Abstract
The overall size and composition of the pool of naive and memory T cells are tightly regulated by homeostatic mechanisms. Recent work has shown that homeostasis of naive T cells is controlled by two factors, self-major histocompatibility complex (MHC)/peptide ligands and a cytokine, interleukin (IL)-7. In particular, contact with these two factors is required for naive CD4+ and CD8+ cells to undergo “homeostatic” proliferation, i.e., proliferation induced as a consequence of severe T cell depletion. In contrast to naive T cells, the factors that drive memory T cells to undergo homeostatic proliferation are poorly understood. To address this issue, purified memory phenotype CD4+ and CD8+ cells from normal mice were adoptively transferred into various gene-knockout mice rendered T cell–deficient by sublethal irradiation. Three findings are reported. First, unlike naive T cells, homeostatic proliferation of memory T cells is largely MHC independent. Second, memory CD8+ cells can utilize either IL-7 or IL-15 to undergo homeostatic proliferation; however, in the absence of both IL-7 and IL-15, homeostatic proliferation fails to occur. Third, unlike memory CD8+ cells, homeostatic proliferation of memory CD4+ cells is independent of IL-7 and IL-15 (also IL-4). Thus, the homeostatic proliferation mechanisms that control memory CD8+ cells and memory CD4+ cells are quite distinct.
Keywords: homeostasis, cytokines, memory, T lymphocytes, lymphopenia
Introduction
The total number of T lymphocytes in the body is tightly controlled by homeostatic mechanisms to remain at a constant level (1–4). Such regulation is manifested by the finding that T cells undergo “homeostatic” proliferation upon adoptive transfer into T cell–depleted syngenic hosts (1–4). Recently, it has been shown that naive T cells require contact with two ligands in order to undergo homeostatic proliferation in T cell–depleted hosts, namely (i) self-MHC/peptide complexes and (ii) the cytokine IL-7, a member of the cytokine family that binds to receptors which share a common γ (γc)* chain (5–10). Continuous contact with self-MHC/peptide and IL-7 is also required for long-term survival of naive T cells in their normal quiescent state (9–19).
Like naive T cells, memory T cells undergo homeostatic proliferation in lymphopenic hosts (14). However, the factors controlling homeostatic proliferation of memory T cells are largely unknown. For CD8+ cells, homeostatic proliferation and survival of these cells is normal in MHC class I–deficient hosts (14, 20), implying that contact with MHC ligands is not required. Whether CD4+ memory cells require MHC contact is less clear. CD4+ memory cells survive well in MHC class II− hosts (21), but whether this also applies to homeostatic proliferation has not been studied.
In addition to proliferating in response to T cell depletion, memory T cells undergo intermittent cell division under normal T cell–sufficient conditions (22–24). Such background turnover presumably reflects the fact that memory T cells are more metabolically active than naive T cells, which rarely divide under normal conditions (22, 25). The periodic turnover of memory T cells is MHC independent, at least for CD8+ cells (14), and occurs without any significant change in the total size of the memory T cell pool, implying that background proliferation is offset by cell death.
For memory CD8+ cells, recent work has shown that both survival and background turnover are controlled by IL-15, another member of the γc cytokine family (26–29). The notion that memory CD8+ cells are controlled by IL-15 stemmed from the observation that memory phenotype CD44hi CD8+ cells selectively express elevated level of CD122, a shared component of the receptors for IL-2 and IL-15, and that IL-15 caused selective proliferation of purified CD44hi CD8+ cells (26). Direct support for this idea came from the finding that background turnover of CD44hi CD8+ cells is inhibited with injection of anti-CD122 mAb though not by anti–IL-2 mAb (28), and that total numbers of CD122hi CD44hi CD8+ cells are selectively reduced in mice deficient in IL-15 or IL-15Rα chain (27, 29). In contrast to memory CD8+ cells, IL-15 appears not to have a role in homeostasis of memory CD4+ cells (26, 29). This could be a reflection of low CD122 expression on memory CD4+ cells (26), but it could also be due to a fundamental difference in the mechanisms regulating the two populations of memory cells. In support of this idea, long-lived memory CD4+ cells, despite expression of the γc chain, can be generated from γc − CD4+ cells (30, 31), implying that γc family of cytokines (IL-2, -4, -7, -9, -15) are dispensable for survival of memory CD4+ cells.
In this paper, we examined the requirements for MHC and γc cytokines for homeostatic proliferation of memory CD4+ and CD8+ cells in syngenic T cell–depleted hosts. As expected from findings on cell survival, memory T cells do not require contact with MHC molecules for homeostatic proliferation. In terms of cytokine requirements, however, the results were unexpected. Thus, based on the results of transferring T cells to cytokine knockout mice, memory CD8+ cells were found to utilize either IL-7 or IL-15 for undergoing efficient homeostatic proliferation in T cell–depleted hosts. Significantly, complete ablation of proliferation of memory CD8+ cells occurred in hosts lacking both IL-7 and IL-15. In contrast to memory CD8+ cells, there was little or no evidence that IL-4, IL-7, and IL-15 controlled homeostatic proliferation of memory CD4+ cells. In addition, competition experiments involving coinjection of large numbers of purified naive or memory T cell populations suggest that homeostatic proliferation of naive CD4+/CD8+ and memory CD8+ cells are regulated by some overlapping components. Homeostatic proliferation of memory CD4+ cells, on the other hand, appears to be regulated independently of other subsets of T cells.
Materials and Methods
Mice and Antibodies.
C57BL/6 (B6), B6.PL, and B6.Ly 5.1 congenic mice were purchased from the breeding colony at The Scripps Research Institute (TSRI). B6.IL-4– (32) and B6.β2m− (33) mice were purchased from The Jackson Laboratory. B6.Ly 5.1+ β2m− mice were purchased from Taconic. B6.Aβ− mice (34) were provided by T. Laufer (University of Pennsylvania, Philadelphia, PA). IL-7− (35), B6.IL-15− (29), B6.IL-7 transgenic (36) mice were gifts from DNAX, Immunex Corp., and R. Ceredig (INSERM), respectively; β2m−K−D− mice (14, 37), generated by F. Lemonnier (Institut Pasteur, Paris, France), were provided by R. Ahmed (Emory University, Atlanta, GA). β2m−Aβ− and IL-7− IL-15− double deficient mice were bred at The Scripps Research Institute. Hybridomas secreting anti–IL-7Rα (A7R34) mAb (38) and anti–IL-7 (M25) mAb (39) (generated by Immunex Corp.) were provided by P. Marrack (National Jewish Medical and Research Center, Denver, CO).
Adoptive Transfer of T Cells.
Naive (CD44lo) and memory (CD44hi) CD4+ and CD8+ cells were obtained as follows. Pooled LN and spleen cells from adult mice at 6–12 mo of age were first depleted of non-T cells by treating with a cocktail of anti-HSA (clone J11D) and anti-Ab (clone 28–16–8S) mAbs plus complement. Cells were then stained with FITC-conjugated anti-CD4 (clone RM4–5; eBioscience), PE-conjugated anti-CD44 (eBioscience), and Cy5-conjugated anti-CD8 (eBioscience), and then sorted for CD44lo or CD44hi CD4+ and CD8+ cells using Becton Dickinson Vantage™ SE. Sorted cells were labeled with CFSE (Molecular Probes) as described previously (7, 40). Aliquots of 1–2 × 106 cells were injected intravenously into mice exposed to 600 cGy whole body irradiation 1 d before cell transfer. After 7 or 8 d, host LN and spleen cells were stained with the appropriate mAbs and analyzed by flow cytometry (7).
