Abstract
Activation of the nuclear factor (NF)-κB transcription complex by signals derived from the surface expressed B cell antigen receptor controls B cell development, survival, and antigenic responses. Activation of NF-κB is critically dependent on serine phosphorylation of the IκB protein by the multi-component IκB kinase (IKK) containing two catalytic subunits (IKKα and IKKβ) and one regulatory subunit (IKKγ). Using mice deficient for protein kinase C β (PKCβ) we show an essential role of PKCβ in the phosphorylation of IKKα and the subsequent activation of NF-κB in B cells. Defective IKKα phosphorylation correlates with impaired B cell antigen receptor–mediated induction of the pro-survival protein Bcl-xL. Lack of IKKα phosphorylation and defective NF-κB induction in the absence of PKCβ explains the similarity in immunodeficiencies caused by PKCβ or IKKα ablation in B cells. Furthermore, the well established functional cooperation between the protein tyrosine kinase Bruton's tyrosine kinase (Btk), which regulates the activity of NF-κB and PKCβ, suggests PKCβ as a likely serine/threonine kinase component of the Btk-dependent NF-κB activating signal transduction chain downstream of the BCR.
Keywords: B cell survival, B cell receptor, signal transduction, IκB kinase complex, Btk
Introduction
Efficient B cell immunity requires mechanisms that guarantee the representation of a diverse immunoglobulin repertoire within the peripheral B cell pool (1). A key feature of peripheral B cells in this context is their ability to persist in the absence of antigenic stimulation. The relatively long life of mature naive B cells in peripheral lymphoid organs of mice and man increases the likelihood for an individual B cell to encounter an antigen and initiate an effective humoral immune response (2). Conversely, reduced peripheral B cell survival is frequently associated with naturally occurring or genetically engineered immunodeficiencies (3, 4).
Survival of peripheral B cells is critically dependent on the surface expression of the B cell antigen receptor (BCR) and its ability to generate a signal. Ablation of surface expressed BCR on peripheral B cells by Cre recombinase-mediated modification of the Ig locus or inducible inactivation of Syk protein kinase gene lead to rapid B cell death (5; and unpublished data). These observations suggest a BCR-dependent signaling cascade responsible for B cell survival in the absence of antigenic stimulation. Furthermore, reduction in the expression level of Bcl-2 protein after the BCR ablation and the rescue from apoptosis of BCR-deficient B cells by Bcl-2 overexpression point to the existence of a signaling axis connecting the BCR and transcription of anti-apoptotic Bcl-2 family of proteins (5). A likely link between the BCR and expression of the anti-apoptotic proteins is suggested by the ability of the BCR to trigger the activation of nuclear factor (NF)-κB, which is known to control the expression of Bcl-2 and Bcl-xL (6). Successful activation of NF-κB and hence transcription of the NF-κB–dependent genes by various ligands requires the phosphorylation of the inhibitory protein IκB. Unphosphorylated IκB is bound to the complex of the transcription activating Rel-subunits and retains these within the cytoplasm. Upon cellular activation, IκB is phosphorylated on serine residues by the multicomponent IκB kinase (IKK) containing two catalytic subunits (IKKα and IKKβ) and one regulatory subunit (IKKγ). Phosphorylation of IκB targets it for ubiquitination and subsequent degradation by the proteasome (7, 8).
Recent studies demonstrated that phosphorylation of IκBα downstream of BCR triggering is regulated by the Bruton's tyrosine kinase (Btk; references 9 and 10). Mutation of Btk results in X-linked agammaglobulinemia (XLA) in humans and X-linked immunodeficiency (Xid) in mice (11). BCR cross-linking induces IκBα phosphorylation in wild-type B cells, whereas Btk-deficient B cells or Xid B cells are unable to support IκBα phosphorylation and efficient activation of NF-κB. Combined with the short life-span and inefficient Bcl-xL induction of Btk-deficient/Xid B cells, these data support an important role of Btk-mediated NF-κB activation in BCR-dependent B cell survival (9, 10, 12).
The substrate specificity of Btk as a tyrosine kinase precludes its direct involvement in the phosphorylation of IKKs or IκBs on serine residues. This, in turn, implies the existence of a Btk-dependent serine kinase involved in the regulation of IKK activity, IκB phosphorylation, and NF-κB activation (9, 10).
Recently, a functional link between Btk and the Ca2+/diacylglycerol (DAG)-dependent serine/threonine protein kinase C β (PKCβ) was demonstrated. B cells of mice deficient for PKCβ display a signaling phenotype similar to Xid B cells (13–15). We therefore hypothesized that PKCβ may be involved in the Btk-mediated NF-κB activation in B cells.
Here we demonstrate that PKCβ selectively controls phosphorylation of IKKα in mature B cells and is required for the efficient BCR-induced IκBα phosphorylation, degradation, and subsequent NF-κB induction. Furthermore, impaired phosphorylation of IKKα and compromised activation of NF-κB correlate with defective BCR-mediated induction of Bcl-xL in B cells, thus providing a mechanistic explanation for the reduced life-span of PKCβ-deficient B cells.
