Abstract
In normal human serum (NHS), axenic promastigotes of Crithidia, Phytomonas, and Leishmania trigger complement activation, and from 1.2 to 1.8 × 105 C3 molecules are deposited per promastigote within 2.5 min. In Leishmania, promastigote C3 binding capacity remains constant during in vitro metacyclogenesis. C3 deposition on promastigotes activated through the classical complement pathway reaches a 50% maximum after ∼50 s, and represents >85% of total C3 bound. In C1q- and C2-deficient human sera, promastigotes cannot activate the classical pathway (CP) unless purified C1q or C2 factors, respectively, are supplemented, demonstrating a requirement for CP factor in promastigote C3 opsonization. NHS depleted of natural anti-Leishmania antibodies cannot trigger promastigote CP activation, but IgM addition restores C3 binding. Furthermore, Leishmania binds natural antibodies in ethylenediaminetetracetic acid (EDTA)-treated NHS; after EDTA removal, promastigote-bound IgM triggers C3 deposition in natural antibody-depleted NHS. Serum collectins and pentraxins thus do not participate significantly in NHS promastigote C3 opsonization. Real-time kinetic analysis of promastigote CP-mediated lysis indicates that between 85–95% of parasites are killed within 2.5 min of serum contact. These data indicate that successful Leishmania infection in man must immediately follow promastigote transmission, and that Leishmania evasion strategies are shaped by the selective pressure exerted by complement.
Keywords: Trypanosomatids, Leishmania, complement opsonization, promastigote lysis, human serum
Introduction
Leishmania protozoa, the etiologic agents of the leishmanioses, are transmitted to vertebrate hosts by the bite of sandflies of the genera Phlebotomus (Old World) and Lutzomyia (New World). Phlebotomine sandflies infect mammals by inoculating promastigotes into hemorrhagic spots created in the skin while probing for a blood meal. In contact with normal human blood, Leishmania promastigotes trigger complement activation and C3 opsonization (1). Analysis of early promastigote–host cell interactions using an ex vivo model of Leishmania human blood infection indicates that after C3 opsonization, promastigotes undergo an immune adherence (IA)* reaction and bind to CR1 erythrocyte receptors (2). Further studies showed that Leishmania-platelet binding, a primitive form of Leishmania-erythrocyte IA, occurs after promastigote contact with blood of nonprimate mammals, confirming that promastigote IA is the first Leishmania interaction with mammalian cells after infection (3). The immune adherent promastigotes are thereafter endocytosed by blood phagocytes, and undergo physiological and phenotypic transformations inside the phagolysosomes, which culminate in the vertebrate-dwelling amastigote forms (4).
During Leishmania host invasion, complement-mediated promastigote killing can compromise parasite survival. Identification of promastigote opsonization by host serum is thus essential to understanding Leishmania infection strategy. Pioneering studies on promastigote opsonization in normal human serum (NHS) indicated that IgM anti-Leishmania antibodies were responsible for promastigote agglutination, classical complement pathway (CP) activation, and parasite killing (5, 6). Despite these data, understanding of the promastigote opsonization mechanism has to date been dominated by the concept that Leishmania spp. promastigotes activate complement in NHS through the alternative pathway (AP), thus lacking antibody involvement (7–9). Exceptions to this rule have been reported for Leishmania donovani promastigotes (8) and axenic metacyclic peanut agglutinin-negative forms of Leishmania major (10), but the view prevails that leishmanias activate complement via the AP (11, 12).
In addition to promastigote-C3 opsonization by the classical and alternative routes, it is also reported that two specific carbohydrate-binding proteins in serum, mannan-binding lectin (MBL) and the acute phase protein C-reactive protein (CRP), bind Leishmania parasites (13–15); they thus could initiate promastigote opsonization through a novel antibody- and C1-independent mechanism, the lectin-mediated pathway.
To clarify this issue, we performed a comprehensive quantitative and kinetic analysis of promastigote opsonization in NHS in near-physiological conditions using promastigote cell binding assays, high opsonizing serum concentrations (25–100%), and short incubation times (≤3 min). The results indicate that binding of natural IgM anti-Leishmania antibodies (NAb) to conserved trypanosomatid epitopes triggers C3 parasite opsonization, and that serum collectins (MBL) and pentraxins (CRP) do not participate significantly in complement activation. In NHS, promastigotes activate complement CP and AP simultaneously, but >85% of promastigote-bound C3 is generated through the CP, indicating that physiological C3 opsonization of Leishmania is activated through the CP in a natural infection. In the early infection period, promastigote lysis by complement parallels the course of C3 deposition (2). As real-time data on this mechanism were lacking, we measured real-time kinetics of promastigote killing in 50% NHS, and show that from 85–95% of stationary culture promastigotes become permeable to propidium iodide in <3 min after serum contact. Human infection by Leishmania is thus an extremely rapid process, and promastigotes must display evasion strategies immediately after inoculation to avoid lysis by complement.
Materials and Methods
Parasites and Cultures.
Trypanosomatids studied were Leishmania donovani Khartoum 1246 (MHOM/SD/43/124), Leishmania amazonensis Maria (MHOM/Br/79/Maria), Leishmania infantum PB75 (MHOM/Fr/LEM75), L. major NIH 173 (MHOM/IR/-/173), Crithidia fasciculata, and Phytomonas characias. Leishmania and Crithidia promastigotes were cultured in RPMI 1640, and Phytomonas in Grace's medium (both from GIBCO BRL/Life Technologies), supplemented with 10% heat-inactivated FCS (Imperial Labs), 2 mM L-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin (complete medium). Cultures were incubated at 26°C. Stationary phase parasites were harvested and handled as described previously (2).
Antibodies and Human Sera.
