Abstract
Natural killer (NK) T cells recognize lipid antigens in the context of the major histocompatibility complex (MHC) class 1–like molecule CD1 and rapidly secrete large amounts of the cytokines interferon (IFN)-γ and interleukin (IL)-4 upon T cell receptor (TCR) engagement. We have asked whether NK T cell activation influences adaptive T cell responses to myelin antigens and their ability to cause experimental autoimmune encephalomyelitis (EAE), a model for multiple sclerosis. While simultaneous activation of NK T cells with the glycolipid α-galactosylceramide (α-GalCer) and myelin-reactive T cells potentiates EAE in B10.PL mice, prior activation of NK T cells protects against disease. Exacerbation of EAE is mediated by an enhanced T helper type 1 (Th1) response to myelin basic protein and is lost in mice deficient in IFN-γ. Protection is mediated by immune deviation of the anti-myelin basic protein (MBP) response and is dependent upon the secretion of IL-4. The modulatory effect of α-GalCer requires the CD1d antigen presentation pathway and is dependent upon the nature of the NK T cell response in B10.PL or C57BL/6 mice. Because CD1 molecules are nonpolymorphic and remarkably conserved among different species, modulation of NK T cell activation represents a target for intervention in T cell–mediated autoimmune diseases.
Keywords: NK T cells, CD1d, experimental autoimmune encephalomyelitis, immunotherapy, α-galactosylceramide
Introduction
Innate pathways of immunity are crucial in the upregulation of costimulatory molecules on antigen presenting cells as well as in providing an initial cytokine milieu necessary for the development of acquired immunity. NK T cells that rapidly secrete cytokines can influence the outcome of immune responses, not only in infectious but also in autoimmune diseases. NK T cells recognize lipid antigens in the context of CD1d molecules, which are nonpolymorphic, MHC class I like, and β2-microglobulin dependent (1, 2). The CD1 proteins are encoded by five distinct genes (isotypes) and can be categorized into two groups: group 1 comprises CD1a, CD1b, and CD1c and is present in humans but not in rodents. Group 2, the CD1d molecule, is primarily expressed by dendritic cells, macrophages, subsets of B cells, thymocytes, and by hepatocytes. CD1e does not belong to either group and may not be functional. Human and murine CD1d share significant sequence homology (1, 2). The main T cell subset associated with CD1d recognition is the NK T cells, most of which express a TCR encoded by an invariant TCR α chain (formed by a Vα14-Jα18 rearrangement in mice and a homologous Vα24-Jα15 rearrangement in humans.) The invariant Vα is coexpressed with one or a few Vβs, although in mice these have very diverse CDR3 regions (3–5).
Activation of NK T cells in vivo promptly induces activation of both innate and adaptive responses and induces activation of NK cells, dendritic cells, T cells, and B lymphocytes, a characteristic of strong adjuvants. Upon activation, NK T cells also secrete large amounts of cytokines characteristic of both type 1 (IFN-γ) and type 2 (IL-4, IL-13) responses (1, 2). Thus, NK T cells have been implicated in various immune responses: in protection against pathogens, such as Leishmania major (6); in tumor immunity (7–9); in tolerance associated with the anterior chamber of eye and tissue grafts (10, 11); and in autoimmunity (12–23). The number as well as cytokine phenotype of NK T cells is altered in several autoimmune experimental animals as well as in humans with diabetes or multiple sclerosis.
α-galactosylceramide (α-GalCer),* a glycolipid originally isolated from a marine sponge, is recognized by nearly all the mouse and human NK T cells with invariant α chain TCR rearrangements (3–5). α-GalCer is a potent activator of NK T cells both in vivo and in vitro. Recently it has been suggested that the recognition of autologous lipids rather than microbial ligands is the primary function of CD1d-restricted T cells (24). Although CD1d-restricted self-antigens have not yet been identified, several observations suggest that CD1d-reactive T cells are autoreactive and that they exhibit the phenotype of activated memory cells (1, 25). In vivo activation of Vα14 NK T cells by administration of α-GalCer in C57BL/6 mice, results in the polarization of adaptive immune response toward Th2 (26, 27).
EAE is a prototypic CD4 T cell–mediated autoimmune disease, characterized by inflammation and demyelination in the central nervous system accompanied by paralysis following immunization with myelin antigens, for example myelin basic protein (MBP) and myelin oligodendrocyte protein (MOG). EAE is considered to be an instructive model for the human demyelinating disease multiple sclerosis (MS) because they both share many pathological and immune dysfunctions (28). A majority of the MBP-primed effector CD4 T cells which mediate EAE in B10.PL mice recognize the NH2-terminal peptide MBPAc1–9 and predominantly use the TCR Vβ8.2 gene segment (29, 30). Generally EAE is transitory or monophasic in B10.PL mice, and most mice spontaneously recover following expansion of regulatory CD4 and CD8 T cells recognizing distinct determinants on the Vβ8.2 chain (31–34). Although the regulation and function of individual cytokines is complex, most experimental observations are consistent with the idea that myelin antigen-reactive Th1 cells are encephalitogenic whereas the antigen-reactive Th2 response is protective (35–37). Th1 cells secrete IL-2 and IFN-γ, whereas Th2 cells secrete IL-4, IL-5, IL-10, and IL-13.
Here we have examined the effect of activation of NK T cells by α-GalCer on the response to myelin antigens and on the course of EAE in B10.PL and in C57BL/6 mice. Our findings demonstrate that the timing of activation of NK T cells by α-GalCer has a dramatic effect on the cytokine secretion profile of MBP-reactive T cells as well as on the clinical course of EAE. While coimmunization of lipid with myelin antigens potentiates disease in B10.PL mice, it prevents EAE in C57BL/6 mice. However, prior immunization prevents EAE in both strains. Using IFN-γ2/− mice and IL-4–depleted mice, we show here that these cytokines are involved in the α-GalCer–mediated modulation of disease. Furthermore α-GalCer immunization does not influence EAE in CD1d-deficient mice. These data suggest that appropriate activation of invariant NK T cells provides a useful target for intervention in autoimmune demyelinating diseases.
Materials and Methods
Mice.
Female B10.PL and C57BL/6 mice were purchased from The Jackson Laboratory. C57BL/6.CD1d−/− mice were originally generated in the laboratory of Dr. L. Van Kaer. B10.PL.CD1d−/− and B10.PLCD1d+/+ mice were bred in our colony. B10.PL.IFN-γ2/− mice were obtained from the laboratory of Dr. Garrison Fathman (Stanford University, Stanford, CA) and bred in our colony. All mice were maintained under specific pathogen-free conditions at the vivarium of the La Jolla Institute for Allergy and Immunology (LIAI).
Reagents.
MBP was purified from guinea pig brains and spinal cords, as described previously (38). MBPAc1–9 (Ac-ASQKRPSQR) was synthesized in our own facility at LIAI. MOG peptide 35–55 (MEVGWYRSPFRVVHLYRNGK) was synthesized by the Biopolymer Facility at Harvard Medical School (Boston, MA). Glycolipids, α-GalCer or KRN7000 and β-GalCer were synthesized by the Pharmaceutical Research Laboratory of Kirin Brewery Co. (Gunma, Japan) as described previously (3). A stock solution of α-GalCer or β-GalCer was diluted to 220 μg/ml in 0.5% polysorbate-20 and 0.9% NaCl (vehicle). PE-labeled mouse CD1d tetramer complexes loaded with α-GalCer were generated as described (39).
Flow Cytometry Analysis.
For the in vivo activation/depletion of NK T cells, mice were injected intravenously with 4.4 μg of α-GalCer in 200 μl PBS or an equivalent volume of PBS, and liver mononuclear cells and splenocytes were collected for staining after 4 h. Cell suspensions (106) were stained for various NK T cell markers using either PE-labeled mCD1d tetramer loaded with α-GalCer or unloaded, or PE-labeled anti-NK1.1 (clone PK136) in combination with FITC-labeled anti-TCR Vβ (clone H57–597), anti-Vβ8.1/8.2 (clone MR5–2), anti-Vβ7 (clone TR310), and anti-Vβ2 clone (B10.6). After staining the cells were analyzed by flow cytometry using the FACScan™ with CELLQuest™ software (BD PharMingen). All antibodies were purchased from BD PharMingen.
