Abstract
Exceptionally germinal center formation can be induced without T cell help by polysaccharide-based antigens, but these germinal centers involute by massive B cell apoptosis at the time centrocyte selection starts. This study investigates whether B cells in germinal centers induced by the T cell–independent antigen (4-hydroxy-3-nitrophenyl)acetyl (NP) conjugated to Ficoll undergo hypermutation in their immunoglobulin V region genes. Positive controls are provided by comparing germinal centers at the same stage of development in carrier-primed mice immunized with a T cell–dependent antigen: NP protein conjugate. False positive results from background germinal centers and false negatives from non-B cells in germinal centers were avoided by transferring B cells with a transgenic B cell receptor into congenic controls not carrying the transgene. By 4 d after immunization, hypermutation was well advanced in the T cell–dependent germinal centers. By contrast, the mutation rate for T cell–independent germinal centers was low, but significantly higher than in NP-specific B cells from nonimmunized transgenic mice. Interestingly, a similar rate of mutation was seen in extrafollicular plasma cells at this stage. It is concluded that efficient activation of hypermutation depends on interaction with T cells, but some hypermutation may be induced without such signals, even outside germinal centers.
Keywords: spleen, plasma cells, DNA mutational analysis, adoptive cell transfer, immunization
Introduction
Immunoglobulin V region gene hypermutation modifies the primary immune repertoire by generating effector and memory cells of high specificity. This process is a feature of B cells activated to proliferate in germinal centers (1, 2); plasma cells in primary extrafollicular responses have at best a low level of V-region mutation in a minority of cells (3–5). The nature of the transmembrane signals that activate hypermutation in germinal center B cells remains largely unknown. Nevertheless, germinal center formation typically is T cell–dependent, although sporadic germinal centers are seen in congenitally athymic animals (6) and mice deficient in both β and δ T cell receptor genes (7, 8). Exceptionally germinal centers can be formed reproducibly in the absence of T cells. These T cell–independent germinal centers appear to be induced by strong multivalent engagement of B cell receptors alone and do not require signaling through CD40 (9). These observations provide an opportunity to test whether the hypermutation process is activated in T cell–independent germinal centers. We have induced T cell–independent germinal centers by immunizing mice that have transgenic B cells specific for (4-hydroxy-3-nitrophenyl)-acetyl (NP) with NP-Ficoll conjugate. These germinal centers grow with the same kinetics and reach the same size as T cell–dependent NP-specific germinal centers induced by immunizing chicken γ-globulin (CGG)-primed mice with NP-CGG (10). While the T cell–dependent germinal centers go on to produce plasma cells and memory cells over a period of weeks, the germinal centers induced with NP-Ficoll collapse by mass B cell apoptosis a few hours after dark and light zones form. It is assumed that this apoptosis results from failure of the T cell–dependent selection of centrocytes.
In this study high affinity NP-specific transgenic B cells were transferred into either nonimmunized, or CGG-primed, congenic wild-type recipients. They were then challenged with NP-Ficoll or NP-CGG, respectively, to induce T cell–independent or T cell–dependent germinal centers. At 4 d after challenge when the germinal centers had reached peak size and had just developed dark and light zones the cells of the germinal center were microdissected from tissue sections and the transgenic V region sequences of the constituent cells determined as established by Jacob et al. (2). Germinal centers formed in response to high dose NP-Ficoll in TCR β and δ deficient mice have been reported to lack V region mutations (8). The same study reported a low level of mutation in NP-Ficoll–induced germinal centers in wild-type mice, but the data do not exclude the possibility that background germinal center cells were responsible for these mutations. We have controlled for mutated sequences from background germinal centers and still find a low but definite level of hypermutation both in germinal centers and surprisingly in plasma cells in extrafollicular foci. These are antigen-induced mutations, for no mutations were found in 63 VH region sequences of NP-specific B cells from nonimmunized quasimonoclonal (QM) mice.
Materials and Methods
Mice, Cell Transfers, and Immunizations.
