Abstract
To examine the widely accepted dogmas that corneal grafts lack passenger leukocytes or cells capable of migrating directly to lymph nodes (LNs), we tracked the migration of corneal graft-derived transgenic green fluorescent protein (GFP; Iab) cells into the draining LNs of allogeneic (Iad) recipients. GFP+ cells were identified in cervical LNs several hours after transplantation, and this traffic was significantly enhanced when grafts were placed in inflamed recipient beds. Draining cells were Iab+, CD45+, and CD11c+, and examination of ungrafted corneas revealed numerous similarly CD45+CD11c+CD3−CD8α− cells that uniformly lacked major histocompatibility complex (MHC) class II expression; transmission electron microscopy confirmed the presence of morphologically similar cells. After transplantation, or placement in culture, these CD11c+ cells became class II+ in a time-dependent manner and were capable of allostimulatory function. However, the stimulatory capacity of these cornea-derived dendritic cells (DCs) was suppressed compared with splenic controls. These results demonstrate for the first time that the cornea is endowed with resident DCs that are universally MHC class II− but that are capable of expressing class II antigen after surgery and migrating to draining LNs of allografted hosts. These data refute the tenet that the cornea is immune privileged due to lack of resident lymphoreticular cells or due to antigenic sequestration from systemic immunity.
Keywords: antigen presentation, dendritic cells, lymph node, MHC antigens, transplantation
Introduction
Corneal transplantation is by far the most common form of solid tissue transplantation with over 40,000 cases performed annually in the United States (1). Uncomplicated corneal grafts performed in nonvascularized “low risk” host beds enjoy a survival rate of >90% under cover of local immune suppression, and represent one of the most successful forms of transplantation. This high success rate is overshadowed by survival rates of well under 50% when grafts are placed in “high-risk” hosts whose recipient bed is inflamed and vascularized, a common finding in patients with a history of ocular inflammation (2).
The general success of allogeneic corneal transplants observed in hosts with uninflamed and nonvascularized graft beds has been ascribed to the fact that the cornea and ocular anterior segment are considered immune privileged sites (3, 4). Features of the ocular anterior segment that have been implicated in immune privilege include expression of a number of immunosuppressive factors by the cornea and iris-ciliary body (3), Fas ligand expression by the cornea (5), and the fact that the cornea itself is avascular and devoid of lymphatics (6). In addition, the central uninflamed cornea has the unique property of not expressing class II MHC (Ia/HLA DR) antigens (7–10). In contrast, other solid organ grafts (e.g., heart, kidney, and skin) are significantly endowed with MHC class II+ dendritic “passenger leukocytes” (11–13) capable of migrating to host lymphoid organs and stimulating T cells directly by presenting donor-derived peptides in the context of donor MHC class II (12, 14). It has been argued, however, that due to the putative absence of corneal bone marrow–derived cells that may serve as APCs (7, 9), allosensitization in corneal transplantation relies almost exclusively on the indirect pathway where host APCs migrate into the graft and present processed allopeptides in the context of recipient class II antigens (15, 16). The normal lack of MHC class II+ cells in the cornea has additionally contributed to the dominant paradigm of corneal alloimmunity asserting that generation of immunity to graft antigens is almost exclusively directed at donor minor (and not MHC) antigens through a T cell response that is self-restricted (17).
There are, nevertheless, several lines of evidence to suggest that donor MHC antigens are relevant in corneal alloimmunity, in particular in the high-risk setting where the degree of inflammation in the graft bed may augment the immune response generated to ocular antigens (18). First, it has been shown that under appropriate inflammatory stimulation, corneal cells are capable of overexpression of class II antigens (10, 19). Second, some degree of direct priming of host T cells has been documented in high-risk transplantation (20), suggesting that inflamed graft beds may afford a more facile access of donor cells to host lymphoid organs, particularly to the draining LN which has recently been shown to be the critical site for induction of corneal alloimmunity (21). However, in spite of data that implicate the potential role of donor MHC in directly priming corneal alloimmunity, the mechanisms by which graft MHC antigens gain access to host lymphoid organs has remained unclear.
Herein we provide direct support for our hypothesis that, contrary to current dogma, donor graft cells expressing MHC class II antigens migrate from the corneal transplant to draining LNs, and the traffic of these donor cells to draining LNs is significantly enhanced in inflamed high-risk host beds compared with low-risk hosts (with avascular recipient beds). Moreover, we demonstrate that these trafficking cells are CD45+CD11c+ cells that are normally universally MHC class II− when resident in the uninflamed cornea but rapidly become MHC class II+ after transplantation or when placed in culture. Finally, we demonstrate that in culture corneal CD11c+ cells spontaneously migrate out of the cornea and progressively acquire MHC class II expression, but have modest allostimulatory capacity compared with dendritic cells (DCs)* derived from the spleen.
Materials and Methods
Mice.
8–10-wk-old mice were used for these experiments; wild-type mice consisted of BALB/c (H-2d) and C57BL/6 (H-2b) strains obtained from our Institute breeding facility or purchased from The Jackson Laboratory. Transgenic mice expressing enhanced green fluorescent protein (GFP) on a C57BL/6 background (strain C57BL/6-TgN[b-act-EGFP]OsbSer H-2b) were provided by Dr. Masaru Okabe (Osaka University, Osaka, Japan; reference 22) and bred in our Institute. Each animal was placed under general anesthesia by intramuscular injections of ketamine (3–4 mg) and xylazine (0.1 mg) for each surgical procedure. All protocols were approved by the Schepens Eye Research Institute Animal Care and Use Committee, and all animals were treated according to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Induction of Corneal Neovascularization for Creation of High-Risk Host Beds.
Three interrupted sutures (11–0 nylon, Sharpoint; Vanguard) were placed in the central cornea of one eye of each recipient BALB/c mouse to induce inflammatory corneal neovascularization, which is also associated with significant lymphangiogenesis, as described previously (18). 2 wk later, when all corneas developed extensive neovascularization, all sutures were removed. The neovascularized corneas then served as high-risk host beds for corneal transplants.
Cauterization of the Corneal Surface.
Mice were anesthetized and placed under the operating microscope. Using the tip of a handheld thermal cautery (Aaron Medical Industries Inc.), six burns were applied to the central 50% of the cornea as previously described in order to induce ingress of (donor) APCs into the central cornea (23). 2 wk later the cauterized corneas were excised and used as corneal grafts.