Blood from healthy donors was allowed to clot in siliconized glass tubes (20°C, 30 min), and serum aliquots stored in liquid nitrogen. Clinical and genetic data of sera from patients with hereditary deficiencies in complement factors C1q (C1qDS) and C2 (C2DS) have been reported elsewhere (16, 17). Anti–human C3α chain mAb SIM27–49, IgG2b, developed in our laboratory, was purified from mouse ascites fluid by HiTrap-protein A treatment. NHS was adsorbed (Ads-NHS) for 30 min on ice with L. donovani or L. amazonensis promastigotes at a ratio of 1 ml of 50% PBS-diluted NHS:109 pelleted promastigotes. Ads-NHS was centrifuged (11,000 g, 3 min), and the supernatant readsorbed in two additional cycles; finally, serum was filtered through a 0.22-μm pore membrane to remove aggregates and used in functional assays. IgM was isolated from NHS by affinity chromatography on a protamine-Sepharose CL4B column by end-over-end mixing of 20 ml 50% H20-diluted NHS with 10 ml packed protamine-Sepharose beads (4 h, 20°C). After incubation, the column was washed and eluted as described (18). The IgM-enriched fraction was dialyzed against PBS, filtered to remove aggregates, stored at 4°C, and used within 24 h. NHS IgG was isolated on a HiTrap Protein G column (Amersham Pharmacia Biotech). Protein content was determined by BCA assay (19). SIM27–49 (25 μg) was labeled with 5 μl of Na 125iodine (carrier-free, 105.36 mCi/ml; Dupont/NEN Life Science Products) in iodogen (Pierce Chemical Co.)-coated tubes as described (2). The [125I]SIM27–49 immune reactive fraction (IRF) was measured by titrating a fixed, limiting amount of radiolabeled antibody against increasing concentrations of NHS-opsonized Leishmania promastigotes, until promastigote-bound C3 epitopes greatly exceeded [125I]SIM27–49 paratopes. The antibody IRF was calculated by direct linear plot (20).
Kinetics of Complement Activation by Leishmania, Crithidia, and Phytomonas Promastigotes in NHS and Mg-EGTA-treated NHS.
50 μl of a 2 × 108/ml promastigote suspension were mixed with 50 μl of 50% NHS or 50% NHS-adjusted to 10 mM EGTA, 7 mM MgCl2 (Mg-EGTA-NHS), and incubated for varying time periods. The reaction was terminated by adding cold PBS containing 2.5% FCS and 0.05% NaN3 (PFS) and the parasites washed twice by centrifugation (11,000 g, 1 min). The cell pellet was resuspended in 200 μl of PFS containing 2 × 105 cpm of [125I]SIM27–49 (specific activity 107 cpm/μg) and incubated 1 h on ice. Promastigotes were then washed twice by centrifugation as above, and bound [125I]SIM27–49 cpm determined. A t50 index (time required for each species to reach 50% maximum C3 binding) was calculated from a plot of percent-normalized promastigote-C3 binding against incubation time.
Complement Activation by Leishmania in C1q- and C2-deficient Sera.
Duplicate 50 μl samples of a 2 × 108/ml L. donovani promastigote suspension were incubated at 37°C with 50 μl of 50% diluted NHS, 50% diluted Mg-EGTA-treated NHS, C1qDS, or C2DS, for 0, 0.5, 3, 5, 7, and 10 min. Conditions for L. amazonensis assay were identical, but to compensate for activity loss during long-term storage, C2DS was used at 60% concentration. Purified C1q (125 μg/ml) and C2 (47,500 CH50 units) were supplemented. After incubation, assay conditions were as described above for complement activation kinetics. Finally, bound [125I]SIM27–49 cpm were determined.
Complement Activation by Leishmania in Ig-supplemented Ads-NHS.
IgM and IgG preparations devoid of complement activity were purified as described above. All IgM and IgG preparations used showed promastigote binding ≥100% of that observed for 25% NHS. L. donovani and L. amazonesis promastigote triggering of complement activation was analyzed in a two-step C3 binding assay. Pelleted L. donovani promastigotes (107) were resuspended in 100 μl of 25% NHS adjusted to 10 mM EDTA (NHS-EDTA), purified IgM adjusted to 10 mM EDTA (IgM-EDTA), or purified IgG adjusted to 10 mM EDTA (IgG-EDTA), and incubated (37°C, 30 s). Promastigotes were then washed twice by centrifugation (11,000 g, 1 min). Promastigote pellets preincubated in NHS-EDTA were resuspended in 100 μl of 25% NHS (positive control) or 25% L. donovani Ads-NHS; promastigotes preincubated in IgM-EDTA or IgG-EDTA were resuspended in 100 μl of 25% of L. donovani Ads-NHS. Tubes were incubated (2 min, 37°C), followed by two centrifugation washes (11,000 g, 1 min). Promastigotes were resuspended in 0.2 ml PFS containing 2 × 105 [125I]SIM27–49 cpm and incubated (1 h, on ice). After two further washes, bound cpm were determined. Control samples for classical and alternative complement pathway activation were not preincubated. To study CP activation, 107 pelleted L. donovani promastigotes were resuspended in 100 μl of 25% NHS, 25% Mg-EGTA-NHS (NHS-EGTA), 25% purified IgM, 25% purified IgG, or 25% L. donovani-Ads-NHS, and incubated (2 min, 37°C). For AP activation, promastigotes were resuspended in 100 μl of 25% NHS, 25% NHS-EGTA, or 25% L. donovani-Ads-NHS, and incubated (15 min, 37°C). Samples were washed twice by centrifugation, promastigotes resuspended in 0.2 ml of PFS containing 2 × 105 cpm [125I]SIM27–49, incubated 1 h on ice, and processed as above. NHS complement activation by L. amazonensis was measured similarly using L. amazonensis-Ads-NHS.
Quantitation of CP-activated Promastigote-C3 Binding during In Vitro Metacyclogenesis.
Cultures were seeded with 105 L. amazonensis promastigotes/ml and cultured (27°C, 17 d). Promastigotes were sampled at days 3, 4, 5, 6, 7, 10, 11, 14, and 17, washed twice by centrifugation in PBS, and opsonized (108 promastigotes/ml in 25% PBS-diluted NHS) at 37°C for 2.5 min. Promastigotes were washed, added to duplicate tubes at concentrations of 0.625, 1.25, 2.5, 5, and 10 × 106, adjusted to 107/0.1 ml with nonopsonized promastigotes, and incubated (2 h, 0°C) with a limiting amount of [125I]SIM27–49. After incubation, promastigotes were washed twice by centrifugation and bound [125I]SIM27–49 cpm determined. Background cpm ([125I]SIM27–49 cpm bound to nonopsonized promastigotes) were subtracted. A single lot of [125I]SIM27–49 (specific activity, 6 × 106 cpm/μg) was used for all experiments. Kd values were calculated by direct linear plot from data of three independent cultures.
To measure CP-activated C3 binding to stationary promastigotes of Leishmania and Crithidia, parasites (108 cells/ml) were incubated in 25% PBS-diluted NHS (37°C, 3 min). The reaction terminated by dilution with PFS (4°C), and C3-opsonized promastigotes washed twice by centrifugation (1,500 g, 15 min). Two duplicate series of tubes containing 105 and 2 × 105 C3-promastigotes were adjusted to 5 × 106 promastigotes/tube with nonopsonized promastigotes. A third series of control tubes contained 5 × 106 nonopsonized promastigotes. Leishmanias were pelleted and resuspended in 0.2 ml PFS containing increasing concentrations of [125I]SIM27–49, until a paratope/ligand ratio of ∼10 was obtained. Samples were incubated on ice to equilibrium (≥3 h). After reaction, parasites were washed twice by centrifugation (11,000 g, 1 min) in PFS and C3-bound [125I]SIM27–49 determined. Functional [125I]SIM27–49 was calculated as input cpm × IRF. SIM27–49 Kd and the number of C3 molecules/promastigote were calculated by direct linear plot and Scatchard analysis (21, 22).
