Abstract
Asthma is a chronic disease characterized by increased airway responsiveness and airway inflammation. The functional role of nitric oxide (NO) and the various nitric oxide synthase (NOS) isoforms in human asthma is controversial. To investigate the role of NO in an established model of allergic asthma, mice with targeted deletions of the three known isoforms of NOS (NOS1, 2, and 3) were studied. Although the inducible (NOS2) isoform was significantly upregulated in the lungs of ovalbumin (OVA)-sensitized and -challenged (OVA/OVA) wild-type (WT) mice and was undetectable in similarly treated NOS2-deficient mice, airway responsiveness was not significantly different between these groups. OVA/OVA endothelial (NOS3)-deficient mice were significantly more responsive to methacholine challenge compared with similarly treated NOS1 and NOS1&3-deficient mice. Airway responsiveness in OVA/OVA neuronal (NOS1)-deficient and neuronal/endothelial (NOS1&3) double-deficient mice was significantly less than that observed in similarly treated NOS2 and WT groups. These findings demonstrate an important function for the nNOS isoform in controlling the inducibility of airway hyperresponsiveness in this model of allergic asthma.
Keywords: nitric oxide, allergen, mice, asthma, nitric oxide synthase
Asthma is an inflammatory disease of the airways characterized by airway obstruction and increased airway responsiveness (1). Animal models of allergic asthma exhibit many of the features of human asthma, including airway hyperresponsiveness, airway inflammation, and increased serum IgE levels (2–7); these models have been useful in elucidating the pathobiology of the asthmatic response. Nitric oxide (NO)1 is a short-lived molecule that has been shown to have a number of important biological functions in various diseases including asthma (8–13). Several studies have clearly demonstrated the presence of increased NO concentrations in the exhaled air of asthmatics (12–14) and normalization of expired NO concentrations after treatment with corticosteroids (15). Despite these observations, the role of NO in asthma remains controversial. It has been suggested that NO is a marker of inflammation (16, 17) and a cytotoxic molecule contributing to epithelial damage (18). NO has also been reported to be a weak bronchodilator (19–21) or to have no effect on airway caliber (22–24). Nitric oxide is produced by a group of enzymes commonly referred to as nitric oxide synthases (NOSs). The enzymes found in endothelial (eNOS, NOS3) and neuronal (nNOS, NOS1) cells are usually constitutively expressed, whereas the form of NOS expressed in numerous cell types including macrophages, endothelial cells, epithelial cells, and neutrophils is inducible and termed iNOS (NOS2) (25–27). Of the three enzyme isoforms identified, only iNOS has been believed to play an important role in asthma (18, 28). It has been shown by immunostaining that iNOS expression is increased in the airways of asthmatics (18), which suggests that iNOS probably contributes most to the increase in exhaled NO concentrations observed in asthma (28). The functional role of nNOS and eNOS in asthma is uncertain (29–31). To understand the actions of the individual NOS isoforms, investigators have used several NOS inhibitors to try to dissect out the role of constitutive and inducible forms of NOS (32); these studies are subject to the problems of NOS inhibitor specificity and concerns about inhibitor uptake, distribution, and metabolism. The availability of mouse strains with targeted deletions of these three NOS isoforms, as either single or double deletions, provides an alternative approach to dissect out the contribution of each NOS isoform in models of disease (32–34). We previously used this strategy to investigate the role of the nNOS gene in regulating baseline airway responsiveness in the mouse (29). Targeted deletion of the nNOS gene in the mouse was associated with a decrease in airway responsiveness and exhaled NO concentrations (29). No other known studies have been carried out to isolate the contribution of the iNOS and eNOS isoforms in either naive animals or a model of allergic asthma in targeted gene knockouts (KOs). Given the significant reduction in airway responsiveness in the nNOS KO mouse and given that several recent genetic studies have reported evidence of linkage of the diagnosis of asthma (35–37) with the region harboring the nNOS gene in humans, we examined the contribution of each NOS isoform to the expression of various facets of airway inflammation and physiology in an established murine model of allergic asthma.
Materials and Methods
Animals.
Neuronal (NOS1-KO), endothelial (NOS3-KO), and double neuronal and endothelial KO mice (NOS1&3-KO) and matched wild-type (WT) controls were bred in a sterile pathogen-free (SPF) barrier facility. These three gene-targeted mutants were produced on a mixed SV129/C57BL/6 background (38–40). The iNOS KO mice (NOS2-KO) were purchased from The Jackson Laboratory and were backcrossed for >10 generations onto a C57BL6/J (B6) background, the controls for the NOS2 KO mice. To control for gender-induced differences in airway reactivity, only male offspring were used for these studies. All mice were 4–5 wk old at entry into the protocol. Mice were housed in isolation cages under SPF conditions. Blood from sentinel animals was routinely tested to ensure their SPF status. All mice were acclimatized for 7–10 d after arrival and were studied at 7–8 wk of age.
In one set of experiments the whole body plethysmographic method (Buxco®) was used to assess airway responsiveness in a different cohort of iNOS KO mice (41). These iNOS KO mice were provided by Drs. J.S. Mudgett (Merck Research Labs., Rahway, NJ), J.D. MacMicking, and C. Nathan (both from Cornell University Medical College, New York, NY) and had been backcrossed into a B6 background. Sex- and age-matched B6 mice were used as controls for the NOS2 group.
Experimental Design.
The gene targeted mutants type (NOS1-KO, NOS3-KO, NOS2-KO, and NOS1&3-KO) and matched WT control mice (on the appropriate genetic background) were all sensitized to chicken OVA (Grade III; Sigma Chemical Co.). Sensitized mice were then randomized to repeated exposure either to an aerosol generated from an OVA solution or to PBS.
Sensitization and Challenge Protocol.
Two protocols were used in this study. In the first set of experiments, all mice were immunized on day 0 via an intraperitoneal injection with 10 μg chicken OVA, mixed with 1 mg Al(OH)3 (alum; J.T. Baker Chemical) in 0.2 ml of PBS as previously described (7). A booster injection was given on day 7 using the identical reagents. Starting 7 d later, mice were exposed either to aerosolized OVA (6% OVA) dissolved in PBS (pH = 7.4) or to PBS alone for 25 min per day for 7 d consecutively. All mice were studied 24 h after the last aerosol (day 21). For the aerosol exposures mice were placed in a plastic chamber (23 × 23 × 11 cm), and the OVA or PBS solution was delivered via an ultrasonic nebulizer (model 5000; DeVilbiss) attached to a port in the mouse chamber.