Corneal Transplantation.
Our standard protocol (21) for murine orthotopic corneal transplantation was used for these studies. Briefly, the center of the donor cornea (from transgenic GFP or wild-type C57BL/6 background mice) was marked with a 2-mm diameter microcurette, and excised with Vannas scissors (Storz Instruments Co.) and placed into chilled PBS. The recipient graft bed was prepared by excising a 1.5-mm site in the central cornea. The donor button was then placed onto either normal avascular (low-risk; n = 5 per time point studied) or neovascularized (high-risk; n = 5 per time point studied) graft beds of BALB/c recipients and secured with eight interrupted 11–0 nylon sutures, followed by application of antibiotic ointment.
Transmission Electron Microscopy.
Freshly excised healthy BALB/c corneas were fixed in Karnovsky solution. After three washes in cacodylate buffer, corneas were postfixed for 1.5 h in 1% osmium tetroxide in the same buffer. Corneas were washed with H2O, stained in aqueous 2% uranyl acetate, dehydrated, and embedded in epon. Sections were cut at 60 Å and a Philips 410 TEM was used for electron microscopy.
Abs.
The immunohistochemical, immunocytochemical, and FACS® staining procedures were performed using the following Abs: purified mouse anti–mouse class II MHC (KH74, anti-Iab); FITC-conjugated mouse anti–mouse class II MHC (AF6–120, anti-Iab); FITC-conjugated mouse anti–mouse class II MHC (39–10–8, anti-Iad); purified rat anti–mouse CD45 (30-F11, pan-leukocyte marker); purified hamster anti–mouse CD11c (HL3, DC marker); PE-conjugated hamster anti–mouse CD11c (HL3); polyclonal rabbit anti-GFP; FITC-conjugated rat anti–mouse CD8α (53–6.7, lymphoid DC marker); FITC-conjugated hamster anti–mouse CD3-e (145–2C11, T lymphocyte marker); rabbit polyclonal anti-keratin 12 (K-12, corneal epithelial cell marker); and rat anti–mouse CD16/32 (2.4G2, FcγIII/II receptor). The secondary antibodies were Cy5-conjugated goat anti–mouse F(ab′)2; Cy3-conjugated donkey anti–rat IgG; Cy5-conjugated goat anti–Armenian hamster IgG; and R-conjugated donkey anti–rabbit IgG. Isotype controls included mouse IgG2a, mouse IgG3, rat IgG2b, rat IgG2a-FITC, hamster IgG, mouse IgG3-FITC, mouse IgG2a-FITC, and hamster IgG-PE. All primary mAbs (except where noted) and isotype matched controls were purchased from BD PharMingen, and second IgG Abs were purchased from Jackson ImmunoResearch Laboratories. The anti–K-12 and anti-GFP Abs were gifts of Dr. Zieske and Dr. Young, respectively, both of the Schepens Eye Research Institute.
Immunofluorescence Confocal Microscopy and Immunocytochemistry.
Normal and grafted corneas (2, 6, 16, 24, 48, and 72 h after grafting) were excised from BALB/c mice. Full thickness corneal tissue or 8-μm frozen sections were fixed in acetone for immunofluorescence staining for CD3, CD8α, CD11c, CD45, donor (Iab) and recipient (Iad)-type class II MHC, and K-12. Draining cervical LNs were collected at specific times (6, 16, 24, 48, and 72 h) after grafting, frozen in liquid nitrogen, and fixed by 4% paraformaldehyde. In some experiments, LN sections (8 μm) were fixed in acetone instead of paraformaldehyde. To block nonspecific staining, sections were blocked with anti-FcR mAb (CD16/CD32) for 30 min before they were immunostained with primary antibodies or isotype-matched control antibodies for 2 h. Thereafter, the sections were incubated with secondary antibodies for 1 h. All staining procedures were performed at room temperature, and each step was followed by three thorough washings in PBS for 5 min each. Finally, the samples were covered with mounting medium (Vector Laboratories) and analyzed using a confocal laser scanning microscope (Leica TCS 4D; Lasertechnik). At least five sections were analyzed for each tissue specimen derived from each animal. Representative data are presented below. To assay for GFP-expressing cells, full thickness corneal tissue or 8-μm frozen sections of cornea or lymph nodes were observed by confocal microscopy without immunofluorescence labeling. In addition, anti-GFP Ab was used for confirmation of GFP expression in the LN. For immunocytochemical studies, cytospin preparations were made from cultured corneal explants' nonadherent cells (see below). The cytospin slides were fixed in chilled acetone for 15 min and cells were stained with anti-CD11c and CD45, with relevant isotype controls after FcR blockade. All experiments were conducted three times, independently.
Isolation of Nonadherent Migrating Corneal Cells.
Corneal buttons were excised from BALB/c mice and placed into a 6-well plate with 10 buttons per well. The corneal buttons were cultured in 2.5 ml of RPMI-1640 with 10% FBS (Hyclone), 10 mM HEPES, 0.1 mM nonessential amino acid, 100 U/ml of penicillin, 100 μg/ml of streptomycin (Biowhittaker), and 10−5 M 2-mercaptoethanol (Sigma-Aldrich) and incubated at 37°C for 1, 3, or 6 d. The nonadherent cells were isolated from the supernatant of the cultures; the collected supernatant was centrifuged and the cells resuspended in PBS. The cells were washed once in PBS, and filtered through a 70-μm nylon cell strainer to remove corneal fragments. These nonadherent cells were assayed for their allosensitizing capacity and analyzed by flow cytometry as described below.
Mixed Lymphocyte Reaction.
The nonadherent corneal cells, harvested as described above, were used as stimulators (105/well in 96-well plate) in the mixed lymphocyte reactions (MLRs). For stimulator controls, fresh splenocytes were collected from naive C57BL/6 mice for syngeneic controls and from naive BALB/c mice for allogeneic controls. The cells were cultured in 5% FBS RPMI-1640 and incubated at 37°C for 90 min. Nonadherent splenic cells were discarded and adherent cells were then washed three times and incubated for 30 min in serum-free RPMI-1640 to promote DC detachment. The detached cells were collected, washed with 10% FBS, and used as stimulator cells in the MLR. Responder cells were splenocytes (2 × 105/well) derived from naive C57BL/6 mice. The cultures were incubated at 37°C in 10% FBS RPMI-1640 and [3H]thymidine was added to each culture (1 μCi/well) after 72 h. After an additional 18 h of incubation, the cells were collected through a multichannel plate harvester (Wallac Inc.) and DNA incorporated [3H]thymidine was counted by scintillation counting and expressed as mean cpm ± SEM of triplicate cultures.