Real-time Kinetics of Leishmania Promastigote Lysis in NHS.
Promastigote cultures of L. major, L. amazonensis, L. donovani, and L. infantum were seeded in triplicate at 106 cells/ml, and cell growth registered daily. Parasites were sampled at mid-log and early stationary growth phase, i.e., 2 d after the end of log-phase growth, and real-time promastigote lysis in 50% pooled NHS was analyzed by measuring propidium iodide (PI) uptake by killed promastigotes in a FACSCalibur™ flow cytometer (Becton Dickinson). The reaction was initiated by addition of 100 μl of undiluted 0.22 μm porefiltered NHS into a tube containing 105 promastigotes, 2 μl PI (0.5 mg/ml; Sigma-Aldrich) and PBS (200 μl final reaction volume), and incubated in a 37°C waterbath during the data acquisition period. Promastigotes were identified and gated at a forward-angle light scatter versus side-angle light scatter. PI emission was collected in the FL2 detector through a 585/42 nm band pass filter. Detector amplification was set to include untreated promastigotes (negative control) between 100–101 intensity. Promastigotes were acquired at a ratio of 250 events over a period of 204.8 s, with a data acquisition interval of 200 ms, and analyzed with CELLQuest™ software (Becton Dickinson). Promastigote PI uptake kinetics was analyzed in a FL2 versus time dot-plot divided into thirteen 15.98 s regions. The percentage of promastigotes incorporating PI over incubation time was quantitated for each region as number of events emitting PI fluorescence >101/total event number (PI emitting plus nonemitting promastigotes). Controls included (a) maximum cell lysis in promastigotes treated (30 min) with acetone:methanol (1:1), (b) pooled NHS, and (c) nonspecific promastigote lysis in 10 mM EDTA-chelated 50% NHS.
Results
Kinetics of NHS Complement Activation by Trypanosomatid Promastigotes.
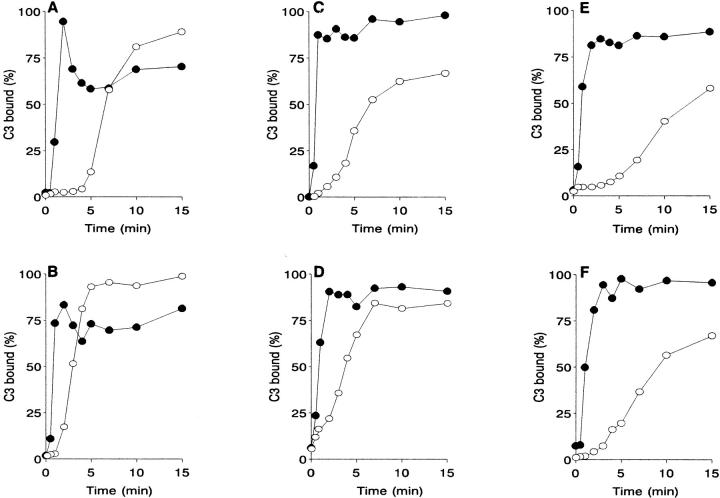
Complement activation by trypanosmatids was analyzed by measuring NHS C3 binding kinetics to four Leishmania promastigote species, L. donovani, L. amazonensis, L. infantum, and L. major, and to promastigotes of two unrelated trypanosomatid genera, C. fasciculata and P. characias. In 25% PBS-diluted NHS, all trypanosomatids studied activate the classical and alternative complement pathways simultaneously. Promastigote-C3 binding via the CP is very rapid, reaching maximum within 2 to 3 min; longer incubations of up to 15 min do not increase CP C3 deposition (Fig. 1). For Leishmania promastigotes, the average time required to deposit 50% of total C3 bound through the CP (CPt 50) is ∼50 s (49.5 ± 7 s), and 53 s for all trypanosomatids analyzed. After CP blockade with Mg-EGTA, promastigote-C3 binding kinetics follows a slower course characteristic of the AP activation mechanism. Trypanosomatid promastigotes differ in their AP activation kinetics. Plotting the percent-normalized promastigote-C3 binding against incubation time rendered a sigmoid curve for each trypanosomatid species, from which an APt 50 index was obtained. Promastigotes can be classified by the APt 50 index as rapid (C. fasciculata and L. amazonensis; APt 50 3–3.5 min), intermediate (L. major, P. characias, and L. infantum; APt 50 4.9–6.5 min), and slow (L. donovani; APt 50 8.5 min) AP activators.
Figure 1.
Kinetics of C3 deposition on trypanosomatid promastigotes in NHS. Promastigotes (107) were incubated at 37°C in 25% NHS or 10 mM EGTA/7 mM MgCl2-treated NHS for 0, 0.5, 1, 2, 3, 4, 5, 7, 10, and 15 min. Parasites were then washed twice by centrifugation (11,000 g, 1 min) in cold PFS, and promastigote-bound C3 measured with anti-human C3α mAb [125I]SIM27–49. C3 binding (mean of duplicate values) is expressed as a percentage of the point of maximum antibody. (•) CP-activated, (○) AP-activated C3 deposition. (A) P. characias (3); (B) C. fasciculata (4); (C) L. major (3); (D) L. amazonensis (4); (E) L: donovani (4); (F) L. infantum (3). In parenthesis, the number of experiments performed for each species.
At CPt 50, the amount of C3 deposited by the AP is at most 13.7% of total C3 binding. The CP-activated C3 deposition rate on the promastigote surface is thus seven times faster than that activated by the AP. This indicates that promastigote C3 deposition by the CP is the functionally relevant mechanism during parasite opsonization in NHS.
Complement Activation Mechanism by Leishmania Promastigotes in NHS.
