Abstract
We recently purified lipoteichoic acid (LTA) from Staphylococcus aureus to more than 99% purity by a novel preparation method and deduced its structure with the first nuclear magnetic resonance (NMR) of a complete LTA. In contrast to Gram-negative lipopolysaccharides, this LTA requires the toll-like receptor (TLR)-2 and not TLR-4 for cytokine induction in monocytes and macrophages. To elucidate the structure–function relationships for LTA from S. aureus, the lipid anchor was prepared by either acidic hydrolysis of native LTA or chemical synthesis (gentiobiosyl-sn-dimyristoylglycerol). Next, a complete LTA molecule with six glycerophosphate units carrying four alanine plus one N-acetyl-glucosamine substituent was synthesized, which displayed the same potency to activate monocytes as native LTA. However, 100–1,000 times higher concentrations of the lipid anchor were required for cytokine induction. It is worthy to note that replacing d-alanine with l-alanine blunted the effect indicating stereoselective recognition. The structure identification of this synthesized and biologically active LTA was proven by NMR and matrix-assisted laser desorption-ionization mass spectrometry. We concluded that the lipid anchor, with its fatty acids, represents an integral part of the immunostimulatory activity of LTA, but requires additional structural components on the polyglycerophosphate backbone.
Keywords: tumor necrosis factor; Gram-positive bacteria; immunity, natural; structure–activity relationship; chemistry, organic
Introduction
The inflammatory responses to Gram-negative and Gram-positive bacteria can hardly be distinguished. However, although most of the immune stimulation caused by Gram-negative bacteria could be attributed to LPS as a general principle, no consensus as to its Gram-positive counterpart was reached during the last decades. LPS has been available in a pure, biologically active form since 1952 (1) and its active principle, the lipid anchor lipid A, was finally proven by chemical synthesis in 1985 (2). More than 45,000 Medline-listed scientific papers since 1966 describe biological activities of LPS.
Lipoteichoic acid (LTA) is found in most Gram-positive bacteria. Like LPS, it is an amphiphilic, negatively charged glycolipid. For three decades there has been intense discussion about whether or not LTA represents a counterpart to LPS. Many studies were performed with commercial preparations of poor quality and the significant activities reported could, in many instances, be traced back to LPS contaminations (3–6). When adequately purified, phenol-extracted LTA from both Staphylococcus aureus (7, 8) and Enterococcus hirae (9) turned out to be essentially inactive in inducing cytokine release as a measure of immunostimulatory activity. Furthermore, a synthetic LTA derivative was found to have no stimulatory activity (10–12). These observations have put into doubt the general role of LTA as an immunostimulator. We have recently shown that many of these concerns resulted from the inappropriate preparation methods used for LTA (13). After the phenol extraction, which is also used for LPS, we obtained partially degraded LTA. A novel, more gentle preparation scheme led to essentially homogenous, biologically active LTA. By means of structural analysis, we could attribute a crucial role to preserved d-alanine substituents of the polyglycerophosphate backbone of LTA (13). As a final proof of the biological activity of LTA, and to allow structure–function analysis, the chemical synthesis of a biologically active LTA based on the structure of the one from S. aureus was performed.
Materials and Methods
Synthesis Strategy for LTA.
