Abstract
Using a single vector targeting strategy, we have generated mice with a combined deficiency of interleukin (IL)-4 and IL-13 to clarify their roles in T helper type 2 (Th2) cell responses. Using immunological challenges normally characterized by a Th2-like response, we have compared the responses of the double-deficient mice with those generated by wild-type, IL-4–deficient, and IL-13–deficient mice. Using a pulmonary granuloma model, induced with Schistosoma mansoni eggs, we demonstrate that although eosinophil infiltration, immunoglobulin E, and IL-5 production are reduced in the IL-4–deficient mice and IL-13–deficient mice, they are abolished only in the combined absence of both cytokines. Furthermore, IL-4/13–deficient animals are severely impaired in their ability to expel the gastrointestinal nematode Nippostrongylus brasiliensis. Unexpectedly, N. brasiliensis–infected IL-4/13–deficient mice developed elevated IL-5 and eosinophilia, indicating that compensatory mechanisms exist for the expression of IL-5, although serum IgE remained undetectable. IL-4/13–deficient mice default to a Th1-like phenotype characterized by the expression of interferon γ and the production of IgG2a and IgG2b. We conclude that IL-4 and IL-13 cooperate to initiate rapid Th2 cell–driven responses, and that although their functions overlap, they perform additive roles.
Keywords: interleukin 4, interleukin 13, T helper type 2 cells, immunoglobulin E, eosinophilia
Although IL-4 is a key cytokine in the development of Th2 cell responses (1, 2), recent studies of IL-4R–mediated signaling pathways have implied the presence of alternative routes for Th2 cell induction (3–6). Indeed, we have recently identified that the closely related cytokine, IL-13, can also effect Th2 development (7), raising the question of how these two cytokines may compensate for one another during immune responses.
Both IL-4 and IL-13 are produced primarily by Th2-like cells (8–10) and mast cells (11), and in vitro assay systems have demonstrated that they share several biological functions. These include inducing human B cells to undergo Ig isotype switching to IgE (12) and regulating inflammatory responses by suppressing TNF-α and IL-1 expression from monocytes and macrophages (12–14). However, there are also specific functional differences between these molecules, most notable of which is the ability of IL-4 to induce T cell proliferation (15). Recent in vivo studies examining the clearance of the parasitic gastrointestinal nematode Nippostrongylus brasiliensis from the intestines of infected cytokine-deficient mice have also demonstrated divergent responses to IL-4 and IL-13. Although IL-4–deficient mice expelled N. brasiliensis worms with similar kinetics to wild-type mice, IL-13–deficient animals or mice treated with an inhibitor of IL-13 did not expel worms as efficiently (16, 17).
Analysis of IL-4 and IL-13 receptor usage has explained certain aspects of the related responses. It is evident that both IL-4 and IL-13 can cross-compete for IL-4Rα, but that only IL-4 binds directly to this receptor chain (18). IL-13 binds to its own primary binding chain (IL-13Rα1), to which IL-4 does not bind, and recruits IL-4Rα into a receptor complex resulting in an increase in binding affinity and the initiation of signal transduction (19, 20). Further differential signaling pathways can be envisaged for the IL-4R, since IL-4 binding may recruit IL-13Rα1 or IL-2Rγc into its active receptor complex (21, 22). This complexity of receptor usage and the potential diversity of signaling pathways combine with the temporal and spatial expression of the individual ligands to create a diversity of possible responses.
The genes encoding IL-4 and IL-13 are closely linked on human chromosome 5 and the syntenic region of mouse chromosome 11, and map to a cytokine gene cluster that also includes IL-5, IL-3, and GM-CSF (23, 24). To study the individual and combined contributions of IL-4 and IL-13 to immune responses, it would be advantageous to study mouse lines in which the expression of both cytokines had been disrupted; however, the close linkage of their genes precludes the generation of such lines by simple interbreeding of IL-4–deficient and IL-13–deficient mice. To circumvent these difficulties, we have used a single vector gene targeting strategy to simultaneously disrupt both the IL-4 and IL-13 genes, thereby allowing us to investigate the potential compensatory roles of these cytokines during Th2-dominated immune responses in vivo. Using a Th2-driven lung granuloma model, we demonstrate that IL-4 and IL-13 can partially compensate for each other and that granuloma formation is abolished only in the absence of both cytokines. Furthermore, we show that although IL-13 is primarily involved in the expulsion of N. brasiliensis, the concomitant disruption of IL-4 further impairs parasite clearance. Interestingly, IL-4/13–deficient mice have demonstrated the existence of compensatory mechanisms that still enable the expression of IL-5 and subsequent eosinophilia after N. brasiliensis infection. We conclude that IL-4 and IL-13 act in concert to initiate rapid Th2-like responses, and that their combined disruption can either abolish such responses or significantly delay their onset, resulting in an inappropriate Th1 response.
Materials and Methods
Targeted Disruption of the Mouse IL-13 and IL-4 Genes in Embryonic Stem Cells.
The single targeting vector consisted of 6 kb of the IL-13 gene providing the 5′ arm of homology and 4.0 kb of the IL-4 gene comprising the 3′ homology. The replacement vector was constructed to insert the neomycin resistance gene into an engineered SalI site in exon 3 of the IL-13 gene. Stop codons in all three frames were inserted 5′ of the selectable marker. The IL-4 region was a HindIII fragment containing exon 4. The targeting vector was linearized and electroporated into E14.1 embryonic stem (ES)1 cells (7). Of 500 G418-resistant clones screened by Southern blot analysis, using a probe made with PCR primers (TGACCACAGGCAGTTTCACCTGC and TTATCATCTCAGCCTCATATACAG), one was found to be correctly targeted. Hybridization with a probe to the neomycin sequence and IL-13 cDNA sequences confirmed the predicted size of the targeted fragment and that only a single integration had occurred. The targeted ES cell clone was microinjected into 3.5-d C57BL/6 blastocysts to generate chimeras. These mice were mated with C57BL/6 mice and transmitted the ES cell genotype through the germline. Mice homozygous for the disrupted IL-4 and IL-13 genes were obtained by interbreeding the heterozygotes. The IL-4/13 gene–targeted, IL-13 gene–targeted (7), and wild-type animals used in the experiments reported below were maintained on a 129 × C57BL/6 (F2) background in a specific pathogen–free environment. IL-4−/− mice (1) had been backcrossed 10 times onto C57BL/6. Wild-type C57BL/6 mice were included as controls in the schistosome egg experiments and N. brasiliensis experiments, and showed similar phenotypes to the wild-type 129 × C57BL/6 (F2) controls. Therefore, for the sake of clarity only the wild-type 129 × C57BL/6 (F2) controls are presented.
