Abstract
Perichondrium in fetal limb is composed of undifferentiated mesenchymal cells. However, the multipotency of cells in this region and the role of perichondrium in bone marrow formation are not well understood. In this report, we purified and characterized perichondrial cells using a monoclonal antibody against activated leukocyte cell adhesion molecule (ALCAM) and investigated the role of perichondrial cells in hematopoietic bone marrow formation. ALCAM is expressed on hematopoietic cells, endothelial cells, bone marrow stromal cells, and mesenchymal stem cells and mediates homophilic (ALCAM–ALCAM)/heterophilic (ALCAM-CD6) cell adhesion. Here we show by immunohistochemical staining that ALCAM is expressed in perichondrium. ALCAM+ perichondrial cells isolated by FACS® exhibit the characteristics of mesenchymal stem cells. ALCAM+ cells can differentiate into osteoblasts, adipocytes, chondrocytes, and stromal cells, which can support osteoclastogenesis, hematopoiesis, and angiogenesis. Furthermore, the addition of ALCAM-Fc or CD6-Fc to the metatarsal culture, the invasion of the blood vessels to a cartilage was inhibited. Our findings indicate that ALCAM+ perichondrial cells participate in vascular invasion by recruiting osteoclasts and vessels. These findings suggest that perichondrium might serve as a stem cell reservoir and play an important role in the early development of a bone and bone marrow.
Keywords: limb bud, stem cell, differentiation, homophilic cell adhesion, angiogenesis
Introduction
Development, remodeling, and regeneration of tissue and organs are dependent on the activity of stem cells. Bone formation in fetal stages, especially endochondral ossification, begins with aggregation of mesenchymal stem cells (MSCs)* to form condensations (1). In the core of mesenchymal condensation, cells differentiate into chondrocytes. However, in the periphery of such condensations, undifferentiated mesenchymal cells form the perichondrium (which is then called periosteum; reference 2). At the time when chondrocyte hypertrophy occurs, the perichondrial cells differentiate into osteoblasts to form the bone collar (3). Just after the calcification of this bone collar, blood vessels penetrate it and the cartilage, bringing the blood supply that will form the hematopoietic bone marrow (BM; reference 4).
In adult stages, MSCs contribute to the maintenance of various tissues. It has been reported that MSCs can be isolated from adult BM (5) and can be induced in vitro and in vivo to differentiate into a variety of mesenchymal tissues such as bone, cartilage, muscle, ligament, tendon, adipose tissue, and stroma. In addition, human BM-derived MSCs can maintain their multipotential capacity and exhibit site-specific differentiation after in utero transplantation in sheep (6). MSCs derived from adult BM has been well characterized and analyzed for its ability to differentiate, whereas it is still unclear how MSCs contribute to in vivo embryonic tissue formation (organogenesis), such as bone and BM. Previous studies indicate that perichondrium contains immature mesenchymal cells and plays a critical role of bone formation (2, 7, 8). Moreover, it is reported that a population of small, relatively agranular cells isolated from fetal rat perichondrium display stem cell characteristics (9). However, evidence supporting multipotency of cells derived from perichondrium has been insufficient.
Recently, several cell surface markers expressed on marrow-derived MSCs have been identified. It has been reported that activated leukocyte cell adhesion molecule (ALCAM; CD166) is expressed on MSCs (5, 10). This expression disappears once the MSCs embark on an osteogenic pathway and begin to express alkaline phosphatase (ALP). These observations indicate that ALCAM may play a role in the progress of osteogenic differentiation, although the precise mechanism of that activity remains to be elucidated (10).
ALCAM was first identified on thymic epithelial cells (11) and activated leukocytes (12). ALCAM is a member of the Ig superfamily and contains five extracellular Ig domains. It is a highly glycosylated type I transmembrane molecule with a short (32-aa) cytoplasmic tail and an observed molecular mass of 105 kD, and it mediates homophilic/heterophilic adhesion (ALCAM-CD6; reference 12). ALCAM-mediated homophilic cell adhesion is tightly regulated through the actin cytoskeleton by the formation of clusters of molecules at the cell surface (13). Uchida et al. (14) identified hematopoietic cell antigen, which is identical to ALCAM, on hematopoietic stem cells (HSCs) and myeloid progenitors. ALCAM is highly homologous to a neuronal protein known as DM-GRASP/SC-1/BEN in chick (15–17), KG-CAM in rat (18), and neurolin in goldfish (19). In addition to being expressed on MSCs and hematopoietic cells, ALCAM is expressed on metastasizing melanoma (20), neuronal cells (16), endothelial cells (21), and hematopoiesis-supporting osteoblastic cells (22). ALCAM is also expressed by subsets of stromal cells in the primary hematopoietic sites that sequentially develop in the human embryo and fetus, i.e., the paraaortic mesoderm, liver, thymus, and BM (23).
In this report, we analyzed the differentiation ability of perichondrial cells, which can be isolated by FACS® using anti-ALCAM mAb. Furthermore, we investigated the role of cell–cell adhesion through ALCAM in vascular invasion to the cartilage, and clarified how perichondrium contributes to bone and BM development.
Materials and Methods
Mice.
Pregnant C57BL/6 mice were purchased from Japan SLC. Embryos at E13.5 were used to dissect the limb, and embryos at E17.5 were used to dissect the metatarsals of hindlimb.
Antibodies.
The mAbs were used in this study: anti–CD31/PECAM-1; anti-CD45 antibody, anti-β1 integrin; anti-α4 integrin; anti-α5 integrin; anti-αv integrin; anti-β3 integrin; anti–intracellular cell adhesion molecule (ICAM)-1; anti–ICAM-2; anti-CD44; anti-Endoglin; anti–platelet-derived growth factor (PDGF) receptor α chain; anti–Thy-1; anti-CD34; anti–c-Kit; anti-CD4; anti-CD8; anti-B220, anti–TER-119; anti–Gr-1; and anti–Mac-1. All were purchased from BD PharMingen. The production of the anti-ALCAM mAb was described previously (21).
Immunohistochemistry.
For histological analysis, dissected forelimbs were fixed 2 h in 4% paraformaldehyde at 4°C, rinsed in PBS, dehydrated through a methanol series, embedded in polyester wax, and sectioned at 8 μm. The procedure for immunohistochemistry of tissue sections was as described previously (24). An anti-ALCAM mAb and an anti–PECAM-1 mAb were used in this assay. The primary antibody was developed with horseradish peroxidase-conjugated anti–rat Ig antibody (Biosource).
Cell Preparation and Cell Sorting.
Dissected limbs were incubated with 2.4 U Dispase® (GIBCO BRL) in PBS at 37°C for 60 min and passed through a 23 G needle. Dispersed cells were washed with DMEM-low glucose (GIBCO BRL) containing 10% FCS (JRH Bioscience). Mononuclear cells were isolated by centrifugation of total limb mononuclear cells (LMCs) on Lymphoprep™ (NYCOMED PHARMA AS.) according to the manufacturer's instructions. The cell-staining procedure for flow cytometry was as described previously (25). The stained cells were analyzed and sorted by FACSvantage™ (Becton Dickinson).
To identify and isolate side population (SP) cells from LMCs, the cells were suspended at 106 cells per ml in 2% FCS/DMEM (staining medium) and preincubated at 37°C for 10 min. The cells were then labeled with 2.5 μg/ml Hoechst 33342 (Molecular Probes) in staining medium at 37°C for 90 min. Verapamil (Sigma-Aldrich) was used at 50 μM and included in the entire Hoechst staining procedure.
Cell Culture.
Fractionated and unfractionated cells were maintained in 10% FCS/DMEM low glucose at 37°C in humidified 5% CO2 air. Inductions of osteogenic and adipogenic differentiation were performed according to the report of Pittenger et al. (5). The procedures for chondrogenic differentiation were described previously (26, 27). For osteogenic induction, cells were cultured for 2 wk in 10% FCS/DMEM low glucose in the presence of 10 mM β-glycerol phosphate (β-GP; Sigma-Aldrich) and 50 μM ascorbic acid (Sigma-Aldrich). Osteoblastic differentiation was evaluated by ALP staining and ALP enzymic activity. Calcium deposition was stained with 1% alizarin red. ALP staining was performed using a histochemical kit (no. 85; Sigma-Aldrich) according to the manufacturer's instructions. ALP activity was measured by a diagnostic kit (no. 245; Sigma-Aldrich). For adipogenic induction, cells were cultured with 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) and 1 μM Dex, 10 mg/ml insulin, and 10% FCS in DMEM low glucose (MDI medium: adipogenic induction medium). After 1 wk of cultivation, media was changed to adipogenic maintenance medium (10% FCS/DMEM low glucose containing 1 μM Dex and 10 mg/ml insulin) for an additional 7 d. Adipogenic differentiation was examined by the accumulation of lipid vesicles using a fluorescence assay based on Nile red staining. Chondrogenic potential was assayed by culturing cells in 10% FCS/DMEM low glucose containing 1 μg/ml rh-BMP-2 (a gift from Yamanouchi Pharmaceutical Co., Tokyo, Japan) for 2 wk at 37°C in humidified 5% CO2 air. After cultivation, cells were stained with alcian blue (28).
For blocking of ALCAM–ALCAM interaction, recombinant human ALCAM/human IgG1 Fc chimeric protein (ALCAM-Fc; R&D Systems) or recombinant mice CD6/human IgG1 Fc chimeric protein (CD6-Fc; R&D Systems) were added to the in vitro cultivation. CD4-Fc was used for control-Fc.
In Vitro Osteoclastogenesis.
BM-derived osteoclast precursor cells (25) were cocultured with ALCAMhigh cells. Cultures were maintained in α-MEM/10% FCS with 10−8 M 1,25-(OH)2D3 and 10−7 M dexamethasone (Dex). Tartrate-resistant acid phosphatase (TRAP) staining and measurement of TRAP-activity were performed on day 7 of coculture. Procedures used for TRAP staining and analysis of TRAP activity were described previously (25).
In Vitro Hematopoiesis.
BM-derived HSCs and fractionated limb-derived cells were cocultured in 10% FCS/RPMI 1640 (GIBCO BRL) containing 10−5 M 2ME (GIBCO BRL), 20 ng/ml IL-6 (PeproTech), 50 U/ml IL-7 (PeproTech), 50 ng/ml stem cell factor (SCF; R&D Systems), and 2 U/ml Erythropoietin (Epo) (a gift from Snow-Brand Milk Products, Tochigi, Japan) at 37°C in humidified 5% CO2 air. After 10 d of cultivation, expanded number of CD45+ cells were counted and the expression of c-Kit and lineage markers were analyzed by FACS®.