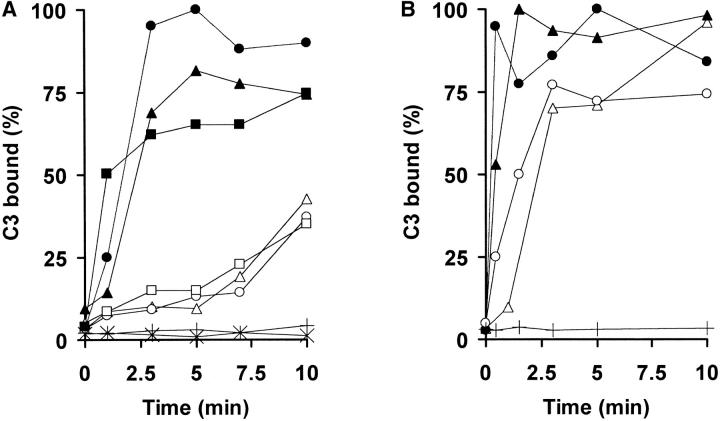
Complement activation by the CP is Ca2+ and Mg2+ ion-dependent, whereas AP activation requires only Mg2+. Differential Ca2+ chelation in Mg-EGTA-treated NHS thus permits identification of the activating pathway (23). To confirm that rapid and slow promastigote-C3 deposition kinetics in NHS are triggered by the CP and the AP, respectively, the C3 binding rate to L. donovani and L. amazonensis promastigotes in NHS or Mg-EGTA–treated NHS was compared with that in sera of individuals congenitally deficient in C1q or C2 early CP factors. L. donovani is a very slow AP activator, allowing clear separation between the CP and AP C3 binding courses (Fig. 1 E). In NHS, C3 deposition on L. donovani promastigotes reaches a plateau after ∼3 min (Fig. 2 A), whereas the C3 binding rate is slower in C1qDS and C2DS, similar to the course followed in Mg-EGTA–treated NHS. After 3 min, the percentage of promastigote-C3 binding in C1qDS and C2DS is only 15.6 and 10.5%, respectively, of that observed in NHS. In contrast, promastigotes incubated in C1qDS and C2DS supplemented with purified C1q and C2 factors, respectively, recover CP C3 binding kinetics and deposit 70.5% (in C1q-supplemented C1qDS) and 72.4% (C2-supplemented C2DS) of total C3 bound in NHS. L. amazonensis is a rapid AP-activating species, and the course of CP and AP C3 binding are closer to each other in time than in L. donovani (Fig. 1 D). Analysis of L. amazonensis promastigote-C3 deposition kinetics in C2DS, and after C2DS supplementation with C2 factor, shows results similar to those in L. donovani (Fig. 2 B). These data indicate that irrespective of the species analyzed, Leishmania promastigotes require early CP factor activity for rapid C3 binding kinetics in NHS.
Figure 2.
Complement activation by Leishmania in C1q- and C2-deficient sera. Promastigotes were incubated in 50% PBS-diluted NHS (•), EGTA-NHS (○), C1qDS (□), C2DS (Δ), C1qDS supplemented with purified C1q (▪), or C2DS supplemented with purified C2 (▴), and incubated at 37°C for 0, 0.5, 3, 5, 7, and 10 min. Promastigote-bound C3 was then measured with [125I]SIM27–49. Results are expressed as a percentage of maximum [125I]SIM27–49 binding. Each point represents the mean of duplicate samples. C1q- and C2 factor-mediated C3 binding was <5% of total C3 bound. (A) L. donovani; (B) L. amazonensis.
Classical Complement Pathway Triggering by Natural Anti-Leishmania Antibodies.
IgM and IgG devoid of complement activity were isolated from NHS by affinity chromatography on protamine-Sepharose, and HiTrap-protein G, respectively. NHS depleted of CP-triggering activity was prepared by adsorption with L. donovani or L. amazonensis promastigotes (Ads-NHS), as described (Materials and Methods). Promastigote activation of the CP was analyzed using a two-stage incubation assay that measures CP C3 deposition within 2 min. IgM, IgG, and Ads-NHS preparations lack the ability to trigger CP C3 deposition on promastigotes (Table I); after longer incubation (10 min), Ads-NHS shows AP C3 deposition capacity (89.5% for L. amazonensis and 66.3% for L. donovani) similar to that seen in NHS-EGTA (86.5% for L. amazonensis and 47% for L. donovani; not shown). Promastigotes preincubated with NHS-EDTA or IgM-EDTA, conditions that prevent promastigote MBL and CRP binding, and subsequently incubated with Ads-NHS, trigger CP C3 deposition. In contrast, promastigotes preincubated in IgG-EDTA contribute only ∼18% of total C3 bound (Table I). All together, these data indicate that in NHS, natural IgM anti-Leishmania antibodies are the main triggering factor for CP activation by promastigotes, and that serum MBL and CRP do not contribute significantly to this process.
Table I.
Classical Complement Pathway Triggering of Promastigote-C3 Opsonization by Natural Anti-Leishmania Antibodies
| Incubation
|
Promastigote bound C3 (%)
|
||
|---|---|---|---|
| 30 s | 120 s |
L. amazonensis
(n) |
L. donovani
(n) |
| PBS | NHS | 100 | 100 |
| PBS | NHS-EGTA | 8.3 ± 1.7 (4) | 3.2 ± 0.4 (4) |
| PBS | IgM | 2.8 ± 1.2 (6) | 3.0 ± 1.4 (6) |
| PBS | IgG | 0.8 ± 0.6 (3) | 0.6 ± 0.6 (4) |
| PBS | Ads-NHS | 15.1 ± 2.0 (15) | 11.4 ± 1.1 (24) |
| NHS-EDTA | NHS | 100 | 100 |
| NHS-EDTA | Ads-NHS | 88.8 ± 4.8 (15) | 71.7 ± 5.3 (26) |
| IgM-EDTA | Ads-NHS | 88.6 ± 2.7 (13) | 93.8 ± 3.6 (13) |
| IgG-EDTA | Ads-NHS | 17.8 ± 1.4 (13) | 17.4 ± 2.9 (13) |
Promastigote triggering of complement activation was analyzed in a two-step binding assay. Promastigotes were preincubated (37°C, 30 s) in 100 μl of PBS, NHS-EDTA, IgM-EDTA, or IgG-EDTA as indicated, washed twice by centrifugation, and incubated (37°C, 120 s) in the conditions indicated. Samples then were processed as described (Materials and Methods). Results are expressed as the percentage of maximum promastigote-bound C3 in NHS (100%) = SEM; (n), number of experiments performed.
Quantitative Analysis of CP Promastigote-C3 Opsonization during In Vitro Metacyclogenesis.
