 2002. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 195:1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan, J.T., B. Ernst, W.C. Kieper, E. LeRoy, J. Sprent, and C.D. Surh. 2002. IL-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory-phenotype CD4+ cells. J. Exp. Med. 195:1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang, X., S. Sun, I. Hwang, D.F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 8:591–599. [DOI] [PubMed] [Google Scholar]
- 11.Yajima, T., H. Nishimura, R. Ishimitsu, T. Watase, D.H. Busch, E.G. Pamer, H. Kuwano, and Y. Yoshikai. 2002. Overexpression of IL-15 in vivo increases antigen-driven memory CD8+ T cells following a microbe exposure. J. Immunol. 168:1198–1203. [DOI] [PubMed] [Google Scholar]
- 12.Lodolce, J.P., D.L. Boone, S. Chai, R.E. Swain, T. Dassopoulos, S. Trettin, and A. Ma. 1998. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 9:669–676. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy, M.K., M. Glaccum, S.N. Brown, E.A. Butz, J.L. Viney, M. Embers, N. Matsuki, K. Charrier, L. Sedger, C.R. Willis, et al. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tough, D.F., and J. Sprent. 1994. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 179:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu, T.S., J.M. Lee, Y.G. Lai, J.C. Hsu, C.Y. Tsai, Y.H. Lee, and N.S. Liao. 2002. Reduced expression of Bcl-2 in CD8+ T cells deficient in the IL-15 receptor α-chain. J. Immunol. 168:705–712. [DOI] [PubMed] [Google Scholar]
- 16.Prlic, M., and S.C. Jameson. 2002. Homeostatic expansion versus antigen-driven proliferation: common ends by different means? Microbes Infect. 4:531–537. [DOI] [PubMed] [Google Scholar]
- 17.Surh, C.D., and J. Sprent. 2002. Regulation of naive and memory T-cell homeostasis. Microbes Infect. 4:51–56. [DOI] [PubMed] [Google Scholar]
- 18.Schluns, K.S., W.C. Kieper, S.C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426–432. [DOI] [PubMed] [Google Scholar]
- 19.Ku, C.C., M. Murakami, A. Sakamoto, J. Kappler, and P. Marrack. 2000. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 288:675–678. [DOI] [PubMed] [Google Scholar]
- 20.Reinhardt, R.L., A. Khoruts, R. Merica, T. Zell, and M.K. Jenkins. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 410:101–105. [DOI] [PubMed] [Google Scholar]
- 21.Masopust, D., V. Vezys, A.L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 291:2413–2417. [DOI] [PubMed] [Google Scholar]
- 22.Lodolce, J.P., P.R. Burkett, D.L. Boone, M. Chien, and A. Ma. 2001. T cell-independent interleukin 15Rα signals are required for bystander proliferation. J. Exp. Med. 194:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lantz, O., I. Grandjean, P. Matzinger, and J.P. Di Santo. 2000. Gamma chain required for naive CD4+ T cell survival but not for antigen proliferation. Nat. Immunol. 1:54–58. [DOI] [PubMed] [Google Scholar]
- 24.Geginat, J., F. Sallusto, and A. Lanzavecchia. 2001. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J. Exp. Med. 194:1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]