Abstract
Interleukin 13 receptor α2 (IL-13Rα2) chain is highly expressed on some tumor cell lines and primary cell cultures. This receptor chain plays an important role in ligand binding and internalization. To determine the functional significance of overexpression of this chain, we stably transfected IL-13Rα2 chain in human breast (MDA-MB-231) and pancreatic (PANC-1) cancer cell lines that naturally do not express this chain. There was no difference in growth between vector only transfected and IL-13Rα2 chain transfected cells in vitro. However, surprisingly, in immunodeficient mice, tumorigenicity was profoundly inhibited in IL-13Rα2 chain overexpressing tumors. Because breast tumors that grew later showed loss of IL-13Rα2 gene expression, lack of tumorigenicity correlated positively with IL-13Rα2 chain expression. Inflammatory cells including neutrophils and macrophages were identified in IL-13Rα2 overexpressing regressing tumors and neutrophils were found to produce IL-13. IL-13 showed a modest antitumor activity to IL-13Rα2 chain overexpressing tumors in vitro and in vivo. Furthermore, IL-13Rα2 chain overexpressing tumors constitutively produced IL-8 that has been shown to have antitumor effect. These results establish a novel function of a cytokine receptor chain and further suggest that the presence of this chain on tumor cells by itself may play a key role in tumorigenicity.
Keywords: tumor antigen, neutrophil infiltration, tumorigenesis, IL-8, cytokine receptor
Introduction
IL-13 is a Th2 cell–derived pleiotropic immune regulatory cytokine (1). It has predominant biological activities on B cells and monocytes. In recent years, the receptors for IL-13 (IL-13R) have been extensively characterized. We have demonstrated that IL-13R may exist as three different forms in different cell types (2–7). Two different chains of the IL-13R, IL-13Rα1 (also known as IL-13Rα′) and IL-13Rα2 (also known as IL-13Rα) have been cloned. The murine and human IL-13Rα1 chain was cloned first (8, 9). This chain binds IL-13 at low level but when coupled with IL-4Rα chain (also known as IL-4Rβ) binds IL-13 with high affinity and mediates IL-13–induced signaling (8–10). The second chain of IL-13R, IL-13Rα2 was cloned from a human renal cell carcinoma cell line (Caki-1). This chain has 50% homology to IL-5R at the DNA level, has a short intracellular domain, and binds IL-13 with ∼50 times higher affinity than IL-13Rα1 chain (11, 12).
We and others have reported that IL-13Rα2 chain is expressed on many human cancer cell lines and primary cell cultures including glioblastoma, AIDS-associated Kaposi's sarcoma, ovarian carcinoma, renal cell carcinoma (13–16), and fibroblast cell lines (6). Interestingly, this receptor chain does not seem to be present or present at extremely low level in normal endothelial cells, immune cells, or certain types of cancer cell lines as assessed by RT-PCR analysis (12–15). The reason for difference in the expression is unknown; however, it is predicted that some correlation between the presence of IL-13Rα2 chain and tumor biology must exist. More recently, we have demonstrated that IL-13Rα2 chain plays an important role in IL-13 binding and internalization (17–19). Although the function of IL-13Rα2 chain is being unraveled, it is still not known why this receptor chain is expressed on the surface of only certain tumor cell lines. Numerous studies in the literature suggest that gene transfer of cytokine or growth factors in tumor cells can inhibit tumorigenicity of murine or human tumors in experimental animals (20–25). In most studies, resident immune cells seemed to play a major role in the loss of tumorigenesis in vivo (20, 21, 23–26). However, several studies have reported that in addition to immune mechanism, in some situations, inflammatory cells such as eosinophils and macrophages play a dominant role in the control of tumor growth (24, 25). Although the mechanism of tumor growth control by inflammatory cells is not completely clear, the growth factor(s) or chemokine(s) produced by these cells may play some role in this process (26–28). One of the chemokine, IL-8, has been shown to play a major role in the control of tumorigenesis most likely through regulation of angiogenesis in developing tumor (20, 21, 26). Despite extensive studies on the role of growth factors, cytokines, and chemokines on tumorigenesis, the role of receptors for these factors has not been extensively studied.
In this study, we have examined the functional significance of IL-13Rα2 chain in two tumor models. We stably transfected human breast cancer cell line, MDA-MB-231 and pancreatic cancer cell line, PANC-1 with IL-13Rα2 chain. Various clones of these cell lines were assessed for IL-13 receptor expression in vitro and in vivo, and the effect of IL-13Rα2 chain on tumorigenicity of these two tumorigenic tumor cell lines was investigated. To our surprise, we found that the overexpression of IL-13Rα2 chain profoundly inhibited tumor development in immunodeficient animals. The mechanism of tumor regression in these two tumor models has been investigated.
Materials and Methods
Recombinant Cytokine, Toxin, and Cell Lines.
Recombinant human IL-4 and IL-13 were produced and purified to homogeneity in our laboratory (29). Recombinant IL-13-Pseudomonas exotoxin A (PE)*38QQR was also produced and purified in our laboratory (30, 31). Human breast cancer (MDA-MB-231) and pancreatic cancer (PANC-1) cell lines were purchased from the American Type Culture Collection. Cells were cultured in RPMI 1640 (MDA-MB-231) or DMEM (PANC-1) containing 10% FBS (Biowhittaker), 1 mM Hepes, 1 mM l-glutamine, 100 μg/ml penicillin, and 100 μg/ml streptomycin (Biowhittaker).
Stable Transfection and Selection.
cDNA encoding human IL-13Rα2 chain was cloned into pME18S mammalian expression vector (11, 32). Plasmid DNA (12 μg/100-mm culture dish) was cotransfected with 1.2 μg of pPUR selection vector (CLONTECH) into semiconfluent cells using GenePORTER transfection reagent (Gene Therapy Systems) according to the manufacturer's instructions. Briefly, cells (2 × 106 cells per 100-mm dish) were incubated with the DNA-GenePORTER mixture for 5 h in DMEM followed by 20-h incubation in fresh DMEM containing 20% FBS and additional 24 h in medium with 10% FBS. At 48 h after the start of transfection, cells were trypsinized and cultured in selection medium that contained 1 μg/ml of puromycin (CLONTECH). Cells were maintained for 4 wk in the same medium, which was replaced every 3 d. Resistant clones isolated with the cloning cylinder (Bel-Art Products) were characterized for IL-13Rα2 chain expression by RT-PCR and radioreceptor binding assays. Finally, three IL-13Rα2-overexpressing clones (termed α2 clone 1, 2, and 3) were selected for further analysis. The vector control (mock) transfected cell lines were used for comparison with IL-13Rα2 transfected cells. To reduce antibiotic side effects, puromycin was removed at least 14 d before experiments were performed.
RT-PCR.
To detect the mRNA expression in cells, total RNA was isolated using TRIZOL reagent (Life Technologies), then RT-PCR was performed using specific primers as described previously (13, 33).
Radioreceptor Binding Assays.
Recombinant human IL-13 was labeled with 125[I] (Amersham Pharmacia Biotech) using IODO-GEN reagent (Pierce Chemical Co.) as described previously (34). The specific activity of the radio-labeled cytokines was estimated to be 12.7 μCi/μg of protein. For binding experiments, 5 × 105 cells in 100 μl binding buffer (RPMI 1640 containing 0.2% human serum albumin and 10 mM Hepes) were incubated with 200 pM 125[I]-IL-13 with or without various concentrations (10 pM to 100 nM) of unlabeled IL-13 at 4°C for 2 h. Cell-bound 125[I]-IL-13 was separated from unbound by centrifugation through a phthalate oil gradient and radioactivity was determined with a γ counter (Wallac). In some experiments, the number of IL-13Rs and binding affinities were calculated using the LIGAND program (35).
Protein Synthesis Inhibition Assay.
The cytotoxic activity of IL-13 toxin was tested as described previously (36). Typically, 104 cells were cultured in leucine-free medium with or without various concentrations of IL-13-PE38QQR for 20–22 h at 37°C. Then 1 μCi of [3H]leucine (NEN Research Products) was added to each well and incubated for an additional 4 h. Cells were harvested and radioactivity incorporated into cells was measured by a β plate counter (Wallac).
Animal Studies.
Athymic nude mice 4 wk old (∼20 g in body weight) were obtained from Frederick Cancer Center Animal Facilities (National Cancer Institute). Animal care was in accordance with the guidelines of NIH Animal Research Advisory Committee. Human breast and pancreatic tumor models were established in the nude mice by subcutaneous injection into the flank. Vector alone and IL-13Rα2 chain cDNA transfected tumor cells were 5 × 106 MDA-MB-231 or 4 × 106 PANC-1 cells in 150 μl of PBS plus 0.2% human serum albumin. Palpable tumors developed within 3–4 d. Tumors were measured by Vernier calipers. In general, five mice were used for each group. In some experiments, mice were injected with 1 μg of IL-13 intratumorally or 0.1 mg of Gr-1 (rat anti–mouse granulocytes antibody; Cedarlane) intraperitoneally.
Histological Analysis.
