Abstract
Activation of tumor-associated CD8+ cytotoxic T lymphocytes (CTLs) often requires antigen representation, e.g., by dendritic cells (DCs), and CD4+ T cell help. Previously, we showed that CTL-mediated tumor immunity required interleukin 4 (IL-4) during the immunization but not effector phase. To determine the source and target cells of IL-4, we performed adoptive T cell transfers using CD4+ and CD8+ T cells from IL-4−/− and IL-4R−/− mice and analyzed CTL generation. Even though necessary for CTL generation, CD4+ T cells did not need to express IL-4 or IL-4R. Surprisingly, CTL generation required IL-4 but not IL-4R expression by CD8+ T cells. As IL-4 (a) was expressed by naive CD8+ T cells within 24 h after antigen encounter, (b) IL-4 induced DC maturation, and (c) CTL development was impaired in T cell–reconstituted IL-4R−/− mice, CD8+ T cell–derived IL-4 appears to act on DCs. We conclude that CD4+ and CD8+ T cells provide different signals for DC activation during CTL generation.
Keywords: tumor vaccination, cytotoxic T lymphocytes, interleukin 4, interleukin 4 (receptor)–deficient mice, cross-priming
Introduction
CTL responses to a variety of different antigens (e.g., viral-, allo-, auto-, and tumor antigens) are induced by cross-priming (1–4). For cross-priming, specialized APCs have to take up and process cell-derived antigens and to present MHC class I–restricted peptides to CD8+ T cells (5). Cross-presenting APCs appear to be bone marrow–derived (4), e.g., dendritic cells (DCs)* (6, 7). CTL responses to cell-derived antigens often depend on CD4+ T cell help for which CD4+ and CD8+ T cells must recognize antigen on the same APC (8, 9). In several models CD4+ T cell help could be replaced by agonistic anti-CD40 antibodies (10–12). The conclusion was that CD4+ T cells activate APCs through CD40/CD40-ligand interactions, so that APCs can activate CTLs.
Previously, we showed that IL-4−/− mice have a defect to develop systemic tumor immunity (13). This defect was associated with impaired CTL and Th1 responses. Application of exogenous IL-4 together with the cells used for immunization restored tumor immunity in IL-4−/− mice, ruling out a developmental defect and pointing to the priming phase during which IL-4 is required. This was firmly established by antibody neutralization of IL-4 in normal mice, showing that anti-IL-4 antibodies abolished tumor immunity when given at the time of immunization but not when given at the time of tumor challenge (13). The question which cells have to produce and respond to IL-4 for CTL generation remained open and was addressed here focusing on cells involved in the above mentioned three cell type interactions (CD4+, CD8+ T cells, and APCs).
IL-4 is mainly produced by CD4+ T cells (Th2 cells) (14) which are associated with humoral immune responses and counteract CTL development (15). Th2 cells are usually detected in the effector phase of an immune response, e.g., after chronic antigen exposure (16), and after prolonged in vitro stimulation in the presence of exogenous IL-4 (15, 16). In addition to CD4+ T cells, CD8+ T cells were also shown to produce IL-4 after in vitro culture for a longer period of time in the presence of exogenous IL-4 (17, 18). A functional role of IL-4 expressed by CD8+ T cells in vivo is not known. DCs cultured in the presence of IL-4 and GM-CSF had a more mature phenotype than those cultured with GM-CSF only (19–21). However, it remained unclear whether IL-4 directly contributed to DC maturation or suppressed the generation of other cells in the culture such as macrophages (19).
CTL priming in T cell–reconstituted SCID mice has been shown (22). By reconstituting SCID mice with T cells from IL-4−/− and IL-4R−/− mice we created experimental conditions so that defined cell types (e.g., CD4+ and CD8+ T cells) could either produce or respond to IL-4. We measured CTLs specific for a tumor rejection antigen of the colon carcinoma CT26, an endogenous retrovirus (23), because CTLs to antigens derived from CT26 cells have been shown to be induced by cross-priming (4, 24). We show that the generation of CTL responses to the CT26-derived tumor antigen requires CD8+ T cell–derived IL-4 which most likely acts on APCs.
Materials and Methods
Mice, Cell Lines, Immunizations, and Depletion of T Cell Subsets.
BALB/c mice were purchased from Charles River. BALB/c-SCID mice were obtained from The Jackson Laboratory and Harlan Winkelmann GmbH. Recombination activating gene (RAG)2-deficient (RAG2−/−) BALB/c mice were purchased from Taconic. IL-4–deficient (IL-4−/−; reference 25), IL-4 receptor α chain–deficient (IL-4R−/−; reference 26), and RAG2−/−/IL-4R−/− double-knockout mice (all congenic to BALB/c) were bred at the animal facilities of our institutions. C57Bl/6 OT-I mice express a transgenic T cell receptor specific for the H2-Kb–restricted peptide ova257–264 derived from chicken ovalbumin (27) and were provided through M. Zenke (MDC Berlin, Berlin, Germany) with kind permission of F. Carbone and W. Heath. OT-I mice were crossed to Rag1−/− mice obtained from The Jackson Laboratory and RAG1-deficient OT-I mice (OT-I × RAG1−/−) were used for the experiments. CT26 is a BALB/c colon carcinoma expressing MHC class I but not MHC class II molecules as determined by cytofluorimetric analysis (data not shown). Renca is a BALB/c renal cell carcinoma cell line. All cell lines were cultured in RPMI-1640 with 10% FCS. For the analysis of tumor immunity, mice were subcutaneously injected with 106 irradiated (100 Gy) CT26 cells and 2 wk later contralaterally challenged with 106 viable tumor cells. Mice that had not developed a tumor within 60 d were scored as tumor free. For depletion of CD4+ and CD8+ T cells, immunized BALB/c mice received a single intraperitoneal injection of 100 μg anti-CD4 (GK1.5) or anti-CD8 (2.43) antibody 2 d before challenge. Depletion of the respective T cell subpopulation was controlled by cytofluorimetric analysis of peripheral blood using Coulter EPICS-XL (Beckman Coulter) and anti-CD4-PE (RM4–5) and anti-CD8α-FITC (53–6.7; BD PharMingen).
Adoptive Transfer of T Lymphocytes and Cytotoxicity Assays.
Single cell suspensions of splenocytes were prepared and red blood cells were removed by NH4Cl treatment. For positive selection of CD4+ or CD8+ T cells, splenocytes were incubated with microbeads specific for CD4 or CD8α (Miltenyi Biotec), respectively, and passed over a MACS column according to the manufacturer's recommendations. For removal of contaminating adherent cells, enriched CD4+ and CD8+ T cells were incubated in Petri dishes for 30 min at 37°C. The purity of each preparation was analyzed by cytofluorimetric analysis as described above and was 93–99%. Before intravenous injection into the tail vein of immunodeficient mice, T cells were washed twice and resuspended in PBS. Each recipient received 2 × 107 CD4+ and 107 CD8+ T cells in a total volume of 200 μl. All experimental groups consisted of 3–5 mice.
