Abstract
Immunoglobulin A (IgA) provides protection against pathogens at mucosal surfaces. Chemotactic responses have been hypothesized to target IgA plasma cells involved in mucosal immune responses. We show here that thymus-expressed chemokine (TECK, CCL25) is a potent and selective chemoattractant for IgA antibody-secreting cells (ASC), efficiently recruiting IgA-producing cells from spleen, Peyer's patches, and mesenteric lymph node. Cells secreting IgA antibody in response to rotavirus, an intestinal pathogen, also respond well. In contrast, IgG– and IgM–ASC respond poorly. Epithelial cells in the small intestines, a principal site of IgA–ASC localization and IgA production in the body, highly and selectively express TECK. The migration of IgA–ASC to the intestinal epithelial cell chemokine TECK may help target IgA-producing cells to the gut wall, thus helping define and segregate the intestinal immune response.
Keywords: homing, migration, chemokine, B cell, isotype
Introduction
Humoral immunity in mucosal tissues, especially the gastrointestinal mucosa, is characterized by local production and secretion of IgA, and the intestinal lamina propria (LP)* harbors many IgA-producing plasma cells initially recruited from circulating antibody-secreting cells (ASC; for a review, see references 1 and 2). In contrast, IgG–ASC predominate in systemic (nonmucosal) chronic inflammatory sites, for example in arthritis or soft tissue inflammation, and IgM–ASC, which produce the initial wave of low affinity antibodies during the primary immune response, are prominent within lymphoid organs (3). Early studies demonstrating mucosal lymphoid-derived ASC migration to factors in mouse colostrum led to the hypothesis that ASC might be targeted to mucosal surfaces by epithelium-derived chemoattractants (4). Since chemoattractant cytokines (chemokines) play major roles in the in vivo homing of other leukocyte subsets to lymphoid and extralymphoid tissues (5, 6), we set out to assess the chemokine responsiveness of cells secreting antibodies of different isotypes.
Materials and Methods
Chemotaxis.
Lymphocytes from mouse spleen, Peyer's patch, mesenteric lymph node, and bone marrow (BM) from male and female C57Bl6/J mice were isolated and migration assays were performed as described previously (7). Briefly, 2 × 106 lymphocytes were placed in 5-μ Transwell inserts (Costar) in wells with medium alone (basal) or medium containing chemokines at concentrations shown previously to be optimal for chemotaxis of various responding BM and peripheral leukocyte populations (7) (100 nM mKC, 100 nM monokine induced by IFN-γ (MIG), 50 nM stromal cell–derived factor (SDF-1α), 500 nM mBLC, 100 nM mMIP-1α, 1 nM mJE, 100 nM mEotaxin, 100 nM hTARC, 100 nM mRANTES, 100 nM hMIP-3α, 100 nM hELC, 100 nM hI-309, or 300 nM thymus-expressed chemokine [TECK]). Chemokines were obtained from Peprotech, Inc. or R&D Systems. None of the populations tested responded to eotaxin or I-309. After 2 h, inserts were removed and the responding population in the bottom wells was quantified as described below. For evaluation of migrated ASC, multiple replicate chemotaxis wells (generally 4–5 per condition in each experiment) were combined for ELISPOT analyses.
Flow Cytometric Analysis.
Follicular B lymphocytes were enumerated using a bead counting method as described previously (7). The mAbs used during this analysis were RA3–6B2 (APC-conjugated rat anti–mouse CD45R/B220; BD PharMingen); 145–2C11 (biotin-conjugated Armenian hamster anti–mouse CD3ɛ; BD PharMingen); AF6–78 (PE-conjugated rat anti–mouse IgMb; BD PharMingen); B3B4 (PE-conjugated rat anti–mouse CD23; BD PharMingen); 11–26c.2a (FITC-conjugated rat anti–mouse IgD; BD PharMingen); and 7G6 (FITC-conjugated rat anti–mouse CD21; BD PharMingen).
ELISPOT Assay.
IgM–ASC, IgG–ASC, and IgA–ASC were identified in conventional ELISPOT assays (8, 9). Nitrocellulose 96-well plates (Multiscreen 96-well filtration plate; Millipore) were coated with goat anti–mouse IgM-, IgG-, and IgA-specific polyclonal antibodies (all from Kirkegaard and Perry) to capture the respective secreted Igs from the ASC during overnight culture. ASC were removed and the captured Ig was detected with HRP-conjugated goat anti–mouse IgM, IgG, or IgA, respectively (Kirkegaard and Perry), and visualized by development with 3-amino-9-ethylcarbazole for 15–20 min to yield a reddish spot where an ASC had been. The number of ASC per well was determined by counting under a dissecting microscope, and the efficiency of migration calculated by comparing the number of spots present in the input population with the number of spots present in the migrated population.
The above protocol was modified to detect rotavirus (RV)-specific IgA–ASC. After chemotaxis of an ASC-enriched population to chemokines, the responding cells were harvested and plated overnight onto nitrocellulose plates precoated with virus-like particles made from baculovirus heterologously expressing bovine RV VP6 and VP2 (8, 9). J. Cohen (Virologie Moleculaire et Cellullaire, Cedex, France) provided the virus-like particles. Plates were washed, incubated with HRP-conjugated goat anti–mouse IgA or IgG polyclonal antibodies, and developed as described above.