Tissues at the site of tumor injection were embedded in OCT compound (Miles) and snap frozen by liquid nitrogen. 5-μ cryostat sections were fixed in 90% ethyl alcohol and stained with hematoxylin and eosin.
Immunofluorescence Assay.
Sections (5 μm) were prepared and stained for neutrophils (Gr-1; BD PharMingen), macrophage (Mac-3; BD PharMingen), or IL-13 (C-19; Santa Cruz Biotechnology, Inc.). Isotype control Abs was used for each corresponding Ab. Slides were fixed in acetone at −20°C for 5 min and air dried. Nonspecific binding was blocked by treatment with 10% serum (goat or rat) for 1 h followed by incubation with Ab or isotype control. Sections were subsequently incubated for 1 h with secondary Ab that had either tetramethylrhodamine isothiocyanate or FITC tag. After three washes with PBS, slides were dried and layered with Vectashield antifluorescence fading mounting medium (Vector Laboratories) and a coverslip. The slides were viewed in a Nikon fluorescence microscope using appropriate filters.
Clonogenic Assay.
The in vitro antitumor activity of IL-13 on MDA-MB-231 and PANC-1 cells transfected with IL-13Rα2 chain or vector only (mock control) was determined by a colony-forming assay as described previously (18).
ELISA for Human IL-8 Expression.
The level of IL-8 protein in culture supernatants was determined by using a quantitative immunometric sandwich enzyme immunoassay (ELISA) kit (Human IL-8/NAP-1 immunoassay kit; BioSource International). The O.D. of the test sample was compared with the standard curve.
Results
IL-13Rα2 Expression in MDA-MB-231 and PANC-1 Cell Lines.
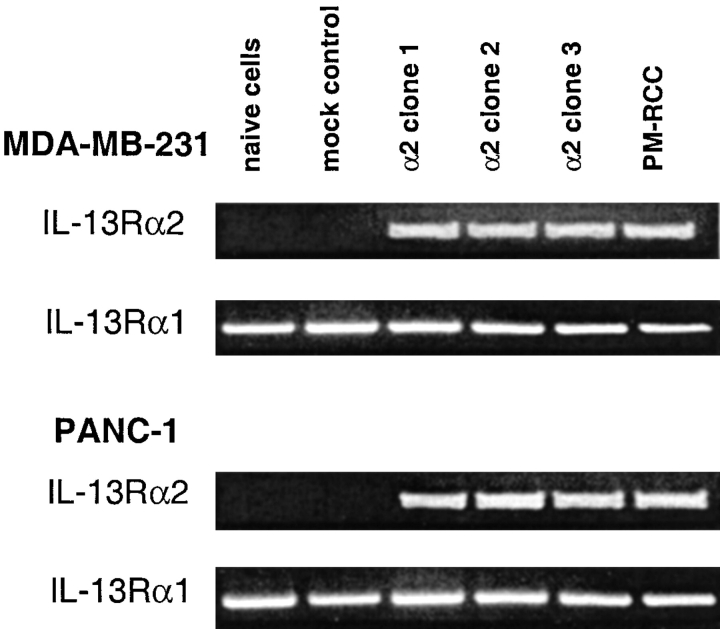
To determine the expression of IL-13R in MDA-MB-231 and PANC-1 cell lines, we first examined the mRNA expression of IL-13R components, IL-13Rα2 and IL -13Rα1 chains by RT-PCR. As shown in Fig. 1, mRNA for IL-13Rα1 chain was present in both cell lines, however they did not express IL-13Rα2 mRNA. In stably transfected cell lines, IL-13Rα2 chain expression was confirmed as these clones expressed abundant mRNA for this chain. PM-RCC cells that express IL-13Rα2 and IL-13Rα1 mRNA served as a positive control (13).
Figure 1.
RT-PCR analysis for IL-13 receptor transcripts in MDA-MB-231 and PANC-1 cell lines. Total RNA (2 μg) from cells transfected with or without IL-13Rα2 chain or vector alone was examined for expression of mRNA for IL-13Rα2 and IL-13Rα1 chains by RT-PCR analysis. The same amount of total RNA from PM-RCC cells served as a positive control.
IL-13 Binding Sites on MDA-MB-231 and PANC-1 Cells Increased After Gene Transfer of IL-13Rα2 Chain.
Then we determined the expression and binding affinity of IL-13R on MDA-MB-231 and PANC-1 cell lines by 125[I]-IL-13 binding assays. As shown in Fig. 2 A, vector only transfected control cells do not express IL-13Rα2 chain and consequently show minimal binding to 125[I]-IL-13. However, stable clones transfected with IL-13Rα2 chain showed highly increased 125[I]-IL-13 binding activity. This binding activity was displaced by excess of unlabeled IL-13. Because IL-13R and IL-4R share two chains with each other, we also examined whether IL-4 can displace IL-13 binding in MDA-MB-231 and PANC-1 cells transfected with IL-13Rα2 chain (7, 32, 37). As shown in Fig. 2 A, IL-4 showed only a little displacement of 125[I]-IL-13 binding. These findings indicate that IL-13Rα2 chain transfected MDA-MB-231 and PANC-1 cells form a type I IL-13 receptors where IL-13Rα2, IL-13Rα1, and IL-4Rα chains coexist (2, 6, 7, 13). To further characterize the IL-13R in IL-13Rα2 chain transfected cells, we performed Scatchard analysis using MDA-MB-231α2 clone 3 and PANC-1α2 clone 2 (Fig. 2 B and C). Both clone cells bound IL-13 in a concentration-dependent manner. Scatchard analysis of the binding data showed a single binding site receptor with a K d value of 1.24 nM (MDA-MB-231α2 clone 3) and 1.39 nM (PANC-1α2 clone 2). The number of IL-13Rs were calculated as 39,700 (MDA-MB-231α2 clone 3) and 37,740 (PANC-1α2 clone 2) IL-13 molecules bound per cell. Since IL-13 binding sites on vector only transfected cells were calculated to be 90 (MDA-MB-231) and 160 (PANC-1) per cell, an increase in IL-13 binding sites in IL-13Rα2 chain transfectants was ∼440-fold and 240-fold higher compared with control cells, respectively.
Figure 2.
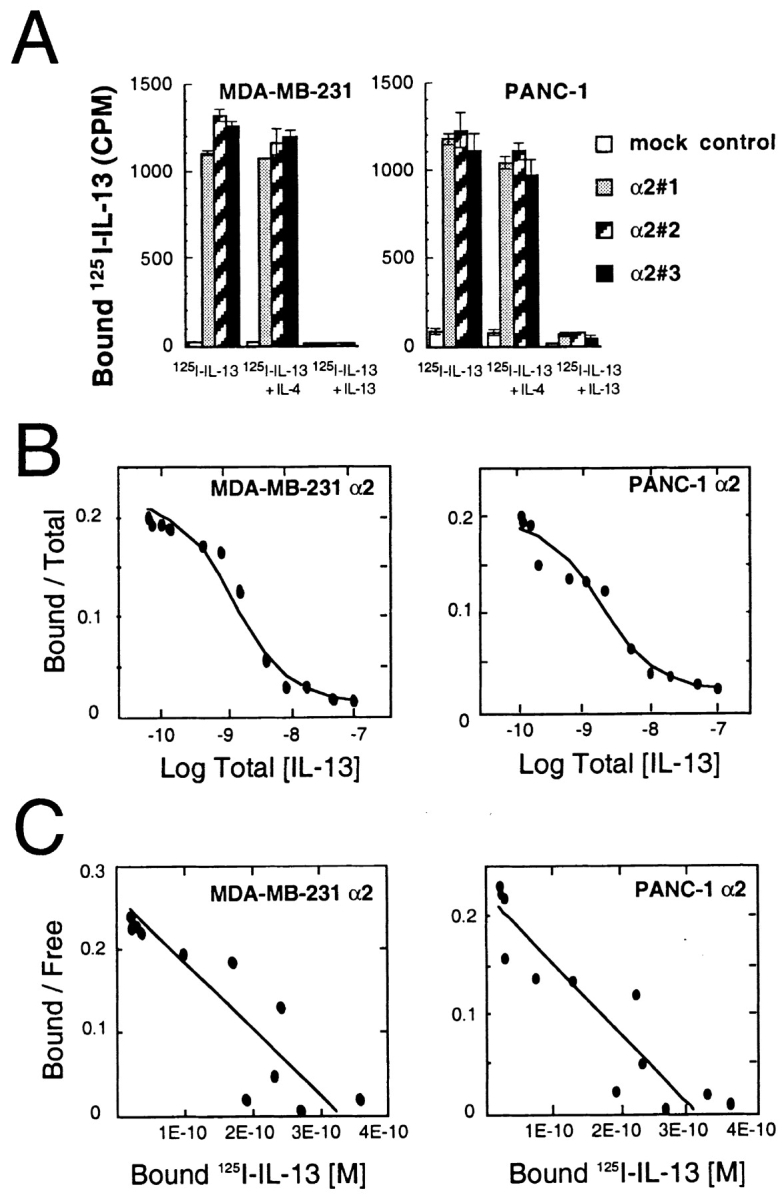
IL-13 binding to MDA-MB-231 and PANC-1 cells. Binding of 125[I]-labeled IL-13 was performed as described in Materials and Methods. (A) Single-point binding assays on cells stably transfected with vector-only or IL-13Rα2 chain. Data were obtained from the mean of duplicate determinations, and the assay was repeated several times. Cells (5 × 105) were incubated at 4°C for 2 h with 200 pM 125[I]-labeled IL-13 with or without 40 nM unlabeled IL-4 or IL-13. Data are means; bars, SD. The displacement curve (B) and Scatchard analysis (C) were generated from the binding data from MDA-MB-231 (α2 clone 3) and PANC-1 (α2 clone 2) cells using LIGAND program (reference 35).