Materials and Methods
Mice.
PKCβ−/− mice on C57BL/6 or 129/Sv genetic background were used for analysis (13). Mice were kept in the animal facility of the Institute for Genetics at the University of Cologne or in the SPF facility of the Laboratory Animal Research Center at The Rockefeller University following the university guide lines. Wild-type C57BL/6 or 129/Sv mice were purchased from The Jackson Laboratory or Charles River Laboratories, respectively.
B Cell Proliferation.
Splenic B cells of >95% purity were isolated as described previously (16). Splenic B cells were stimulated in vitro with 1.2 μg/ml goat anti–mouse IgM F(ab′)2 (Jackson ImmunoResearch Laboratories), 7.5 μg/ml anti-CD38 (17), or 5.25 μg/ml anti-RP105 (18) alone or in combination with 25 U/ml recombinant mouse IL-4 (Genzyme) as described previously (16). Labeling of cells with 5-(and 6-)-carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE; Molecular Probes) for measurement of the proliferative responses was performed as described (19). The decline in CFSE fluorescence as measure of B cell proliferation was determined by FACS® analysis.
Cell Survival Assay.
Purified splenic B cells were cultured in the absence or presence of 25 U/ml recombinant mouse IL-4 (Genzyme) for various times. Cells were washed once with ice-cold Annexin V binding buffer (10 mM Hepes, pH 7.5, 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2) and cell pellets were stained with Annexin V (Roche) and 7-aminoactinomycin D (7AAD; Sigma-Aldrich) as described (5).
Western Blotting Analysis and EMSA.
The expression level of Bcl-2 and Bcl-xL proteins were analyzed by Western blotting. Splenic B cells were purified as described previously (16). 2 × 106 purified B cells were cultured with 10 μg/ml F(ab′)2 fragment goat anti-IgM (Jackson ImmunoResearch Laboratories) for indicated time. Cells were harvested and lysed by 1% NP-40 containing buffer as described previously (16). Anti-Bcl-2 (Neomarkers) and anti-Bcl-xL (Cell Signaling Technology) were used for this analysis. The loading of the protein was controlled by Western blotting of anti-α-Actin (Oncogene Research Products).
For the analysis of Rel family protein expression, the protein lysate of purified splenic B cells from PKCβ−/− and control mice were analyzed by Western blotting with antibodies against RelA, RelB, c-Rel, and p52 (Santa Cruz Biotechnology, Inc.). To analyze the IKKα/IKKβ/IKKγ complex, purified splenic B cells were lysed 1% CHAPS containing buffer. After the centrifugation and removal of the insoluble fraction, the protein lysates were precleared by rabbit Ig and protein A-sepharose. The IKK complex was immunoprecipitated with anti-IKKα antibody (Cell Signaling Technology) or control rabbit Ig followed by the incubation with protein A–sepharose. Sepharose beads were washed three times with lysis buffer and subjected to SDS-PAGE and transferred onto PVDF membrane. The membrane was incubated with anti-IKKα (Upstate Biotechnology), anti-IKKβ (Upstate Biotechnology), or anti-IKKγ antibodies (Santa Cruz Biotechnology, Inc.).
NF-κB activation was studied after the stimulation with 20 μg/ml goat anti–mouse IgM F(ab′)2 (Jackson ImmunoResearch Laboratories) or 10 μg/ml anti-CD40 (3/23; BD PharMingen) using cytoplasmic and nuclear extracts prepared as described (19). Degradation and phosphorylation of IκBα protein were measured by Western blotting with anti-IκBα antibody (Santa Cruz Biotechnology, Inc.) and anti-phospho IκBα (Ser32; Cell Signaling Technology). The phosphorylation of IKKα and IKKβ was analyzed by the antibody against phospho-specific form of IKKα (Ser180) and IKKβ (Ser181; Cell Signaling Technology). For the loading control, the membranes were stripped and reprobed with anti-IKKα and anti-IKKβ antibodies (Upstate Biotechnology). DNA-binding activity of NF-κB was analyzed by electrophoretic mobility shift assay (EMSA) as described (19). For the supershift, antibodies against p50, RelA and c-Rel (Santa Cruz Biotechnology, Inc.) were added before the binding reaction with DNA. A mutant oligonucleotide was used to test the specificity of DNA binding. The protein loading was determined by Oct-1 EMSA. All quantifications were done with NIH image software.