Flow Cytometry.
The nonadherent cells were blocked by anti-FcR mAb (CD16/CD32) before cells were labeled with FITC-conjugated mouse anti-Iad and PE-conjugated hamster anti–mouse CD11c. For isotype controls, the cells were labeled with FITC-conjugated mouse IgG3 and PE-conjugated hamster anti-mouse CD11c. Cells were washed and analyzed using an Epics XL flow cytometer (Beckman Coulter). The analysis was done by gating on CD11c-positive cells using appropriate isotype and cell culture controls to adjust color compensation and gating parameters. The proportion of CD11c+ cells that were also Ia positive was quantified. Nonadherent cells of parallel spleen cell cultures were used as controls to evaluate relative MHC class II expression. The splenic cultures were established with initially adherent cells from naive BALB/c mice incubated in culture for 90 min, washed, and then 2.5 ml of 10% FBS RPMI-1640 was added to the cultures. Supernatants were collected and the harvested nonadherent cells were treated identically as nonadherent cells derived from corneal explants. Cultures were incubated for 1, 3, or 6 d.
Data Analysis.
Student's t test was used to compare the number of GFP-positive corneal donor cells migrating to the host LN at different time points, and for comparing [3H]thymidine uptake in MLR experiments. Chi-square test for trend analysis was performed to evaluate changes in Ia expression by cornea-derived DCs. P value of <0.05 was considered significant.
Results
GFP+ Cells Are Detected in Ipsilateral Draining Cervical LNs after Corneal Transplantation.
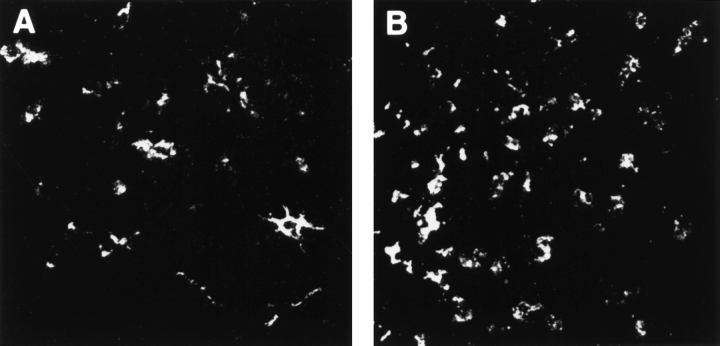
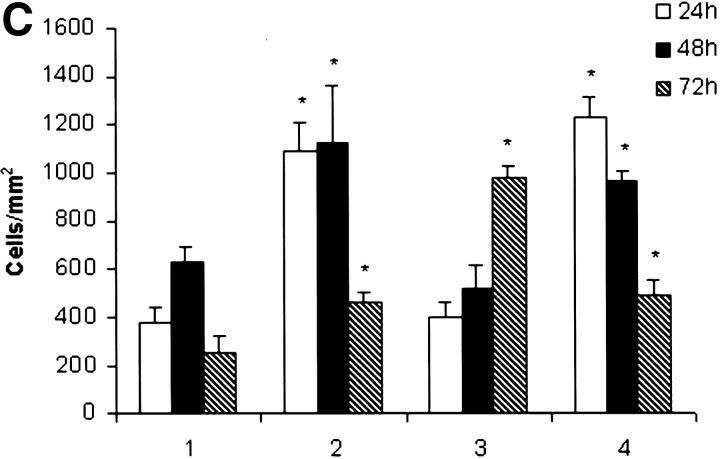
LNs were harvested at various time points after corneal transplantation and examined under confocal microscopy for detection, localization, and quantification of GFP+ cells. Control slides, in which primary antibody was omitted or replaced by an irrelevant antibody of the same isotype, or LNs contralateral from the side of the surgery, did not show any reactivity. Controls with anti-GFP Ab were used to confirm the results of specimens examined for GFP+ cells. In the low-risk uninflamed setting, GFP+ cells were clearly evident as early as 24 h after grafting in the ipsilateral cervical LNs (Fig. 1 A). GFP expression was patchy and localized largely to the parafollicular areas of the LN with a density of 375 ± 66 cells/mm2 at 24 h (Fig. 1 C; group 1). The number of GFP+ cells increased to 633 ± 63 cells/mm2 at 48 h, and decreased to 275 ± 90 cells/mm2 by 72 h after transplantation. In the case of normal donor tissue grafted into inflamed high-risk beds, GFP+ cell traffic to ipsilateral host LNs reached a mean density of 1,090 ± 115 cells/mm2 at 24 h (Fig. 1 B), and remained consistently higher than the traffic observed in low-risk grafting (Fig. 1 C; group 2). Draining LNs of cauterized donor corneas (thus manipulated to enrich with donor APCs before grafting) transplanted into uninflamed host beds also first showed evidence for GFP+ cells at 24 h after transplantation (403 ± 66 cells/mm2), with increasing numbers of cells detected at the later time points studied (Fig. 1 C, group 3). When cauterized donor corneas were grafted into high-risk lymphatic-rich beds, GFP+ cells were found as early as 6 h after transplantation, and peaked at 24 h (1,167 ± 76 cells/mm2; Fig. 1 C, group 4).
Figure 1.
GFP+ cells are detected in ipsilateral draining cervical lymph nodes after corneal transplantation. Host draining cervical LN (LN) were analyzed at different time points after transplantation of transgenic GFP+ grafts. Draining LNs from low-risk corneal grafts (A) exhibited a low number of GFP+ cells at 24 h after transplantation. In contrast, LNs draining high-risk grafts (B) exhibited a significantly higher number of GFP+ cells. Original magnification, (A and B) 400×. Mean numbers of GFP+ cells present in ipsilateral LN at 24, 48, and 72 h after transplantation are depicted in C: (1) normal cornea grafted into nonvascularized bed; (2) normal cornea grafted into inflamed and vascularized high-risk bed; (3) cauterized (APC-enriched) cornea grafted into nonvascularized bed; and (4) cauterized cornea grafted into high-risk bed. Mean ± SD (cells/mm2) from cervical LNs of five mice for each group/time point are compared. * denotes statistically significant (P < 0.05) difference compared with the same time points in group 1.