For mice studied using the whole body plethysmographic method of assessing airway responsiveness (Buxco®), mice were immunized on day 0 via an intraperitoneal injection with 20 μg chicken OVA mixed with 2 mg Al(OH)3. A booster injection was given on day 7 using 10 μg chicken OVA mixed with 1 mg Al(OH)3. All mice were exposed either to aerosolized OVA (3% OVA) or PBS for 10 min on days 14, 15, and 16, studied on day 17 (airway responsiveness measured by whole body plethysmography), and killed on day 18 (for bronchoalveolar lavage [BAL] and harvesting of tissues).
Determination of Anti-OVA IgE Antibody Serum Titers.
OVA-specific IgE levels were measured by capture ELISA as previously described (42). ELISA plates were coated with a purified anti– mouse IgE mAb (PharMingen) at a concentration of 2 μg/ml and blocked with PBS/10% FCS. Serum samples were diluted in PBS/10% FCS and incubated in the wells for 2 h. After washing with 0.05% PBS/Tween 20, biotinylated OVA (10 μg/ml) was added to the wells and incubated for 1 h. The plates were washed with PBS/Tween 20 followed by the addition of avidin alkaline phosphatase (Sigma Chemical Co.) for 1 h. The plates were then washed with PBS/Tween 20 and distilled water, before the addition of the phosphatase substrate. The plates were allowed to develop for 30 min and read in an ELISA plate reader at 405 nm.
Assessment of In Vitro Pulmonary NOS Activity.
Pulmonary NOS activity was measured using a modification of an in vitro [3H]l-arginine to [3H]l-citrulline conversion assay that has been described previously (43). Mouse lungs were kept frozen (−80°C) until the day of assay when they were homogenized in 10 vol of 50 mM potassium phosphate (pH 7.4). A 50-μl aliquot was incubated at 37°C for 15 min in 100 μl of incubation buffer (50 mM potassium phosphate, 60 mM l-valine, 1 mg/ml BSA, 1 mM NADPH, 10 μM FAD, 10 μM tetrahydrobiopterin, 30 μM [2,3-3H]l-arginine [200 counts/min/pmol], and 1.2 mM MgCl2; pH 7.4). In the quantification of calcium-dependent NOS activity, indicating both constitutive calcium-dependent forms (i.e., nNOS and eNOS) and therefore termed cNOS, calmodulin (100 nM) and CaCl2 (1 mM) were added to the incubation buffer. In contrast, EDTA (1.2 mM) and ethylene glycol-bis(b-aminoethyl ether) N,N,N′,N′-tetraacetic acid (EGTA; 1 mM) were added to the incubation buffer when measuring calcium-independent (iNOS) NOS activity. A third incubation condition of EDTA/ EGTA and l-N G-nitro-arginine methyl ester (l-NAME; 1 mM) was used to account for nonspecific radiation and nonspecific metabolism (i.e., non-NOS-mediated conversion) of [3H]l-arginine. The reaction was terminated by the addition of 500 μl of ice-cold stop buffer (4°C; 100 mM Hepes and 12 mM EDTA, pH 5.5) and 2 ml of 50% Dowex 50W (200–400, 8% cross-linked, Na+ form, pH 7.0) in water in order to remove any excess [3H]l-arginine. Samples were centrifuged for 20 min at 600 g and 0.5 ml of the supernatant was added to 4.5 ml of scintillation fluid; radioactivity was measured by liquid scintillation counting (Beckman Scientific Instruments). cNOS activity was calculated as the difference between the calcium–calmodulin sample (total NOS activity) and the EDTA–EGTA sample. iNOS activity was defined as the l-NAME–inhibitable proportion of the activity found in the samples containing EDTA/EGTA.
Measurement of Airway Responsiveness.
Airway responsiveness was measured by two different methods in our study. In the first set of experiments airway responsiveness was measured in anesthetized mice using a sealed constant mass plethysmograph as previously described (7, 29, 44–46). In brief, dose–response curves to methacholine (Sigma Chemical Co.) were obtained 24 h after the last aerosol exposure of either OVA or PBS by administering sequentially increasing doses of methacholine intravenously (33–3,300 μg/kg) in a 20–35-μl volume. From the relationship between the administered dose and pulmonary resistance (RL), the effective dose required to increase RL to 200% of control values (ED200RL), was determined by log-linear interpolation. ED200RL is an index of airway responsiveness.
In a second set of experiments, an alternative method of measuring airway responsiveness was adopted. This was done to confirm the results of a specific experiment and to see if the results could be reproduced in unanesthetized mice. The whole body plethysmograph system was therefore used to measure airway responsiveness in these experiments (47, 48). The day after the last allergen challenge, each mouse was placed in a chamber and box pressure/time wave form was analyzed to yield the indicator of airflow obstruction, Penh. PBS or methacholine was given by aerosol in increasing concentrations through an inlet of the chamber for 1 min and readings were taken for 9 min at each dose step. Penh values averaged for 5 min after each nebulization were evaluated.
Bronchoalveolar Lavage.
After the termination of the experiments, BAL was performed on mice in each of the four treatment groups for NOS gene KO and WT strains. 2 ml of PBS with 0.6 mM EDTA was instilled into the lungs and retrieved using gentle suction. The lavage was centrifuged at 2,000 g for 10 min, the supernatant was separated from the cell pellet, and aliquots were frozen at −70°C for cytokine analysis. The cell pellets were resuspended in Hank's balanced salt medium (JRH Biosciences) and slides were prepared by spinning samples at 800 rpm for 10 min (Cytospin 2; Shandon). Total cell counts were made in a hemocytometer and differentials were prepared by cytospin and stained with Wright-Giemsa stain. The investigator counting the cells was blinded to the treatment groups.
Measurement of Eosinophil Peroxidase and Protein in Bronchoalveolar Lavage Fluid.
Eosinophil peroxidase (EPO) levels in the lavage were measured colorimetrically as previously described (2, 49). 100 μl of sample or standard, porcine EPO (ExOxEmis Corp.) were pipetted, in duplicate, into the wells of a 96-well plate (Cell Wells™; Corning) followed by 100 μl of assay reaction mixture containing 0.05 M Tris buffer [Tris(hydroxymethyl)aminomethane; Trizma®; Sigma Chemical Co.], 0.1 μl 30% H2O2 (Fisher Scientific Co.), 0.015% Triton X-100 (Sigma Chemical Co.), pH 8.0, and 0.05 M ortho-phenylenediamine (Sigma Chemical Co.). The plate was incubated in the dark for 30 min and the reaction was terminated with 50 μl of 4 M H2SO4 per well and then read on a plate reader (Spectramax Model 340; Molecular Devices Corp.) at 490 nm. A BCA protein assay (Pierce Scientific) was used to quantitate lavage protein. All regression analysis was performed using the Softmax Pro software (Spectramax Plate Reader Model 340; Molecular Devices Corp.).
Histological Evaluation.