Results and Discussion
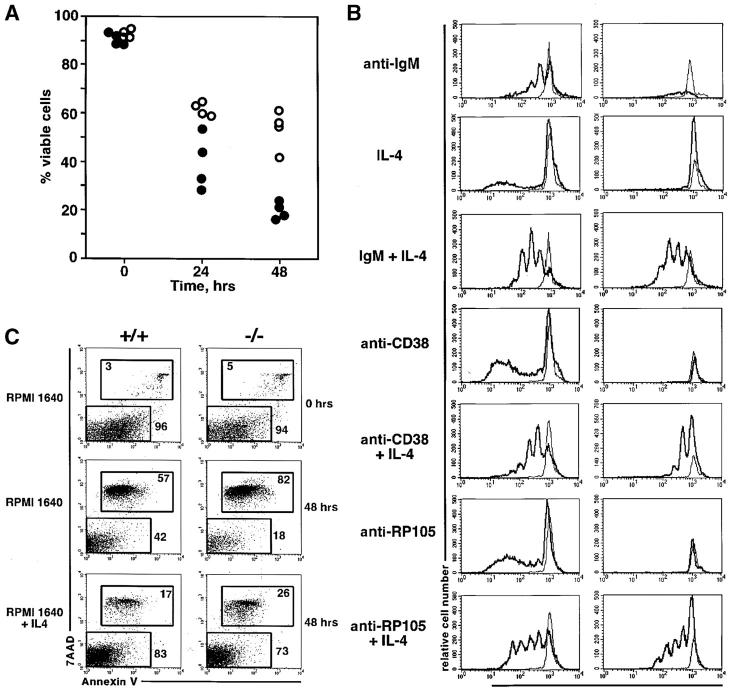
B cells deficient for Btk or expressing a mutant form of the Btk protein are characterized by drastically reduced life-span in vitro and in vivo (12). Similar properties are characteristic for PKCβ-deficient B cells. Incubation of ex vivo isolated splenic PKCβ-deficient B cells in vitro in the absence of exogenously added agonists of B cell survival is accompanied by a rapid decline of cell viability (Fig. 1 A). Similar to B cells with impaired Btk function, the PKCβ-deficient B cells are characterized by drastically decreased proliferative responses to the antibody-mediated cross-linking of BCR, or to the polyclonal activation by anti-CD38 or anti-RP105 antibodies (Fig. 1 B). Addition of the general B cell survival factor IL-4 (20) to the culture medium promotes B cell survival (Fig. 1 C) and increases the fraction of dividing cells (Fig. 1 B). These data suggest that accelerated death of PKCβ-deficient B cells in vitro is likely causing defective proliferative responses of PKCβ-deficient B cells to various stimuli in vitro.
Figure 1.
Impaired survival and proliferation of PKCβ-deficient B cells in vitro. (A) Accelerated cell death of PKCβ−/− B cells cultured without stimulation. Percentage of cell viability of purified splenic B cells is plotted for wild-type (129/Sv) control (open circles) and PKCβ−/− (filled circles) B cells. Each dot represents cells isolated from an individual mouse. (B) Impaired proliferative responses of PKCβ−/− B cells in vitro in the absence of IL-4 survival signal. Purified splenic B cells of wild-type (left column) or PKCβ−/− mice (right column) were labeled with CFSE and incubated for 3 d in the presence or absence of the indicated stimuli (see Materials and Methods for details). The thick line indicates the level of CFSE florescence in cells upon stimulation, whereas the thin line indicates the CFSE label of nonstimulated cells. Reduction in the CFSE fluorescence is proportional to the number of cell divisions. (C) IL-4 promotes survival of both wild-type and PKCβ−/− B cells. FACS® analysis of Annexin-V in combination of 7AAD staining of wild-type (left column) and PKCβ−/− (right column) B cells cultured in complete RPMI with or without IL-4. Note the increase of live cells annexin-V and 7AAD negative in the presence of IL-4. Numbers indicate percentages of gated cells.
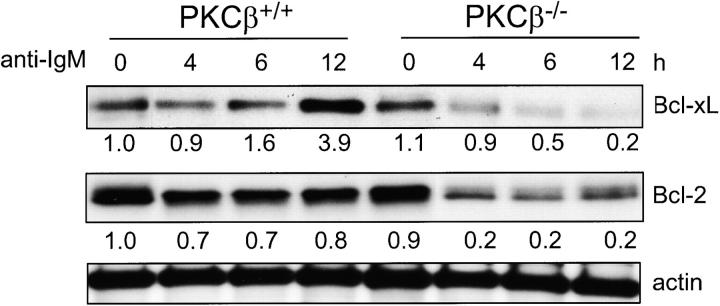
Poor survival of PKCβ-deficient B cells in vitro correlates with their inability to up-regulate expression of the anti-apoptotic protein Bcl-xL upon stimulation with anti-IgM (Fig. 2) . Incubation of wild-type splenic B cells with anti-IgM results in progressive increase in the expression levels of the Bcl-xL (Fig. 2). Similar treatment of the PKCβ-deficient B cells has a negative impact on the Bcl-xL expression level (Fig. 2). The inability of the surface expressed BCR to promote the expression of the anti-apoptotic Bcl-xL and Bcl-2 proteins likely causes the rapid death of the PKCβ-deficient B cells.