In Vitro Angiogenesis.
E9.5 mouse paraaortic splanchnopleural mesoderm (P-Sp)-derived cells were used as endothelial precursor cells. These cells were cocultured with ALCAMhigh cells in the presence or absence of 10 ng/ml vascular endothelial growth factor (VEGF) for 10 d. Before cocultivation, ALCAMhigh cells were induced or not induced to undergo mesenchymal lineage-specific differentiation (osteo-, adipo-, and chondrogenic differentiation). After cocultivation, cells were fixed and stained with anti–PECAM-1 mAb.
Metatarsal Culture.
Metatarsal culture was performed as described previously (29, 30). In brief, the three middle metatarsals of each hindlimb of E17.5 embryo were dissected and cultured for 5 d on Millicell® culture plate insert (Millipore) on 1 ml of BGJb medium (GIBCO BRL) supplemented with 10% FCS. Control-Fc, ALCAM-Fc, or CD6-Fc was added to a final concentration of 20 μg/ml. After cultivation, metatarsals were fixed and processed for polyester wax sections. Each section was stained with anti–PECAM-1 MoAb.
RT-PCR Analysis.
Isolated RNA was reverse transcribed using an RT-PCR kit (CLONTECH). The cDNAs were amplified using an Advantage polymerase mix (CLONTECH) in a GeneAmp PCR system model 9700 (Perkin-Elmer) for 25–30 cycles. Sequences of gene specific primers for RT-PCR were as follows: 5′-angiopoietin (Ang)-1 (CAGTGGCTGCAAAAACTTGA); 3′-Ang-1 (TCTGCACAGTCTCGAAATGG); 5′-Ang-2 (CACACTGACCTTCCCCAACT); 3′-Ang-2 (TGGTGTCTCTCAGTGCCTTG); 5′-VEGF (CTTTACTGCTGTACCTTCACCATGC); 3′-VEGF (AACAAGGCTCACAGTGATTTTCTGG); 5′-basic fibroblast growth factor (bFGF) (CCC-AAGCGGCTCTACTGCAAGAAC); 3′-bFGF (GGTCCCG-TTTTGGATCCGAGTTTAT); 5′-ChM-1 (CACAAGAAGA-CCACACAGCGAACCT); 3′-ChM-1 (CTTTCAGCACAT-TTGCACACAGTGG); 5′-mOC: TAGTGAACAGACTCCGGCGCTACCTT; 3′-mOC: AGCTCGTCACAAGCAGGGTTAAGCTC; 5′-PPARγ: GCTGATGCACTGCCTATGAGC-ACTT; 3′-PPARγ: GAAGCCTGATGCTTTATCCCCACAG; 5′-mSOX9: GGAAGATAAGTTCCCCGTGTGCATC; 3′-mSOX9: ATGTGAGTCTGTTCCGTGGCCTCTT; 5′-mcol2: CAGAAAGGAGAACCTGGAGATATCA; 3′-mcol2: TCTCCCTTGTCACCACGATCACCTC; 5′-mG3PDH (TGAAGGTCGGTGTGAACGGATTTGGC); and 3′-mG3PDH (CATGTAGGCCTAGAGGTCCACCAC). Each cycle consisted of 30 s denaturation at 94°C, and 4 min annealing/extension at 70°C.
Results
Purification of ALCAM-Positive Perichondrial Cells.
To investigate the expression of ALCAM at the site of endochondral ossification, we analyzed the ALCAM expression pattern by immunohistochemistry. In E13.5 limb, high levels of ALCAM expression were restricted to the periphery of cartilage (perichondrium) (Fig. 1 A a and b; black arrowheads). In addition, ALCAM+ endothelial cells were distributed in the direction of the midshaft of cartilage (Fig. 1 A a–c; arrows). PECAM-1+ endothelial cells were seen close to the ALCAM+ perichondrium (Fig. 1 A c and d). FACS® analysis showed the proportion of PECAM-1− CD45−ALCAM+ cells was 8.2 ± 2.5% in freshly prepared LMCs. After 2 d of cultivation, the percentage of PECAM-1−CD45−ALCAMhigh cells increased to 87.4 ± 4.0% (Fig. 1 B). For further analysis, total LMCs were precultured for 2 d and PECAM-1−CD45−ALCAMhigh (hereafter ALCAMhigh) and PECAM-1−CD45−ALCAMlow/− (hereafter ALCAMlow/−) cells were sorted and used for a mesenchymal differentiation assay. Both ALCAMhigh cells and ALCAMlow/− cells were spreading on the dish, and the remarkable difference was not seen in 12 h after plating. After 4 d of cultivation, in the ALCAMlow/− cells, the number of swollen cells were increased as cultivation progressed (Fig. 1 C c). ALCAMhigh cell seemed to be a homogeneous population in shape (Fig. 1 C d). These data suggest that cells in perichondrium region express ALCAM and that ALCAM expression could be useful for isolation of a perichondrium fraction.
Figure 1.
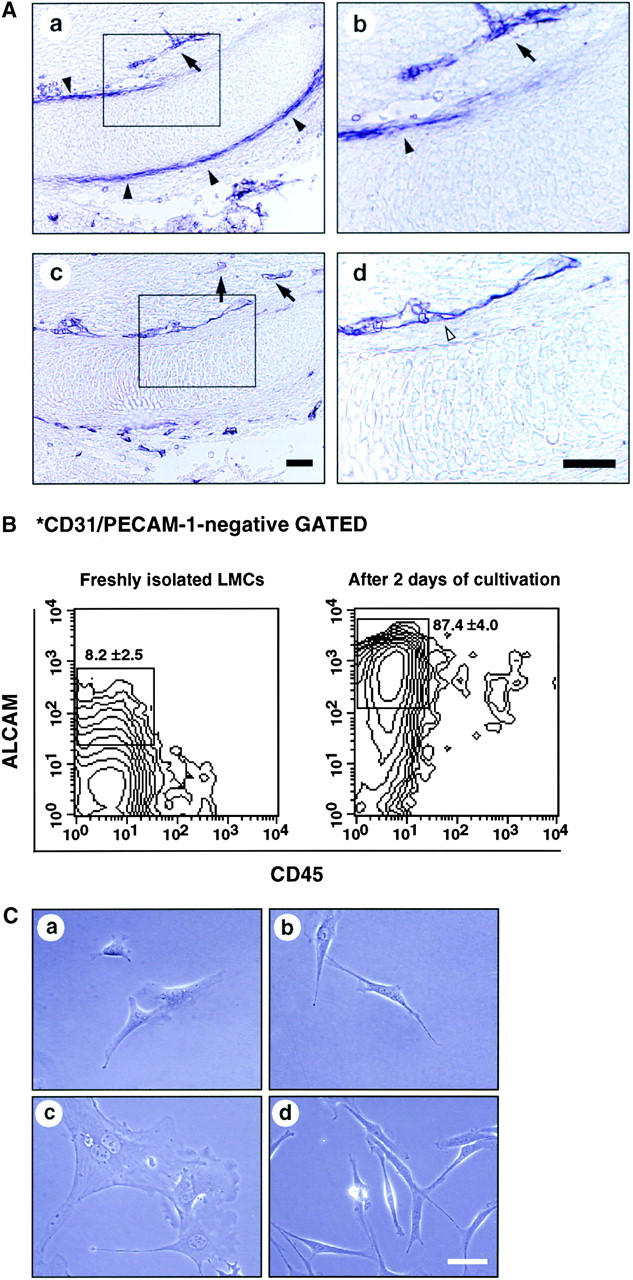
Perichondrial cells express ALCAM. (A) Longitudinal sections of E13.5 forelimb were stained with anti-ALCAM mAb (a and b) and anti–PECAM-1 mAb (c and d). ALCAM+ cells (black arrowheads) are seen in the perichondrium region. PECAM-1+ endothelial cells (white arrowheads) were detected surrounding cartilage and ALCAM-expressing perichondrium. ALCAM+ endothelial cells distributed toward the perichondrium (allows). b and d are higher magnifications of enclosed boxes in a and c, respectively. Scale bar, 100 μm. (B) LMCs derived from E13.5 limb were stained with anti-CD45, anti–PECAM-1, and anti-ALCAM mAbs and then fractionated by FACS®. PECAM-1− cells were gated and examined for expression of CD45 and ALCAM by contour blot. The percentages of ALCAM+ cells are representative of triplicate experiments. (C) Morphology of sorted ALCAMhigh (a and b) and ALCAMlow/− cells (c and d). (a and c) 12 h after plating. (b and d) 4 d after plating. Scale bar, 50 μm.
Characterization of Limb-derived ALCAMhigh Cells.
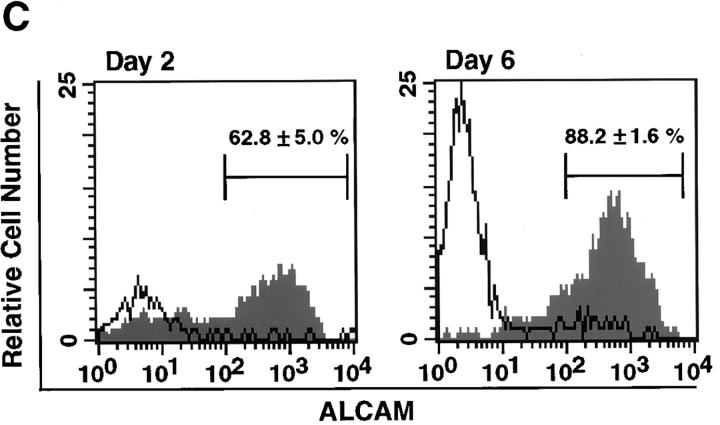
ALCAM expression was increased in LMCs after 2 d of cultivation as shown in Fig. 1 B. There are two possibilities for such an increase in ALCAM expression. One is that ALCAM+ cells have a higher proliferation activity than ALCAM− cells in freshly prepared LMCs, and the other is that freshly isolated ALCAM− cells express ALCAM. To differentiate between these two possibilities we compared cell growth between ALCAM+ and ALCAM− cells that were sorted from freshly prepared LMCs. The expanded cell number derived from freshly isolated ALCAM+ cells was 2.5 times greater than from freshly isolated ALCAM− cells after 4 d of cultivation (Fig. 2 A). Then, to know whether the cell adhesion through ALCAM regulate cell growth, we added ALCAM-Fc and CD6-Fc to cultivation of ALCAM+ cells, and examined those effects on cell proliferation. Fig. 2 B a shows the comparison of cell proliferation among the cells cultured in the presence of control-Fc, ALCAM-Fc, or CD6-Fc. Fig. 2 B b shows the comparison of the number of cells in each experimental day. The number of adherent cells in 0.5 d after plating did not have the difference in each group, indicating that ALCAM-Fc and CD6-Fc did not inhibit initial cell attachment. In the fifth day of cultivation, as compared with the cells that added control-Fc, the number of the expanded cells was reduced ∼33.3 ± 12.5% and 56.0 ± 7.7% by the addition of 20 μg/ml ALCAM-Fc and 20 μg/ml CD6-Fc, respectively (Fig. 2 B). These data suggest that ALCAM is working to support the cell proliferation in perichondrium. Next, we showed that freshly isolated ALCAM− cells from LMCs became to express ALCAM in the culture. After cultivation, 62.8 ± 5.0% and 88.2 ± 1.6% of the cells expressed ALCAM on days 2 and 6, respectively (Fig. 2 C).