Mice were removed from the plethysmograph while under surgical anesthesia and killed by cervical dislocation. Blood was collected by cardiac puncture and the lungs were removed from the thoracic cavity and inflated with pH balanced 4% formaldehyde fixative (pH 7.4). A sagittal block of the whole left lung was dehydrated and embedded in paraffin and 5-μm sections were stained with hematoxylin and eosin and examined by light microscopy. Sections were examined for the presence of perivascular and peribronchiolar infiltrates by an investigator blinded to the treatment exposure or genotype groups.
Statistical Analysis.
Computations were performed with the JMP® 3.1.5 (SAS Institute Inc.) statistical package. A Tukey-Kramer HSD multiple comparison test was used to assess differences among the four treatment groups. For nonparametric data, differences between groups were analyzed using the Wilcoxon rank sum test. When appropriate, results are expressed as means ± SEM, and unless otherwise stated were considered statistically significant at the P < 0.05 level.
Results
Basal Expression of Pulmonary NOS Activity.
In naive WT mice exposed to neither PBS nor OVA, basally expressed total pulmonary NOS activity was detectable at a low level (0.45 ± 0.08 pmol citrulline/mg/min), of which 75 ± 9% was accounted for by iNOS activity. In WT mice sensitized to OVA, but only challenged with aerosolized PBS, there was no change in total NOS activity (0.44 ± 0.12 pmol citrulline/mg/min, P = NS versus naive WT) or iNOS activity (80 ± 12% of total NOS, P = NS versus naive WT) (Fig. 1).
Figure 1.

Assessment of calcium-dependent (cNOS, eNOS, and nNOS activity) and calcium-independent (iNOS activity) pulmonary NOS activity in OVA/PBS and OVA/OVA WT and NOS-deficient mice. Calcium-dependent (top) and -independent (bottom) NOS activity was measured in whole lung preparations as described in Materials and Methods. Data represents means ± SEM. # P < 0.05 compared with OVA/PBS, same genotype. ¶ P < 0.05 compared with WT, same treatment. Black bars, OVA/OVA; hatched bars, OVA/PBS.
Basally expressed levels of total NOS activity were not significantly different in NOS3-KO (eNOS knockout), NOS1-KO (nNOS knockout), NOS1&3-KO (nNOS and eNOS double knockout), or NOS2-KO (iNOS knockout) mice in comparison with WT mice (analysis of variance on ranks, P = 0.23). The absolute level of cNOS activity (i.e., activity that was attributable to eNOS and nNOS) was not affected in the single cNOS KO strains, NOS1-KO and NOS3-KO, in comparison with the WT strain, but was significantly reduced in the double cNOS knockout (NOS1&3-KO) mice (0.0 ± 0.0 versus 0.08 ± 0.02 pmol citrulline/mg/min in naive WT, P < 0.05). The proportion of total NOS activity characterized as iNOS activity was not significantly different among WT, NOS1-KO, and NOS3-KO mice, but was increased in NOS1&3-KO mice (100 ± 0% of total NOS, P < 0.05 versus WT), and was markedly reduced in NOS2-KO mice to a level not different from zero (11 ± 6% of total NOS, P < 0.01 versus WT).
Effects of Antigen Sensitization and Acute Antigen Exposure on Pulmonary NOS Activity.
Aerosol OVA exposure in OVA-sensitized (OVA/OVA) WT mice was associated with markedly increased total NOS activity (1.76 ± 0.30 pmol citrulline/mg/min, P < 0.01 versus naive WT), due completely to an increase in iNOS activity (99 ± 1% of total NOS, P < 0.05 versus WT) (Fig. 1). OVA sensitization and challenge was associated with similar increases in total NOS activity and iNOS activity in NOS1-KO, NOS3-KO, and NOS1&3-KO mice. In contrast, OVA sensitization and challenge in NOS2-KO mice was not associated with any increase in total NOS (0.17 ± 0.06 versus 0.33 ± 0.10 pmol citrulline/mg/min in naive NOS2-KO, P = NS) or iNOS activity (0.08 ± 0.03 versus 0.04 ± 0.02 pmol citrulline/mg/min in naive NOS2-KO, P = NS).
Airway Responsiveness in Mice Sensitized with OVA and Challenged with Either OVA or PBS.
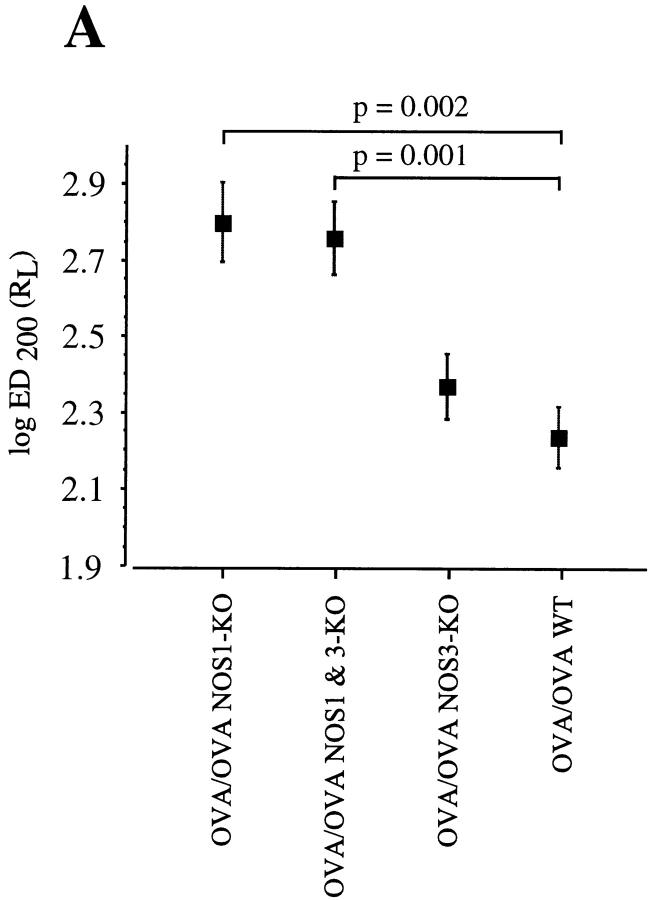
Airway responsiveness was measured in anesthetized OVA/OVA or OVA/PBS-treated mice. Airway responsiveness, expressed as the logED200RL, was measured in OVA/OVA NOS2-KO (n = 7), OVA/OVA WT (SV129/B6, n = 10), OVA/OVA NOS3-KO (n = 12), OVA/OVA NOS1&3-KO (n = 10), OVA/OVA NOS1-KO (n = 11), and OVA/OVA WT (B6, n = 15 mice) (Fig. 2, A and B). Analysis of airway reactivity (assessed by the logED200RL) revealed no significant differences between the OVA/OVA NOS2-KO and OVA/OVA WT (on a B6 background) groups (P = 0.31, Fig. 2 B). There was a significant induction in allergen-induced airway hyperresponsiveness for both the NOS2-KO and WT (B6) groups when compared with their respective OVA/PBS control groups (P < 0.05). The OVA/PBS NOS2-KO group was significantly less responsive than the OVA/PBS WT (B6) group (P < 0.001, data not shown). When the methacholine-induced airway responses were analyzed in the OVA/OVA NOS1-KO and OVA/OVA NOS1&3-KO groups, there were no significant differences between the two groups (P = 0.76). When the OVA/OVA NOS1-KO airway responses were compared with the OVA/OVA NOS3-KO mice, the OVA/OVA NOS1-KO mice were significantly less responsive to methacholine challenge (P = 0.0062). Similarly, when the OVA/OVA NOS1&3-KO airway responses were compared with the OVA/OVA NOS3-KO mice, the OVA/OVA NOS1&3-KO mice were significantly less responsive to methacholine challenge (P = 0.016). Therefore, the OVA/OVA NOS1&3-KO and OVA/OVA NOS1-KO groups were the most hyporesponsive of the OVA/OVA groups, whereas the OVA/OVA WT (B6 or SV129/B6) and OVA/OVA