For induction of CTLs, mice were immunized twice with 106 irradiated (100 Gy) CT26 cells in a 2-wk interval. 7–10 d after the second immunization, single-cell suspensions of red blood cell–depleted spleen cells were prepared. To evaluate reconstitution efficacy, spleen cells from mice of one experimental group were pooled, counted, and the percentage of CD4+ and CD8+ T cells was determined by cytofluorimetric analysis as described above. Splenocytes were cultured at 2 × 106/ml in RPMI-1640 plus 10% FCS, penicillin/streptomycin, MEM, and 2-ME (50 μM) in the presence of 100 ng/ml of the H2-Ld–restricted peptide AH1 (SPSYVYHQF) which is derived from an activated endogenous retrovirus in CT26 cells (23). After 6–7 d of culture, responder cells were harvested, washed twice and incubated with 51Cr (1 mCi/ml) (NEN Life Science Products)-labeled target cells at different E/T cell ratios. After an incubation period of 4.5 h, supernatants were assayed for radioactivity on a gamma counter (Top count; Packard Instrument Co.). The percent lysis was calculated as [(sample cpm − spontaneous cpm)/(maximal cpm − spontaneous cpm)] × 100%. Spontaneous release was below 22% in all experiments. CT26-specific lysis represents the difference between the percent lysis of CT26 cells and the percent lysis of Renca cells. AH1-specific lysis represents the difference between percent lysis of Renca cells loaded with 1 μg/ml of AH1 and Renca cells loaded with the same amount of the H2-Ld–restricted, β-galactosidase–derived peptide TPHPARIGL. Due to elevated levels of NK cell activity in SCID mice, lysis of Renca cells varied in the course of the experiments between 12–48%. CT26-specific lysis and the corresponding AH1-specific lysis are only shown if the difference between the percent lysis of CT26 cells and the percent lysis of Renca cells was at least 40% (at an E/T ratio of 10:1).
Stimulation of OT-I × RAG1−/− Splenocytes and Detection of IL-4 mRNA by Quantitative Reverse Transcription PCR.
Single cell suspensions of red blood cell-depleted splenocytes from OT-I × RAG1−/− mice were cultured in RPMI-1640 plus 10% FCS, penicillin/streptomycin, MEM, and 2-ME (50 μM) at 1.5 × 106/ml in the presence or absence of 5 μg/ml peptide ova257–264 (SIINFEKL). Cells were then harvested and total RNA was extracted using the Invisorb Spin Cell RNA Kit (Invitek) according to the manufacturer's instructions. Quantitative reverse transcription (RT)-PCR was performed using an ABI PRISM 7700 detection system and software (PerkinElmer). Primer and probe sequences for HPRT and IL-4 were used as published (28): HPRT sense 5′-CTGGTGAAAAGGACCTCTCG-3′, HPRT antisense 5′-TGAAGTACTCATTATAGTCAAGGGCA-3′, HPRT probe JOE-5′-TGTTGGATACAGGCCAGACTTTGTTGGAT-3′-6-carboxytetramethylrhodamine (Tamra); IL-4 sense 5′-AGATCATCGGCATTTTGAACG-3′, IL-4 antisense 5′-TTTGGCACATCCATCTCCG-3′, IL-4 probe 6-carboxyfluorescein (FAM)-5′-TCACAGGAGAAGGGACGCCATGC-3′-Tamra. The IL-4 primers were intron spanning. Therefore, amplification as a result of DNA contamination could be excluded. Cycling conditions were 20 min at 48°C (cDNA Synthesis), 10 min at 95°C, followed by 40 repeats of 95°C for 15 s and 60°C for 1 min. RNA from J558L, J558L transfected with the IL-4 gene (J558L-IL-4), and RNA from unstimulated lymphocytes established specificity of primer pairs and probes and were used for standard curves. RNA samples in the absence of reverse transcriptase were included in all experiments and did not give PCR signals excluding contamination with genomic DNA. IL-4 and HPRT transcripts were analyzed in one reaction (multiplex analysis). IL-4 transcripts were normalized to HPRT abundance. ΔRn is determined by the sequence detector software as: ΔRn = (Rn+) − (Rn−), where Rn+ = emission intensity of reporter/emission intensity of quencher at any given time in a reaction tube, and Rn− = emission intensity of reporter/emission intensity of quencher before PCR amplification in that same reaction tube.
Generation of DCs, Coculture with IL-4, and FACS® Analysis.
Bone marrow cells were cultured at 2 × 106/ml in RPMI-1640 containing 10% FCS, penicillin/streptomycin, MEM, 2-ME (50 μM), and 10% supernatant from NIH cells transfected to secrete murine GM-CSF (unpublished data). The final GM-CSF concentration was 10–20 ng/ml. At day 6, 50 ng/ml of recombinant mouse IL-4 (BD PharMingen) were added. To prevent maturation of DCs due to mechanical manipulation (29, 30), cells were not replated. Instead, half of the medium was replaced every second day. At day 12 of culture, DCs were harvested, washed, and incubated in PBS containing 10% mouse serum and 2 mM EDTA for 20 min. This preincubation was followed by the addition of monoclonal antibodies against mouse CD11c (HL3), I-Ad/I-Ed (2G9), B7.2 (GL1), and CD40 (HM-40–3; all BD PharMingen). Cytofluorimetric analysis was performed as described above.
Results
CD4+ T Cell–dependent, CTL-mediated Tumor Immunity Is Impaired in IL-4−/− and IL-4R−/− Mice.