RT-PCR Analysis of CC Chemokine Receptor 9 mRNA in Splenic ASC Subsets.
3,000–4,000 splenic IgA and IgG ASC were isolated based on their unique cell surface phenotype (B220lo/−, IgG+ or IgA+, and negative for myeloid and T cell antigens), as described previously (10).
Splenocytes were initially enriched for ASC by streptavidin-conjugated magnetic bead (Dynal) depletion of cells stained with biotinylated rat anti–mouse Thy-1.2 (Clone 53–2.1; BD PharMingen), IgD (Clone 11–26; Southern Biotechnology, Inc.), and CD11b/MAC-1 (Clone M1/70; American Type Culure Collection). ASC enriched splenocytes were then stained for FACS® sorting with biotinylated rat anti–mouse Thy-1.2, IgD, and CD11b/MAC-1, and CD45R/B220-PE (BD PharMingen), in combination with either rat anti– mouse IgA–FITC (Clone C10–3; BD PharMingen), IgG1–FITC+IgG2a/2b-FITC+IgG3–FITC (Clones A85–1, R2–40, and R40–82, respectively; BD PharMingen), or rat IgG2a–FITC as a negative control (Clone R-35–95; BD PharMingen). In one experiment, anti–mouse CD11c was added to this depleting antibody cocktail to exclude possible dendritic cell contamination. Biotinylated antibodies were visualized with streptavidin-Cy-Chrome (BD PharMingen) and FITC-staining was amplified using the Alexa Fluor 488 Signal Amplification kit for Fluorescein-conjugated probes (Molecular Probes). The sort gate for ASC was set for B220lo/−, Thy-1.2−, IgD−, CD11b/Mac-1− ± CD11c− large lymphocytes and the FITC amplified background was determined using rat IgG2a-FITC stained cells. Sort gates for IgA+ and IgG+ ASC were identical. Sorted cells (generally 3,500–5,000 IgA+ or IgG+ ASC phenotype cells) were lysed in guanidinium buffer and the total RNA isolated by phenol/chloroform extraction. The RNA was reverse transcribed using the Advantage RT-for-PCR kit (CLONTECH). PCR and Southern Blot analysis were performed as described previously (11). The intron-spanning PCR primers for mouse CC chemokine receptor (CCR)9 were 5′-CCTCTCCTTACAGACCCAGAC-3′ and 5′-GTCATGGTCTTCACTCTTGTGC-3′. As a loading control, PCR products for mouse GAPDH were created using intron-spanning PCR primers that were 5′-CCATGTTTGTGATGGGTGTG-3′ and 5′-CCTTCTTGATGTCATCATAC-3′.
Isolation of and Chemotaxis by LP Lymphocytes.
LP lymphocytes (LPLs) were isolated from normal murine small intestine as described in reference 12. Briefly, 4–5 small intestines were cleared of Peyer's patches, cut open longitudinally, cut into short 5-mm segments, and washed at room temperature with vigorous shaking four times in divalent cation-free HBSS supplemented with 5 mM EDTA, 25 mM Hepes, and 2.5% antibiotic-antimycotic (Sigma-Aldrich) to remove epithelial cells and intraepithelial lymphocytes until no more shedding occurred. Intestines were then washed twice in RPMI 1640 with 10% FCS/15 mM Hepes/2.5% antibiotic-antimycotic. LPLs were isolated by shaking the intestinal pieces in the RPMI 1640 supplemented with 20% BCS/25 mM Hepes/antibiotic-antimycotic and 300 U/ml collagenase Type VIII (Sigma-Aldrich) for three 40-min sessions. At the end of each 40-min incubation, released cells were immediately washed in RPMI 1640/10% BCS containing penicillin-streptomycin to remove and neutralize the collagenase. LPLs were allowed to recover in RPMI 1640/10% FCS containing penicillin-streptomycin in a CO2 incubator for 2 h before any analysis. Migration of LPLs was performed in RPMI 1640 supplemented with 0.5% BSA instead of serum to decrease background migration of lymphocytes. Responding cells were stained as outlined in the figure legend.
ASC Enrichment for Chemotaxis Assays.
Mesenteric lymph node cells were harvested 10 d after conventional oral inoculation of mice with RV (8, 9). The cell preparation was enriched for ASC (B220lo/neg, IgD−, nonT cells) by precoating goat anti–rat IgG-conjugated Dynalbeads (Dynal) with rat anti–mouse CD4, CD8, and IgD antibodies (all FITC conjugated) individually. The individually coated beads were combined, washed, and incubated with the cell preparation for 45 min at 4°C. CD4+, CD8+, and IgD+ cells were removed with a magnet and the unbound ASC-enriched population was transferred and placed in culture for 1 h at 37°C to recover before assay.