High numbers of naive and memory phenotype bystander T cells were obtained as follows. LN cells from young B6 mice (for naive T cells) or from IL-7 transgenic mice (for memory CD8+ cells) were depleted of non-T cells as described above and panned for T cells using plates coated with both anti-CD4 (clone RL172) and anti-CD8 (clone 3.168) mAbs (7). Purified T cells were stained with biotinylated anti-CD44 (eBioscience) mAb followed by FITC-conjugated streptavidin (Jackson ImmunoResearch Laboratories), followed by biotinylated magnetic microbeads (Miltenyi Biotec). These cells were then passed through MACS® LS separation columns (Miltenyi Biotec) to either collect CD44lo eluent cells or bound CD44hi cells. All FACS® and column-purified cells were >98% pure.
FACS® Analysis.
LN or spleen cells were stained for donor cells as described previously (7). Thy-1.1+ donor cells were detected by staining with biotinylated OX-7 (BD PharMingen) followed by Cy5-conjugated streptavidin (Jackson ImmunoResearch Laboratories) plus PE-conjugated anti-CD4 (eBioscience). Ly 5.1+ donor cells were detected by staining with Cy5-conjugated A20–1.7 (41) plus PE-conjugated anti-CD4 (eBioscience). Simultaneous detection of both Thy-1.1+ and Ly 5.1+ cells was achieved by staining with biotinylated OX-7 followed by PE-conjugated streptavidin (Jackson ImmunoResearch Laboratories) plus Cy5-conjugated A20–1.7 and PE-Cy5–conjugated anti-CD4 (eBioscience). To determine expression of cytokine receptors on T cells, LN cells from B6.PL mice were stained with either PE-conjugated anti–IL-2Rβ (BD PharMingen), biotinylated anti–IL-7Rα (clone A7R34) followed by PE-conjugated streptavidin, or anti-γc (clone 4G3; BD PharMingen) followed by biotinylated anti–rat IgG (Jackson ImmunoResearch Laboratories) followed by PE-conjugated streptavidin; the cells were then stained with FITC-labeled anti-CD44 and either Cy5-labeled anti-CD4 or anti-CD8 (eBioscience). PE- or unconjugated rat IgG Ab (Jackson ImmunoResearch Laboratories) was used for background staining.
Generation of Bone Marrow Chimeras.
A mixture of 3.5 × 106 B6.Ly 5.1+ β2m− and 1.5 × 106 B6 bone marrow (BM) cells were injected into lethally irradiated (1,000 cGy) B6 mice that also received 100 μl of PK136 (anti-NK1.1) (42) ascites 1 d before. β2m– T cells were obtained from these chimeras 3–6 mo later.
Results
Unless stated otherwise, the memory T cells used in this study were obtained by sorting for polyclonal CD44hi CD4+ and CD44hi CD8+ cells prepared from normal B6.PL (Thy-1.1+) mice at 6–12 mo of age; sorted polyclonal B6.PL or B6.Ly 5.1+ CD44lo cells were used as a source of naive T cells. Proliferation of CFSE-labeled donor T cells injected intravenously into irradiated B6 (Thy-1.2+ Ly 5.2+) hosts was analyzed 7–8 d later by double-staining for Thy-1.1 and/or Ly 5.1 and CD4 to identify donor CD4+ and CD8+ (CD4−) cells. In some experiments a mixture of CFSE-labeled B6.PL CD44hi CD4+/CD8+ and B6.Ly 5.1+ CD44lo CD4+/CD8+ cells were injected and triple stained for Thy-1.1, Ly 5.1, and CD4 to identify all four populations of donor cells. All hosts were T cell–depleted by exposure to a light dose of whole body irradiation (600 cGy) 1 d before donor cell injection.
The results discussed below refer to T cell proliferation, as measured by CFSE dilution. With regard to cell recoveries, total numbers of donor cells recovered from spleen and pooled LN of hosts injected with CD44lo cells were generally in the order of 20–40% of the injected cell numbers if the cells proliferated strongly and 5–10% if the cells remained largely in interphase; recoveries from recipients of CD44hi cells were about twofold lower. Recoveries of CD8+ cells were usually twofold higher than CD4+ cells. For cells transferred to IL-7− hosts, cell recoveries were more variable than for other gene-knockout hosts and may have reflected that the IL-7− hosts were backcrossed only twice to a B6 background.
Proliferation of Memory T Cells in T Cell–depleted Syngenic Hosts.
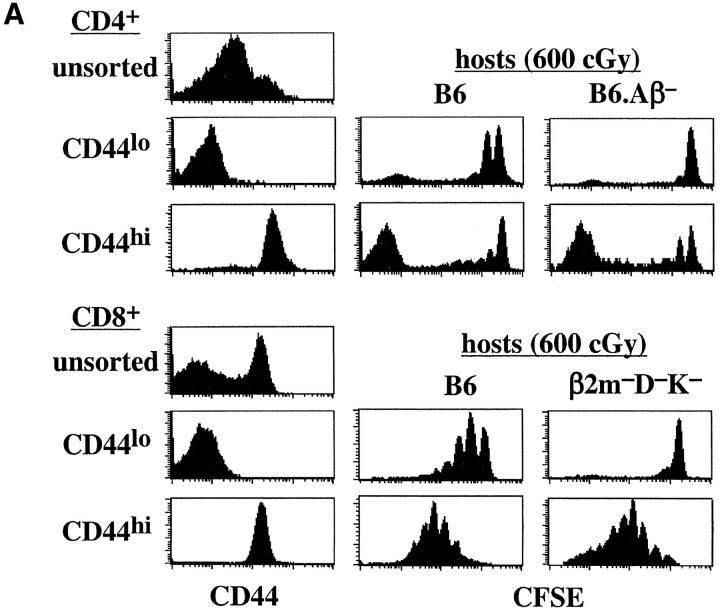
As shown previously for naive T cells (7), sorted CD44lo CD4+ and CD8+ cells underwent slow homeostatic proliferation during the 8-d period after injection into syngenic T cell–depleted B6 hosts (Fig. 1 A). Homeostatic proliferation was also evident for sorted memory phenotype CD44hi CD4+ and CD8+ cells during this period, but with faster kinetics of proliferation than for naive T cells (Fig. 1 A). Interestingly, proliferation of CD44hi CD4+ cells yielded two distinct populations, a fast-dividing subset that became CFSE− and a population of cells that either did not divide or underwent only 1–3 rounds of cell division. Homeostatic proliferation of memory CD8+ cells, on the other hand, was more homogeneous, as reported previously (14). Thus, CD44hi CD8+ cells uniformly underwent 2–6 rounds of cell division (Fig. 1 A); very few cells divided extensively, i.e., became CFSE−. In general, CD44hi CD8+ cells underwent 2–3 more rounds of cell division than CD44lo CD8+ cells.
Figure 1.