Measurement of NK T Cell Response to α-GalCer.
For proliferative response to α-GalCer, splenocytes were collected at days 3, 5, and 10 after lipid administration and cultured in vitro in the presence of 100 ng/ml of α-GalCer. Proliferation was determined by incorporation of [3H]thymidine as described earlier (31). For comparison of the proliferative and cytokine secretion profiles, splenocytes from naive B10.PL, C57BL/6, and C57BL/6.CD1d−/− mice were used in the presence of different concentrations of the glycolipid. Supernatants from 14 or 48 h cultures were used in ELISA assays to measure cytokine secretion as described previously (40).
Measurement of Myelin Antigen-specific Proliferative Response and Cytokine Secretion.
For the measurement of response to MBP or MOG peptides, mice were immunized subcutaneously with 100 μg of MBPAc1–9 or MOG35–55 emulsified in CFA. α-GalCer was injected intraperitoneally either at the same time in a coimmunization or 1 wk earlier in the preimmunization protocol. Lymph nodes or spleens of mice were removed after 9–10 d. Lymph node cells (5 × 105/well) and splenocytes (8 × 105/well) were cultured with the indicated peptides in HL-1 media (Hyclone) supplemented with 50 μM 2-ME (Sigma-Aldrich), 0.1 U penicillin-streptomycin (Life Technologies), and 2 mM L-glutamine. After 14 or 48 h the culture supernatants were collected for standard sandwich ELISA assays to measure the IFN-γ or IL-4 cytokine production as described (40). Proliferation was assayed by addition of 1 μCi [3H]TdR (ICN Biomedicals) for the last 16 h of a 5 d culture. Cells were harvested and uptake of radioactivity was measured with the Betaplate reader (PerkinElmer).
Induction and Clinical Evaluation of EAE.
Female mice, 6–15 wk of age were immunized once subcutaneously with 100 or 300 μg of MBP or MOG35–55 peptide emulsified in IFA (DIFCO) supplemented with attenuated M. tuberculosis (DIFCO) to 5 mg/ml. 0.15 μg of pertussis toxin (PTx; List Biological Laboratories, Inc.) was injected twice in 200 μl saline intraperitoneally 24 and 72 h later. Mice were observed daily for signs of EAE for 30 to 50 d. The average disease score for each group was calculated by averaging the maximum severity of all of the affected animals in the group. Also, the maximum disease score of each animal is given in the parentheses. Disease severity was scored on a 5-point scale, as described earlier (31): 1, flaccid tail; 2, hind limb weakness; 3, hind limb paralysis; 4, whole body paralysis; 5, moribund or death.
Immunization with α-GalCer or β-GalCer. In the coimmunization protocol, 4.4 μg of α-GalCer dissolved in 200 μl PBS was given intraperitoneally at the time of EAE induction. For the prior-immunization protocol, 4.4 μg of α-GalCer in 200 μl of either PBS or vehicle was given (either three times, 1 wk apart, or once as indicated) 1 wk before the induction of EAE. In some experiments, 4.4 μg of β-GalCer in 200 μl of vehicle was injected as control.
Liver Mononuclear Cell Purification.
Liver monocytes were isolated as described previously (39). For purification of liver monocytes, liver was excised after perfusion through the left ventricle of the heart. Liver tissue was then pressed through a 70-μm cell strainer (Becton Dickinson) in RPMI 1640 (Hyclone) supplemented with 5% FCS equilibrated to room temperature before use. Liver cells were suspended in 40% isotonic Percoll (Amersham Pharmacia Biotech) underlaid with 60% isotonic Percoll, and spun for 25 min at 900 g at room temperature. The fat layer and the bottom layer of 60% Percoll solution were discarded. Cells in the remaining layer were washed twice to remove Percoll before use.
Results
Characterization of NK T Cells in B10.PL (H-2u) Mice.
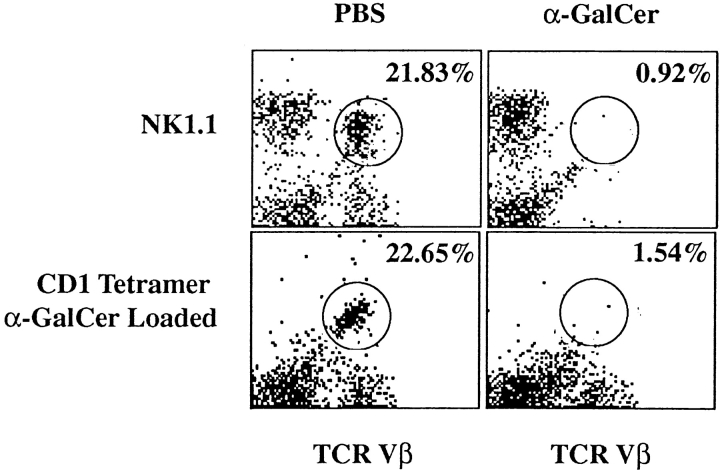
NK T cells from B10.PL mice were characterized for the expression of various cell surface markers and for their activation in vitro and in vivo in response to the glycolipid α-GalCer. Single cell suspensions from liver and spleen of B10.PL mice were stained for typical markers associated with NK T cells as well as with mCD1d-tetramers loaded with α-GalCer and analyzed by flow cytometry. In parallel, mice were injected with α-GalCer or vehicle and 4 h later assessed for the presence of NK T cells in the liver and spleen. The flow cytometry profiles of lymphocytes stained with a pan-TCR Vβ chain antibody and with either the NK1.1 marker or the mCD1 tetramer loaded with α-GalCer are shown in Fig. 1. The percentages of different populations in vehicle or α-GalCer–immunized mice are compiled in Table I. Similar to the data reported in C57BL/6 mice (39), NK T cells in B10.PL mice are NK1.1+ and constitute 20–30% of cells in the liver and 1–2% in the spleen. The majority of liver NK T cells stain with α-GalCer tetramer and predominantly utilize TCR Vβ8.2, Vβ7, and Vβ2 gene segments. They display an activated memory T cell phenotype since they are CD44+, CD69+, and CD62L− (unpublished data).
Figure 1.
Flow cytometry profiles of the NK T cell population in B10.PL mice immunized either with PBS or α-GalCer. Mononuclear cells from the livers and spleens of mice immunized with PBS or with α-GalCer were stained with PE-labeled mCD1d/α-GalCer tetramers or with PE-labeled anti-NK1.1 antibodies in combination with FITC-labeled pan anti-TCR Vβ mAb. Numbers indicate the percentage of NK.1.1+TCR Vβ+ or tetramer+TCR Vβ+ cells in individual mice. These data are representative of two independent experiments.
Table I.
Cell Surface Markers Expressed by the NK T Cell Population in B10.Pl Mice
| Spleen
|
Liver
|
|||
|---|---|---|---|---|
| Markers | PBS | α-GalCer | PBS | α-GalCer |
| MCD1d-tetramer | 2.56 | 1.78 | 18.67 | 0.1 |
| MCD1d-tetramer plus TCR | 1.78 | 1.41 | 22.65 | 1.54 |
| NK1.1 | 3.37 | 3.22 | 41.62 | 36.96 |
| NK1.1 plus TCR | 1.39 | 0.8 | 21.83 | 0.92 |
| NK1.1 plus Vβ8.1/2 | 0.11 | 0.05 | 10.64 | 1.77 |
| NK1.1 plus Vβ7 | 0.8 | 0.53 | 4.13 | 0.38 |
| NK1.1 plus Vβ2 | 0.05 | 0.11 | 2.36 | n/d |
| Unloaded tetramer | 1.02 | 1.08 | 0.65 | 0.44 |
Monocytes from the liver and spleens of each PBS or α-GalCer immunized mouse were stained and analyzed by flow cytometry as described in Materials and Methods. These data are representative of two independent experiments.