GFP+ Donor Cells Migrate Centrifugally Out of the Corneal Graft into the Recipient Bed.
Based on the finding of significant GFP+ cells in the draining cervical LNs after corneal transplantation, we evaluated corneal specimens at various time points after surgery to examine whether GFP+ cells could be seen emigrating out of the grafts. When specimens were examined at 24 h (or later) after transplantation we observed GFP+ cells migrating centrifugally out into the wild-type recipient beds (Fig. 2). Confocal microscopy showed that this phenomenon was present for 360 degrees around the grafts, and was particularly marked for grafts placed in inflamed high-risk beds.
Figure 2.
GFP+ donor cells are evident migrating centrifugally out of the corneal graft into the recipient bed. Confocal microscopic analysis performed on transgenic GFP+ grafts (green fluorescence) placed onto wild-type hosts (shown as black background) revealed migration of donor-derived GFP+ cells into the recipient bed. Dashed line demarcates the graft-host margin. * depicts GFP+ cells. Original magnification, 160×.
High-risk Corneal Transplantation Leads to Significantly Enhanced Traffic of MHC Class II+ Donor Cells to Draining LNs.
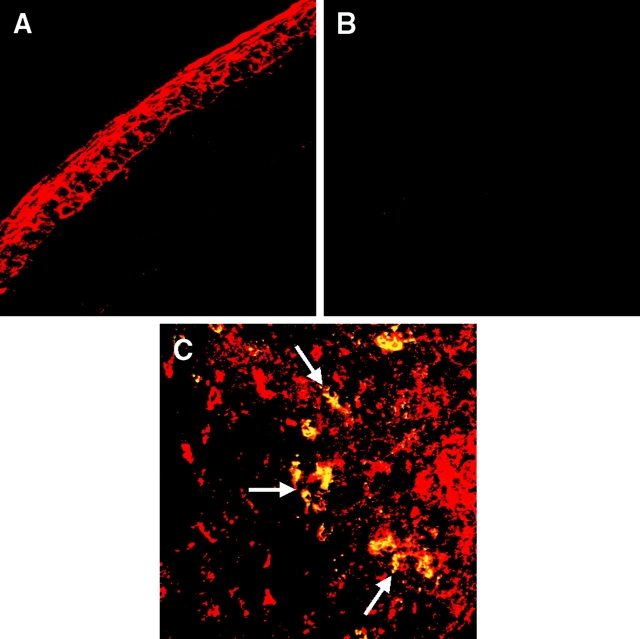
To ensure that the GFP detection in the draining LNs was a reflection of intact donor-derived cells migrating to LNs, we performed immunostaining for donor-type MHC class II (anti-Iab Ab) and GFP (anti-GFP Ab). Results clearly demonstrated the presence of significant numbers of intact Iab+ cells in the draining LNs of hosts of both low-risk (Fig. 3 A) and high-risk (Fig. 3 B) corneal grafts. Donor Iab+ cells colocalized strongly with GFP expression (Fig. 3 C). In contrast, we did not detect colocalization of graft-derived GFP and host Iad+ cells at the early time points studied; instead, the large numbers of host Iad+ cells in the LNs were GFP− (Fig. 3 D) suggesting that mobilization of host APCs capable of processing corneal antigen and migrating to draining LNs had not occurred by the time of our analyses.
Figure 3.
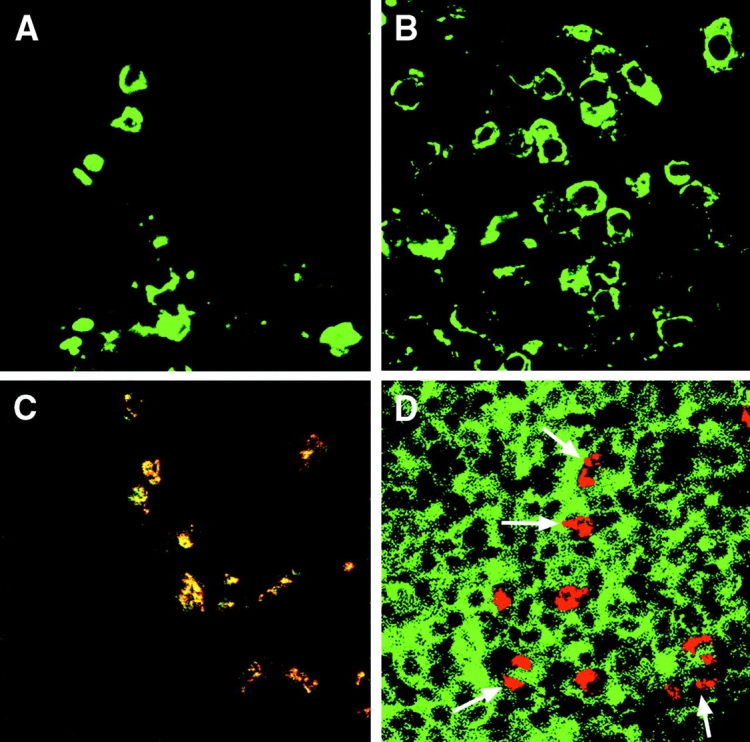
High-risk corneal transplantation is associated with enhanced traffic of MHC class II+ donor cells to draining LNs. Cervical LNs from BALB/c hosts grafted with C57BL/6 background mice were procured and immunostained for detection of donor (Iab) and host (Iad) derived MHC class II (green) and GFP (red) expression. Clusters of Iab+ cells were clearly visible in the draining LNs in low-risk hosts (A); significantly higher numbers of cells were detected in the high-risk setting (B). Graft-derived GFP colocalized with donor-type Iab (yellow; C); in contrast, GFP (red) did not colocalize with host class II+ (Iad; green) cells (D). Arrows demarcate the parafollicular areas of the LNs. Representative data at 48 h; original magnifications 1,000× (A and B) and 400× (C and D).
Donor-derived Cells in the Draining LNs Are Bone Marrow Derived.