Homeostatic proliferation of naive and memory T cells in T cell–depleted syngenic hosts. (A) LN and spleen cells from B6.PL mice were sorted for naive (CD44lo) or memory phenotype (CD44hi) CD4+ and CD8+ T cells. Small numbers (106 cells per mouse) of CFSE-labeled CD44lo or CD44hi cells were intravenously injected into irradiated (600 cGy) B6, B6. H2-Aβ−, and β2m−K−D− mice; proliferation of donor cells was analyzed 7 d later by flow cytometry after staining host LN and spleen cells for Thy-1.1 and CD4. Shown are CD44 profiles on the sorted donor cells before injection (left column), and the CFSE profiles on gated donor CD4+ (Thy-1.1+ CD4+) and CD8+ (Thy-1.1+ CD4−) cells in the LN in the indicated hosts (right columns). Similar results were found in the spleen. (B) Small numbers (106 cells per mouse) of sorted CFSE-labeled CD44lo or CD44hi T cells were injected into two groups of irradiated (600 cGy) B6, B6.β2m−, B6.Aβ−, and B6.β2m− Aβ− mice; proliferation of donor cells was analyzed 7 d later. The injected donor cells were deficient in MHC class I molecules to prevent rejection by B6.β2m−Aβ− mice. MHC class I–deficient donor cells were obtained from mixed BM chimeras generated by reconstituting lethally irradiated B6 mice with a mixture of β2m-deficient B6.Ly 5.1+ and normal B6 BM cells as described in Materials and Methods. Shown are representative CFSE profiles on gated donor CD44lo or CD44hi CD4+ (Ly5.1+ CD4+) cells in the host LN. All host mice were pretreated with anti-NK1.1 (clone PK136) mAb to prevent rejection of β2m− donor cells. Similar results were obtained from two other experiments.
Role of MHC in Homeostatic Proliferation.
As reported previously (5–8), homeostatic proliferation of naive CD44lo CD4+ and CD8+ cells was minimal in irradiated hosts lacking MHC class II (B6.Aβ−) and MHC class I (β2m−K−D−) molecules, respectively (Fig. 1 A). In marked contrast to naive T cells, homeostatic proliferation of memory CD44hi T cells did not require contact with MHC molecules. Thus, proliferation of CD44hi CD4+ cells was as prominent in MHC class II− hosts as in normal B6 hosts (Fig. 1 A). Likewise, confirming previous findings (14), CD44hi CD8+ cells proliferated extensively in both MHC class I− and normal hosts.
For CD44hi CD4+ cells, the strong homeostatic proliferation of these cells in irradiated MHC class II− hosts also applied in combined MHC class I−II− (B6.β2m−Aβ2) hosts (Fig. 1 B). Likewise, CD44hi CD8+ cells proliferated well in both MHC class I− and MHC class I− II− hosts (data not shown). Thus, for memory CD44hi T cells, homeostatic proliferation was independent of both MHC class I and MHC class II molecules. Note that, because of unexplained rejection of T cells in combined MHC I−II− hosts, the T cells used in Fig. 1 B were raised in BM chimeras (see Materials and Methods).
Role of γc Cytokines in Homeostatic Proliferation of Memory T Cells.
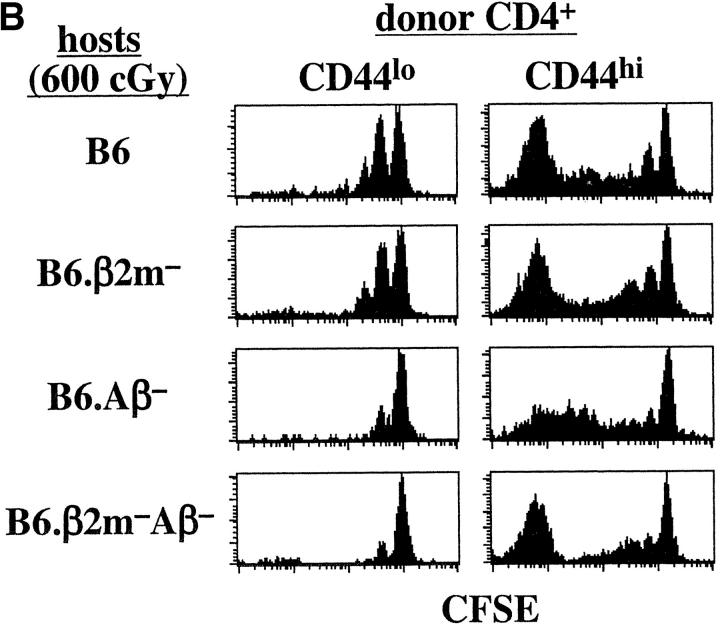
To assess the role of γc cytokines, we examined homeostatic proliferation in IL-4−, IL-7−, and IL-15− hosts. Confirming previous findings (9, 10), homeostatic proliferation of naive CD44lo CD4+ and CD8+ cells was largely ablated in IL-7− hosts but retained in IL-4− and IL-15− hosts (Fig. 2) . Homeostatic proliferation of naive T cells was thus strongly dependent on IL-7 but relatively independent of IL-4 and IL-15. The results for memory T cells were somewhat different.
Figure 2.
Homeostatic proliferation of naive and memory phenotype T cells in cytokine-deficient mice. Small numbers (106 cells per mouse) of CFSE-labeled sorted B6.Ly 5.1 CD44lo, and CD44hi T cells were injected into groups of irradiated (600 cGy) B6, B6.IL-4−, IL-7−, and B6.IL-15− mice and analyzed 7 d later for proliferation of donor cells in host lymphoid tissues. Shown are CFSE profiles of gated donor CD44lo or CD44hi CD4+ (Ly5.1+ CD4+) and CD8+ (Ly5.1+ CD4−) cells in host LN 7 d after transfer. Data are representative of two separate experiments using a total of four mice for each host type.
For CD44hi CD4+ cells, proliferation of these cells in IL-4−, IL-7−, and IL-15− hosts was almost as marked as in normal B6 hosts. This finding, which was seen in several other experiments (Figs. 3 B and 4), indicated that IL-4, IL-7, and IL-15 played little if any role in homeostatic proliferation of CD44hi CD4+ cells.
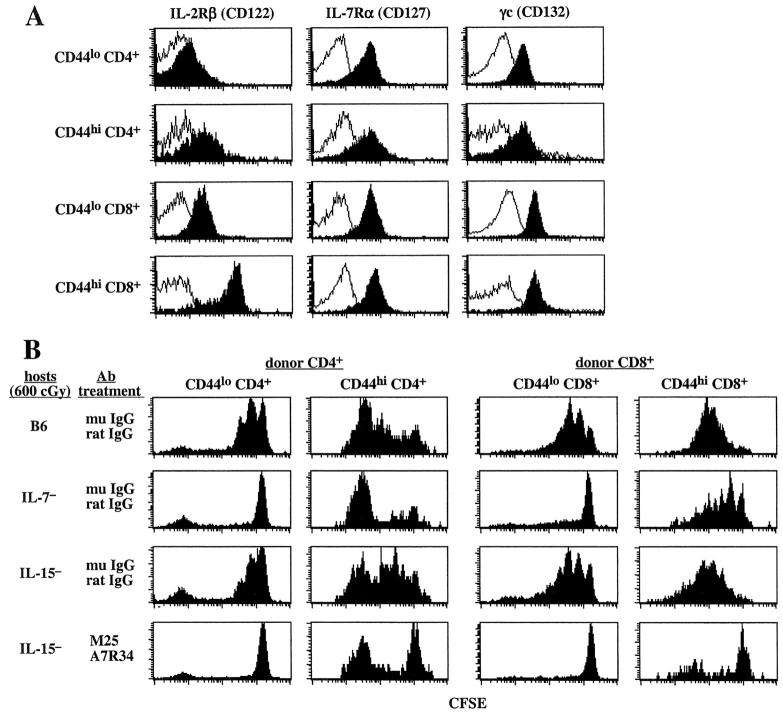
Figure 3.