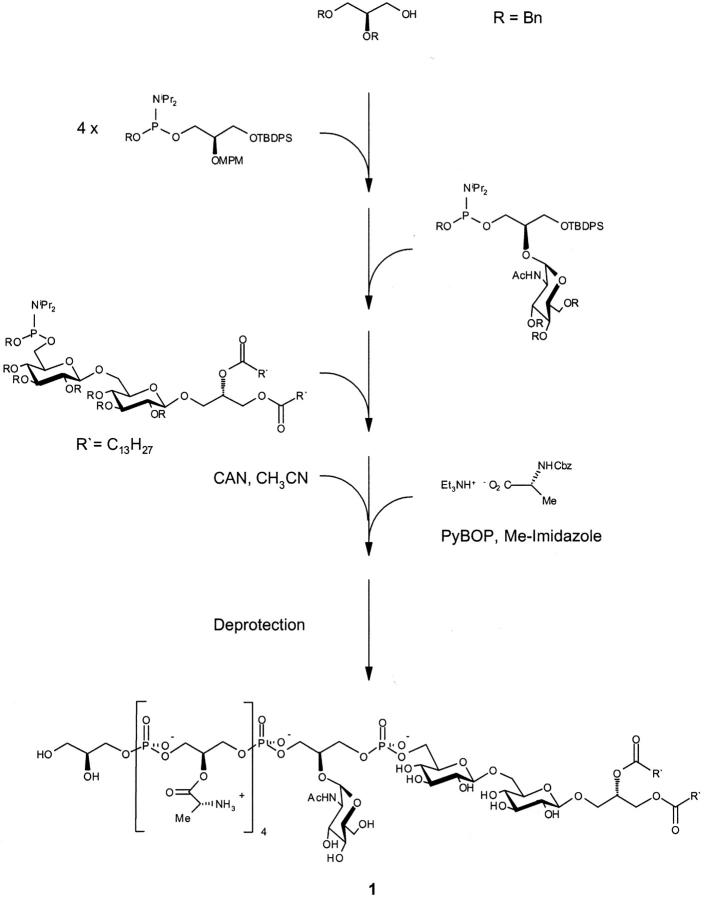
To confirm the structural assignment and the biological effect, compound 1, having six glycerol phosphate residues, was planned as the synthetic target. Thus, the proportion of the substituents d-Ala (66%), d-GlcNAc (17%), and hydrogen (17%) at the 2-O position of the glycerol moiety corresponds approximately to the distributions found in the natural product, i.e., d-Ala (70%), d-GlcNAc (15%), and hydrogen (17%), respectively (13). Compound 1 was assembled with the five building blocks shown in Fig. 1 based on a carefully selected protective group pattern. The phosphorous esters were obtained based on the phosphitamide methodology. The hydrolytically highly labile d-Ala residues were attached in the second to last step, which was immediately followed by deprotection (hydrogenolytic removal of all 21 benzyl groups) and purification by hydrophobic interaction chromatography on octyl Sepharose (13), thus providing compound 1 in good overall yield. The structure was confirmed by nuclear magnetic resonance (NMR) and mass spectrometry (MS) data (Fig. 2) .
Figure 1.
Scheme of the synthesis strategy for LTA analogue 1 from S. aureus.
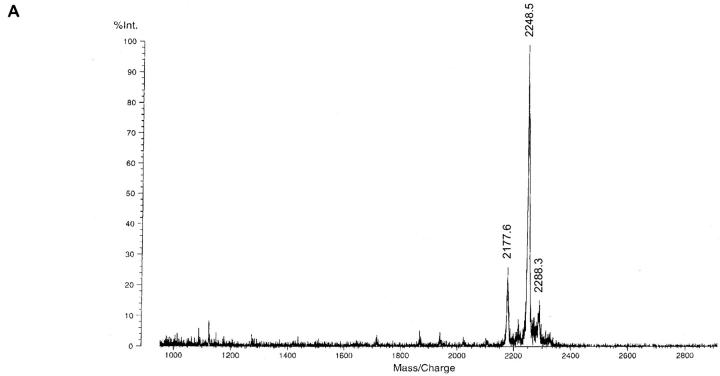
Figure 2.
Identification of synthetic LTA by MALDI-TOF MS and 1H-NMR. (A) Part of negative ion MALDI-TOF mass spectrum of synthetic LTA (calculated molecular weight: 2,248.9). In this mode, different ionization of the single-charged, deprotonated LTA molecule (M−H)− = 2,248.5, i.e., ([M+K] − 2H)− = 2,288.3 and prompt fragmentation of a d-alanine group (Ala) ([M−Ala]−H)− = 2,177.6 caused peak diversity. (B) The target structure (upper) and the confirmation by 1H-NMR spectrum (lower) are shown. The different signals were assigned to structural components by color coding.
LTA Structure Analysis.
NMR experiments were performed at 600.13 MHz (1H) and 300 K. The NMR spectra were related to 3-(trimethylsilyl) 3,3,2,2-tetradeuteropropionic acid Na salt (d4-TSPA). Homonuclear assignments were taken from double-quantum filtered correlation, total correlation, rotating frame Overhauser enhancement, and nuclear Overhauser effect spectra. 13C assignments were based on heteronuclear multiple-quantum correlation. Mass spectra were performed by gas chromatography-mass spectrometry (GC-MS) and matrix-assisted laser desorption-ionization time-of-flight (MALDI-TOF) MS.
Deacylation of Native LTA.
Hydrolysis of LTA from S. aureus (13) was performed at 37°C for 2 h with 0.1 M NaOH. Fatty acids of LTA were determined by GC-MS (Hewlett-Packard) as the respective methyl esters after methanolysis using 2 M HCl in methanol for 7 h at 85°C. The water phase was neutralized with NaHCO3 and freed of salts by dialysis in Spectrapor tubing with a molecular mass cut-off of 1,000 daltons.