Figure 2.
Reduced expression of Bcl-xL and Bcl-2 after IgM cross-linking on B cells of PKCβ−/− compared with control mice. Splenic B cells were isolated from PKCβ−/− and PKCβ+/+ mice and incubated with 10 μg/ml anti-IgM for the indicated time (h). Bcl-xL and Bcl-2 protein expression was analyzed by Western blot analysis. Equal protein loading was controlled by anti-actin Western blotting. For quantification, band intensities were first normalized to the respective actin signal and then calculated as fold-change relative to unstimulated PKCβ+/+, which was set to 1.0.
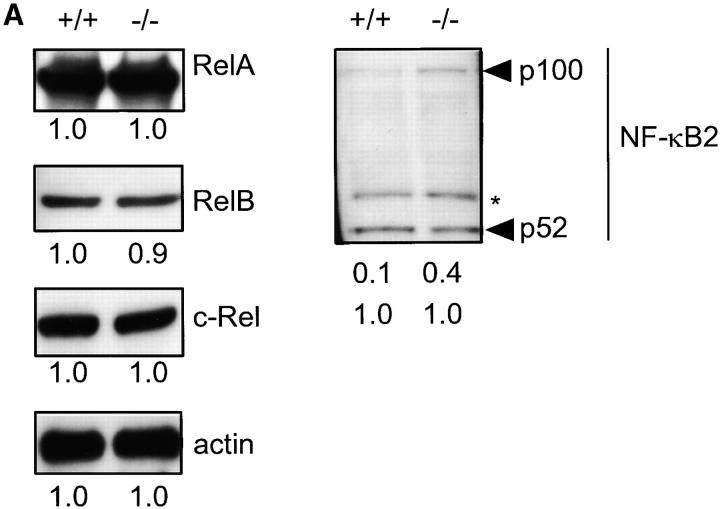
Expression of Bcl-xL in B cells is dependent on the activity of the NF-κB transcription factor (6, 21, 22), thus suggesting a possible involvement of PKCβ in NF-κB regulation. Members of the NF-κB/Rel family of proteins include RelA, c-Rel, RelB p50/NF-κB1, and p52/NF-κB2 (7). Deficiency in PKCβ does not affect expression levels of RelA, c-Rel, and RelB in splenic B cells (Fig. 3 A). NF-κB2 is synthesized as a large precursor (p100) that requires proteolytic processing to produce p52 (7). The relative amount of unprocessed p100 NF-κB2 precursors is increased in PKCβ-deficient B cells compared with control B cells (Fig. 3 A).
Figure 3.
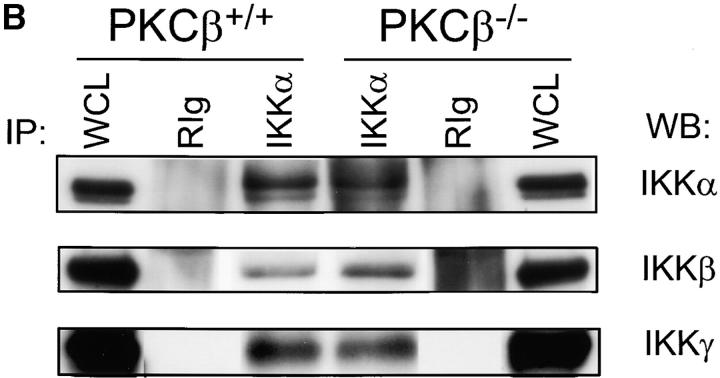
Expression of Rel family proteins and IKKα/IKKβ/IKKγ complex in splenic B cells of PKCβ−/− (−/−) and PKCβ+/+ (+/+) mice. (A) The amount of RelA, RelB, and c-Rel (left panel) as well as NF-κB2 (p100 and p52, right panel) were examined by sequential Western blotting. The actin blot (left panel, bottom) was used for quantification as described in Fig. 2. For NF-κB2 both p100 and p52 are quantified in relation to p52 of +/+ lysates. The asterisk (*) indicates a nonspecific band. (B) Normal composition of the IKKα/IKKβ/IKKγ signalosome complex in PKCβ−/− B cells. CHAPS lysates of splenic B cells from PKCβ−/− and PKCβ+/+ mice were immunoprecipitated with anti-IKKα antibody (IKKα) or control rabbit immunoglobulin (RIg). Whole cell lysates (WCL) are shown as blotting control. Membranes were sequentially probed with IKKα (top), IKKβ (middle), and IKKγ (bottom) antibodies.