Specific pathogen free C57BL/6 mice (Harlan) and QM (quasimonoclonal) mice were maintained in the University of Birmingham animal unit. QM mice are hemizygous for a targeted insertion of a rearranged NP-specific V-D-J (VH17.2.25-DSP2.3-JH4) heavy chain segment and a targeted deletion of the JH region on the other allele; their Jκ loci are deleted (11). QM mice were crossed onto C57BL/6. The F1 hybrids were tissue typed for the presence of a single NP-specific heavy chain transgene (QM donors) or the deletion of the JH region (recipient mice).
For experiments on T cell–dependent germinal centers mice were primed with 50 μg CGG (Jackson ImmunoResearch Laboratories) in alum with 109 killed Bordetella pertussis bacteria intraperitoneally (10). 5 wk later recipient mice received intravenously 106 QM donor splenocytes. 1 d later mice were immunized intraperitoneally with 50 μg soluble NP18-CGG. For T cell–independent germinal centers, unprimed recipients of QM donor splenocytes were given intraperitoneally 30 μg NP32-Ficoll (Biosearch Technologies) 1 d after cell transfer. Spleens were taken 4 d after the last immunization.
Immunohistology.
Frozen spleen sections (5-μm thick) were stained for NP-binding, the QM IgH transgene idiotype, peanut agglutinin binding, IgD, and BCl-6 expression as described (9, 10).
Ig V Region Gene Analysis.
In each experiment, four different NP-specific germinal centers were microdissected using micromanipulator needles (5). Each germinal center was scraped from four different adjacent spleen sections and the V region DNA was amplified separately by nested PCR. Methods are described (4, 5). Primers for the V186.2 family response in C57BL/6 wild-type mice are described (4). Primers for the NP-specific 17.2.25 gene were TTCAGAGGTTCAGCTGCAGCAGT and CTYACCTGAGGAGACDGTGA for the first PCR and primers described (11) for the nested PCR. After digestion, V region genes were amplified as described (5) using PfuTurbo DNA polymerase (Stratagene). Several negative controls for each DNA isolation and amplification step were included. PCR products were inserted in pCR-Blunt II-TOPO vector (Invitrogen), cloned, and sequenced (MWG-Biotech AG). Mutations were verified by comparing sense and antisense strand sequence. PfuTurbo polymerase is inactivated by a heat labile monoclonal antibody. Thermal cycling denatures the antibody and releases fully active Pfu polymerase. The error rate of Pfu DNA polymerase is 1–2 × 10−6 misincorporations/bp (5). To control for the fidelity of our method patches of around 50 cells were scraped from a QM mouse kidney section and the transgene was amplified, cloned, and sequenced; 23 sequences isolated showed no mutations.
The background mutation rate in QM B cells was tested by amplifying V region genes of single NP-specific B220+ splenocytes from an 8-mo-old nonimmunized QM mouse. Splenocytes were stained with FITC B220 and NP-phycoerythrin as described (9). Using the cloning unit of a FACS®, individual cells from the 50% of the B cells staining strongest for NP were sorted into wells of a 96-well PCR plate containing lysis buffer. V region genes of these single cells were amplified by nested PCR; 64 wells produced a 358-bp product. One row of eight wells without cells did not produce a specific product. The products of positive wells were sequenced without cloning.
Statistical Analysis of V Region Sequences.
Mutations were grouped into replacement and silent mutations in framework region (FR) and CDR as defined by Kabat (12). Where the number of replacement mutations is (R) and the number of silent mutations (S) the percentage of replacement mutations was calculated as R/(R + S). The percentage of replacement mutations expected from random mutagenesis was calculated by taking the sum of R and the sum of S for each base expected to occur after a random base exchange and then making the same calculation as above. The expected replacement mutation rate for the CDR3 of the wild-type sequences was calculated from the averages of R and S of the codons of each proposed nonmutated founder sequence. The statistical significance of the differences between mutation rates was calculated using a χ2 test.
Results
Hypermutation in Ig V Region Genes Is Well Developed in Hapten-specific Germinal Centers 4 d after Carrier-primed Mice Are Challenged with Hapten-Protein.