It is believed that axenic Leishmania cultures give rise to two developmental promastigote forms, log phase complement-sensitive and stationary phase complement-resistant (24–28). The question arises whether these differences in Leishmania susceptibility to complement can be ascribed to variation in the CP activating capacity of promastigote developmental stages. We thus measured CP C3 deposition on L. amazonensis promastigotes taken from different growth phases of in vitro metacyclogenesis. Promastigotes were sampled daily from triplicate cultures, and each sample individually opsonized in 25% NHS. Promastigote-bound C3 was titrated against [125I]SIM 27–49, and Kd values of the C3-opsonized promastigote populations calculated. Kd values (the number of C3-opsonized promastigotes that bind 50% of [125I]SIM 27–49 cpm) are very similar, 1.0 ± 0.07 × 106 promastigotes/ml (mean ± SEM), irrespective of the culture growth phase. Differential susceptibility of axenic promastigote populations to complement thus cannot be ascribed to variations in their capacity to fix C3. The number of CP-activated C3 molecules bound to stationary phase L. amazonensis, L. donovani, and C. fasciculata promastigotes was calculated from Langmuir saturation isotherm data. On a per cell basis, L. amazonensis promastigotes bound 1.2 × 105, L. donovani 1.8 × 105, and C. fasciculata 1.3 × 105 C3 molecules.
Real-time Kinetics of Leishmania Promastigote Lysis in NHS.
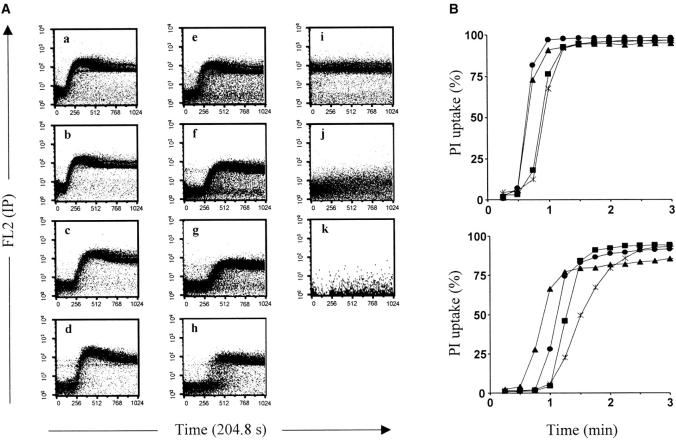
Promastigotes of four Leishmania species were obtained from axenic cultures in log phase and the early stationary phase, and kinetic data on complement-mediated lysis determined by measuring PI-stained cells. Dot plots show real-time kinetics of log phase (Fig. 3 A, a–d) and early stationary phase (Fig. 3 A, e–h) promastigote killing in 50% NHS, as well as controls (Fig. 3 A, i–k). Log phase leishmania begin to incorporate PI within 30 s of serum contact, and all cells are PI-labeled by 60 s (Fig. 3 B, top panel). Log phase promastigotes are thus highly susceptible to human complement. In contrast, PI uptake kinetics by early stationary phase promastigotes is slower; it begins within 30–60 s of serum contact, and is complete by ∼2.5 min (Fig. 3 B, bottom panel). Stationary phase promastigotes show greater interspecies variability in PI uptake kinetics and percent killing (Fig. 3 B, bottom panel) than log phase promastigotes. In stationary phase L. major and L. amazonensis promastigotes, 15 and 10% of the population, respectively, show a degree of complement resistance (Fig. 3 A, e–f). The precise size of this population is difficult to calculate as, in the presence of NHS, promastigote cell volume and refractile properties are altered, blurring the distinction between promastigotes, cell debris, and NHS background signal. Stationary phase L. donovani and L. infantum promastigotes appear to be more complement susceptible (Fig. 3 A, g and h), and no motile promastigotes were observed under the microscope. Lysis of log and early stationary phase promastigotes is gradual in all four species (Fig. 3 A, a–g); the lytic mechanism differs for L. infantum stationary phase promastigotes in that the entire promastigote population undergoes sudden, simultaneous death, with no intermediate stages (Fig. 3 A, h). These results indicate that in NHS, Leishmania promastigote lysis is an extremely rapid reaction.
Figure 3.
Real-time kinetics of Leishmania promastigote lysis in NHS. Promastigotes were taken from log phase and early stationary phase growth cultures, and the course of parasite lysis measured in 50% NHS as described (Materials and Methods). (A) FL2 against time dot plots of log phase (a–d), and stationary phase (e–h) promastigote PI uptake in 50% NHS, as well as controls (i–k). L. major (a and e); L. amazonensis (b and f); L. donovani (c and g); L. infantum (d and h); maximum PI uptake control (i); background PI uptake control in EDTA-NHS (j); and nonspecific NHS fluorescence (k). (B) Promastigote PI uptake kinetics derived from dot plot data. Top panel: log phase promastigotes. Lower panel: early stationary phase promastigotes. L. major (▴); L. amazonensis (•); L. donovani (▪); L. infantum (*).
Discussion
To infect their hosts, Leishmania promastigotes must circumvent the cytolytic effects of complement. Identification of the reaction mechanisms that govern promastigote opsonization could thus aid in comprehending Leishmania evasion strategies of host innate immunity. The mechanism of promastigote opsonization in NHS is the subject of controversy; some data indicate that promastigotes activate complement via the CP (2, 5, 6), but the dominant view is that Leishmania activation of complement is antibody independent, i.e., lectin- or AP-mediated (7–15). To elucidate this mechanism, we studied four aspects of complement-parasite interaction at near-physiological conditions, (a) the kinetics of promastigote-C3 binding in NHS and in EGTA-NHS, (b) the requirement for early CP factors for C3 deposition, (c) the ability of anti-Leishmania NAb to trigger C3 deposition in Ads-NHS, and (d) complement leishmaniacidal activity by measuring the real-time kinetics of promastigote killing.
In 25% NHS, promastigotes of all trypanosomatid species studied activate classical and alternative complement pathways simultaneously (Fig. 1). Promastigote-C3 deposition is extremely rapid; the reaction is complete after 2 to 3 min, at which time 86–93% of fixed C3 has been activated through the CP. The contribution of the AP to promastigote opsonization is much smaller, ranging from 7.1–13.7%. Promastigote C3 binding is traditionally measured after 15 min incubation (9, 10) or longer (30–60 min) (7, 8, 11, 12, 28). CP C3 deposition can be detected only in a narrow time window that lasts but a few minutes (Fig. 1); after this time, classical and alternative pathway kinetics merge, and only AP-dependent activity is observed. This may explain why promastigote CP C3 deposition has passed unnoticed.
AP-mediated C3 deposition has a slower time course than the CP (Fig. 1). In AP triggering, no specific recognition mechanism is involved, and both the kinetics and the amount of C3 bound are directly related to the structure of the promastigote surface. The AP pathway permits differentiation of trypanosomatids by their C3 binding kinetics and APt 50 values, disclosing the large instraspecies variability that exists within the subgenus Leishmania. Crithidia and Leishmania are genetically more distant than amphibians and mammals (29), yet larger AP kinetic differences are found among Leishmania species than among parasites of Leishmania, Crithidia and Phytomonas.