The data above demonstrated traffic of donor-derived cells to host LNs. As many nucleated, including corneal, cells are capable of expressing MHC class II antigens under the appropriate inflammatory conditions (10, 19), we tested whether the origin of the migrating cells is corneal (i.e., epithelial) or bone marrow derived. Resident corneal epithelial cells can be differentiated from bone marrow and lymphoreticular cells by their failure to express the pan-leukocyte marker CD45, and their specific expression of keratin-12 (24).
Our results showed that whereas corneal cells exhibited K-12 expression (Fig. 4 A), there was no K-12 expression in any of the LNs assayed at the time points studied (Fig. 4 B). To establish the bone marrow derivation of the migrating cells, we performed double staining on the specimens to colocalize CD45 and donor Ia expression (corneal keratocytes and epithelial cells do not express CD45). Examination of draining LNs from grafts revealed significant numbers of CD45+ cells in the LNs, and a subpopulation of these cells expressing donor Iab (Fig. 4 C). Similar data were derived when double staining was performed for donor-type Ia and CD11c. Most donor cells localized to the parafollicular areas of the draining LNs. No CD3+ cells expressing donor Ia were observed at any of the time points studied.
Figure 4.
Donor-derived cells in the draining LNs are bone marrow derived. The normal cornea exhibited strong expression of keratin 12 (K-12) (A). However, examination of the draining LNs after corneal transplantation did not exhibit any sign of K-12+ cells at any time point studied (B). Numerous CD45+ (red) cells were seen in the LNs and double staining for donor Iab (green) exhibited colocalization (yellow) mostly in the parafollicular areas (arrows) of the draining LNs (C). No purely green cell was observed, confirming that all donor MHC class II+ cells are bone marrow derived (CD45+). Original magnifications 400× (A), 160× (B), and 400× (C).
Resident Corneal MHC Class II–negative Bone Marrow–derived Cells Express MHC Class II after Transplantation-induced Inflammation.
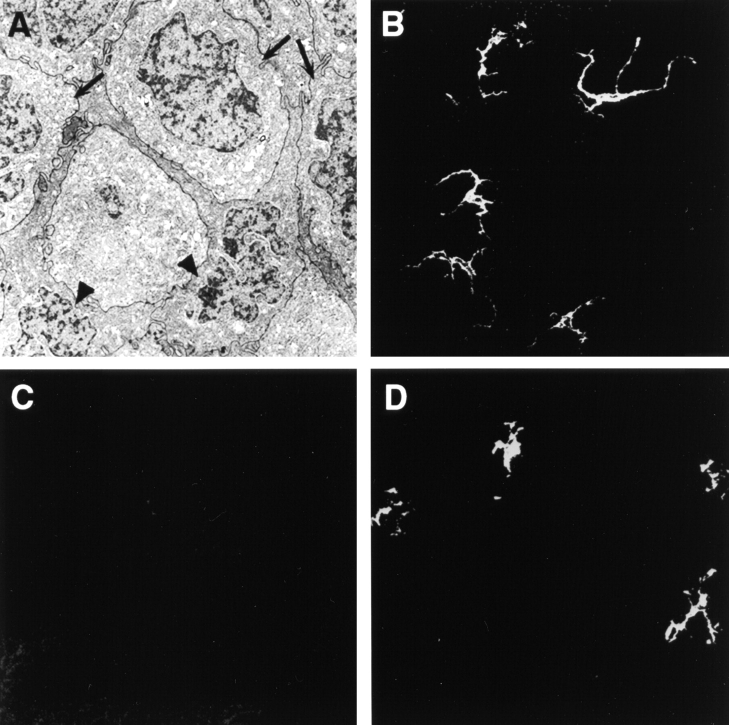
The finding of bone marrow–derived donor class II+ cells in the draining LN led us to focus on the origin of these cells in the corneal graft. Transmission electron microscopy (TEM) demonstrated presence of numerous DCs interdigitating among the corneal epithelial cells (Fig. 5 A). Cells with similar morphology were also identified in the corneal stroma. Confocal analysis of the same specimens revealed numerous cells that were CD45+ and CD11c+ (Fig. 5 B), but uniformly failed to express class II antigens (Fig. 5 C), demonstrating that central areas of corneal tissue procured for grafting are indeed universally and normally MHC class II negative. However, within 24 h after surgical inflammation these cells became positive for Iab (Fig. 5 D). The coexpression by both grafted corneas and draining LNs of CD45+CD11c+Iab+ cells suggested that corneal grafts have an endowment of resident MHC class II–negative bone marrow–derived cells that may, under appropriate stimulation, express Ia and traffic to draining LNs. The donor CD11c+ cells in the cornea were uniformly CD8α− and CD3− in all specimens studied.
Figure 5.
Resident corneal CD11c+ cells upregulate expression of MHC class II after surgical inflammation. The center of normal uninflamed corneas, corresponding to areas procured for transplantation, contains numerous DCs (arrow heads) interdigitating between epithelial cells (arrows) as seen by TEM (A). These cells express CD11c (B), but uniformly fail to express MHC class II (Iab) antigens (C). Within 24 h of surgery, these DCs begin to coexpress Iab (D). Original magnifications 7,500× (A); 400× (B–D).
Cultured Corneal DCs Have Modest Allostimulatory Capacity.
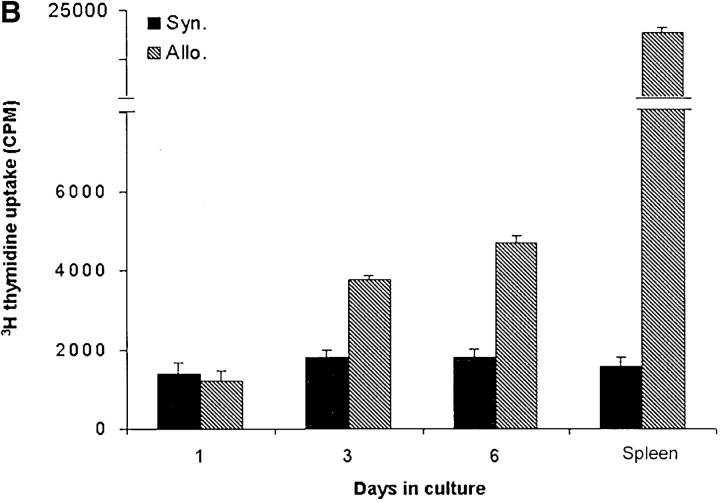
The above experiments, based on in situ confocal studies, provided evidence for resident corneal MHC class II− DCs capable of trafficking to draining LNs as more mature MHC class II–expressing cells. To further phenotype and study the function of these cells in vitro, normal BALB/c-derived corneas were excised and placed in culture as described in Materials and Methods. Using standard techniques of harvesting DCs in culture, the nonadherent floating cells were procured and stained for CD11c demonstrating ample expression of this DC marker (Fig. 6 A); similar results were obtained for CD45 (data not shown). These corneal cells, or equal number of splenic CD11c+ cells, were then harvested and used as stimulators in MLR assays with allogeneic responder C57BL/6 splenic cells; syngeneic C57BL/6-derived corneal or splenic cells served as syngeneic stimulator controls (Fig. 6 B). Results demonstrate that 1 d-cultured allogeneic corneal cells have no demonstrable allostimulatory capacity; however, modest stimulation of C57BL/6 responders was observed with BALB/c corneal cells cultured for 3 d (stimulation index, 2.1) or 6 d (stimulation index, 2.6) but not with syngeneically derived corneal cells (Fig. 6 B; P = 0.01). However, the stimulatory capacity of these corneal cells was significantly lower than that observed for allogeneic splenic controls (stimulation index, 13.5; P = 0.001).