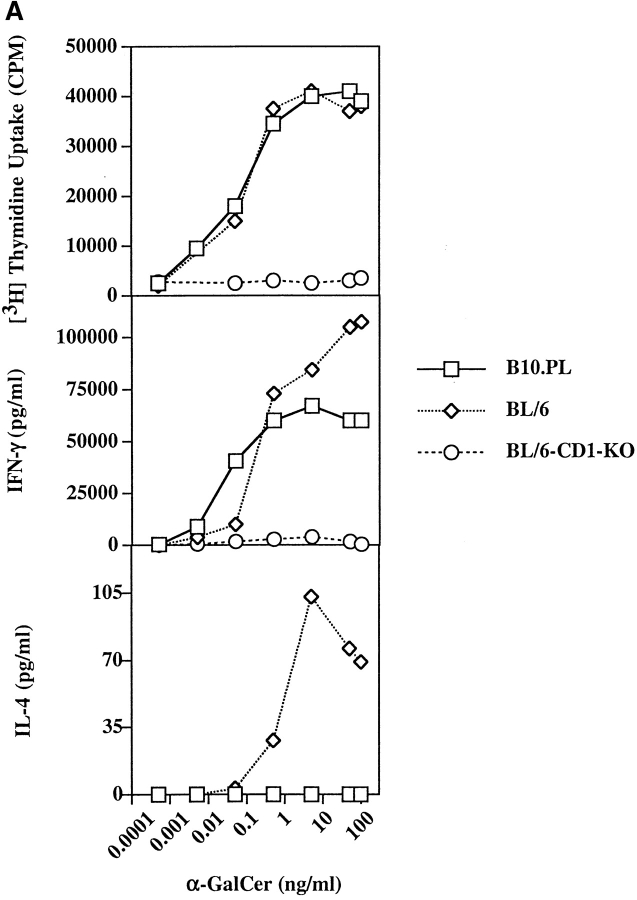
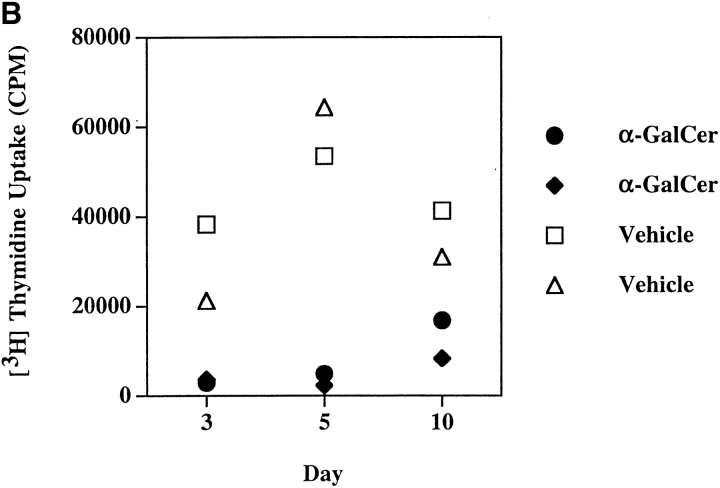
The kinetics of α-GalCer–dependent activation and subsequent depletion of NK T cells in B10.PL mice is also similar to that reported in C57BL/6 mice (39). As shown in Fig. 1 and Table I, most tetramer-reactive NK T cells in the liver disappear within 4 h after injection with α-GalCer. As splenic NK T cells from naive mice proliferate in response to in vitro challenge with α-GalCer, we wanted to examine their proliferative response in mice at different times following a single injection of α-GalCer. As shown in Fig. 2 B, proliferative responses to α-GalCer are absent in mice injected 3 and 5 d earlier. However, by day 10 proliferation is partially restored, suggesting reconstitution of the NK T cell population. Next we compared in vitro proliferation and cytokine production in response to α-GalCer in both B10.PL and C57BL/6 mice. As shown in Fig. 2 A, proliferative response and IFN-γ secretion were very similar in both mice. However a significant difference between the strains was found in IL-4 secretion, which was significantly reduced in B10.PL compared with C57BL/6 mice.
Figure 2.
In vitro response of splenocytes to α-GalCer. (A) Splenocytes from naive B10.PL, C57BL/6, and C57BL/6.CD1−/− mice were cultured with graded concentrations of α-GalCer for measurement of proliferation and cytokine secretion. Proliferative response and IFN-γ secretion is shown in individual mice. A representative of three experiments is shown. (B) Groups of B10.PL mice (two in each group) were immunized either with α-GalCer or with vehicle, and proliferation in response to an in vitro recall with 100 ng of α-GalCer was determined 3, 5, or 10 d later. Proliferative responses in individual mice are shown. These data are representative of two independent experiments.
Coimmunization of α-GalCer and MBP Results in Skewing of Anti-MBP Response Toward Th1 in B10.PL Mice.
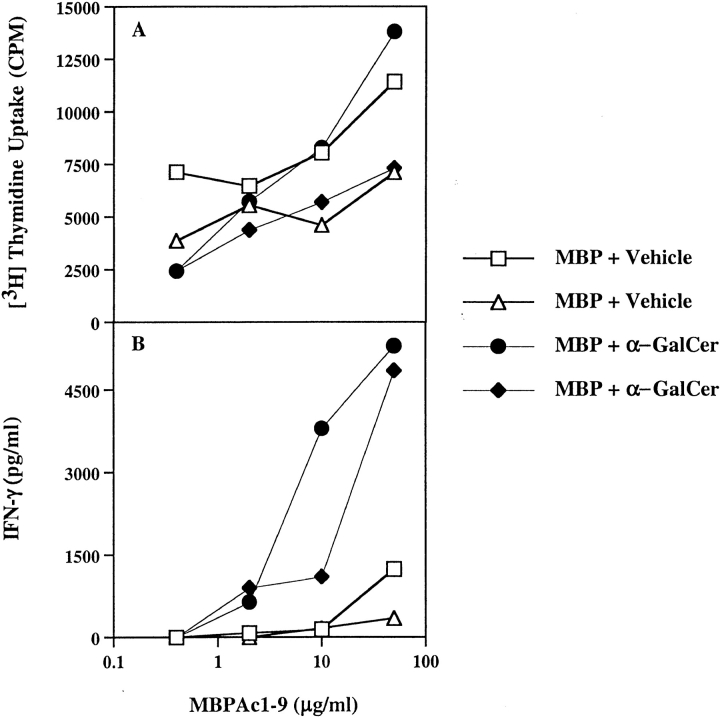
As the cytokine microenvironment is one of the crucial factors in differentiation of naive CD4 T cells in a type 1 or 2 direction, we wanted to determine whether rapid secretion of both IFN-γ and IL-4 after activation of NK T cells by α-GalCer can influence the anti-MBP T cell response. Groups of B10.PL mice were injected intraperitoneally with 4.4 μg of α-GalCer in vehicle or with vehicle only and simultaneously with a subcutaneous immunization of 100 μg MBP emulsified in CFA. 10 d later draining lymph nodes were assayed for proliferation and cytokine production in response to an in vitro challenge with the dominant determinant of MBP, Ac1–9 (Fig. 3). Although there was no significant difference in the proliferative recall with Ac1–9, IFN-γ secretion was enhanced 4–5-fold by MBPAc1–9–reactive T cells in the animals coimmunized with α-GalCer. There was no detectable difference in the secretion of IL-4 in these cultures. Proliferative responses and cytokine production in response to purified protein derivative of mycobacterium (PPD) were comparable in both groups of mice (proliferation ranged from 65,765 to 128,392 cpm, while IFN-γ levels were from 2,033–3,500 pg/ml). These data suggested that activation of NK T cells at the time of priming of MBP-reactive T cells in B10.PL mice results in further skewing of the anti-MBP response toward a predominant Th1 phenotype.
Figure 3.
Coimmunization of α-GalCer with MBP promotes antigen-specific Th1 responses in B10.PL mice. Groups of B10.PL mice (two in each) were immunized either with α-GalCer or with PBS/vehicle at the time of challenge with MBP emulsified in CFA. 9 d later lymph node cells were cultured with graded concentrations of MBPAc1–9. Proliferation (A) and IFN-γ secretion (B) are shown. IL-4 production was below the level of detection, and response to PPD was similar in both groups of mice. These data are representative of two independent experiments.
Coimmunization of α-GalCer with MBPAc1–9 Exacerbates EAE in B10.PL Mice.