The first evidence for hypermutation was looked for in early T cell–dependent germinal centers in wild-type mice. CGG-primed mice were challenged intraperitoneally with soluble NP-CGG (10). The immediate availability of T cell help in carrier-primed mice results in cognate interaction of NP-specific B cells with T cells within 12 h of immunization with NP-conjugated to the carrier protein CGG (10). Carrier-specific Ig quickly removes antigen from the circulation ensuring only a single cohort of antigen-specific B cells is recruited into the response. Hapten-specific virgin B cells dominate the follicular response (10, 13, 14). At 96 h after immunization, when germinal centers have reached peak size, cells were microdissected from NP-specific germinal centers identified immunohistologically and DNA extracted from the cells was amplified, cloned, and sequenced. The V region sequences are shown in Figs. 1 and 2. Stained adjacent tissue sections confirmed these IgD−, NP-binding B cell clusters in the follicles bind peanut agglutinin and express BCl-6.
Figure 1.
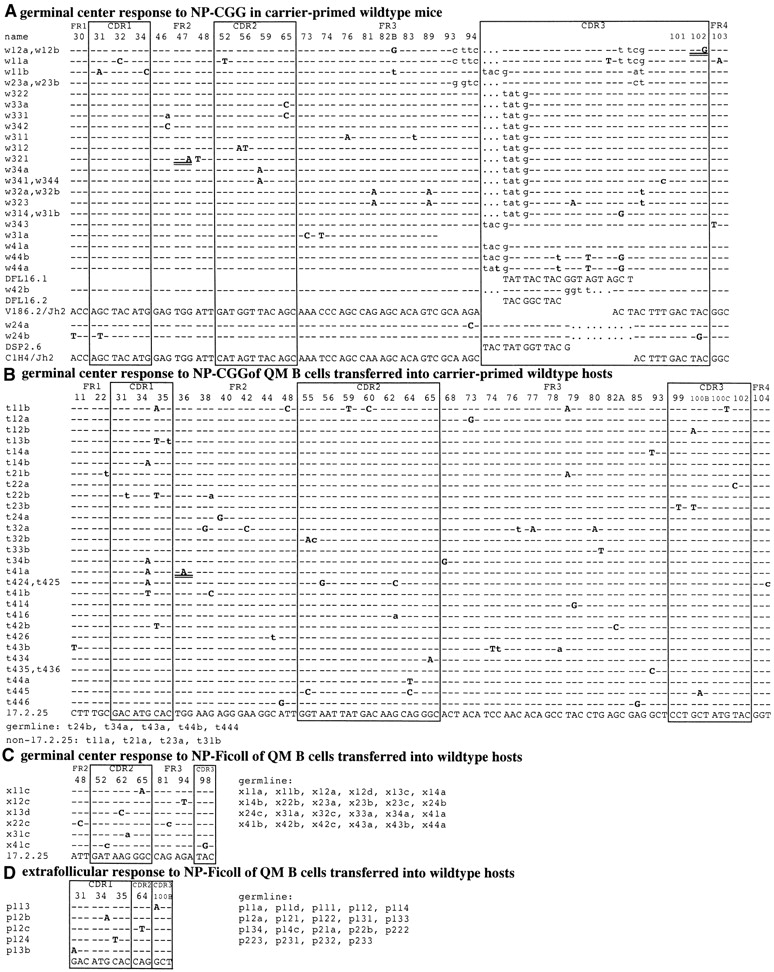
Sequence data from micromanipulated germinal center cells 4 d after immunization with (A) NP-CGG in CGG-primed C57Bl/6 mice, (B) NP-CGG in CGG-primed recipients of QM B cells, (C) NP-Ficoll in recipients of QM B cells, and (D) from extrafollicular plasmablast foci 4 d after NP-Ficoll in recipients of QM B cells. Specific germinal centers were identified by staining for (A) NP-specific cells and IgD, or in (B and C) QM mouse idiotype (17.2.25) and IgD. Sequence names identify the experiment (first letter), the individual germinal center or plasmablast focus (first number), tissue section (second number), and clone number (last number or letter). Only codons with mutations and in (A) all codons of CDR3 are shown; hyphens indicate identity with the germline sequence. Mutations are shown in bold (lower case for silent mutations, upper case for replacement mutations, and double-underlined for premature stop codon mutations). Sequence names of nonmutated germline transgene sequences are listed at the bottom of each section to indicate the number of nonmutated sequences. Nontransgenic sequences (derived from the C57Bl/6 wild-type allele) are listed as non-17.2.25 sequences and were not analyzed further. These sequence data are available from GenBank/EMBL/DDBJ under accession no. (A) AJ307411–AJ307439, (B) AJ310462–AJ310465, AJ307470–AJ307498, (C) AJ307499–AJ307504, and (D) AJ307505–AJ307507, AJ417976, AJ417977.
