Abstract
Cognate interaction of chemokine receptor CCR7 on lymphocytes with its ligands CCL19 and CCL21 expressed on high endothelial venules (HEVs) is essential for effective migration of T and B cells across HEVs into secondary lymphoid organs. Plt mice, which lack expression of CCL19 and CCL21-ser, both ligands for CCR7 on HEVs, as well as CCR7-deficient mice, have a defective cell migration and reduced homing of lymphocytes. FTY720, a novel immunosuppressant, causes a reduction of lymphocytes in peripheral blood and tissues and their sequestration into lymphoid tissues. In this study we demonstrate that FTY720 rescues the homing defect in both CCR7−/− mice and plt mice. After FTY720 treatment, the number of CD4+ and CD8+ T cells as well as B cells in peripheral blood is reduced while pertussis toxin–sensitive homing into peripheral lymph nodes, mesenteric lymph node, and Peyer's patches is increased. Immunohistology demonstrates that FTY720 enables these cells to enter lymphoid tissue through HEVs. Thus, our data suggest an alternative G-αi-dependent, CCR7-CCL19/CCL21-independent mechanism for lymphocyte homing through HEVs which is strongly augmented in the presence of FTY720.
Keywords: lymphocyte migration, chemokine receptor, T cell, B cell, lymphoid organs
Introduction
Migration of T and B lymphocytes into secondary lymphoid organs, encounter with antigen-presenting cells, and subsequent release into the bloodstream and peripheral tissues is required for the induction of an antigen-specific immune response. The homing of lymphocytes into LNs is tightly regulated and follows defined pathways. While memory T cells enter LNs by afferent lymphatic vessels, naive T cells as well as B cells migrate directly from the blood stream into secondary lymphoid tissue by extravasation through specialized high endothelial venules (HEVs; reference 1). This process is controlled at a molecular level by cellular interactions of adhesion molecules such as selectins and integrins and involves regulation of cellular trafficking by chemokines and their receptors (2). Chemokines are a family of small chemoattractant proteins that bind to G protein–coupled receptors expressed on target cells, thus allowing these cells to follow chemokine concentration gradients into selected tissues. Chemokines guide lymphocytes to secondary lymphoid organs and to specific microenvironments within these organs, such as T cell zone, follicles, etc., thereby coordinating the appropriate interactions of T cells, dendritic cells (DCs), and B cells required for the initiation of an immune response (3, 4).
Specifically, it has been shown that the chemokine receptor CCR7 and its ligands, the chemokines EBV-induced molecule 1 ligand chemokine (ELC/CCL19) and secondary lymphoid tissue chemokine (SLC/CCL21) play a decisive role in the migration of T and B cells through HEVs into LNs and to specialized microenvironments within these tissues. (3, 5). CCL19 and CCL21 are expressed on HEVs in Peyer's patches (PPs) and peripheral LNs (PLNs) as well as on stroma cells and DCs within the T cell zones of LNs, PPs, and spleen (6, 7). Analysis of transgenic CCR7-deficient mice (CCR7−/− mice) revealed significantly increased numbers of T cells in peripheral blood, while the number of T cells in LNs is reduced. It could be demonstrated that the migration of DCs, T, and B cells into PLNs and PPs is impaired (3). Similarly, a mutant mouse, the plt (paucity of LN T cells) mouse, shows increased numbers of T cells in peripheral blood and reduced numbers of T cells in LNs. These mice fail to express detectable levels of CCL19 and CCL21-ser in lymphatic organs and suffer from a homing defect resembling the defect of CCR7−/− mice (5).
FTY720 (2-amino-2-[2-(4-octylphenyl) ethyl] propane-1,3-diol) is a novel immunosuppressant that is thought to act by accelerating lymphocyte homing into LNs and PPs and sequestering circulating lymphocytes within these tissues (8, 9). FTY720 is a synthetic immunosuppressant obtained by chemical modification of a product isolated from filamentous fungus Isaria sinclairii and shows potent immunosuppressive effects in skin and cardiac allograft models (10). Treatment with FTY720 induces a marked reduction of peripheral blood lymphocytes, especially T cells (9).
Regulation of lymphocyte trafficking by chemokines is to a certain degree redundant: some chemokines bind several chemokine receptors, while some receptors have several, apparently functionally equivalent ligands. Furthermore, lymphocyte trafficking is also regulated by and dependent upon interactions of various adhesion molecules. How these mechanisms depend on one another and to what degree they are redundant is not entirely clear. In this study we show that FTY720 treatment of CCR7−/− mice and plt mice restores the lymphocyte homing defect in a pertussis toxin–sensitive manner, thus pointing to an alternative, CCR7-CCL19/CCL21-independent mechanism for lymphocyte migration via HEVs into secondary lymphoid tissue.