To identify the mechanism that triggers Leishmania opsonization in NHS, we used sera congenitally deficient in C1q or C2 complement factors. Promastigote CP C3 binding kinetics in C1q- or C2-deficient sera is restored by addition of purified C1q or C2, respectively (Fig. 2); this result supports the involvement of early CP factors in promastigote C3 opsonization. Parasite killing in C4- or C2-deficient sera (8, 12), or in normal serum depleted of natural anti-Leishmania antibodies (9, 11), has been taken as proof that this reaction is AP mediated. In these studies, earliest parasite lysis was measured after 15-min incubation, when AP-mediated promastigote killing is fully active. These experiments consequently provide no information on the role of the CP in the lytic mechanism, for which promastigote killing must be recorded in the early opsonization period (Fig. 1).
Candidate molecules in NHS to initiate parasite activation of the CP include anti-Leishmania NAb (2, 5, 6), MBL (13), and CRP (14, 15). Complement triggering was analyzed in a two-stage incubation assay that measures the ability of promastigote-bound IgM or IgG to initiate CP activity in Ads-NHS; this preparation lacks the ability to trigger CP C3 deposition. Promastigotes preincubated in NHS-EDTA or IgM-EDTA bind natural IgM anti-Leishmania antibodies; after a second incubation in Ads-NHS, they respectively deposit 88.8 and 88.6% of total C3 deposited in NHS (Table I). In contrast, promastigotes preincubated with IgG-EDTA fix only 17.8% of total C3 bound (Table I). Triggering of CP C3 deposition by calcium-chelated NHS and protamine-isolated IgM fractions (Table I) indicates that MBL and CRP do not participate in the reaction, as their activity is calcium-dependent (14, 30). A basic requirement for the physiological Leishmania opsonization mechanism in NHS is that C3 deposition must be complete within 2–3 min of incubation at 37°C. MBL and CRP are reported to bind to the Leishmania surface (13–15), although these assays were done in far from physiological conditions (1 h in the cold). Average human plasma concentrations of MBL and CRP are 1,000- (∼10−10 M [31]) and 200-fold (∼0.5 × 10−9 M [14]) lower, respectively, than that of natural IgM anti-Leishmania antibodies (∼1.6 × 10−7 M [2]). It thus appears unlikely that they mediate the promastigote opsonization kinetics shown in Fig. 1. In chronic infections, MBL concentrations can increase 2- to 3-fold (32) and those of CRP up to 300-fold (33); MBL may thus act as an opsonin (34) and modulate disease progression (35).
Given sufficient time and suitable temperature conditions in vitro, Leishmania promastigotes can probably activate human complement via classical, alternative, and lectin pathways. Under quasi-physiological conditions, however, Leishmania opsonization is extremely rapid, and it is unlikely that MBL or CRP can account for this mechanism. We thus maintain that in NHS, natural IgM anti-Leishmania antibodies are the main trigger of CP activation and promastigote opsonization.
It has been proposed that parasite infective success relies on their capacity to establish multiple interactions with host cell receptors (36). The velocity of parasite opsonization and the massive C3 deposition suggest that promastigote-bound C3 has a pivotal role in the parasite–host interaction. Leishmania C3 binding is a prerequisite for promastigote IA (2, 3), receptor-mediated parasite binding to macrophages and endocytosis (37–39), and intracellular Leishmania survival (40). To correlate C3 deposition levels with parasite infectivity, we analyzed promastigote-C3 binding during the L. amazonensis life cycle, and found that promastigotes maintain a stable, CP-mediated C3 binding capacity throughout in vitro metacyclogenenesis. Similar data, although measuring AP-mediated C3 deposition, have been reported for L. donovani, L. major, and L. amazonensis (9–11), although others found higher C3 binding to stationary promastigotes (37). Leishmania promastigotes in axenic cultures are believed to recapitulate the developmental sequence in the insect vector (41); promastigotes in the vector gut may thus behave as do the axenic promastigotes, and maintain invariant C3 binding capacity. Assuming that promastigote-bound C3 is responsible for receptor-mediated parasite binding to host cells and endocytosis, it is plausible that all C3-opsonized promastigotes egested from the sandfly gut into the blood pool have a similar probability of invading host leukocytes. In this case, the presence of infective metacyclic promastigotes would not be a necessary condition for infectivity.
Despite the abundant literature on promastigote killing by complement, time course studies of CP-mediated promastigote lysis at near-physiological conditions are lacking. Here we show real-time kinetic analyses of this mechanism (Fig. 3). Three aspects to be emphasized are the extreme velocity of the promastigote lytic reaction, the striking sensitivity of log phase Leishmania spp. populations to complement, and the differential complement susceptibility among stationary phase Leishmania spp promastigote species. In all four species tested, PI uptake by stationary phase promastigotes begins within 30–60 s of serum contact and is complete by 2.5 min (Fig. 3 B, bottom panel), confirming previous results (2). L. major promastigotes incorporate PI more rapidly (Fig. 3 A, e), although this does not correlate with greater complement sensitivity. Using flagellar motility as a viability criterion, 15% (L. major) and 10% (L. amazonensis) of the promastigotes were viable after 2.5 min in 50% NHS. The data thus indicate that these species show a degree of complement resistance, and support the idea (24) that axenic Leishmania cultures give rise to developmental parasite forms that are resistant to human complement within the physiological infection period.
In contrast, L. donovani and L. infantum are highly sensitive to complement, which is also the case for promastigotes of all species tested in exponential growth (Fig. 3 B, top panel). From these data, we estimate that from 85% (stationary phase) to 100% (log phase) Leishmania spp. promastigotes are killed by complement after 2.5 min in human blood. To survive, Leishmania promastigotes must invade host cells within this period. Assuming a sandfly blood meal of 0.3 μl, and sandfly thoracic midgut promastigote body volume of 4 μm3 (42), each sandfly bite would inoculate at most 75 parasites. Estimating that at least 90% will be killed within 2.5 min, and that human blood phagocytes engulf promastigotes at a granulocyte:monocyte ratio of 2:1, in each bite, <4% of promastigotes inoculated (5 promastigotes) would enter a safe monocyte haven. It is thus clear that, during early stages of leishmaniosis transmission, serum opsonins exert very strong selective pressure on Leishmania, a mechanism that has presumably contributed to shaping the parasite's host evasion strategies.