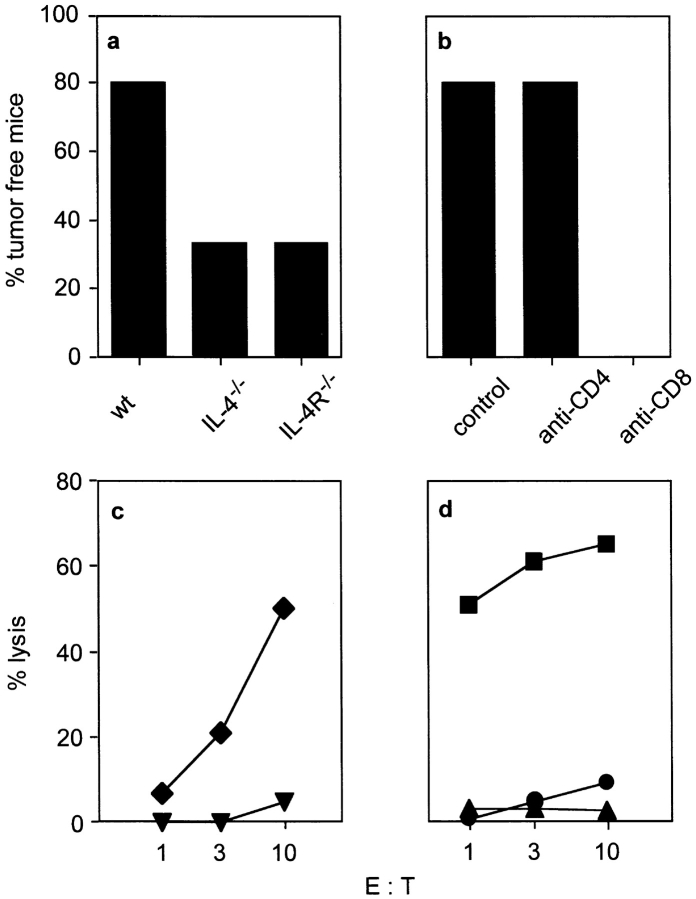
First, we analyzed whether IL-4R−/− mice have a similar phenotype as IL-4−/− mice with regard to the generation of tumor immunity. Both knockout strains were generated with BALB/c ES cells and can therefore be considered as congenic BALB/c lines (25, 26), thus minimizing the problem of unwanted alloresponses. Immunization with irradiated cells of the BALB/c colon carcinoma CT26 protected 80% of wild-type (wt) mice but only 33% of IL-4−/− and IL-4R−/− mice from a lethal tumor challenge showing that IL-4−/− and IL-4R−/− mice are similarly impaired to develop tumor immunity (Fig. 1 a). To analyze which T cell subset was required in the effector phase, wt mice were immunized as before and depleted of either CD4+ or CD8+ T cells beginning 2 d before challenge with CT26 cells. CD4+ T cells were not required as effector cells for tumor rejection in this model, whereas CD8+ T cells were essential (Fig. 1 b). In addition to the capability of CD8+ T cells to mediate rejection of CT26 tumors in vivo, CTLs derived from immunized wt mice specifically lysed CT26 cells after 1 wk of in vitro restimulation with the CT26-derived peptide AH1 (Fig. 1 c). Intracellular cytokine staining showed that CT26-reactive CTLs secreted IFN-γ but not IL-4 (data not shown), indicating that conventional CTLs were involved in tumor cell destruction. Next we tested, whether CD4+ T cells contribute to CTL generation in T cell–reconstituted SCID mice, as we wanted to use this system for subsequent experiments to analyze the role of IL-4 for CTL generation. SCID mice were reconstituted with CD4+ and CD8+ T cells or CD8+ T cells alone, immunized with CT26 cells and subsequently analyzed for CTL activity. Mice reconstituted with both T cell subsets contained CTLs after immunization, whereas no CTL activity could be detected in mice reconstituted only with CD8+ T cells (Fig. 1 d). Therefore, CTL activation to CT26 cells (MHC class I+, class II−) requires help from CD4+ T cells, which thus have to recognize tumor-derived antigens on MHC class II+ APCs. As CTL generation against CT26-derived antigens requires cross-presentation by host APCs (4, 24), it is likely that CD4+ T cell–dependent CTL priming in our model also required both T cell subsets to interact with the same APC as described previously in other experimental systems (8, 9).
Figure 1.
CD4+ T cell–dependent, CTL-mediated tumor immunity is impaired in IL-4−/− and IL-4R−/− mice. (a) The indicated mice (5–6/group) were immunized with 106 irradiated CT26 cells, contralaterally challenged with 106 viable CT26 cells 2 wk later, and tumor growth was monitored. One out of three experiments with similar results is shown. (b) BALB/c mice (10/group) were immunized and challenged with CT26 as described in panel a and tumor growth was monitored. 2 d before challenge, mice were depleted of CD4+ or CD8+ T cells. For a and b, the percentage of tumor free mice 60 d after challenge is shown. (c) To determine tumor-specific CTL activity in vitro, BALB/c mice (five/group) were immunized twice with 106 irradiated CT26 cells in a 2-wk interval or left untreated. 7 to 10 d after the second immunization, spleen cells were restimulated for 7 d with the CT26-derived peptide AH1. CTL activity against CT26 was determined for immunized (diamonds) and nonimmunized BALB/c mice (triangles) at different E:T ratios. One representative experiment out of three is shown. (d) To test whether the generation of anti-CT26 CTLs was CD4+ T cell dependent, SCID mice (four/group) were reconstituted with either sorted BALB/c CD4+ and CD8+ T cells or CD8+ T cells only. Recipients were immunized or left untreated and analyzed for CTL activity as described in c. CTL activities of immunized CD4wt/CD8wt-recipients (squares), immunized CD8wt-recipients (triangles), and nonimmunized CD4wt/CD8wt-recipients (circles) was measured against CT26.
CTL Generation Does Not Require IL-4 or IL-4R Expression by CD4+ T Cells.
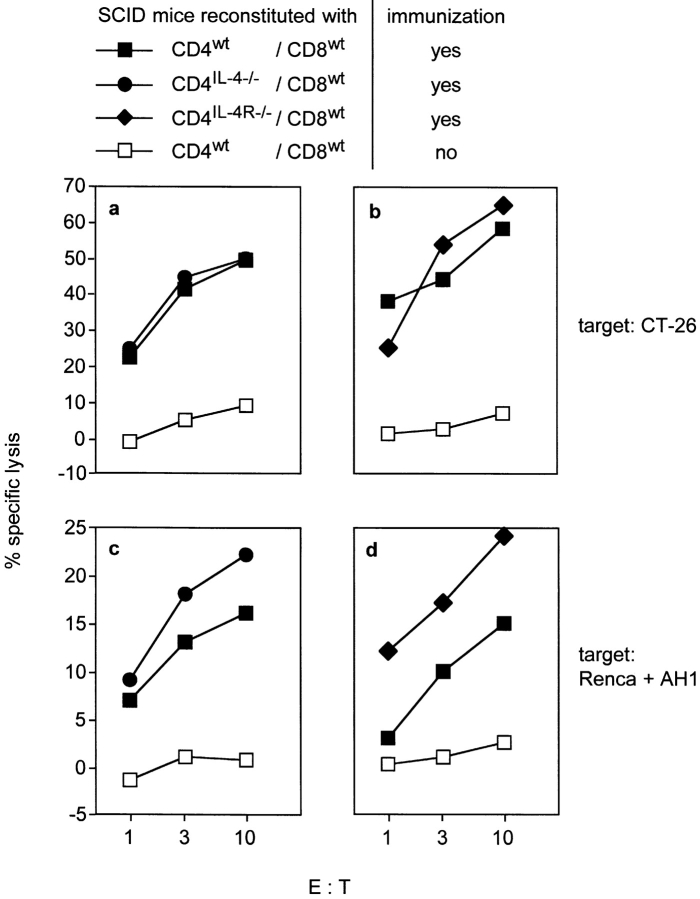
Having demonstrated that CTLs against CT26 can be primed in T cell–reconstituted SCID mice, we next transferred combinations of purified CD4+ or CD8+ T cells that were selectively defective for IL-4 or IL-4R expression. We analyzed first, whether CD4+ T cell–derived IL-4 was necessary for the generation of CTLs. SCID mice were reconstituted with CD4+ T cells from IL-4−/− mice (CD4IL-4−/−) and CD8+ T cells from wt mice (CD8wt) and immunized with CT26 cells. Control mice received CD4wt and CD8wt cells and were immunized or left untreated. Spleen cells from nonimmunized mice showed no cytotoxicity. In contrast, spleen cells from immunized CD4wt/CD8wt and CD4IL-4−/−/CD8wt recipients specifically lysed CT26 cells (Fig. 2 a) and Renca cells pulsed with AH1 peptide (Fig. 2 c). This experiment excluded CD4+ T cells as the critical source of IL-4, although CD4+ T helper cell function could require IL-4 from another cell type. To analyze this, we used CD4+ T cells from IL-4R−/− mice (CD4IL-4R−/−) and CD8wt cells to reconstitute SCID mice. Splenocytes from immunized CD4IL-4R−/−/CD8wt and CD4wt/CD8wt recipients but not from nonimmunized CD4wt/CD8wt mice showed specific cytotoxicity (Fig. 2, b and d). Therefore, while CTL generation was dependent on CD4+ T cell help (Fig. 1 d), this help was not associated with the production or consumption of IL-4 by CD4+ T cells.