Materials and Methods
CCR7− / − mice on a mixed Balb/c-129SV background and corresponding wild-type mice as well as plt mice on a C57BL6 background and C57BL6 mice were maintained under specific pathogen-free conditions. FTY720 (Novartis) was dissolved in distilled water and administered orally by gavage or in drinking water. Control animals received the vehicle only. CFDA-SE (carboxyfluorecein diacetate succinimidyl ester [CFSE]) was obtained from Molecular Probes. The antibodies used for flow cytometry and immunohistology have been described (3, 4).
Cell Counts and Flow Cytometry.
Peripheral blood was collected by retro-orbital venous plexus sampling into tubes containing sodium citrate. Erythrocytes were removed by NH4Cl lysis. Single cell suspensions of lymphoid organs were prepared by mincing and passing through nylon mesh. Cell counts were determined using a Neubauer chamber. Differential cell counts of CD4+, CD8+, B220+, CD11b+, and CD43+ cells were analyzed by flow cytometry.
To analyze migration patterns of lymphocytes in vivo, CFDA-SE–labeled lymphocytes were injected into recipient animals and their tissue distribution was then evaluated by immunohistology or differential flow cytometry of single cell suspension of isolated organs. Lymphocytes were isolated by preparing single cell suspensions from spleen and MLNs of either CCR7−/− or wild-type mice. Cells were labeled for 6 min at 37°C with 50 μM CFDA-SE. Washed cells were suspended in RPMI medium and treated in vitro with FTY720 and/or pertussis toxin for the indicated time periods at 37°C, washed, counted, and injected into retro-orbital venous plexus of recipient mice. Recipient mice were killed after the indicated time periods and lymphoid organs were removed and single cell suspensions were prepared. Differential cell counts were analyzed by flow cytometry using antibodies specific for CD4, CD8, or B220. Based on the green fluorescence of CFDA-SE labeling and the far red labeling of antibodies, the percentage of transferred CD4+ T cells, CD8+ T cells, or B cells was determined.
To determine the localization of transferred cells within lymphoid tissues, lymphoid organs were removed from recipient mice, embedded in Tissue-Tek® (Vogel Giessen) and snap frozen on liquid nitrogen. Cryostat sections (8-μm thick) on slides were stained for immunohistology as described earlier (3, 4).
Results and Discussion
Hallmark of the action of FTY720 is a reduction of lymphocytes in peripheral blood and tissues and their sequestration into lymphoid tissues (9). As CCR7−/− mice have a defective cell migration and reduced homing of lymphocytes (3), we were interested whether FTY720 has an effect on the homing of CCR7-deficient lymphocytes.
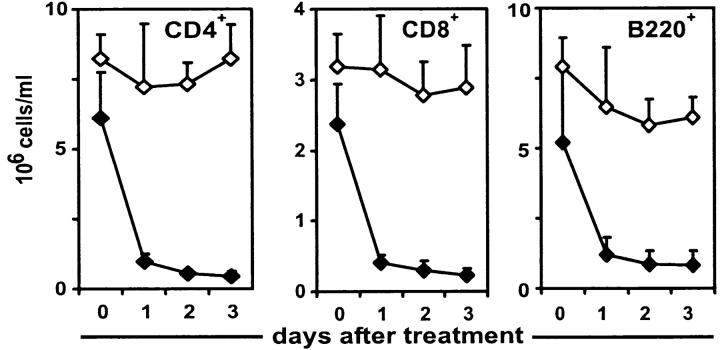
To test whether the FTY720-induced reduction of peripheral lymphocytes is dependent on CCR7-mediated homing into lymphoid organs, CCR7−/− mice and wild-type controls were given FTY720 orally over a period of 72 h and the numbers of CD4+ T cells, CD8+ T cells, and B220+ cells in peripheral blood were determined by cell counting and flow cytometry. Surprisingly, the lymphocyte homing defect of CCR7−/− mice was alleviated by FTY720 treatment. Compared with wild-type mice, the reduction of lymphocytes in peripheral blood of CCR7−/− mice follows delayed kinetics and is not as complete: whereas in wild-type mice a nearly complete disappearance of CD4+ T cells could be observed after 24 h of FTY720 treatment, CD4+ cell counts in CCR7−/− mice were continually reduced to ∼10% over a period of 72 h (Fig. 1 A). At the same time flow cytometry with staining of CD43 and CD11b revealed neutrophil and macrophage populations were not affected by FTY720 (data not shown). Analysis of the short term effects of FTY720 treatment confirmed that both CD4+ T cells and B cells of wild-type mice left the bloodstream noticeably faster than those of CCR7−/− mice (Fig. 1 B), thus suggesting both a CCR7-dependent as well as an independent component of FTY-mediated homing of lymphocytes.