Figure 2.
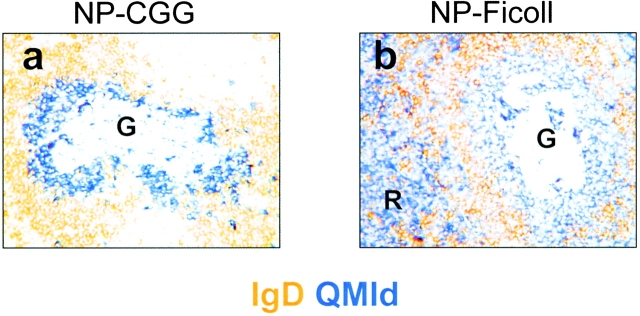
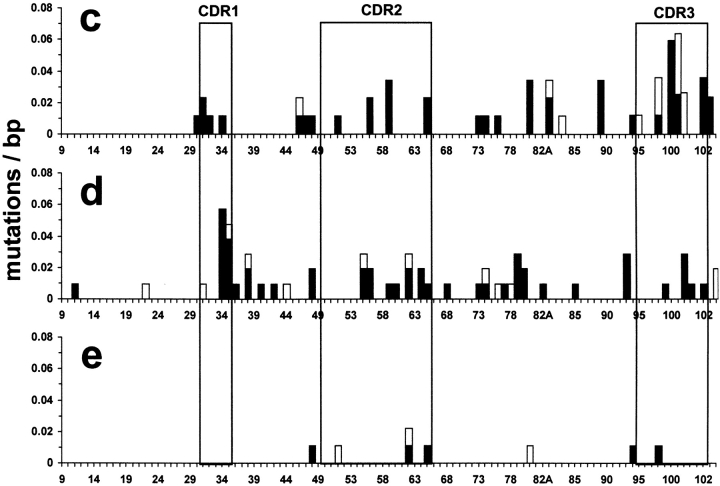
(a) Section of a spleen 4 d after a CGG-primed recipient of QM B cells had been immunized with NP-CGG. A germinal center containing transgene-expressing B cells, which are stained blue has been microdissected; the dissected area is marked G. Note the very few transgenic cells in the follicular mantle, identified by its IgD-expressing cells stained brown. (b) A similarly stained section of spleen 4 d after a recipient of QM B cells had been immunized with NP-Ficoll; R marks the abundant red pulp plasma cells. (c–e) Frequency and distribution of mutations in the V region gene after different immunization schedules. Sequence data, as in Fig. 1, are derived from germinal center cells after immunization with (c) NP-CGG in CGG-primed C57Bl/6 mice, (d) NP-CGG in CGG-primed recipients of QM B cells, and (e) NP-Ficoll in recipients of QM B cells. Solid bars show replacement mutations, open bars indicate silent mutations.
Sequences from four germinal centers were analyzed taking cells from four sections for each germinal center, which were amplified independently to ensure sequences were obtained from multiple sources. Clonally related sequences were frequently seen in the same germinal center. Most of the sequences were based on the canonical V186.2-DFL16.1-JH2 combination; three from germinal center 3 appear to represent second-generation mutations (Fig. 3). The overall mutation frequency was 5.9 mutations/kbp (Table I), resembling that found in day 10 T cell–dependent germinal centers in primary splenic responses to hapten-protein (1, 4, 15, 16). Of the 29 sequences, 3 were nonfunctional with premature stop codons, which were clearly preselection cells. There was no evidence of selection against replacement mutations in the framework region (Fig. 2, Table I).
Figure 3.