Role of IL-7 and IL-15 for homeostatic proliferation of naive and memory phenotype T cells. (A) Expression of IL-2Rβ (CD122), IL-7Rα (CD127), and γc (CD132) on CD44lo or CD44hi T cells. LN cells from a young B6.PL mouse were stained for the indicated cytokine receptors and also for CD44, CD4, and CD8. Shown are expression levels of the indicated cytokine receptors (black) and background control (white) on gated CD44lo, CD44hi CD4+, and CD8+ cells. (B) Blocking IL-7 in IL-15− mice suppresses homeostatic proliferation of CD44hi CD8+ cells. A mixture of CFSE-labeled sorted B6.Ly 5.1+ CD44lo T cells (106 cells per mouse) and B6.PL CD44hi T cells (106 cells per mouse) were injected into irradiated (600 cGy) B6, IL-7−, and IL-15− mice. Host mice were either treated with a mixture of control mouse and rat IgG or with a cocktail of anti–IL-7Rα (clone A7R34) and anti–IL-7 (clone M25) mAbs. Cocktails containing 500 ug of each antibody were intraperitoneally injected every other day for a total of four times starting 1 d before cell transfer. Shown are CFSE profiles of donor CD44loCD4+ (Ly 5.1+ CD4+), CD44lo CD8+ (Ly5.1+ CD4–), CD44hi CD4+ (Thy1.1+ CD4+), or CD44hi CD8+ (Thy1.1+ CD4−) cells in the host LN 7 d after transfer. One other experiment showed similar results.
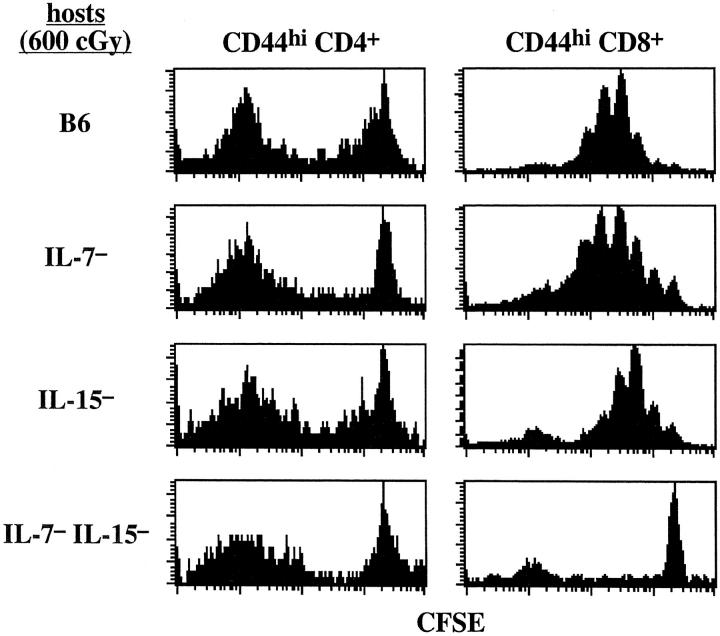
As for CD44hi CD4+ cells, homeostatic proliferation of CD44hi CD8+ cells occurred in all three knockout hosts (Fig. 2). However, the extent of proliferation in these hosts was variable. In some experiments (3 out of 6), proliferation of CD44hi CD8+ cells was considerably reduced in IL-7− hosts (Fig. 3 B) but this was not an invariable finding (Figs. 2 and 4) . Likewise, proliferation in IL-15− hosts was reduced in some experiments (2 out of 6; Fig. 4), but not in others (Fig. 2 and 3 B). Proliferation in IL-4− hosts, however, was not reduced (Fig. 2 and data not shown).
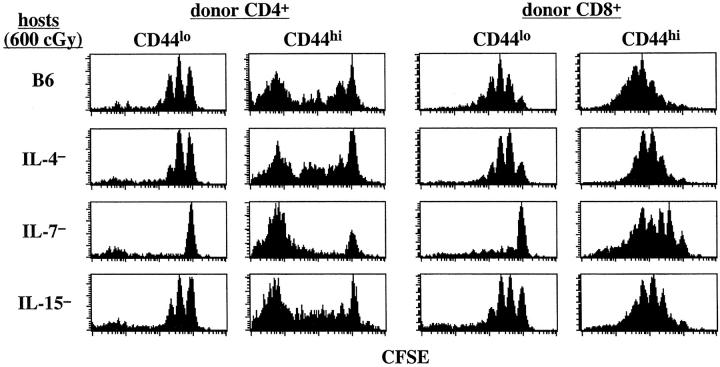
Figure 4.
Mice deficient in both IL-7 and IL-15 fail to support homeostatic proliferation of memory phenotype CD8+ cells but support efficient homeostatic proliferation of memory phenotype CD4+ cells. Small numbers (106) of sorted and CFSE-labeled B6.PL CD44hi T cells were transferred into irradiated B6, IL-7−, IL-15−, and combined IL-7− IL-15− mice; proliferation of donor cells was examined 7 d later. Shown are CFSE profiles of gated donor CD44hi CD4+ and CD44hi CD8+ cells in the LN. Similar results were obtained from two other experiments.
Roles for Both IL-7 and IL-15 in Homeostatic Proliferation of Memory CD8+ Cells.
The above results suggest that homeostatic proliferation of CD44hi CD8+ cells is relatively independent of IL-4, IL-7, and IL-15. However, an alternative possibility is that proliferation is controlled by multiple cytokines rather than by a single cytokine. Here, we considered the idea that proliferation could be controlled by both IL-7 and IL-15, the presence of IL-15 in IL-7− hosts compensating for the lack of IL-7, and vice versa in IL-15− hosts. This idea seemed plausible because (i) IL-7 clearly played a decisive role for homeostatic proliferation of naive T cells and (ii) IL-15 is known to control the background proliferation of CD44hi CD8+ cells in nonirradiated hosts (Introduction). In addition, CD44hi CD8+ cells show high expression of receptors for both IL-7 and IL-15. Thus, as shown in Fig. 3 A, expression of IL-7Rα, and also γc, was as prominent on CD44hi CD8+ cells as on other T cells. Likewise, expression of CD122, an important component of the receptor for IL-15 (and IL-2), was prominent on CD44hi CD8+ cells, in fact far higher on these cells than on other T cell subsets (reference 26 and Fig. 3 A).
If either IL-7 or IL-15 can control homeostatic proliferation of CD44hi CD8+ cells, depletion of both cytokines would be expected to prevent proliferation. To test this prediction, T cells were transferred to irradiated IL-15− hosts; responsiveness to IL-7 in these hosts was then blocked by injecting the mice with a mixture of anti–IL-7 (M25) and anti–IL-7Rα (A7R34) mAbs. As shown in Fig. 3 B, this treatment totally inhibited proliferation of naive CD44lo CD4+ and CD8+ T cells, thus indicating that mAb treatment was effective in blocking responsiveness to IL-7. Significantly, the same finding applied to CD44hi CD8+ cells. Thus, proliferation of these cells was near normal in control Ab-treated IL-15− hosts (relative to B6 hosts) but markedly reduced in IL-15− hosts given anti–IL-7/7Rα mAbs. The latter treatment had little or no effect on proliferation of CD44hi CD4+ cells.
The above data thus provided strong support for the notion that homeostatic proliferation of CD44hi CD8+ cells (but not CD44hi CD4+ cells) is under the joint control of two cytokines, IL-7 and IL-15. Further support for this idea came from the finding that homeostatic proliferation of CD44hi CD8+ cells was virtually abolished in combined IL-7− IL-15− hosts (Fig. 4). Proliferation of CD44hi CD4+ cells in these hosts, by contrast, was unimpaired.
Competition between Naive and Memory T Cells for Cytokines.