In Vitro Growth Characteristics and Stability of IL-13Rα2 Chain Expression in Transfected Cells.
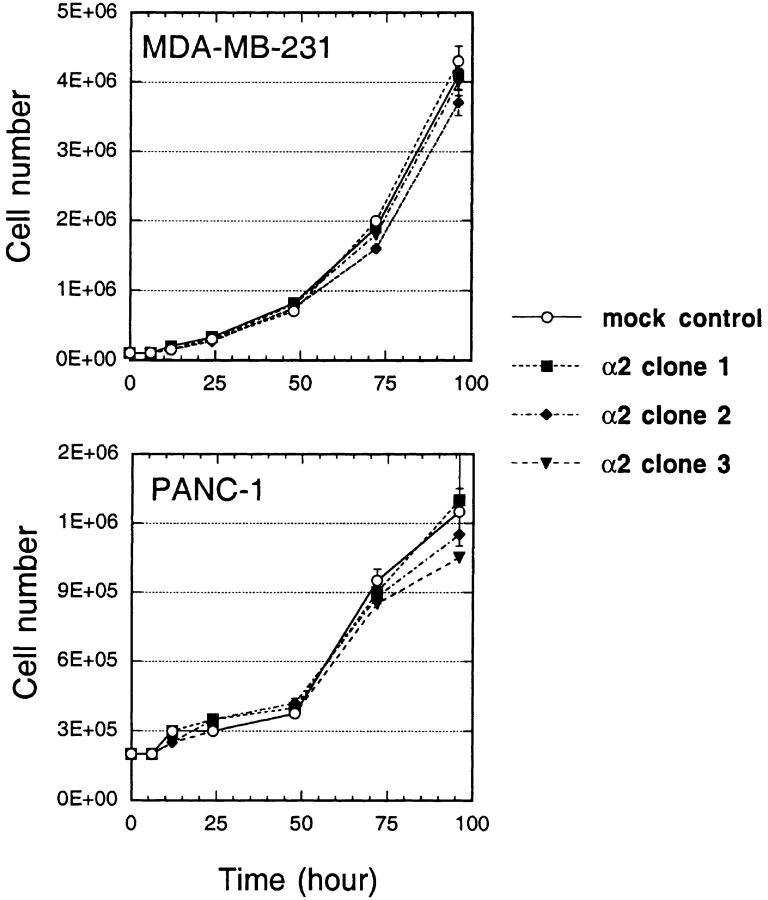
To determine the effect of IL-13Rα2 chain on in vitro cell growth characteristics of MDA-MB-231 and PANC-1 cells, vector alone and IL-13Rα2 chain transfected cloned cells were cultured in petri dishes and viable cell number determined at various time points. As shown in Fig. 3, three clones each of both MDA-MB-231 and PANC-1 cell lines grew in a similar manner in vitro. There was no difference in growth rate between vector only transfected control cells and IL-13Rα2 chain transfected cells.
Figure 3.
In vitro cell growth of MDA-MB-231 and PANC-1 tumor cell clones. To examine in vitro cell growth of MDA-MB-231 and PANC-1 cells transfected with IL-13Rα2 chain or vector alone, cloned cells (2 × 105) were cultured in 100-mm petri dishes and were harvested by trypsin after 0–96 h, and then cell number was counted by Trypan blue staining. The results were represented as means ± SD of triplicate determinations, and the assay was repeated several times.
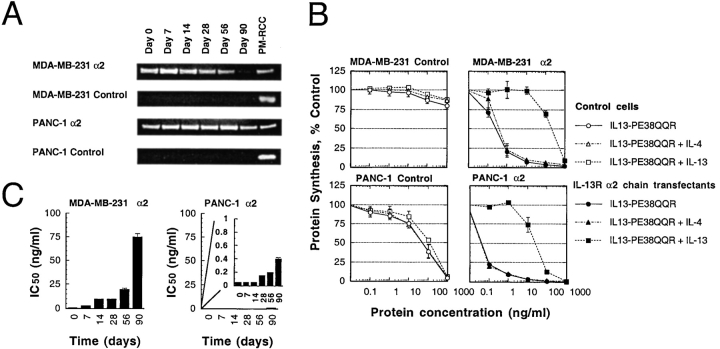
To determine the in vitro stability of gene expression, we cultured vector only transfected control cells and IL-13Rα2 chain transfected cells (MDA-MB-231α2 clone 3 and PANC-1α2 clone 2) for a 90-d period after removal of antibiotics. During the culture, cells were split twice (MDA-MB-231) or once (PANC-1) a week. At various time points, total RNA was extracted and mRNA expression for IL-13Rα2 chain was assessed by RT-PCR. In MDA-MB-231α2 clone, IL-13Rα2 gene expression decreased in a time-dependent manner, and on day 90 very faint mRNA expression was detected (Fig. 4 A). However, in case of PANC-1α2 clone, IL-13Rα2 chain expression remained at high level until day 90, suggesting this clone expresses this chain very stably.
Figure 4.
In vitro stability of IL-13Rα2 chain expression on MDA-MB-231 and PANC-1 clones. (A) Total RNA (2 μg) extracted from cells transfected with IL-13Rα2 chain or vector alone at various time point after removal of antibiotic was examined for the expression of IL-13Rα2 chain mRNA by RT-PCR analysis. The same amount of total RNA from PM-RCC cells served as a positive control. (B and C) After removal of antibiotic (B, day 0 and C, days 0–90), cells transfected with IL-13Rα2 chain or vector only were cultured with various concentrations of IL-13-PE38QQR (0–1,000 ng/ml) with or without IL-4 or IL-13 (2 μg/ml). The results were represented as means ± SD (n = 4). The concentration of IL-13-PE38QQR causing 50% inhibition of protein synthesis was calculated (IC50).
To further confirm the expression of IL-13Rα2 chain on plasma membranes of cells transfected with this chain, we used a chimeric protein composed of IL-13 and a truncated form of PE, IL-13 toxin (IL-13-PE38QQR). This molecule was found to be potently cytotoxic to IL-13R-positive solid tumor cells (30, 31, 38–40). It has been found that IL-13 toxin will only bind and be cytotoxic to cells that express high levels of IL-13R on cell surface. As shown in Fig. 4 B, just after removal of antibiotic, in both MDA-MB-231 and PANC-1 cells, cytotoxic activity of IL-13 toxin dramatically increased after gene transfer of IL-13Rα2 chain. IC50 (the protein concentration required for the inhibition of protein synthesis by 50%) in MDA-MB-231 and PANC-1 cell lines decreased from >1,000 ng/ml to 0.25 ng/ml and from 60 ng/ml to <0.1 ng/ml, respectively. These cytotoxic activities in cells transfected with IL-13Rα2 chain were neutralized by IL-13 not by IL-4, indicating the cytotoxicity mediated by IL-13 toxin is specific. The stability of IL-13Rα2 chain expression in MDA-MB-231 and PANC-1 cells transfected with this chain was also assessed by determining cytotoxicity mediated by IL-13 toxin using cells cultured for same period as Fig. 4 A. As shown in Fig. 4 C, IC50s increased in a time-dependent manner in MDA-MB-231 cells transfected with IL-13Rα2 chain. However, IC50 remained significantly lower than observed in vector only transfected cells (Fig. 4 B). On day 90, the IC50 increased to ∼75 ng/ml correlating with significant decrease in IL-13Rα2 gene expression. On the other hand, PANC-1 cells transfected with IL-13Rα2 chain maintained high sensitivity to IL-13 toxin throughout the 90-d period (<1 ng/ml). These results were consistent with RT-PCR results (Fig. 4 A), suggesting that the transgene expression in PANC-1 cells transfected with IL-13Rα2 chain remained much stable than MDA-MB-231 transfectants. After 90 d of culture, both cell lines maintained their rapid growth characteristics as observed on day 0 (data not shown).
Overexpression of IL-13Rα2 Chain on the Cell Surface Inhibits Tumorigenicity of MDA-MB-231 and PANC-1 Tumors.