Figure 1.
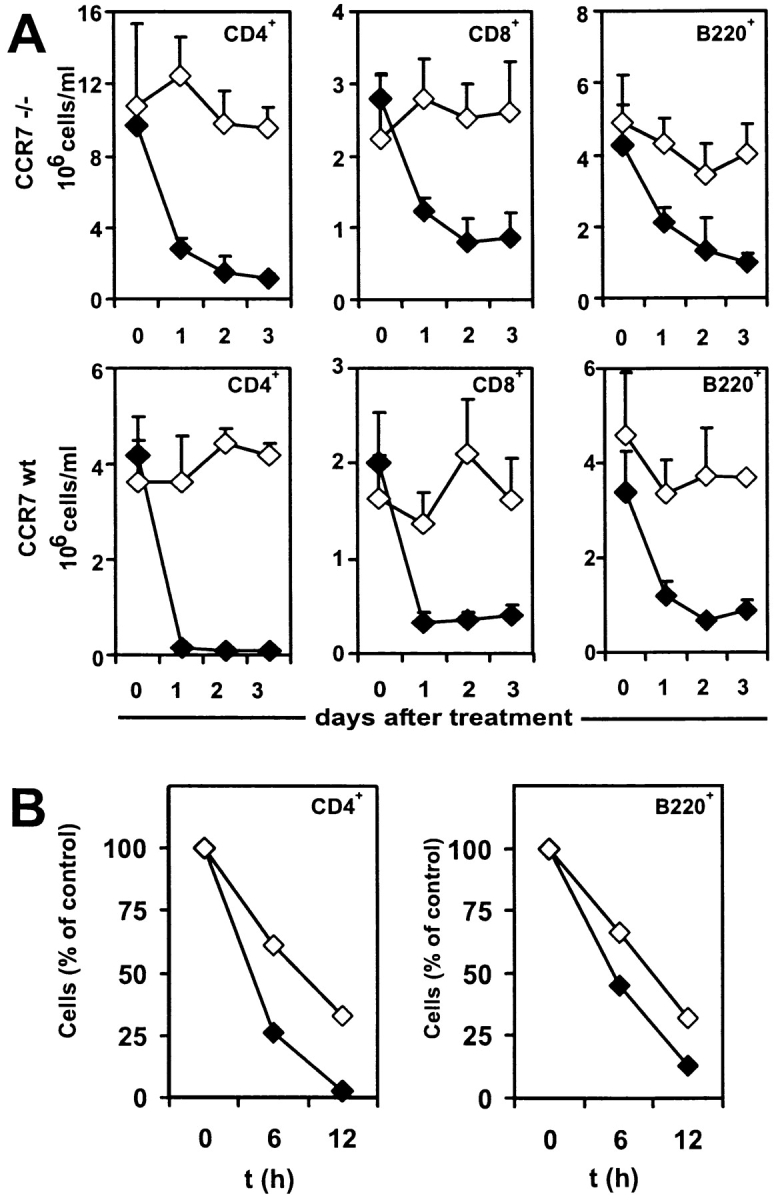
FTY720 treatment reduces lymphocyte counts in CCR7−/− mice. Peripheral blood was taken before FTY720 administration and at indicated time points afterwards and analyzed by cell counting and flow cytometry. (A) CCR7−/− and wild-type mice were given FTY720 in drinking water (2 μg/ml; closed symbols) or water only (open symbols). Mean values of five animals each are represented and error bars indicate standard deviation. (B) CCR7−/− and wild type mice were given FTY720 orally once by gavage (6 μg/animal) and then in drinking water (2 μg/ml) or vehicle only for control animals. Mean values of four animals each are represented, and cell number is expressed as percentage of matching untreated control animals.
To analyze homing of lymphocytes to lymphatic tissue in CCR7−/− compared with wild-type mice, animals were treated with FTY720 and differential cell counts in secondary lymphoid organs were analyzed. The homing of CD4+ T cells was affected the most by FTY720. A dramatic, two- to threefold increase in the number of CD4+ T cells in PLNs and PPs could be observed after FTY720 treatment of CCR7−/− mice (Fig. 2). In wild-type mice the numbers of CD4+ T cells in PLN increased by ∼50%. This lesser increase compared with CCR7−/− mice is probably due to the fact that the PLNs of wild-type mice harbor much larger numbers of lymphocytes than CCR7−/− mice (Fig. 2). In terms of absolute numbers of increased CD4+ T cells in LNs the effects of FTY720 in CCR7−/− mice and wild-type mice were on a similar scale: on average 5.8 ± 1.7 × 105 CD4+ T cells/LN migrated into PLNs of CCR7−/− mice, 6.4 ± 3.4 × 105 CD4+ T cells/LN migrated into PLNs of wild-type mice (average of six PLN each from three animals ± SD). On CD8+ T cells the effects of FTY720 were similar, though not quite as strong as on CD4+ T cells.