Figure 6.
CD11c+ cells migrating out of cultured corneal explants have modest allostimulatory capacity. Corneal explants were placed in culture, and the nonadherent cells harvested and stained for CD11c expression; representative data from 3 d-cultured explants shown (A). 105 cells harvested from BALB/c (allogeneic) or C57BL/6 (syngeneic) corneal explants kept in culture for 1, 3, or 6 d were used as stimulators for 2 × 105 C57BL/6 splenocytes in MLRs; splenic cells were used as control stimulators (B). Data demonstrate modest allostimulatory capacity by corneal cells cultured for 3 d or longer.
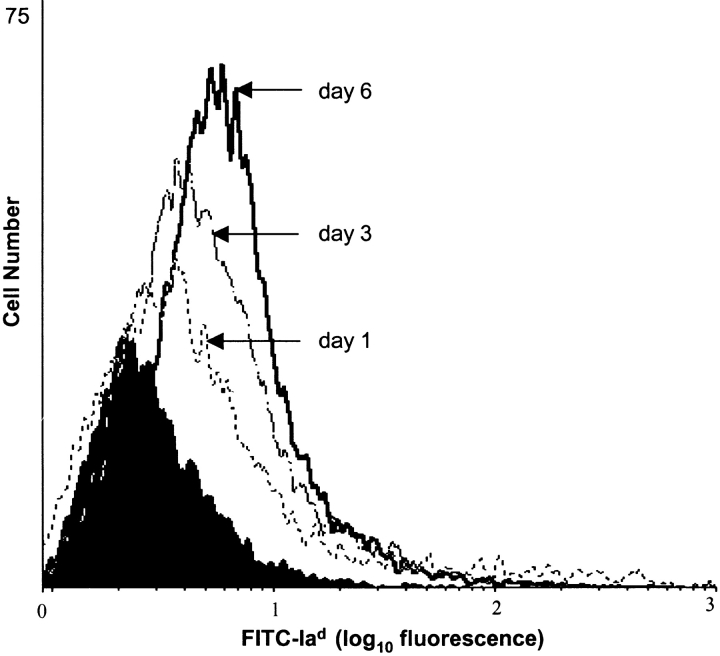
To further phenotype these cells, normal corneas were excised, placed in culture, and incubated for different periods. The nonadherent cells were harvested, and subjected to two-color staining (CD11c-PE and Iad-FITC) for flow cytometric analysis. Similarly derived splenic nonadherent cells were used as controls. 84% of splenic CD11+ cells were Ia+ compared with 39% of corneal CD11C+ cells after 1 d of culture. Prolonged culture of the cells led to 47 and 64% Ia-positivity at days 3 and 6, respectively (Fig. 7; P for trend <0.05), suggesting that the differential capacity of these DCs to stimulate allogeneic responders based on culture time is due at least in part to their relative expression of MHC class II.
Figure 7.
Prolonged culturing of corneal CD11c+ cells leads to enhanced MHC class II (Iad) expression. Phenotypic analysis of cultured CD11c+ corneal DCs by flow cytometry using two-color staining (CD11c-PE and Iad-FITC) at days 1, 3, and 6 reveals progressive expression (logarithmic scale) of MHC class II with increasing culture periods. Shaded area shows isotype control.
Discussion
The dual requirement for intimate APC–T cell interaction, and a microenvironment which facilitates expansion of primed T cells, is afforded by lymphoid organs that drain APCs from the periphery and bring them in close proximity to naive T cells (13). In solid organ transplantation the concept that access of donor-derived cells to afferent lymphatics and consequently to draining LNs is a critical facet of alloimmunity was introduced by Barker and Billingham several decades ago through their skin graft studies (25). Years later, Lechler and Batchelor showed that the key observation of Barker and Billingham was related to the migration of MHC class II+ resident donor interstitial cells to draining LNs, and defined these “passenger leukocytes” as a critical component of graft immunogenicity (26).
However, the application of these general concepts of transplantation immunology to the case of corneal alloimmunity has been unclear, and met with considerable resistance for several reasons. First, the putative lack of any donor-derived passenger leukocytes has been cited as a critical facet of corneal alloimmunity (7–9, 15). Second, the lack under normal (uninflamed) circumstances of any corneal lymphatics (6) has led to a focus on antigen and APC traffic via the oculosplenic axis (through the venous circulation) rather than the more “conventional” route of APC traffic to draining LNs through lymphatics (27). However, there is now ample evidence to suggest that draining LNs are a major reservoir of alloreactive T cells in corneal transplantation (20, 21); in fact we have recently shown that generation of corneal alloimmunity is critically dependent on a functional ocular-lymphatic axis, and that lymphadenectomy leads to indefinite survival of corneal grafts (21). We propose, therefore, that at issue is not whether there is access of corneal graft antigens to draining LNs, but rather the source and route of these antigens.