NOS2-KO groups were the most responsive (Fig. 2, A and B). There were no significant differences between the OVA/PBS control groups (on a B6 or SV129/B6 background) with the single exception that the OVA/PBS NOS1&3-KO group was significantly less responsive to methacholine challenge when compared with the OVA/PBS WT (SV129/B6) group (P = 0.004) (data not shown). Inducible NOS (iNOS) is upregulated in cases of human allergic asthma (18, 28) and is believed to represent the main source of increased expired NO, a molecule believed to modulate airway function (50). The lack of a difference between the OVA/OVA NOS2-KO and OVA/OVA WT (B6) groups was unexpected and prompted us to carry out a second set of experiments to confirm these results using a different methodology for measuring airway obstruction and a modified sensitization and challenge protocol. As there were no discernible differences in airway reactivity after 7 d of daily allergen exposures, we reasoned that a shorter more acute exposure may reveal a difference in airway responsiveness between the OVA/OVA NOS2-KO and OVA/OVA WT groups. In this second set of experiments, Penh, an index of bronchoconstriction, was measured using a whole body plethysmograph system in awake OVA/OVA NOS2-KO (n = 14) and OVA/OVA WT (n = 13) mice. Analysis of aerosol methacholine dose–response curves similarly revealed no significant differences in the degree of airway responsiveness between the OVA/OVA NOS2-KO and OVA/OVA WT groups, confirming the earlier data obtained by measuring RL (Fig. 3). These results indicate that our observation of no difference in airway responsiveness between WT and NOS2-KO mice is not an artifact of our specific protocols.
Figure 2.
(A) Airway responsiveness measured as ED200RL in anesthetized OVA/OVA WT (SV129/B6) and NOS1-, NOS3-, and NOS1&3-deficient mice (bred on a SV129/B6 background). Airway responses were measured from the methacholine dose–response curves. The dose necessary to cause a doubling of lung resistance was calculated by log linear interpolation. (B) Airway responses measured in anesthetized OVA/OVA WT (B6) and OVA/OVA NOS2-deficient mice bred on a C57BL/6 (B6) background. The log ED200RL values represent an index of airway responsiveness. There were no significant differences between the OVA/OVA WT (B6) and OVA/ OVA NOS2-deficient mice. Numerically lower values are indicative of increased airway responsiveness. Data represents mean log ED200RL values ± SEM.
Figure 3.
Airway responsiveness measured by whole body plethysmography in awake WT (B6) and NOS2-KO mice. Penh, an index of airway obstruction, was calculated from the box pressure/time wave form after aerosolization of increasing doses of methacholine. Numerically higher values of Penh are indicative of increased airway obstruction. Dose– response curves are shown for OVA- and PBS-challenged WT and NOS2-KO mice. Data represents mean Penh values ± SEM.
Effects of Antigen Sensitization and Acute Antigen Exposure on BAL Total Cell Counts and Differentials.
Aerosol OVA exposure in all OVA-sensitized mice was associated with a significant increase in total cell counts in BAL fluid compared with OVA-sensitized and PBS-challenged mice. The BAL total cell counts did not differ among the WT, NOS2-KO, NOS1-KO, NOS3-KO, and NOS1&3-KO OVA/PBS control groups (data not shown). The BAL total cell counts (× 103 cells ± SEM) for the WT (SV129/B6) (n = 10), NOS2-KO (n = 7), NOS1-KO (n = 11), NOS3-KO (n = 16), and NOS1&3-KO (n = 9) OVA/OVA groups were 642 ± 99.7, 843 ± 125, 904 ± 149, 883 ± 104, 531 ± 228 × 103 cells, respectively. There were no significant differences in the BAL total cell counts among the OVA-sensitized and -challenged mice groups. There were no significant differences in the proportions of macrophages, neutrophils, lymphocytes, and eosinophils in the OVA/OVA exposed mice among the treatment groups (Table I). Differential cell counts in the OVA/PBS groups revealed no significant differences in the proportion of macrophages, neutrophils, lymphocytes, and eosinophils (Table I).
Table I.
Differential Cell Counts Obtained on BAL Fluid from iNOS, nNOS, eNOS, and Double Neuronal and Endothelial KO Mice and WT (SV129/B6) Mice Treatment Groups
| Genotype | Treatment | n | Differential cell counts (mean ± SEM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macrophages | Neutrophils | Lymphocytes | Eosinophils | |||||||||
| % | ||||||||||||
| NOS2-KO | OVA/OVA | 6 | 15.2 ± 1.9 | 5.2 ± 1.6 | 8.7 ± 1.4 | 70.8 ± 3.4 | ||||||
| NOS2-KO | OVA/PBS | 5 | 98.2 ± 0.7 | 0.6 ± 0.4 | 1.2 ± 0.6 | 0.0 ± 0.0 | ||||||
| P = * | 0.008 | 0.02 | 0.008 | 0.006 | ||||||||
| WT | OVA/OVA | 7 | 16.3 ± 1.8 | 4.4 ± 1.0 | 5.1 ± 0.9 | 74.1 ± 2.1 | ||||||
| WT | OVA/PBS | 5 | 99.2 ± 0.4 | 0.4 ± 0.3 | 0.4 ± 0.3 | 0.0 ± 0.0 | ||||||
| P = * | 0.006 | 0.005 | 0.005 | 0.004 | ||||||||
| NOS1-KO | OVA/OVA | 6 | 15 ± 2.3 | 3.3 ± 0.5 | 4.8 ± 0.7 | 77.0 ± 3.1 | ||||||
| NOS1-KO | OVA/PBS | 5 | 98.2 ± 0.6 | 0.2 ± 0.2 | 1.4 ± 0.5 | 0.2 ± 0.2 | ||||||
| P = * | 0.008 | 0.007 | 0.016 | 0.007 | ||||||||
| NOS3-KO | OVA/OVA | 6 | 16.8 ± 0.5 | 3.7 ± 0.6 | 4.8 ± 0.8 | 74.7 ± 1.1 | ||||||
| NOS3-KO | OVA/PBS | 5 | 98.4 ± 0.7 | 0.2 ± 0.2 | 1.0 ± 0.3 | 0.4 ± 0.4 | ||||||
| P = * | 0.008 | 0.007 | 0.0096 | 0.007 | ||||||||
| NOS1,3-KO | OVA/OVA | 7 | 19 ± 1.2 | 3.14 ± 0.3 | 5.0 ± 0.5 | 73.6 ± 1.5 | ||||||
| NOS1,3-KO | OVA/PBS | 5 | 98.8 ± 0.4 | 0.2 ± 0.2 | 1.0 ± 0.5 | 0.0 ± 0.0 | ||||||
| P = * | 0.005 | 0.005 | 0.005 | 0.004 | ||||||||
P values are for OVA/OVA versus OVA/PBS.