Figure 2.
Characterization of perichondrial cells. (A) Comparison of proliferation between freshly isolated ALCAM+ and ALCAM− cells. ALCAM+ and ALCAM− cells were sorted from freshly isolated LMCs and plated into 48-well plates at 1.5 × 104 cells per well. Cell numbers were determined on days 1–4. (B) Effects of ALCAM-Fc and CD6-Fc on the cell proliferation. ALCAM+ cells were sorted from freshly prepared LMCs, and cultured for 5 d in the presence of various concentrations of Fc-chimeric proteins. The number of expanded cells was counted on days 0.5, 3, and 5. (a) The ratio of cell growth. The cell number of the 0.5 d of culture in the presence of control-Fc was set at 1.0. (b) The relative cell numbers on each day of culture. The cell number in cultivation with control-Fc in each culture period was set as 1.0. The data shown represent the mean ±SD. (C) ALCAM− cells were isolated from freshly prepared LMCs, and the expression of ALCAM was examined on days 2 and 6 of cultivation. The percentages of ALCAM+ cells in each panel are representative of triplicate experiments. (D) Flow cytometric characterization of ALCAMhigh cells. The expression of cell surface markers indicated on panels (a–l) was examined by FACS®.
To further characterize ALCAMhigh cells, we analyzed the expression of several cell surface markers, which were known to be expressed (β1, α5, αv, β3 integrins, ICAM-1, PDGFR, Endoglin, CD44, and Thy-1) or not expressed (α4 integrin, ICAM-2, and CD34) in human BM-derived MSCs (5). As shown in Fig. 2 D, ALCAMhigh cells expressed β1, α5, αv, β3 integrins, ICAM-1, PDGFR, and CD44 but did not express CD49d, ICAM-2, and CD34. These expression patterns suggest that ALCAMhigh cells represent a fairly homogeneous population at least for β1, α5, αv, β3 integrins, ICAM-1, PDGFR, and CD44.
ALCAM Expression in the Limb SP Cell Fraction.
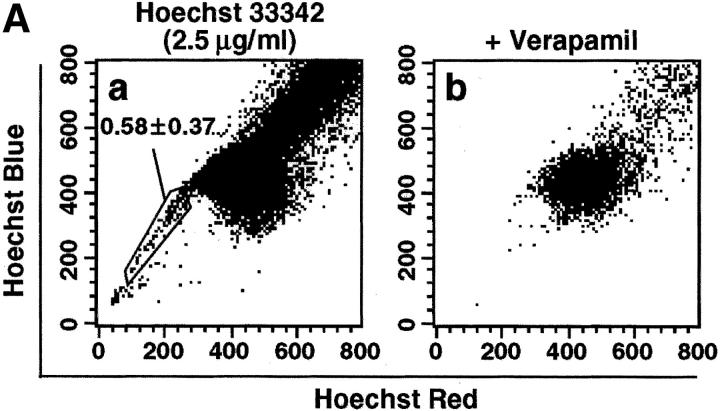
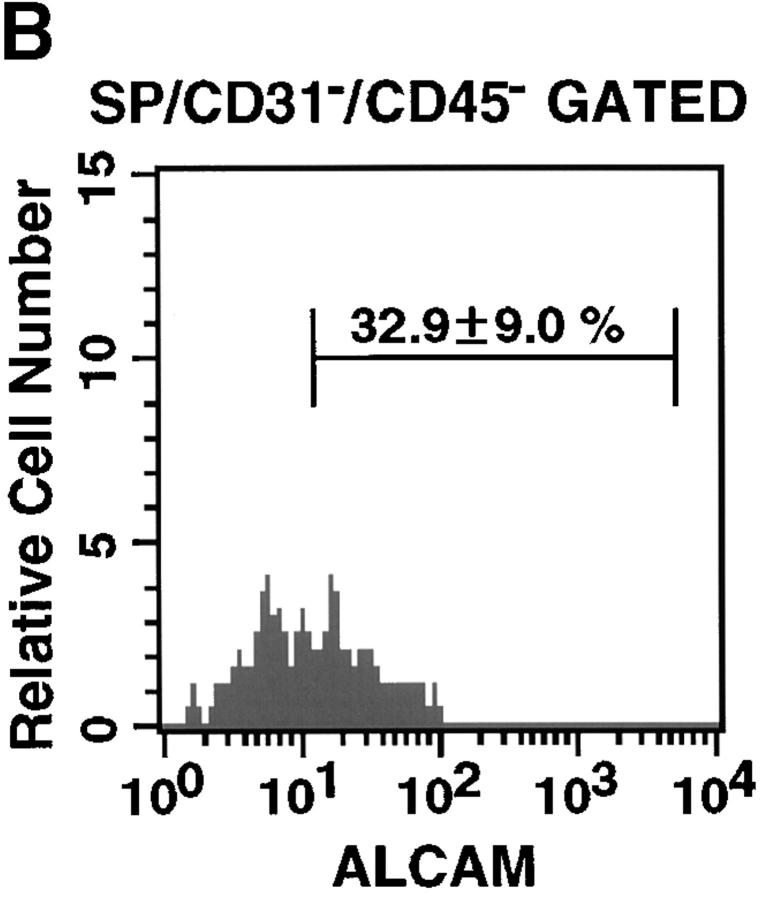
To analyze ALCAM expression on stem cell fraction in limb bud, we examined limb SP cells. SP cells are known to be pluripotent stem cells. To identify SP cells in the limb, initial studies were conducted to establish optimal Hoechst dye staining conditions (data not shown). Incubation of LMCs with 2.5 μg/ml of Hoechst 33342 for 90 min consistently identified a population similar to that of murine BM SP cells (Fig. 3 A a, and data not shown). SP cells represented 0.58 ± 0.37% in freshly prepared LMCs. Goodell et al. had previously shown that the Hoechst SP profile in murine BM was blocked by staining in the presence of verapamil, indicating that the faint staining of SP cells was at least partially due to the efflux of Hoechst dye mediated by ABC transporters (31). Hoechst staining of LMCs was also sensitive to verapamil (Fig. 3 A b). The percentages of ALCAM+ cells in PECAM-1−CD45− gated SP cells were 32.9 ± 9.0% (Fig. 3 B). Thus, ALCAM+ cells were enriched about fourfold in SP cell population.
Figure 3.
Limb SP cells express ALCAM. (A) LMCs were stained with Hoechst 33342 and analyzed for expression of ALCAM, PECAM-1, and CD45 in the SP cell fraction. (a) Hoechst 33342 staining and emission pattern of LMCs. SP cells were indicated in the enclosed region. (b) Hoechst 33342 staining and emission pattern of LMCs in the presence of verapamil. (B) A fluorescence histogram shows the ALCAM staining profile of the SP gated with the PECAM-1−CD45− fraction. The percentages of ALCAM+ cells are representative of triplicate experiments.
Osteo-, Adipo-, and Chondrogenic Differentiation from ALCAMhigh Cells.
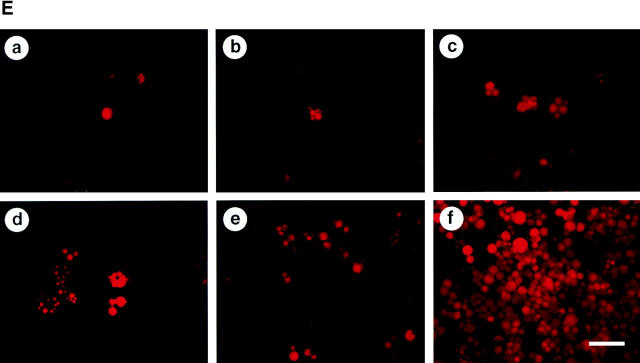
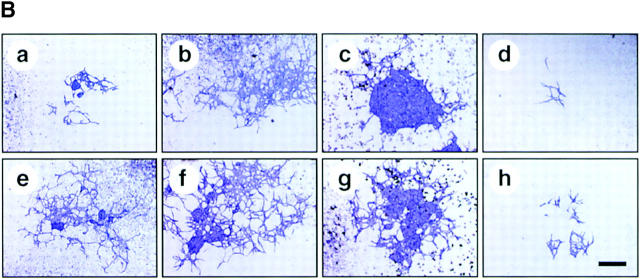
To determine whether perichondrial cells could differentiate into multiple mesenchymal lineages, we examined osteoblast, adipocyte, and chondrocyte differentiation from total LMCs, ALCAMhigh, and ALCAMlow/− cells. As shown in Fig. 4 A, ALCAMhigh cells but not ALCAMlow/− cells differentiated efficiently into ALP+ osteoblastic cells in the presence of β-GP and ascorbic acid. Moreover, after 2 wk of cultivation, alizarin red-positive calcium deposition was observed in ALCAMhigh cells in the presence of β-GP and ascorbic acid (Fig. 4 B). Strong calcium deposition was observed in the corner of culture plate (Fig. 4 B c). ALCAMhigh cells support osteoclast formation from mouse BM precursor cells. In particular, when ALCAMhigh cells directed to the osteoblasts, it reacted well to 1,25-(OH)2D3 and Dex, and higher TRAP activity was seen as compared with the cells without precultivation for osteogenic induction (Fig. 4 C). TRAP+ multinucleated cells were formed on osteoblast differentiation induced ALCAMhigh cells in the presence of 1,25-(OH)2D3 and Dex (Fig. 4 D, arrowheads). Regarding adipogenic differentiation, fluorescence microscopy showed that a large number of cells cultured in MDI medium contained lipid droplets in ALCAMhigh fraction, while lipid accumulation in total LMCs and ALCAMlow/− cells was very low (Fig. 4 E). Chondrogenic phenotype was evaluated by staining with alcian blue, which stains unmineralized cartilaginous matrix. In the presence of BMP-2, large amounts of alcian blue stained extracellular matrix were observed in ALCAMhigh cells (Fig. 4 F f). Although, both unfractionated LMCs and ALCAMlow/− cells may contain chondrocytes or prechondrocytes, only LMCs differentiated into chondrocytes, while ALCAMlow/− cells did not. In addition, the rate of alcian blue positive cartilage matrix synthesis by LMCs was lower than ALCAMhigh cells (Fig. 4 F d). The morphology of the cells from ALCAMlow/− fraction became elongated shape in the presence of BMP-2 (Fig. 4 F e). In addition, differentiations of osteoblast, adipocyte, and chondrocyte from ALCAMhigh cells were confirmed by the specific gene expressions corresponding to each linage. As shown in Fig. 6 A, after inductions of osteoblasts, adipocytes, and chondrocytes, ALCAMhigh cells express osteocalcin, PPAR-γ, SOX9, and type II collagen, respectively. Altogether, these data suggest that ALCAMhigh cells can differentiate into osteoblasts, adipocytes, and chondrocytes.