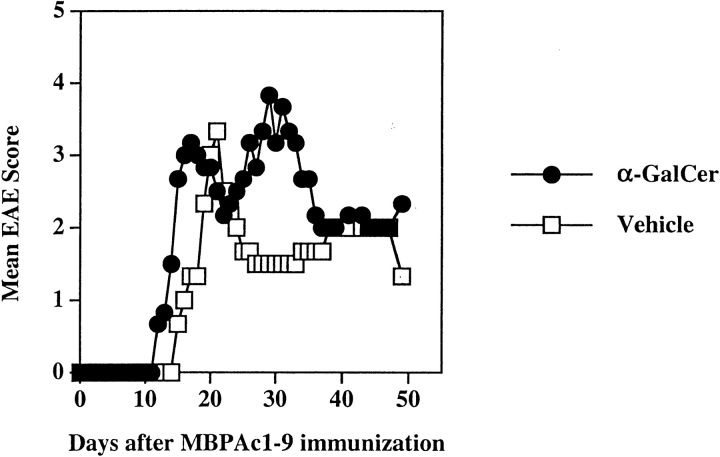
Generally, further polarization of MBPAc1–9–reactive T cells toward the Th1 phenotype exacerbates EAE, while their Th2 polarization ameliorates clinical signs of the disease (36, 37). As we found further Th1-skewing of the MBP response following coimmunization with α-GalCer and MBP, we next examined whether the course of EAE is altered. Groups of B10.PL mice were injected intraperitoneally with α-GalCer in vehicle or with vehicle alone and at the same time immunized with MBPAc1–9/CFA/PT for the induction of EAE and monitored daily for clinical paralysis. As shown in Fig. 4 and Table II, the control (vehicle/PBS) group had a normal disease course with an average disease score of 2.9 and a mean day of onset 13.2 d after Ac1–9 immunization. In the α-GalCer–immunized group, mice contracted much more severe and chronic EAE with an average disease score of 4.5. 1 in 10 mice died from paralysis in the control group whereas 5/8 mice did not recover and died from EAE in the α-GalCer–coimmunized group. Thus activation of NK T cells with α-GalCer concomitant with the MBP/Ac1–9-immunization results in exacerbated disease.
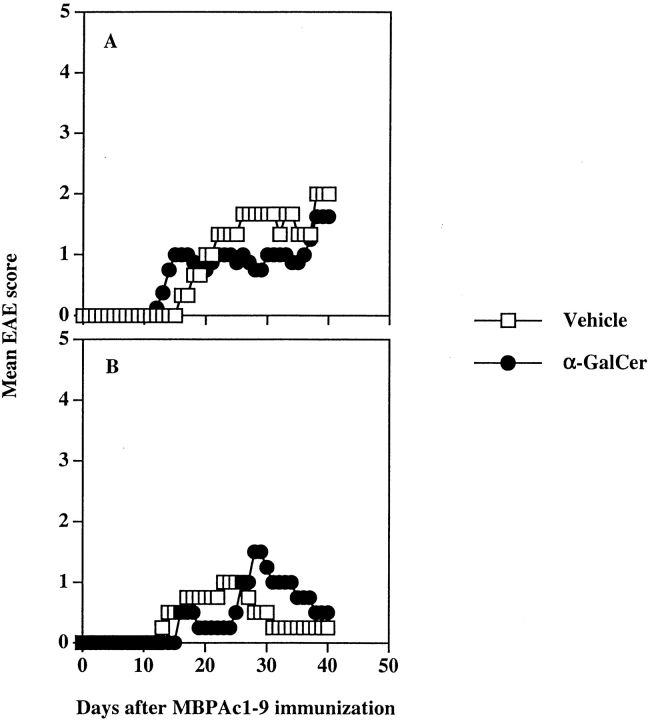
Figure 4.
Coimmunization of α-GalCer with MBP exacerbates EAE in B10.PL mice. EAE was induced following the coimmunization protocol as described in Materials and Methods. The mean clinical disease course of mice (four in each group) in both vehicle and α-GalCer–immunized groups is shown. These data are representative of two independent experiments. A summary of all independent experiments is provided in Table II.
Table II.
Modulation of EAE by α-GalCer in B10.PL Mice
| Treatment | No. animals with EAE/total no. of animals (individual maximal score) |
Mean disease score |
Mean day of onset |
|---|---|---|---|
| Vehicle/PBS | 9/10 (5,4,4,4,3,3,3,2,1,0) | 2.9 | 13.2 |
| Coimmunization with α-GalCer |
8/8 (5,5,5,5,5,4,4,3) | 4.5 | 12.3 |
| Preimmunization with α-GalCer (1×) |
4/8 (4,3,1,1,0,0,0,0) | 1.1 | 11.5 |
| Preimmunization with α-GalCer (3×) |
3/9 (4,1,1,0,0,0,0,0) | 0.7 | 13.2 |
| Preimmunization with β-GalCer (1×) |
4/4 (4,3,3,3) | 3.2 | 12.7 |
| Preimmunization with α-GalCer (3×) plus anti-IL-4 |
6/6 (5,4,4,3,3,3) | 3.3 | 11.2 |
| Preimmunization with α-GalCer (1×) plus control Ig |
3/4 (2,1,1,0) | 1.1 | 11.7 |
Groups of age-matched B10.PL mice were subcutaneously injected with MBPAc1-9/CFA/PT for the induction of EAE and immunized intraperitoneally with glycolipids in the vehicle or with vehicle only, according to coimmunization or preimmunization protocols described in Material and Methods. For the depletion of IL-4, a neutralizing anti-IL-4 monoclonal antibody 11B11 (1 mg/mouse) or a control Rat Ig was administered. Clinical symptoms of EAE were monitored daily.
Preimmunization with α-GalCer Results in Decreased Secretion of IFN-γ by MBP-reactive T Cells and Prevention of EAE in B10.PL Mice.
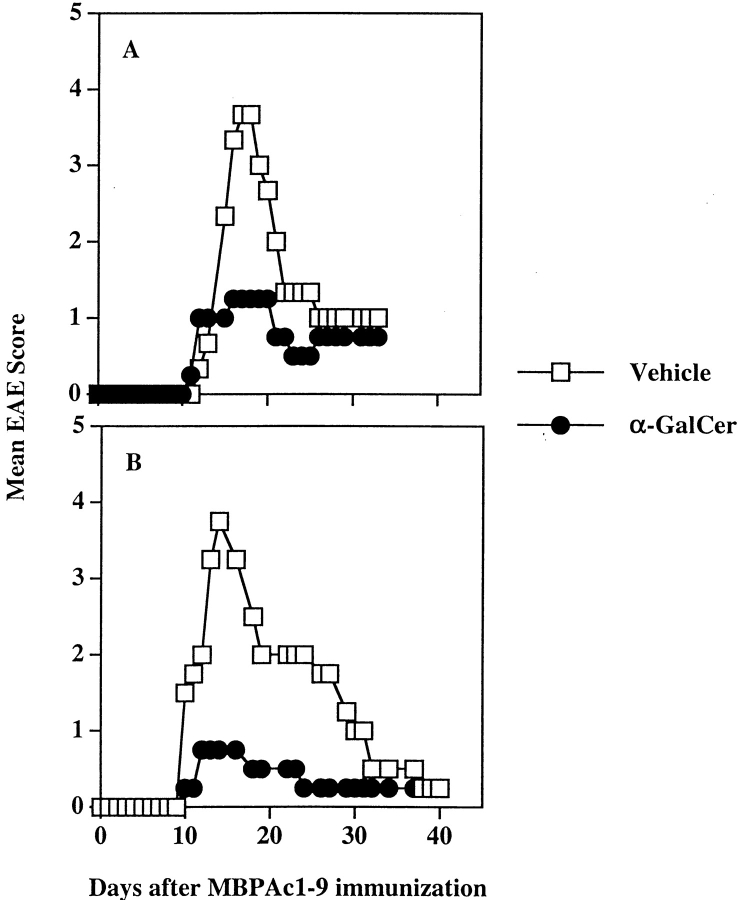
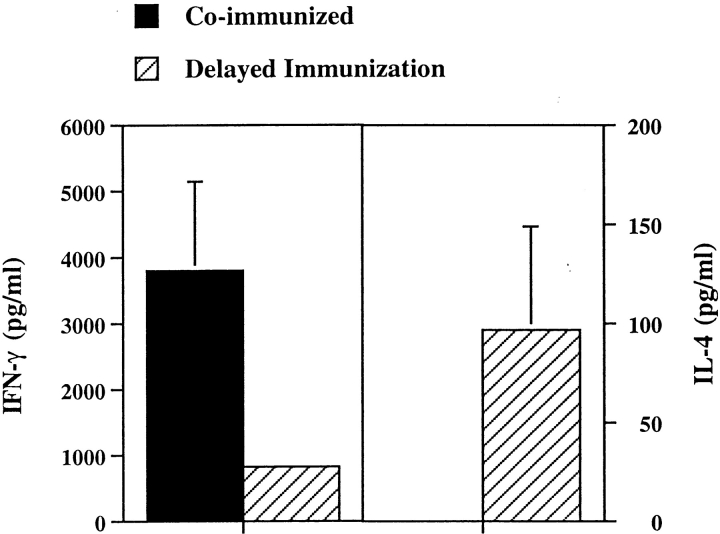
As shown in Fig. 1 and Table I, most NK T cells disappear after a single injection with α-GalCer. Furthermore, previous studies have shown that multiple injections of α-GalCer in C57BL/6 mice results in an enhanced production of IL-4 by the remaining population of T cells (26). Similarly multiple injections of α-GalCer protects NOD mice from diabetes (41–43). To determine whether the timing of the NK T cell activation (coimmunization versus preimmunization with α-GalCer) is important in modulating EAE, two sets of experiments were performed. In the first set, groups of mice were given a single injection of α-GalCer 5–7 d before MBPAc1–9/CFA/PT challenge. In the second set, groups of mice were given three weekly injection of α-GalCer and 1 wk after the last injection, they were challenged with MBPAc1–9/CFA/PT for induction of EAE. All mice were monitored for symptoms of disease daily. As shown in Fig. 5, A and B, and Table II, both α-GalCer preimmunized groups of mice contracted monophasic disease of less severity (mean disease score of 1.2 in mice immunized once and 0.7 in mice immunized three times) in comparison to mice in the control group with mean disease score of 2.9. In contrast, prior immunization of B10.PL mice with β-GalCer which binds to CD1d but does not activate NK T cells, has a negligible influence on the course of EAE (Table II). Thus, coimmunization exacerbates disease while preimmunization significantly suppresses EAE. It is apparent that the timing of activation of NK T cells by α-GalCer affects the nature of the modulation of EAE. Next we examined the secretion of IFN-γ and IL-4 by MBPAc1–9–reactive T cells in mice coimmunized or preimmunized with α-GalCer. As shown in Fig. 6, while there was a significant decrease in the secretion of IFN-γ by MBPAc1–9–reactive T cells in the preimmunized group, IL-4 secretion was moderately enhanced, compared with the coimmunized group of animals. These data suggest that prior activation of NK T cells by α-GalCer prevents EAE by decreasing IFN-γ secretion and enhancing IL-4 secretion by the subsequent anti-MBP response.