Defective NF-κB2 processing has been previously found in B cells deficient for IKKα or expressing a catalytically inactive form of IKKα, which is a component of the tri-member IκB kinase complex IKKα/IKKβ/IKKγ (23). To test whether PKCβ controls NF-κB2 processing through the regulation of IKKα expression or activation we have analyzed the expression and activation state of IKKs in PKCβ-deficient splenic B cells. Both IKKα and IKKβ, as well as the regulatory component of the IκB kinase complex, IKKγ, are expressed in PKCβ-deficient B cells at wild-type levels (Fig. 3 B, WCL lanes). Immunoprecipitation of IKKα or IKKβ from PKCβ-deficient and control B cell lysates results in coprecipitation of IKKβ/IKKγ or IKKα/IKKγ, respectively (Fig. 3 B, and data not shown). These data suggest that expression of IKKα and formation of the IκB kinase complex is not controlled by PKCβ in B cells.
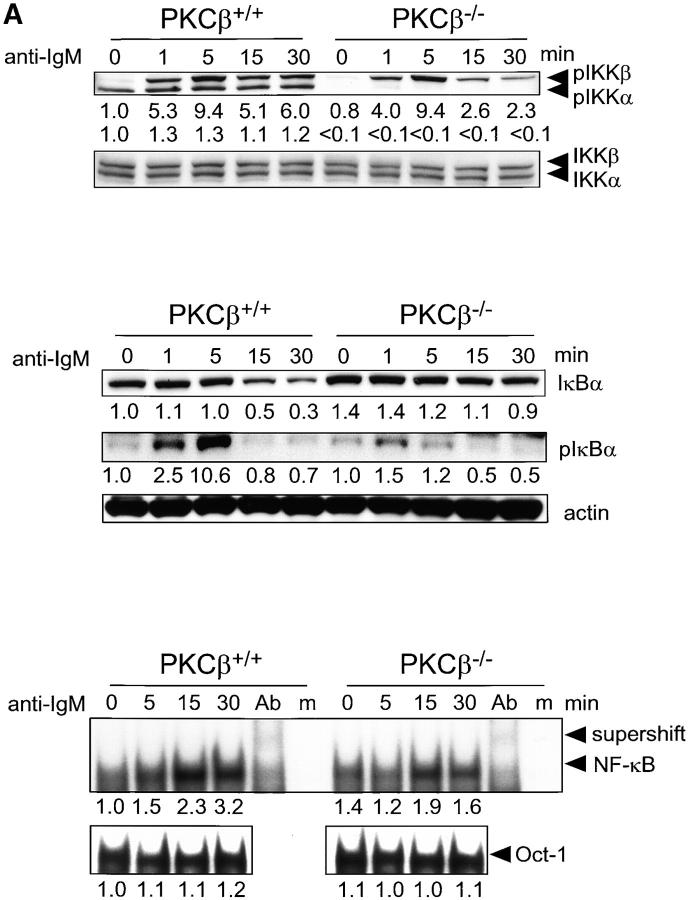
Activation of IKKα and IKKβ is controlled by their phosphorylation in the activation loop of the kinase domain at serine residues 176/180 and 177/181, respectively (24). Hence, the levels of phospho-IKKα (Ser-180) and phospho-IKKβ (Ser-181) reflect indirectly the fraction of activated IKKs within a cell. In wild-type B cells more phospho-IKKα than phospho-IKKβ can be detected. Incubation of the wild-type B cells with anti-IgM results in a drastic increase of the amount of phospho-IKKβ while the phospho-IKKα levels remain relatively stable in the course of B cell stimulation (Fig. 4 A, top panel). Deficiency in PKCβ has a dramatic impact on the phosphorylation state of IKKα and to a lesser extent on IKKβ. Phospho-IKKα is virtually absent both in nonstimulated and anti-IgM treated PKCβ-deficient B cells (Fig. 4 A, top panel). Moreover, the duration of IgM-mediated phosphorylation of IKKβ is diminished in PKCβ-deficient B cells compared with the wild-type B cells (Fig. 4 A, top panel). Considering the essential role of serine phosphorylation in IKKα and IKKβ activation, these results reveal PKCβ as a key regulatory serine/threonine kinase connecting the BCR and IKK activation.