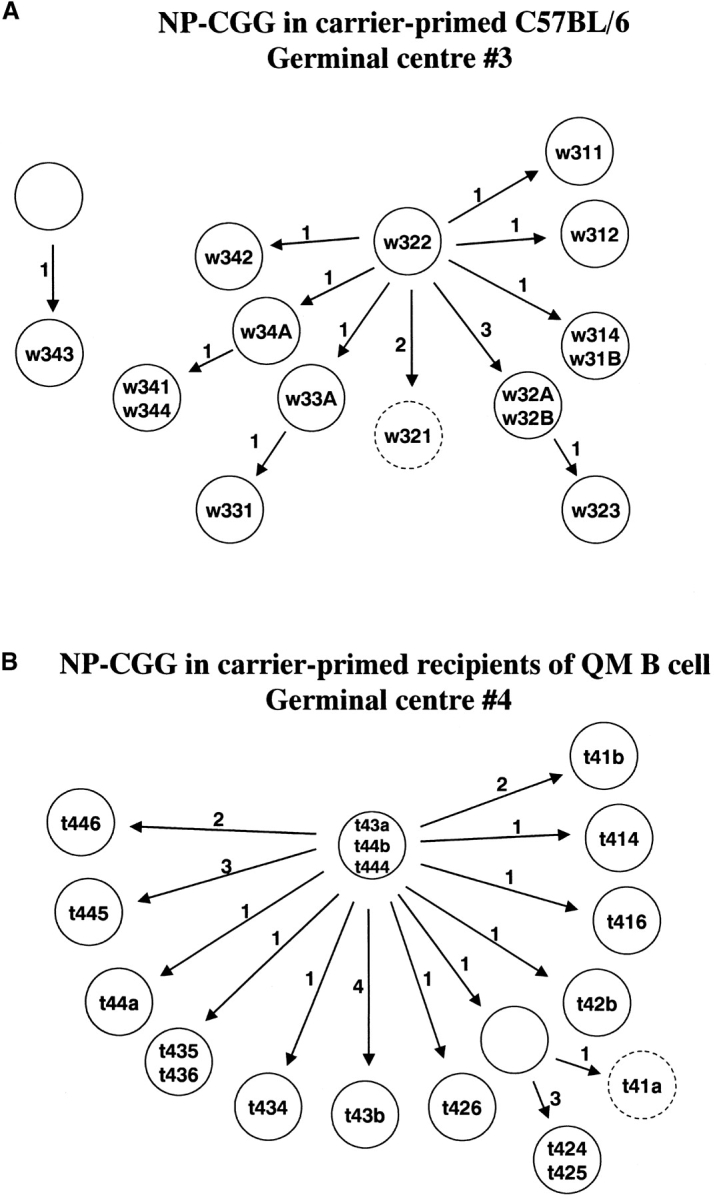
Genealogical relationships between sequences from T cell–dependent germinal centers. (a) Sequences of germinal center 3 from the carrier-primed wild-type mice immunized with NP-CGG. (b) Sequences of germinal center 4 from carrier-primed recipients of QM B cells immunized with NP-CGG. Sequence names, assigned as in Fig. 1, are shown in the circles. Numbers beside the arrows indicate the number of new mutations introduced. Predicted intermediates are shown as circles without a sequence name. Sequences with premature stop codons have dotted circles.
Table I.
Summary of Frequency of Replacement, Silent, and Overall Mutations
| R | S | Percent replacementa | Observed/expected | Mutations/kbp | |
|---|---|---|---|---|---|
| NP-CGG immunization of CGG-primed C57BL/6 (29 sequences) | |||||
| FR (76%)b | 17 | 3 | 85% | 1.12 | 3.3 |
| CDR (85%) | 24 | 8 | 75% | 0.88 | 11.5 |
| NP-CGG immunization of CGG-primed recipients of QM B cells (35 sequences)c | |||||
| FR (77%) | 21 | 8 | 72% | 0.95 | 4.0 |
| CDR (82%) | 27 | 4 | 87% | 1.06 | 9.0 |
| NP-Ficoll immunization of recipients of QM cells, germinal center cells (30 sequences) | |||||
| FR (77%) | 2 | 1 | 67% | 0.87 | 0.5 |
| CDR (82%) | 3 | 2 | 60% | 0.73 | 1.7 |
| NP-Ficoll immunization of recipients of QM cells, red pulp plasma cells (24 sequences) | |||||
| FR (77%) | 0 | 0 | 0% | − | 0 |
| CDR (82%) | 5 | 0 | 100% | 1.22 | 2.1 |
| Nonactivated NP-binding B cells of a QM mouse (63 sequences) | 0 | ||||
| 0 | 0 | 0% | − | 0 | |
Summary of number of replacement (R) and silent mutations (S) and mutation frequencies in the framework and CDR regions.
