Abstract
We analyzed the interaction between human peripheral blood natural killer (NK) cells and monocyte-derived immature dendritic cells (DC). Fresh NK cells were activated, as indicated by the induced expression of the CD69 antigen, and their cytolytic activity was strongly augmented by contact with lipopolysaccharide (LPS)-treated mature DC, or with immature DC in the presence of the maturation stimuli LPS, Mycobacterium tuberculosis or interferon (IFN)-α. Reciprocally, fresh NK cells cultured with immature DC in the presence of the maturation stimuli strongly enhanced DC maturation and interleukin (IL)-12 production. IL-2–activated NK cells directly induced maturation of DC and enhanced their ability to stimulate allogeneic naive CD4+ T cells. The effects of NK cells were cell contact dependent, although the secretion of IFN-γ and TNF also contributed to DC maturation. Within peripheral blood lymphocytes the reciprocal activating interaction with DC was restricted to NK cells, because the other lymphocyte subsets were neither induced to express CD69, nor induced to mature in contact with DC. These data demonstrated for the first time a bidirectional cross talk between NK cells and DC, in which NK cells activated by IL-2 or by mature DC induce DC maturation.
Keywords: dendritic cell maturation, cytotoxicity, natural killer cells, cytokines, human
Introduction
NK cells are effector cells of innate resistance that are able to lyse a variety of cells, including tumor and virus-infected cells, spontaneously in the absence of Ag-specific recognition and clonal expansion (1). The cytolytic activity of NK cells is augmented by IL-2 and, to a lesser extent, by other cytokines, including IL-12 and IFN-α. Activated NK also acquire the capacity to lyse target cells that are resistant to resting NK cells, and produce cytokines, particularly IFN-γ. IFN-γ–producing NK cells regulate innate resistance by activating phagocytic cells and priming APC for IL-12 production, thus shaping adaptive immunity toward a Th-1 response (2).
Dendritic cells (DC)* augment the cytotoxic functions of NK cells (3–5). DC are APC with the unique ability to induce primary immune responses. Resting immature DC reside in all tissues. Signals inducing maturation of DC include microbial products and interaction with activated T lymphocytes, which signal through binding of their Ag receptor to peptide–MHC class II complexes, and through costimulatory molecules. Mature DC accumulate long lasting, MHC–peptide complexes on their surface, upregulate costimulatory molecules, secrete cytokines, and migrate to secondary lymphoid tissues where they trigger specific T cell responses (6).
In this study, we analyzed the interaction between human peripheral blood NK cells and monocyte-derived DC upon the activation/maturation of either cell type, and found a potent, activating cross talk between NK cells and DC.
Materials and Methods
Cells and Cell Culture.
All reagents used in this study were tested for low endotoxin contamination using the Limulus amoebocyte assay (Associates of Cape Cod). PBMC were from healthy human donors and PBL were from PBMC after monocyte depletion by plastic adherence. DC were obtained by 6-d culture of plastic-adherent PBMC in medium with 12 ng/ml IL-4 (108 U/mg; CLB) and 50 ng/ml GM-CSF (1.107 U/mg; Novartis). For NK cell purification, frozen aliquots of PBMC were thawed and depleted of T and B lymphocytes and monocytes by magnetic depletion using FITC–anti-CD3, –anti-CD19, and –anti-CD14 mAb, goat anti-FITC–magnetic microbeads, BS separation columns, and a VarioMACS magnet (Miltenyi Biotec). After depletion, FITC-stained cells were less than 1%, and CD16+/CD56+ NK cells were from 85 to 95%. NK cells were depleted by magnetic depletion using FITC–anti-CD16 and –anti-CD56 mAb. NK cells (0.25 × 106/well) and DC (0.1 × 106/well) were cultured together or separately in 48-well plates for 18 h in or out of the presence of the following stimuli: LPS (1 μg/ml; Sigma-Aldrich); heat-killed Mycobacterium tuberculosis cell suspension (H37Rv strain, 1.5 × 106 CFU/ml; provided by L. Fattorini, Istituto Superiore di Sanità, Rome, Italy); IFN-α, (1,000 U/ml; Schering-Plough Laboratory for Immunological Research); IL-2 (400 U/ml; provided by Chiron Corp.). Where indicated, immature DC were incubated for 18 h in the presence or the absence of LPS or IFN-α, and washed before coculture with resting NK cells for an additional 18 h. IL-2–activated or untreated NK cells, PBL, and NK-depleted PBL were obtained by 18-h (NK) or 48-h (PBL and NK-depleted PBL) treatment with or without IL-2 (250 U/ml), and washed before culture for an additional 18 h with immature DC. The following neutralizing mAb: B133.3, anti–IFN-γ and B154.2, anti-TNF, were used as ascites, 1:200.
Cell-mediated Cytotoxicity Assay.
NK cells and DC, cultured alone or together, were washed and incubated at different effector–target ratios in U-bottomed microtiter plates, with 5 × 103 51Cr-labeled Daudi cells for 4 h.