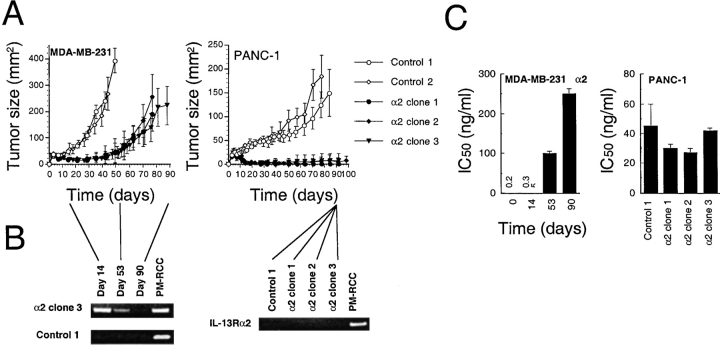
To investigate in vivo growth characteristics of breast and pancreatic tumor cells that overexpress IL-13Rα2 chain, MDA-MB-231 and PANC-1 cell clones transfected with IL-13Rα2 chain or vector alone were implanted subcutaneously into the flank of nude mice. As shown in Fig. 5 A, MDA-MB-231 vector only transfected control tumor cells grew rapidly requiring sacrifice of these animals between days 44 and 50 because of heavy tumor burden. MDA-MB-231 tumors expressing IL-13Rα2 chain also started growing as fast as control tumors initially; however, they stopped growing completely and tumor remained same in size (∼20 mm2) until days 40–46. After that, they started growing again gradually and reached the size of 160–220 mm2 on day 70–82. In additional experiments, cells derived from different IL-13Rα2 chain-expressing clones grew into tumors at a similar slower pace compared with control cells (data not shown). On the other hand, despite linear tumor growth of PANC-1 vector only transfected control cells, PANC-1 tumors expressing IL-13Rα2 chain stopped growing after they established as palpable tumors between days 3 and 5. Then, these tumors started diminishing. Between days 11 and 16, almost all the tumors disappeared completely. Between days 21 and 32, some animals had palpable tumors (1/5 mice of clone 1, 2/5 mice of clone 2, and 1/5 mice of clone 3), however, they never grew beyond the size of 20 mm2.
Figure 5.
In vivo MDA-MB-231 and PANC-1 tumor growth and stability of IL-13Rα2 chain expression. (A) Nude mice were implanted subcutaneously with 5 × 106 MDA-MB-231 or 4 × 106 PANC-1 cloned cells on day 0. Palpable tumors developed within 3–4 d. The results were represented as means ± SD (n = 5). (B and C) Tumors were resected from mice on days 14, 53, and 90 (MDA-MB-231) or day 90 (PANC-1), then tumors were minced and cells were cultured for three passages. (B) Total RNA was extracted and mRNA expression for IL-13Rα2 chain was assessed by RT-PCR. (C) Cells were cultured with various concentrations of IL-13-PE38QQR (0–1,000 ng/ml) and protein synthesis inhibition assays were performed, and the concentration of IL-13-PE38QQR at which IC50 occurred was calculated. The results were represented as means ± SD (n = 4).
To assess the in vivo stability of IL-13Rα2 chain expression, MDA-MB-231 and PANC-1 tumors were resected at various time points (MDA-MB-231, days 14, 53, and 90) or on day 90 (PANC-1; vector only transfected control and IL-13Rα2 chain transfected clones that had very small tumors). Tumors were minced and treated with 10 μg/ml collagenase, 1 mg/ml hyaluronidase, and 0.5 mg/ml DNase (Sigma-Aldrich), washed with PBS for two times and cultured in culture medium containing 10% FBS. After three passages, contaminating tumor debris and blood cells were removed, and then total RNA was extracted and mRNA expression for IL-13Rα2 chain was assessed by RT-PCR. As shown in Fig. 5 B, in MDA-MB-231 tumors derived from IL-13Rα2 chain transfected clone 3, the expression level of IL-13Rα2 chain mRNA was found to be decreased in a time-dependent manner with almost complete disappearance on day 90. Whereas all the clones derived from PANC-1 tumors on day 90 showed no or very low level expression of IL-13Rα2 chain.
To further analyze the IL-13Rα2 chain expression on resected tumors, using three-passage-cultured cells, cytotoxic activity of IL-13 toxin was examined. As shown in Fig. 5 C, cells derived from MDA-MB-231 tumors (IL-13Rα2 chain transfectants clone 3) showed the decreasing sensitivities to IL-13 toxin in a time-dependent manner. On day 90, when IL-13Rα2 gene expression was significantly diminished, the IC50 rose to ∼250 ng/ml. The IC50 still remained at least four times lower compared with vector only transfected control cells (IC50 > 1,000 ng/ml). Cells cultured from PANC-1 IL-13Rα2 chain transfected clones-derived tumors on day 90 showed significantly less sensitivity to IL-13 toxin compared with in vitro cultured PANC-1 IL-13Rα2 chain transfected cells (IC50 < 1 ng/ml; Fig. 4 C), and the IC50 ranged between 27 and 42 ng/ml. Sensitivity of PANC-1 cells cultured from vector only transfected control clone-derived tumor to IL-13 toxin was similar to IL-13Rα2 chain transfected clones-derived tumors (IC50 = 45 ng/ml). These results were consistent with RT-PCR results and indicate that loss of IL-13Rα2 chain in vivo correlated with loss in sensitivity to IL-13 toxin.
Infiltration of Inflammatory Cells Was Observed at Early Time Point After Implantation of MDA-MB-231 Tumors Overexpressing IL-13Rα2 Chain.
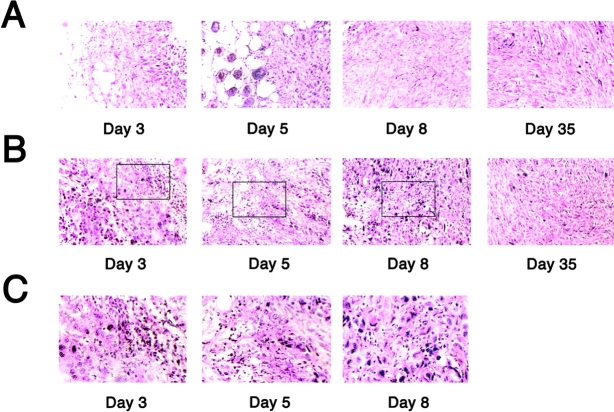
To investigate the mechanism by which tumorigenicity of IL-13Rα2 chain transfected tumors was inhibited, both vector-only transfected and IL-13Rα2 chain transfected MDA-MB-231 tumors were resected from mice on days 3, 5, 8, and 35 after the implantation, and then histological study of the tumor was performed after H&E staining. As shown in Fig. 6 A, vector-only transfected control cells developed breast tumors without infiltration of inflammatory cells. On the other hand, MDA-MB-231 tumor transfected with IL-13Rα2 chain displayed a marked cellularity (Fig. 6 B and C). The cell infiltration was dominant in connective tissue around the tumor on day 3, however, as the time progressed (days 5 and 8), inflammatory cells were observed infiltrating inside the tumor mass. Both vector-only and IL-13Rα2 chain transfected tumors resected on day 35 displayed limited level of inflammatory cells. PANC-1 tumors could not be analyzed on days 8 and 35 because tumor had completely regressed. The population of infiltrating cells differed among tumors, and neutrophils were determined to be dominant cell type (Table I).
Figure 6.
MDA-MB-231 tumors overexpressing IL-13Rα2 chain display cellular infiltration. MDA-MB-231 tumors originated from clones transfected with vector alone (A) or IL-13Rα2 chain (B and C) were resected from mice on day 3, 5, 8, and 35 after the implantation, and then histological study of the tumor was performed by H&E staining (B, original magnification: × 200 and C, original magnification: ×400). Picture C shows the higher power view of squared area in B.
Table I.
Population of Infiltrating Cells as Determined by Histological Examination
| Time (d) | Control | IL-13Rα2 chain transfectants
|
||
|---|---|---|---|---|
| Lymphocytes | Macrophages | Neutrophils | ||
| MDA-MB-231 | ||||
| Day 3 | − | +a | + | ++ |
| Day 5 | − | + | ++ | ++ |
| Day 8 | − | + | − | + |
| Day 35 | − | − | − | + |
| PANC-1 | ||||
| Day 5 | − | + | + | + |
Infiltrating cells observed in day 3 tumors were mainly in the connective tissue around the tumor section.
++, moderate; +, mild; −, not detectable.
Immunofluorescence Assays Showed Production of IL-13 from Neutrophils in IL-13Rα2 Chain Overexpressing Tumors.