Figure 2.
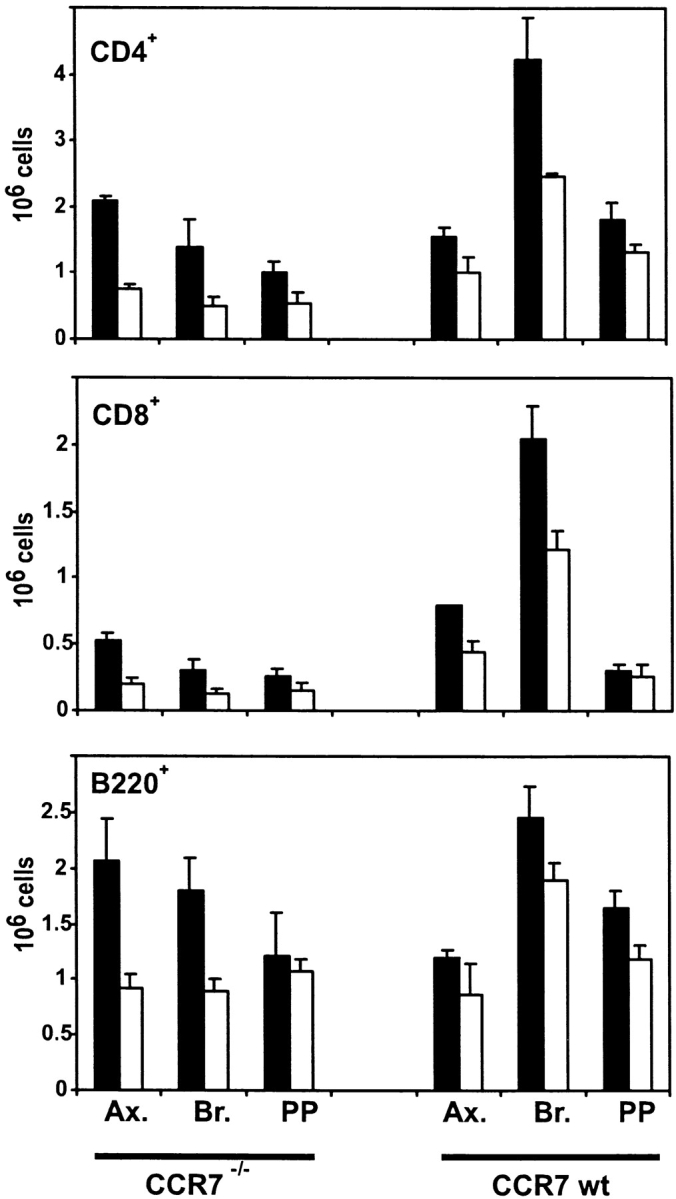
FTY720 treatment increases homing of lymphocytes into PLNs and Peyer's patches in CCR7−/− mice. CCR7−/− and wild-type mice were given FTY720 (black bars) orally once by gavage (6 μg/animal) and then in drinking water (2 μg/ml) or vehicle only (white bars). After 18 h axilary LN (Ax.), brachial LN (Br.), and Peyer's patches (PP) were removed by dissection, and differential cell counts from single cell suspensions were determined by cell counting and flow cytometry. Mean values of six organs from three animals ± SD are shown.
To determine whether CCR7-deficient lymphocytes migrate into LNs through HEVs or afferent lymphatics, splenocytes from CCR7−/− mice were labeled with CFSE, treated with FTY720 or medium only ex vivo, and injected intravenously into wild-type recipient mice. Counterstaining of HEVs on histological sections revealed that labeled, FTY720-treated CCR7-deficient donor cells appeared in lymphoid tissue surrounding HEVs (Fig. 3 A) while untreated cells were largely prevented from entering lymphoid organs (Fig. 3 B). Furthermore, the migration of labeled, CCR7-deficient donor cells through the endothelial walls could be visualized, thus demonstrating that FTY720 enables lymphocytes to enter lymphoid tissue through HEVs independently of CCR7 (Fig. 3 A, insert). As the number of lymphocytes in PLNs and PPs was increased by FTY720 treatment, it was of interest to see if the structure and distribution of lymphocytes within these organs was altered. Within the scope of our experiments the microarchitecture of LNs and spleen apparently remained unaltered after FTY720 treatment (compare Fig. 3, C and D). Likewise, no changes in lymphoid organ structure after FTY720 treatment could be detected in wild-type mice (not shown). This suggests that although FTY720 can restore lymphocyte trafficking via CCR7-independent mechanisms it is not sufficient to substitute the requirement for CCR7 for the maintenance of normal LN architecture.