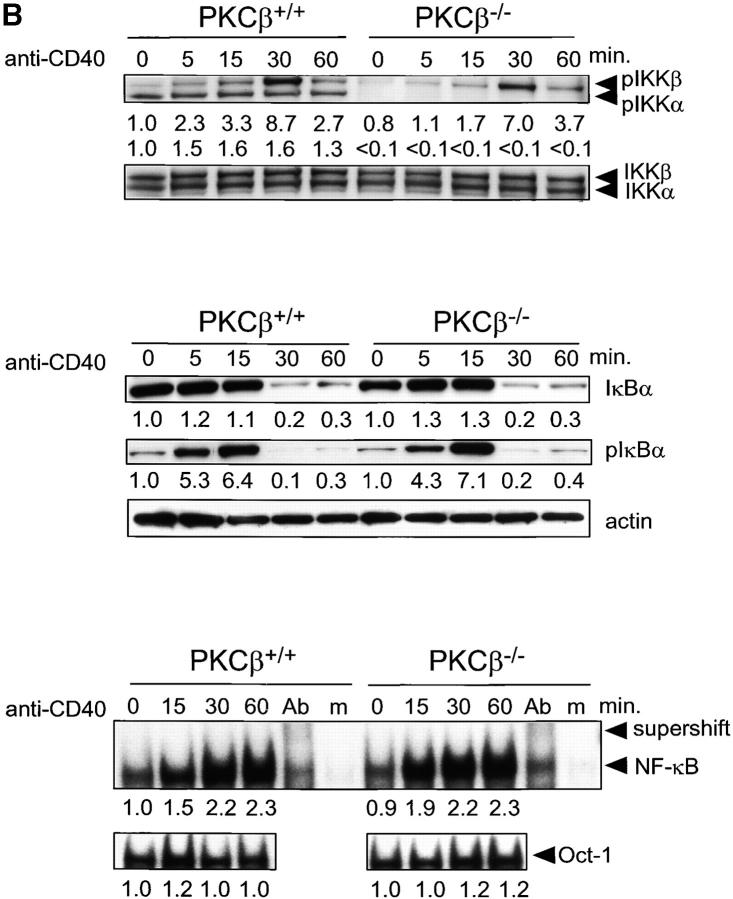
Figure 4.
Impaired activation and NF-κB signaling in PKCβ−/− B cells after BCR cross-linking. Absence of IKKα activation and shortened IKKβ activation in PKCβ−/− B cells. Splenic B cells of PKCβ−/− and PKCβ+/+ mice were stimulated with 20 μg/ml of anti-IgM antibody (A, top panel) or 10 μg/ml anti-CD40 (B, top panel) for the indicated time (min). Western blot analysis of cytoplasmic extracts were done simultaneously with antibodies specific for phospho-Ser180 and phospho-Ser181 of IKKα and IKKβ, respectively (top panel). The membrane was stripped and reprobed with antibodies recognizing nonphosphorylated IKKα and IKKβ. For quantification, band intensities of the phosphorylation site–specific blots were first normalized to the respective signal of unphosphorylated protein and then calculated as fold-change relative to unstimulated PKCβ+/+, which was set to 1.0. Inefficient degradation and phosphorylation of IκBα after IgM cross-linking on PKCβ−/− B cells (A, middle panel) and no alteration in IkBa phosphorylation and degradation after CD40 stimulation (B, middle panel). Splenic B cells were treated as in A. IκBα expression (top) and Ser32 phosphorylation (middle) were analyzed by Western blotting. Equal protein loading was controlled by anti-actin Western blotting (bottom). Quantification was done as in Fig. 2. Reduced DNA binding activity of NF-κB in PKCβ−/− B cells after BCR cross-linking (A, bottom panel) and no alteration after CD40 stimulation (B, bottom panel). EMSA was performed with nuclear extracts of splenic B cells treated as in above. The specificity of NF-κB shift was confirmed by supershift with antibodies for p50, RelA, and c-Rel (Ab) and by using mutant oligonucleotides (m). For quantification, signal intensities were first normalized to the respective signal of an Oct-1 EMSA done with the same nuclear lysates and then calculated as fold-change relative to unstimulated PKCβ+/+, which was set to 1.0.
In wild-type B cells, the BCR-mediated activation of IKKα is followed by phosphorylation of IκBα. This in turn leads to IκBα degradation and translocation of the Rel proteins to the nucleus. Deficiency in PKCβ and ensuing defective IKKα and IKKβ phosphorylation result in impaired IκBα phosphorylation and degradation (Fig. 4 A, middle panel). Congruently, activation of NF-κB is reduced in PKCβ-deficient B cells (Fig. 4 A, bottom panel).
To test whether impaired NF-κB activation in PKCβ-deficient B cells is specific for BCR-mediated signals, we tested stimulation of PKCβ-deficient B cells through CD40, which in wild-type B cells also leads to NF-κB activation. The analysis of IKKα and IKKβ phosphorylation in PKCβ-deficient B cells stimulated through CD40 reveals a paucity in IKKα phosphorylaytion similar to that observed after BCR engagement (Fig. 4 B, top panel). However, the CD40-mediated phosphorylation and degradation of IκBα, as well as the induction of NF-κB DNA-binding activity, are not impaired in PKCβ-deficient B cells (Fig. 4 B, middle and bottom panel). As a member of the TNF receptor family, CD40-mediated NF-κB activation is thought to require a different set of signaling molecules compared with BCR-mediated NF-κB activation, namely TRAF2, 3, 5, and 6 (25). Possibly in CD40/TRAF-mediated NF-κB activation IKKβ activation by itself is sufficient, or other kinases, like NIK, can substitute for IKKα (26). In agreement with the normal induction of Rel family proteins CD40 stimulation alone improves the survival of PKCβ-deficient B cells to wild-type levels (data not shown). This is also consistent with the finding that the CD40-mediated induction of anti-apoptotic A1 is not impaired in IKKα-deficient B cells (27). In contrast to IgM stimulation, CD40-mediated proliferative responses are only mildly impaired in PKCβ-deficient B cells (13), which is also seen in IKKα-deficient B cells (27). In conclusion, the PKCβ-mediated regulation of NF-κB activation is largely specific for BCR-mediated signaling and has only a minor impact on CD40 mediated B cell activation.