Preparation of CD4+ T Cells.
Splenocytes were cultured on plastic tissue culture plates for 1 h at 37°C to remove macrophages. Nonadherent cells were incubated with biotinylated anti–I-Ab antibody (clone AF6-120.1; Becton Dickinson), biotinylated anti-CD8 antibody (clone 53-6.7; Becton Dickinson) and biotinylated anti-B220 antibody (clone RA3-6B2; Becton Dickinson), and streptavidin magnetic beads (MACS®; Miltenyi Biotec) followed by magnetic field separation to remove MHC class II, CD8, and B220-expressing cells. Cell purity was determined using FITC-labeled anti-CD4 and PE-labeled anti-CD8 antibodies and was generally 90–95% CD4+ cells. Purified cells were cultured on anti-CD3ε antibody–coated plates (10 μg/ml of clone 2C11; Becton Dickinson) plus anti-CD28 antibody (1 μg/ml of clone 37.51; Becton Dickinson) in the presence of exogenous cytokines or anticytokine antibody as indicated. IL-2 (10 ng/ml; R&D Systems) was added to all cultures. Th2 cell differentiation was promoted in the presence of 100 ng/ml IL-4 (R&D Systems) and anti–IFN-γ antibody (10 μg/ml of clone XMG1.2; Becton Dickinson). Cells were cultured for 5 d, washed, and resuspended at 106 cells/ml for 24 h in the presence of anti-CD3. Supernatants were analyzed by cytokine ELISA performed as above.
Granuloma Formation.
Synchronous pulmonary granulomas were induced by intravenous injection of mice with Schistosoma mansoni eggs. S. mansoni eggs were isolated from the livers of infected mice as described (25). Mice were sensitized to schistosome eggs by intraperitoneal injection of 5,000 live eggs. 2 wk later, sensitized and naive mice, six mice per group, were injected intravenously with 5,000 eggs to induce synchronous pulmonary granulomas. 15 d after intravenous egg injection, mice were killed, serum was recovered, and the draining mediastinal lymph nodes were removed. The lungs were inflated with formol saline and processed for histology. The size (diameter on hematoxylin and eosin [H&E]-stained sections) and cell composition (percentage of eosinophils on Giemsa-stained sections) of the granuloma surrounding individual eggs were measured with an ocular micrometer using a double blind protocol by an investigator not involved in the study; >100 individual granulomas were analyzed per group. Cells were prepared from pooled mediastinal lymph nodes from each group and processed for cell culture as described (25). 3 × 106 cells/ml were cultured and restimulated with 10 μg/ml of soluble egg antigen. IL-4, IL-5, IL-10, and IFN-γ were assayed using ELISA. Egg antigen–specific IgE, IgG1, and IgG2a isotype responses were measured using ELISA as described (26). Statistical analysis was performed using analysis of variance (ANOVA) and Dunnett's test; P < 0.05 was considered significant.
N. brasiliensis Infection.
Individual mice were inoculated subcutaneously with 400 viable third-stage N. brasiliensis larvae.
OVA Immunization.
8–10-wk-old mice were immunized intraperitoneally with 100 μg of OVA adsorbed to aluminium hydroxide (OVA/alum) with subsequent boost injections with 100 μg of OVA/alum after 10 and 20 d. Serum samples were assayed for Ig isotypes.
ELISA Assays.
Serum Igs were assayed using sandwich ELISA. 96-well plates were coated with anti-Ig isotype capture mAbs, and bound Ig of diluted serum samples was detected using biotinylated anti-Ig isotype detection mAbs (Becton Dickinson). Concentrations were calculated using purified Ig isotypes as standards (Becton Dickinson). OVA-specific ELISAs were performed by coating 96-well plates with OVA at 2.5 μg/ml; bound Ig of diluted serum samples was detected using biotinylated anti-Ig isotype detection mAbs (Becton Dickinson). Cytokine ELISA also used the sandwich format, with capture and detection antibodies purchased from Becton Dickinson. ELISAs were performed according to Becton Dickinson's ELISA protocol. The IL-13 ELISA was purchased from R&D Systems.
Results
Generation of Mice Double-deficient for IL-4 and IL-13.