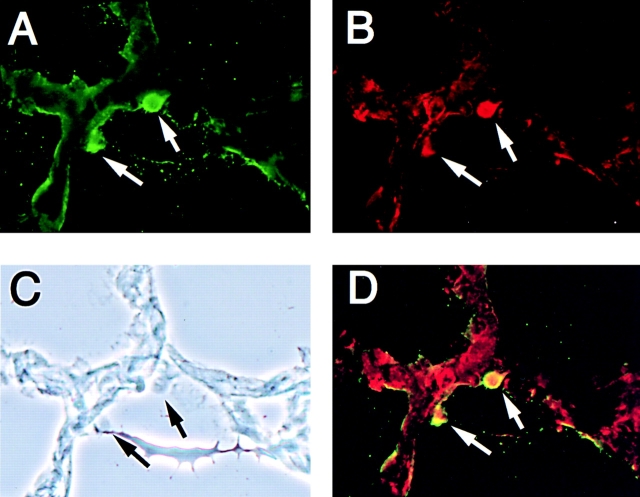
To further characterize the infiltrating cells, immunofluorescence assays were performed on frozen tissue sections obtained from subcutaneously growing MDA-MB-231 IL-13Rα2 overexpressing tumors. As shown in Fig. 7, staining with antibody to IL-13 (C-19, Fig. 7 A), neutrophils (Gr-1, Fig. 7 B) and macrophages (Mac-3, data not shown) in IL-13Rα2 chain transfected tumors on day 5 showed infiltration of cells that produce IL-13. On the other hand, mock-transfected control tumors and IL-13Rα2–transfected tumors after 35 d of growth did not show detectable levels of staining for IL-13, neutrophils, or macrophages (data not shown). Interestingly, we found colocalization of IL-13 and neutrophils but not macrophages which indicates that IL-13 is produced from neutrophils (Fig. 7 D). On the other hand, macrophages did not produce IL-13. These results suggest that infiltrating neutrophils are most probably involved in the inhibition of tumorigenicity by gene transfer of IL-13Rα2 chain.
Figure 7.
Immunofluorescence assay on IL-13Rα2 chain overexpressing MDA-MB-231 tumors. Immunofluorescence assays using Abs for IL-13 (C-19, A) or neutrophils (Gr-1, B) were performed on frozen tissue sections on tumors resected on day 5 after subcutaneous implantation of MDA-MB-231 cells. (C) Phase contrast. (D) Superimposed image of A and B. Original magnification: ×400.
IL-13 Showed Modest Antitumor Activity in IL-13Rα2 Chain Overexpressing Tumors In Vitro and In Vivo.
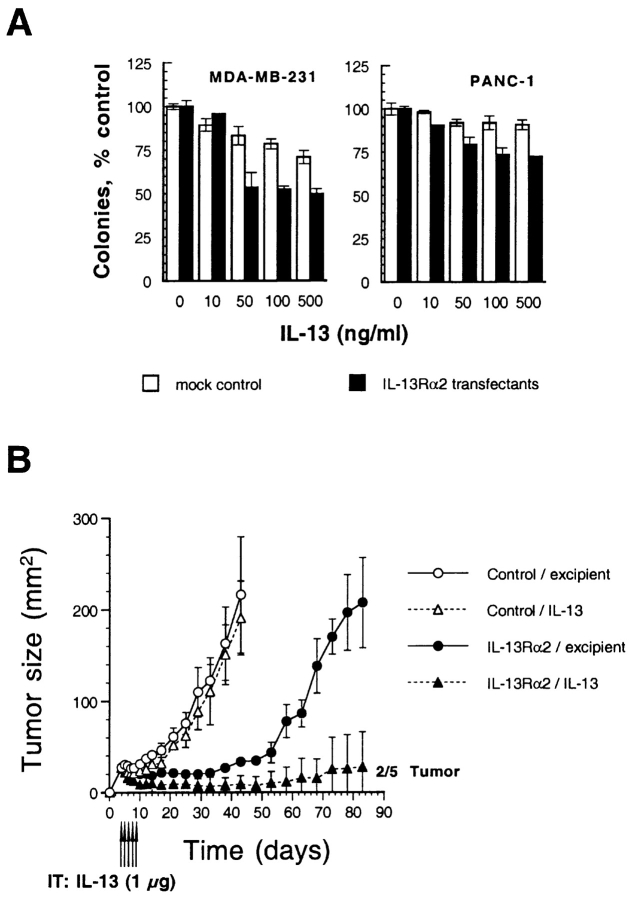
Because IL-13 production from neutrophils was observed in IL-13Rα2 overexpressing tumors, we hypothesized that IL-13 itself may have direct antitumor effect or is involved in the inhibition of tumorigenicity. To address this issue, we first performed in vitro colony formation assay in MDA-MB-231 and PANC-1 cells transfected with vector only (mock control) or IL-13Rα2 chain. As shown in Fig. 8 A, in both cell lines, IL-13 showed a modest inhibitory effect on colony formation in IL-13Rα2 transfectants (50% inhibition in MDA-MB-231 cells and 28% inhibition in PANC-1 cells at 50–500 ng/ml of IL-13). Although IL-13 showed slight inhibitory effect on colony formation in mock control MDA-MB-231 cells, the effect was more pronounced in IL-13Rα2 transfectants.
Figure 8.
Antitumor activity of IL-13 in IL-13Rα2 chain overexpressing tumors in vitro and in vivo. (A) Vector-only or IL-13Rα2 chain transfected cells (500 cells per group) were allowed to adhere in Petri dishes, and the medium was replaced with medium containing various concentrations (0–500 ng/ml) of IL-13. Cells were cultured for 9 d, and colonies consisting of at least 50 cells were scored after staining with crystal violet. Data are means of triplicate determinations; bars, SD. (B) Vector-only or IL-13Rα2 chain transfected MDA-MB-231 cells (5 × 106) were implanted in the flank of nude mice on day 0 and were treated with intratumoral administration of either excipient or IL-13 (1 μg) from days 4 to 8 (total five injections). The results were represented as means ± SD (n = 5).
To assess in vivo antitumor effect of IL-13 in IL-13Rα2 overexpressing tumors, we then directly injected IL-13 (1 μg) in MDA-MB-231 tumor (mock control and IL-13Rα2 transfectants) bearing nude mice intratumorally from days 4 to 8 (total five injections). As shown in Fig. 8 B, in control tumors, IL-13 did not show significant effect and tumors grew as fast as tumors without any IL-13 injections (Fig. 5 A). On the other hand, in IL-13Ra2 overexpressing tumors, IL-13 induced regression of tumors during the injection period, and two out of five tumors were completely disappeared by day 33. Although in the rest of the mice tumors started growing eventually, the mean size of the tumors remained very small (28 mm2) compared with excipient-injected control tumors (207 mm2) by day 83 after implantation of the tumors. These results suggest that IL-13 produced by neutrophils should be considered to play a role in the inhibition of tumorigenesis in vivo in IL-13Rα2 chain overexpressing tumors.
Depletion of Neutrophils in IL-13Rα2 Chain Overexpressing Tumor Bearing Nude Mice Showed Initial Growth of Tumors.
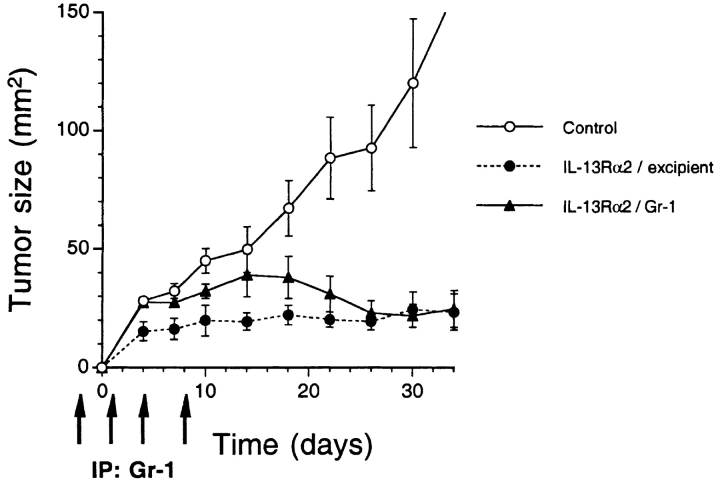
To assess whether neutrophils by itself may have some potential role in the inhibition of tumor growth, we depleted neutrophil in nude mice by intraperitoneal injections of antineutrophil antibody (Gr-1) and IL-13Rα2 chain transfected MDA-MB-231 cells were implanted. During the injection period, blood samples were taken from mice and absence of neutrophils was confirmed (data not shown). As shown in Fig. 9, during the depletion period IL-13Rα2 chain transfected tumor grew better than excipient-only injected tumors. These tumors kept on growing by day 14 after implantation, then began to regress once antibody injection was stopped. Finally, these tumors regressed to the mean tumor size of control IL-13Rα2 tumors (20–23 mm2) when the experiment was terminated. These data indicate that neutrophils play a partial role in the inhibition of tumor growth.
Figure 9.
Administration of the antibody to neutrophil in nude mice enhances the tumor growth of IL-13Rα2 chain overexpressing tumor. Vector-only or IL-13Rα2 chain transfected MDA-MB-231 cells (5 × 106) were implanted in the flank of nude mice on day 0 and were treated with intraperitoneal administration of either excipient or Gr-1 (0.1 mg) on days −1, 1, 4, and 8 (total four injections). The results were represented as means ± SD (n = 5).
High Levels of IL-8 Was Constitutively Released from IL-13Rα2 Chain Overexpressing MDA-MB-231 Tumor Cells.