In previous studies, it was found that homeostatic proliferation of naive T cells could be markedly inhibited by coinjecting large numbers of unlabeled “bystander” T cells (7, 43). The mechanism of inhibition was not established, though competition for MHC–peptide ligands was excluded. In light of the above results, the inhibition of naive T cells could reflect competition for cytokines, notably, IL-7. If so, comparable inhibition might apply to memory CD44hi CD8+ cells, but only if the competing cells removed both IL-7 and IL-15. In the experiments discussed below, we tested this idea by examining whether bulk populations of T cells could inhibit proliferation of memory CD44hi CD8+ cells; inhibition of naive T cells was used as a control.
For naive T cells, our previous data showing inhibition of proliferation by bulk populations of T cells referred to unseparated T cells, i.e., a mixture of naive and memory cells. As shown in Fig. 5 A, proliferation of CFSE-labeled CD44lo CD8+ cells in either B6 hosts (group 1) or IL-15− hosts (group 2) was strongly inhibited by coinjection of a large dose (3–4 × 107) of purified CD44lo T cells. Since proliferation of CD44lo T cells is selectively dependent on IL-7 (see above), bulk populations of CD44lo T cells, being IL-7Rhi, presumably inhibit proliferation by binding and removing IL-7, thus depriving the coinjected naive CFSE-labeled T cells from contact with IL-7.
Figure 5.
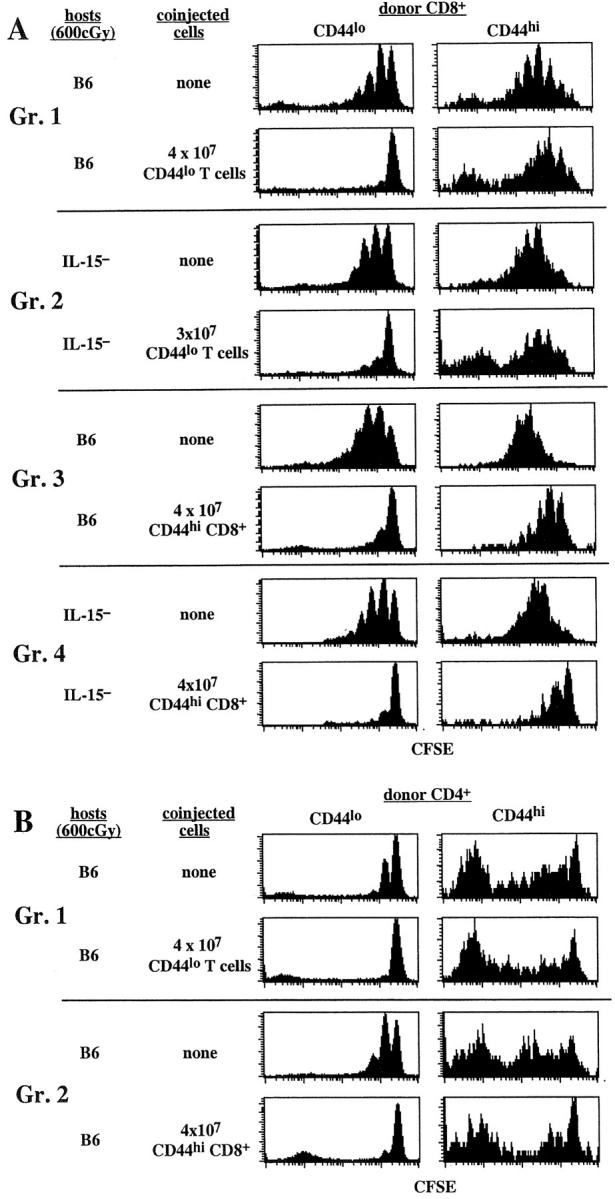
Competition between naive and memory phenotype T cells for factors driving homeostatic proliferation. (A) Coinjection of large numbers of CD44lo T cells fails to inhibit homeostatic proliferation of CD44hi CD8+ cells, but coinjection of large numbers of CD44hi CD8+ cells blocks homeostatic proliferation of CD44lo T cells. A mixture of CFSE-labeled sorted B6.Ly 5.1+ CD44lo T cells (106 cells per mouse) and B6.PL CD44hi T cells (106 cells per mouse) was injected into a group of irradiated (600 cGy) B6 or B6.IL-15− mice. Half of the hosts were then injected with a large number (3–4 × 107) of magnetic bead-purified B6 CD44lo T cells or CD44hi CD8+ cells obtained as described in Materials and Methods. CD44hi CD8+ cells were obtained from B6.IL-7 transgenic mice. Donor cells in the host LN and spleen were examined 7 d later. Shown are CFSE profiles on gated donor CD44lo and CD44hi CD8+ cells. Note that each group represents separate experiments; hence, representative control proliferations of CFSE-labeled donor cells in the absence of coinjected bystander cells is shown for each group. Results are representative of two to three independent experiments. (B) Failure of bystander naive T cells or CD44hi CD8+ cells to diminish proliferation of CD44hi CD4+ cells. Representative data on gated donor CD44lo and CD44hi CD4+ cells from some of the experiments described in A are shown.
Despite being able to absorb IL-7, bulk populations of CD44lo T cells, being CD122lo, would presumably have only limited capacity to remove IL-15. Hence, one would not expect these cells to inhibit proliferation of memory CD44hi CD8+ cells, i.e., cells that can utilize either IL-7 or IL-15 for proliferation. In agreement with this prediction, despite strongly inhibiting proliferation of naive T cells, bulk populations of unlabeled CD44lo T cells failed to inhibit proliferation of CFSE-labeled memory CD44hi CD8+ cells in irradiated B6 mice (group 1). Here, proliferation of CD44hi CD8+ cells was presumably driven by IL-15, IL-7 having been removed by absorption. If so, quite different results would be expected in IL-15− hosts, i.e., a situation where, according to the above results (Figs. 3 and 4), proliferation is driven solely by IL-7. Here, we predicted that IL-15− hosts given bulk populations of CD44lo T cells would lack IL-7 as well as IL-15 and would thus fail to allow proliferation of CFSE-labeled CD44hi CD8+ cells. Surprisingly, this was not the case; in fact, despite minimal proliferation of CFSE-labeled CD44lo T cells, proliferation of CD44hi CD8+ cells in IL-15− hosts given bulk populations of CD44lo T cells was almost as high as in control B6 mice (group 2).
One explanation for this unexpected finding is that, after injection of bulk populations of naive CD44lo T cells, these cells localized in the T cell–dependent areas of the lymphoid tissues and thus caused local absorption of IL-7 in these sites but not in other sites. Since proliferation of naive T cells is largely restricted to the T cell–dependent areas, one can envisage that local absorption of IL-7 in these areas is sufficient to block proliferation of naive T cells. However, the situation for CD44hi CD8+ cells could be different. Thus, because of less stringent requirements for contact with professional APC (14), homeostatic proliferation of CD44hi CD8+ cells might be able to occur outside the T cell–dependent areas. If so, local absorption of IL-7 only in the T cell–dependent areas would not block proliferation of CD44hi CD8+ cells.