Native LTA Anchor Preparation.
After hydrolyzing 220 mg LTA from S. aureus (13) with 5 ml hydrofluoric acid (48%) at 2°C for 42 h, 150 ml distilled water and 35 ml saturated NaHCO3 were added. The lyophilisate was resuspended in 70 ml H2O/CH2Cl2/MeOH (3:3:1) and centrifuged at 3,200 rpm for 7 min. The pellet was washed with CH2Cl2 and the water layer with CH2Cl2/MeOH (3:1). The collected organic phases were evaporated under vacuum at 40°C to a volume of ∼15 ml and lyophilized. An aliquot of the lyophilisate (10 mg) was resolved in 200 μl methanol and subjected to a high performance thin layer chromatography plate silica gel 60 without fluorescent indicator using the solvent system CHCl3/MeOH/H2O (65:25:4). The Rf of the intact glycolipid was 0.68 (0.42 for anchor with one fatty acid). The substance line was scratched off and filtered over a glass filter (pore size 4) under vacuum after suspending with thin layer chromatography solvent. Finally, the solution was evaporated and lyophilized.
Murine Peritoneal Cell Preparation.
Male C3H/HeN and C3H/HeJ mice were purchased from Charles River Laboratories. The animals received humane care in accordance with the National Institutes of Health guidelines and the legal requirements in Germany. Mice were put to sleep by terminal pentobarbital anesthesia (Narcoren™; Merial) and 10 ml of ice-cold PBS (GIBCO BRL) were injected into the peritoneal cavity. Animals were shaken gently and the lavage liquid was transferred to siliconized glass tubes (Vacutainer®; Becton Dickinson). After centrifugation, cells were resuspended in RPMI 1640 medium (BioWhittaker) containing 10% FCS (Boehringer) and 100 IU/ml penicillin/streptomycin (Biochrom), and transferred to 96-well cell culture plates (105 cells/well). For the determination of cytokine induction by LPS, native LTA, or synthetic glycolipids, cells were stimulated immediately with pyrogen-free saline as solvent (Braun) at 37°C, 5% CO2 in a humidified atmosphere overnight.
Cytokine Induction Assay.
Cytokine release of human whole blood was determined as previously described (15) by incubating 800 μl isotonic sodium chloride solution, 200 μl human heparinized whole blood, and 10 μl stimuli. TNF-α was measured by sandwich ELISA (Endogen).
Results and Discussion
For LPS, the key role of fatty acids in binding to cellular receptors and the activation of monocyte/macrophages is well established (14). When LTA from S. aureus was deacylated by alkaline hydrolysis, the material failed to induce any cytokine release in human whole blood. It is worthy to note that the deacylated molecule was not an antagonist of native LTA (2,330 ± 700 pg/ml TNF-α inducible by 1 μg/ml native LTA alone vs. 2,380 ± 430 pg/ml TNF-α inducible with the addition of 10 μg deacylated LTA; n = 4, NS), which indicates that it had lost its ability to compete for the cellular binding site.
This finding indicated that the lipid anchor of LTA represents a key component of its biological activity. Therefore, the lipid anchor was prepared by hydrofluoric acid hydrolysis and purified by thin layer chromatography. The resulting material did stimulate cytokine release in human whole blood but microgram/milliliter quantities of the LTA glycolipid were required (Fig. 3 A), whereas 600-fold less native, complete LTA sufficed for cytokine induction. Next, the lipid anchor was synthesized. GC-MS analysis of the native anchor showed a considerable heterogeneity of fatty acids in the anchor (13) as ∼50% of the fatty acids were methylated. The most frequent fatty acids found were tetradecanoic acids (>36%), which were chosen for the lipid anchor synthesis in the nonbranched form. The synthetic molecule displayed activities similar to the natural isolate (Fig. 3 A). The relative role of different fatty acids will require further studies. Interestingly, when structurally very similar compounds were tested, i.e., lactosyl disaccharides that were ether-linked to fatty acids, we found a similar induction of cytokine release to the lipid anchor (unpublished data). Furthermore, when the racemic mixture of both stereoisomers of the LTA anchor was compared with the pure isomer, no major difference in cytokine induction was observed (Fig. 3 A). In conclusion, the pattern recognition of the lipid anchor is relatively loose, allowing a high degree of variability of the anchor structure.
Figure 3.