Effects of Antigen Sensitization and Acute Antigen Exposure on OVA Specific IgE Levels.
Antigen sensitization and challenge induced a significant increase in serum levels of OVA-specific IgE in the WT, NOS2-KO, NOS1-KO, NOS3-KO, and NOS1&3-KO groups compared with OVA-sensitized and PBS-challenged groups. OVA-specific IgE levels reported as absorbance values (OD 405 nm) measured in the OVA/OVA WT, NOS2-KO, NOS1-KO, NOS3-KO, and NOS1&3-KO groups were 0.71 ± 0.10, 0.61 ± 0.14, 0.43 ± 0.16, 0.93 ± 0.25, and 0.79 ± 0.32, respectively. There were no significant differences among the OVA/ OVA groups. The values for the absorbance (OD 405 nm) measured in the OVA/PBS WT, NOS2-KO, NOS1-KO, NOS3-KO, and NOS1&3-KO groups were 0.03 ± 0.01, 0.14 ± 0.05, 0.05 ± 0.03, 0.11 ± 0.06, and 0.03 ± 0.03, respectively. Similarly, there were no significant differences among the OVA/PBS control groups. When the OVA/OVA groups were compared with their matched OVA/PBS control groups, there were significant within genotype differences attributable to sensitization (P < 0.05) for all groups with the exception of the NOS1-KO group, which approached but did not achieve statistical significance (P = 0.052).
Effects of Antigen Sensitization and Acute Antigen Exposure on BAL EPO and Total Protein Levels.
BAL EPO and total protein levels were evaluated as fluid phase indicators of the inflammatory response to allergen challenge. There were no significant differences in either EPO or total protein levels within the OVA/OVA or OVA/PBS groups (data not shown).
Histology Findings.
Examination of fixed lung tissue from the OVA-sensitized and -challenged groups revealed peribronchial and perivascular accumulation of eosinophils, granulocytes, and mononuclear cells. No differences in the degree of airway inflammation or presence of inflammatory infiltrates were noted between the OVA/OVA WT and NOS-deficient mice (all NOS KOs). Similarly, the histological findings in the OVA-sensitized and PBS-challenged groups were unremarkable with no observable differences between the KO and WT mice.
Discussion
Systemic sensitization and aerosol challenge with allergen in mice reproduces many of the phenotypic features of human asthma, including increased airway hyperresponsiveness, airway inflammation, and increased antigen-specific IgE. The role of NO in human asthma or animal models thereof is unclear, as is the relative contribution of each of the NOS isoforms. NO has been reported to exhibit both beneficial and deleterious effects in asthma. It has been reported to be a weak bronchodilator (19–21); however, others have reported no effect on airway tone (22– 24). Still others have reported that the formation of NO may have deleterious effects due to its reported cytotoxic effects (18), and finally it has been reported that NO may serve as a marker of inflammation (16, 17, 51).
To determine the specific contribution of each of the NOS isoforms in asthma, we studied mice with targeted deletions of the endothelial, neuronal, inducible, and double endothelial and neuronal NOS isoforms using an established model of allergic asthma in the mouse (7, 52). The use of gene KO mice is advantageous in isolating the specific contribution of each NOS isoform. Indeed, the use of NOS inhibitors to dissect out the role of constitutive and inducible NOS is subject to problems of inhibitor specificity and pharmacokinetic concerns.
Because iNOS has been shown to be upregulated in asthma and is believed to represent the major source of NO in the lung (13, 53, 54), we reasoned that iNOS would play a pivotal role in either relaxing or exacerbating the allergen induced airway hyperresponsiveness. Airway responsiveness is classically assessed by analyzing dose– response relationships in which a bronchoconstrictor is administered in increasing doses while monitoring the effects of the agonist on mechanical indices of pulmonary obstruction such as pulmonary resistance (RL) (2, 7, 44, 45). This index of airflow obstruction represents the pressure loss in phase with airflow and is analogous to measures of pulmonary resistance in human subjects. Our data show that iNOS is significantly upregulated in the lung tissue of the WT mice sensitized and challenged with OVA, a finding reported in asthmatics (9, 18). Despite this upregulation, there were no significant differences in airway responsiveness, airway inflammation, or cellular recruitment into the airway space when compared with the NOS2-KO OVA-challenged treatment group. In this regard our data do not agree with the recent findings of Xiong et al., who demonstrated a significant decrease in eosinophilia and suppression of allergic inflammation in NOS2 KO mice immunized and challenged with OVA (55). The discrepancy in their results and our own is likely due to significant differences in the immunization and challenge protocols (55). The immunization and challenge protocol used by Xiong et al. resulted in ∼90% eosinophilia in the BAL in their WT OVA/OVA group, considerably higher than the proportion reported by us and others in models of allergen- induced airway inflammation in the mouse (7, 52, 56–59).
The lack of a significant difference in the pulmonary resistance (RL) response to methacholine between the NOS2-KO OVA/OVA and WT (B6) OVA/OVA mice prompted us to repeat these experiments using unanesthetized mice studied in a whole body plethysmograph. This method (47, 60) confirmed our previous observation whereby the NOS2-KO OVA/OVA and WT OVA/ OVA mice exhibited increased, albeit similar degrees of airway hyperresponsiveness. In this regard our data are in agreement with the findings of Xiong et al., who also demonstrated that NOS2-KO mice did not have diminished airway hyperresponsiveness when compared with WT mice exposed to an OVA sensitization and challenge protocol (55). Thus, our data and those of others clearly indicate that allergen-induced airway hyperresponsiveness is fully expressed in the absence of iNOS.