IL-8 has been shown to have potent antitumor effect in several tumor types including lung cancer, ovarian cancer, and prostate cancer (20, 21). To explore whether this chemokine is released from IL-13Rα2 chain overexpressing tumor cells, we extracted RNA from MDA-MB-231 cells transfected with vector only (mock control) or IL-13Rα2 chain after treatment with IL-13 (50 ng/ml) for 24 h. By serial twofold dilutions of RNA samples, we found that IL-8 gene is constitutively expressed in MDA-MB-231 mock control cells, and approximately fourfold upregulation of this gene was observed after stimulation with IL-13 (Fig. 10 A). To our surprise, in IL-13Rα2 chain transfected MDA-MB-231 cells, IL-8 gene expression was two- to fourfold higher compared with mock control cells. To further confirm these results, we performed ELISA assays for human IL-8 in MDA-MB-231 derived culture supernatants. As shown in Fig. 10 B, after stimulation with IL-13 (50 ng/ml), IL-8 production from the mock control cells was increased in a time-dependent manner. In IL-13Rα2 chain transfected MDA-MB-231 cells, constitutive release of IL-8 was ∼1.75-fold higher compared with mock control cells. IL-8 release level from IL-13Rα2 chain transfectants was also augmented in a time-dependent manner. These results suggest that IL-8 is constitutively produced from IL-13Rα2 chain overexpressing tumor cells and this production level is upregulated after stimulation with IL-13.
Figure 10.
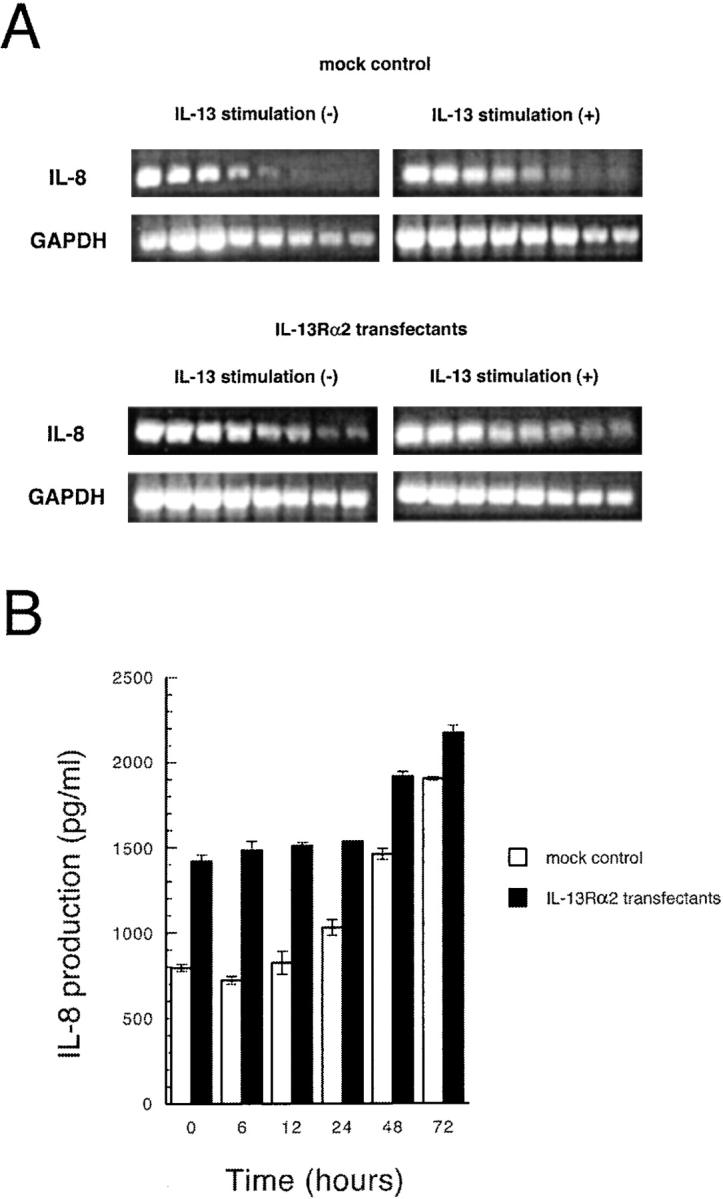
IL-8 production from MDA-MB-231 cells transfected with vector only or IL-13Rα2 chain. (A) Total RNA was extracted from MDA-MB-231 cells transfected with vector only (mock control) or IL-13Rα2 chain after treatment with IL-13 (50 ng/ml) for 24 h. Then IL-8 and GAPDH mRNA levels in serial twofold dilutions of RNA were measured by RT-PCR. GAPDH mRNA was measured to normalize the amount of mRNA in each group. (B) after stimulation with IL-13 (50 ng/ml) at various time periods (0–72 h), culture supernatant from MDA-MB-231 cells (5 × 105 cells each in 6-well plates; mock control and IL-13Rα2 transfectants) were collected and applied to ELISA plate to assess IL-8 production. The results were represented as means ± SD (n = 3).
Discussion
In this study, we demonstrate that overexpression of IL-13Rα2 chain inhibits tumorigenicity of human breast and pancreatic tumor cells in nude mice. Although there was no difference in growth between vector only transfected cells and IL-13Rα2 chain transfected cells in vitro, in nude mice, tumorigenicity was impaired in IL-13Rα2 chain overexpressing tumors. The presence of inflammatory cells, particularly neutrophils were found to correlate with the lack of tumorigenicity or significant reduction in tumor growth rate. Neutrophils were found to produce IL-13 and IL-13 had modest antitumor effect in IL-13Rα2 overexpressing tumors. Moreover, IL-13Rα2 overexpressing tumor cells produced high level of IL-8 which has been shown to reduce tumorigenicity in several tumor models. To our knowledge this is the first report that implicates cytokine receptor chain in tumorigenesis.
It is of interest to note that stability of IL-13Rα2 chain expression and tumor growth in nude mice correlated extremely well. MDA-MB-231 tumors transfected with IL-13Rα2 chain did not begin to grow until ∼day 50, after which they grew gradually. This dormancy and growth of tumors in vivo correlated with IL-13Rα2 chain expression in vitro and in vivo. MDA-MB-231 cells transfected with IL-13Rα2 chain showed decrease in IL-13Rα2 gene expression in a time-dependent manner in vitro. Similarly gene expression decreased disappearing completely on day 90 in vivo. The decrease in gene expression was further confirmed by cytotoxicity assay. IL-13-PE38QQR was highly cytotoxic to MDA-MB-231 IL-13Rα2 overexpressing cells initially. However, as the gene expression decreased, the cytotoxicity decreased. This decrease in cytotoxicity was evident in vitro–cultured cells and on tumor cells obtained from in vivo–growing tumors. In contrast to MDA-MB-231cells, PANC-1 tumor cell clones transfected with IL-13Rα2 chain showed very limited growth in nude mice, which was consistent with in vitro strong expression of this chain throughout 90-d culture. However, when residual tumor was harvested on day 90, no detectable expression of IL-13Rα2 chain was observed as assessed by RT-PCR and IL-13-PE38QQR–mediated cytotoxic assays. Since pancreatic tumor cells transfected with IL-13Rα2 chain did not form tumors, earlier time points could not be evaluated for the expression of IL-13Rα2 gene. It is possible that IL-13Rα2 gene was lost in last few days of tumor growth. These results suggest that in general, presence of IL-13Rα2 chain in MDA-MB-231 and PANC-1 tumor cells controls and prevents tumor growth. When cells loose this receptor chain, they acquire tumorigenicity in nude mice.
To reveal the mechanism by which IL-13Rα2 chain inhibited tumorigenicity in MDA-MB-231 and PANC-1 tumors, we performed histological examinations and immunofluorescence assays. We observed infiltration of inflammatory cells, particularly neutrophils and macrophages during early period of slow tumor growth suggesting that these cells may play an important role in the inhibition of tumorigenicity caused by overexpression of IL-13Rα2 chain. Moreover, we found that infiltrated neutrophils produced IL-13 that had modest antitumor effect on IL-13Rα2 chain overexpressing tumor in vitro and in vivo. IL-13 has been shown to be produced by B cells, T cells, and monocytes (41). Infiltrating inflammatory cells also produce Th2 cytokines (42, 43). In addition IL-13 has been shown to inhibit in vitro growth of renal cell carcinoma and breast tumor cells (44, 45). Gene transfer of IL-13 in tumor cells has been shown to inhibit tumorigenicity of mastocytoma cells in animal model (22). Thus, these reports support our findings that IL-13 secreted by infiltrating inflammatory cells may at least partially inhibit tumor growth of IL-13Rα2 chain overexpressing tumors. We also observed rapid initial growth of IL-13Rα2 chain transfected tumor in neutrophil depleted mice similar to control mice, and when administration of antineutrophil antibody stopped, the tumor growth decreased similar to nonneutrophil depleted mice. Because neutrophils, particularly polymorphonuclear leukocytes are known to enhance antitumor immune responses against cancers (23, 46, 47), neutrophils along with IL-13 may play some role in the inhibition of growth of IL-13Rα2 chain overexpressing tumors. Alternatively, IL-13 produced by neutrophils may induce additional factor(s) from tumor cells that may further inhibit tumor growth of IL-13Rα2 chain overexpressing tumors.