Immunofluorescence Assay.
Expression of the antigens CD86, CD83, CD80, HLA-DR, and CCR7 was detected on DC by direct (PE-conjugated anti-CD86 and anti-CD80, FITC-conjugated anti-CD83, and D1-12 mabs), or indirect (anti-CCR7 mAb) immunofluorescence and flow cytometry (Epics XL; Beckman Coulter). CD69 antigen expression was detected on PBL by double staining with PE-Cy5–conjugated anti-CD69 (Immunotech) mAb and FITC-conjugated anti-CD3, or anti-CD16 plus anti-CD56 mAbs. In DC–NK cell cocultures, viable DC were distinguished from NK cells or dead DC by side and forward scatter parameters. Percent DC recovery was calculated as the number of viable DC after culture with NK cells, relative to DC cultured alone.
Detection of IFN-γ and IL-12 p70 and p40.
These cytokines were quantitated in cell culture supernatants by RIA using the following mAb: B133.1 and B133.5 for IFN-γ; 12H4 and C8.6 for IL-12 p70; and C11.79 and C8.6 for IL-12 p40. Recombinant human IFN-γ (specific activity 2 × 107 U/mg; provided by Roussel Uclaf), IL-12 p70, and p40 were used as reference standards.
Naive CD4+ T Lymphocyte Proliferation.
DC were cultured for 18 h with medium, LPS, or with IL-2–activated or untreated NK. NK cells were removed from DC by magnetic depletion. After irradiation (3,000 rads), DC were cultured at graded numbers with allogeneic PBL (0.8 × 105 cells/well) that had been depleted of CD45RO+, CD8+, CD16+ and, CD56+ by mAbs and goat anti–mouse IgG conjugated magnetic beads. Proliferation was evaluated after 5-d culture by 3H-TdR uptake.
Results
Activation and Enhancement of Cytotoxic Activity in NK Cells Cultured with DC in the Presence of Different Stimuli.