Figure 5.
Preimmunization with α-GalCer protects B10.PL mice from EAE. EAE was induced following the preimmunization protocol as described in Materials and Methods. Groups of mice (four in each) were immunized either once (A) or three times (B) with α-GalCer, and 1 wk after the last immunization, they were immunized with MBP/CFA/PT for the induction of EAE. A summary of all independent experiments is given in Table II.
Figure 6.
Preimmunization with α-GalCer decreases IFN-γ production and increases IL-4 production by MBPAc1–9–reactive T cells in B10.PL mice. Groups of B10.PL mice (two in each) were immunized either with α-GalCer or with PBS three times, 1 wk apart, 1 wk before challenge with MBPAc1–9 emulsified in CFA. 9 d later lymph node cells were cultured with Ac1–9 and supernatants were collected 48 h later for cytokine secretion. These data are representative of three experiments.
α-GalCer–mediated Exacerbation and Protection from EAE Is Lost in Mice Deficient in IFN-γ and IL-4, Respectively.
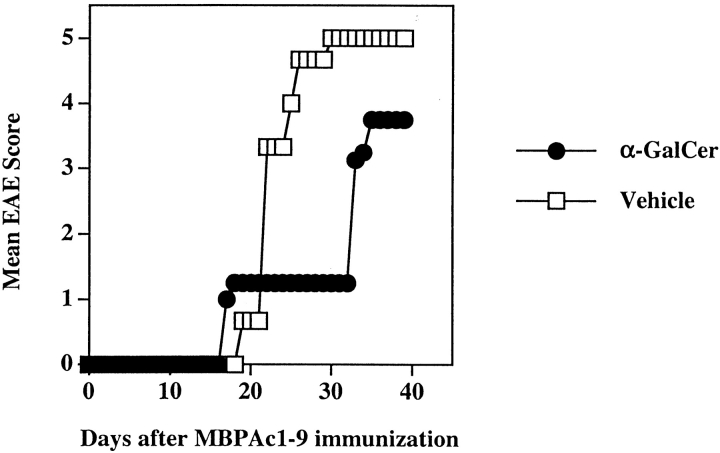
As shown in Figs. 3 and 6, while coimmunization of α-GalCer with MBP enhances IFN-γ secretion, preimmunization leads to decreased IFN-γ and moderately enhanced IL-4 secretion by MBP-reactive T cells. We have used B10.PL-IFN-γ2/− and anti–IL-4–treated B10.PL mice to examine the role of IFN-γ and IL-4 in α-GalCer–mediated potentiation and protection from EAE, respectively. MBP-induced EAE in B10.PL-IFN-γ2/− mice is usually lethal, and nearly all mice fail to recover and die from paralysis. The NK T cell population and its activation/depletion kinetics was characterized in these mice and found to be similar to the wild type B10.PL mice (unpublished data). As shown in Fig. 7, coimmunization of α-GalCer with MBPAc1–9 in B10.PL-IFN-γ2/− mice fails to potentiate EAE. In fact α-GalCer–treated mice contracted less severe disease (mean disease score 3.1), compared with mice in the control group (mean disease score, 4.9). 9 out of 10 mice died from paralysis in the control group, whereas 5/10 mice succumbed to death in the α-GalCer–coimmunized group. To examine the involvement of IL-4 in protecting mice against EAE using the preimmunization protocol, groups of B10.PL mice were injected with anti–IL-4 mAb (11B11, 1 mg per mouse) or control Ig (Table II). The protective effect of prior immunization with α-GalCer was lost in the absence of IL-4 in vivo. These data indicate that α-GalCer–dependent potentiation and protection of EAE is mediated by IFN-γ and IL-4, respectively.
Figure 7.
IFN-γ is required for the α-GalCer-mediated potentiation of EAE in B10.PL mice. Groups of B10.PL.IFN-γ2/− (four in each) were coimmunized for EAE with MBPAc1–9 and α-GalCer as described in Materials and Methods. Mean clinical disease scores in both vehicle and α-GalCer immunized groups are shown. These data are representative of two independent experiments.
Both the Potentiating and Protective Effects of α-GalCer Immunization on EAE Require CD1d Antigen Presentation.
As prior immunization of B10.PL mice with β-GalCer, which binds to CD1d but does not activate NK T cells, has negligible influence on the course of EAE (Table II), we wanted to determine whether modulation of EAE by α-GalCer requires the CD1d antigen presentation pathway. We have generated B10.PL.CD1d+/+ and B10.PL.CD1d−/− mice by backcrossing CD1d-deficient C57BL/6 mice onto the B10.PL strain. As shown in Fig. 8, coimmunization (A) or preimmunization (B) with α-GalCer had no effect on the course of disease in B10.PL.CD1d−/− mice and clinical symptoms of EAE in both vehicle- and α-GalCer–immunized mice were very similar. These data provide strong evidence that potentiation or protection from EAE by α-GalCer is mediated by the CD1d-dependent activation of Vα14+ NK T cells.
Figure 8.
Potentiation and protection from EAE by α-GalCer in B10.PL mice are dependent upon the CD1d antigen presentation pathway. Groups of B10.PL.CD−/− mice (six in each) were injected with α-GalCer and MBP following the coimmunization (A) or prior-immunization (B) protocol as described in Materials and Methods. The mean clinical disease scores in each group are shown.
Coimmunization of α-GalCer with MOG35–55 Prevents EAE in C57BL/6 Mice.