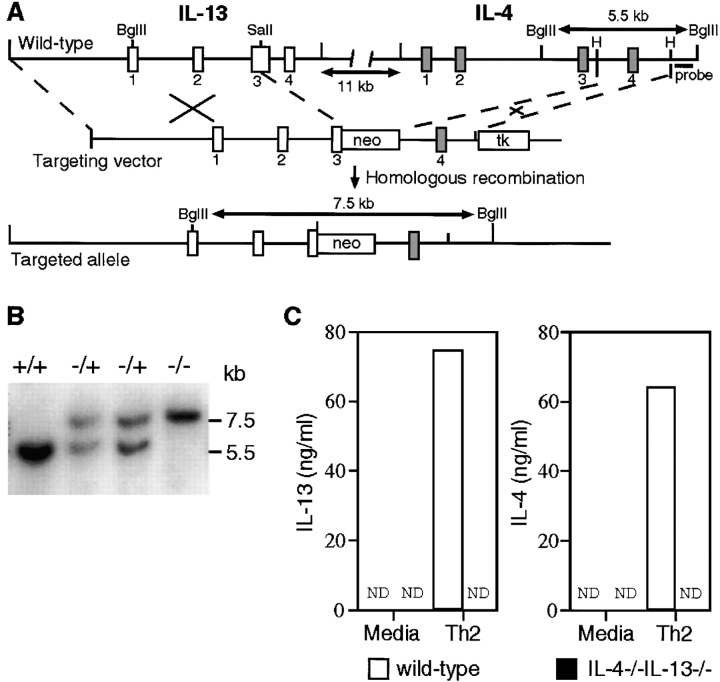
Since the IL-4 and IL-13 genes are closely linked on mouse chromosome 11 (∼11 kb apart), it would be impractical to attempt to mate IL-13–deficient mice with IL-4–deficient mice in order to generate a crossover event between these two genes. Therefore, we have used a single targeting construct to exploit their juxtaposition and simultaneously target both genes. The mouse IL-4 and IL-13 genes are transcribed in the same orientation, with the IL-13 gene lying upstream of the IL-4 gene (A.N.J. McKenzie, unpublished data). The targeting vector comprised a 5′ arm of homology derived from the IL-13 gene, and a 3′ arm of homology derived from the IL-4 gene positioned on either side of the neomycin resistance cassette (Fig. 1 A). The resulting homologous recombination event excises ∼15 kb of intervening sequence. Genotyping of wild-type (IL-4+/+ IL-13+/+), heterozygous (IL-4+/−IL-13+/−), and homozygous null (IL-4−/−IL-13−/−) mice is shown in Fig. 1 B. The IL-4−/−IL-13−/− mice were healthy and displayed no overt phenotypic abnormalities. Analysis of the IL-4−/−IL-13−/− mice failed to detect IL-4 or IL-13 RNA transcripts from activated lymphocytes using reverse transcriptase PCR assays (data not shown), and ELISAs also failed to identify IL-4 or IL-13 protein in supernatants from spleen-derived CD4+ T cells cultured under Th2 cell differentiation conditions (Fig. 1 C). To determine the relative in vivo roles of IL-4 and IL-13, we have assessed the immune responses of the IL-4−/−IL-13−/− animals, in combination with IL-4–deficient mice (IL-4−/−) and IL-13–deficient mice (IL-13−/−), to a range of immunological challenges that normally provoke a Th2 phenotype.
Figure 1.
Simultaneous inactivation of the IL-4 and IL-13 genes by homologous recombination. (A) The structure of the two loci, the single targeting vector, and the predicted homologous recombination event are shown. Targeted disruption results in the deletion of the 15-kb region extending from within exon 3 of the IL-13 locus (white boxes) to a HindIII site in intron 3 of the IL-4 locus (gray boxes). H, HindIII; neo, neomycin resistance cassette; tk, thymidine kinase cassette. (B) Southern blotting of F2 tail genomic DNA. The indicated probe detects a 5.5-kb BglII fragment in the wild-type IL-4 gene and a 7.5-kb fragment as a result of the correct homologous recombination event between the IL-4 and IL-13 loci. (C) Analysis of IL-4 and IL-13 expression by CD4+ T cells. CD4+ T cells purified from two pooled spleens from two wild-type or two IL-4−/− IL-13−/− mice were stimulated under conditions promoting the expression of Th2 cytokines. Supernatants were analyzed by ELISA. Data are representative of three repeat experiments. ND, not detected.
Induction of Synchronous Pulmonary Granuloma Formation Using S. mansoni Eggs Is Abolished Only in the Combined Absence of IL-4 and IL-13.
To determine the relative in vivo contribution of IL-4 and IL-13 in a Th2 cytokine–mediated inflammatory response, we used a model system in which synchronous pulmonary granuloma formation is induced around S. mansoni eggs (27). In this model, a cellular granulomatous response develops around parasite eggs that lodge in the lungs after their intravenous injection into mice. This inflammatory response is characterized by the high-level expression of Th2 cytokines (28).
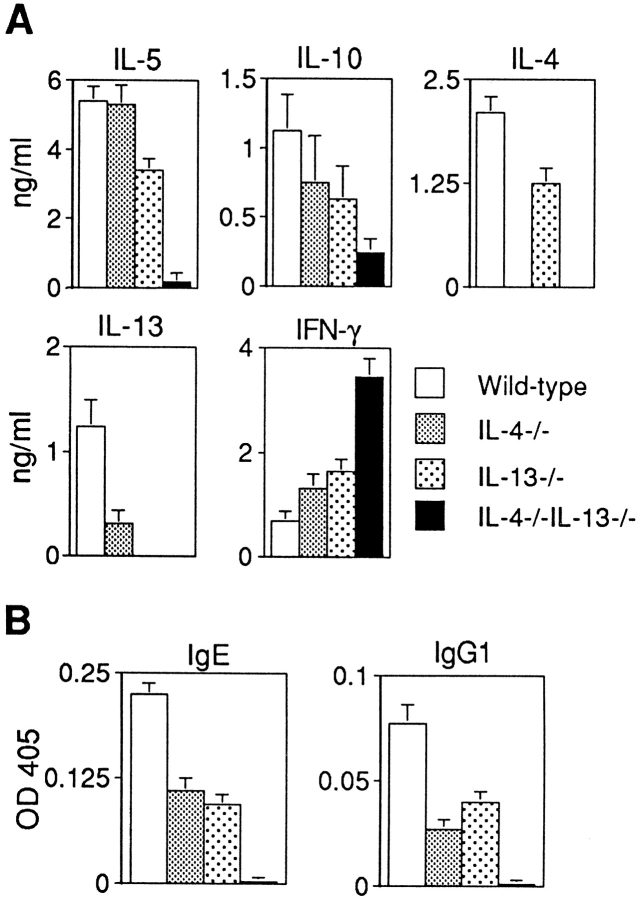
We observed a profound inability of IL-4−/−IL-13−/− mice to develop the Th2 cell–mediated inflammatory response normally generated during synchronous pulmonary granuloma formation in response to schistosome egg immunization (Fig. 2 A). This was in marked contrast to the response generated by wild-type mice in which large eosinophil-rich granulomas formed around eggs lodged in the lung alveoli (Fig. 2, A and B). Furthermore, this wild-type response was associated with expression of IL-4, IL-5, IL-10, and IL-13 by cells recovered from the draining mediastinal lymph nodes of the lung (Fig. 3 A) and the generation of increased titers of egg antigen–specific IgE and IgG1 (Fig. 3 B). By contrast, the IL-4−/−IL-13−/− mice did not develop granulomas (Fig. 2 A), and eosinophil infiltration was virtually absent, although some monocyte infiltration was evident (Fig. 2 B). Correlating with this, schistosome egg–challenged IL-4/13–deficient mice also expressed very low levels of IL-5 (Fig. 3 A), the primary cytokine in the induction of eosinophil differentiation (29). IL-10 production was also impaired, but there was a significant increase in the expression of IFN-γ (Fig. 3 A). Antigen-specific IgE was also not detected in the serum of IL-4−/−IL-13−/− mice, and antigen-specific IgG1 was virtually absent (Fig. 3 B).