The pattern of defective NF-κB activation is very similar in B cells of PKCβ-deficient and Xid mice (9, 10). As PKCβ can act as a negative regulator of Btk, the similar effect of both mutations on NF-κB activation appears paradoxical. However, increased or decreased Btk-mediated signaling may have the same outcome, as shown by the severe Xid-like phenotype of mice expressing a constitutively active Btk mutant (28, 29). We think that in addition to its inhibitory role for Btk activation, PKCβ may also act downstream of Btk to mediate the activation of IKKs. It remains to be seen whether PKCβ regulates IKKs through direct phosphorylation. As the activation loop serine residues of IKKα or IKKβ are not part of a PKC consensus phosphorylation site, other serine/threonine residues of IKKα and IKKβ may serve as direct substrates for PKCβ. Interestingly, a low stringency scan for common PKC phosphorylation sites returns several potential sites for IKKα, but none for IKKβ (30). Alternatively, PKCβ may regulate IKKs indirectly through an intermediate kinase that would be directly controlled by PKCβ. Regardless of the exact mechanism of the PKCβ involvement in IKKs phosphorylation, the data presented reveal PKCβ as a novel component of the NF-κB signaling axis responsible for the survival and activation of B cells after BCR cross-linking.
Acknowledgments
We thank Michel Nussenzweig and Tsuneyasu Kaisho for the valuable discussion.
This study was supported by grants from Systemic Lupus Erythematosus foundation (K. Saijo), The Rockefeller University's Women & Science Fellowship Program (I. Mecklenbräuker), The Rockefeller University Presidential Fellowship (C. Schmedt), National Institutes of Health (C. Schmedt, A. Santana, A. Tarakhovsky), and The Irene Diamond Fund (A. Tarakhovsky).
References
- 1.Rajewsky, K. 1996. Clonal selection and learning in the antibody system. Nature. 381:751–758. [DOI] [PubMed] [Google Scholar]
- 2.Fulcher, D.A., and A. Basten. 1997. B cell life span: a review. Immunol. Cell Biol. 75:446–455. [DOI] [PubMed] [Google Scholar]
- 3.Conley, M.E., and M.D. Cooper. 1998. Genetic basis of abnormal B cell development. Curr. Opin. Immunol. 10:399–406. [DOI] [PubMed] [Google Scholar]
- 4.Satterthwaite, A., and O. Witte. 1996. Genetic analysis of tyrosine kinase function in B cell development. Annu. Rev. Immunol. 14:131–154. [DOI] [PubMed] [Google Scholar]
- 5.Lam, K.P., R. Kuhn, and K. Rajewsky. 1997. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 90:1073–1083. [DOI] [PubMed] [Google Scholar]
- 6.Karin, M., and A. Lin. 2002. NF-κB at the crossroads of life and death. Nat. Immunol. 3:221–227. [DOI] [PubMed] [Google Scholar]
- 7.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621–663. [DOI] [PubMed] [Google Scholar]
- 8.Silverman, N., and T. Maniatis. 2001. NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dev. 15:2321–2342. [DOI] [PubMed] [Google Scholar]
- 9.Petro, J.B., S.M. Rahman, D.W. Ballard, and W.N. Khan. 2000. Bruton's tyrosine kinase is required for activation of IκB kinase and nuclear factor κB in response to B cell receptor engagement. J. Exp. Med. 191:1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajpai, U.D., K. Zhang, M. Teutsch, R. Sen, and H.H. Wortis. 2000. Bruton's tyrosine kinase links the B cell receptor to nuclear factor κB activation. J. Exp. Med. 191:1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan, W.N. 2001. Regulation of B lymphocyte development and activation by Bruton's tyrosine kinase. Immunol. Res. 23:147–156. [DOI] [PubMed] [Google Scholar]
- 12.Anderson, J.S., M. Teutsch, Z. Dong, and H.H. Wortis. 1996. An essential role for Bruton's tyrosine kinase in the regulation of B-cell apoptosis. Proc. Natl. Acad. Sci. USA. 93:10966–10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leitges, M., C. Schmedt, R. Guinamard, J. Davoust, S. Schaal, S. Stabel, and A. Tarakhovsky. 1996. Immunodeficiency in protein kinase Cbeta-deficient mice. Science. 273:788–791. [DOI] [PubMed] [Google Scholar]