NK cells after an 18-h culture in the presence or absence of DC, mediated minimal cytotoxicity against the NK-resistant Daudi cell line (Fig.1 A). In the absence of DC, heat-killed M. tuberculosis, and IFN-α, but not LPS, modestly enhanced NK cell–mediated cytotoxicity (Fig. 1 A). However, NK cell cytotoxicity was highly augmented after an 18-h culture with autologous (Fig. 1 A) or allogeneic (unpublished data) DC in the presence of M. tuberculosis, IFN-α, or LPS. Identical results were obtained with two other tumor target cell lines (unpublished data). LPS-pretreated DC, but not IFN-α–pretreated DC, induced strong cytotoxic activity in cocultured NK cells (Fig. 1 B). When PBL were cultured with LPS-pretreated DC, CD69 activation antigen was induced in most NK cells (CD16+, CD56+) but not in T cells (Fig. 1 C). Separation of NK cells and DC by a porous membrane during the culture in the presence of LPS completely abrogated the induction of cytotoxic activity, whereas neutralizing anti–IL-12 or anti–IL-2 mAb did not prevent activation of NK cell cytotoxicity, or CD69 expression (unpublished data).
Figure 1.
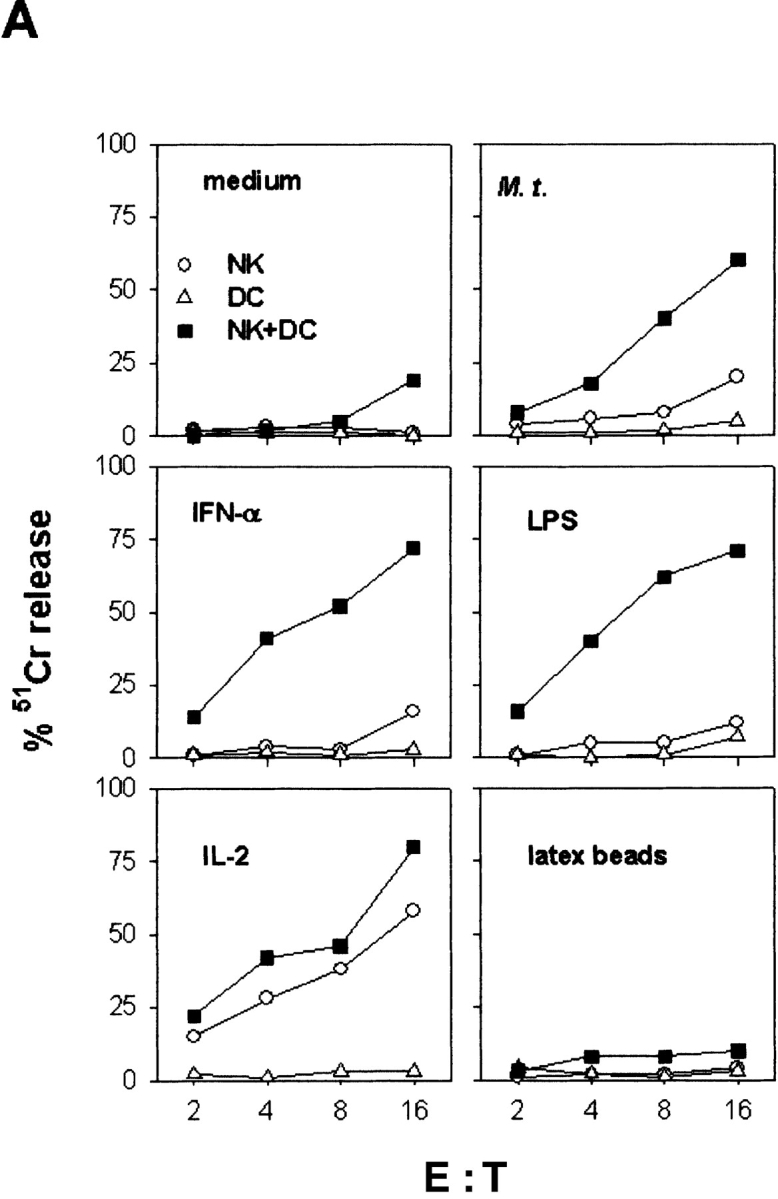
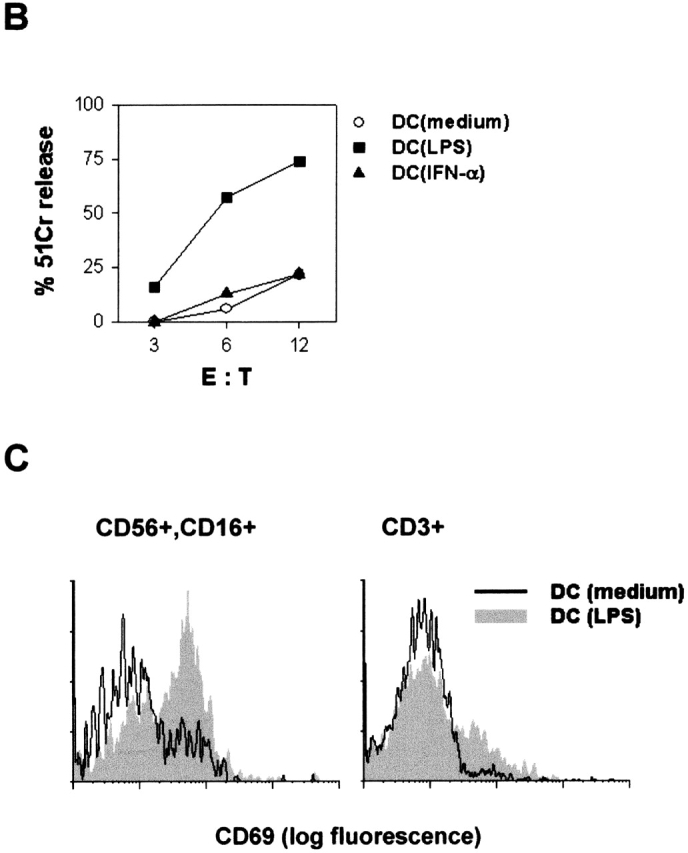
Activation and generation of cytotoxic activity in NK cells cultured with DC. (A) Purified NK cells and DC were cultured for 18 h separately or together in medium, or in the presence of killed M. tuberculosis (M. t.), IFN-α, LPS, IL-2, or latex particles. (B) DC were cultured for 18 h in medium, or with LPS or IFN-α, and washed before 18-h coculture with fresh NK cells. Cytolytic activity (A and B) was evaluated against the Daudi cell line. (C) DC were cultured for 18 h in medium or with LPS and washed before a 18-h coculture with PBL; CD69 expression was evaluated on CD56+, CD16+, or on CD3+ PBL by double immunofluorescence.
Cytokine Production and DC Activation in Cultures of NK Cells and DC.