Percentage of replacement mutations of all observed mutations.
Brackets show expected percentage of replacement mutations for random mutagenesis.
Data for the VH 17.2.25 gene of QM B cells were analyzed.
Comparison of Hypermutation in T Cell–dependent and T Cell–independent Germinal Centers.
To study synchronized T cell–dependent and T cell–independent germinal centers NP-specific B cells from QM mice were transferred into CGG-primed, or nonprimed congenic mice. Primed recipients were immunized intraperitoneally with NP-CGG; nonprimed recipients received NP-Ficoll intraperitoneally. NP-specific germinal centers positive for the transgenic heavy chain idiotype were microdissected 96 h later and the transgenic V region sequences determined (Fig. 2). This transfer system ensures there are no transgenic non-B cells in germinal centers and that B cells of background germinal centers do not carry the transgene.
39 sequences were obtained from four idiotype-positive T cell–dependent germinal centers (Figs. 1 B and 2 d). 35 represented the 17.2.25 gene of QM B cells and of these, 30 contained mutations, with an overall mutation frequency of 5.6/kbp. In contrast, the 30 sequences from NP-Ficoll–induced germinal centers showed far fewer mutations (Figs. 1 C and 2 e). All the sequences were based on 17.2.25 and 24 of the 30 were nonmutated. The frequency of mutation was only 0.9/kbp compared with 5.6/kbp at the equivalent stage of the response to NP-CGG (Table I).
Hypermutation in Extrafollicular Plasmablasts at Similar Levels to T Cell–independent Germinal Centers.
Two clusters of idiotype-positive extrafollicular plasma cells, from four adjacent sections from recipients of QM B cells 4 d after immunization with NP-Ficoll, showed levels of hypermutation similar to that in germinal centers induced by T cell–independent antigens; 5 of 24 sequences contained single mutations (Fig. 1 D and Table I), corresponding to 0.7 mutations per kbp. This low mutation rate is significantly above the background of mutation in B cells from nonimmunized QM mice, for no mutations were recorded in 63 sequences from individual NP-specific B220+ QM mouse splenic B cells (P < 0.0005). Thus, there is a significant low level of hypermutation in QM mouse B cells induced by NP-Ficoll either to form germinal centers or to grow as plasmablasts in the splenic red pulp.
Discussion
The most obvious conclusion to be drawn from this study is that the hypermutation mechanisms are activated by signals delivered by T cells. These might be delivered in the T zone when B cells first make cognate interaction with primed T cells (10, 14, 15). This interaction can induce germinal center formation or extrafollicular growth as plasmablasts. Alternatively, T cells might deliver their effect in follicles by factor production or direct interaction with B cells. Some T cells activated in primary responses migrate to follicles where they proliferate (17). These are critical in selecting centrocytes in mature germinal centers (9), but it is unknown if they influence B cells during germinal center formation. Follicular dendritic cells do not appear to be critical for hypermutation. Lymphotoxin α and β deficient animals lack follicular dendritic cells and do not produce germinal centers in follicles, but form ectopic germinal centers where there is some hypermutation (18, 19). Hypermutation can occur in germinal centers when there is no antigen on follicular dendritic cells (20).
The present study shows hypermutation is underway by the time centrocyte selection starts. The extent of hypermutation in day 4 germinal centers suggests hypermutation was already underway during exponential growth. Theoretically it would be more efficient to have a proliferative phase before the onset of hypermutation (21). This would be sufficiently served by delaying hypermutation until the clone size was 32 to 64 cells, reached at ∼1.5 d into germinal center formation. Exponential growth continues until some 104 cells have been produced at around day 4 (13). Understanding of the exact way B cells are induced by T cells to form germinal centers will be critical in identifying how B cells are induced to start hypermutation of the Ig V region genes. Candidate signals include those delivered through CD40 (22) and Ox-40-ligand (23).