Figure 3.
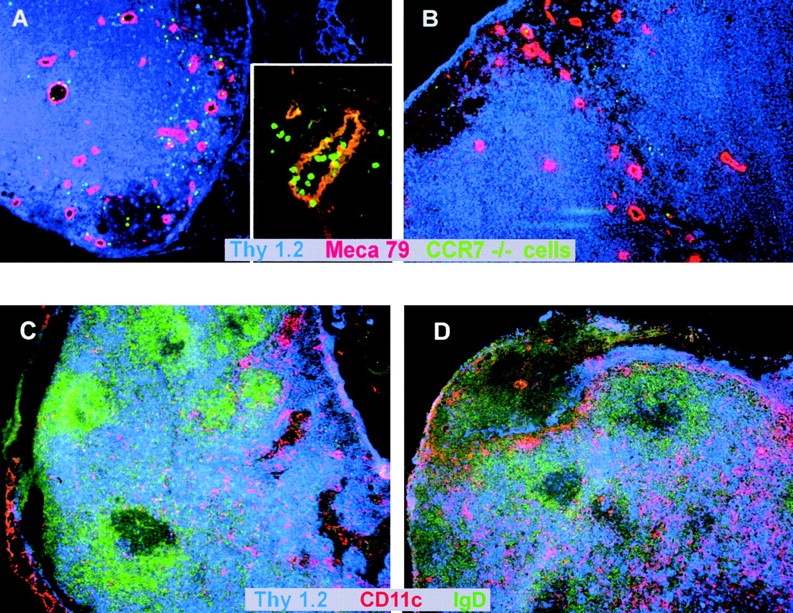
(A and B) CCR7−/− lymphocytes are able to migrate through HEVs into secondary lymphoid tissue after FTY720 treatment. Splenocytes from a CCR7−/− donor animal were labeled with CFDA-SE. Cells were treated ex vivo with FTY720 (0.5 μM, 37°C, 2 h) or incubated in medium only (37°C, 2 h) and injected into wild-type recipient mice. After 2.5 h, LNs were obtained by dissection and cryostat sections were prepared. HEVs were stained with MECA-79 mAb and counterstaining was against CD90 (Thy1). (A) FTY720-treated CCR7−/− cells migrate through HEVs (insert, 40× original magnification) and into surrounding tissue (large panel, 10× original magnification). (B) Untreated CCR7−/− cells do not migrate into tissue surrounding HEVs (10× magnification). Representative results of independent experiments with four recipient animals each (treated and untreated) are shown. (C and D) Increased numbers of T cells migrate into PLNs of CCR7−/− mice but their tissue distribution is not altered after FTY720 treatment. CCR7−/− mice were treated with FTY720 (2 μg/ml) (C) or vehicle only (D) for 10 d. LNs were obtained by dissection and cryostat sections were prepared. T cells were stained with anti-Thy 1-Cy5, B cells were stained with anti-IgD-FITC, and DCs were stained with CD11c-biotin/streptavidin-Cy3.
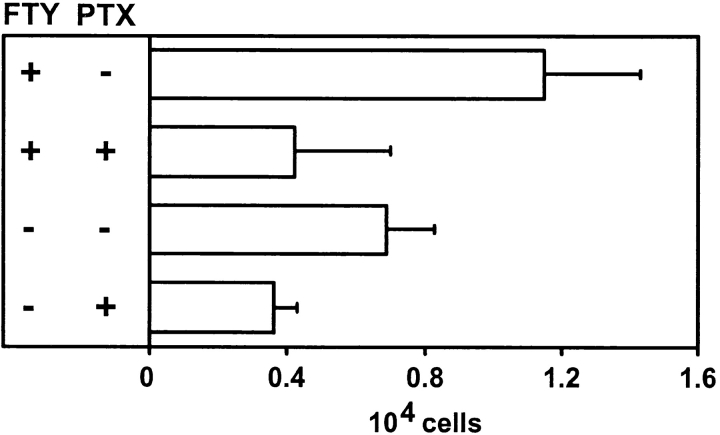
To test whether CCR7-independent homing of lymphocytes is mediated by G protein–coupled receptors, splenocytes from CCR7−/− mice were labeled with CFSE, treated ex vivo with FTY720 and/or pertussis toxin, and injected intravenously into CCR7−/− recipient mice that had been given a single oral dose of FTY720 or vehicle control. After 4 h the lymphoid organs of recipient mice were removed and percentage of labeled infiltrated CD4+ cells in peripheral LNs was determined by flow cytometry. After pertussis toxin treatment the number of infiltrated cells in FTY720-treated recipient animals was reduced from ∼1.2 × 104 cells/LN to 0.4 × 104 cells and in vehicle treated animals from 0.8 × 104 cells to 0.4 × 104 cells (Fig. 4). These data suggest that FTY720 induces lymphocyte homing mediated by G-αi–coupled receptors.