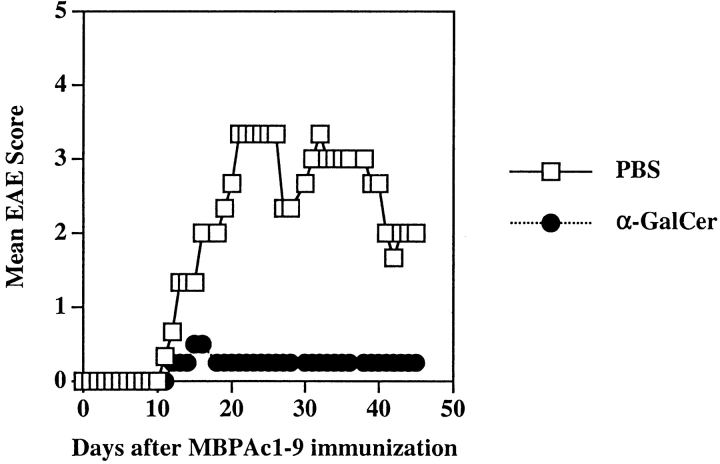
As shown in Fig. 2, IL-4 production in splenocytes from C57BL/6 mice in response to an in vitro stimulation with α-GalCer is more pronounced than in B10.PL mice. As C57BL/6 is relatively resistant to MBP-induced EAE, we have tested whether coimmunization of α-GalCer with MOG35–55 is able to influence the disease course. Groups of C57BL/6 mice were injected intraperitoneally with α-GalCer in vehicle or with vehicle alone and at the same time immunized with MOG35–55/CFA/PT for the induction of EAE. As shown in Fig. 9 and Table III, mice in the α-GalCer–immunized group were significantly protected from EAE: 9/10 mice in the vehicle-immunized group contracted severe disease (mean disease score 3.1), whereas 2/10 mice developed milder disease (mean disease score 0.3) in the α-GalCer–treated group. Furthermore α-GalCer-mediated prevention of EAE in C57BL/6 mice also requires the CD1d presentation pathway, as the course of EAE in CD1d-deficient mice is not influenced by coimmunization with α-GalCer (Table III). It is interesting that the anti-MOG response in C57BL/6 mice following coimmunization with α-GalCer becomes skewed in a Th2 direction (43a; and data not shown).
Figure 9.
Coimmunization of α-GalCer with MOG35–55 prevents EAE in C57BL/6 mice. Groups of BL/6 mice (four in each) were immunized with MOG35–55 peptide for EAE induction following the coimmunization protocol with α-GalCer. The mean clinical disease scores for both vehicle and α-GalCer immunized groups are shown. Summary of all experiments is provided in Table III. Monocytes from the liver and spleens of each PBS or α-GalCer immunized mouse were stained and analyzed by flow cytometry as described in Materials and Methods. These data are representative of two independent experiments.
Table III.
Modulation of EAE by α-GalCer in C57BL/6 and C57BL/6.CD1d−/− Mice
| Treatment | No. animals with EAE/total no. of animals (individual maximal score) |
Mean disease score | Mean day of onset |
|---|---|---|---|
| C57BL/6 mice | |||
| Vehicle/PBS | 9/10 (5,5,4,4,4,4,3,1,1,0) | 3.1 | 14.4 |
| Coimmunization with α-GalCer | 2/10 (2,1,0,0,0,0,0,0,0,0) | 0.3 | 13.5 |
| C57BL/6.CD1d−/− mice | |||
| Vehicle/PBS | 10/12 (4,3,3,3,3,2,2,2,1,1,0,0) | 2.0 | 14.9 |
| Coimmunization with α-GalCer | 7/8 (4,3,2,2,2,2,1,0) | 2.0 | 16.6 |
Groups of age-matched mice were subcutaneously immunized with 300 μg of MOG35–55 emulsified in CFA and at the same time administered with 4.4 μg of α-GalCer in vehicle or with the vehicle only, as described in Materials and Methods. Clinical symptoms of EAE were monitored daily.
Discussion
EAE in the B10.PL mouse represents one of the best-characterized models of antigen-induced T cell–mediated autoimmune disease. Although a great deal is known about different myelin antigens, autoreactive T cells, and cytokines involved in the pathogenesis of EAE in these animals, little is known about the role of NK T cells. Here we have demonstrated that activation of Vα14 NK T cells by α-Gal-Cer in the context of CD1d alters the cytokine profile of T cells reactive to myelin antigens and their ability to induce EAE. Interestingly, administration of α-GalCer can either potentiate or prevent disease, depending upon the nature of the NK T cell response in the strain examined. While IFN-γ secretion is involved in exacerbation, IL-4 plays an important role in protection. These findings suggest that NK T cells play an important role in the pathogenesis of EAE and that these cells are useful targets for intervention in autoimmune diseases of the CNS.
Do NK T cells play a role in the pathogenesis of EAE or MS in the absence of lipid immunization? Earlier observations have suggested low numbers of NK T cells in EAE-susceptible SJL/J mice (22). Consistent with this idea, it has been recently reported that a significant decrease in the Vα24JαQ NK T cell population occurs during clinical remission in MS, but not in autoimmune/inflammatory diseases affecting peripheral nerves or in chronic inflammatory demyelinating polyneuropathy (14). It is interesting that the severity of EAE in both C57BL/6.CD1d−/− (H2b) and B10.PL.CD1d−/− (H-2u) mice is reduced in comparison to wild-type mice. Furthermore, we have found the presence of CD1d-α-GalCer tetramer+ T cells in CNS-infiltrates during the normal course of EAE in animals injected with MBPAc1–9 in the absence of α-GalCer (unpublished data). Thus, NK T cells are potentially involved in pathogenesis of autoimmune demyelination and could have a positive or negative influence. A recent demonstration related to the presence of NK1.1+, DX5+ T cell population and its regulatory role in EAE is consistent with this notion (44). In diabetes patients IL-4 secretion by NK T cells has been found to be defective (13). These observations further suggest a potential role of NK T cells in autoimmune conditions in humans.
C57BL/6 mice are relatively resistant to MBP-induced EAE. CD1d-restricted NK T cells in EAE-susceptible B10.PL (H-2u) and relatively resistant C57BL/6 (H-2b) mice are very similar with respect to cell number and TCR usage. As in C57BL/6 mice, NK T cells in B10.PL mice predominantly utilize the TCR Vβ8.2 gene segment. After in vivo activation with α-GalCer, Vα14+ NK T cells in B10.PL mice using all Vβ gene segments disappear in a similar fashion as reported by others (39). However, although there is no significant strain difference in proliferation or in IFN-γ production in response to α-GalCer, IL-4 secretion is much lower in splenocytes from B10.PL mice in comparison to C57BL/6 mice. This difference in IL-4 secretion upon CD1d-dependent recognition of α-GalCer does not appear to be related to MHC difference, as in another H-2u strain, PL/J, IL-4 secretion levels are similar to those in C57BL/6 (unpublished data). Our data has shown that coimmunization of α-GalCer with MBP in B10.PL mice results in further skewing of the anti-MBP response toward Th1 and exacerbation of EAE, whereas coimmunization of α-GalCer with MOG35–55 protects C57BL/6 mice from disease. Our preliminary data and the accompanying paper (43a) suggest that protection is mediated by immune deviation of the anti-MOG response. In both strains, prior immunization with α-GalCer leads to reduced IFN-γ secretion and increased IL-4 secretion by MBP-reactive T cells and subsequent protection from EAE. Collectively, these data suggest that the balance between IFN-γ and IL-4 secretion in response to α-GalCer determines whether the activation of Vα14+ NK T cells will enhance or diminish disease.
It is well established that invariant Vα14+ NK T cells rapidly secrete IFN-γ as well as IL-4 upon stimulation in vitro and in vivo (3–5). α-GalCer-activated NK T cells also activate NK cells, B cells and dendritic cells as well as conventional T cells (27, 45–48). Consistent with their secretion of both IFN-γ and IL-4, NK T cells have been implicated in either augmenting or suppressing antitumor responses (7–9). Similarly NK T cells also have been implicated in effective immunity against pathogens (6, 49, 50). It has been suggested that IFN-γ secretion by NK cells is dependent upon the activation of NK T cells (45). It is possible that in B10.PL mice α-GalCer administration activates NK T cells followed by activation of NK cells, which contributes to the early IFN-γ–rich milieu, thus skewing the MBP response toward Th1 and the potentiation of EAE. Our observation that the loss of potentiation of EAE in mice deficient in either IFN-γ or in the CD1d pathways of antigen presentation indicates that even if NK cells eventually may become involved, the initial response is mediated by Vα14+ NK T cells.