Figure 2.
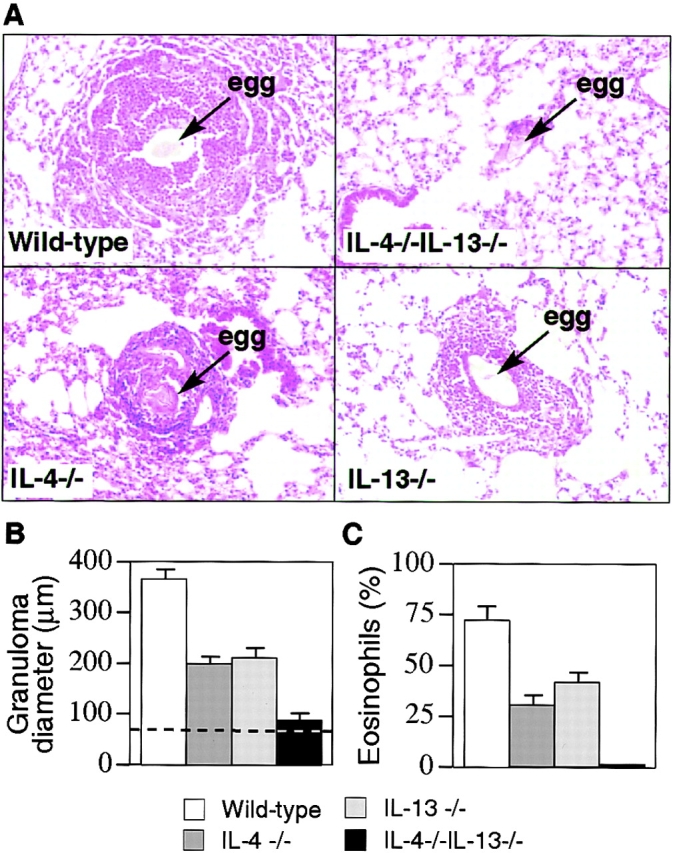
Analysis of pulmonary inflammatory response. (A) Morphological analysis of granuloma formation in wild-type, IL-4−/−, IL-13−/−, and IL-4−/−IL-13−/− mice. Cohorts of six mice were sensitized to S. mansoni eggs by intraperitoneal injection of 5,000 live eggs. After 15 d, these mice were injected intravenously with 5,000 eggs to induce synchronous pulmonary granuloma. Mice were killed 15 d later. Lung sections were stained with H&E. Original magnification: ×100. (B) Determination of granuloma diameters in immunized mice. Lung sections were stained with H&E, and at least 100 individual granulomas were measured per group. Dashed line indicates the mean diameter of an egg. (C) Eosinophil counts. After granuloma formation, the percent composition of eosinophils in the granulomas was determined from Giemsa-stained lung sections. Representative data from two repeat experiments (six to eight mice per group). Data are presented as means ± SD.
Figure 3.
Cytokine and Ig responses to pulmonary challenge. (A) Cytokine responses from activated lymph node cells. Draining mediastinal lymph node cells were stimulated with soluble egg antigen, and supernatants were assayed for cytokines by ELISA. (B) Antigen-specific serum IgE and IgG1 after granuloma formation. Egg antigen–specific antibody isotypes were assayed by ELISA. Representative data from two repeat experiments using six to eight mice per group. Data are presented as means ± SD.
We also assessed how IL-4–deficient mice and IL-13– deficient mice responded to schistosome egg challenge. Although granuloma size and eosinophil infiltration were impaired in both single cytokine–deficient IL-4−/− and IL-13−/− mice, compared with wild-type (P < 0.001; Fig. 2, A–C), they continued to develop a Th2 response with the expression of IL-5 and the infiltration of eosinophils (Fig. 2 C and Fig. 3 A). Significantly, the granuloma response observed in both of the single cytokine gene–disruption mouse lines was substantially greater than that formed in the doubly targeted mice (Fig. 2, A and B). It is also noteworthy that both the IL-4−/− and IL-13−/− mice continued to produce egg antigen– specific IgE and IgG1, although the levels of these Ig isotypes were reduced relative to wild-type mice (Fig. 3 B).
Thus, the pulmonary granulomatous model demonstrates that mice deficient in either IL-4 or IL-13 are still capable of mounting a Th2-like response, albeit reduced compared with wild-type mice, but that simultaneous disruption of IL-4 and IL-13 results in abrogation of the granulomatous response. This demonstrates that IL-4 and IL-13 perform compensatory roles that are essential in combination for the successful development of a Th2 cell–driven inflammatory response, and that in their absence the response becomes dominated by the Th1 cell cytokine IFN-γ (Fig. 3 A).
Expulsion of the Gastrointestinal Parasitic Nematode N. brasiliensis Is Further Impaired in the Combined Absence of IL-4 and IL-13.