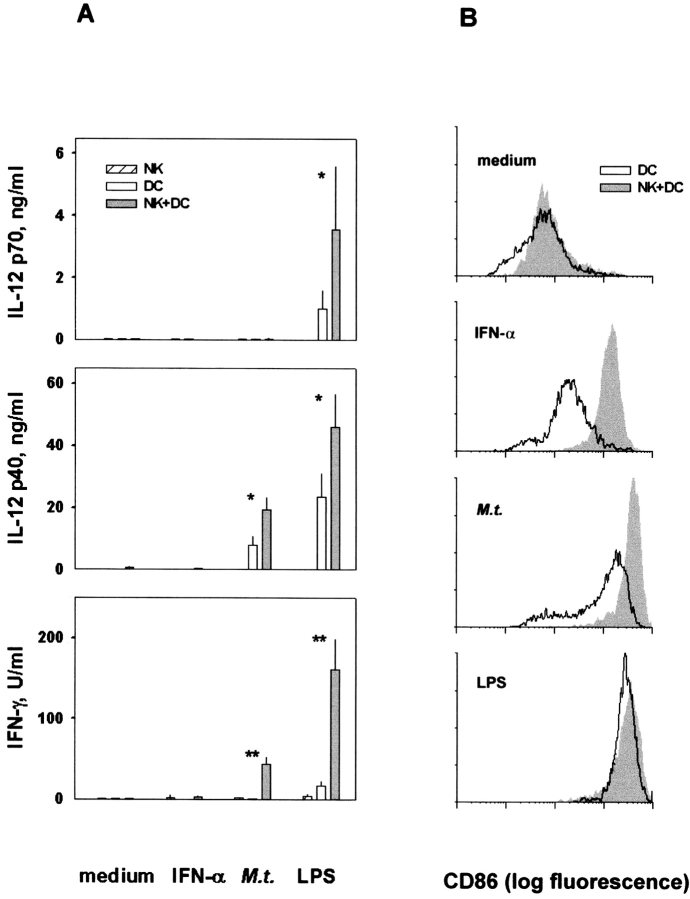
The production of IL-12 heterodimer (p70), IL-12 free heavy chain (p40), and IFN-γ was analyzed in cultures of NK cells and/or DC (Fig. 2 A). DC produced IL-12 p70 after stimulation with LPS, but not with M. tuberculosis or IFN-α. The presence of NK cells in culture with DC significantly increased IL-12 p70 production after stimulation with LPS, but not with M. tuberculosis and IFN-α. DC stimulated with either LPS or M. tuberculosis produced considerable amounts of the free p40 chain, and the presence of NK cells increased its production. IFN-γ was observed in supernatants of LPS-, and to a lesser extent, M. tuberculosis–treated NK–DC cocultures, whereas it was almost undetectable in supernatants of NK cells or DC cultured alone, irrespective of the stimulus (Fig. 2 A). A neutralizing anti–IL-12 mAb almost completely abrogated IFN-γ production (unpublished data). In IFN-α–treated NK–DC cocultures, IFN-γ was undetectable.
Figure 2.
Cytokine production and CD86 expression on DC in mixed culture of NK cells and DC. Fresh NK cells and DC were cultured separately or together in the absence of any stimulus (medium), or in the presence of IFN-α, killed M. tuberculosis (M. t.), or LPS. (A) IL-12 p70, p40, and IFN-γ were measured in culture supernatants. Two or one asterisks indicate P < 0.005 or P < 0.05, respectively, by Student's t test, using two-tailed distribution of paired samples, comparing cytokine production by cells cultured separately or together. (B) CD86 expression was determined on DC.
LPS induced strong upregulation of CD86 antigen on DC (Fig. 2 B). M. tuberculosis and IFN-α induced lower levels of CD86 antigen than LPS, whereas IL-2 and latex beads had no effect (unpublished data). In the absence of maturation stimuli, the addition of fresh NK cells to DC cultures had little or no effect, but in the presence of IFN-α, M. tuberculosis, or LPS, NK cells strongly enhanced CD86 expression (Fig. 2 B). The NK cell enhancement of LPS-induced CD86 expression was more evident when suboptimal concentrations of LPS were used (unpublished data).
IL-2–activated NK Cells Induce the Functional Maturation of DC.
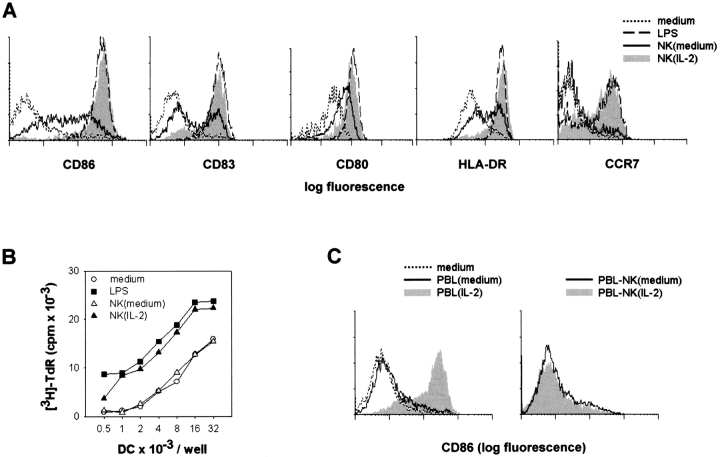
The enhanced expression of CD86 and IL-12 in NK–DC mixed cultures, in conditions in which both NK cells and DC are activated, suggests that not only do mature DC activate NK cells, but also that activated NK cells induce maturation of DC. To dissociate the two phenomena, NK cells were preactivated with IL-2. Immature DC cultured for 18 h with IL-2–activated NK cells, but not with untreated NK cells, strongly expressed several maturation markers (Fig. 3 A). After elimination of NK cells, these DC induced proliferation of allogeneic naive CD4+ T cells (Fig. 3 B), at levels comparable to those induced by LPS-treated mature DC.
Figure 3.