Figure 2.
The generation of CT26-specific CTLs does not require IL-4- or IL-4R-expression by CD4+ T cells. SCID mice were reconstituted with sorted CD8+ T cells from wt mice and sorted CD4+ T cells from wt, IL-4−/− or IL-4R−/− mice, respectively. Immunizations and in vitro restimulation of spleen cells were done as described in Fig. 1. CT26-specific (a and b) and AH1-specific CTL activity (c and d) of CD4IL-4−/−/CD8wt- (•) (a and c), CD4IL-4R−/−/CD8wt- (♦) (b and d), and CD4wt/CD8wt-recipients (▪) (a–d) was measured at various E:T ratios. Percent specific lysis represents the difference between percent lysis of CT26 cells and Renca cells (a and b) or the difference between percent lysis of AH1 peptide-loaded and control peptide loaded Renca cells (c and d). Splenocytes from nonimmunized CD4wt/CD8wt-recipients served as negative control (□).
IL-4 but Not IL-4R Expression by CD8+ T Cells Is Required for CTL Generation.
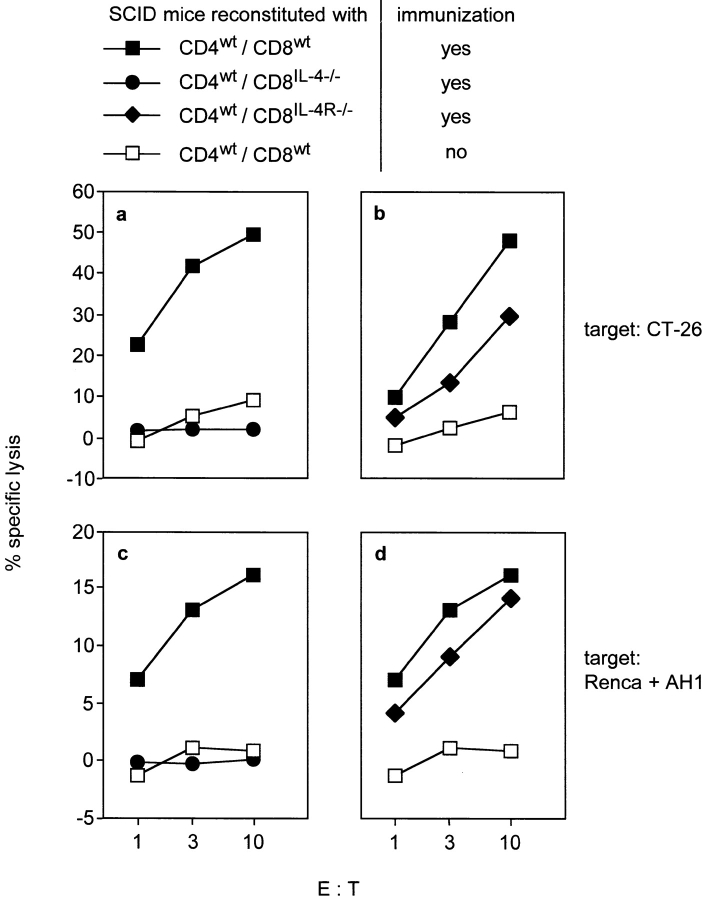
To investigate whether the generation of CTLs required CD8+ T cells to produce IL-4, SCID mice were reconstituted with CD8IL-4−/− and CD4wt cells and CTL activity was determined as above. Remarkably, CTLs could not be detected in CD4wt/CD8IL-4−/−-recipients, while spleen cells from immunized CD4wt/CD8wt-controls specifically lysed target cells (Fig. 3, a and c). Next we analyzed, whether CD8+ T cell–derived IL-4 acted in an autocrine or paracrine fashion. We cotransferred CD8IL-4R−/− and CD4wt cells to SCID mice and determined CTL activity after immunization with CT26 cells. Splenocytes of CD4wt/CD8IL-4R−/−-recipients showed specific lysis, although it was slightly reduced compared with splenocytes from CD4wt/CD8wt-control mice (Fig. 3, b and d). These results demonstrate that CTL activation required IL-4 from CD8+ T cells which acted primarily in a paracrine fashion.
Figure 3.
IL-4- but not IL-4R-expression by CD8+ T cells is required for the generation of CT26-specific CTLs. SCID mice were reconstituted with sorted CD4+ T cells from wt mice and sorted CD8+ T cells from wt, IL-4−/−, or IL-4R−/− mice, respectively. Recipients were immunized with CT26 and CT26-specific (a and b) as well as AH1-specific CTL activity (c and d) of CD4wt/CD8IL-4−/−- (•) (a and c), CD4wt/CD8IL-4R−/−- (♦) (b and d), and CD4wt/CD8wt-recipients (▪) (a–d) was determined as described in Fig. 2. Splenocytes from nonimmunized CD4wt/CD8wt-recipients served as negative control (□). The same controls are shown in a and Fig. 2 a and in c and d because these experiments were done in parallel.
Naive CD8+ T Cells Upregulate IL-4 mRNA within 24 h of Antigen Stimulation.
Due to the low precursor frequency of AH1-specific CD8+ T cells, we used T cell receptor transgenic OT-I mice (27) to determine whether naive CD8+ T cells can express IL-4 in response to primary antigen-encounter. To exclude IL-4 expression from CD4+ T cells, spleen cells from RAG1-deficient OT-I mice (OT-I × RAG1−/−) were cultured with or without their cognate, ovalbumin-derived peptide ova257–265 (SIINFEKL). 24 h later, IL-4 mRNA levels were determined by quantitative RT-PCR. Cycle threshold analysis revealed linear amplification of IL-4 mRNA in peptide stimulated splenocytes, while mRNA levels from nonstimulated splenocytes never attained linear amplification (Fig. 4 a). HPRT gene expression analyzed in parallel showed a similar amplification profile with or without peptide stimulation (Fig. 4 b). We therefore conclude that there is little, if any, IL-4 mRNA in unstimulated splenocytes, while IL-4 mRNA is induced upon antigenic stimulation of CD8+ OT-I cells. Thus, naive CD8+ T cells express IL-4 within 24 h after antigenic stimulation. This finding correlates with the failure to generate CTLs after adoptive transfer of CD8IL-4−/− cells (Fig. 3 a) and suggests that IL-4 production by classical CD8+ T cells can contribute to CTL generation. However, IL-4-expression by AH1-specific CD8+ T cells remains to be shown.