Figure 4.
FTY720-induced homing of CCR7−/− T cells is pertussis toxin sensitive. Splenocytes from CCR7−/− donor animals were labeled with CFDA-SE. Cells were treated ex vivo with FTY720 (0.5 μM, 37°C, 3 h), and/or pertussis toxin (PTX; 20 ng/ml, 37°C, 3 h) or incubated in medium only (37°C, 3 h) and injected into wild-type recipient mice. Recipient mice receiving FTY720 treated cells were given FTY720 once orally by gavage (6 μg/animal) 2 h before transfer of labeled cells. Recipient mice receiving cells not treated with FTY720 were given vehicle only by gavage. 4 h after the transfer, LNs were obtained by dissection and the number of CD4+ CFSE stained T cells was determined by cell counting and flow cytometry. Mean values of six LNs from three animals are shown, error bars indicate SD.
Plt mice are characterized by a lack of expression in secondary lymphoid organs and on HEVs of the chemokines CCL19 and CCL21-ser both of which are ligands for CCR7 and on HEV. As we observed that FTY720 induces lymphocyte homing independently of CCR7, it was of interest to determine whether this effect was also independent of the known ligands of CCR7. Plt mice and wild-type controls were given FTY720 orally over a period of 72 h and the numbers of CD4+ T cells, CD8+ T cells, and B220+ cells in peripheral blood were determined by differential cell counts. The number of CD4+, CD8+, and B220+ lymphocytes were markedly decreased after FTY720 treatment in peripheral blood of plt mice with a similar kinetic as that of CCR7-deficient mice while cell counts in untreated controls remained constant (Fig. 5). The similar kinetics and level of reduction of peripheral lymphocyte counts in plt and CCR7−/− mice lead us to the assumption that FTY720 acts in both these animal models in a similar fashion, although it has not yet been proven formally that FTY720 accelerates lymphocyte homing in plt mice as well. Taken together, these results demonstrate that FTY720 promotes lymphocyte homing in a CCR7-CCL19/CCL21-independent manner.
Figure 5.
FTY720 treatment reduces lymphocyte counts in plt mice. Plt mice were given FTY720 in drinking water (2 μg/ml; closed symbols) or water only (open symbols). Peripheral blood was taken before FTY720 administration and at indicated time points afterwards and analyzed by cell counting and flow cytometry. Mean values of three animals each are represented and error bars indicate SD.
Treatment of CCR7−/− and plt mice with FTY720, a novel immunosuppressant, resulted in a marked decrease of all lymphocytes in peripheral blood. Compared with wild-type mice subjected to the same treatment protocol, the reduction of lymphocytes in CCR7-deficient and plt mice followed slower kinetics. Also, the overall level of reduction in wild-type mice was higher than in CCR7−/− mice. Thus, FTY720-induced lymphocyte migration seems to have a CCR7-dependent as well as a CCR7-independent component. Similarly, it could be demonstrated that after FTY720 treatment homing into secondary lymphoid tissues was increased in CCR7−/− mice. These observations are in accordance with previous studies, showing that FTY720 induces homing of lymphocytes into PLNs and PPs, but does not impede the generation of an immune response within secondary lymphoid organs (11, 12). Our observations suggest that aside from accelerating lymphocyte homing FTY720 does not induce gross alterations of the architecture of secondary lymphoid organs. Taken together, these findings imply that FTY720 acts indeed by sequestering lymphocytes in secondary lymphoid organs, thereby preventing their migration toward sites of infection and allograft rejection.
Furthermore, the data show that the FTY720-induced, CCR7-independent homing into PLNs occurs at the HEVs. Homing of naive T cells and B cells into these tissues is thought to proceed in a multistep fashion requiring the interactions of selectins, integrins, and chemokine receptors with their corresponding ligands. As yet, the molecular target of FTY720 is unknown. Homing of CD62L+ (L-selectin) T cells into PPs is increased by FTY720 but expression levels of CD62L, CD49d (VLA4), and CD11a (LFA1) were not altered by FTY720 (9). The fact that FTY720-induced, CCR7-independent homing into PLNs is sensitive to pertussis toxin demonstrates that FTY720 acts in a specific, receptor-dependent manner. As FTY720 is an amphiphilic molecule, it cannot be excluded that unspecific lipophilic interactions of the hydrocarbon moiety of FTY720 with components of the cell membrane also contribute to the mechanisms of this drug (10). Using pertussis toxin as an inhibitor, it was also revealed that there is a basal, CCR7-independent trafficking of lymphocytes through HEVs, which is mediated by G-αi-coupled receptors. Along with the different kinetics of lymphocyte homing of CCR7−/− and wild-type mice, this shows there are additional mechanisms responsible for lymphocyte homing, in addition to CCR7-mediated homing into secondary lymphoid organs. This is also underlined by the fact that the effects of FTY720 treatment vary for different cell populations: CD4+ T cells are affected the most, B cells to a lesser degree. Interestingly, both in CCR7−/− and plt mice the overall number of B cells in secondary lymphoid organs is not reduced, as opposed to T cells (3, 5). B cells are known to enter LNs through HEVs, but apparently are able to do so independently of CCR7 and CCL19/ CCL21. Perhaps this CCR7-independent mechanism is amplified by FTY720 treatment, which would also explain the observed increase in B cell homing.