- 14.Chan, V.W., I. Mecklenbrauker, I. Su, G. Texido, M. Leitges, R. Carsetti, C.A. Lowell, K. Rajewsky, K. Miyake, and A. Tarakhovsky. 1998. The molecular mechanism of B cell activation by toll-like receptor protein RP-105. J. Exp. Med. 188:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang, S.W., M.I. Wahl, J. Chu, J. Kitaura, Y. Kawakami, R.M. Kato, R. Tabuchi, A. Tarakhovsky, T. Kawakami, C.W. Turck, et al. 2001. PKCbeta modulates antigen receptor signaling via regulation of Btk membrane localization. EMBO J. 20:5692–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Texido, G., I.H. Su, I. Mecklenbrauker, K. Saijo, S.N. Malek, S. Desiderio, K. Rajewsky, and A. Tarakhovsky. 2000. The B-cell-specific Src-family kinase Blk is dispensable for B-cell development and activation. Mol. Cell. Biol. 20:1227–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita, Y., K. Miyake, Y. Kikuchi, K. Takatsu, S. Noda, A. Kosugi, and M. Kimoto. 1995. A monoclonal antibody against a murine CD38 homologue delivers a signal to B cells for prolongation of survival and protection against apoptosis in vitro: unresponsiveness of X-linked immunodeficient B cells. Immunology. 85:248–255. [PMC free article] [PubMed] [Google Scholar]
- 18.Miyake, K., Y. Yamashita, M. Ogata, T. Sudo, and M. Kimoto. 1995. RP105, a novel B cell surface molecule implicated in B cell activation, is a member of the leucine-rich repeat protein family. J. Immunol. 154:3333–3340. [PubMed] [Google Scholar]
- 19.Meclenbräuker, I., K. Saijo, N.Y. Zheng, M. Leitges, and A. Tarakhovsky. 2002. Protein kinase Cδ controls self-antigen–induced B-cell tolerance. Nature. 416:860–865. [DOI] [PubMed] [Google Scholar]
- 20.Mori, M., S.C. Morris, T. Orekhova, M. Marinaro, E. Giannini, and F.D. Finkelman. 2000. IL-4 promotes the migration of circulating B cells to the spleen and increases splenic B cell survival. J. Immunol. 164:5704–5712. [DOI] [PubMed] [Google Scholar]
- 21.Baichwal, V.R., and P.A. Baeuerle. 1997. Activate NF-kappa B or die? Curr. Biol. 7:R94–R96. [DOI] [PubMed] [Google Scholar]
- 22.Wu, M., H. Lee, R.E. Bellas, S.L. Schauer, M. Arsura, D. Katz, M.J. FitzGerald, T.L. Rothstein, D.H. Sherr, and G.E. Sonenshein. 1996. Inhibition of NF-kappaB/Rel induces apoptosis of murine B cells. EMBO J. 15:4682–4690. [PMC free article] [PubMed] [Google Scholar]
- 23.Senftleben, U., Y. Cao, G. Xiao, F.R. Greten, G. Krahn, G. Bonizzi, Y. Chen, Y. Hu, A. Fong, S.C. Sun, and M. Karin. 2001. Activation by IKKα of a second, evolutionary conserved, NF-κ B signaling pathway. Science. 293:1495–1499. [DOI] [PubMed] [Google Scholar]
- 24.Delhase, M., M. Hayakawa, Y. Chen, and M. Karin. 1999. Positive and negative regulation of IκB kinase activity through IKKβ subunit phosphorylation. Science. 284:309–313. [DOI] [PubMed] [Google Scholar]
- 25.Arch, R.H., R.W. Gedrich, and C.B. Thompson. 1998. Tumor necrosis factor receptor-associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes Dev. 12:2821–2830. [DOI] [PubMed] [Google Scholar]
- 26.Garceau, N., Y. Kosaka, S. Masters, J. Hambor, R. Shinkura, T. Honjo, and R.J. Noelle. 2000. Lineage-restricted function of nuclear factor κB-inducing kinase (NIK) in transducing signals via CD40. J. Exp. Med. 191:381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaisho, T., K. Takeda, T. Tsujimura, T. Kawai, F. Nomura, N. Terada, and S. Akira. 2001. IκB kinase α is essential for mature B cell development and function. J. Exp. Med. 193:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dingjan, G.M., A. Maas, M.C. Nawijn, L. Smit, J.S. Voerman, F. Grosveld, and R.W. Hendriks. 1998. Severe B cell deficiency and disrupted splenic architecture in transgenic mice expressing the E41K mutated form of Bruton's tyrosine kinase. EMBO J. 17:5309–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maas, A., G.M. Dingjan, F. Grosveld, and R.W. Hendriks. 1999. Early arrest in B cell development in transgenic mice that express the E41K Bruton's tyrosine kinase mutant under the control of the CD19 promoter region. J. Immunol. 162:6526–6533. [PubMed] [Google Scholar]
- 30.Yaffe, M.B., G.G. Leparc, J. Lai, T. Obata, S. Volinia, and L.C. Cantley. 2001. A motif-based profile scanning approach for genome-wide prediction of signaling pathways. Nat. Biotechnol. 19:348–353. [DOI] [PubMed] [Google Scholar]