IL-2–activated NK cells induce DC maturation. (A and B) DC were cultured for 18 h in medium only or in the presence of LPS, IL-2–activated [NK(IL-2)] or untreated [NK(medium)] NK cells. (A) Cells were washed and DC expression of CD86, CD83, CD80, HLA-DR, and CCR7 was analyzed by immunofluorescence. (B) DC, depleted of NK cells and irradiated, were cultured for 5 d with allogeneic naive CD4+ PBL, and 3H-TdR uptake was determined. (C) CD86 expression was determined on DC after an 18-h culture in the absence of any stimulus, or with PBL, or NK-depleted PBL (PBL–NK), either IL-2–activated or untreated.
The number of DC recovered at the end of the 18-h culture with IL-2–activated NK cells (62 ± 13% average recovery ± SD, n = 10) was lower than after coculture with untreated NK cells (92 ± 13), suggesting that the DC maturation induced by IL-2–activated NK cells is accompanied by the lysis of a variable proportion of DC.
PBL treated for 48 h with IL-2 also induced CD86 expression on DC (Fig. 3 C). However, depletion of NK cells from IL-2–activated PBL completely abolished this effect, demonstrating that among PBL, only NK cells are activated by IL-2 to induce DC maturation.
Both Cell to Cell Interaction and Cytokines are Responsible for the Induction of DC Maturation by NK Cells.
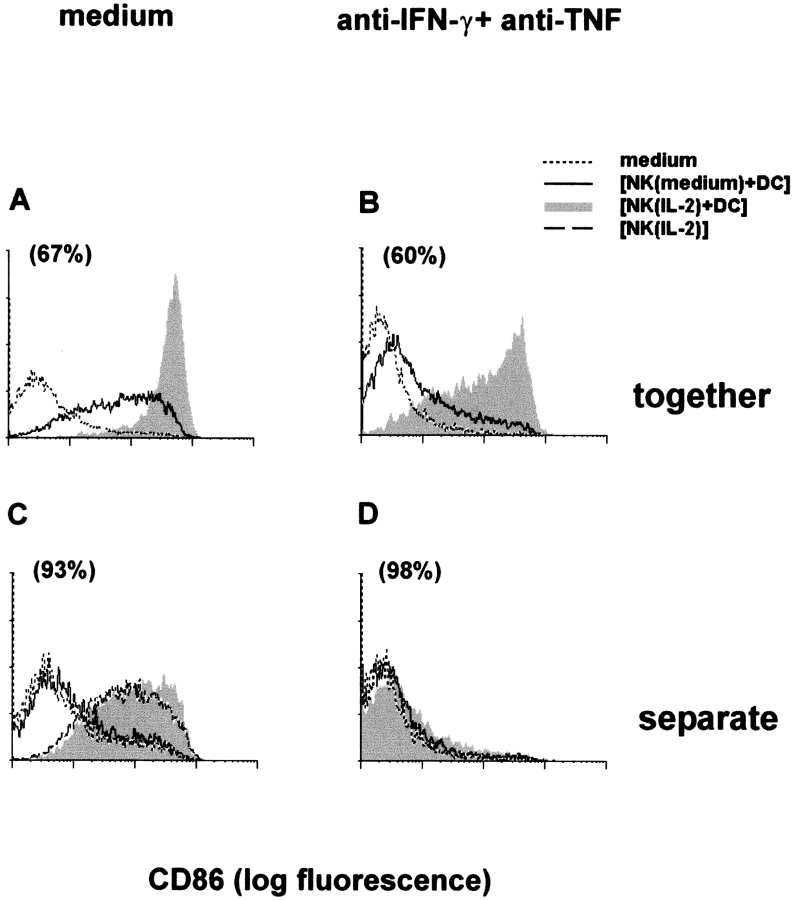
Maturation was evaluated on DC cultured with IL-2–activated or untreated NK cells in the same well or across a porous membrane. Strong CD86 expression was induced on DC cultured in contact with IL-2–activated NK cells (Fig. 4 A), whereas a much lower expression was induced on DC exposed across a porous membrane to IL-2–activated NK cells, either mixed with DC or not (Fig. 4 C).
Figure 4.
Contact between IL-2–activated NK cells and immature DC is required for induction of CD86 on DC. CD86 was evaluated on DC cultured for 18 h in the same well (A and B) with IL-2–activated [NK(IL-2)] or untreated [NK(medium)] NK cells, or across a porous membrane (C and D) from IL-2–activated NK cells and DC, untreated NK cells and DC, or IL-2–activated NK cells (C) in the absence (A and C) or in the presence (B and D) of neutralizing anti–IFN-γ plus anti-TNF mAb. CD86 expression in DC cultured in the absence of NK cells (medium) is indicated by dotted lines. Numbers in brackets indicate percent recovery of DC after culture with IL-2–activated NK cells in comparison to DC cultured alone.