Other chemokine receptors possibly involved in CCR7 independent homing of T and B lymphocytes include CXCR3, CXCR4 (which is of specific interest since it is also expressed by naive T cells), as well as CCR5 and CCR6. While these chemokine receptors are expressed by T and B cells, none of their known ligands are expressed on HEVs. CXCL9 (Mig), CXCL10 (IP-10), and CXCL11 (I-TAC), all ligands for CXCR3, are IFN-inducible chemokines attracting T cells to inflammatory sites (13). SDF-1, the chemokine recognized by CXCR4 seems not to be expressed on HEVs (our unpublished observation), and the main ligands for CCR5, CCL3 (macrophage inflammatory protein [MIP]-1α), CCL4 (MIP-1β), CCL5 (RANTES [regulated upon activation, normal T cell expressed and secreted]), are involved in inflammatory processes and are also not found on HEVs. Similarly, CCL20 (Mip-3α, LARC), which is bound by CCR6 also directs inflammatory cell migration but is not known to be involved in lymphocyte homing to LNs (14). This suggests, that FTY720 might act on the chemokine system as a whole rather than on a specific chemokine receptor. This conclusion is also supported by the following observations: (a) while the effect of FTY720 on lymphocyte chemotaxis in vitro is most pronounced toward CCL19 and CCL21, it has also been shown that the chemotaxis to other chemokines is also increased by FTY720 (unpublished data), and (b) although FTY720 treatment augments lymphocyte chemotaxis toward CCL19/CCL21 in vitro (unpublished data), it shows an even more dramatic effect in vivo in mice lacking CCR7 or CCL19 and CCL21.
Alternatively, mechanisms independent of the chemokine system might be involved. A host of other receptors mediate cell motility, such as Sphingosine-1 phosphate receptors, opioid receptors, and others which share G-αi–coupled signaling as a common denominator (15, 16). Among these, Sphingosine-1 phosphate receptors are of special interest, as some of them, such as EDG-6, are expressed specifically by various lymphocyte subsets and are known to mediate activation of lymphocytes (17).
These findings might have potentially interesting consequences for further therapeutic uses of FTY720: it is known that some cytotoxic CCR7-negative effector T cells are unable to enter secondary lymphoid organs, and that these organs are sites of persistence and active replication of lymphotropic viruses, such as HIV,CMV, EBV, or measles virus (18, 19, 20). Apparently, these viruses are able to utilize these sites as part of their immune evasion strategy (18, 21). It would be of interest to see if FTY720 treatment enables effector T cells to reenter into secondary lymphoid organs and if these cells mount an antiviral immune response at the site of viral replication. Further studies are required to find out whether the immunosuppressive effect of FTY720 is solely due to the sequestration of lymphocytes, or whether it interferes with additional lymphocyte activation pathways.
In summary, our data revealed a novel G-αi–coupled, CCR7-CCL19/CCL21–independent mechanism of lymphocyte homing through HEVs which is strongly enhanced in the presence of the immunosuppressant FTY720. This sheds new light on the mechanism through which FYT720 acts. At the same time, it poses new questions which need to be addressed to understand how different mechanisms cooperate to regulate the homing of various lymphocyte subsets.
Acknowledgments
We thank I. Goldberg, M. Manoharan, and B. Schreiber for expert technical assistance.