Results and Discussion
We initially analyzed ASC from spleen, a central filtering and collecting organ for circulating immune cells that comprises plasma cells of each isotype. Spleen cells were isolated, placed in an upper Transwell chamber, and allowed to migrate to individual chemokines placed in the bottom well. ASC in the starting population and in the bottom well after migration to specific chemokines or control media were enumerated by conventional ELISPOT assays, and the percent of ASC migrated was determined. The migration of naive follicular B cells, a population that circulates through secondary lymphoid tissues but not the gut wall, is presented for comparison.
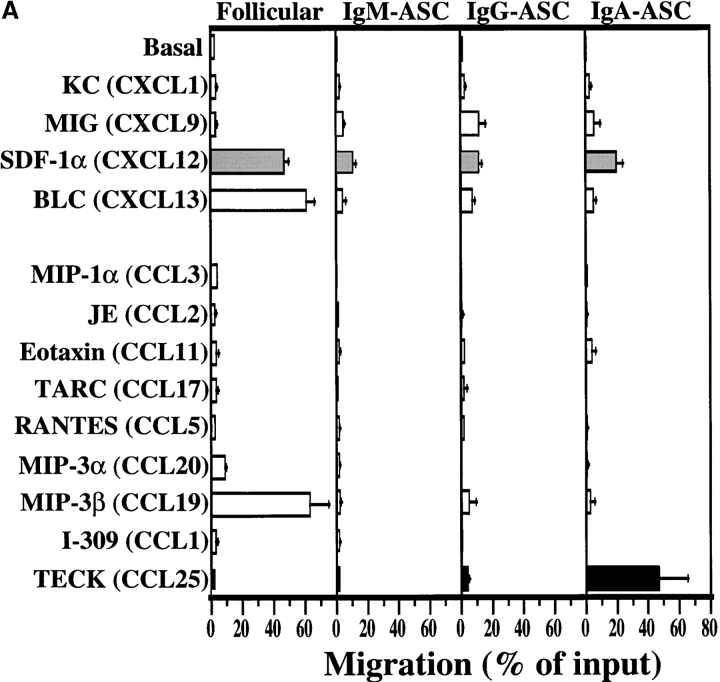
Fig. 1 A shows that IgM-ASC failed to respond well to any chemokine tested, although they migrated above background to SDF-1α, (CXCL12), a widely expressed chemokine ligand for CXC chemokine receptor (CXCR)4 that is active on many lymphocyte subsets. IgG–ASC (Fig. 1 A) displayed a detectable but weak SDF-1α response, as well, but also migrated significantly to MIG (CXCL9), a ligand for CXCR3. CXCR3 and its ligands have been implicated in inflammatory T cell migration (13); thus, migration to MIG may help IgG–ASC enter diverse tissue sites of inflammation.
Figure 1.
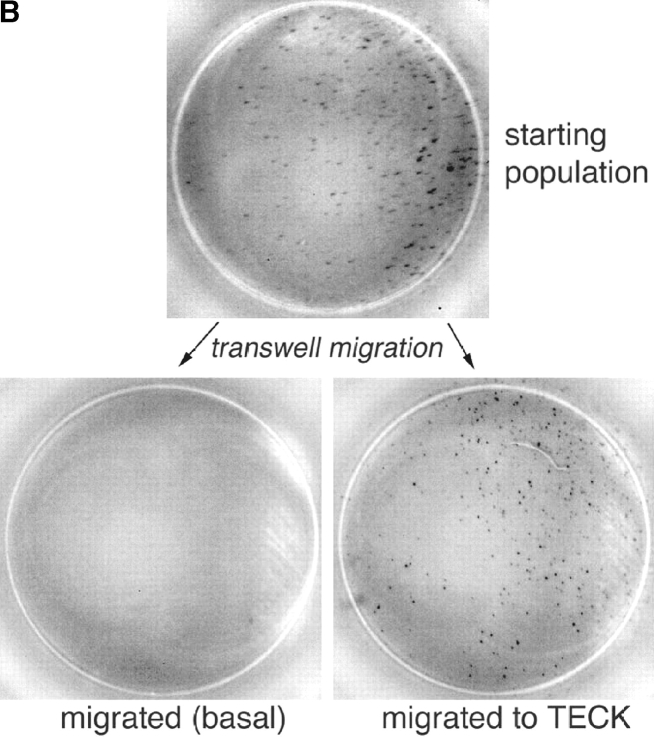
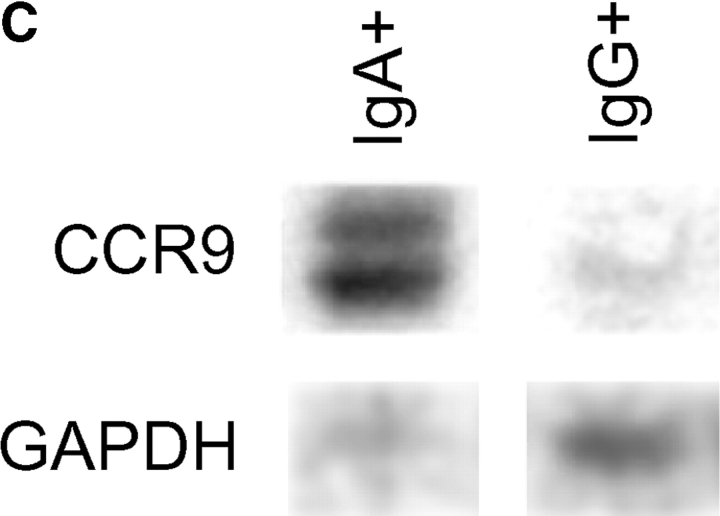
TECK selectively attracts IgA ASC. (A) Mouse splenocytes were migrated to optimal chemokine concentrations as described in Materials and Methods. Follicular B lymphocytes were identified by flow cytometry as B220+/CD3ɛ−/IgMlo/IgDhi or B220+/CD3ɛ−/CD21int/CD23int cells. IgM–ASC, IgG–ASC, and IgA–ASC were identified in conventional ELISPOT assays (reference 9). The average migration from 7 to 9 experiments is presented with SE. The frequency of IgM–, IgG–, and IgA–ASC in the spleen was 110 ± 30, 50 ± 10, and 60 ± 20 per 106 total cells plated, respectively. (B) ELISPOT assays illustrating IgA-ASC (spots) in the starting spleen cell population, and among cells migrated to TECK. In contrast, no IgA–ASC was detected in cells that migrated spontaneously with only medium in the attractant well (basal migration). (C) Sorted splenic IgA+ (but not IgG+) ASC express mRNA for CCR9. CCR9 message was abundant in IgA–ASC in 3/3 experiments and was weakly detected in IgG–ASC in 1/3 experiments. The RT-PCR product of a housekeeping gene, GAPDH message is illustrated as a loading control.