Figure 4.
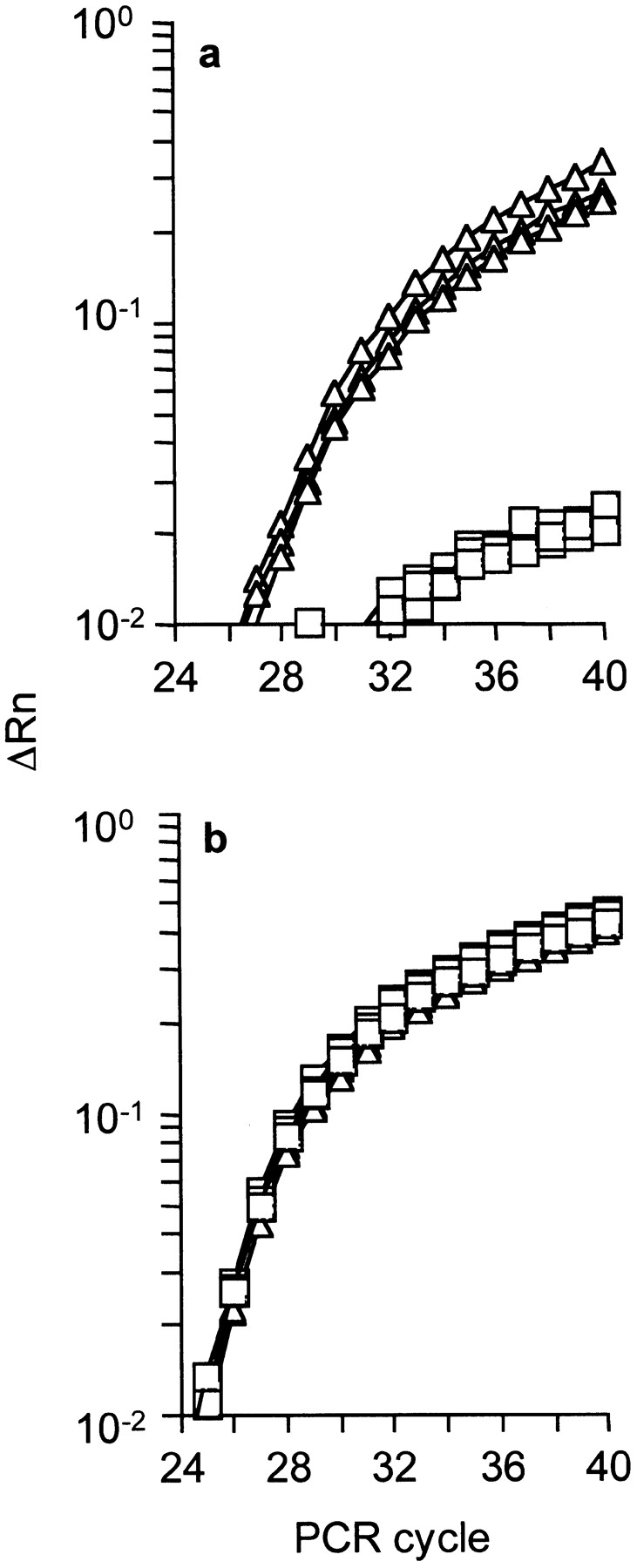
Naive CD8+ T cells upregulate IL-4 gene expression within 24 h of antigen stimulation. Spleen cells from OT-I × RAG1−/− mice were incubated for 24 h in the presence (Δ) or absence (□) of 5 μg/ml peptide ova257–264 (SIINFEKL) and the production of IL-4 mRNA was evaluated by quantitative RT-PCR. Amplification plots for IL-4 (a) and hypoxanthine guanine phosphoribosyltransferase (HPRT) (b) are shown for each RNA sample, in triplicates. IL-4 transcripts were normalized to HPRT abundance and analyzed in one reaction (multiplex analysis). One representative out of three experiments is shown. ΔRn represents changes of emission of reporter dye versus quenching dye over the course of the PCR reaction.
IL-4 Is a Maturation Factor for Immature DCs.
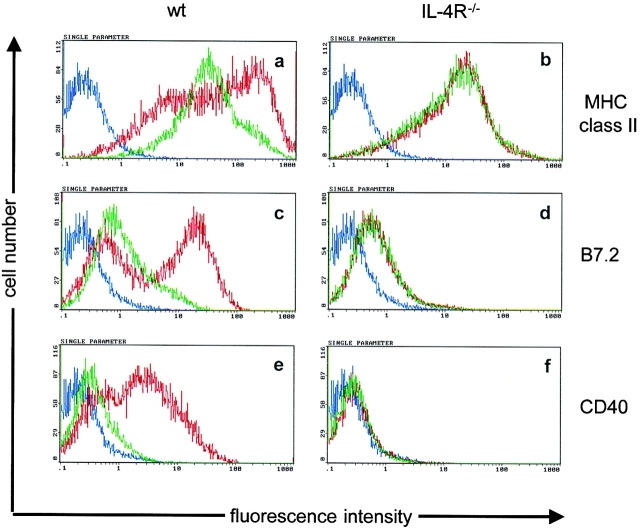
In our experimental system, DCs were the likely APCs, as (a) the recipient mice lacked B cells and (b) macrophages are less efficient in CTL cross-priming compared with DCs (31–33). To test whether IL-4 directly activates DCs, bone marrow cells from IL-4R+/+ and IL-4R−/− mice were cultured for 6 d in the presence of GM-CSF, when the majority of nonadherent cells were immature DCs (low MHC class II, low B7.2, low CD40). Subsequently, the cells were exposed to IL-4 or left untreated and CD11c+ cells were analyzed for the expression of MHC class II, B7.2, and CD40 (Fig. 5). IL-4–induced maturation of IL-4R+/+ DCs was already visible 2 d after IL-4 exposure and is shown after a 6 d exposure time by the strong upregulation of MHC class II, B7.2, and CD40 (Fig. 5, a, c, and e). Maturation of DCs from IL-4R−/− mice was not observed at this time (Fig. 5, b, d, and f) ruling out that IL-4–unrelated stimuli, e.g., LPS, were responsible for the maturation of IL-4R+/+ DCs. LPS induced similar activation of IL-4R+/+ and IL-4R−/− DCs demonstrating that DCs from IL-4R−/− mice are not generally defective to become activated (data not shown). Although it has previously been demonstrated that the addition of IL-4 to human peripheral blood mononuclear cells and murine bone marrow cells cultured with GM-CSF results in the generation of DCs with enhanced immunostimulatory capacity (19–21), our data show that IL-4 induces maturation of differentiated immature DCs.
Figure 5.
IL-4 induces maturation of DCs. Day 6 bone marrow–derived DCs from BALB/c (a, c, and e) and IL-4R−/− mice (b, d, and f) were either treated with 50 ng/ml recombinant mouse IL-4 (red lines) or left untreated (green lines). 6 d later, CD11c+ cells were analyzed for the expression of MHC class II (a and b), B7.2 (c and d) and CD40 (e and f). Isotype-matched controls are shown in blue. One representative out of two experiments is shown.