Figure 4.
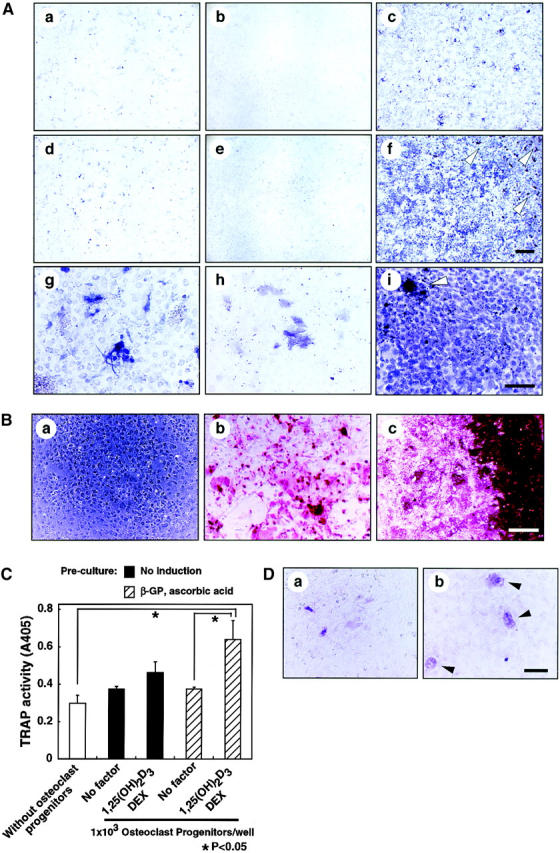
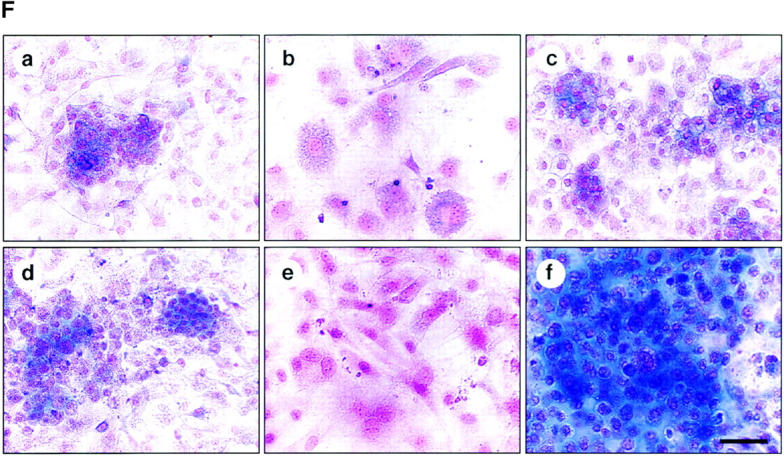
Osteoblasts, adipocytes, and chondrocyte differentiation. (A) Total LMCs (a, d, and g), ALCAMlow/− cells (b, e, and h), and ALCAMhigh cells (c, f, and i) were induced (d–i) or not-induced (a–c) to undergo osteogenic differentiation. After 2 wk, ALP staining was performed. Panels g–i show high power fields of d–f, respectively. Scale bars, 1 mm (a–f), 200 μm (g–i). (B) The region of alizarin red positive calcium deposition in ALCAMhigh cells, in which osteogenic differentiation was induced for 2 wk. (a) no factor; (b and c) β-GP and ascorbic acid. (b) The center of culture plate. (c) The corner of the culture plate. (C) TRAP activities were measured on day 7 of cocultivation of BM derived osteoclast precursor cells and ALCAMhigh cells, which were induced (hatched bar) or not induced (solid bar) to undergo osteogenic differentiation in the presence or absence of 1,25-(OH)2D3 and Dex. The data shown represents the mean ±SD. (D) Results of TRAP staining of cocultured BM-derived osteoclast precursor cells and ALCAMhigh cells, in which osteogenic differentiation was induced. TRAP-positive multinucleated cells are indicated by arrowheads. (a) no factor; (b) in the presence of 1,25-(OH)2D3 and Dex. Scale bar, 100 μm. (E) Total LMCs (a and d) ALCAMlow/− cells (b and e), and ALCAMhigh cells (c and f) were induced to undergo adipogenic differentiation (as described in Materials and Methods) and stained with Nile red. (a–c) control medium; (d–f) MDI medium. Scale bar, 200 μm. (F) Alcian blue staining after chondrogenic induction. Total LMCs (a and d), ALCAMlow/− cells (b and e), and ALCAMhigh cells (c and f) were induced to undergo chondrogenic differentiation as described in Materials and Methods. (a–c) no factor; (d–f) in the presence of BMP-2. Scale bar, 100 μm.
Figure 6.
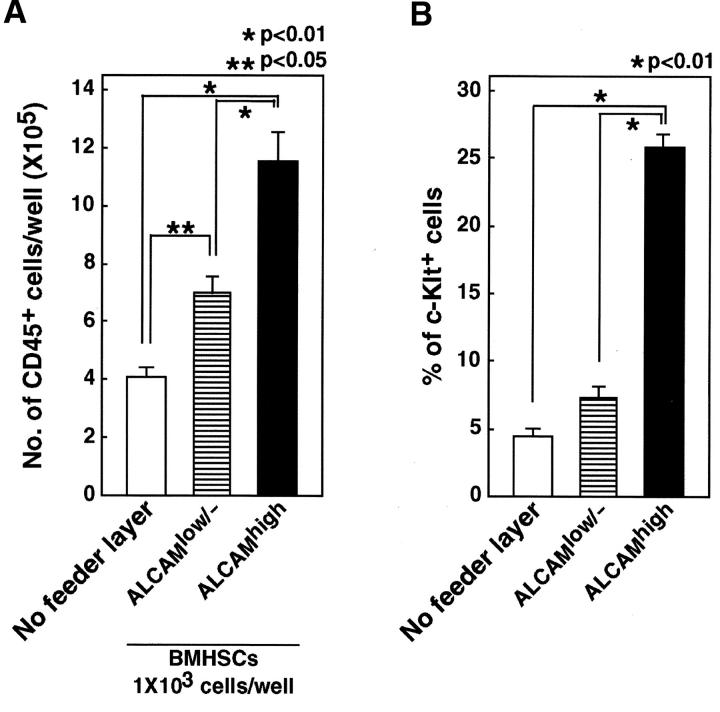
ALCAMhigh cells support hematopoiesis. Total of 103 Lin-c-Kit+Sca-1+ cells were cultured without feeder layer or cocultured with ALCAMhigh or ALCAMlow/− cells in the presence of SCF, IL-6, IL-7, and EPO. After 8 d of cultivation, the number of CD45+ cell was scored (A), and expression of c-Kit was analyzed by FACS® (B). The data shown represents the mean ±SD.
ALCAM-mediated Homophilic Adhesion Plays a Part in Osteogenic Differentiation.
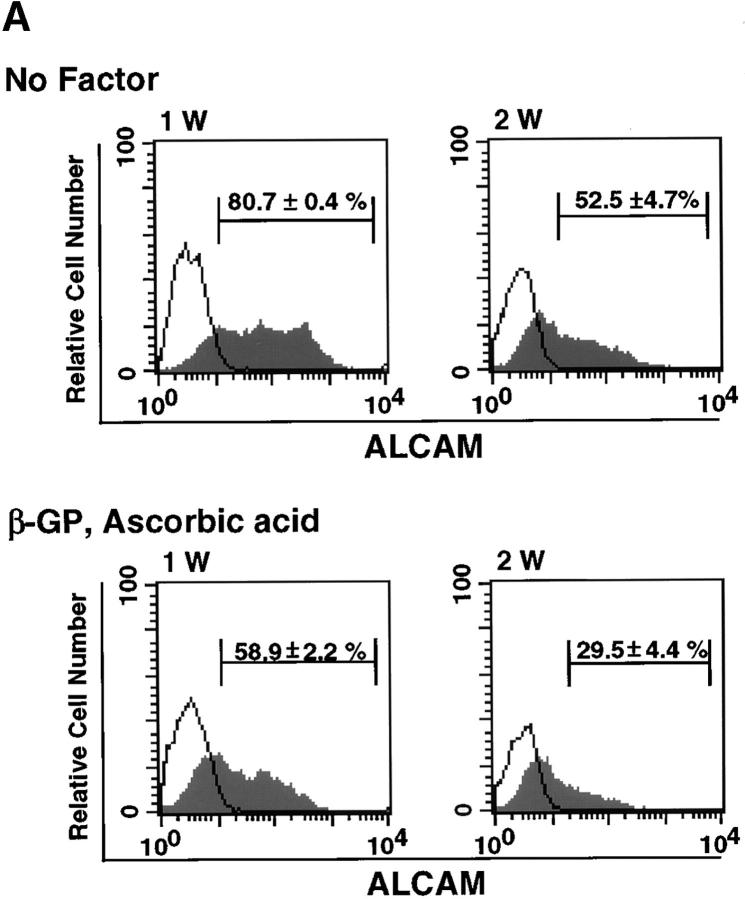
We used FACS® to analyze changes in expression of ALCAM during osteoblastic differentiation. The expression of ALCAM was decreased with longer cultivation in β-GP and ascorbic acid (58.9 ± 2.2% for 1 wk of cultivation and 29.5 ± 4.4% for a 2-wk period) (Fig. 5 A, bottom panel). Although a similar decrease was also observed in a culture without factors, the overall percentage of ALCAM+ cells was higher than in culture with factors (80.7 ± 0.4% after 1 wk of cultivation and 52.5 ± 4.7% after 2 wk) (Fig. 5 A, top panel). Next, we analyzed how ALCAM-mediated cell–cell adhesions participate in mesenchymal differentiation of ALCAMhigh cells. To do so, ALCAM-Fc or CD6-Fc were added to cultures undergoing osteogenic differentiation to block ALCAM-mediated cell adhesion. After 2 wk of cultivation, ALCAM-Fc or CD6-Fc slightly enhanced ALP expression in ALCAMhigh cells with or without β-GP and ascorbic acid (Fig. 5 B and C). We propose that blocking of ALCAM-ALCAM interaction works auxiliary osteogenic differentiation and may facilitate migration of osteoprogenitor cells into cartilage.