The most dramatic chemotactic responses, however, were displayed by IgA–ASC (Fig. 1 A, right panel). These IgA-producing cells respond well to SDF-1 α (a chemokine whose receptor is expressed by most leukocytes), but also to TECK (CCL25). In control experiments, incubation with TECK had no effect on the numbers of IgA–ASC detected or on the amount of IgA produced per ASC during overnight cultures (data not shown), indicating that the ASC arrived in the bottom chemoattractant wells by migration. A representative ELISPOT well illustrating IgA–ASC migration to TECK is presented as Fig. 1 B. Consistent with their efficient response to TECK, sorted IgA+ (but not IgG+) ASC express abundant messenger RNA for the TECK receptor, CCR9 (Fig. 1 C; note the presence of two bands, indicating expression of both CCR9 splice variants).
Unlike most circulating naive and memory lymphocytes including follicular (IgD+) B cells (5, 7) (illustrated in Fig. 1), neither IgA– nor IgG–ASC migrated to the lymphoid tissue-expressed CCR7 ligands ELC/CCL19 (Fig. 1) or SLC/CCL21 (data not shown), chemokines implicated in homing to lymphoid organs. These results are consistent with recent studies showing that ASC phenotype (B220int/CD138+) splenocytes from alum/nitrophenyl chicken γ-globulin-immunized mice migrate to SDF-1α but not ELC or SLC (14). Similarly, Wehrli et. al. has suggested that early ASC phenotype cells can migrate to ELC and SDF-1α, but that they largely lose these responses in association with exit from their lymphoid sites of generation (15). Thus most ASC may be programmed to migrate to extralymphoid sites, rather than recirculate through secondary lymphoid tissues.
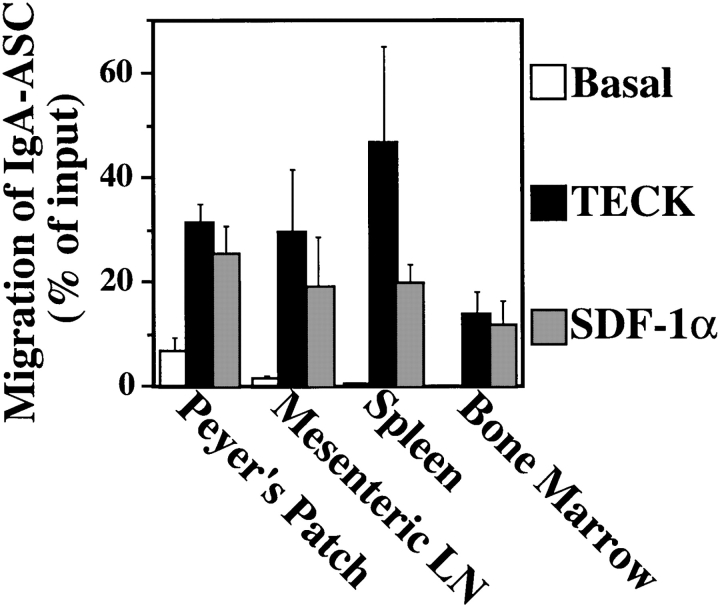
IgA–ASC are thought to be induced in the intestine-associated Peyer's patch and mesenteric lymph nodes. They then travel via the lymphatic system through the thoracic duct into the bloodstream. Many localize to or pass through the spleen on their way to populating the intestinal LP (16, 17). Some IgA-ASC also migrate to and reside in the BM, where they contribute to systemic IgA production (18). As shown in Fig. 2, TECK responsiveness is a common feature of IgA–ASC harvested from Peyer's patches and mesenteric lymph nodes, as well as spleen. On average, BM IgA–ASC responded relatively less well to TECK (although the difference observed is not statistically significance) (Fig. 2). In fact, migration of BM IgA–ASC to TECK and SDF was variable and in some experiments was significantly less than migration by IgA–ASC from MLN or spleen. These differences may reflect variability in the proportion of terminally differentiated plasma cells in the BM (see below), where mature plasma cells are expected to be present at a higher frequency than in lymphoid organs.