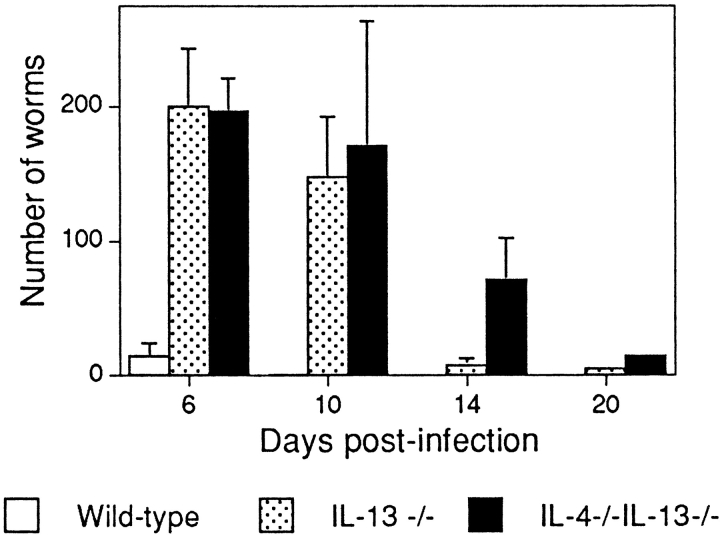
Immunological responses to gastrointestinal parasitic worm infections are also characterized by the expression of Th2 cytokines (30). Using N. brasiliensis as a model, recent studies have shown that although IL-4–deficient mice expel these worms almost as efficiently as wild-type animals (16, 17), IL-13–deficient mice display impaired expulsion kinetics (16). We have infected the IL-4−/− IL-13−/− mice with N. brasiliensis and compared the kinetics of worm expulsion with that of wild-type and IL-13−/− animals (Fig. 4). As expected, the wild-type mice expelled their worms rapidly, with complete expulsion by day 10 post- infection (p.i.), whereas the expulsion of worms from the IL-13−/− mice was delayed beyond 10 d (Fig. 4). Significantly, the combined ablation of both cytokines further delayed the expulsion of N. brasiliensis, with substantially more worms present at day 14 p.i., even when compared with IL-13−/− animals (Fig. 4). Thus, although IL-13 is apparently the primary cytokine regulating N. brasiliensis expulsion, IL-4 does play an additional role in this process. Interestingly, we have also found that treatment of IL-13−/− mice with recombinant IL-4 results in the rapid expulsion of worms from these animals (data not shown). Thus, once again there is a dual effect of removing both IL-4 and IL-13, indicating that these cytokines act in combination to initiate a potent Th2 response.
Figure 4.
Analysis of infection with N. brasiliensis. Determination of worm burdens. Cohorts of five mice were infected with 400 viable third-stage N. brasiliensis larvae and killed at the times indicated to obtain intestinal worm counts. Representative data from two repeat experiments. Data are presented as means ± SD.
IL-4/13–deficient Mice Fail to Express IgE, But Exhibit Delayed IL-5 Expression and Eosinophilia in Response to N. brasiliensis Infection.
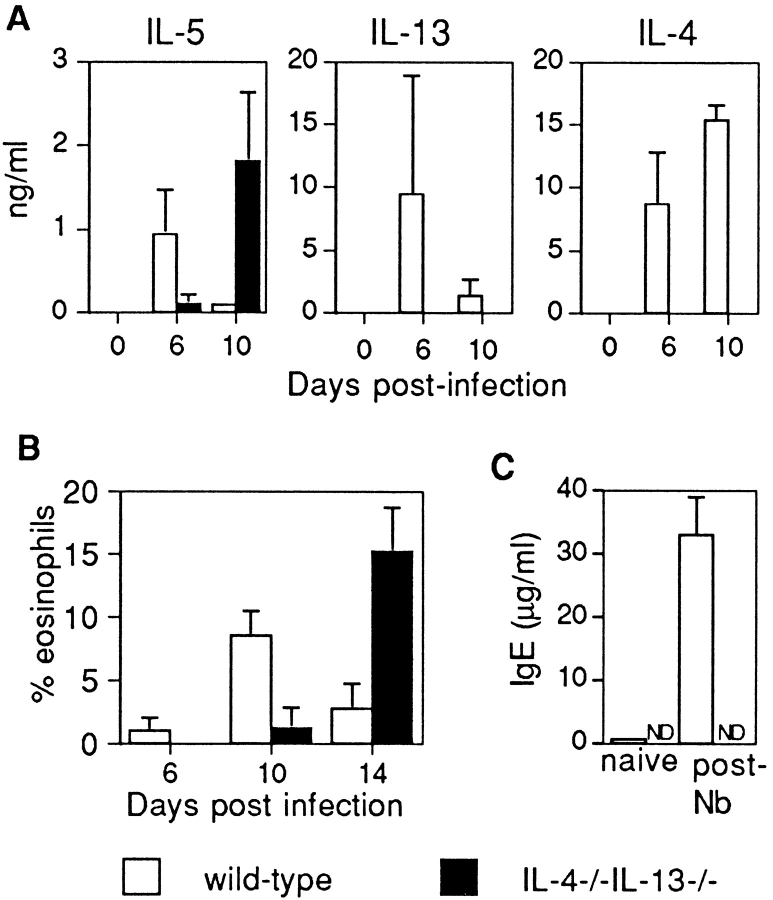
The normal immune response to N. brasiliensis is characterized by Th2 cytokine expression, elevated levels of IgE expression, and eosinophilia (Fig. 5, A–C). Indeed, wild-type animals developed a profound eosinophilia by day 10 p.i. (Fig. 5 B), and total serum IgE had increased by ∼100-fold by day 14 p.i. (Fig. 5 C). Unexpectedly, we found that IL-5 expression and eosinophilia were still induced in the IL-4−/−IL-13−/− mice p.i. with N. brasiliensis, although their production was significantly delayed (Fig. 5, A and B). However, we failed to detect IgE expression in the serum of the IL-4−/−IL-13−/− mice (Fig. 5 C), and levels of worm antigen–specific IgG1 were also undetectable (data not shown). Thus, we conclude that although IL-4 and IL-13 are required for the rapid initiation of Th2-like responses, alternative IL-4/IL-13–independent processes compensate for their loss and facilitate the expression of IL-5 and the coordinate development of eosinophilia.
Figure 5.
Analysis of cytokines, eosinophilia, and IgE in response to N. brasiliensis infection. (A) Cytokine expression from Con A–stimulated mesenteric lymph node cells from wild-type and IL-4−/−IL-13−/− animals after infection with N. brasiliensis. Lymph node cells (2 × 106 cells/ml) were cultured for 24 h in the presence of Con A (2 μg/ml). Supernatants were analyzed by cytokine ELISA. Representative data from two repeat experiments are shown. (B) Blood eosinophilia after N. brasiliensis infection. Peripheral blood was sampled at the times indicated, and the percentage of eosinophils was determined from blood smears stained with Giemsa. (C) Total serum IgE expression from wild-type and IL-4−/−IL-13−/− animals pre- and post- (day 14) infection with N. brasiliensis (Nb) assessed by ELISA. ND, not detected. Representative data from two repeat experiments using five mice per group. Data are presented as means ± SD.
Antigen-specific Antibody Responses Are Biased away from IgE and IgG1 Production and towards IgG2a and IgG2b in IL-4/13–deficient Mice.