We found high level of IL-8 production from IL-13Rα2 chain overexpressing MDA-MB-231 cells. IL-8 has been shown to regulate angiogenesis and matrix metalloproteinase 9 (MMP-9) productions in tumors consequently reducing tumorigenicity and metastatic potential (20, 21). It is therefore possible that IL-8 secreted by IL-13Rα2 chain overexpressing tumor cells results in the regulation of tumorigenicity. However, Arenberg et al. observed that by injecting neutralizing antibody to IL-8 in SCID mice results in regression of non-small cell lung cancer tumor xenografts (26). Thus, IL-8 can produce contrasting effect in different tumor models. In our RT-PCR and ELISA results, IL-13 was found to further augment IL-8 expression in IL-13Rα2 transfected tumors. Taken together, our results suggest that the mechanism involved in the loss of in vivo tumorigenicity of IL-13Rα2 chain overexpressing tumor cells involve infiltration of IL-13 producing neutrophils which in turn augment IL-8 production from tumor cells for an autocrine growth inhibition.
Although there seems to be no reports that show inhibition of tumorigenicity by overexpression of one cytokine receptor subunit on the cell surface, it has been reported that some cell-surface antigens, e.g., a proliferation-inducing ligand (APRIL), heregulin (HRG), and transforming growth factor β inhibit tumorigenesis in certain types of cancers (48–50). Furthermore, cytokines like IL-4, IL-8, and IL-13 produced by inflammatory cells such as neutrophils, macrophages, and/or eosinophils have been shown to inhibit tumorigenesis (20–22, 24, 25, 42). Our results also suggest involvement of infiltrating inflammatory cells in the inhibition of tumorigenesis caused by IL-13Rα2 chain overexpression. In our previous studies, we generated several IL-13Rα2 chain transfected cancer cell clones including head and neck and prostate cancer cells, however, in vivo tumorigenicity was not impaired in these cases (31, 40). Because IL-13Rα2 chain expression level was lower in these cases, it is possible that the loss of tumorigenicity by IL-13Rα2 chain overexpression depends on the IL-13Rα2 expression level. Alternatively, various tumor types behave differently. These possibilities are being investigated.
The significance of constitutive expression of IL-13Rα2 chain in certain tumor cell types is still not known. In murine model, extracellular domain of IL-13Rα2 chain is secreted by cells and has been detected in serum and urine of mice. However, extracellular domain of this chain has not been detected in body fluids of humans (12). Based on murine studies it has been proposed that extracellular domain of IL-13Rα2 chain serves as a decoy and possibly a IL-13 carrier protein. In contrast, here we show a unique function of IL-13Rα2 chain which inhibits tumorigenicity of breast and pancreatic tumor cells. We hypothesize that IL-13Rα2 chain may control vigorous growth of tumor cells in vivo. Tumor cells may grow faster if level of expression was decreased or this chain was not expressed at all. Alternatively, constitutive expression of IL-13Rα2 chain in tumor cells may recruit Th2 cells that predominantly produce IL-13, which may help escape immune surveillance and tumor development in immunocompetent hosts. Additional studies are warranted to further unravel the mechanism of inhibition of tumor growth and exact function of IL-13Rα2 chain in the context of tumor biology.
Acknowledgments
We thank Dr. Bharat H. Joshi for providing IL-13 and IL-13-PE38QQR, and Ms. Pamela Dover for the procurement of reagents and laboratory supplies; Dr. Ken Ishii for technical assistance in immunofluorescent microscopy; Drs. Bernard Fox and Karen Elkins for helpful suggestions in neutrophil depletion experiments; and Drs. Elizabeth Shores and Abdur Razzaque of CBER/FDA for critical reading of this manuscript.
Footnotes
Abbreviation used in this paper: PE, Pseudomonas exotoxin A.
References
- 1.Minty, A., P. Chalon, J.M. Derocq, X. Dumont, J.C. Guillemot, M. Kaghad, C. Labit, P. Leplatois, P. Liauzun, B. Miloux, et al. 1993. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 36:248–251. [DOI] [PubMed] [Google Scholar]
- 2.Obiri, N.I., W. Debinski, W.J. Leonard, and R.K. Puri. 1995. Receptor for interleukin 13: interaction with interleukin 4 by a mechanism that does not involve the common γ chain shared by receptors for interleukins 2, 4, 7, 9, and 15. J. Biol. Chem. 270:8797–8804. [DOI] [PubMed] [Google Scholar]
- 3.Murata, T., N.I. Obiri, W. Debinski, and R.K. Puri. 1997. Human ovarian carcinoma cell lines express IL-4 and IL-13 receptors: comparison between IL-4- and IL-13-induced signal transduction. Int. J. Cancer. 70:230–240. [DOI] [PubMed] [Google Scholar]
- 4.Obiri, N.I., P. Leland, T. Murata, W. Debinski, and R.K. Puri. 1997. The IL-13 receptor structure differs on various cell types and may share more than one component with IL-4 receptor. J. Immunol. 158:756–764. [PubMed] [Google Scholar]
- 5.Obiri, N.I., T. Murata, W. Debinski, and R.K. Puri. 1997. Modulation of interleukin (IL)-13 binding and signaling by the γc chain of the IL-2 receptor. J. Biol. Chem. 272:20251–20258. [DOI] [PubMed] [Google Scholar]
- 6.Murata, T., S.R. Husain, H. Mohri, and R.K. Puri. 1998. Two different IL-13 receptor chains are expressed in normal human skin fibroblasts, and IL-4 and IL-13 mediate signal transduction through a common pathway. Int. Immunol. 10:1103–1110. [DOI] [PubMed] [Google Scholar]
- 7.Murata, T., N.I. Obiri, and R.K. Puri. 1998. Structure of and signal transduction through interleukin-4 and interleukin-13 receptors. Int. J. Mol. Med. 1:551–557. [DOI] [PubMed] [Google Scholar]
- 8.Aman, M.J., N. Tayebi, N.I. Obiri, R.K. Puri, W.S. Modi, and W.J. Leonard. 1996. cDNA cloning and characterization of the human interleukin 13 receptor α chain. J. Biol. Chem. 271:29265–29270. [DOI] [PubMed] [Google Scholar]
- 9.Hilton, D.J., J.G. Zhang, D. Metcalf, W.S. Alexander, N. Nicola, and T.A. Willson. 1996. Cloning and characterization of a binding subunit of the interleukin-13 receptor that is a component of the interleukin-4 receptor. Proc. Natl. Acad. Sci. USA. 93:497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miloux, B., P. Laurent, O. Bonnin, J. Lupker, D. Caput, N. Vita, and P. Ferrara. 1997. Cloning of the human IL-13Rα1 chain and reconstitution with the IL-4Rα of a functional IL-4/IL-13 receptor complex. FEBS Lett. 401:163–166. [DOI] [PubMed] [Google Scholar]
- 11.Caput, D., P. Laurent, M. Kaghad, J.M. Lelias, S. Lefort, N. Vita, and P. Ferrara. 1996. Cloning and characterization of a specific interleukin (IL)-13 binding protein structurally related to the IL-5 receptor α chain. J. Biol. Chem. 271:16921–16926. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson, D.D., M.J. Whitters, L.J. Fitz, T.Y. Neben, H. Finnerty, S.L. Henderson, R.M. O'Hara, Jr., D.R. Beier, K.J. Turner, C.R. Wood, and M. Collins. 1998. The murine IL-13 receptor α2: molecular cloning, characterization, and comparison with murine IL-13 receptor α1. J. Immunol. 161:2317–2324. [PubMed] [Google Scholar]
- 13.Murata, T., N.I. Obiri, W. Debinski, and R.K. Puri. 1997. Structure of IL-13 receptor: analysis of composition in cancer and immune cells. Biochem. Biophys. Res. Commun. 238:90–94. [DOI] [PubMed] [Google Scholar]
- 14.Husain, S.R., and R.K. Puri. 2000. Interleukin-13 fusion cytotoxin as a potent targeted agent for AIDS-Kaposi's sarcoma xenograft. Blood. 95:3506–3513. [PubMed] [Google Scholar]
- 15.Joshi, B.H., G.E. Plautz, and R.K. Puri. 2000. IL-13 receptor α chain: a novel tumor associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res. 60:1168–1172. [PubMed] [Google Scholar]
- 16.Liu, H., B.S. Jacobs, J. Liu, R.A. Prayson, M.L. Estes, G.H. Barnett, and B.P. Barna. 2000. Interleukin-13 sensitivity and receptor phenotypes of human glial cell lines: non-neoplastic glia and low-grade astrocytoma differ from malignant glioma. Cancer Immunol. Immunother. 49:319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawakami, K., J. Taguchi, T. Murata, and R.K. Puri. 2001. Interleukin-13 receptor α2 chain: an essential component for binding and internalization but not for IL-13 induced signal transduction through STAT6 pathway. Blood. 276:2673–2679. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami, K., B.H. Joshi, and R.K. Puri. 2000. Sensitization of cancer cells to interleukin 13-Pseudomonas exotoxin-induced cell death by gene transfer of interleukin 13 receptor α chain. Hum. Gene Ther. 11:1829–1835. [DOI] [PubMed] [Google Scholar]