We evaluated whether IFN-γ and TNF mediate the DC maturation effect of NK cells. IL-2–activated NK cells produced IFN-γ (from undetectable to 200 U/ml in seven different experiments), whereas TNF was always below the limit of detection. Immature DC spontaneously secreted TNF (from undetectable to 1.1 ng/ml). In cocultures, TNF was detected by intracytoplamic immunofluorescence in DC, but not in resting or in IL-2–activated NK, and its expression was increased in comparison to DC cultured alone (unpublished data). CD86 expression in DC treated for 18 h with human recombinant IFN-γ (1,000 or 100 U/ml) or TNF (20 ng/ml) was negligible, whereas it was induced by the combination of the two cytokines, although at a much lower level than that induced by IL-2–activated NK cells (unpublished data). A combination of neutralizing anti–IFN-γ and anti-TNF mAb only partially decreased CD86 expression in DC cultured with IL-2–activated NK cells (Fig. 4 B). CD86 expression in DC exposed across a porous membrane to IL-2–activated NK–DC cocultures, in contrast, was completely abrogated by the mAb (Fig. 4 D). Results using neutralizing anti–IFN-γ and anti-TNF mAb separately indicated a predominant role of TNF in DC maturation (unpublished data).
Discussion
In this paper we demonstrate for the first time a bidirectional cross talk between NK cells and DC resulting in activation of both cell types. In the presence of a maturation stimulus, DC activate NK cells that in turn strongly enhance DC maturation. Previous reports in murine (3) and human (4, 5) models demonstrated that DC enhance the cytolytic activity of resting NK cells. However, the requirement for direct NK–DC contact, soluble factors, and DC maturation varies in the different studies. We show that human DC, matured by microbial stimuli, induce in resting NK cells the expression of the CD69 activation antigen and a strong cytolytic activity against NK-resistant target cells. This effect depends on cell to cell contact, whereas it is independent from endogenous production of IL-12 or IL-2.
In the mixed cultures of NK cells and DC in the presence of DC maturation stimuli, not only were NK cells activated, but also a much stronger DC maturation was observed than in the absence of NK cells. The presence of NK cells increased both the expression of the costimulatory molecule CD86 on DC and the production of IL-12. The effect of NK cells was particularly dramatic when relatively inefficient maturation inducers such as IFN-α and M. tuberculosis or suboptimal concentrations of LPS were used. In particular, it is of interest to note that IFN-α alone was almost inefficient in inducing either activation of NK cell cytotoxicity or DC maturation, but very potent in the mixed cultures, suggesting a positive feedback loop that augments both NK activation and DC maturation.
To dissect the mechanism of DC maturation mediated by activated NK cells in the absence of other maturation stimuli, we used IL-2–activated NK cells. IL-2, a strong activator of NK cells even in the absence of accessory cells such as DC, does not act directly on DC-inducing maturation. IL-2–activated NK cells induced DC maturation at a similar extent as LPS, as indicated by increased expression of several maturation markers (CD80, CD83, CD86, HLA-DR, and CCR7) and ability to induce an allogeneic T lymphocyte response. Although activated T cells also induce DC maturation, NK cells are the only resting cell type within peripheral blood that can be rapidly activated by IL-2 or mature DC to mediate this function.
Direct cell to cell contact between IL-2–activated NK cells and DC is necessary for a strong CD86 expression in DC. This effect does not require IFN-γ and TNF production, because a combination of neutralizing antibodies able to inhibit much higher concentrations of the two cytokines than those produced in the mixed cultures only marginally inhibited DC maturation. However, TNF, produced by DC, and IFN-γ, produced by IL-2–activated NK cells, variably contribute to enhance induction of DC maturation. High concentrations of recombinant IFN-γ and TNF synergized to induce DC maturation, but never to the extent induced by IL-2–activated NK cells or by LPS. It remains to be evaluated whether other soluble factor(s) might be required.
The ability of cytokine-activated NK cells to lyse immature DC much more efficiently than the mature ones (7, 8, and unpublished data) could suggest that the observed, increased expression of maturation markers after culture of DC with IL-2–activated NK cells were due to the selective survival of a proportion of mature DC, already present within the population or induced by the released cytokines. However, an analysis of the high percent recovery of the DC cocultured with the activated NK cells, and the low number of mature DC in the population even after exposure to the soluble factors released by the NK–DC cultures, clearly shows that a selective lysis of immature DC may only minimally contribute to the strongly increased CD86 expression observed in DC after coculture with IL-2–activated NK cells. Because exposure to necrotic cells induces DC maturation (9), exposure to the DC killed by IL-2–activated NK cells might contribute to the maturation of bystander DC. Indeed expression of CD86 antigen was induced on immature DC cultured in the presence of up to 70% DC killed by repeated freezing and thawing. However, that concentration of necrotic cells was much higher than that observed in the mixed cultures, and the level of CD86 induced in these conditions was much lower than that induced by IL-2–activated NK cells, suggesting only a secondary role, if any, for necrotic DC uptake in the mechanism of NK cell–induced DC maturation.