IL-4R Expression by Non-T, Non-B Cells Is Essential for CTL Generation.
The observations that CTL generation did not require CD4+ and CD8+ T cells to express IL-4R and that IL-4 activated immature DCs indicated a paracrine effect of CD8+ T cell–derived IL-4 on APCs during CTL generation. To test this, CD4wt and CD8wt cells were transferred to RAG2−/−/IL-4R−/− or RAG2−/−/IL-4R+/+ mice. Both groups were immunized with CT26 cells and analyzed for CT26-specific CTLs. As shown in Fig. 6, CTL generation was strongly impaired in RAG2−/−/IL-4R−/− mice, whereas CTL activity in RAG2−/−/IL-4R+/+ mice was comparable to that observed in the previous experiments. These results indicate that host APCs require IL-4R expression to activate CTLs. Additionally, these data support that CTLs to CT26-derived antigens are induced by cross-priming (4, 24) as direct priming should have occurred independently of IL-4R expression on host cells.
Figure 6.
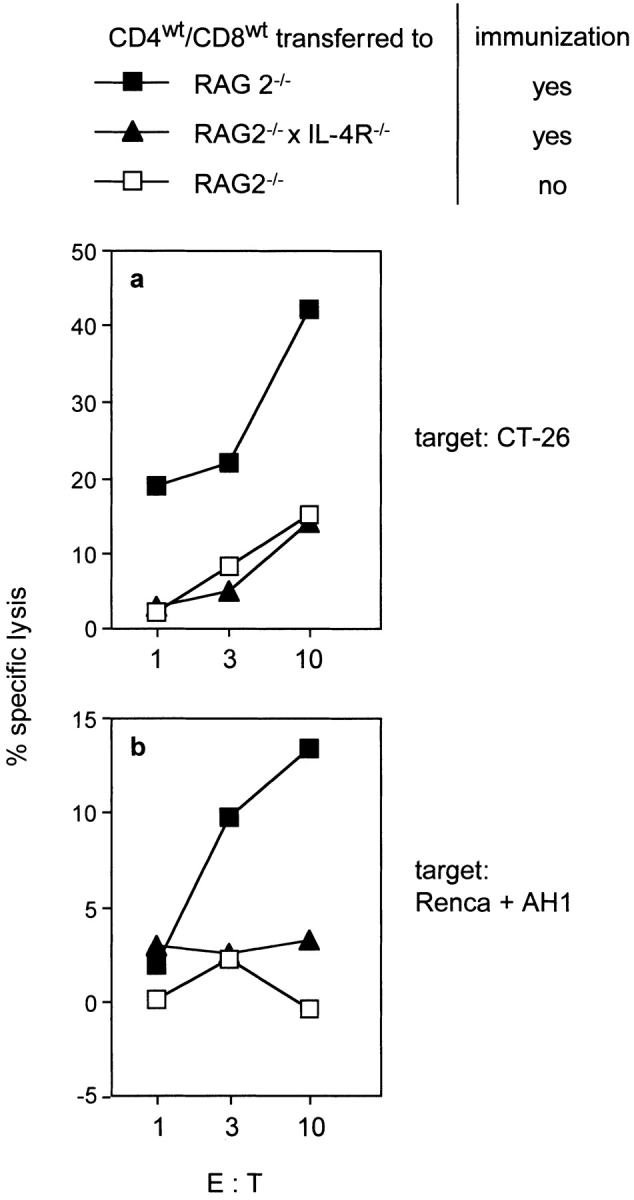
IL-4R-expression by non-T, non-B cells is required for the generation of CT26-specific CTLs. CD4+ and CD8+ T cells from wt mice were transferred to RAG2−/−/IL-4R+/+ (▪) and RAG2−/−/IL-4R−/− mice (▴). The recipients were immunized with CT26 and CT26-specific (a) as well as AH1-specific CTL activity (b) was determined as described in Fig. 2. Splenocytes from reconstituted, nonimmunized RAG2−/−/IL-4R+/+ recipients served as negative control (□).
It should be noted that T cell recovery from the reconstituted mice in the above experiments was similar regardless of the phenotype of the donor T cells or recipient mice. In Table I, the numbers of recovered splenocytes from reconstituted mice and the percentage of CD4+ and CD8+ T cells for each experimental situation are shown. Thus, differences in CTL generation did not result from different reconstitution efficacy.
Table I.
Recovery of Transferred T Cells Is Similar Regardless of the T Cell or Recipient Phenotype
| Transferred T cells
|
Splenocytes recovered
|
|||||
|---|---|---|---|---|---|---|
| CD4+ | CD8+ | Recipients | Immunization | Percent CD4+ | Percent CD8+ | Cell number |
| wt | wt | SCID | − | 9.9 | 5.1 | 8.9 × 107 |
| wt | wt | SCID | + | 18.0 | 9.4 | 10.5 × 107 |
| IL-4−/− | wt | SCID | + | 22.0 | 9.5 | 9.6 × 107 |
| IL-4R−/− | wt | SCID | + | 13.2 | 13.3 | 10.1 × 107 |
| wt | IL-4−/− | SCID | + | 16.3 | 10.5 | 13.5 × 107 |
| wt | IL-4R−/− | SCID | + | 16.3 | 9.8 | 11.3 × 107 |
| wt | wt | RAG2−/−/IL-4R+/+ | − | 18.9 | 9.0 | 8.3 × 107 |
| wt | wt | RAG2−/−/IL-4R+/+ | + | 19.1 | 7.0 | 8.1 × 107 |
| wt | wt | RAG2−/−/IL-4R−/− | + | 18.2 | 9.2 | 5.5 × 107 |
Lymphocyte-deficient mice were reconstituted with CD4+ and CD8+ T cells from the indicated donors and were left untreated or subsequently immunized with CT26 cells as described in Materials and Methods. 10 d after the second immunization, splenocytes from five recipients per group were pooled and counted. The percentage of CD4+ and CD8+ T cells was determined by cytofluorimetric analysis. The data shown for SCID and RAG2−/− mice, respectively, represent one experiment in which the indicated T lymphocyte combinations were transferred in parallel. The lower percentage of CD4+ and CD8+ T cells in nonimmunized compared to immunized SCID mice was not observed in five further experiments.
Discussion
Previously we showed that CTL- and Th1-associated tumor immunity required IL-4 during the priming phase (13). Here we demonstrated that the generation of CTL responses to a tumor-derived antigen (the peptide AH1 derived from an endogenous retrovirus activated in CT-26 cells) required CD8+ T cells to produce IL-4 which most likely acted on APCs. The data (summarized in Table II) are discussed with regard to the dual role which IL-4 can play during T cell-responses and the possible regulatory role of CD8+ T cells producing IL-4 during cross-priming.
Table II.
Summary of Results
| Transferred T cells
|
||||
|---|---|---|---|---|
| CD4+ | CD8+ | Recipients | n a | Lysis |
| wt | wt | IL-4R+/+ | 6 | Yes |
| − | wt | IL-4R+/+ | 2 | No |
| IL-4−/− | wt | IL-4R+/+ | 2 | Yes |
| IL-4R−/− | wt | IL-4R+/+ | 4 | Yes |
| wt | IL-4−/− | IL-4R+/+ | 2 | No |
| wt | IL-4R−/− | IL-4R+/+ | 3 | Yes |
| wt | wt | IL-4R−/− | 3 | No |
n represents the number of independent experiments performed with groups of 3–5 immunized recipient mice.