Figure 5.
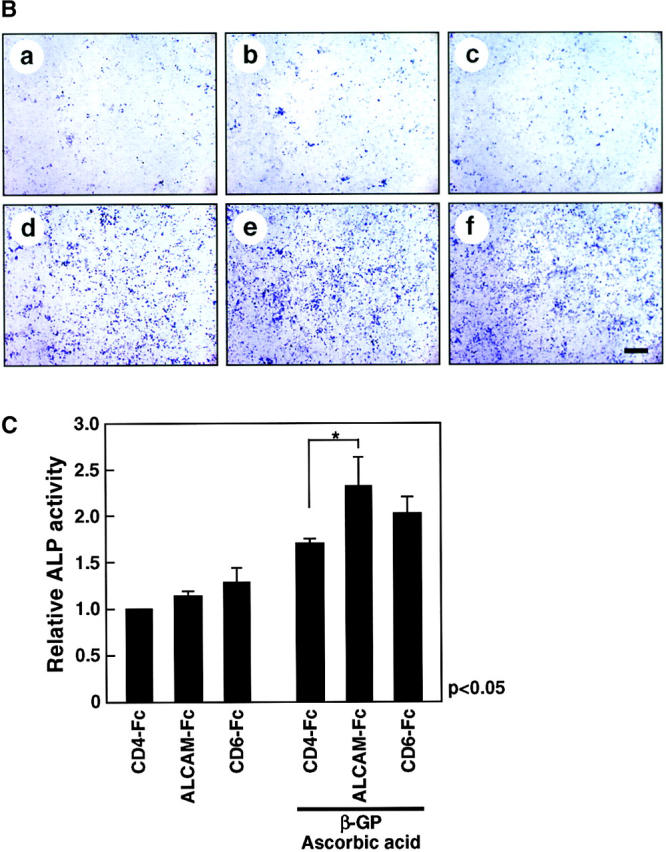
ALCAM-mediated homophilic adhesion plays a role in osteogenic differentiation. (A) Changes in expression of ALCAM were examined by FACS® after 1 wk (1 W) and 2 wk (2 W) of osteoblastic differentiation. The percentages of ALCAM+ cells in each panel represent triplicate experiments. (B) Osteogenic differentiation of ALCAMhigh cells was induced in the presence of control-Fc (a and d), ALCAM-Fc (b and e), or CD6-Fc (c and f). After 2 wk of cultivation, all cells were stained by ALP. (a–c) No factor; (d–f) in the presence of β-GP and ascorbic acid. Scale bar, 1 mm. (C) Relative ALP-activities were measured on day 14 of cultivation. ALP-activity from in the presence of control-Fc alone was set at 1.0. The data shown represents the mean ±SD.
Development of Stromal Cells that Support Hematopoiesis.
We previously reported that ALCAM-transfected endothelial cells support hematopoiesis (21). The number of immature nonadherent cells was significantly greater in coculture of fetal liver HSCs with ALCAM-transfected endothelial cells compared with coculture with vector-transfected cells. Moreover, lethally irradiated recipient mice were repopulated by hematopoietic cells cocultured with ALCAM-transfected endothelial cells, but not with vector-transfected cells. These data suggest that ALCAM might be involved with maintenance of HSCs or expansion of primitive hematopoietic progenitor cells. In this study, we analyzed whether ALCAMhigh cells can support hematopoietic cell differentiation and growth. BM HSCs were cultured without feeder layer cells or cocultured with ALCAMhigh or ALCAMlow/− cells in the presence of SCF, IL-6, IL-7, and Epo for 8 d. After cocultivation, the number of CD45+ cells was counted and the expressions of c-Kit and hematopoietic lineage markers (CD4, CD8, B220, TER-119, Gr-1, and Mac-1) were analyzed (Table I). The number of CD45+ cells was significantly greater in coculture with ALCAMhigh cells compared with culture without feeder layer or coculture with ALCAMlow/− cells (Fig. 6 A). Moreover, ALCAMhigh cells efficiently maintained c-Kit expression (25.8 ± 0.9%) as compared with ALCAMlow/− cells (7.3 ± 0.8%) or without feeder layer (4.5 ± 0.5%) (Fig. 6 B). It indicated that ALCAMhigh cells maintain immature phenotype of HSCs. Further, these data were corresponding to our previous report (21). The percentages of B220+ B cells and TER-119+ erythroid lineages in ALCAMhigh cells (B220+: 11.8 ± 2.0%, TER-119+: 6.1 ± 5.2%) were also higher than ALCAMlow/− cells (B220+: 4.9 ± 1.6%, TER-119+: 2.8 ± 1.0%). The expressions of Mac-1 and Gr-1 were not significantly changed between ALCAMhigh and ALCAMlow/− cells. After BM cavity formation, osteoblasts consist of a part of hematopoietic microenvironment in BM (32). Therefore, we also examined hematopoiesis on ALCAMhigh cells that induced to undergo osteoblastic differentiation. On osteoblastic feeder layer, Mac-1+ cells were developed well (93.3 ± 2.4%) as compared with ALCAMhigh cells (72.3 ± 4.2%). In addition, the percentage of B220+ cells was reduced on osteoblastic feeder layer (1.3 ± 0.3%).
Table I.
ALCAMhigh Cells Support the Differentiation of Hematopoietic Cells
| Percentage of positive cells
|
|||
|---|---|---|---|
| ALCAMlow/− | ALCAMhigh | ALCAMhigh/osteogenic | |
| CD4 | 1.2 ± 0.5 | 1.0 ± 0.1 | 7.7 ± 1.8 |
| CD8 | 0.3 ± 0.2 | 3.8 ± 0.5 | 6.0 ± 1.2 |
| B220 | 4.9 ± 1.6 | 11.8 ± 2.0 | 1.3 ± 0.3 |
| TER-119 | 2.8 ± 1.0 | 6.1 ± 5.2 | 2.5 ± 0.5 |
| Mac-1 | 76.3 ± 7.7 | 72.3 ± 4.2 | 93.3 ± 2.4 |
| Gr-1 | 83.9 ± 12.1 | 62.3 ± 5.8 | 69.0 ± 1.1 |
BM-derived Lin−c-Kit+Sca-1+ HSCs (500 cells per well) were cocultured with ALCAMlow/−, ALCAMhigh cells, or osteogenic differentiation induced ALCAMhigh cells (ALCAMhigh/osteogenic) in the presence of SCF, IL-6, IL-7, and Epo. After 8 d of cultivation, the expression of lineage markers was analyzed by FACS®. The data shown represents the mean ±SD.
Angiogenesis Supporting Activity of Each Lineage.
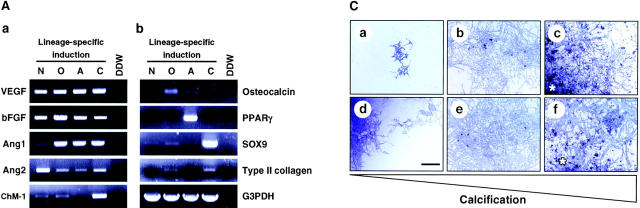
We analyzed angiogenesis supporting ability of perichondrial cells. First, we examined the expression of angiogenic factors in ALCAMhigh cells. ALCAMhigh cells were induced to undergo osteo- adipo-, and chondrogenic differentiation and the expression of Ang1, Ang2, VEGF, bFGF, and chondromodulin-1 (ChM-1) was examined by RT-PCR (Fig. 7 A a). Differentiations of osteoblast, adipocyte, and chondrocyte from ALCAMhigh cells were confirmed by the specific gene expressions, such as osteocalcin (for osteoblast), PPARγ (for adipocyte), αI type II collagen and SOX9 (for chondrocyte) (Fig. 7 A b). Before cultivation, ALCAMhigh cells highly expressed VEGF, bFGF, and Ang2, and faintly expressed Ang1 and ChM-1. The expression of Ang1 was increased and Ang2 was decreased during osteogenic, adipogenic, and chondrogenic differentiation. bFGF expression was slightly increased after osteogenic induction. In chondrocytes, expression of ChM-1, a known inhibitor of angiogenesis, was increased. We further analyzed whether ALCAMhigh cells support endothelial colony formation. E9.5 P-Sp–derived cells were cultured on various feeder layers in the presence or absence of VEGF for 10 d. It is known that the P-Sp region contains hematopoietic stem/progenitor cells and endothelial progenitor cells or common progenitors, hemangioblasts (33). Osteoblast and adipocyte layers can support angiogenesis from P-Sp. There was no significant difference in the number of the colonies formed on osteoblast and adipocyte layers (data not shown). Although the mechanisms are not known, vascular network formation on osteoblast layers developed well, while on adipocyte layers, vascular sheet formation was detected. On the other hand, chondrocyte layers did not support angiogenesis, possibly due to the expression of ChM-1. Exogenous VEGF enhanced endothelial cell colony formation in each feeder layer (Fig. 7 B).
Figure 7.