The interaction of immature DC with IL-2–activated NK cells results in either maturation or cell death. The mechanisms that determine the choice between death and maturation when DC are exposed to activated NK cells remain to be evaluated. At higher NK–DC ratios than those used here to study DC maturation, predominant DC death rather than maturation was indeed observed. Receptors mediating the NK cell–mediated lysis of DC (10, 11) might also be responsible for induction of DC maturation. TNF family ligands are involved in NK cell–mediated target lysis (12, 13) and in the maturation of DC induced by activated T cells (14–17). The ability of the neutralizing anti-TNF mAb to partially prevent DC maturation might be partly due to inhibition of membrane TNF, reportedly expressed in NK cells (13, 18) in addition to the neutralization of soluble TNF, released from DC. Expression of CD40 on DC has been reported to contribute, at least in part, to lysis of DC by cytokine-activated NK cells (7). However, the presence of a neutralizing anti-CD40 mAb during cocultures of IL-2–activated NK cells with DC, affected neither CD86 expression nor DC recovery (unpublished data). This result, together with the absence of CD40 ligand on IL-2–activated NK cells (unpublished data), suggests that other receptors may play a role in DC activation. TNF-related activation-induced cytokine was expressed at similar levels on both resting and activated NK cells (unpublished data), suggesting that it does not play a determinant role in DC maturation induced by IL-2–activated NK cells. CD134, which mediates interactions between T lymphocytes and mature OX40L-expressing DC (17), increases in NK cells after activation with IL-2 (unpublished data), suggesting that it may be a candidate in mediating the interaction between NK cells and DC.
The efficient cross talk between NK cells and DC is analogous to that between activated T lymphocytes and DC, and allows the reciprocal transfer of an activation state between NK cells and DC. It would be expected that the interaction between NK cells and DC is regulated in order to prevent unnecessary activation of self-reactive or bystander cells. Indeed, minimal cooperation was detected between resting NK cells and immature DC, whereas the potent activating interaction between resting NK cells and mature DC, and the reciprocal one between activated NK cells and immature DC, may be functionally relevant in different stages of the immune response. After the encounter with a pathogen, DC mature and induce activation of resting NK cells. A different type of precursor DC, the plasmacytoid DC, upon virus infection produces IFN-α (19, 20) that in the presence of DC enhances the activation of NK cells. NK cells are not only effector cells of innate resistance, but also regulatory cells in both innate resistance and adaptive immunity. They have been shown in both humans and mice to be required for resistance to leishmaniasis, for the induction of a Th-1 response by IL-12, and to regulate B cell responses (21). Whereas the innate effector functions of NK cells are dependent on their cytolytic activity, their regulatory functions have been ascribed principally to their ability to produce cytokines early after infection, particularly IFN-γ, thus favoring activation of phagocytic cells, IL-12 production from APC, and development of Th-1 cells that produce pro-inflammatory cytokines. We now show that NK cells activated either by IL-2 or by DC exposed to microbial products or IFN-α, directly induce DC maturation and strikingly enhance the effect of microbial maturation stimuli. Thus, early during an infection, before antigen-specific T cells are expanded, NK cells may become activated and amplify the maturation of DC induced by microbial products or by virus-induced IFN, facilitating the activation and expansion of antigen-specific naive T cells. The activated NK cells could also lyse tumor cells and virus-, bacteria-, or parasite-infected cells and provide immature DC with antigenic cellular material to be internalized and presented to T cells upon maturation. At later stages of the response, after IL-2 release by antigen-specific Th-1 cells, IL-2–activated NK cells directly inducing the maturation of DC might play a powerful role in enhancing the specific immune response. Conversely, an excessive immune response might be limited by the mechanisms that regulate the choice between maturation and lysis of DC. Activated NK cells when present in sufficient number may induce death rather than activation, particularly in immature DC. Therefore, depending on the stage and on the intensity of the immune response, the cross talk between NK cells and DC may result in either the initiation/amplification or the shutting off of antigen presentation.
Acknowledgments
We thank Christophe Caux for critically reading the manuscript and the Centro Trasfusionale, Policlinico G.B. Rossi, for buffy coats.
This work was supported by the Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST) 40% project “Infiammazione: biologia e clinica,” University of Verona, and Azienda Ospedaliera di Verona.
Footnotes
Abbreviation used in this paper:DC, dendritic cells.