Despite extensive investigation of the potential mechanisms for V region–directed hypermutation, these have not yet been identified. Mice and humans deficient in the activation-induced cytidine deaminase form large T cell–dependent germinal centers but have minimal hypermutation and Ig class switching (24). This suggests a mechanistic link between Ig class switching and hypermutation. Switching to IgG3 is induced in extrafollicular responses to low dose NP-Ficoll where neither germinal centers (25) nor hypermutation (3, 5) are induced, indicating switching can occur in the absence of hypermutation.
The low level of V-region mutation in red pulp plasma cells differs significantly from the 63 sequences obtained from nonimmunized QM mouse NP-specific B cells that were all not mutated. Maizels and Bothwell failed to find V region mutations in NP-specific hybridomas derived from mice immunized with NP-Ficoll (3). Similarly, in a small study of NP-specific plasma cells in B6 mice immunized with NP-Ficoll, 20 V region sequences amplified by nested PCR had no mutations (5), but this sample is too small to exclude the chance absence of mutated cells. It may be that the exceptionally strong signal provided by NP-Ficoll to the QM transgenic B cells is alone sufficient to induce some hypermutation in follicular B blasts as well as in plasmablasts. Patients with deficiency in CD40L do not have germinal centers, but they have a CD27+ memory B cell population, which is unswitched, but contains normal numbers of mutations in its Ig variable region genes (26). Further studies of plasma cells generated in extrafollicular antibody responses are required. These will have to avoid admixture of cells passing through the red pulp after leaving germinal centers (5), or the recruitment of mutated memory cells into the response.
NP-Ficoll immunization induces germinal centers with apparently normal histology, containing dark and light zones, they express BCl-6 and GL-7, bind peanut agglutinin, and have downregulated IgD expression (9). Consequently, the signals that induce these phenotypic changes are not sufficient to induce high level hypermutation. The model for TI and TD germinal center formation described in this study provides an opportunity to compare gene expression between the germinal centers formed in these two conditions; this may identify molecules involved in Ig V region-directed hypermutation.
Acknowledgments
We are grateful to Marilia Cascalho and Matthias Wabl for enabling us to use QM mice.
This work was supported by grants from the British Medical Research Council.
C.G. Vinuesa's present address is John Curtin School of Medical Research, Australian National University, Canberra, 2601 ACT, Australia.
References
- 1.Berek, C., A. Berger, and M. Apel. 1991. Maturation of the immune response in germinal centers. Cell. 67:1121–1129. [DOI] [PubMed] [Google Scholar]
- 2.Jacob, J., G. Kelsoe, K. Rajewsky, and U. Weiss. 1991. Intraclonal generation of antibody mutants in germinal centres. Nature. 354:389–392. [DOI] [PubMed] [Google Scholar]
- 3.Maizels, N., and A.L. Bothwell. 1985. The T cell-independent immune response to the hapten N.P. uses a large repertoire of heavy chain genes. Cell. 43:715–720. [DOI] [PubMed] [Google Scholar]
- 4.Jacob, J., and G. Kelsoe. 1992. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath–associated foci and germinal centers. J. Exp. Med. 176:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sze, D.M.-Y., K.-M. Toellner, C. García de Vinuesa, D.R. Taylor, and I.C.M. MacLennan. 2000. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J. Exp. Med. 192:813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuurman, H.J., E.B. Bell, K. Gartner, H.J. Hedrich, A.K. Hansen, B.C. Kruijt, P. de Vrey, R. Leyten, S.J. Maeder, R. Moutier, et al. 1992. Comparative evaluation of the immune status of congenitally athymic and euthymic rat strains bred and maintained at different institutes: 2. Athymic rats. J. Exp. Anim. Sci. 35:33–48. [PubMed] [Google Scholar]
- 7.O'Leary, P. 2000. T cell influences in antibody responses to lipopolysaccharides and polysaccharides. University of Birmingham, Birmingham, UK. 213 pp.