Figure 2.
IgA–ASC in Peyer's patch, mesenteric lymph nodes, and spleen migrate well to TECK. Lymphocytes were harvested from the indicated lymphoid organs and the migration of IgA–ASC present in these organs to medium (Basal) or medium containing 300 nM mTECK or 50 nM hSDF-1 α was assessed as described in Materials and Methods. The data is the mean ± SEM from 3 to 9 individual experiments. The frequency of IgA–ASC in the Peyer's patch, mesenteric lymph node, spleen, and BM were 70 ± 40, 90 ± 50, 60 ± 20, and 25 ± 10 per 106 total cells plated, respectively. The fold-increase in specific migration over basal migration to TECK and SDF by IgA–ASC from Peyer's patch was 4.7 and 3.8, respectively; from mesenteric lymph node was 19.8 and 12.6, respectively, and from spleen was 63.8 and 31.8, respectively. BM–IgA ASC had zero (below detectable) basal migration precluding presentation of a fold-increase over basal.
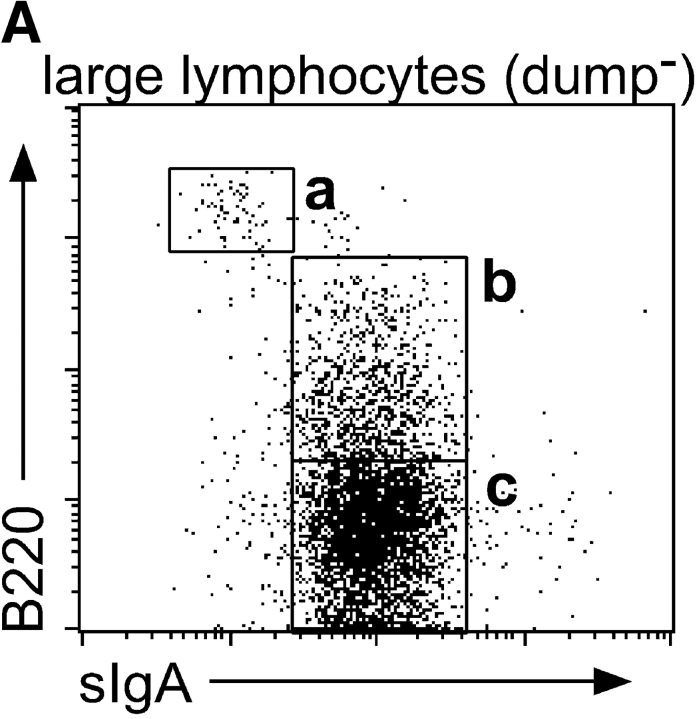
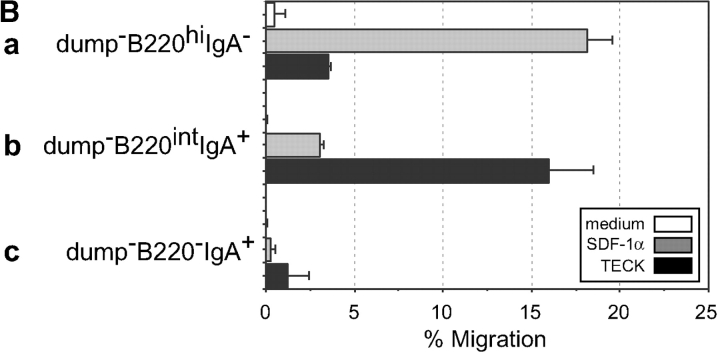
The development of naive B cells into differentiated IgA–ASC is associated with the gain of surface IgA expression and a progressive decrease in B220 levels; few if any ASC express B220 at the high levels seen on naive or germinal center B cells, and many terminally differentiated tissue plasma cells are B220neg (19). The gut LP is a major site of IgA–ASC recruitment and the relative high percentage of IgA–ASC in this organ allowed us to address the B220 levels of the TECK-responsive IgA–ASC. (The cytological and histochemical terms “plasmablasts” and “plasma cells” have been used inconsistently in reference to ASC expressing varying levels of B220 [references 14, 15, 19, and 20), and will not be used here). Fig. 3 A illustrates that both IgA+/B220intermediate (B220int) and IgA+/B220neg large lymphocytes are found in the LP, and we have confirmed that both of these populations comprise IgA–ASC (unpublished data). Fig. 3 B demonstrates that the IgA+/B220int LP IgA–ASC population responds much better to TECK than IgA+/B220neg ASC. The LP IgA+/B220neg ASC population had also lost its responsiveness to SDF-1α (similar to a B220neg/CD138hi lymph node population described by Wehrli et. al., reference 15). Splenic TECK-responsive IgA–ASC were also restricted to the IgA+/B220int but not IgA+/B220neg ASC populations (data not shown). IgA+/B220neg ASC may represent cells that have terminally homed to the site of their final residence with no further need for migratory receptors.