References
- 1.Trinchieri, G. 1989. Biology of natural killer cells. Adv. Immunol. 47:187–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trinchieri, G. 1995. Natural killer cells wear different hats: effector cells of innate resistance and regulatory cells of adaptive immunity and hematopoiesis. Semin. Immunol. 7:83–88. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez, N.C., A. Lozier, C. Flament, P. Ricciardi-Castagnoli, D. Bellet, M. Suter, M. Perricaudet, T. Tursz, E. Maraskovsky, and L. Zitvogel. 1999. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune response in vivo. Nat. Med. 5:405–411. [DOI] [PubMed] [Google Scholar]
- 4.Yu, Y., M. Hagihara, K. Ando, B. Gansuvd, H. Matsuzawa, T. Tsuchiya, Y. Ueda, H. Inoue, T. Hotta, and S. Kato. 2001. Enhancement of human cord blood CD34+ cell-derived NK cell cytotoxicity by dendritic cells. J. Immunol. 166:1590–1600. [DOI] [PubMed] [Google Scholar]
- 5.Nishioka, Y., N. Nishimura, Y. Suzuki, and S. Sone. 2001. Human monocyte-derived and CD83(+) blood dendritic cells enhance NK cell-mediated cytotoxicity. Eur. J. Immunol. 31:2633–2641. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.J. Liu, B. Pulendran, and K Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811. [DOI] [PubMed] [Google Scholar]
- 7.Carbone, E., G. Terrazzano, G. Ruggiero, D. Zanzi, A. Ottaiano, C. Manzo, K. Kärre, and S. Zappacosta. 1999. Recognition of autologous dendritic cells by human NK cells. Eur. J. Immunol. 29:4022–4029. [DOI] [PubMed] [Google Scholar]
- 8.Wilson, J.L., L.C. Heffler, J. Charo, A. Scheynius, M.T. Bejarano, and H.G. Ljunggren. 1999. Targeting of human dendritic cells by autologous NK cells. J. Immunol. 163:6365–6370. [PubMed] [Google Scholar]
- 9.Sauter, B., M.A. Albert, L. Francisco, M. Larsson, S. Somersan, and N. Bhardwaj. 2000. Consequences of cell death: exposure to necrotic tumor cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers, B.J., M. Salcedo, and H.G. Ljunggren. 1996. Triggering of natural killer cells by the costimulatory molecule CD80 (B7-1). Immunity. 5:311–317. [DOI] [PubMed] [Google Scholar]
- 11.Geldhof, A.B., M. Moser, L. Lespagnard, K. Thielemans, and P. De Baetselier. 1998. Interleukin-12-activated natural killer cells recognize B7 costimulatory molecules on tumor cells and autologous dendritic cells. Blood. 91:196–206. [PubMed] [Google Scholar]
- 12.Carbone, E., G. Ruggiero, G. Terrazzano, C. Palomba, C. Manzo, S. Fontana, H. Spits, K. Karre, and S. Zappacosta. 1997. A new mechanism of NK cell cytotoxicity activation: the CD40–CD40 ligand interaction. J. Exp. Med. 185:2053–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kashii, Y., R. Giorda, R.B. Herberman, T.L. Whiteside, and N.L. Vujanovic. 1999. Constitutive expression and role of the TNF family ligands in apoptotic killing of tumor cells by human NK cells. J. Immunol. 163:5358–5366. [PubMed] [Google Scholar]
- 14.Ridge, J.P., F. Di Rosa, and P.A. Matzinger. 1998. Conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 393:474–478. [DOI] [PubMed] [Google Scholar]
- 15.Anderson, D.M., E. Maraskovsky, W.L. Billingsley, W.C. Dougall, M.E. Tometsko, E.R. Roux, M.C. Teepe, R.F. DuBose, D. Cosman, and L.A. Galibert. 1997. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 390:175–179. [DOI] [PubMed] [Google Scholar]
- 16.Rescigno, M., V. Piguet, B. Valzasina, S. Lens, R. Zubler, L. French, V. Kindler, J. Tschopp, and P. Ricciardi-Castagnoli. 2000. Fas engagement induces the maturation of dendritic cells (DCs), the release of interleukin (IL)-1b, and the production of interferon γ in the absence of IL-12 during DC-T cell cognate interactions: a new role for Fas ligand in inflammatory responses. J. Exp. Med. 192:1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, A.I., A.J. McAdam, J.E. Buhlmann, S. Scott, M.L. Lupher, Jr., E.A. Greenfield, P.R. Baum, W.C. Fanslow, D.M. Calderhead, G.J. Freeman, and A.H. Sharpe. 1999. Ox40-ligand has a critical costimulatory role in dendritic cell: T cell interactions. Immunity. 11:689–698. [DOI] [PubMed] [Google Scholar]
- 18.Caron, G., Y. Delneste, J.P. Aubry, G. Magistrelli, N. Herbault, A. Blaecke, A. Meager, J.Y. Bonnefoy, and P. Jeannin. 1999. Human NK cells constitutively express membrane TNF-alpha (mTNFalpha) and present mTNFalpha-dependent cytotoxic activity. Eur. J. Immunol. 29:3588–3595. [DOI] [PubMed] [Google Scholar]
- 19.Siegal, F.P., N. Kadowaki, M. Shodell, P.A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y.J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science. 284:1835–1837. [DOI] [PubMed] [Google Scholar]
- 20.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919–923. [DOI] [PubMed] [Google Scholar]
- 21.Scott, P, and G. Trinchieri. 1995. The role of natural killer cells in host-parasite interactions. Curr. Opin. Immunol. 7:34–40. [DOI] [PubMed] [Google Scholar]