Analysis of markers of inflammation (total protein and EPO) in the BAL fluid revealed no significant differences in our data set between the NOS2-KO and WT OVA/ OVA groups. Additionally, there were no significant differences in the levels of OVA-specific IgE between any of the OVA-sensitized and -challenged treatment groups. This is not surprising given that NO has no reported effects on antigen presentation and processing. These findings indicate that the iNOS isoform is not important in the genesis of airway inflammation in this allergic model of asthma.
When airway responsiveness was ascertained in the NOS3-KO, NOS1-KO, and NOS1&3-KO mice, the NOS1-KO and NOS1&3-KO OVA/OVA groups exhibited increased airway responsiveness when compared with their respective controls (OVA/PBS), yet there was significantly less enhancement of airway responses secondary to OVA exposure than in NOS2-KO and WT mice. The similar degree of diminished airway hyperresponsiveness among the NOS1-KO and NOS1&3-KO mice, and the observation of a more intermediate degree of airway hyperresponsiveness in the NOS3-KO OVA/OVA mice, indicate that the nNOS isoform is the one principally responsible for the OVA-induced enhancement of airway responsiveness. The reduction in allergen-induced airway hyperresponsiveness in NOS1-KO mice is interesting given that nNOS has previously been shown to contribute to baseline airway responsiveness (29). Although the results of our study do not determine the mechanism(s) by which nNOS is modulating airway responsiveness, the neuronal-derived NO may directly effect airway smooth muscle tone. NO has been implicated in nonadrenergic, noncholinergic (NANC)-mediated airway relaxation (30, 31). Indeed, our data are intriguing given recent studies that have established linkage of the diagnosis of asthma to a region that maps near the human nNOS gene (35–37). Aside from the differences in airway responsiveness, the NOS1-KO and NOS1&3-KO OVA/OVA groups did not differ in their degree of airway inflammation, levels of OVA-specific IgE, or recruitment of inflammatory cells into the BAL. These findings suggest a noninflammatory link between the nNOS gene and airway hyperresponsiveness in this murine model of allergic asthma.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants HL56383 and AI01245. G.T. De Sanctis is supported by a grant from the NIH (HL36110) and a Partners Investigator Nesson Award. P. Huang is an established Investigator of the American Heart Association and was funded by National Institute of Neurological Disorders and Stroke Grant NS33335. H. Grasemann is supported by a grant from the Deutsche Forschungsgemeinschaft.
Abbreviations used in this paper
- BAL
bronchoalveolar lavage
- cNOS
constitutive NOS
- eNOS
endothelial NOS
- EPO
eosinophil peroxidase
- iNOS
inducible NOS
- KO
knockout
- nNOS
neuronal NOS
- NO
nitric oxide
- NOS
nitric oxide synthase
- RL
pulmonary resistance
- SPF
sterile pathogen-free
- WT
wild-type
Footnotes
The analysis of lung resistance was performed using software provided by Andrew Jackson (Biomedical Engineering at Boston University, Boston, MA).
References
- 1.National Asthma Education Program. Expert Panel Report. Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute. J Allergy Clin Immunol. 1991;88:425–534. [PubMed] [Google Scholar]
- 2.Wolyniec WW, De Sanctis GT, Nabozny G, Torcellini C, Haynes N, Joetham A, Gelfand EW, Drazen JM, Noonan TC. Reduction of antigen-induced airway hyperreactivity and eosinophilia in ICAM-1-deficient mice. Am J Respir Cell Mol Biol. 1998;18:777–785. doi: 10.1165/ajrcmb.18.6.3056. [DOI] [PubMed] [Google Scholar]
- 3.Padrid PA, Mathur M, Li X, Herrmann K, Qin Y, Cattamanchi A, Weinstock J, Elliott D, Sperling AI, Bluestone JA. CTLA4Ig inhibits airway eosinophilia and hyperresponsiveness by regulating the development of Th1/ Th2 subsets in a murine model of asthma. Am J Respir Cell Mol Biol. 1998;18:453–462. doi: 10.1165/ajrcmb.18.4.3055. [DOI] [PubMed] [Google Scholar]
- 4.Haczku A, Takeda K, Hamelmann E, Oshiba A, Loader J, Joetham A, Irvin C, Kikutani H, Gelfand EW. CD23 deficient mice develop allergic airway hyperresponsiveness following sensitization with ovalbumin. Am J Respir Crit Care Med. 1997;156:1945–1955. doi: 10.1164/ajrccm.156.6.9701087. [DOI] [PubMed] [Google Scholar]
- 5.Hogan SP, Mould A, Kikutani H, Ramsay AJ, Foster PS. Aeroallergen-induced eosinophilic inflammation, lung damage, and airways hyperreactivity in mice can occur independently of IL-4 and allergen-specific immunoglobulins. J Clin Invest. 1997;99:1329–1339. doi: 10.1172/JCI119292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kung TT. Pulmonary eosinophilia and inflammation in allergic mice. Lab Anim Sci. 1998;48:61–63. [PubMed] [Google Scholar]
- 7.De Sanctis GT, Wolyniec WW, Green FH, Qin S, Jiao A, Finn PW, Noonan T, Joetham AA, Gelfand E, Doerschuk CM, Drazen JM. Reduction of allergic airway responses in P-selectin-deficient mice. J Appl Physiol. 1997;83:681–687. doi: 10.1152/jappl.1997.83.3.681. [DOI] [PubMed] [Google Scholar]
- 8.Nijkamp FP, Folkerts G. Nitric oxide and bronchial hyperresponsiveness. Arch Int Pharmacodyn Ther. 1995;329:81–96. [PubMed] [Google Scholar]
- 9.Hamid Q, Springall DR, Riveros-Moreno V, Chanez P, Howarth P, Redington A, Bousquet J, Godard P, Holgate S, Polak JM. Induction of nitric oxide synthase in asthma. Lancet. 1993;342:1510–1513. doi: 10.1016/s0140-6736(05)80083-2. [DOI] [PubMed] [Google Scholar]
- 10.Yeadon M, Price R. Induction of calcium-independent nitric oxide synthase by allergen challenge in sensitized rat lung in vivo. Br J Pharmacol. 1995;116:2545–2546. doi: 10.1111/j.1476-5381.1995.tb17204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renzi, P.M., N. Sebastiao, A.S. al Assaad, A. Giaid, and Q. Hamid. 1997. Inducible nitric oxide synthase mRNA and immunoreactivity in the lungs of rats eight hours after antigen challenge. Am. J. Respir. Cell Mol. Biol. 17:36–40. [DOI] [PubMed]