Leishmania has been called a “parasite of paradox” for its ability to dwell in macrophages, the cell committed to its destruction (43); we would further justify this appellation. Complement protects vertebrates against protozoan invasion of the blood through the dual mechanisms of IA opsonic activity and the lytic cascade. Members of the Muridae family, such as the mouse and the hamster, have very low complement lytic activity against Leishmania, and may rely on IA opsonic activity to fend off infection (our unpublished data). In these genera, IA opsonic activity enhances promastigote phagocytosis and macrophage infection, which may explain the reservoir role of these rodents for Leishmania. In contrast, humans have a strong complement system and a potent IA mechanism; after inoculation, promastigote survival is linked to IA and endocytosis. We suggest that appropriation of IA-mediated opsonophagocytosis is the principal Leishmania strategy to evade the innate host response. Its capacity to transform the vertebrate innate protective response into the key to host invasion provides an additional motive for the sobriquet “parasite of paradox.”
Acknowledgments
The authors thank M. Díez for excellent technical assistance; N. Sánchez and M.A. Ortega for blood extraction, Drs. R. Molina (Serv. Parasitología, Centro Nacional de Microbiología) for sandfly bloodmeal data, A. Orfao (Serv. General de Citometría, Universidad de Salamanca, Spain) and J.M. Ligos (BD, Spain) for advice on real-time cytofluorometric analysis; and C. Mark for editorial assistance, critical reading and sound advice on the manuscript.
This work was supported by grants 08.2/0006/97 from the Comunidad Autónoma de Madrid, PM99-0012 from the Programa Nacional de Salud (Ministerio de Educación y Cultura), and institutional funds from the Centro Nacional de Microbiología, Instituto de Salud Carlos III.
Footnotes
Abbreviations used in this paper: Ads-NHS, adsorbed NHS; AP, alternative complement pathway; C1qDS, serum deficient in C1q complement component; C2DS, serum deficient in C2 complement factor; CP, classical complement pathway; CRP, C-reactive protein; IA, immune adherence; IRF, immune reactive fraction; MBL, mannan-binding lectin; NAb, natural antibody; PI, propidium iodide.
References
- 1.Mosser, D.M., and A. Brittingham. 1997. Leishmania, macrophages and complement: a tale of subversion and exploitation. Parasitology. 115:S9–S23. [DOI] [PubMed] [Google Scholar]
- 2.Domínguez, M., and A. Toraño. 1999. Immune adherence-mediated opsonophagocytosis: the mechanism of Leishmania infection. J. Exp. Med. 189:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domínguez, M., and A. Toraño. 2001. Leishmania immune adherence reaction in vertebrates. Parasite Immunol. 23:259–265. [DOI] [PubMed] [Google Scholar]
- 4.Handman, E. 2000. Cell biology of Leishmania Adv. Parasitol. 44:2–39. [DOI] [PubMed] [Google Scholar]
- 5.Pearson, R.D., and R.T. Steigbigel. 1980. Mechanism of lethal effect of human serum upon Leishmania donovani. J. Immunol. 125:2195–2201. [PubMed] [Google Scholar]
- 6.Navin, T.R., E.C. Krug, and R.D. Pearson. 1989. Effect of immunoglobulin M from normal human serum on Leishmania donovani promastigote agglutination, complement-mediated killing, and phagocytosis by human monocytes. Infect. Immun. 57:1343–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosser, D.M., and P.J. Edelson. 1984. Activation of the alternative complement pathway by Leishmania promastigotes: parasite lysis and attachment to macrophages. J. Immunol. 132:1501–1505. [PubMed] [Google Scholar]
- 8.Mosser, D.M., S.K. Burke, E.E. Coutavas, J.F. Wedgwood, and P.J. Edelson. 1986. Leishmania species: mechanisms of complement activation by five strains of promastigotes. Exp. Parasitol. 62:394–404. [DOI] [PubMed] [Google Scholar]
- 9.Puentes, S.M., D.M. Dwyer, P.A. Bates, and K.A. Joiner. 1989. Binding and release of C3 from Leishmania donovani promastigotes during incubation in normal human serum. J. Immunol. 143:3743–3749. [PubMed] [Google Scholar]
- 10.Puentes, S.M., D.L. Sacks, R.P. da Silva, and K.A. Joiner. 1988. Complement binding by two developmental stages of Leishmania major promastigotes varying in expression of a surface lipophosphoglycan. J. Exp. Med. 167:887–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nunes, A.C., and F.J. Ramalho-Pinto. 1996. Complement resistance of Leishmania amazonensis promastigotes is independent of parasite proteases and lysis of sensitive forms in not due to natural antibodies in normal human serum. Braz. J. Med. Biol. Res. 29:1633–1640. [PubMed] [Google Scholar]
- 12.Noronha, F.S.M., A.C. Nunes, K.T. Souza, M.N. Melo, and F.J. Ramalho-Pinto. 1998. Differential sensitivity of New World Leishmania spp promastigotes to complement-mediated lysis: correlation with the expression of three parasite polypeptides. Acta Trop. 69:17–29. [DOI] [PubMed] [Google Scholar]
- 13.Green, P.J., T. Feizi, M.S. Stoll, S. Thiel, A. Prescott, and M.J. McConville. 1994. Recognition of the major cell surface glycoconjugates of Leishmania parasites by the human serum mannan-binding protein. Mol. Biochem. Parasitol. 66:319–328. [DOI] [PubMed] [Google Scholar]
- 14.Culley, F.J., R.A. Harris, P.M. Kaye, P.W.J. McAdam, and J.G. Raynes. 1996. C-reactive protein binds to a novel ligand on Leishmania donovani and increases uptake into human macrophages. J. Immunol. 156:4691–4696. [PubMed] [Google Scholar]
- 15.Bee, A., F.J. Culley, I.S. Alkahalife, K.B. Bodman-Smith, J.G. Raynes, and P.A. Bates. 2001. Transformation of Leishmania mexicana metacyclic promastigotes to amastigote-like forms mediated by binding of human C-reactive protein. Parasitology. 122:521–529. [DOI] [PubMed] [Google Scholar]
- 16.Leyva-Cobián, F., I. Moneo, F. Mampaso, M. Sánchez-Bayle, J.L. Ecija, and A. Bootello. 1981. Familial C1q deficiency associated with renal and cutaneous disease. Clin. Exp. Immunol. 44:173–180. [PMC free article] [PubMed] [Google Scholar]
- 17.Sobel, A.T., M. Moisy, G. Hirbec, A. Tournesac, J.P. Berry, P. Mannoni, A.P. Peltier, and G. Lagrue. 1979. Hereditary C2 deficiency associated with non-systemic glomerulonephritis. Clin. Nephrol. 12:132–136. [PubMed] [Google Scholar]
- 19.18. Hudson, L., and F.C. Hay. 1989. Practical Immunology. 3rd ed. Blackwell, Oxford. 319–321.