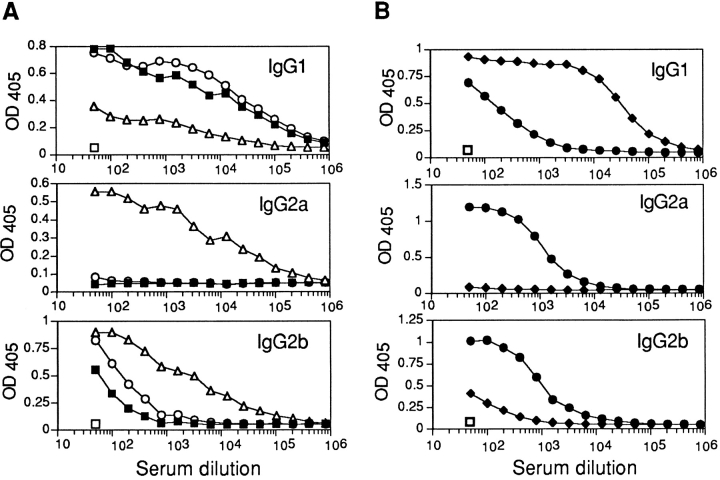
Analysis of total serum Ig isotypes demonstrated that like the IL-4–deficient animals, mice lacking both IL-4 and IL-13 had 10–50-fold lower levels of serum IgG1 and undetectable levels of IgE, whereas other serum isotypes remained similar to wild-type (data not shown). However, due to the specific roles of Th2 cell cytokines in regulating humoral immune responses, we have also analyzed the antigen-specific Ig responses of wild-type, IL-4−/−, IL-13−/−, and IL-4−/−IL-13−/− mice immunized with the protein antigen OVA complexed to alum. As shown in Fig. 6 A, the IL-4−/−IL-13−/− animals are severely impaired in their ability to mount an IgG1 response, a deficiency also apparent in IL-4–deficient animals (Fig. 6 B). By contrast, IL-13−/− animals develop a normal IgG1 response to antigen challenge (Fig. 6 A). Thus, the regulation of antigen-specific IgG1 responses appears to require IL-4, but not IL-13. However, cumulative roles for IL-4 and IL-13 in the generation of the antibody responses are suggested by the highly biased IgG2a and IgG2b responses evoked upon immunization of the IL-4−/−IL-13−/− animals (Fig. 6 A). These isotype profiles are typical of a Th1 response and are significantly elevated.
Figure 6.
Antigen-specific Ig response to OVA after immunization. (A) IL-13–deficient (○) and IL-4/13–deficient (▵) mice (shown against preimmune [□] and wild-type [▪] mice); (B) IL-4–deficient (•) mice (shown against preimmune [□] and C57BL/6 [♦] mice). Cohorts of four animals were immunized intraperitoneally with 100 μg of OVA adsorbed to alum with subsequent boost injections of OVA/alum after 10 and 20 d. Serum samples were assayed by ELISA for Ig isotypes. Representative data from two repeat experiments are shown.
Discussion
The generation and analysis of IL-4/13–deficient mice has enabled us to demonstrate conclusively that these cytokines cooperate in the development of Th2 cell–mediated immune responses. Due to the close proximity of the IL-4 and IL-13 genes, we used a single vector targeting strategy to disrupt the expression of both cytokine genes, thereby allowing us to dissect the potential compensatory roles played by these cytokines. To define their functional importance, we have used several model antigenic challenges that are normally characterized by Th2-like responses.
We have found that the Th2-like characteristics of synchronous pulmonary granuloma formation, including eosinophil infiltration and IgE production, are only abolished when both IL-4 and IL-13 are removed, whereas disruption of each individual cytokine resulted in only partial abrogation of the response. Thus, our data demonstrate for the first time that IL-13 also plays a significant role in granuloma formation and, since neither cytokine is fully able to compensate for the absence of the other, they illustrate a clear functional specificity for IL-4 and IL-13 in the development of the response. Even more significantly, the combined disruption of IL-4 and IL-13 results in the almost complete abolition of the Th2-driven granuloma response, demonstrating the additive roles of these two cytokines in the generation of Th2 responses. In the double-deficient mice, the virtual absence of eosinophil infiltration, antigen-specific IgE, and antigen-specific IgG1 is replaced by enhanced expression of IFN-γ and the upregulation of IgG2a (data not shown), both indicative of Th1-like responses. These data generated using the ligand-deficient mice also clarify any ambiguity raised by the results that have been generated using signal transducer and activator of transcription (Stat)6-deficient and IL-4Rα–deficient mice in which pulmonary granuloma responses were reported to be more significantly reduced than in the IL-4–deficient mice (31–34).
While previous studies have demonstrated that IL-13 plays a unique and dominant role in the efficient expulsion of N. brasiliensis, the double-deficient mice have enabled us to identify an additive role for IL-4 in this process. These data are supportive of studies in which the exogenous administration of IL-4 induced worm expulsion (35) and our own experiments showing that exogenous IL-4 can induce rapid expulsion of N. brasiliensis from the intestines of IL-13–deficient mice (data not shown). However, since IL-4–deficient mice expel N. brasiliensis worms normally (16, 17), the most significant defect in the clearance of these parasites is the absence of IL-13.
Interestingly, although Th2 responses to N. brasiliensis infection are significantly delayed in the IL-4/13–deficient mice, our experiments have also identified alternative, IL-4– and IL-13–independent mechanisms for IL-5 production and eosinophilia in IL-4/13–deficient mice. Thus, even in the absence of IL-4 and IL-13, a response can develop Th2-like characteristics. The mechanism underlying the development of IL-5–producing cells is unclear. Such cells have been recognized in IL-4–deficient mice (2) and IL-4Rα– deficient mice (3) infected with N. brasiliensis, but normally only constitute a minor population when evaluated at 7 d p.i. This is also the case in the IL-4/13–deficient mice, where IL-5 expression at day 6 p.i. is very low. However, IL-5 levels in the double-deficient mice become highly elevated by day 10 p.i., indicating that, although significantly delayed, IL-5–producing cells can receive sufficient costimulation to expand in the absence of IL-4 and IL-13 signals. Since this phenomenon was not observed in the lung response to schistosome eggs, it may indicate a unique feature of the intestinal response to nematode infection.