The role of IL-4 in α-GalCer–mediated protection from EAE is clearly highlighted by the fact that prior immunization with the lipid is protective in the presence but not in the absence of IL-4. It is likely that IL-4 secretion by both NK T and MBP-reactive T cells is important for prevention of EAE. Although IL-4 is involved in this protection, it is not yet clear whether repeated immunization polarizes NK T cells for the production of IL-4, as suggested by earlier data (26). Our findings do not support this possibility, as when the final α-GalCer injection of a series is given at the same time as MBP immunization, B10.PL mice still develop exacerbated EAE (unpublished data). If NK T cells themselves were deviated to produce IL-4, their activation should deviate the anti-MBP response and protect mice from disease. It remains possible that repeated activation of NK T cells alters antigen-presentation in vivo. We are currently investigating the mechanism by which repeated injection of α-GalCer deviates the anti-MBP response.
Our data collectively suggest that the mode of activation of NK T cells by α-GalCer is decisive in determining the Th1/Th2 balance among MBP-reactive T cells and hence their ability to cause EAE. Therefore for intervention in Th1-mediated autoimmune conditions, it may be desirable to activate NK T cells by other lipid ligands or by altered lipid ligands in a manner that enhances secretion of IL-4. For example, it has recently been shown that NK T cells predominantly secrete IL-4 upon activation with an altered α-GalCer molecule containing truncated sphingosine residues (51). The potential therapeutic value of targeting NK T cells for treatment of autoimmune conditions has also recently been shown in NOD mice, where administration of α-GalCer results in immune deviation of the anti-islet response and protection from diabetes (41–43).
As CD1d molecules, unlike classical MHC molecules, are nonpolymorphic and are remarkably conserved among different species, insight into the role of the NK T cell population will be extremely valuable in the development of HLA-independent therapeutic approaches for autoimmune conditions. Therefore studies of the role of lipid-reactive T cells and their modulatory influence on adaptive immunity has implications for the pathophysiology as well as for the development of potential therapies for intervention in T cell–mediated diseases.
Acknowledgments
The authors thank Dr. Luc Van Kaer for sharing information prior to publication, Dr. Randle Ware for critical comments on the manuscript, and Dr. Eli Sercarz for support and encouragement.
V. Kumar was supported by grants from the National Multiple Sclerosis Society and the Arthritis Foundation. This is publication #452 from LIAI.
Footnotes
Abbreviations used in this paper: α-GalCer, α-galactosylceramide; EAE, experimental autoimmune encephalomyelitis; MBP, myelin basic protein; MOG, myelin oligodendrocyte protein; MS, multiple sclerosis.
References
- 1.Bendelac, A., M.N. Rivera, S.H. Park, and J.H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535–562. [DOI] [PubMed] [Google Scholar]
- 2.Porcelli, S.A., and R.L. Modlin. 1999. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 17:297–329. [DOI] [PubMed] [Google Scholar]
- 3.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, et al. 1997. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 278:1626–1629. [DOI] [PubMed] [Google Scholar]
- 4.Spada, F.M., Y. Koezuka, and S.A. Porcelli. 1998. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J. Exp. Med. 188:1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brossay, L., M. Chioda, N. Burdin, Y. Koezuka, G. Casorati, P. Dellabona, and M. Kronenberg. 1998. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 188:1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishikawa, H., H. Hisaeda, M. Taniguchi, T. Nakayama, T. Sakai, Y. Maekawa, Y. Nakano, M. Zhang, T. Zhang, M. Nishitani, et al. 2000. CD4(+) v(alpha)14 NKT cells play a crucial role in an early stage of protective immunity against infection with Leishmania major. Int. Immunol. 12:1267–1274. [DOI] [PubMed] [Google Scholar]
- 7.Smyth, M.J., K.Y. Thia, S.E. Street, E. Cretney, J.A. Trapani, M. Taniguchi, T. Kawano, S.B. Pelikan, N.Y. Crowe, and D.I. Godfrey. 2000. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 191:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moodycliffe, A.M., D. Nghiem, G. Clydesdale, and S.E. Ullrich. 2000. Immune suppression and skin cancer development: regulation by NKT cells. Nat. Immunol. 1:521–525. [DOI] [PubMed] [Google Scholar]
- 9.Terabe, M., S. Matsui, N. Noben-Trauth, H. Chen, C. Watson, D.D. Donaldson, D.P. Carbone, W.E. Paul, and J.A. Berzofsky. 2000. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat. Immunol. 1:515–520. [DOI] [PubMed] [Google Scholar]
- 10.Sonoda, K.H., M. Exley, S. Snapper, S.P. Balk, and J. Stein-Streilein. 1999. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J. Exp. Med. 190:1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong, S., and L. Van Kaer. 1999. Immune privilege: keeping an eye on natural killer T cells. J. Exp. Med. 190:1197–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumida, T., A. Sakamoto, H. Murata, Y. Makino, H. Takahashi, S. Yoshida, K. Nishioka, I. Iwamoto, and M. Taniguchi. 1995. Selective reduction of T cells bearing invariant V alpha 24J alpha Q antigen receptor in patients with systemic sclerosis. J. Exp. Med. 182:1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson, S.B., S.C. Kent, K.T. Patton, T. Orban, R.A. Jackson, M. Exley, S. Porcelli, D.A. Schatz, M.A. Atkinson, S.P. Balk, et al. 1998. Extreme Th1 bias of invariant Valpha24JalphaQ T cells in type 1 diabetes. Nature. 391:177–181. [DOI] [PubMed] [Google Scholar]
- 14.Illes, Z., T. Kondo, J. Newcombe, N. Oka, T. Tabira, and T. Yamamura. 2000. Differential expression of NK T cell V alpha 24J alpha Q invariant TCR chain in the lesions of multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J. Immunol. 164:4375–4381. [DOI] [PubMed] [Google Scholar]
- 15.Kojo, S., Y. Adachi, H. Keino, M. Taniguchi, and T. Sumida. 2001. Dysfunction of T cell receptor AV24AJ18+, BV11+ double-negative regulatory natural killer T cells in autoimmune diseases. Arthritis Rheum. 44:1127–1138. [DOI] [PubMed] [Google Scholar]
- 16.Gausling, R., C. Trollmo, and D.A. Hafler. 2001. Decreases in interleukin-4 secretion by invariant CD4(−)CD8(−)V alpha 24J alpha Q T cells in peripheral blood of patients with relapsing- remitting multiple sclerosis. Clin. Immunol. 98:11–17. [DOI] [PubMed] [Google Scholar]
- 17.Gombert, J.M., A. Herbelin, E. Tancrede-Bohin, M. Dy, C. Carnaud, and J.F. Bach. 1996. Early quantitative and functional deficiency of NK1+-like thymocytes in the NOD mouse. Eur. J. Immunol. 26:2989–2998. [DOI] [PubMed] [Google Scholar]
- 18.Baxter, A.G., S.J. Kinder, K.J. Hammond, R. Scollay, and D.I. Godfrey. 1997. Association between alphabetaTCR+ CD4−CD8− T-cell deficiency and IDDM in NOD/Lt mice. Diabetes. 46:572–582. [DOI] [PubMed] [Google Scholar]