The Dual Role of IL-4.
There is good evidence that IL-4 produced by CD4+ T cells (Th2 cells) has adverse effects on cellular immune responses, e.g., counteracts Th1-development or CTL-responses (15). The requirement of IL-4 for CTL generation appears to be in disagreement with the opposing effects of Th2 cells on CTL development. This apparent discrepancy can be resolved, however, if one considers that (a) in our model, CTL responses did not require CD4+ T cells to produce or respond to IL-4 and (b) Th2 cells are usually detected late in immune responses, long after T cell priming has occurred, e.g., after prolonged antigen exposure in vivo (16) or after in vitro activation of CD4+ T cells in the presence of exogenous IL-4 (15, 16). Late IL-4 derived from CD4+ T cells stands in contrast to early IL-4 which supports CTL generation. We showed previously by IL-4 neutralization in normal mice that the generation of tumor immunity required IL-4 in the priming phase only, e.g., at the time of immunization. In the effector phase, e.g., at the time of tumor challenge, IL-4 was not needed (13). In the current study we showed that not only the time point but also the cellular source of IL-4 is important for CTL generation. Thus, our results reveal a defined function for IL-4–producing CD8+ T cells in vivo. It is known that CD8+ T cells can produce IL-4 (Tc2 cells) after exposure to IL-4 in vitro (17, 18). Because CTLs against CT26 produced IFN-γ but no detectable IL-4 (data not shown) and IL-4 was dispensable in the effector phase, it appears that the early IL-4 from CD8+ T cells was necessary for the generation of typical CTLs. This assumption is supported by the observation that CTL generation did not require IL-4R expression by CD8+ T cells which probably would have been necessary for the development of Tc2 cells. Correlating with the requirement for CD8+ T cell–derived IL-4 for CTL generation in vivo, quantitative RT-PCR analysis showed that naive CD8+ OT-I cells, similar to naive CD4+ T cells (28, 34), can upregulate IL-4 gene expression within 24 h after T cell receptor stimulation in vitro. It is therefore likely that IL-4 produced by CD8+ T cells in the priming phase of the antitumor response contributed to CTL generation. IL-4 from CD4+ T cells could not substitute for CD8+ T cell–derived IL-4, although CD4+ T cells were essential for CTL generation during the priming phase. Several findings suggest that APCs have to respond to IL-4 during CTL generation: (a) CD4+ and CD8+ T cells did not need to express IL-4R, (b) IL-4 induced expression of MHC class II, B7.2, and CD40 by immature DCs, and (c) CTLs did not develop in T cell–reconstituted IL-4 R−/− recipient mice. Additionally, IL-4 overexpression in mice increases rather than decreases IFN-γ (35, 36) and IL-12 production (37) and IL-4 induces IL-12 expression by DCs (37). The latter finding has been explained by a negative feedback mechanism counteracting Th2 responses. An alternative explanation is that early IL-4 production by CD8+ T cells, in contrast to the central role of late IL-4 from CD4+ T cells during Th2 responses, is necessary for Th1 responses to certain antigens. Both, impaired Th1 responses in IL-4−/− mice (13, 38, 39) and the observation that CD8+ T cells activate DCs in vivo (40) support this assumption. However, we cannot yet exclude other target cells that respond to IL-4 and contribute to the generation of antitumor CTLs.
A Regulatory Role of IL-4–producing CD8 + T Cells during Cross-priming. IL-4−/− mice mount normal CTL responses against viruses (41) that directly activate APCs (40), suggesting that cytokine-mediated amplification signals are particularly required for the generation of immune responses against weak antigens, e.g. tumor or autoantigens, in order to allow DC activation and subsequent T cell priming. CTLs to antigens derived from CT26 cells have been shown to be induced by cross-priming (4, 24). This is supported here, because direct CTL activation by CT-26 cells used for immunization should have occurred in IL-4R−/− recipient mice reconstituted with CD4wt/CD8wt T cells. During cross-priming, CD4+ T cells activate APCs that present exogenous (cell-derived) antigens to both CD4+ T helper cells and CD8+ CTLs (8, 9). The data presented here suggest that CTL generation requires CD4+ and CD8+ T cells to provide qualitatively different signals to APCs. In this model, CD8+ T cells produce IL-4 and most likely induce the maturation of DCs. In response to IL-4, DCs produce a chemokine that attracts naive CD4+ T cells (42) which may provide another signal to APCs that is unrelated to IL-4. Only both signals together ensure CTL generation. Together, the data presented here suggest an as yet unappreciated regulatory function for CD8+ T cells in the priming phase of CD4+ T cell–dependent, CTL-mediated antitumor immune responses. Whether APCs receive the primary activation signal from CD4+ or CD8+ T cells cannot be decided from this study. However, parallel experiments suggest that CD8+ T cell/APC interactions precede those between CD4+ T cells and APCs (unpublished data).
Acknowledgments
We thank G. Baukus, M. Rösch, and C. Westen for excellent technical assistance, and M. Mohaupt for GM-CSF–producing cells.
This work was supported by grants from the Deutsche Krebshilfe Mildred-Scheel-Stiftung e.V. (10-1535-BL2), the Deutsche Forschungsgemeinschaft (SFB 506), and the Bundesministerium für Bildung und Forschung (BMBF-01KV9911).
T. Schüler and T. Kammertoens contributed equally to this work.
Footnotes
Abbreviations used in this paper: DC, dendritic cell; RAG, recombination activating gene; RT, reverse transcription; wt, wild-type.