To date, IgE responses by IL-4/13–deficient mice to a range of antigenic challenges have remained below the level of detection in ELISA assays, although IgE responses were detected in individual cytokine–deficient mice. These findings support previous studies which have identified IL-13 and IL-4 as alternative cytokines in the regulation of IgE expression (1, 2, 7, 36). Furthermore, in the combined absence of IL-4 and IL-13, the Ig response becomes more characteristic of a Th1 cell response, illustrating the additive roles played by these cytokines in the regulation of Ig expression. This was clearly demonstrated by assessing the antigen-specific Ig isotype response generated against protein antigen OVA immunization in the presence of the Th2- inducing adjuvant alum. Although the expression of IgG2a and IgG2b was only minimally enhanced after the individual disruption of IL-13, there was a significant increase when both IL-13 and IL-4 are cotargeted, indicating that at least in the regulation of Ig expression IL-4 appears able to compensate more effectively for the loss of IL-13 than IL-13 does after the disruption of IL-4.
The development and analysis of mice with a combined deficiency for IL-4 and IL-13 expression have clearly demonstrated that IL-4 does not act in isolation in the development of Th2 cell responses. It is noteworthy that IL-4 and IL-13 share the α chain of the IL-4 receptor (37) and consequently signal through related pathways, including Stat6 (38). Our results now explain why IL-4Rα–deficient mice and Stat6-deficient mice display more severe phenotypic differences than were reported for the IL-4–deficient mice (3–5, 17). It is apparent that IL-4 and IL-13 act in conjunction to ensure the rapid onset of a Th2-like response and that in their combined absence the vestiges of the Th2 response are abolished or significantly delayed.
Th2 cell–driven responses, particularly IgE and eosinophilia, are instrumental in disease processes, including allergies, asthma, and helminth infections (29, 30, 39, 40). Indeed, recent studies have identified that IL-13 is also a major mediator in experimental models of allergic asthma (41, 42). Our findings have obvious implications for the development of therapeutic strategies for cytokine/anticytokine modulation of immune reactions. Thus, to control the initiation of Th2 cell responses and the abolition of IgE production, it appears that it will be necessary to inactivate both IL-4 and IL-13, and that even then default mechanisms may allow the development of eosinophilia. The IL-4/ 13–deficient mice will prove an important tool for dissecting the intimate interaction of these cytokines in numerous disease states.
Acknowledgments
The authors would like to thank Sarah Bell, Helen Jolin, David Matthews, and Michael Townsend for critical reading of this manuscript, and Emma Richardson for technical assistance.
Abbreviations used in this paper
- ES
embryonic stem
- H&E
hematoxylin and eosin
- p.i.
post-infection
- Stat
signal transducer and activator of transcription
Footnotes
Address correspondence and requests for materials to Andrew N.J. McKenzie, MRC Laboratory of Molecular Biology, Hills Road, Cambridge, CB2 2QH, UK. Phone: 44-1223-402377; Fax: 44-1223-412178; E-mail: anm@mrc-lmb.cam.ac.uk
References
- 1.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4-deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 2.Kopf M, Le Gros G, Bachmann M, Lamers C, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 3.Barner M, Mohrs M, Brombacher F, Kopf M. Differences between IL-4Rα-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr Biol. 1998;8:669–672. doi: 10.1016/s0960-9822(98)70256-8. [DOI] [PubMed] [Google Scholar]
- 4.Shimoda K, van Deursen J, Sangster M, Sarawar S, Carson R, Tripp R, Chu C, Quelle F, Nosaka T, Vignali D, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 5.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi N, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan M, Schindler U, Smiley S, Grusby M. Stat6 is required for mediating responses to IL-4 and for the development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 7.McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, Murray R, McKenzie ANJ. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9:423–432. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 8.Brown K, Zurawski S, Mosmann T, Zurawski G. A family of small inducible proteins secreted by leukocytes are members of a new super-family that includes leukocyte and fibroblast-derived inflammatory agents, growth factors and indicators of various activation processes. J Immunol. 1989;142:679–687. [PubMed] [Google Scholar]
- 9.Mosmann T, Coffman R. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;5:429–459. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 10.McKenzie A, Culpepper J, de Waal R, Malefyt, Briere F, Punnonen J, Aversa G, Sato A, Dang W, Cocks B, Menon S, et al. Interleukin-13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proc Natl Acad Sci USA. 1993;90:3735–3739. doi: 10.1073/pnas.90.8.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burd PR, Thompson WC, Max EE, Mills FC. Activated mast cells produce interleukin 13. J Exp Med. 1995;181:1373–1380. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Punnonen J, Aversa G, Cocks B, McKenzie A, Menon S, Zurawski G, de Waal R, Malefyt, de Vries J. Interleukin-13 induces interleukin-4-independent IgG4 and IgE synthesis and CD23 expression by human B-cells. Proc Natl Acad Sci USA. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doherty M, Kastelein K, Menon S, Andrade S, Coffman R. Modulation of murine macrophage function by IL-13. J Immunol. 1993;151:7151–7160. [PubMed] [Google Scholar]
- 14.de Waal Malefyt, R., C. Figdor, R. Huijbens, S. Mohan-Peterson, B. Bennett, J. Culpepper, W. Dang, G. Zurawski, and J. de Vries. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. J Immunol. 1993;151:6370–6381. [PubMed] [Google Scholar]