To assess this model, we examined whether proliferation of CD44hi CD8+ cells could be blocked by coinjecting bulk populations of CD44hi CD8+ cells, i.e., cells that presumably localize in the same sites as the proliferating cells. Since purifying large numbers of CD44hi CD8+ cells from normal mice was impractical, we used IL-7 transgenic mice (36) as donors. Numbers of CD44hi CD8+ cells in these mice are greatly expanded, presumably through contact with high levels of IL-7 (44). However, by all parameters tested, these cells (which do not themselves synthesize IL-7) are identical to normal T cells, both in terms of phenotype and requirements for homeostatic proliferation (44). As shown in Fig. 5 A, coinjecting large numbers of CD44hi CD8+ cells into IL-15− hosts did indeed markedly inhibit proliferation of CFSE-labeled CD44hi CD8+ cells (group 4). Interestingly, substantial inhibition of proliferation of CD44hi CD8+ cells also occurred in B6 hosts (group 3). Here, being CD122hi as well as IL-7Rhi, the bulk population of CD44hi CD8+ cells presumably inhibited proliferation by depleting both IL-7 and IL-15.
The above data thus indicate that, unlike CD44lo cells, bulk populations of CD44hi CD8+ cells caused strong inhibition of proliferation of CD44hi CD8+ cells, presumably because both the competing and responding cells localize in the same tissue sites. Under these conditions, the competing cells cause local removal of both IL-7 and IL-15 (or just IL-7 in IL-15– hosts) and thus deprive the proliferating T cells from contact with these two cytokines. In control experiments, bulk populations of CD44hi CD8+ cells failed to impair proliferation of CD44hi CD4+ cells (Fig. 5 B). This finding was expected because, as discussed earlier (Fig. 4), proliferation of CD44hi CD4+ cells appears to be IL-7 and IL-15 independent.
Discussion
In previous studies, it was shown that homeostatic proliferation of naive T cells is driven by self-MHC ligands and requires contact with IL-7 (5–10). Memory phenotype T cells also undergo homeostatic proliferation (14) but the requirements for stimulating these cells are largely unknown. For CD8+ cells, the prior observation that homeostatic proliferation of CD44hi CD8+ cells occurred in MHC class I− hosts raised the question whether proliferation of memory phenotype T cells is driven by cytokines. As shown here, homeostatic proliferation of memory phenotype CD8+ cells is indeed cytokine dependent and is controlled by two different γc cytokines, IL-7 and IL-15. By contrast, γc cytokines do not appear to be involved in proliferation of memory phenotype CD4+ cells. Before discussing memory phenotype T cells, it is important to consider the role of cytokines in controlling proliferation of naive T cells.
For naive phenotype CD44lo T cells, our results confirm that TCR contact with self-MHC/peptide ligands and exposure to IL-7 are both essential for homeostatic proliferation of these cells (5–10). Thus, homeostatic proliferation of purified polyclonal CD44lo T cells was very limited in MHC− hosts and IL-7− hosts but unimpaired in IL-4− and IL-15− hosts. Confirming previous findings with unseparated T cells (5–10), coinjection of bulk populations of purified CD44lo T cells or CD44hi CD8+ cells greatly reduced homeostatic proliferation of naive T cells. The simplest explanation for this finding is that, by homing to the T cell–dependent areas of the lymphoid tissues, the coinjected T cells caused local depletion of IL-7: naive T cells making contact with self-MHC ligands on dendritic cells in the T cell–dependent areas were starved of IL-7 and failed to proliferate. Here, it is notable that, unlike T cells, bulk populations of B cells fail to inhibit homeostatic proliferation of naive T cells (7). This finding may reflect that, although IL-7R expression on B cells is high, B cells fail to localize in the T cell–dependent areas and for this reason fail to deplete IL-7 in these areas.
For memory phenotype T cells, prior evidence that homeostatic proliferation of these cells is MHC independent was limited to the finding that proliferation of CD44hi CD8+ cells was able to occur in MHC class I− hosts (14). In extending this finding, we show here that homeostatic proliferation is MHC independent for both CD4+ and CD8+ memory phenotype cells. Moreover, these cells undergo efficient homeostatic proliferation in the combined absence of both class I and II MHC molecules, indicating that even suboptimal signaling through TCR recognition of the “opposite” class of MHC molecules (e.g., class I for CD4+ cells) is not required for homeostatic proliferation.
Recent work has shown that, under normal T cell–sufficient conditions, homeostasis of CD8+ memory phenotype T cells is controlled by IL-15 (26–29). The dependency of CD44hi CD8+ cells on IL-15 applies both to cell survival and turnover and is restricted to a subset of cells (∼70% of CD44hi CD8+ cells) expressing a high density of CD122 (IL-2Rβ) (unpublished data), an important component of the receptor for both IL-15 and IL-2 (45). IL-15− (29) and IL-15Rα− (27) mice show reduced numbers of CD44hi CD8+ cells and, at least for IL-15− mice, are virtually devoid of CD122hi cells (unpublished data). Likewise, CD122hi CD8+ cells from normal mice disappear rapidly and fail to divide after transfer to IL-15− hosts (unpublished data). In light of these findings, we expected that homeostatic proliferation of memory CD8+ cells would be very limited in T cell–depleted IL-15− hosts. Surprisingly, however, homeostatic proliferation of CD44hi CD8+ cells was clearly apparent in irradiated IL-15− hosts, and also in IL-4− and IL-7− hosts. The key finding was that homeostatic proliferation of CD44hi CD8+ cells was abolished in combined IL-15− IL-7− hosts. Therefore, the implication is that, under T cell–depleted conditions, IL-7 and IL-15 are functionally interchangeable and either of these cytokines is able to induce homeostatic proliferation of CD44hi CD8+ cells. This contrasts sharply with the situation in normal T cell–sufficient mice where only IL-15 appears to be important.
Although CD44hi CD8+ cells are highly sensitive to IL-15 (26–29), the observation that IL-7 can drive homeostatic proliferation of these cells is rather unexpected because there is very little precedent for the notion that IL-7 has a role in memory CD8+ cell homeostasis. Nevertheless, it was recently reported that CD8+ OT-I TCR transgenic cells deficient in expression of IL-7Rα were much less efficient than wild-type OT-I cells in generating memory CD8+ cells upon adoptive transfer into normal B6 hosts, despite a similar level of expansion during the primary response to antigen (9). However, because IL-7 is crucial for thymopoiesis, the few T cells that are generated in IL-7Rα− mice may be abnormal in their ability to convert to memory cells and/or respond to IL-15. Furthermore, if IL-7 was able to compensate for the absence of IL-15 under normal T cell–sufficient conditions, one would expect both IL-15− and IL-15R− mice to possess normal numbers of CD44hi CD8+ cells, which is not the case.
If IL-7 is not required for maintaining CD44hi CD8+ cells in normal mice, why is IL-7 important for controlling homeostatic proliferation of CD44hi CD8+ cells in T cell–depleted hosts? Different levels of IL-7 in these two situations could be a key factor. Thus, in normal mice, local absorption of IL-7 by the large numbers of T cells residing in the T cell areas may keep IL-7 at a low level. In T cell–depleted mice, by contrast, reduced absorption of IL-7 by T cells may cause the local concentration of IL-7 to rise to a level sufficient to stimulate T cells undergoing homeostatic proliferation. Support for this idea is provided by the finding that basal levels of IL-7 are elevated in T cell–depleted conditions in humans, i.e., after BM transplantation, chemotherapy, and at late stages of HIV infection (46–48). Thus far, however, our attempts to adapt the highly sensitive immunoassay used to detect IL-7 in humans has been unsuccessful for measuring mouse IL-7 levels.