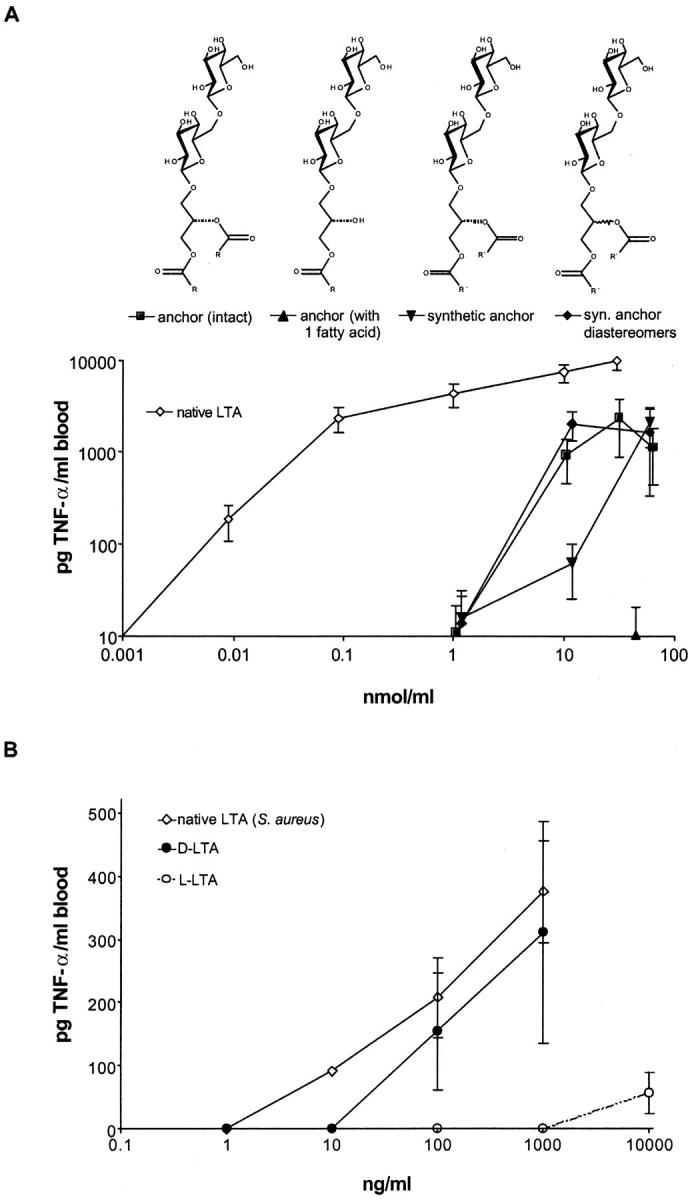
TNF-α induction by LTA anchor derivatives and synthetic LTA. (A) The fission products of native LTA from S. aureus, intact anchor (▪), partially deacylated anchor (▴), and the synthetic compounds (▾, pure diastereomer and ♦, diastereomeric mixture) were used at the concentrations indicated to induce TNF-α release in human whole blood in comparison to native LTA from S. aureus (⋄). As a comparison, 10 ng/ml LPS (Salmonella abortus equi) induced 7,620 ± 3,670 pg/ml TNF-α. Data are given as means ± SEM of whole blood incubations of three donors. (B) Human whole blood was incubated in the presence of synthetic LTA carrying d-alanine (•) or l-alanine (○) substituents, or native LTA (⋄) at the concentrations indicated.