- 12.Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J. 1993;6:1368–1370. [PubMed] [Google Scholar]
- 13.Kharitonov, S.A., D. Yates, D.R. Springall, L. Buttery, J. Polak, R.A. Robbins, and P.J. Barnes. 1995. Exhaled nitric oxide is increased in asthma. Chest. 107:156S–157S. (Suppl.). [DOI] [PubMed]
- 14.Massaro AF, Mehta S, Lilly CM, Kobzik L, Reilly JJ, Drazen JM. Elevated nitric oxide concentrations in isolated lower airway gas of asthmatic subjects. Am J Respir Crit Care Med. 1996;153:1510–1514. doi: 10.1164/ajrccm.153.5.8630594. [DOI] [PubMed] [Google Scholar]
- 15.Baraldi E, Azzolin NM, Zanconato S, Dario C, Zacchello F. Corticosteroids decrease exhaled nitric oxide in children with acute asthma. J Pediatr. 1997;131:381–385. doi: 10.1016/s0022-3476(97)80062-5. [DOI] [PubMed] [Google Scholar]
- 16.Lundberg JO. Airborne nitric oxide: inflammatory marker and aerocrine messenger in man. Acta Physiol Scand Suppl. 1996;633:1–27. [PubMed] [Google Scholar]
- 17.Silkoff PE, McClean PA, Slutsky AS, Caramori M, Chapman KR, Gutierrez C, Zamel N. Exhaled nitric oxide and bronchial reactivity during and after inhaled beclomethasone in mild asthma. J Asthma. 1998;35:473–479. doi: 10.3109/02770909809071000. [DOI] [PubMed] [Google Scholar]
- 18.Saleh D, Ernst P, Lim S, Barnes PJ, Giaid A. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: effect of inhaled glucocorticoid. FASEB J. 1998;12:929–937. [PubMed] [Google Scholar]
- 19.Vaali K, Li L, Redemann B, Paakkari I, Vapaatalo H. In-vitro bronchorelaxing effects of novel nitric oxide donors GEA 3268 and GEA 5145 in guinea-pigs and rats. J Pharm Pharmacol. 1996;48:1309–1314. doi: 10.1111/j.2042-7158.1996.tb03941.x. [DOI] [PubMed] [Google Scholar]
- 20.Kacmarek RM, Ripple R, Cockrill BA, Bloch KJ, Zapol WM, Johnson DC. Inhaled nitric oxide. A bronchodilator in mild asthmatics with methacholine- induced bronchospasm. Am J Respir Crit Care Med. 1996;153:128–135. doi: 10.1164/ajrccm.153.1.8542105. [DOI] [PubMed] [Google Scholar]
- 21.Gwyn DR, Lindeman KS, Hirshman CA. Inhaled nitric oxide attenuates bronchoconstriction in canine peripheral airways. Am J Respir Crit Care Med. 1996;153:604–609. doi: 10.1164/ajrccm.153.2.8564105. [DOI] [PubMed] [Google Scholar]
- 22.Pfeffer KD, Ellison G, Robertson D, Day RW. The effect of inhaled nitric oxide in pediatric asthma. Am J Respir Crit Care Med. 1996;153:747–751. doi: 10.1164/ajrccm.153.2.8564128. [DOI] [PubMed] [Google Scholar]
- 23.Roger N, Barbera JA, Farre R, Cobos A, Roca J, Rodriguez-Roisin R. Effect of nitric oxide inhalation on respiratory system resistance in chronic obstructive pulmonary disease. Eur Respir J. 1996;9:190–195. doi: 10.1183/09031936.96.09020190. [DOI] [PubMed] [Google Scholar]
- 24.Taylor DA, McGrath JL, O'Connor BJ, Barnes PJ. Allergen-induced early and late asthmatic responses are not affected by inhibition of endogenous nitric oxide. Am J Respir Crit Care Med. 1998;158:99–106. doi: 10.1164/ajrccm.158.1.9709091. [DOI] [PubMed] [Google Scholar]
- 25.Robbins RA, Springall DR, Warren JB, Kwon OJ, Buttery LD, Wilson AJ, Adcock IM, Riveros-Moreno V, Moncada S, Polak J, et al. Inducible nitric oxide synthase is increased in murine lung epithelial cells by cytokine stimulation. Biochem Biophys Res Commun. 1994;198:835–843. doi: 10.1006/bbrc.1994.1119. [DOI] [PubMed] [Google Scholar]
- 26.Watkins DN, Peroni DJ, Basclain KA, Garlepp MJ, Thompson PJ. Expression and activity of nitric oxide synthases in human airway epithelium. Am J Respir Cell Mol Biol. 1997;16:629–639. doi: 10.1165/ajrcmb.16.6.9191464. [DOI] [PubMed] [Google Scholar]
- 27.Liu SF, Haddad EB, Adcock I, Salmon M, Koto H, Gilbey T, Barnes PJ, Chung KF. Inducible nitric oxide synthase after sensitization and allergen challenge of Brown Norway rat lung. Br J Pharmacol. 1997;121:1241–1246. doi: 10.1038/sj.bjp.0701242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yates DH, Kharitonov SA, Thomas PS, Barnes PJ. Endogenous nitric oxide is decreased in asthmatic patients by an inhibitor of inducible nitric oxide synthase. Am J Respir Crit Care Med. 1996;154:247–250. doi: 10.1164/ajrccm.154.1.8680689. [DOI] [PubMed] [Google Scholar]
- 29.De Sanctis GT, Mehta S, Kobzik L, Yandava C, Jiao A, Huang PL, Drazen JM. Contribution of type I NOS to expired gas NO and bronchial responsiveness in mice. Am J Physiol. 1997;273:L883–L888. doi: 10.1152/ajplung.1997.273.4.L883. [DOI] [PubMed] [Google Scholar]
- 30.Tucker JF, Brave SR, Charalambous L, Hobbs AJ, Gibson A. L-NG-nitro arginine inhibits non-adrenergic, non-cholinergic relaxations of guinea-pig isolated tracheal smooth muscle. Br J Pharmacol. 1990;100:663–664. doi: 10.1111/j.1476-5381.1990.tb14072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li CG, Rand MJ. Evidence that part of the NANC relaxant response of guinea-pig trachea to electrical field stimulation is mediated by nitric oxide. Br J Pharmacol. 1991;102:91–94. doi: 10.1111/j.1476-5381.1991.tb12137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feder LS, Stelts D, Chapman RW, Manfra D, Crawley Y, Jones H, Minnicozzi M, Fernandez X, Paster T, Egan RW, et al. Role of nitric oxide on eosinophilic lung inflammation in allergic mice. Am J Respir Cell Mol Biol. 1997;17:436–442. doi: 10.1165/ajrcmb.17.4.2845. [DOI] [PubMed] [Google Scholar]
- 33.Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, Peitzman AB, Billiar TR, Tweardy DJ. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998;187:917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan IA, Matsuura T, Kasper LH. Inducible nitric oxide synthase is not required for long-term vaccine- based immunity against Toxoplasma gondii. . J Immunol. 1998;161:2994–3000. [PubMed] [Google Scholar]