Figure 3.
B220int but not B220−IgA+ ASC phenotype cells in LP migrate to TECK. LPLs were stained for B220, IgA, and for excluded lineage markers (a dump cocktail or anti-Thy1.1, NK1.1, IgD, and CD11c). (A) Large lymphocytes were gated for dump negativity and plotted for B220 versus IgA expression. The indicated gates were used to determine migration efficiency of the different populations. (B) LPLs were allowed to migrate to medium (white bars), 100 nM hSDF-1 α (gray bars), or 250 nM mTECK (black bars). Responding cells were stained as above and the migration by the indicated cell population to chemokine presented. Migration of a B220hi, IgAneg population (presumed blasting B cells) is shown for comparison.
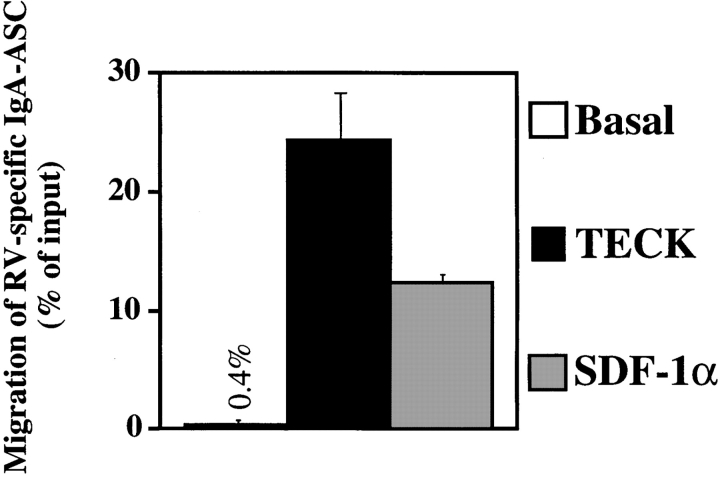
We next asked whether cells producing antibody in response to a well-defined intestinally restricted pathogen could also migrate to TECK. RV, a double-stranded RNA virus, exclusively infects the small intestine villous epithelium in humans and mice, causing diarrhea (21). RV is responsible for up to a million childhood deaths per year. Protection against RV in immunodeficient mice can be conferred by transfer of immune B cells, and immunity correlates with anti-RV IgA but not anti-RV IgG levels in the mouse model (22, 23). Mesenteric lymph node cells were harvested from mice 10 d after RV infection, and cells migrating to TECK or SDF-1α were tested for the isotype of anti-RV antibody production by modified (antigen-specific) ELISPOT assay (8, 9). RV-specific IgA-ASC represent ∼10–12% of total IgA ASC in mesenteric nodes at this time (9). Fig. 4 shows that these RV-specific IgA–ASC migrate strongly to TECK. In contrast, in an experiment in which mesenteric node IgG–ASC were assessed in parallel, RV-specific IgG-ASC did not respond to TECK above background (data not shown). The frequency of RV-specific IgA–ASC migration to TECK was comparable to that of the total IgA–ASC population in the same experiments. We conclude that IgA–ASC induced by small intestinal infection with RV display efficient chemotaxis to TECK.
Figure 4.
RV-specific IgA–ASC migrate to TECK. Mesenteric lymph node cells from RV-infected mice were harvested, enriched for ASC, and allowed to migrate to medium (Basal) or medium containing optimal chemotactic concentrations of mTECK (300 nM) or SDF-1α (50 nM). RV-specific IgA–ASC were identified and enumerated as described in Materials and Methods. The data is the mean ± range from two independent experiments.
Migration to the widely expressed chemokine SDF-1α is a property shared with most mature leukocytes, and many other cell types as well (24). Such a general response may contribute to motility within tissues or retention within a tissue (14), but is unlikely to control differential tissue and subset-specific cell homing. In contrast, recent studies of TECK tissue expression patterns reveal that it is critically positioned to contribute to IgA–ASC localization in the gut. TECK mRNA is highly expressed in the thymus, where it is hypothesized to participate in T cell development (25, 26). In the periphery however, TECK mRNA is largely restricted to the gastrointestinal tract, especially the small intestinal mucosa (27, 28). In situ hybridization and immunohistochemistry reveal TECK expression by epithelial cells in the jejunum, duodenum and ileum, especially but not exclusively in the crypt regions near the base of intestinal villi (28, 29). This region is enriched in MAdCAM-1+ venules supporting extravasation of homing lymphocytes, as well. Additional chemoattractants, possibly including SDF-1α (30), may support further migration and dispersal of ASC throughout the intestinal LP. In contrast, TECK is poorly expressed by nonintestinal epithelial tissues (28). Thus, the studies presented here lead to the hypothesis that selective expression of TECK by intestinal epithelial cells may serve to efficiently recruit IgA–ASC to the gastrointestinal LP, and it will be of interest to assess the role of TECK in circulating ASC interactions with LP venules, and in diapedesis into the LP, in future in situ studies. Interestingly, TECK is expressed at lower levels in the colon than in the small intestines. It could contribute to ASC migration there as well, but other chemoattractants may play parallel roles in the homing of IgA–ASC to the colon and other mucosal tissues.