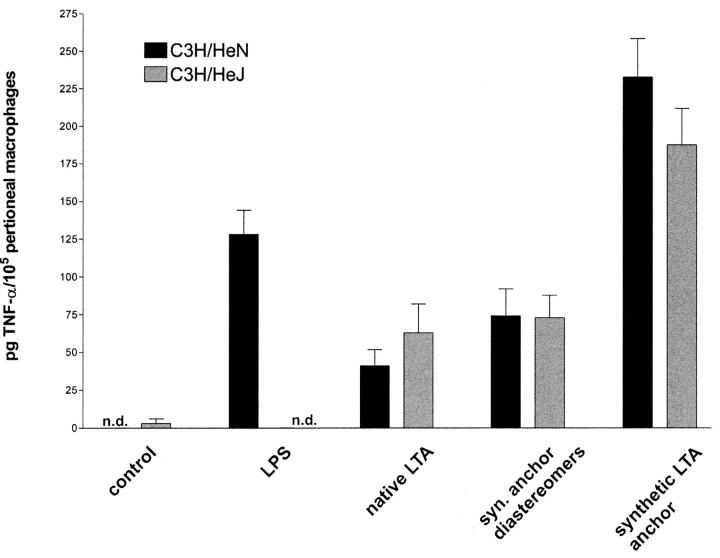
The fact that the lipid anchor alone was a factor of 100–1,000 less potent in inducing cytokine release than native LTA (Fig. 3 A), prompted the synthesis of a complete LTA molecule. The synthesis strategy is depicted in Fig. 1. The assembly of the five building blocks resulted in milligram quantities of LTA, which was purified by hydrophobic interaction chromatography. The identity of the molecule was shown by NMR and MS (Fig. 2). The material was negative in the Limulus test for Gram-negative endotoxin (i.e., <10 pg LPS/mg LTA; Endosafe Limulus amebocyte lysate [LAL] Endochrome; Charles River Laboratories), excluding an LPS contamination and demonstrating that the LAL test does not detect this Gram-positive stimulus. The combination of analytical methods excludes any contamination by more than 1% and the LAL test largely excludes LPS contamination. The latter notion is supported by the finding that macrophages from LPS-nonresponsive C3H/HeJ mice responded similarly to those of LPS-responsive C3H/HeN mice to both the synthetic lipid anchor and native LTA (Fig. 4) .
Figure 4.
Toll-like receptor dependence of TNF-α induction by synthetic LTA anchor. TNF-α induction in murine peritoneal cells (105 in 220 μl) by stimulation with 100 ng/ml LPS, 10 μg/ml native LTA, or 28 μg/ml synthetic glycolipids. LPS-nonresponsive C3H/HeJ (gray bars) are shown in comparison to C3H/HeN cells (black bars). n.d., not detectable. Data are given as means ± SEM of a group of four mice.
The resulting compound was as potent in inducing cytokine formation in human whole blood as the native molecule (Fig. 3 B). We concluded that the structure of LTA from S. aureus suggested (13), and indeed represents, an immunostimulatory molecule. Although the natural compounds termed LTA exist as a rather heterogeneous class of apparently related molecules, the synthetic compounds appear to reflect key structural requirements for immune activation. We confirmed the key role of d-Ala substituents by synthesizing the l-Ala derivative, which made the compound less active by a factor of 100 (Fig. 3 B).
The biological activity of native LTA has been characterized recently in several studies (13, 15–19). The fact that the synthetic LTA behaved very similarly with regard to potency and pattern of cytokine induction as well as toll-like receptor use in vitro and in vivo (15, 16), suggests that it mirrors a major component of the biological isolate which, however, does not necessarily translate to all activities of the biological isolates.
Taken together, these studies show that LTA represents a Gram-positive endotoxin, i.e., a nonsecreted toxin of bacteria as originally defined. We suggest to broaden the use of the term endotoxin in this sense, to cover Gram-negative lipopolysaccharides, Gram-positive LTAs, and further immunostimulatory structures of the bacterial cell wall yet to be identified. Furthermore, we suggest to term the lipid anchor of LTA as “lipid B” in analogy to the name “lipid A” carried by the LPS anchor. Additional studies will be required to distinguish the different activities of LPS and LTA and define the effects of heterogeneities of the LTA molecule.
Acknowledgments
The authors are indebted to Professor Werner Fischer, who pioneered LTA research and introduced us to the field. The valuable help of Leonardo Cobianchi, Susanne Deininger, Isabel Diterich, Anke Friemel, Jürgen Kley, Ina Seuffert, and Stephanie Traub is gratefully appreciated. We thank Sonja von Aulock for help with the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
References
- 1.Westphal, O., O. Lüderitz, and F. Bister. 1952. Über die Extraktion von Bakterien mit Phenol/Wasser. Z. Naturforsch. 7:148–155. [Google Scholar]
- 2.Galanos, C., O. Luederitz, E.T. Rietschel, O. Westphal, H. Brade, L. Brade, M. Freudenberg, U. Schade, M. Imoto, and H. Yoshimura. 1985. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur. J. Biochem. 148:1–5. [DOI] [PubMed] [Google Scholar]