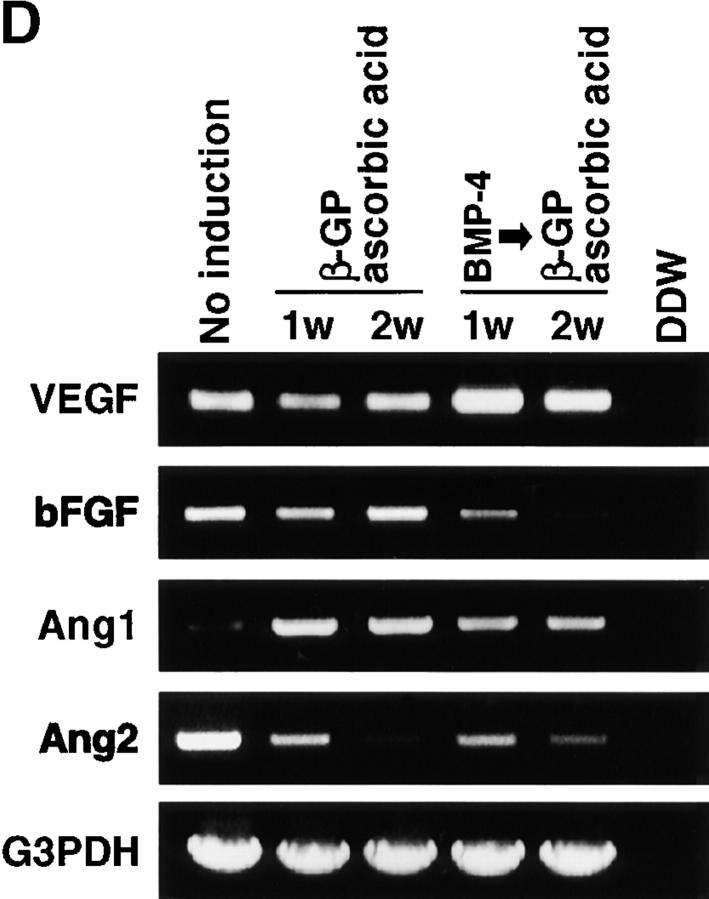
ALCAMhigh cells support growth and differentiation of endothelial cells. (A) Expression of VEGF, bFGF, Ang1, Ang2, and ChM-1 was analyzed by RT-PCR after induction of mesenchymal lineages (a). The expression of mesenchymal lineage markers (b). N, not induced; O, osteogenic; A, adipogenic; and C, chondrogenic induction. (B) Cocultivation of E9.5 P-Sp–derived cells and ALCAMhigh cells in the presence (e–h) or absence (a–d) of VEGF. The cells of feeder layers were not induced (a and e) or induced to undergo osteoblast (b and f) adipocyte (c and g), and chondrogenic (d and h) induction. After 10 d of cultivation, endothelial cells were verified by PECAM-1 reactivity. Scale bar, 500 μm. (C) Differences in angiogenesis supportive activity between calcified or not-calcified osteogenic differentiation. ALCAMhigh cells were precultured in β-GP and ascorbic acid for 1 wk (not-calcified condition, b and e) or rh-BMP-4 for 1 d, followed by treatment with β-GP and ascorbic acid for an additional 6 d (calcified condition, c and f). P-Sp–derived cells were cocultured on each feeder layer in the presence (c–f) or absence (a–c) of VEGF. After 10 d of cocultivation, cells were fixed and stained anti–PECAM-1 mAb. Asterisks indicate a region where calcium deposition appears to occur. Scale bar, 500 μm. (D) Expression of VEGF, bFGF, Ang1, and Ang2 in calcified or not calcified osteogenic induction analyzed by RT-PCR.
As shown in Fig. 7 C, we compared endothelial network formation between cultivations on calcified or noncalcified osteoblast layers. When ALCAMhigh cells were cultured for 1 wk in the presence of β-GP and ascorbic acid (noncalcified osteoblast layer), fine endothelial network formation was observed in the presence or absence of VEGF. By contrast, cultivation of ALCAMhigh cells for 1 d in the presence of BMP-4 followed by 6 d in β-GP and ascorbic acid to promote osteogenic differentiation resulted in strong calcium deposition (calcified osteoblast layers). On the calcified osteoblast layers, branches of PECAM-1+ networks were thicker than on noncalcified osteoblast layers.
To analyze differences in endothelial cell-supporting activity between calcified and noncalcified conditions, the expression of VEGF, bFGF, Ang1, and Ang2 was examined. As shown in Fig. 7 D, the expression of bFGF was downregulated on calcified osteoblasts after treatment with a combination of BMP-4, β-GP, and ascorbic acid. In contrast, VEGF expression was upregulated after treatment with the same combination compared with treatment with β-GP and ascorbic acid only. Although other factors may promote endothelial network formation, higher expression of VEGF and lower expression bFGF in the calcified feeder layer are likely related to making thicker branching of endothelial networks.
Function of ALCAM in Vascular Invasion.
To analyze the role of ALCAM-mediated cell-cell interaction for invasion of blood vessels into cartilage, we performed metatarsal culture as a model of the vascular invasion, and considered the function of ALCAM-Fc and CD6-Fc. E17.5 mouse three middle metatarsals of hindlimb were dissected and cultured in 10% FCS/BGJb medium in the presence of Fc-chimeric protein. After 5 d of cultivation, metatarsals were fixed and processed for polyester wax sections. Each section was stained with anti–PECAM-1 mAbs. Before cultivation, blood vessels enclosed the cartilage, but there were no invaded PECAM-1+ vessels (Fig. 8 A). The addition of control-Fc to metatarsal culture, there are many blood vessels that invaded into cartilage were observed (Fig. 8 B). In contrast, blocking of ALCAM-ALCAM interaction by the addition of ALCAM-Fc and CD6-Fc inhibit vascular invasion into the cartilage (Fig. 8 C and D). Although it is known well that vascular invasion is mainly controlled by angiogenic factors, it is assumed that ALCAM-mediated homophilic cell adhesion may support endothelial cells to adhere to cartilages.
Figure 8.
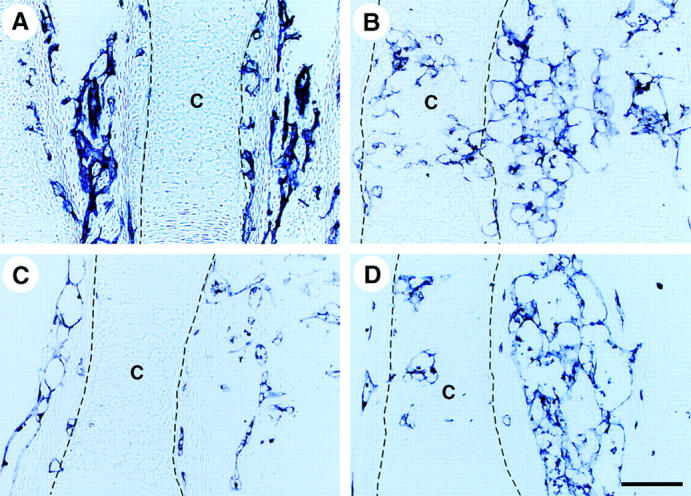
ALCAM-Fc and CD6-Fc inhibit vascular invasion into cartilage. PECAM-1 immunohistochemical staining of metatarsal culture sections. E17.5 mouse three middle metatarsals of hind limb were dissected and cultured for 5 d in 10% FCS/BGJb medium in the presence of Fc-chimeric protein (A) before cultivation. (B) control-Fc. (C) CD6-Fc. (D) ALCAM-Fc. The dotted line indicates the margin of cartilage. Scale bar, 100 μm. Representative data from independent three experiments are shown.
Discussion
Our results provide further characterization of perichondrial cells in mouse fetal limb and clarify the functions of ALCAM for bone and BM formation. Perichondrial cells were isolated by FACS® using an anti-ALCAM mAb. These cells exhibited the characteristics of MSCs and were directed into restricted terminal differentiation pathways, such as osteogenic, adipogenic, chondrogenic, and stromal lineages (Fig. 9 A).
Figure 9.
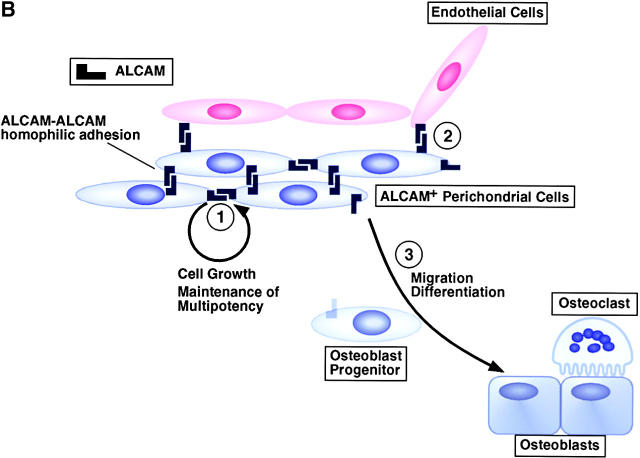
Model of multipotency of ALCAM+ perichondrial cells and the function of ALCAM in the perichondrium. (A) ALCAM+ perichondrial cells differentiate into osteo-, adipo-, chondro-, and stromal cell lineages and support osteoclastogenesis, hematopoiesis, and angiogenesis. VEGF, bFGF, and Ang1, which are expressed by stromal cells, osteoblasts, adipocytes, and hypertrophic chondrocytes, promote angiogenesis. ChM-1, which is expressed by immature chondrocytes, inhibits angiogenesis. Osteoblasts support osteoclastogenesis by the expression of RANKL and M-CSF. (B, 1) ALCAM-mediated homophilic cell-cell adhesion supports cell growth and maintenance of multipotency. (2) ALCAM-mediated cell adhesion between perichondrial cells and endothelial cells attracts vessels to cartilage. (3) Loss of ALCAM–ALCAM interaction or downregulation of ALCAM assists osteogenic differentiation and may facilitate migration of osteoprogenitor cells into cartilage.
Multipotential Nature of ALCAMhigh Cells.
Previous experiments have shown that MSCs isolated from adult BM were characterized by their potential to proliferate in culture, by their multilineage differentiation ability and by the presence of a consistent set of marker proteins (5, 34). In this paper, we analyzed embryonic MSCs. Although the adult mesenchymal progenitor cells may be phenotypically different from embryonic ones, the former follows the early ontogeny. Data presented here suggests that the multipotential nature of perichondrial cells fits with many of the functional requirements described for MSCs (35, 36). ALCAM expression was localized to the perichondrium and endothelial cells. PECAM-1− gating excluded ALCAM+ endothelial cells of fetal limb (Fig. 1). ALCAMhigh cells showed multiple differentiation capacity, such as osteo-, adipo-, chondrogenic, and stromal differentiation (Figs. 4–7). Although total LMCs consisted largely of chondrocytes, the rate of cartilage matrix synthesis without BMP-2 was lower than ALCAMhigh cells. It is thought that LMC was not able to maintain differentiation ability of a chondrocyte during precultivation. Our results strongly suggest that MSCs exist in the perichondrium, and existence of MSCs in perichondrial region may be reasonable for both bone collar formation and supplement of osteoprogenitor cells into cartilage during endochondral ossification.