Our results provide the first demonstration that ASC respond to chemokines and demonstrate further the specialization of chemokine responses can be associated, at the population level, with ASC specialization in terms of isotype expression. The selective recruitment of IgA–ASC by the intestinal chemokine TECK not only provides a mechanism for the recruitment and/or retention of mucosal ASC in the intestinal wall as originally hypothesized by Lamm (4), but may also provide a paradigm in which tissue-selective chemokine expression by specialized epithelia helps determine the character of local humoral and cellular immune responses.
Acknowledgments
We thank June Twelves and Lusijah Rott for technical assistance and expertise. E.P. Bowman, E.J. Kunkel, and J. Pan were recipients of Arthritis Foundation Postdoctoral Fellowships, and K.R. Youngman was supported by NRSA DK10022.
N.H. Lazarus was supported by the National Institutes of Health (NIH) Training grant 5 T32 AI07290. Supported in part by NIH grants AI37832, GM37734, AI47822 to E.C. Butcher, 5R37AI121362-16 to H.B. Greenberg and VA Merit Review Awards to E.C. Butcher and H.B. Greenberg.
E.P. Bowman, N.A. Kuklin, K.R. Youngman, N.H. Lazarus, and E.J. Kunkel contributed equally to this manuscript.
E.P. Bowman's present address is Department of Immunology, DNAX Research Institute, 901 California Ave., Palo Alto, CA 94304-1104.
Footnotes
Abbreviations used in this paper: ASC, antibody-secreting cells; BM, bone marrow; CCR, CC chemokine receptor; CXCR, CXC chemokine receptor; LP, lamina propria; LPL, LP lymphocyte; MIG, monokine induced by IFN-γ; RV, rotavirus; SDF, stromal cell–derived factor; TECK, thymus-expressed chemokine.
References
- 1.Brandtzaeg, P., I.N. Farstad, F.E. Johansen, H.C. Morton, I.N. Norderhaug, and T. Yamanaka. 1999. The B-cell system of human mucosae and exocrine glands. Immunol. Rev. 171:45–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogra, P., J. Mestecky, M. Lamm, W. Strober, J. Bienenstock, and J. McGhee. 1998. Mucosal Immunology. 2nd edition. P. Ogra, J. Mestecky, M. Lamm, W. Strober, J. Bienenstock, and J. McGhee, editors. Academic Press, San Diego. 1–766.
- 3.Picker, L., and M. Siegelman. 1999. Lymphoid Tissues and Organs. 4th edition. Fundamental Immunology. W. Paul, editor. Lippincott-Raven, Bethesda. 449–531.
- 4.Czinn, S.J., and M.E. Lamm. 1986. Selective chemotaxis of subsets of B lymphocytes from gut-associated lymphoid tissue and its implications for the recruitment of mucosal plasma cells. J. Immunol. 136:3607–3611. [PubMed] [Google Scholar]
- 5.Campbell, J.J., and E.C. Butcher. 2000. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr. Opin. Immunol. 12:336–341. [DOI] [PubMed] [Google Scholar]
- 6.Zlotnik, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity. 12:121–127. [DOI] [PubMed] [Google Scholar]
- 7.Bowman, E.P., J.J. Campbell, D. Soler, Z. Dong, N. Manlongat, D. Picarella, R.R. Hardy, and E.C. Butcher. 2000. Developmental switches in chemokine response profiles during B cell differentiation and maturation. J. Exp. Med. 191:1303–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco, M.A., and H.B. Greenberg. 1997. Immunity to rotavirus in T cell deficient mice. Virology. 238:169–179. [DOI] [PubMed] [Google Scholar]
- 9.Williams, M.B., J.R. Rose, L.S. Rott, M.A. Franco, H.B. Greenberg, and E.C. Butcher. 1998. The memory B cell subset responsible for the secretory IgA response and protective humoral immunity to rotavirus expresses the intestinal homing receptor, α4β7. J. Immunol. 161:4227–4235. [PubMed] [Google Scholar]
- 10.Smith, K.G., T.D. Hewitson, G.J. Nossal, and D.M. Tarlinton. 1996. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur. J. Immunol. 26:444–448. [DOI] [PubMed] [Google Scholar]
- 11.Pan, J., E.J. Kunkel, U. Gosslar, N. Lazarus, P. Langdon, K. Broadwell, M.A. Vierra, M.C. Genovese, E.C. Butcher, and D. Soler. 2000. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J. Immunol. 165:2943–2949. [DOI] [PubMed] [Google Scholar]
- 12.Harriman, G.R., E. Hornqvist, and N.Y. Lycke. 1992. Antigen-specific and polyclonal CD4+ lamina propria T-cell lines: phenotypic and functional characterization. Immunology. 75:66–73. [PMC free article] [PubMed] [Google Scholar]