- 19.Kawakami, K., F. Takeshita, and R.K. Puri. 2001. Identification of distinct roles for a dileucine and a tyrosine internalization motif in the interleukin-13 binding component IL-13 receptor α2 chain. J. Biol. Chem. 276:25114–25120. [DOI] [PubMed] [Google Scholar]
- 20.Lee, L.F., R.P. Hellendall, Y. Wang, J.S. Haskill, N. Mukaida, K. Matsushima, and J.P.Y. Ting. 2000. IL-8 reduced tumorigenicity of human ovarian cancer in vivo due to neutrophil infiltration. J. Immunol. 164:2769–2775. [DOI] [PubMed] [Google Scholar]
- 21.Inoue, K., J.W. Slaton, B.Y. Eve, S.J. Kim, P. Perrotte, M.D. Balbay, S. Yano, M. Bar-Eli, R. Radinsky, C.A. Pettaway, and C.P.N. Dinney. 2000. Interleukin 8 expression regulates tumorigenicity and metastasis in androgen-independent prostate cancer. Clin. Cancer Res. 6:2104–2119. [PubMed] [Google Scholar]
- 22.Lebel-Binay, S., B. Laguerre, F. Quintin-Colonna, H. Conjeaud, M. Magazin, B. Miloux, F. Pecceu, D. Caput, P. Ferrara, and D. Fradelizi. 1995. Experimental gene therapy of cancer using tumor cells engineered to secrete interleukin-13. Eur. J. Immunol. 25:2340–2348. [DOI] [PubMed] [Google Scholar]
- 23.Musiani, P., A. Allione, A. Modica, P.L. Lollini, M. Giovarelli, F. Cavallo, F. Belardelli, G. Forni, and A. Modesti. 1996. Role of neutrophils and lymphocytes in inhibition of a mouse mammary adenocarcinoma engineered to release IL-2, IL-4, IL-7, IL-10, IFN-α, IFN-γ, and TNF-α. Lab. Invest. 74:146–157. [PubMed] [Google Scholar]
- 24.Tepper, R.I., R.L. Coffman, and P. Leder. 1992. An eosinophil-dependent mechanism for antitumor effect of interleukin-4. Science. 257:548–551. [DOI] [PubMed] [Google Scholar]
- 25.Tepper, R.I., P.K. Pattengale, and P. Leder. 1989. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 57:503–512. [DOI] [PubMed] [Google Scholar]
- 26.Arenberg, D.A., S.L. Kunkel, P.J. Polverini, M. Glass, M.D. Burdick, and R.M. Strieter. 1996. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J. Clin. Invest. 97:2792–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koshiba, T., R. Hosotani, Y. Miyamoto, J. Ida, S. Tsuji, S. Nakajima, M. Kawaguchi, H. Kobayashi, R. Doi, T. Hori, et al. 2000. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin. Cancer Res. 6:3530–3535. [PubMed] [Google Scholar]
- 28.Mantovani, A. 1999. The chemokine system: redundancy for robust outputs. Immunol. Today. 20:254–257. [DOI] [PubMed] [Google Scholar]
- 29.Oshima, Y., B.H. Joshi, and R.K. Puri. 2000. Conversion of interleukin-13 into a high affinity agonist by a single amino acid substitution. J. Biol. Chem. 275:14375–14380. [DOI] [PubMed] [Google Scholar]
- 30.Debinski, W., N.I. Obiri, I. Pastan, and R.K. Puri. 1995. A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J. Biol. Chem. 270:16775–16780. [DOI] [PubMed] [Google Scholar]
- 31.Kawakami, K., M. Kawakami, B.H. Joshi, and R.K. Puri. 2001. Interleukin-13 receptor-targeted cancer therapy in an immunodeficient animal model of human head and neck cancer. Cancer Res. 61:6194–6200. [PubMed] [Google Scholar]
- 32.Murata, T., J. Taguchi, and R.K. Puri. 1998. Interleukin-13 receptor α′ but not α chain: a functional component of interleukin-4 receptors. Blood. 91:3884–3891. [PubMed] [Google Scholar]
- 33.Ameixa, C., and J.S. Friedland. 2000. Down-regulation of interleukin-8 secretion from Mycobacterium tuberculosis-infected monocytes by interleukin-4 and -10 but not by interleukin-13. Infect. Immunity. 69:2470–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obiri, N.I., G.G. Hillman, G.P. Haas, S. Sud, and R.K. Puri. 1993. Expression of high affinity interleukin-4 receptors on human renal carcinoma cells and inhibition of tumor growth in vitro by interleukin-4. J. Clin. Invest. 91:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munson, P.J., and D. Rodbard. 1980. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 107:220–239. [DOI] [PubMed] [Google Scholar]
- 36.Puri, R.K., M. Ogata, P. Leland, G.M. Feldman, and I. Pastan. 1991. Expression of high affinity IL4 receptors on murine sarcoma cells and receptor mediated cytotoxicity of tumor cells to chimeric protein between IL-4 and Pseudomonas exotoxin. Cancer Res. 51:3011–3017. [PubMed] [Google Scholar]
- 37.Kawakami, K., P. Leland, and R.K. Puri. 2000. Structure, function, and targeting of interleukin 4 receptors on human head and neck cancer cells. Cancer Res. 60:2981–2987. [PubMed] [Google Scholar]
- 38.Puri, R.K., P. Leland, N.I. Obiri, S.R. Husain, R.J. Kreitman, G.P. Haas, I. Pastan, and W. Debinski. 1996. Targeting of interleukin-13 receptor on human renal cell carcinoma cells by a recombinant chimeric protein composed of interleukin-13 and a truncated form of Pseudomonas exotoxin A (PE38QQR). Blood. 87:4333–4339. [PubMed] [Google Scholar]
- 39.Husain, S.R., N.I. Obiri, P. Gill, T. Zheng, I. Pastan, W. Debinski, and R.K. Puri. 1997. Receptor for interleukin-13 on AIDS-associated Kaposi's sarcoma cells served as a new target for a potent Pseudomonas exotoxin-based chimeric toxin protein. Clin. Cancer Res. 3:151–156. [PubMed] [Google Scholar]
- 40.Kawakami, K., S.R. Husain, R.K. Bright, and R.K. Puri. 2001. Gene transfer of interleukin-13 receptor α2 chain dramatically enhances the antitumor effect of IL-13 receptor-targeted cytotoxin in human prostate cancer xenografts. Cancer Gene Ther. 8:861–868. [DOI] [PubMed] [Google Scholar]
- 41.Brown, K.D., S.M. Zurawski, T.R. Mosmann, and G. Zurawski. 1989. A family of small inducible proteins secreted by leukocytes are members of a new superfamily that induces leukocyte and fibroblast derived inflammatory agents, growth factors, and indicators of various activation processes. J. Immunol. 142:679–687. [PubMed] [Google Scholar]
- 42.Brandt, E., G. Woerly, A.B. Younes, S. Loiseau, and M. Capron. 2000. IL-4 production by human polymorphonuclear neutrophils. J. Leukoc. Biol. 68:125–130. [PubMed] [Google Scholar]
- 43.Loetscher, P., M. Seitz, M. Baggiolini, and B. Moser. 1996. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J. Exp. Med. 184:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obiri, N.I., S.R. Husain, W. Debinski, and R.K. Puri. 1996. Interleukin 13 inhibits growth of human renal cell carcinoma cells independently of the p140 interleukin 4 receptor chain. Clin. Cancer Res. 2:1743–1749. [PubMed] [Google Scholar]
- 45.Serve, H., E. Oelmann, A. Herweg, D. Oberberg, S. Serve, B. Reufi, C. Mucke, A. Minty, E. Thiel, and W.E. Berdel. 1996. Inhibition of proliferation and clonal growth of human breast cancer cells by interleukin 13. Cancer Res. 56:3583–3588. [PubMed] [Google Scholar]
- 46.Seino, K., N. Kayagaki, K. Ohumura, and H. Yagita. 1997. Antitumor effect of locally produced CD95 ligand. Nat. Med. 3:165–170. [DOI] [PubMed] [Google Scholar]
- 47.Yamashiro, S., H. Kamohara, J.M. Wang, D. Yang, W.H. Gong, and T. Yoshimura. 2001. Phenotypic and functional change of cytokine-activated neutrophils: inflammatory neutrophils are heterogeneous and enhance adaptive immune responses. J. Leukoc. Biol. 69:698–704. [PubMed] [Google Scholar]
- 48.Rennert, P., P. Schneider, T.G. Cachero, J. Thompson, L. Trabach, S. Hertig, N. Holler, F. Qian, C. Mullen, K. Strauch, et al. 2000. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J. Exp. Med. 192:1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinstein, E.J., and P. Leder. 2000. The extracellular region of heregulin is sufficient to promote mammary gland proliferation and tumorigenesis but not apoptosis. Cancer Res. 60:3856–3861. [PubMed] [Google Scholar]
- 50.Oft, M., K.H. Heider, and H. Beug. 1998. TGFβ signaling is necessary for carcinoma cell invasiveness and metastasis. Curr. Biol. 8:1243–1252. [DOI] [PubMed] [Google Scholar]