- 19.Falcone, M., B. Yeung, L. Tucker, E. Rodriguez, and N. Sarvetnick. 1999. A defect in interleukin 12-induced activation and interferon gamma secretion of peripheral natural killer T cells in nonobese diabetic mice suggests new pathogenic mechanisms for insulin-dependent diabetes mellitus. J. Exp. Med. 190:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mieza, M.A., T. Itoh, J.Q. Cui, Y. Makino, T. Kawano, K. Tsuchida, T. Koike, T. Shirai, H. Yagita, A. Matsuzawa, et al. 1996. Selective reduction of V alpha 14+ NK T cells associated with disease development in autoimmune-prone mice. J. Immunol. 156:4035–4040. [PubMed] [Google Scholar]
- 21.Hammond, K.J., D.G. Pellicci, L.D. Poulton, O.V. Naidenko, A.A. Scalzo, A.G. Baxter, and D.I. Godfrey. 2001. CD1d-restricted NKT cells: an interstrain comparison. J. Immunol. 167:1164–1173. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimoto, T., A. Bendelac, J. Hu-Li, and W.E. Paul. 1995. Defective IgE production by SJL mice is linked to the absence of CD4+, NK1.1+ T cells that promptly produce interleukin 4. Proc. Natl. Acad. Sci. USA. 92:11931–11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Vliet, H.J., B.M. von Blomberg, N. Nishi, M. Reijm, A.E. Voskuyl, A.A. van Bodegraven, C.H. Polman, T. Rustemeyer, P. Lips, A.J. van den Eertwegh, et al. 2001. Circulating V(alpha24+) Vbeta11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin. Immunol. 100:144–148. [DOI] [PubMed] [Google Scholar]
- 24.Shinkai, K., and R.M. Locksley. 2000. CD1, tuberculosis, and the evolution of major histocompatibility complex molecules. J. Exp. Med. 191:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gumperz, J.E., C. Roy, A. Makowska, D. Lum, M. Sugita, T. Podrebarac, Y. Koezuka, S.A. Porcelli, S. Cardell, M.B. Brenner, and S.M. Behar. 2000. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 12:211–221. [DOI] [PubMed] [Google Scholar]
- 26.Burdin, N., L. Brossay, and M. Kronenberg. 1999. Immunization with alpha-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J. Immunol. 29:2014–2025. [DOI] [PubMed] [Google Scholar]
- 27.Singh, N., S. Hong, D.C. Scherer, I. Serizawa, N. Burdin, M. Kronenberg, Y. Koezuka, and L. Van Kaer. 1999. Cutting edge: activation of NK T cells by CD1d and alpha-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 163:2373–2377. [PubMed] [Google Scholar]
- 28.Paterson, P.Y. 1980. Autoimmune diseases of myelin. Prog. Clin. Biol. Res. 49:19–36. [PubMed] [Google Scholar]
- 29.Kumar, V., D.H. Kono, J.L. Urban, and L. Hood. 1989. The T-cell receptor repertoire and autoimmune diseases. Annu. Rev. Immunol. 7:657–682. [DOI] [PubMed] [Google Scholar]
- 30.Urban, J.L., V. Kumar, D.H. Kono, C. Gomez, S.J. Horvath, J. Clayton, D.G. Ando, E.E. Sercarz, and L. Hood. 1988. Restricted use of T cell receptor V genes in murine autoimmune encephalomyelitis raises possibilities for antibody therapy. Cell. 54:577–592. [DOI] [PubMed] [Google Scholar]
- 31.Kumar, V., and E.E. Sercarz. 1993. The involvement of T cell receptor peptide-specific regulatory CD4+ T cells in recovery from antigen-induced autoimmune disease. J. Exp. Med. 178:909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar, V., K. Stellrecht, and E. Sercarz. 1996. Inactivation of T cell receptor peptide-specific CD4 regulatory T cells induces chronic experimental autoimmune encephalomyelitis (EAE). J. Exp. Med. 184:1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar, V., and E. Sercarz. 1998. Induction or protection from experimental autoimmune encephalomyelitis depends on the cytokine secretion profile of TCR peptide-specific regulatory CD4 T cells. J. Immunol. 161:6585–6591. [PubMed] [Google Scholar]
- 34.Kumar, V. 1998. TCR peptide-reactive T cells and peripheral tolerance to myelin basic protein. Res. Immunol. 149:827–834. [DOI] [PubMed] [Google Scholar]
- 35.Zamvil, S.S., and L. Steinman. 1990. The T lymphocyte in experimental allergic encephalomyelitis. Annu. Rev. Immunol. 8:579–621. [DOI] [PubMed] [Google Scholar]
- 36.O'Garra, A., L. Steinman, and K. Gijbels. 1997. CD4+ T-cell subsets in autoimmunity. Curr. Opin. Immunol. 9:872–883. [DOI] [PubMed] [Google Scholar]
- 37.Weiner, H.L. 2001. Oral tolerance: immune mechanisms and the generation of Th3-type TGF- beta-secreting regulatory cells. Microbes Infect. 3:947–954. [DOI] [PubMed] [Google Scholar]
- 38.Bhardwaj, V., V. Kumar, I.S. Grewal, T. Dao, P.V. Lehmann, H.M. Geysen, and E.E. Sercarz. 1994. T cell determinant structure of myelin basic protein in B10.PL, SJL/J, and their F1S. J. Immunol. 152:3711–3719. [PubMed] [Google Scholar]
- 39.Matsuda, J.L., O.V. Naidenko, L. Gapin, T. Nakayama, M. Taniguchi, C.R. Wang, Y. Koezuka, and M. Kronenberg. 2000. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar, V., V. Bhardwaj, L. Soares, J. Alexander, A. Sette, and E. Sercarz. 1995. Major histocompatibility complex binding affinity of an antigenic determinant is crucial for the differential secretion of interleukin 4/5 or interferon gamma by T cells. Proc. Natl. Acad. Sci. USA. 92:9510–9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharif, S., G.A. Arreaza, P. Zucker, Q.S. Mi, J. Sondhi, O.V. Naidenko, M. Kronenberg, Y. Koezuka, T.L. Delovitch, J.M. Gombert, et al. 2001. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat. Med. 7:1057–1062. [DOI] [PubMed] [Google Scholar]
- 42.Wang, B., Y.B. Geng, and C.R. Wang. 2001. CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes. J. Exp. Med. 194:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong, S., M.T. Wilson, I. Serizawa, L. Wu, N. Singh, O.V. Naidenko, T. Miura, T. Haba, D.C. Scherer, J. Wei, et al. 2001. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat. Med. 7:1052–1056. [DOI] [PubMed] [Google Scholar]
- 43a.Singh, A.K., M.T. Wilson, S. Hong, D. Olivares-Villagómez, C. Du, A.K. Stanic, S. Joyce, S. Sriram, Y. Koezuka, and L. Van Kaer. 2001. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J. Exp. Med. 194:1801–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fritz, R.B., and M.L. Zhao. 2001. Regulation of experimental autoimmune encephalomyelitis in the C57BL/6J mouse by NK1.1+, DX5+, alpha beta+ T cells. J. Immunol. 166:4209–4215. [DOI] [PubMed] [Google Scholar]
- 45.Carnaud, C., D. Lee, O. Donnars, S.H. Park, A. Beavis, Y. Koezuka, and A. Bendelac. 1999. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163:4647–4650. [PubMed] [Google Scholar]
- 46.Kitamura, H., K. Iwakabe, T. Yahata, S. Nishimura, A. Ohta, Y. Ohmi, M. Sato, K. Takeda, K. Okumura, L. Van Kaer, et al. 1999. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 189:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eberl, G., P. Brawand, and H.R. MacDonald. 2000. Selective bystander proliferation of memory CD4+ and CD8+ T cells upon NK T or T cell activation. J. Immunol. 165:4305–4311. [DOI] [PubMed] [Google Scholar]
- 48.Kitamura, H., A. Ohta, M. Sekimoto, M. Sato, K. Iwakabe, M. Nakui, T. Yahata, H. Meng, T. Koda, S. Nishimura, et al. 2000. alpha-galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell. Immunol. 199:37–42. [DOI] [PubMed] [Google Scholar]
- 49.Kumar, H., A. Belperron, S.W. Barthold, and L.K. Bockenstedt. 2000. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J. Immunol. 165:4797–4801. [DOI] [PubMed] [Google Scholar]
- 50.Exley, M.A., N.J. Bigley, O. Cheng, S.M. Tahir, S.T. Smiley, Q.L. Carter, H.F. Stills, M.J. Grusby, Y. Koezuka, M. Taniguchi, and S.P. Balk. 2001. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. J. Leukoc. Biol. 69:713–718. [PubMed] [Google Scholar]
- 51.Miyamoto, K., S. Miyake, and T. Yamamura. 2001. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 413:531–534. [DOI] [PubMed] [Google Scholar]