- 13.Zlotnik, A., J. Morales, and J.A. Hedrick. 1999. Recent advances in chemokines and chemokine receptors. Crit. Rev. Immunol. 19:1–47. [PubMed] [Google Scholar]
- 14.Hargreaves, D.C., P.L. Hyman, T.T. Lu, V.N. Ngo, A. Bidgol, G. Suzuki, Y.R. Zou, D.R. Littman, and J.G. Cyster. 2001. A coordinated change in chemokine responsiveness guides plasma cell movements. J. Exp. Med. 194:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wehrli, N., D.F. Legler, D. Finke, K.M. Toellner, P. Loetscher, M. Baggiolini, I.C. MacLennan, and H. Acha-Orbea. 2001. Changing responsiveness to chemokines allows medullary plasmablasts to leave lymph nodes. Eur. J. Immunol. 31:609–616. [DOI] [PubMed] [Google Scholar]
- 16.Cebra, J.J., P.J. Gearhart, R. Kamat, S.M. Robertson, and J. Tseng. 1977. Origin and differentiation of lymphocytes involved in the secretory IgA responses. Cold Spring Harb. Symp. Quant. Biol. 41:201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott, M.R., and J. Bienenstock. 1979. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J. Immunol. 122:1892–1898. [PubMed] [Google Scholar]
- 18.Benner, R., F. Meima, G.M. Van der Meulen, and W. van Ewijk. 1974. Antibody formation in mouse bone marrow. III. Effects of route of priming and antigen dose. Immunology. 27:747–760. [PMC free article] [PubMed] [Google Scholar]
- 19.Kamata, T., F. Nogaki, S. Fagarasan, T. Sakiyama, I. Kobayashi, S. Miyawaki, K. Ikuta, E. Muso, H. Yoshida, S. Sasayama, and T. Honjo. 2000. Increased frequency of surface IgA-positive plasma cells in the intestinal lamina propria and decreased IgA excretion in hyper IgA (HIGA) mice, a murine model of IgA nephropathy with hyperserum IgA. J. Immunol. 165:1387–1394. [DOI] [PubMed] [Google Scholar]
- 20.Ardavin, C., P. Martin, I. Ferrero, I. Azcoitia, F. Anjuere, H. Diggelmann, F. Luthi, S. Luther, and H. Acha-Orbea. 1999. B cell response after MMTV infection: extrafollicular plasmablasts represent the main infected population and can transmit viral infection. J. Immunol. 162:2538–2545. [PubMed] [Google Scholar]
- 21.Franco, M.A., and H.B. Greenberg. 1999. Immunity to rotavirus infection in mice. J. Infect. Dis. 179:S466–S469. [DOI] [PubMed] [Google Scholar]
- 22.Feng, N., J.W. Burns, L. Bracy, and H.B. Greenberg. 1994. Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J. Virol. 68:7766–7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burns, J.W., M. Siadat-Pajouh, A.A. Krishnaney, and H.B. Greenberg. 1996. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 272:104–107. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani, A. 1999. The chemokine system: redundancy for robust outputs. Immunol. Today. 20:254–257. [DOI] [PubMed] [Google Scholar]
- 25.Vicari, A.P., D.J. Figueroa, J.A. Hedrick, J.S. Foster, K.P. Singh, S. Menon, N.G. Copeland, D.J. Gilbert, N.A. Jenkins, K.B. Bacon, and A. Zlotnik. 1997. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity. 7:291–301. [DOI] [PubMed] [Google Scholar]
- 26.Campbell, J.J., J. Pan, and E.C. Butcher. 1999. Cutting edge: developmental switches in chemokine responses during T cell maturation. J. Immunol. 163:2353–2357. [PubMed] [Google Scholar]
- 27.Zabel, B.A., W.W. Agace, J.J. Campbell, H.M. Heath, D. Parent, A.I. Roberts, E.C. Ebert, N. Kassam, S. Qin, M. Zovko, et al. 1999. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J. Exp. Med. 190:1241–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunkel, E.J., J.J. Campbell, G. Haraldsen, J. Pan, J. Boisvert, A.I. Roberts, E.C. Ebert, M.A. Vierra, S.B. Goodman, M.C. Genovese, et al. 2000. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment. Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wurbel, M.A., J.M. Philippe, C. Nguyen, G. Victorero, T. Freeman, P. Wooding, A. Miazek, M.G. Mattei, M. Malissen, B.R. Jordan, et al. 2000. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur. J. Immunol. 30:262–271. [DOI] [PubMed] [Google Scholar]
- 30.Agace, W.W., A. Amara, A.I. Roberts, J.L. Pablos, S. Thelen, M. Uguccioni, X.Y. Li, J. Marsal, F. Arenzana-Seisdedos, T. Delaunay, et al. 2000. Constitutive expression of stromal derived factor-1 by mucosal epithelia and its role in HIV transmission and propagation. Curr. Biol. 10:325–328. [DOI] [PubMed] [Google Scholar]