- 3.Gao, J.J., Q. Xue, E.G. Zuvanich, K.R. Haghi, and D.C. Morrison. 2001. Commercial preparations of lipoteichoic acid contain endotoxin that contributes to activation of mouse macrophages in vitro. Infect. Immun. 69:751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawamura, N., N. Imanishi, H. Koike, H. Nakahara, L. Phillips, and S. Morooka. 1995. Lipoteichoic acid-induced neutrophil adhesion via E-selectin to human umbilical vein endothelial cells (HUVECs). Biochem. Biophys. Res. Commun. 217:1208–1215. [DOI] [PubMed] [Google Scholar]
- 5.Sugiyama, A., R. Arakaki, T. Ohnishi, N. Arakaki, Y. Daikuhara, and H. Takada. 1996. Lipoteichoic acid and interleukin 1 stimulate synergistically production of hepatocyte growth factor (scatter factor) in human gingival fibroblasts in culture. Infect. Immun. 64:1426–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morath, S., A. Geyer, I. Spreitzer, C. Hermann, and T. Hartung. 2002. Structural decomposition and heterogeneity of commercial lipoteichoic acid preparations. Infect. Immun. 70:938–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keller, R., W. Fischer, R. Keist, and S. Bassetti. 1992. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect. Immun. 60:3664–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusunoki, T., E. Hailman, T.S. Juan, H.S. Lichenstein, and S.D. Wright. 1995. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J. Exp. Med. 182:1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suda, Y., H. Tochio, K. Kawano, H. Takada, T. Yoshida, S. Kotani, and S. Kusumoto. 1995. Cytokine-inducing glycolipids in the lipoteichoic acid fraction from Enterococcus hirae ATCC 9790. FEMS Immunol. Med. Microbiol. 12:97–112. [DOI] [PubMed] [Google Scholar]
- 10.Fukase, K., T. Matsumoto, N. Ito, T. Yoshimura, S. Kotani, and S. Kusumoto. 1992. Synthetic study on lipoteichoic acid of gram positive bacteria. I. Synthesis of proposed fundamental structure of Streptococcus pyogenes lipoteichoic acid. Bull. Chem. Soc. Jpn. 65:2643–2654. [Google Scholar]
- 11.Fukase, K., T. Yoshimura, S. Kotani, and S. Kusumoto. 1994. Synthetic study on lipoteichoic acid of gram positive bacteria. II. Synthesis of proposed fundamental structure of Enterococcus hirae lipoteichoic acid. Bull. Chem. Soc. Jpn. 67:473–482. [Google Scholar]
- 12.Oltvoort, J.J., C.A.A. van Boeckel, J.H. de Koning, and J.H. van Boom. 1982. A simple approach to the synthesis of a membrane teichoic acid fragment of Staphylococcus aureus Recl. Trav. Chim. Pays-Bas. 101:87–91. [Google Scholar]
- 13.Morath, S., A. Geyer, and T. Hartung. 2001. Structure–function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 193:393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buck, R.A., J. Cai, P.D. Eleazer, R.H. Staat, and H.E. Hurst. 2001. Detoxification of endotoxin by endodontic irrigants and calcium hydroxide. J. Endod. 27:325–327. [DOI] [PubMed] [Google Scholar]
- 15.Lehner, M.D., S. Morath, K.S. Michelsen, R.R. Schumann, and T. Hartung. 2001. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J. Immunol. 166:5161–5167. [DOI] [PubMed] [Google Scholar]
- 16.Hermann, C., I. Spreitzer, N.W. Schroder, S. Morath, M.D. Lehner, W. Fischer, C. Schutt, R.R. Schumann, and T. Hartung. 2002. Cytokine induction by purified lipoteichoic acids from various bacterial species—role of LBP, sCD14, CD14 and failure to induce IL-12 and subsequent IFN-γ release. Eur. J. Immunol. 32:541–551. [DOI] [PubMed] [Google Scholar]
- 17.Opitz, B., N.W. Schroder, I. Spreitzer, K.S. Michelsen, C.J. Kirschning, W. Hallatschek, U. Zahringer, T. Hartung, U.B. Gobel, and R.R. Schumann. 2001. Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-kB translocation. J. Biol. Chem. 276:22041–22047. [DOI] [PubMed] [Google Scholar]
- 18.Michelsen, K.S., A. Aicher, M. Mohaupt, T. Hartung, S. Dimmeler, C.J. Kirschning, and R.R. Schumann. 2001. The role of toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCs). Peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J. Biol. Chem. 276:25680–25686. [DOI] [PubMed] [Google Scholar]
- 19.van de Wetering, J.K., M. van Eijk, L.M. van Golde, T. Hartung, J.A. van Strijp, and J.J. Batenburg. 2001. Characteristics of surfactant protein A and D binding to lipoteichoic acid and peptidoglycan, 2 major cell wall components of gram-positive bacteria. J. Infect. Dis. 184:1143–1151. [DOI] [PubMed] [Google Scholar]