The observation that homeostatic proliferation of CD44hi CD8+ cells involves both IL-7 and IL-15 provides an explanation for the inhibition of proliferation induced by coinjection of normal T cells. Here, the surprising finding was the capacity of bulk populations of T cells to block homeostatic proliferation of CD44hi CD8+ cells applied only to CD44hi CD8+ inhibitors and not to CD44lo inhibitors. For inhibition mediated by CD44hi CD8+ cells, our suggestion is that, via IL-7R and CD122, these cells caused depletion of both IL-7 and IL-15 and thus prevented the proliferating T cells from contacting these cytokines. Whereas CD44hi CD8+ cells are IL-7Rhi CD122hi, CD44lo cells are IL-7Rhi CD122lo. Hence, via removal of IL-7, we expected that coinjection of CD44lo cells would block homeostatic proliferation of CD44hi CD8+ cells, though only in IL-15− hosts. Yet, no inhibition was observed, even though CD44lo cells were injected in large numbers (3–4 × 107). One explanation for this finding is that, cell-for-cell, CD44lo cells are less efficient than CD44hi cells at absorbing IL-7. Although this possibility has not been excluded, we favor the notion that CD44lo cells fail to reach the sites of CD44hi CD8+ cell proliferation. Thus, it would seem likely that, unlike naive T cells, proliferation of CD44hi CD8+ cells occurs not only in the T cell–dependent areas but also in other sites, e.g., the splenic white pulp, as well as in nonlymphoid tissues. Hence, we envisage that the inhibitory T cells need to reach these multiple sites in order to cause effective depletion of both IL-7 and IL-15; only CD44hi and not CD44lo inhibitors reach these sites.
The capacity of two different cytokines, IL-7 and IL-15, to promote homeostatic proliferation of CD44hi CD8+ cells raises the question whether other γc cytokines contribute to this process. This possibility would seem unlikely because proliferation was almost undetectable in combined IL-7− IL-15− hosts. However, the basal levels of other γc cytokines in these mice may be too low to compensate for the lack of IL-7 and IL-15. Therefore, a key issue is whether the failure of CD44hi CD8+ cells to undergo homeostatic proliferation in IL-7− IL-15− hosts can be overcome by injecting other γc cytokines, e.g., IL-4 or IL-9. We are in the process of testing this possibility.
If we did find that homeostatic proliferation of CD44hi CD8+ cells can be driven by a spectrum of γc cytokines, these cytokines might also have the potential to control the normal homeostasis of CD44hi CD8+ cells in T cell–sufficient mice. As discussed earlier, normal homeostasis of CD44hi CD8+ cells seems to be under the sole control of a single cytokine, IL-15, though joint exposure to another γc cytokine, IL-2, is known to be inhibitory (28). However, the decisive influence of IL-15 on CD44hi CD8+ cells in normal mice could simply indicate that levels of other γc cytokines in vivo are too low to contribute to homeostasis. If so, the question arises whether the strong dependence of CD44hi CD8+ cells on IL-15 can be overcome by raising in vivo levels of other γc cytokines. In support of this idea we have found that levels of CD44hi CD8+ cells, including CD122hi cells, increase substantially in IL-7 transgenic mice and are also prominent in IL-15− IL-7 transgenic mice (44). Therefore, the implication is that CD44hi CD8+ cells are only dependent on IL-15 when the concentration of IL-7 is low; if levels of IL-7 are raised to above physiological levels, IL-15 is no longer required. Whether raising levels of other γc cytokines can substitute for IL-15 has not been studied.
In marked contrast to CD8+ cells, we found no evidence that γc cytokines are required for homeostatic proliferation of memory phenotype CD4+ cells. This finding is in agreement with the report that memory CD4+ cells can be generated from γc − precursors (30, 31). Since homeostatic proliferation of CD44hi CD4+ cells was unimpeded in MHC class I−II− hosts, proliferation is apparently both MHC independent and γc cytokine independent. Whether proliferation of CD44hi CD4+ cells is controlled by non-γc cytokines and/or by chemokines is unclear, although it is notable that proliferation was not blocked by coinjecting either CD44lo T cells or CD44hi CD8+ cells. Whether bulk populations of CD44hi CD4+ cells (which are difficult to prepare in large numbers) can block proliferation of CD44hi CD4+ cells has not been studied.
Acknowledgments
We are grateful to Drs. P. Marrack for sending us M25 and A7R34 hybridomas, L. Bradley for providing large quantities of A7R34 and M25 antibodies for in vivo studies, and J. Andersson for sending the IL-7 transgenic mice. We also thank B. Bondi-Boyd, J. Kuhns, B. Marchand, M. Chan, and D. Kim for various support. This is publication number 14507-IMM from TSRI.
This work was supported by U.S. Public Health Service grants AI21487, CA38355, and AI46710 (to J. Sprent) and AI41079, AI45809, and AG20186 (to C.D. Surh). J.T. Tan and W.C. Kieper are supported by U.S. Public Health Service Institute National Research Service Awards HL07196 and AI07244, respectively. C.D. Surh is a Scholar of the Leukemia and Lymphoma Society.
B. Ernst's current address is Ludwig Institute for Cancer Research, Chemin des Boveresses 155, CH-1066 Epalinges, Switzerland.
Footnotes
Abbreviations used in this paper: γc, common γ chain; BM, bone marrow.
References
- 1.Freitas, A.A., and B.B. Rocha. 1993. Lymphocyte lifespan: homeostasis, selection and competition. Immunol. Today. 14:25–29. [DOI] [PubMed] [Google Scholar]
- 2.Bell, E.B., and S.M. Sparshott. 1997. The peripheral T-cell pool: regulation by non-antigen induced proliferation? Sem. Immunol. 9:347–353. [DOI] [PubMed] [Google Scholar]
- 3.Goldrath, A.W., and M.J. Bevan. 1999. Selecting and maintaining a diverse T-cell repertoire. Nature. 402:255–262. [DOI] [PubMed] [Google Scholar]
- 4.Surh, C.D., and J. Sprent. 2000. Homeostatic T cell proliferation. How far can T cells be activated to self-ligands? J. Exp. Med. 192:F9–F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viret, C., F.S. Wong, and C.A. Janeway, Jr. 1999. Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity. 10:559–568. [DOI] [PubMed] [Google Scholar]
- 6.Kieper, W.C., and S.C. Jameson. 1999. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proc. Natl. Acad. Sci. USA. 96:13306–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst, B., D.-S. Lee, J.M. Chang, J. Sprent, and C.D. Surh. 1999. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 11:173–181. [DOI] [PubMed] [Google Scholar]
- 8.Goldrath, A.W., and M.J. Bevan. 1999. Antagonist ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 11:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schluns, K.S., W.C. Kieper, S.C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426–432. [DOI] [PubMed] [Google Scholar]
- 10.Tan, J.T., E. Dudl, E. LeRoy, R. Murray, J. Sprent, K.I. Weinberg, and C.D. Surh. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. USA. 98:8732–8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vivien, L., C. Benoist, and D. Mathis. 2001. T lymphocytes need IL-7 but not IL-4 or IL-6 to survive in vivo. Int. Immunol. 13:763–768. [DOI] [PubMed] [Google Scholar]