Our data demonstrate that there is ample traffic of donor MHC class II+ cells to draining LNs after corneal transplantation, and this traffic is significantly greater emanating from high-risk as compared with low-risk grafts. We show that the origin of these draining cells are resident bone marrow-derived (CD45+) dendritic (CD11c+) cells in the cornea that are normally universally MHC class II− but that express class II antigen in vivo after surgical inflammation, or after in vitro culturing. Our data demonstrating migration of MHC class II− DCs from the graft have similarities to the experimental work of Roake et al. (14) in which they depleted Ia+ cells from the heart with systemic administration of LPS and grafted such manipulated hearts into allogeneic hosts showing localization of donor-derived Ia+ cells in host spleens, thereby demonstrating that phenotypically normal (Ia+) passenger leukocytes may be derived from progenitor or immature (Ia−) DCs. However, the distinctive feature of the cornea, as demonstrated by our data, is that all of the trafficking donor DCs begin as MHC class II− cells even without any prior systemic manipulation.
Previous studies that have focused on the distribution of ocular surface APCs (7–10, 15), including from our group (23), have made reference to these cells being MHC class II+ in the corneoscleral limbus (the vascularized area adjacent to the cornea) where they are recruited from the intravascular compartment. It remains unknown whether the CD11c+ cells described in this study represent a progenitor DC population that does not express class II unless stimulated to do so, or whether their expression of MHC class II antigen is in fact actively suppressed once they reside in the cornea in response to one or more of the immunosuppressive factors secreted by this tissue (3). If so, the concept of corneal “immune privilege” based on the putative absence of APC populations in the cornea (7) may well need to be restated as being due rather to the suppressed expression of class II by the numerous CD45+CD11c+ cells in the cornea.
Similar to our data demonstrating acquisition of MHC class II by corneal DCs in culture, Larsen et al. have demonstrated that migration of epidermal Langerhans cells from skin transplants and explants is likewise associated with upregulation in MHC class II expression which coincides with augmented immunostimulatory capacity of these cells (28). However, our data differ from those of Larsen et al. in one important facet; whereas they demonstrate that prolonged culturing of epidermal LCs leads to a higher stimulatory capacity than freshly procured epidermal LC or splenic cell controls, our data clearly show that corneal DCs have a suppressed immunostimulatory capacity compared with spleen cells even after prolonged culturing and acquisition of higher class II expression. This dampened sensitizing capacity, along with the fact that corneal DCs are normally universally MHC class II−, stresses the fact that in spite of certain similarities between the cornea and the skin, the cornea indeed retains certain unique immunological features. These differences likely also explain the dominant role of the indirect pathway in corneal allosensitization as we have recently confirmed (29). However, direct sensitization may become operative in the high-risk corneal transplant setting given both the higher trafficking of donor DCs to draining LNs and the augmented expression of MHC class II by donor DCs in response to inflammation at the graft site. There is some indirect evidence for this hypothesis in the literature where it has been shown that MHC-disparate corneal grafts experimentally manipulated to be enriched with donor-derived APCs (before transplantation) are rejected at a significantly accelerated fashion as compared with grafts without such enrichment, suggesting that donor corneal APCs may become operative as passenger leukocytes (30). In addition, recent data (unpublished data) from our group based on the enzyme-linked immunospot (ELISPOT) assay demonstrate that directly primed alloreactive T cells are in fact elicited by corneal grafts, as early as 72 h after transplantation in the absence of any demonstrable indirect response, emphasizing that the dominant role played by the indirect pathway of sensitization should not be understood as an exclusive one. These observations are in accord with our data demonstrating numerous donor-derived but no host-derived DCs in draining LNs early after transplantation, suggesting that mobilization of host APCs takes longer than trafficking of donor cells to lymphoid organs. Hence in the aggregate, there is evidence that both the direct and indirect pathways of sensitization may concur in corneal transplantation, similar to what has been demonstrated in other organ grafts such as the skin (31), with the relative contribution of each pathway based on multiple host and time-dependent factors.
Finally, the long-standing concept of “ocular antigenic sequestration” which holds that the principal reason behind the generally dampened immune response to eye-derived antigens is the segregation of these antigens from systemic immunity (32) still holds a dominant place in ocular immunology. The data presented herein, however, suggest that this tenet as it applies to the cornea is at best a relative, and not an absolute, concept as donor cells and antigens clearly are capable of having ample access to host lymphoid tissues and in fact lead to a chimeric state as has been described for other solid organ grafts (11).
In summary, our data provide evidence for the first time of resident bone marrow–derived CD11c+ cells in the cornea, and demonstrate significant plasticity in these cells' capacity to express class II antigen and effect T cell stimulation depending on the microenvironment in which they reside. Whether other immune privileged organs are similarly endowed with large numbers of MHC class II− CD11c+ cells, and to what extent this phenotype promotes immunologic quiescence or tolerance, is so far unknown and deserves further investigation.
Acknowledgments
The authors acknowledge the contribution of our colleagues at the Schepens Eye Research Institute, Drs. Nancy Joyce and Wayne Streilein in providing helpful suggestions, Dr. James Zieske for provision of anti-keratin 12 Abs, and Dr. Michael Young for provision of anti-GFP antibodies. In addition, Mrs. Pat Pearson provided invaluable help in the corneal TEM studies.
This work was supported by Public Health Service-National Institutes of Health grants EY12963 (M.R. Dana) and EY10752 (A.W. Taylor); Research to Prevent Blindness Special Scholar Award (M.R. Dana); Fight for Sight (M.R. Dana); and the Massachusetts Lions Eye Research Fund (M.R. Dana).
Y. Liu and P. Hamrah contributed equally to this work.
Footnotes
Abbreviations used in this paper: DC, dendritic cell; GFP, green fluorescent protein; MLR, mixed lymphocyte reaction; TEM, transmission electron microscopy.