- 35.Barnes KC, Neely JD, Duffy DL, Freidhoff LR, Breazeale DR, Schou C, Naidu RP, Levett PN, Renault B, Kucherlapati R, et al. Linkage of asthma and total serum IgE concentration to markers on chromosome 12q: evidence from Afro-Caribbean and Caucasian populations. Genomics. 1996;37:41–50. doi: 10.1006/geno.1996.0518. [DOI] [PubMed] [Google Scholar]
- 36.Thomas NS, Wilkinson J, Holgate ST. The candidate region approach to the genetics of asthma and allergy. Am J Respir Crit Care Med. 1997;156:S144–S151. doi: 10.1164/ajrccm.156.4.12-tac-13. [DOI] [PubMed] [Google Scholar]
- 37.The Collaborative Study on the Genetics of Asthma (CSGA) A genome-wide search for asthma susceptibility loci in ethnically diverse populations. Nat Genet. 1997;15:389–392. doi: 10.1038/ng0497-389. [DOI] [PubMed] [Google Scholar]
- 38.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 39.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 40.Son H, Hawkins RD, Martin K, Kiebler M, Huang PL, Fishman MC, Kandel ER. Long-term potentiation is reduced in mice that are doubly mutant in endothelial and neuronal nitric oxide synthase. Cell. 1996;87:1015–1023. doi: 10.1016/s0092-8674(00)81796-1. [DOI] [PubMed] [Google Scholar]
- 41.MacMicking, J.D., C. Nathan, G. Hom, N. Chartrain, D.S. Fletcher, M. Trumbauer, K. Stevens, Q.W. Xie, K. Sokol, N. Hutchinson, et al. 1995. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 81:641–650. (See published erratum 81(7):following 1170.) [DOI] [PubMed]
- 42.MacLean JA, Sauty A, Luster AD, Drazen JM, De Sanctis GT. Antigen-induced airway hyperresponsiveness, pulmonary eosinophilia and chemokine expression in B cell-deficient mice. Am J Respir Cell Mol Biol. 1999;20:379–387. doi: 10.1165/ajrcmb.20.3.3291. [DOI] [PubMed] [Google Scholar]
- 43.Scott JA, Machoun M, McCormack DG. Inducible nitric oxide synthase and vascular reactivity in rat thoracic aorta: effect of aminoguanidine. J Appl Physiol. 1996;80:271–277. doi: 10.1152/jappl.1996.80.1.271. [DOI] [PubMed] [Google Scholar]
- 44.De Sanctis GT, Merchant M, Beier DR, Dredge RD, Grobholz JK, Martin TR, Lander ES, Drazen JM. Quantitative locus analysis of airway hyperresponsiveness in A/J and C57BL/6J mice. Nat Genet. 1995;11:150–154. doi: 10.1038/ng1095-150. [DOI] [PubMed] [Google Scholar]
- 45.De Sanctis GT, Itoh A, Green FH, Qin S, Kimura T, Grobholz JK, Martin TR, Maki T, Drazen JM. T-lymphocytes regulate genetically determined airway hyperresponsiveness in mice. Nat Med. 1997;3:460–462. doi: 10.1038/nm0497-460. [DOI] [PubMed] [Google Scholar]
- 46.Martin TR, Gerard NP, Galli SJ, Drazen JM. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol. 1988;64:2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- 47.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 48.Corry DB, Grunig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, Rennick DM, Locksley RM. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med. 1998;4:344–355. [PMC free article] [PubMed] [Google Scholar]
- 49.Strath M, Warren DJ, Sanderson CJ. Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J Immunol Methods. 1985;83:209–215. doi: 10.1016/0022-1759(85)90242-x. [DOI] [PubMed] [Google Scholar]
- 50.Barnes PJ. Nitric oxide and airway disease. Ann Med. 1995;27:389–393. doi: 10.3109/07853899509002592. [DOI] [PubMed] [Google Scholar]
- 51.Gaston B, Sears S, Woods J, Hunt J, Ponaman M, McMahon T, Stamler JS. Bronchodilator S-nitrosothiol deficiency in asthmatic respiratory failure. Lancet. 1998;351:1317–1319. doi: 10.1016/S0140-6736(97)07485-0. [DOI] [PubMed] [Google Scholar]
- 52.Krinzman SJ, De Sanctis GT, Cernadas M, Mark D, Wang Y, Listman J, Kobzik L, Donovan C, Nassr K, Katona I, et al. Inhibition of T cell costimulation abrogates airway hyperresponsiveness in a murine model. J Clin Invest. 1996;98:2693–2699. doi: 10.1172/JCI119093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Persson MG, Zetterstrom O, Agrenius V, Ihre E, Gustafsson LE. Single-breath nitric oxide measurements in asthmatic patients and smokers. Lancet. 1994;343:146–147. doi: 10.1016/s0140-6736(94)90935-0. [DOI] [PubMed] [Google Scholar]
- 54.Kharitonov SA, Yates DH, Barnes PJ. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med. 1996;153:454–457. doi: 10.1164/ajrccm.153.1.8542158. [DOI] [PubMed] [Google Scholar]
- 55.Xiong Y, Karupiah G, Hogan SP, Foster PS, Ramsay AJ. Inhibition of allergic airway inflammation in mice lacking nitric oxide synthase 2. J Immunol. 1999;162:445–452. [PubMed] [Google Scholar]
- 56.Lambert LE, Berling JS, Kudlacz EM. Characterization of the antigen-presenting cell and T cell requirements for induction of pulmonary eosinophilia in a murine model of asthma. Clin Immunol Immunopathol. 1996;81:307–311. doi: 10.1006/clin.1996.0194. [DOI] [PubMed] [Google Scholar]
- 57.Kung TT, Jones H, Adams GK, III, Umland SP, Kreutner W, Egan RW, Chapman RW, Watnick AS. Characterization of a murine model of allergic pulmonary inflammation. Int Arch Allergy Immunol. 1994;105:83–90. doi: 10.1159/000236807. [DOI] [PubMed] [Google Scholar]
- 58.Krinzman SJ, De Sanctis GT, Cernadas M, Kobzik L, Listman JA, Christiani DC, Perkins DL, Finn PW. T cell activation in a murine model of asthma. Am J Physiol. 1996;271:L476–L483. doi: 10.1152/ajplung.1996.271.3.L476. [DOI] [PubMed] [Google Scholar]
- 59.MacLean JA, Sauty A, Luster AD, Drazen JM, De Sanctis GT. Antigen-induced airway hyperresponsiveness, pulmonary eosinophilia, and chemokine expression in B cell-deficient mice. Am J Respir Cell Mol Biol. 1999;20:379–387. doi: 10.1165/ajrcmb.20.3.3291. [DOI] [PubMed] [Google Scholar]
- 60.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig BB, Pereira P, Pretolani M. Requirement for γδ T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]