References
- 1.Sigal, L.J., S. Crotty, R. Andino, and K.L. Rock. 1999. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 398:77–80. [DOI] [PubMed] [Google Scholar]
- 2.Bevan, M.J. 1976. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 143:1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurts, C., W.R. Heath, F.R. Carbone, J. Allison, J.F. Miller, and H. Kosaka. 1996. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 184:923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang, A.Y., P. Golumbek, M. Ahmadzadeh, E. Jaffee, D. Pardoll, and H. Levitsky. 1994. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 264:961–965. [DOI] [PubMed] [Google Scholar]
- 5.Heath, W.R., and F.R. Carbone. 1999. Cytotoxic T lymphocyte activation by cross-priming. Curr. Opin. Immunol. 11:314–318. [DOI] [PubMed] [Google Scholar]
- 6.den Haan, J.M., S.M. Lehar, and M.J. Bevan. 2000. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192:1685–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurts, C., M. Cannarile, I. Klebba, and T. Brocker. 2001. Dendritic cells are sufficient to cross-present self-antigens to CD8 T cells in vivo. J. Immunol. 166:1439–1442. [DOI] [PubMed] [Google Scholar]
- 8.Keene, J.A., and J. Forman. 1982. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J. Exp. Med. 155:768–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett, S.R., F.R. Carbone, F. Karamalis, J.F. Miller, and W.R. Heath. 1997. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 186:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridge, J.P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 393:474–478. [DOI] [PubMed] [Google Scholar]
- 11.Bennett, S.R., F.R. Carbone, F. Karamalis, R.A. Flavell, J.F. Miller, and W.R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 393:478–480. [DOI] [PubMed] [Google Scholar]
- 12.Schoenberger, S.P., R.E. Toes, E. van der Voort, R. Offringa, and C.J. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 393:480–483. [DOI] [PubMed] [Google Scholar]
- 13.Schüler, T., Z. Qin, S. Ibe, N. Noben-Trauth, and T. Blankenstein. 1999. T helper cell type 1-associated and cytotoxic T lymphocyte-mediated tumor immunity is impaired in interleukin 4-deficient mice. J. Exp. Med. 189:803-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosmann, T.R., H. Cherwinski, M.W. Bond, M.A. Giedlin, and R.L. Coffman. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348–2357. [PubMed] [Google Scholar]
- 15.Abbas, A.K., K.M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature. 383:787–793. [DOI] [PubMed] [Google Scholar]
- 16.O'Garra, A. 1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 8:275–283. [DOI] [PubMed] [Google Scholar]
- 17.Seder, R.A., J.L. Boulay, F. Finkelman, S. Barbier, S.Z. Ben-Sasson, G. Le Gros, and W.E. Paul. 1992. CD8+ T cells can be primed in vitro to produce IL-4. J. Immunol. 148:1652–1656. [PubMed] [Google Scholar]
- 18.Sad, S., R. Marcotte, and T.R. Mosmann. 1995. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 2:271–279. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayordomo, J.I., T. Zorina, W.J. Storkus, L. Zitvogel, C. Celluzzi, L.D. Falo, C.J. Melief, S.T. Ildstad, W.M. Kast, A.B. Deleo, and M.T. Lotze. 1995. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat. Med. 1:1297–1302. [DOI] [PubMed] [Google Scholar]
- 21.Labeur, M.S., B. Roters, B. Pers, A. Mehling, T.A. Luger, T. Schwarz, and S. Grabbe. 1999. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J. Immunol. 162:168–175. [PubMed] [Google Scholar]
- 22.Monach, P.A., H. Schreiber, and D.A. Rowley. 1993. CD4+ and B lymphocytes in transplantation immunity. II. Augmented rejection of tumor allografts by mice lacking B cells. Transplantation. 55:1356–1361. [PubMed] [Google Scholar]
- 23.Huang, A.Y., P.H. Gulden, A.S. Woods, M.C. Thomas, C.D. Tong, W. Wang, V.H. Engelhard, G. Pasternack, R. Cotter, D. Hunt, et al. 1996. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc. Natl. Acad. Sci. USA. 93:9730–9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, A.Y., A.T. Bruce, D.M. Pardoll, and H.I. Levitsky. 1996. In vivo cross-priming of MHC class I-restricted antigens requires the TAP transporter. Immunity. 4:349–355. [DOI] [PubMed] [Google Scholar]
- 25.Noben-Trauth, N., G. Köhler, K. Burki, and B. Ledermann. 1996. Efficient targeting of the IL-4 gene in a BALB/c embryonic stem cell line. Transgenic Res. 5:487–491. [DOI] [PubMed] [Google Scholar]
- 26.Noben-Trauth, N., L.D. Shultz, F. Brombacher, J.F. Urban, Jr., H. Gu, and W.E. Paul. 1997. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc. Natl. Acad. Sci. USA. 94:10838–10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogquist, K.A., S.C. Jameson, W.R. Heath, J.L. Howard, M.J. Bevan, and F.R. Carbone. 1994. T cell receptor antagonist peptides induce positive selection. Cell. 76:17–27. [DOI] [PubMed] [Google Scholar]
- 28.Grogan, J.L., M. Mohrs, B. Harmon, D.A. Lacy, J.W. Sedat, and R.M. Locksley. 2001. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 14:205–215. [DOI] [PubMed] [Google Scholar]
- 29.Gallucci, S., M. Lolkema, and P. Matzinger. 1999. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5:1249–1255. [DOI] [PubMed] [Google Scholar]
- 30.Inaba, K., S. Turley, T. Iyoda, F. Yamaide, S. Shimoyama, and R. Steinman. 2000. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J. Exp. Med. 191:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brossart, P., and M.J. Bevan. 1997. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 90:1594–1599. [PMC free article] [PubMed] [Google Scholar]
- 32.Albert, M.L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 392:86–89. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez, A., A. Regnault, M. Kleijmeer, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat. Cell. Biol. 1:362–368. [DOI] [PubMed] [Google Scholar]
- 34.Kamogawa, Y., L.A. Minasi, S.R. Carding, K. Bottomly, and R.A. Flavell. 1993. The relationship of IL-4- and IFN gamma-producing T cells studied by lineage ablation of IL-4-producing cells. Cell. 75:985–995. [DOI] [PubMed] [Google Scholar]
- 35.Platzer, C., G. Richter, K. Überla, H. Hock, T. Diamantstein, and T. Blankenstein. 1992. Interleukin-4-mediated tumor suppression in nude mice involves interferon-gamma. Eur. J. Immunol. 22:1729–1733. [DOI] [PubMed] [Google Scholar]
- 36.Platzer, C., G. Richter, K. Überla, W. Müller, H. Blocker, T. Diamantstein, and T. Blankenstein. 1992. Analysis of cytokine mRNA levels in interleukin-4-transgenic mice by quantitative polymerase chain reaction. Eur. J. Immunol. 22:1179–1184. [DOI] [PubMed] [Google Scholar]
- 37.Hochrein, H., M. O'Keeffe, T. Luft, S. Vandenabeele, R.J. Grumont, E. Maraskovsky, and K. Shortman. 2000. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J. Exp. Med. 192:823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mencacci, A., S.G. Del, E. Cenci, C.F. d'Ostiani, A. Bacci, C. Montagnoli, M. Kopf, and L. Romani. 1998. Endogenous interleukin 4 is required for development of protective CD4+ T helper type 1 cell responses to Candida albicans. J. Exp. Med. 187:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bagley, J., T. Sawada, Y. Wu, and J. Iacomini. 2000. A critical role for interleukin 4 in activating alloreactive CD4 T cells. Nat. Immunol. 1:257–261. [DOI] [PubMed] [Google Scholar]
- 40.Ruedl, C., M. Kopf, and M.F. Bachmann. 1999. CD8+ T cells mediate CD40-independent maturation of dendritic cells in vivo. J. Exp. Med. 189:1875–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bachmann, M.F., H. Schorle, R. Kühn, W. Müller, H. Hengartner, R.M. Zinkernagel, and I. Horak. 1995. Antiviral immune responses in mice deficient for both interleukin-2 and interleukin-4. J. Virol. 69:4842–4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adema, G.J., F. Hartgers, R. Verstraten, E. de Vries, G. Marland, S. Menon, J. Foster, Y. Xu, P. Nooyen, T. McClanahan, et al. 1997. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature. 387:713–717. [DOI] [PubMed] [Google Scholar]