References
- 1.The Collaborative Corneal Transplantation Studies Research Group. 1992. The collaborative corneal transplantation studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch. Ophthalmol. 110:1392–1403. [PubMed] [Google Scholar]
- 2.Mader, T.H., and R.D. Stulting. 1991. The high-risk penetrating keratoplasty. Ophthalmol. Clin. North Am. 4:411–426. [Google Scholar]
- 3.Streilein, J.W. 1999. Regional immunity and ocular immune privilege. Chem. Immunol. 73:11–38. [DOI] [PubMed] [Google Scholar]
- 4.Sonoda, Y., and J.W. Streilein. 1992. Orthotopic corneal transplantation in mice-evidence that the immunogenic rules of rejection do not apply. Transplantation. 54:694–704. [DOI] [PubMed] [Google Scholar]
- 5.Griffith, T.S., T. Brunner, S.M. Fletcher, D.R. Green, and T.A. Ferguson. 1995. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 270:1189–1192. [DOI] [PubMed] [Google Scholar]
- 6.Collin, H.B. 1966. Corneal lymphatics in alloxan vascularized rabbit eyes. Invest. Ophthalmol. Vis. Sci. 5:1–13. [PubMed] [Google Scholar]
- 7.Jager, M.J. 1992. Corneal Langerhans cells and ocular immunology. Reg. Immunol. 4:186–195. [PubMed] [Google Scholar]
- 8.Peeler, J.S., and J.Y. Niederkorn. 1986. Antigen presentation by Langerhans cells in vivo: donor-derived Ia+ Langerhans cells are required for induction of delayed-type hypersensitivity but not for cytotoxic T lymphocyte responses to alloantigens. J. Immunol. 136:4362–4371. [PubMed] [Google Scholar]
- 9.Streilein, J.W., G.B. Toews, and P.R. Bergstresser. 1979. Corneal allografts fail to express Ia antigens. Nature. 282:320–321. [DOI] [PubMed] [Google Scholar]
- 10.Pepose, J.S., K.M. Gardner, M.S. Nestor, R.Y. Foos, and T.H. Pettit. 1985. Detection of HLA class I and II antigens in rejected human corneal allografts. Ophthalmology. 92:1480–1484. [DOI] [PubMed] [Google Scholar]
- 11.Thomson, A.W., L. Lu, N. Murase, A.J. Demetris, A.S. Rao, and T.E. Starzl. 1995. Michrochimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells. 13:622–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austyn, M.A., and C.P. Larsen. 1990. Migration patterns of dendritic leukocytes. Implications for transplantation. Transplantation. 49:1–7. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 14.Roake, J.A., A.S. Rao, P.J. Morris, C.P. Larsen, D.F. Hankins, and J.M. Austyn. 1995. Systemic lipopolysaccharide recruits dendritic cell progenitors to nonlymphoid tissues. Transplantation. 59:1319–1324. [PubMed] [Google Scholar]
- 15.Sano, Y., B.R. Ksander, and J.W. Streilein. 2000. Langerhans cells, orthotopic corneal allografts, and direct and indirect pathways of T-cell allorecognition. Invest. Ophthalmol. Vis. Sci. 41:1422–1431. [PubMed] [Google Scholar]
- 16.Shoskes, D.A., and K.J. Wood. 1994. Indirect presentation of MHC antigens in transplantation. Immunol. Today. 15:32–38. [DOI] [PubMed] [Google Scholar]
- 17.Sano, Y., B.R. Ksander, and J.W. Streilein. 1996. Minor H, rather than MHC, alloantigens offer the greater barrier to successful orthotopic corneal transplantation in mice. Transpl. Immunol. 4:53–56. [DOI] [PubMed] [Google Scholar]
- 18.Dana, M.R., and J.W. Streilein. 1996. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest. Ophthalmol. Vis. Sci. 37:2485–2494. [PubMed] [Google Scholar]
- 19.Young, E., W.J. Stark, and R.A. Prendergast. 1985. Immunology of corneal allograft rejection: HLA-DR antigens on human corneal cells. Invest. Ophthalmol. Vis. Sci. 26:571–574. [PubMed] [Google Scholar]
- 20.Ksander, B.R., Y. Sano, and J.W. Streilein. 1996. Role of donor-specific cytotoxic T cells in rejection of corneal allografts in normal and high-risk eyes. Transpl. Immunol. 4:49–52. [DOI] [PubMed] [Google Scholar]
- 21.Yamagami, S., and M.R. Dana. 2001. The critical role of draining lymph nodes in corneal allosensitization. Invest. Ophthalmol. Vis. Sci. 42:1293–1298. [PubMed] [Google Scholar]
- 22.Okabe, M., M. Ikawa, K. Kominami, T. Nakanishi, and Y. Nishimune. 1997. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 407:313–319. [DOI] [PubMed] [Google Scholar]
- 23.Dekaris, I., S.N. Zhu, and M.R. Dana. 1999. TNF-α regulates corneal Langerhans cell migration. J. Immunol. 162:4235–4239. [PubMed] [Google Scholar]
- 24.Liu, C.Y., G. Zhu, A. Westenhausen-Larson, R. Converse, C.W. Kao, T.T. Sun, and W.W. Kao. 1993. Cornea-specific expression of K-12 keratin during mouse development. Curr. Eye Res. 12:963–974. [DOI] [PubMed] [Google Scholar]
- 25.Barker, C.F., and R.E. Billingham. 1968. The role of afferent lymphatics in the rejection of skin homografts. J. Exp. Med. 128:197–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lechler, R.I., and J.R. Batchelor. 1982. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J. Exp. Med. 155:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Streilein, J.W. 1996. Ocular immune privilege and the Faustian dilemma. The Proctor lecture. Invest. Ophthalmol. Vis. Sci. 37:1940–1950. [PubMed] [Google Scholar]
- 28.Larsen, C.P., R.M. Steinman, M. Wimer-Pack, D.F. Hankins, P.J. Morris, and J.M. Austyn. 1990. Migration and maturation of Langerhans cells in skin transplants and explants. J. Exp. Med. 172:1483–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boisgerault, F., Y. Liu, N. Anosova, E. Ehrlich, M.R. Dana, and G. Benichou. 2001. Role of CD4+ and CD8+ T cells in allorecognition. Lessons from corneal transplantation. J. Immunol. 167:1891–1899. [DOI] [PubMed] [Google Scholar]
- 30.Ross, J., D. Callanan, H. Kunz, and J.Y. Niederkorn. 1991. Evidence that the fate of class II-disparate corneal grafts is determined by the timing if class II expression. Transplantation. 51:532–536. [DOI] [PubMed] [Google Scholar]
- 31.Fedoseyeva, E.V., F. Zhang, P.L. Orr, D. Levin, H.J. Buncke, and G. Benichou. 1999. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J. Immunol. 162:6836–6842. [PubMed] [Google Scholar]
- 32.Duke-Elder, S., and E.S. Perkins. 1966. Inflammations of the uveal tract: Uveitis. Chapter III. System of Ophthalmology. Duke-Elder, editor. St. Louis, Mosby, Vol. IX.