G. Henning and L. Ohl contributed equally to this work.
References
- 1.Mackay, C.R., W.L. Marston, and L. Dudler. 1990. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J. Exp. Med. 171:801–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Springer, T.A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 76:301–314. [DOI] [PubMed] [Google Scholar]
- 3.Förster, R., A. Schubel, D. Breitfeld, E. Kremmer, I. Renner-Müller, E. Wolf, and M. Lipp. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 99:23–33. [DOI] [PubMed] [Google Scholar]
- 4.Förster, R., E.A. Mattis, E. Kremmer, E. Wolf, G. Brem, and M. Lipp. 1996. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 87:1037–1047. [DOI] [PubMed] [Google Scholar]
- 5.Nakano, H., S. Mori, H. Yonekawa, H. Nariuchi, A. Matsuzawa, and T. Kakiuchi. 1998. A novel mutant gene involved in T-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. Blood. 91:2886–2895. [PubMed] [Google Scholar]
- 6.Campbell, J.J., E.P. Bowman, K. Murphy, K.R. Youngman, M.A. Siani, D.A. Thompson, L. Wu, A. Zlotnik, and E.C. Butcher. 1998. 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3beta receptor CCR7. J. Cell Biol. 141:1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitfeld, D., L. Ohl, E. Kremmer, J. Ellwart, F. Sallusto, M. Lipp, and R. Förster. 2000. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192:1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinkmann, V., D. Pinschewer, K. Chiba, and L. Feng. 2000. FTY720: a novel transplantation drug that modulates lymphocyte traffic rather than activation. Trends Pharmacol. Sci. 21:49–52. [DOI] [PubMed] [Google Scholar]
- 9.Yanagawa, Y., Y. Masubuchi, and K. Chiba. 1998. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats, III. Increase in frequency of CD62L-positive T cells in Peyer's patches by FTY720-induced lymphocyte homing. Immunology. 95:591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiuchi, M., K. Adachi, T. Kohara, M. Minoguchi, T. Hanano, Y. Aoki, T. Mishina, M. Arita, N. Nakao, M. Ohtsuki, et al. 2000. Synthesis and immunosuppressive activity of 2-substituted 2-aminopropane-1,3-diols and 2-aminoethanols. J. Med. Chem. 43:2946–2961. [DOI] [PubMed] [Google Scholar]
- 11.Pinschewer, D.D., A.F. Ochsenbein, B. Odermatt, V. Brinkmann, H. Hengartner, and R.M. Zinkernagel. 2000. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J. Immunol. 164:5761–5770. [DOI] [PubMed] [Google Scholar]
- 12.Yanagawa, Y., Y. Hoshino, H. Kataoka, T. Kawaguchi, M. Ohtsuki, K. Sugahara, and K. Chiba. 1999. FTY720, a novel immunosuppressant, prolongs rat skin allograft survival by decreasing T-cell infiltration into grafts. Transplant. Proc. 31:1227–1239. [DOI] [PubMed] [Google Scholar]
- 13.Hancock, W.W., W. Gao, V. Csizmadia, K.L. Faia, N. Shemmeri, and A.D. Luster. 2001. Donor-derived IP-10 initiates development of acute allograft rejection. J. Exp. Med. 193:975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook, D.N., D.M. Prosser, R. Förster, J. Zhang, N.A. Kuklin, S.J. Abbondanzo, X.D. Niu, S.C. Chen, D.J. Manfra, M.T. Wiekowski, et al. 2000. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 12:495–503. [DOI] [PubMed] [Google Scholar]
- 15.Wang, F., J.R. Van Brocklyn, J.P. Hobson, S. Movafagh, Z. Zukowska-Grojec, S. Milstien, and S. Spiegel. 1999. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J. Biol. Chem. 274:35343–35350. [DOI] [PubMed] [Google Scholar]
- 16.Ruff, M.R., S.M. Wahl, S. Mergenhagen, and C.B. Pert. 1985. Opiate receptor-mediated chemotaxis of human monocytes. Neuropeptides. 5:363–366. [DOI] [PubMed] [Google Scholar]
- 17.Gräler, M.H., G. Bernhardt, and M. Lipp. 1998. EDG6, a novel G-protein-coupled receptor related to receptors for bioactive lysophospholipids, is specifically expressed in lymphoid tissue. Genomics. 53:164–169. [DOI] [PubMed] [Google Scholar]
- 18.Chen, G., P. Shankar, C. Lange, H. Valdez, P.R. Skolnik, L. Wu, N. Manjunath, and J. Lieberman. 2001. CD8 T cells specific for human immunodeficiency virus, Epstein-Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood. 98:156–164. [DOI] [PubMed] [Google Scholar]
- 19.Soderberg-Naucler, C., and J.Y. Nelson. 1999. Human cytomegalovirus latency and reactivation - a delicate balance between the virus and its host's immune system. Intervirology. 42:314–321. [DOI] [PubMed] [Google Scholar]
- 20.Sallusto, F., D. Lenig, R. Förster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712. [DOI] [PubMed] [Google Scholar]
- 21.Champagne, P., G.S. Ogg, A.S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G.P. Rizzardi, S. Fleury, M. Lipp, et al. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 410:106–111. [DOI] [PubMed] [Google Scholar]