- 12.Tanchot, C., F.A. Lemonnier, B. Pérarnau, A.A. Freitas, and B. Rocha. 1997. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 276:2057–2062. [DOI] [PubMed] [Google Scholar]
- 13.Nesic, D., and S. Vukmanovic. 1998. MHC class I is required for peripheral accumulation of CD8+ thymic emigrants. J. Immunol. 160:3705–3712. [PubMed] [Google Scholar]
- 14.Murali-Krishna, K., L.L. Lau, S. Sambhara, F. Lemonnier, J. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 286:1377–1381. [DOI] [PubMed] [Google Scholar]
- 15.Takeda, S., H.-R. Rodewald, H. Arakawa, H. Bluethmann, and T. Shimizu. 1996. MHC class II molecules are not required for survival of newly generated CD4+ T cells but affect their long-term life span. Immunity. 5:217–228. [DOI] [PubMed] [Google Scholar]
- 16.Brocker, T. 1997. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J. Exp. Med. 186:1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirberg, J., A. Berns, and H. von Boehmer. 1997. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex-encoded molecules. J. Exp. Med. 186:1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rooke, R., C. Waltzinger, C. Benoist, and D. Mathis. 1997. Targeted complementation of MHC class II deficiency by intrathymic delivery of recombinant adenoviruses. Immunity. 7:123–134. [DOI] [PubMed] [Google Scholar]
- 19.Boursalian, T.E., and K. Bottomly. 1999. Survival of naive CD4 T cells: roles of restricting versus selecting MHC class II and cytokine milieu. J. Immunol. 162:3795–3801. [PubMed] [Google Scholar]
- 20.Lau, L.L., B.D. Jamieson, T. Somasundaram, and R. Ahmed. 1994. Cytotoxic T-cell memory without antigen. Nature. 369:648–652. [DOI] [PubMed] [Google Scholar]
- 21.Swain, S.L., H. Hu, and G. Huston. 1999. Class II-independent generation of CD4 memory T cells from effectors. Science. 286:1381–1383. [DOI] [PubMed] [Google Scholar]
- 22.Tough, D.F., and J. Sprent. 1994. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 179:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmerman, C., K. Brduscha-Riem, C. Blaser, R.M. Zinkernagel, and H. Pircher. 1996. Visualization, characterization, and turnover of CD8+ memory T cells in virus-infected hosts. J. Exp. Med. 183:1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruno, L., H. von Boehmer, and J. Kirberg. 1996. Cell division in the compartment of naive and memory T lymphocytes. Eur. J. Immunol. 26:3179–3184. [DOI] [PubMed] [Google Scholar]
- 25.von Boehmer, H., and K. Hafen. 1993. The life span of naive α/β T cells in secondary lymphoid organs. J. Exp. Med. 177:891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, X., S. Sun, I. Hwang, D.F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 8:591–599. [DOI] [PubMed] [Google Scholar]
- 27.Lodolce, J.P., D.L. Boone, S. Chai, R.E. Swain, T. Dassopoulos, S. Trettin, and A. Ma. 1998. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 9:669–676. [DOI] [PubMed] [Google Scholar]
- 28.Ku, C.C., M. Murakami, A. Sakamoto, J. Kappler, and P. Marrack. 2000. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 288:675–678. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy, M.K., M. Glaccum, S.N. Brown, E.A. Butz, J.L. Viney, M. Embers, N. Matsuki, K. Charrier, L. Sedger, C.R. Willis, et al. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lantz, O., I. Grandjean, P. Matzinger, and J.P. Di Santo. 2000. γ chain required for naive CD4+ T cell survival but not for antigen proliferation. Nat. Immunol. 1:54–58. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima, H., E.W. Shores, M. Noguchi, and W.J. Leonard. 1997. The common cytokine receptor gamma chain plays an essential role in regulating lymphoid homeostasis. J. Exp. Med. 185:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhn, R., K. Rajewsky, and W. Muller. 1991. Generation and analysis of interleukin-4 deficient mice. Science. 254:707–710. [DOI] [PubMed] [Google Scholar]
- 33.Zijlstra, M., M. Bix, N.E. Simister, J.M. Loring, D.H. Raulet, and R. Jaenisch. 1990. β2-microglobulin deficient mice lack CD4−8+ cytotoxic T cells. Nature. 344:742–746. [DOI] [PubMed] [Google Scholar]
- 34.Grusby, M.J., R.S. Johnson, V.E. Papaioannou, and L.H. Glimcher. 1991. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 20:1417–1420. [DOI] [PubMed] [Google Scholar]
- 35.von Freeden-Jeffry, U., P. Vieira, L.A. Lucian, T. McNeil, S.E.G. Burdach, and R. Murray. 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mertsching, E., C. Burdet, and R. Ceredig. 1995. IL-7 transgenic mice: analysis of the role of IL-7 in the differentiation of thymocytes in vivo and in vitro. Int. Immunol. 7:401–414. [DOI] [PubMed] [Google Scholar]
- 37.Perarnau, B., M.F. Saron, B.R. San Martin, N. Bervas, H. Ong, M.J. Soloski, A.G. Smith, J.M. Ure, J.E. Gairin, and F.A. Lemonnier. 1999. Single H2Kb, H2Db and double H2KbDb knockout mice: peripheral CD8+ T cell repertoire and anti-lymphocytic choriomeningitis virus cytolytic responses. Eur. J. Immunol. 29:1243–1252. [DOI] [PubMed] [Google Scholar]
- 38.Sudo, T., S. Nishikawa, N. Ohno, N. Akiyama, M. Tamakoshi, and H. Yoshida. 1993. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc. Natl. Acad. Sci. USA. 90:9125–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grabstein, K.H., T.J. Waldschmidt, F.D. Finkelman, B.W. Hess, A.R. Alpert, N.E. Boiani, A.E. Namen, and P.J. Morrissey. 1993. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J. Exp. Med. 178:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyons, A.B., and C.R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods. 171:131–137. [DOI] [PubMed] [Google Scholar]
- 41.Shen, F.-W. 1981. Monoclonal antibodies to mouse lymphocyte differentiation alloantigens. Monoclonal Antibodies and T-Cell Hybridomas: Perspectives and Technical Advances. G.J. Hämmerling, U. Hämmerling, and J.F. Kearney, editors. Elsevier/North-Holland, Amsterdam. pp. 25–31.
- 42.Koo, G.C., and J.R. Peppard. 1984. Establishment of monoclonal anti-Nk-1.1 antibody. Hybridoma. 3:301–303. [DOI] [PubMed] [Google Scholar]
- 43.Dummer, W., B. Ernst, E. LeRoy, D.-S. Lee, and C.D. Surh. 2001. Autologous regulation of naive T cell homeostasis with the T cell compartment. J. Immunol. 166:2460–2468. [DOI] [PubMed] [Google Scholar]
- 44.Kieper, W.C., J.T. Tan, B. Bondi-Boyd, L. Gapin, J. Sprent, R. Ceredig, and C.D. Surh. 2002. Overexpression of interleukin (IL)-7 leads to IL-15–independent generation of memory phenotype CD8+ T cells. J. Exp. Med. 195:1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugamura, K., H. Asao, M. Kondo, N. Tanaka, N. Ishii, K. Ohbo, M. Nakamura, and T. Takeshita. 1996. The interleukin-2 receptor γ chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu. Rev. Immunol. 14:179–205. [DOI] [PubMed] [Google Scholar]
- 46.Napolitano, L.A., R.M. Grant, S.G. Deeks, D. Schmidt, S.C. De Rosa, L.A. Herzenberg, B.G. Herndier, J. Andersson, and J.M. McCune. 2001. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat. Med. 7:73–79. [DOI] [PubMed] [Google Scholar]
- 47.Bolotin, E., G. Annett, R. Parkman, and K. Weinberg. 1999. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 23:783–788. [DOI] [PubMed] [Google Scholar]
- 48.Fry, T.J., E. Connick, J. Falloon, M.M. Lederman, D.J. Liewehr, J. Spritzler, S.M. Steinberg, L.V. Wood, R. Yarchoan, J. Zuckerman, et al. 2001. A potential role for interleukin-7 in T-cell homeostasis. Blood. 97:2983–2990. [DOI] [PubMed] [Google Scholar]