- 19.Smith, P.K., R.I. Krohn, G.T. Hermanson, A.K. Mallia, F.H. Gartner, M.D. Provenzano, E.K. Fujimoto, N.M. Goeke, B.J. Olson, and D.C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76–85. [DOI] [PubMed] [Google Scholar]
- 20.Partridge, C.S., D.W. Britton, and K.D. Bagshawe. 1985. Use of the direct linear plot to estimate, in an immunoglobulin solution, the proportion which is specific monoclonal antibody against an unknown cell surface antigen. J. Immunol. Methods. 78:95–101. [DOI] [PubMed] [Google Scholar]
- 21.Eisenthal, R., and A. Cornish-Bowden. 1974. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem. J. 139:715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ways, J.P., and P. Parham. 1983. The binding of monoclonal antibodies to cell-surface molecules. A quantitative analysis with immunoglobulin G against two alloantigenic determinants of the human transplantation antigen HLA-A2. Biochem. J. 216:423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine, D.P., S.R. Marney, Jr., D.G. Colley, J.S. Sergent, and R.M. Des Prez. 1972. C3 shunt activation in human serum chelated with EGTA. J. Immunol. 109:807–809. [PubMed] [Google Scholar]
- 24.Franke, E.D., P.B. McGreevy, S.P. Katz, and D.L. Sacks. 1985. Growth cycle-dependent generation of complement-resistant Leishmania promastigotes. J. Immunol. 134:2713–2718. [PubMed] [Google Scholar]
- 25.Bandyopadhyay, P., D.K. Ghosh, A. De, K.N. Ghosh, P.P. Chaudhuri, P. Das, and A. Bhattacharya. 1991. Metacyclogenesis of Leishmania spp: species-specific in vitro transformation, complement resistance, and cell surface carbohydrate and protein profiles. J. Parasitol. 77:411–416. [PubMed] [Google Scholar]
- 26.Howard, M.K., G. Sayers, and M.A. Miles. 1987. Leishmania donovani metacyclic promastigotes: transformation in vitro, lectin agglutination, complement resistance, and infectivity. Exp. Parasitol. 64:147–156. [DOI] [PubMed] [Google Scholar]
- 27.Bates, P.A., and L. Tetley. 1993. Leishmania mexicana: induction of metacyclogenesis by cultivation of promastigotes at acidic pH. Exp. Parasitol. 76:412–423. [DOI] [PubMed] [Google Scholar]
- 28.Barral-Netto, M., S.B. Roters, I. Sherlock, and S.G. Reed. 1987. Destruction of Leishmania mexicana amazonensis promastigotes by normal human serum. Am. J. Trop. Med. Hyg. 37:53–56. [DOI] [PubMed] [Google Scholar]
- 29.Shaw, J. 1994. The meeting of two worlds: eco-epidemiology and molecules. Mem. Inst. Oswaldo Cruz. 89:7–9 (Abstr. [DOI] [PubMed] [Google Scholar]
- 30.Drickamer, K. 1999. C-type lectin-like domains. Curr. Opin. Struct. Biol. 9:585–590. [DOI] [PubMed] [Google Scholar]
- 31.Lu, J., S. Thiel, H. Wiedemann, R. Timpl, and K.B.M. Reid. 1990. Binding of the pentamer/hexamer forms of mannan-binding protein to zymosan activates the proenzyme C1r2C1s2 complex, of the classical pathway of complement, without involvement of C1q. J. Immunol. 144:2287–2294. [PubMed] [Google Scholar]
- 32.Thiel, S., U. Holmskov, L. Hviid, S.B. Laursen, and J.C. Jensenius. 1992. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin. Exp. Immunol. 90:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pepys, M.B. 1981. C-reactive protein fifty years on. Lancet. 1:653–656. [DOI] [PubMed] [Google Scholar]
- 34.Peters, C., M. Kawakami, M. Kaul, T. Ilg, P. Overath, and T. Aebischer. 1997. Secreted proteophosphoglycan of Leishmania mexicana amastigotes activates complement by triggering the mannan binding lectin pathway. Eur. J. Immunol. 27:2666–2672. [DOI] [PubMed] [Google Scholar]
- 35.De Miranda Santos, I.K.F., C.H.N. Costa, H. Krieger, M.Y. Feitosa, D. Zurakowski, B. Fardin, R.B.B. Gomes, D.L. Weiner, D.A. Harn, R.A.B. Ezekowitz, and J. Epstein. 2001. Mannan-binding lectin enhances susceptibility to visceral leishmaniasis. Infect. Immun. 69:5212–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Handman, E. 2001. Leishmania virulence: it's a knock out! Trends Parasitol. 17:60. [DOI] [PubMed] [Google Scholar]
- 37.Wozencraft, A.O., and J.M. Blackwell. 1987. Increased infectivity of stationary-phase promastigotes of Leishmania donovani: correlation with enhanced C3 binding capacity and CR3-mediated attachment to host macrophages. Immunology. 60:559–563. [PMC free article] [PubMed] [Google Scholar]
- 38.Mosser, D.M., T.A. Springer, and M.S. Diamond. 1992. Leishmania promastigotes require opsonic complement to bind to the human leukocyte integrin Mac-1 (CD11b/CD18). J. Cell Biol. 116:511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenthal, L.A., F.S. Sutterwala, M. Kehrli, and D.M. Mosser. 1996. Leishmania major-human macrophage interactions: cooperation between Mac-1 (CD11b/CD18) and complement receptor type 1 (CD35) in promastigote adhesion. Infect. Immun. 64:2206–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosser, D.M., and P.J. Edelson. 1987. The third component of complement (C3) is responsible for the intracellular survival of Leishmania major. Nature. 327:329–331. [DOI] [PubMed] [Google Scholar]
- 41.Sacks, D.L. 1989. Metacyclogenesis in Leishmania promastigotes. Exp. Parasitol. 69:100–103. [DOI] [PubMed] [Google Scholar]
- 42.Warburg, A., and Y. Schlein. 1986. The effect of post-bloodmeal nutrition of Phlebotomus papatasi on the transmission of Leishmania major. Am. J. Trop. Med. Hyg. 35:926–930. [DOI] [PubMed] [Google Scholar]
- 43.Chang, K.-P. 1990. Cell biology of Leishmania. Modern Parasite Biology. D.J. Wyler, editor. W. H. Freeman & Co, New York. 79–90.