- 15.Yokota T, Otsuka T, Mosmann T, Bachereau J, DeFrance T, Blanchard D, de Vries J, Lee F, Arai K. Isolation and characterization of a human interleukin cDNA clone, homologous to B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities. Proc Natl Acad Sci USA. 1986;83:5894–5898. doi: 10.1073/pnas.83.16.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenzie G, Bancroft A, Grencis R, McKenzie A. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol. 1998;8:339–342. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- 17.Urban J, Noben-Trauth N, Donaldson D, Madden K, Morris S, Collins M, Finkelman F. IL-13, IL-4R and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. . Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 18.Zurawski S, Vega F, Huyghe B, Zurawski G. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO (Eur Mol Biol Organ) J. 1993;12:3899–3905. doi: 10.1002/j.1460-2075.1993.tb05927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilton D, Zhang J-G, Metcalf D, Alexander W, Nicola N, Wilson T. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc Natl Acad Sci USA. 1996;93:497–501. doi: 10.1073/pnas.93.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aman J, Tayebi N, Obiri N, Puri R, Modi W, Leonard W. cDNA cloning and characterization of the human interleukin 13 receptor α chain. J Biol Chem. 1996;271:29265–29270. doi: 10.1074/jbc.271.46.29265. [DOI] [PubMed] [Google Scholar]
- 21.Obiri N, Debinski W, Leonard W, Puri R. Receptor for interleukin 13: interaction with interleukin 4 by a mechanism that does not involve the common gamma chain shared by receptors for interleukins 2, 4, 7, 9 and 15. J Biol Chem. 1995;270:8797–8804. doi: 10.1074/jbc.270.15.8797. [DOI] [PubMed] [Google Scholar]
- 22.Miloux B, Laurent P, Bonnin O, Lupker J, Caput D, Vita N, Ferrara P. Cloning of the human IL-13Rα1 chain and reconstitution with the IL-4Rα of a functional IL-4/IL-13 receptor complex. FEBS Lett. 1996;401:163–166. doi: 10.1016/s0014-5793(96)01462-7. [DOI] [PubMed] [Google Scholar]
- 23.McKenzie A, Li X, Largaespada D, Sato A, Atsushi K, Zurawski S, Doyle E, Milatovich A, Francke U, Copeland N, et al. Structural comparison and chromosomal location of the human and mouse IL-13 genes. J Immunol. 1993;150:5436–5444. [PubMed] [Google Scholar]
- 24.Frazer KA, Ueda Y, Zhu Y, Gifford VR, Garofalo MR, Mohandas N, Martin CH, Palazzolo MJ, Cheng JF, Rubin EM. Computational and biological analysis of 680 kb of DNA sequence from the human 5q31 cytokine gene cluster. Genome Res. 1997;7:495–512. doi: 10.1101/gr.7.5.495. [DOI] [PubMed] [Google Scholar]
- 25.Fallon PG, Smith P, Dunne DW. Type 1 and type 2 cytokine-producing mouse CD4+ and CD8+ T cells in acute Schistosoma mansoniinfection. Eur J Immunol. 1998;28:1408–1416. doi: 10.1002/(SICI)1521-4141(199804)28:04<1408::AID-IMMU1408>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 26.Fallon PG, Dunne DW. Tolerization of mice to Schistosoma mansoniegg antigens causes elevated type 1 and type 2 cytokine responses and increased mortality in acute infection. J Immunol. 1999;162:4122–4132. [PubMed] [Google Scholar]
- 27.Warren KS, Domingo EO. Granuloma formation around Schistosoma mansoni, S. haematobium, and S. japonicumeggs. Am J Trop Med Hyg. 1970;19:291–304. doi: 10.4269/ajtmh.1970.19.292. [DOI] [PubMed] [Google Scholar]
- 28.Wynn TA, Eltoum I, Cheever AW, Lewis FA, Gause WC, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. . J Immunol. 1993;151:1430–1440. [PubMed] [Google Scholar]
- 29.Sanderson CJ. IL-5, eosinophils, and disease. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- 30.Finkelman F, Shea-Donohue T, Goldhill J, Sullivan C, Morris S, Madden K, Gause W, Urban J. Cytokine regulation of host defence against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 31.Jankovic, D., M.C. Kullberg, P. Caspar, A.W. Cheever, N. Noben-Trauth, and A. Sher. 1998. Induction of egg pathology during Schistosoma mansoni infection requires IL-4 receptor, but not IL-4 expression. Second Woods Hole Immunoparasitology Meeting Summary Book. 2:52 (Abstr.)
- 32.Kaplan MH, Whitfield JR, Boros DL, Grusby MJ. Th2 cells are required for the Schistosoma mansoniegg-induced granulomatous response. J Immunol. 1998;160:1850–1856. [PubMed] [Google Scholar]
- 33.Pearce EJ, Cheever A, Leonard S, Covalesky, Fernandez-Botran R, Kohler G, Kopf M. Schistosoma mansoniin IL-4-deficient mice. Int Immunol. 1996;8:435–444. doi: 10.1093/intimm/8.4.435. [DOI] [PubMed] [Google Scholar]
- 34.Metwali A, Elliott D, Blum AM, Li J, Sandor ML, Lynch R, Noben-Trauth N, Weinstock JV. The granulomatous response in Schistosomiasis mansonidoes not switch to Th1 in IL-4-deficient C57BL/6 mice. J Immunol. 1996;157:4546–4553. [PubMed] [Google Scholar]
- 35.Urban JF, Maliszewski CR, Madden KB, Katona IM, Finkelman FD. Interleukin-4 treatment can cure established gastrointestinal nematode infections in immunocompetent and immunodeficient mice. J Immunol. 1995;154:4675–4684. [PubMed] [Google Scholar]
- 36.Emson CL, Bell SE, Jones A, Wisden W, McKenzie ANJ. Interleukin (IL)-4–independent induction of immunoglobulin (Ig)E, and perturbation of T cell development in transgenic mice expressing IL-13. J Exp Med. 1998;188:399–404. doi: 10.1084/jem.188.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zurawski S, Chomarat P, Djossou O, Bidaud C, McKenzie A, Miossec P, Banchereau J, Zurawski G. The primary binding subunit of the human interleukin-4 receptor is also a component of the interleukin-13 receptor. J Biol Chem. 1995;270:13869–13878. doi: 10.1074/jbc.270.23.13869. [DOI] [PubMed] [Google Scholar]
- 38.Lin J-X, Migone T-S, Tsang M, Friedmann M, Weatherbee J, Zhou L, Yamauchi A, Bloom E, Meitz J, John S, Leonard W. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13 and IL-5. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 39.Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 40.Marsh DG, Neely JD, Breazeale DR, Ghosh B, Freidhoff LR, Ehrlich-Kautzky E, Schou C, Krishnaswamy G, Beaty TH. Linkage analysis of IL4 and other chromosome 5q31.1 markers and total serum immunoglobulin E concentrations. Science. 1994;264:1152–1156. doi: 10.1126/science.8178175. [DOI] [PubMed] [Google Scholar]
- 41.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 42.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]