In addition, recent studies have described methods for identifying pluripotent stem cells, also called SP cells, from many tissues based on Hoechst 33342-dye efflux (37, 38). Purified SP cells derived from adult skeletal muscle, for example, have the capacity to differentiate into all major hematopoietic lineages after transplantation into lethally irradiated mice (39). In our studies, Hoechst staining showed that ALCAM+ cells existed in SP cells in fetal limb (Fig. 3 B), indicating that pluripotent stem cells exist in fetal limb. Altogether, these results suggest that ALCAM is a good marker of MSCs in developing limb. The data demonstrate that cells of the perichondrium layer contain MSCs and that sorted ALCAMhigh cells are a fairly homogeneous population (Fig. 2 C). Further clonal assays may be necessary to fully characterize perichondrial cells. In addition, Reyes et al. (40) reported that human BM-derived mesodermal progenitor cells (MPCs) that can differentiated into not only mesenchymal lineage (osteoblasts, adipocytes, chondrocytes, and skeletal muscle cells) but also endothelial cells. It is interesting that the analysis of the plasticity of MSCs in perichondrium.
Function of ALCAM in MSCs. It is known that maintenance of undifferentiated nature and differentiation of the cell is closely related to cell density (40). To establish and maintain the MSCs, some cell density or cell–cell interaction might be required. Thus, we take notice of a cell adhesion molecule, or ALCAM. We have previously reported that homophilic adhesion through ALCAM is involved with maintenance of HSCs or expansion of primitive hematopoietic progenitor cells. In addition, ALCAM plays a critical role in growth of ALCAM+ endothelial precursor cells (21). ALCAMhigh cells but not ALCAMlow/− cells could differentiated into multiple lineage differentiation (Fig. 4).
We hypothesize that ALCAM-mediated homophilic cell adhesion plays a role in the maintenance of multipotency of perichondrial cells. Furthermore, the freshly isolated ALCAM+ cells showed higher proliferative activities than the freshly isolated ALCAM− cells (Fig. 2 A), and the proliferation of ALCAM+ cells was partially inhibited by the addition of ALCAM-Fc and CD6-Fc (Fig. 2 B), it was suggested that the ALCAM-mediated cell–cell interaction works auxiliary to the proliferation of perichondrial cells (Fig. 9 B). ALCAM expression increased in ALCAM− cells which were isolated from freshly prepared LMCs, and decreased after induction of osteogenic differentiation in ALCAMhigh cells (Figs. 2 C and 5 A). Since osteoblast is one of the terminally differentiated cells in mesenchymal lineages, we suppose that these changes of ALCAM expression are irreversible in perichondrial cells.
In addition, when ALCAM-mediated homophilic interaction was blocked by addition of ALCAM-Fc or CD6-Fc, the osteoblastic differentiation from ALCAMhigh cells was promoted (Fig. 5 B). Bruder et al. (10) also reported that addition of ALCAM Fab fragments to human MSCs undergoing osteogenic differentiation in vitro elevated the level and accelerated the onset of ALP expression and mineral deposition during osteogenesis. Although it is not known whether the ALCAM-mediated cell–cell interaction has controlled osteoblastic differentiation directly or indirectly, our data suggests that interfering with ALCAM–ALCAM interaction supports to differentiation of MSCs into osteoblasts (Fig. 9 B). Altogether, our results suggest the possibility that ALCAM-mediated homophilic cell adhesion assists to both maintaining the multipotentiality and lineage specific differentiation of MSCs of the perichondrium.
Contribution of Perichondrial Cells to Bone and BM Formation.
Final goal of this investigation is to understand the mechanisms of bone and bone marrow formation through the analyses for the differentiation of mesenchymal cells, angiogenesis, and hematopoiesis. During endochondral ossification, MSCs in the perichondrium may play multiple roles. In the mouse humerus, the process of differentiation of osteoblasts in the perichondrium is initiated at approximately E13.5 and deposition of the bone collar takes place until E14.5 (41). Ossification begins with invasion of the calcified hypertrophic cartilage by capillaries, accompanied by apoptosis of terminal hypertrophic chondrocytes, degradation of cartilage matrix, and deposition of bone matrix by osteoblasts. Remodeling of bone matrix by osteoclasts results in a cavity filled with vessels containing hematopoietic cells. During this process, osteoblast progenitor cells are supplied by the perichondrium. Loss of ALCAM–ALCAM interaction may promote both osteoblastic differentiation and migration of osteoprogenitor cells into the inner periosteal layer (Fig. 9 B).
The process of vascular invasion is mainly regulated by an angiogenic factor–dependent pathway (42). It is well known that VEGF, bFGF, and Ang1 enhance angiogenesis and that VEGF and bFGF synergistically control angiogenesis (43). Ang2 is an antagonist of Ang1. Hypertrophic cartilage may produce angiogenic activators, such as VEGF, whereas other types of cartilage produce angiogenic inhibitors such as ChM-1 (44). A delicate balance between the rate of formation of calcified cartilage and its vascularization must be maintained in order for bone development to proceed normally. Recently, it was reported that VEGF, Ang1, and Ang2 were coexpressed in hypertrophic chondrocytes, osteoblasts, osteoclasts, and perichondrium in human neonatal ribs (45). Furthermore, the expression of these factors is highest at sites of bone collar formation and colocalizes with ALP expression (45). Such distribution of Angs and VEGF indicate that these factors might play key roles in the regulation of vascular invasion into cartilage during endochondral ossification. As shown in Fig. 7 A, expression of Ang1 increased and Ang2 decreased during lineage-specific differentiation. Moreover, after induction of osteoblastic differentiation, ALCAMhigh cells supported formation of fine vascular networks. In addition, vascular networks on calcified osteoblast layers were thicker than on noncalcified osteoblast layers. This observation is consisted with the idea that vascular invasion occurs after calcification of bone collar. Takeda et al. (46) reported that cbfa1, also known as AML3 or Pebp2αA, is expressed in the perichondrium and regulates osteoblast differentiation, and cbfa1-deficient mice do not show chondrocyte hypertrophy and vascular invasion. The expression pattern of cbfa1 corresponds to the expression of VEGF, Ang1, and Ang2, which suggests the possibility that osteoblastic differentiation of MSCs regulates the expression of angiogenic factors. In addition, ALCAMhigh cells support osteoclast formation. Osteoclasts allow blood capillaries to penetrate into cartilage and lead to formation of the marrow space. May-Grünwald Giemsa staining revealed that most of ALCAM+ hematopoietic cells (PECAM-1−CD45+ALCAM+ cells) in cultivated LMCs (Fig. 1 B, right panel) were the monocyte-macrophage lineage (data not shown). It is possible that these monocyte-macrophage lineage cells differentiate into osteoclasts. Furthermore, the metatarsal culture revealed that ALCAM-Fc and CD6-Fc inhibit vascular invasion into the cartilage (Fig. 8 C and D). The homophilic cell adhesion through ALCAM between perichondrial cells and endothelial cells may promote blood vessels to adhere to a cartilage (Fig. 8 B). In addition, ALCAM was found to be involved in capillary tube formation and hemangioblast differentiation (21). These observations suggest that perichondrium supplies osteoblast progenitor cells and stromal cells, and plays a positive role in vascular invasion through not only the production of angiogenesis-inducible factors but also promotion of adhesion of endothelial cells to cartilage.
In conclusion, we have identified ALCAM+ MSCs in perichondrium and clarified the role of ALCAM in early bone development. The perichondrium might serve as a MSC reservoir and play a critical role in endochondral ossification and BM formation in fetal limb.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, and Culture of Japan.
Footnotes
Abbreviations used in this paper: β-GP, β-glycerol phosphate; ALCAM, activated leukocyte cell adhesion molecule; ALP, alkaline phosphatase; Ang, angiopoietin; bFGF, basic fibroblast growth factor; BM, bone marrow; Epo, erythropoietin; HSC, hematopoietic stem cell; ICAM, intracellular cell adhesion molecule; LMC, limb mononuclear cell; MSC, mesenchymal stem cell; PDGF, platelet-derived growth factor; P-Sp, paraaortic splanchnopleural mesoderm; SCF, stem cell factor; SP, side population; TRAP, tartrate-resistant acid phosphatase; VEGF, vascular endothelial growth factor.
References
- 1.Hinchcliffe, J.R., and D.R. Johnson. 1990. The development of the vertebrate limb. Clarendon Press, Oxford. 1–300.
- 2.Horton, W.A. 1993. Cartilage Morphology. Extracellular Matrix and Heritable Disorders of Connective Tissue. P.M. Royce and B. Steinman, editors. Alan R. Liss, New York. pp. 73–84.
- 3.Caplan, A.I., and D.G. Pechak. 1987. The cellular and molecular embryology of bone formation. Bone and Mineral Research. W.A. Peck, editor, Elsevier, New York. pp. 117–183.
- 4.Baron, R.E. 1996. Anatomy and ultrastructure of bone. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, third edition. M.J. Favus, editor, Lippincott-Raven, New York. pp. 3–10.