- 8.Lentz, V., and T. Manser. 2001. Germinal centers can be induced in the absence of T cells. J. Immunol. 167:15–20. [DOI] [PubMed] [Google Scholar]
- 9.García de Vinuesa, C., M. Cook, J. Ball, M. Drew, Y. Sunners, M. Cascalho, M. Wabl, G.G.B. Klaus, and I.C.M. MacLennan. 2000. Germinal centers without T cells. J. Exp. Med. 191:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toellner, K.-M., A. Gulbranson-Judge, D.R. Taylor, D.M.-Y. Sze, and I.C.M. MacLennan. 1996. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J. Exp. Med. 183:2303–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cascalho, M., A. Ma, S. Lee, L. Masat, and M. Wabl. 1996. A quasi-monoclonal mouse. Science. 272:1649–1652. [DOI] [PubMed] [Google Scholar]
- 12.Kabat, E., T. Wu, H. Perry, K. Gottesman, and C. Foeller. 1991. Sequences of Proteins of Immunological Interest. National Institutes of Health, Bethesda, MD. 2597 pp.
- 13.Liu, Y.J., J. Zhang, P.J.L. Lane, E.Y.T. Chan, and I.C.M. MacLennan. 1991. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur. J. Immunol. 21:2951–2962. [DOI] [PubMed] [Google Scholar]
- 14.Jacob, J., R. Kassir, and G. Kelsoe. 1991. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 173:1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob, J., J. Przylepa, C. Miller, and G. Kelsoe. 1993. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J. Exp. Med. 178:1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, Z., S.B. Koralov, M. Gendelman, M.C. Carroll, and G. Kelsoe. 2000. Humoral immune responses in Cr2−/− mice: enhanced affinity maturation but impaired antibody persistence. J. Immunol. 164:4522–4532. [DOI] [PubMed] [Google Scholar]
- 17.Gulbranson-Judge, A., and I. MacLennan. 1996. Sequential antigen-specific growth of T cells in the T zones and follicles in response to pigeon cytochrome c. Eur. J. Immunol. 26:1830–1837. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto, M., S.F. Lo, C.J. Carruthers, J. Min, S. Mariathasan, G. Huang, D.R. Plas, S.M. Martin, R.S. Geha, M.H. Nahm, and D.D. Chaplin. 1996. Affinity maturation without germinal centres in lymphotoxin-alpha-deficient mice. Nature. 382:462–466. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto, M., S. Mariathasan, M.H. Nahm, F. Baranyay, J.J. Peschon, and D.D. Chaplin. 1996. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science. 271:1289–1291. [DOI] [PubMed] [Google Scholar]
- 20.Hannum, L.G., A.M. Haberman, S.M. Anderson, and M.J. Shlomchik. 2000. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J. Exp. Med. 192:931–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kepler, T.B., and A.S. Perelson. 1993. Cyclic re-entry of germinal center B cells and the efficiency of affinity maturation. Immunol. Today. 14:412–415. [DOI] [PubMed] [Google Scholar]
- 22.Kawabe, T., T. Naka, K. Yoshida, T. Tanaka, H. Fujiwara, S. Suematsu, N. Yoshida, T. Kishimoto, and H. Kikutani. 1994. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1:167–178. [DOI] [PubMed] [Google Scholar]
- 23.Walker, L.S., A. Gulbranson-Judge, S. Flynn, T. Brocker, C. Raykundalia, M. Goodall, R. Forster, M. Lipp, and P. Lane. 1999. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J. Exp. Med. 190:1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muramatsu, M., K. Kinoshita, S. Fagarasan, S. Yamada, Y. Shinkai, and T. Honjo. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 102:553–563. [DOI] [PubMed] [Google Scholar]
- 25.Garcia de Vinuesa, C., P. O'Leary, D.M. Sze, K.M. Toellner, and I.C. MacLennan. 1999. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur. J. Immunol. 29:1314–1323. [DOI] [PubMed] [Google Scholar]
- 26.Weller, S., A. Faili, C. Garcia, M.C. Braun, F.F. Le Deist, G.G. de Saint Basile, O. Hermine, A. Fischer, C. Reynaud, and J. Weill. 2001. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc. Natl. Acad. Sci. USA. 98:1166–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]