- 5.Pittenger, M.F., A.M. Mackay, S.C. Beck, R.K. Jaiswal, R. Douglas, J.D. Mosca, M.A. Moorman, D.W. Simonetti, S. Creig, and D.R. Marshak. 1999. Multilineage potential of adult human mesenchymal stem cells. Science. 284:143–147. [DOI] [PubMed] [Google Scholar]
- 6.Liechty, K.W., T.C. MacKenzie, A.F. Shaaban, A. Radu, B. Moseley AnneNarie, R. Deans, D.R. Marshak, and A.W. Flake. 2000. Human mesenchymal stem cells engraft and demonstrate site specific differentiation after in utero transplantation in sheep. Nat. Med. 6:1282–1286. [DOI] [PubMed] [Google Scholar]
- 7.Chung, U.I., B. Lanske, K. Lee, E. Li, and H. Kronenberg. 1998. The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc. Natl. Acad. Sci. USA. 95:13030–13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Osch, G.J., S.W. Van Der Veen, E.H. Burger, and H.L. Verwoerd-Verhoef. 2000. Chondrogenic potential of in vitro multiplied rabbit perichondrium cells cultured in alginate beads in defined medium. Tissue Eng. 6:321–330. [DOI] [PubMed] [Google Scholar]
- 9.Ghilzon, R., C.A. McCulloch, and R. Zohar. 1999. Stromal mesenchymal progenitor cells. Leuk. Lymphoma. 32:211–221. [DOI] [PubMed] [Google Scholar]
- 10.Bruder, S.P., N.S. Ricalton, R.E. Boynton, T.J. Connolly, N. Jaiswal, J. Zaia, and F.P. Barry. 1998. Mesenchymal stem cell surface antigen SB-10 corresponds to activated leukocyte cell adhesion molecule and is involved in osteogenic differentiation. J. Bone Miner. Res. 13:655–663. [DOI] [PubMed] [Google Scholar]
- 11.Patel, D.D., S.F. Wee, L.P. Whichard, M.A. Bowen, J.M. Pesando, A. Aruffo, and B.F. Haynes. 1995. Identification and characterization of a 100-kD ligand for CD6 on human thymic epithelial cells. J. Exp. Med. 181:1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowen, M.A., D.D. Parel, X. Li, B. Modrell, A.R. Malacko, W.C. Wang, H. Marquardt, M. Neubauer, J.M. Pesando, U. Francke, et al. 1995. Cloning, mapping, and characterization of activated leukocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J. Exp. Med. 181:2213–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelissen, J.M.D.T., I.M. Peters, B.G. de Grooth, Y. van Kooyk, and C.G. Figdor. 2000. Dynamic regulation of activated leukocyte cell adhesion molecule-mediated homotypic cell adhesion through the actin cytoskeleton. Mol. Biol. Cell. 11:2057–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida, N., Z. Yang, J. Combs, O. Pourquie, M. Nguyen, R. Ramanathan, J. Fu, A. Welply, S. Chen, G. Weddell, et al. 1997. The characterization, molecular cloning, and expression of a novel hematopoietic cell antigen from CD34+ human bone marrow cells. Blood. 89:2706–2716. [PubMed] [Google Scholar]
- 15.Burns, F.R., S. von Kannen, L. Guy, J.A. Raper, J. Kamholz, and S. Chang. 1991. DM-GRASP, a novel immunoglobulin superfamily axonal surface protein that supports neurite extension. Neuron. 7:209–220. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka, H., T. Matsui, A. Agata, M. Tomura, I. Kubota, K.C. McFarland, B. Kohr, A. Lee, H.S. Phillips, and D.L. Shelton. 1991. Molecular cloning and expression of a novel adhesion molecule, SC1. Neuron. 7:535–545. [DOI] [PubMed] [Google Scholar]
- 17.Pourquie, O., C. Corbel, J.P. Le Caer, J. Rossier, and N.M. Le Douarin. 1992. BEN, a surface glycoprotein of the immunoglobulin superfamily, is expressed in a variety of developing systems. Proc. Natl. Acad. Sci. USA. 89:5261–5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peduzzi, J.D., M.H. Irwin, and E.E. Geisert, Jr. 1994. Distribution and characteristics of a 90 kDa protein, KG-CAM, in the rat CNS. Brain Res. 640:296–307. [DOI] [PubMed] [Google Scholar]
- 19.Paschke, K.A., F. Lottspeich, and C.A. Stuermer. 1992. Neurolin, a cell surface glycoprotein on growing retinal axons in the goldfish visual system, is reexpressed during retinal axonal regeneration. J. Cell Biol. 117:863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degen, W.G., L.C. van Kempen, E.G. Gijzen, J.J. van Groningen, Y. van Kooyk, H.P. Bloemers, and G.W. Swart. 1998. MEMD, a new cell adhesion molecule in metastasizing human melanoma cell lines, is identical to ALCAM (activated leukocyte cell adhesion molecule). Am. J. Pathol. 152:805–813. [PMC free article] [PubMed] [Google Scholar]
- 21.Ohneda, O., K. Ohneda, F. Arai, J. Lee, T. Miyamoto, Y. Fukushima, D. Dowbenko, L.A. Lasky, and T. Suda. 2001. ALCAM (CD166): its role in hematopoietic and endothelial development. Blood. 98:2134–2142. [DOI] [PubMed] [Google Scholar]
- 22.Nelissen, J.M.D.T., R. Torensma, M. Pluyter, G.J. Adema, R.A. Raymakers, Y. van Kooyk, and C.G. Figdor. 2000. Molecular analysis of the hematopoiesis supporting osteoblastic cell line U2-OS. Exp. Hematol. 28:422–432. [DOI] [PubMed] [Google Scholar]
- 23.Cortés, F., F. Deschaseaux, N. Uchida, M.C. Labastie, A.M. Friera, D. He, P. Charbord, and B. Péault. 1999. HCA, an immunoglobulin-like adhesion molecule present on the earliest human hematopoietic precursor cells, is also expressed by stromal cells in blood-forming tissues. Blood. 93:826–837. [PubMed] [Google Scholar]
- 24.Takakura, N., T. Watanabe, S. Suenobu, Y. Yamada, T. Noda, Y. Ito, M. Satake, and T. Suda. 2000. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 102:199–209. [DOI] [PubMed] [Google Scholar]
- 25.Arai, F., T. Miyamoto, O. Ohneda, T. Inada, T. Sudo, K. Brasel, T. Miyata, D.M. Anderson, and T. Suda. 1999. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor κB (RANK) receptors. J. Exp. Med. 190:1741–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shukunami, C., Y. Ohta, M. Sakuda, and Y. Hiraki. 1998. Sequential progression of the differentiation program by bone morphogenetic protein-2 in chondrogenic cell line ATDC5. Exp. Cell Res. 241:1–11. [DOI] [PubMed] [Google Scholar]
- 27.Valcourt, U., M.C. Ronzierè, P. Winker, V. Rosen, D. Herbage, and F. Mallein-Gerin. 1999. Different effects of bone morphogenetic protein 2, 4, 12, and 13 on the expression of cartilage and bone markers in the MC615 chondrocyte cell line. Exp. Cell Res. 251:264–274. [DOI] [PubMed] [Google Scholar]
- 28.Weston, A.D.,V. Rosen, R.A. Chandraratna, and T.M. Underhill. 2000. Regulation of skeletal progenitor differentiation by the BMP and retinoid signaling pathways. J. Cell Biol. 148: 679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blavier, L., and J.M. Delaissé. 1995. Matrix metalloproteinases are obligatory for the migration of preosteoclasts to the developing marrow cavity of primitive long bones. J. Cell Sci. 108:3649–3659. [DOI] [PubMed] [Google Scholar]
- 30.Engsig, M.T., Q.J. Chen, T.H. Vu, A.C. Pedersen, B. Therkidsen, L.R. Lund, K. Henriksen, T. Lenhard, N.T. Foged, Z. Werb, and J.M. Delaissé. 2000. Matrix metalloproteinases 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J. Cell Biol. 151:879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou, S., J.D. Schuetz, K.D. Bunting, A.-M. Colapietro, J. Sampath, J.J. Morris, I. Lagutina, G.C. Grosveld, M. Osawa, H. Nakauchi, and B.P. Sorrentino. 2001. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 7:1028–1034. [DOI] [PubMed] [Google Scholar]
- 32.Taichman, R.S., and S.G. Emerson. 1998. The role of osteoblasts in the hematopoietic microenvironment. Stem Cells. 16:7–15. [DOI] [PubMed] [Google Scholar]
- 33.Takakura, N., X.L. Huang, T. Naruse, I. Hamaguchi, D.J. Dumont, G.D. Yancopoulous, and T. Suda. 1998. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity. 9:677–686. [DOI] [PubMed] [Google Scholar]
- 34.Dennis, J.E., A. Merriam, A. Awadallah, J.U. Yoo, B. Johnstone, and A.I. Caplan. 1999. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J. Bone Miner. Res. 14:700–709. [DOI] [PubMed] [Google Scholar]
- 35.Owen, M. 1985. Lineage of osteogenic cells and their relationship to the stromal system. Bone and Mineral Research, Vol. 3. W.A. Prck, editor. Elsevier, Amsterdam. pp. 1–25.
- 36.Caplan, A.I. 1991. Mesenchymal stem cells. J. Orthop. Res. 9:641–650. [DOI] [PubMed] [Google Scholar]
- 37.Goodell, M.A., K. Brose, G. Paradis, A.S. Conner, and R.C. Mulligan. 1996. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 183:1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodell, M.A., M. Rosenzweig, H. Kim, D.F. Marks, M. DeMaria, G. Paradis, S.A. Grupp, C.A. Sieff, R.C. Mulligan, and R.P. Johnson. 1997. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat. Med. 3:1337–1345. [DOI] [PubMed] [Google Scholar]
- 39.Jackson, K.A., T. Mi, and M.A. Goodell. 1999. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc. Natl. Acad. Sci. USA. 96:14482–14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reyers, M., T. Lund, T. Lenvik, D. Aguiar, L. Koodie, and C.M. Verfaille. 2001. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 98:2615–2625. [DOI] [PubMed] [Google Scholar]
- 41.St-Jacques, B., M. Hammerschmidt, and A.P. McMahon. 1999. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 13:2072–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerber, H.P., T.H. Vu, A.M. Ryan, J. Kowalski, Z. Werb, and N. Ferrara. 1999. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 5:623–628. [DOI] [PubMed] [Google Scholar]
- 43.Pepper, M.S., N. Ferrara, L. Orci, and R. Montesano. 1992. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem. Biophys. Res. Commun. 189:824–831. [DOI] [PubMed] [Google Scholar]
- 44.Hiraki, Y., H. Inoue, K. Iyama, A. Kamizono, M. Ochiai, C. Shukunami, S. Iijima, F. Suzuki, and J. Kondo. 1997. Identification of chondromodulin I as a novel endothelial cell growth inhibitor. Purification and its localization in the avascular zone of epiphyseal cartilage. J. Biol. Chem. 272:32419–32426. [DOI] [PubMed] [Google Scholar]
- 45.Horner, A., S. Bord, A.W. Kelsall, N. Coleman, and J.E. Compston. 2001. Tie2 ligands angiopoietin-1 and angiopoietin-2 are coexpressed with vascular endothelial cell growth factor in growing human bone. Bone. 28:65–71. [DOI] [PubMed] [Google Scholar]
- 46.Takeda, S., J.P. Bonnamy, M.J. Owen, P. Ducy, and G. Karsenty. 2001. Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 15:467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]