Abstract
Transgenic mice carrying a 380-kb region of the human immunoglobulin (Ig) λ light (L) chain locus in germline configuration were created. The introduced translocus on a yeast artificial chromosome (YAC) accommodates the most proximal Igλ variable region (V) gene cluster, including 15 Vλ genes that contribute to >60% of λ L chains in humans, all Jλ-Cλ segments, and the 3′ enhancer. HuIgλYAC mice were bred with animals in which mouse Igκ production was silenced by gene targeting. In the κ−/− background, human Igλ was expressed by ∼84% of splenic B cells. A striking result was that human Igλ was also produced at high levels in mice with normal κ locus. Analysis of bone marrow cells showed that human Igλ and mouse Igκ were expressed at similar levels throughout B cell development, suggesting that the Igλ translocus and the endogenous κ locus rearrange independently and with equal efficiency at the same developmental stage. This is further supported by the finding that in hybridomas expressing human Igλ the endogenous L chain loci were in germline configuration. The presence of somatic hypermutation in the human Vλ genes indicated that the Igλ-expressing cells function normally. The finding that human λ genes can be utilized with similar efficiency in mice and humans implies that L chain expression is critically dependent on the configuration of the locus.
Keywords: human Igλ translocus, light chain expression levels, pre-B cell activation, V gene usage, hypermutation
The light chain component of the Ig protein is encoded by two separate loci, Igκ and Igλ. The proportion of antibodies containing κ or λ L chains varies considerably between different species (1–3); in mice the κ/λ ratio is 95:5, compared with 60:40 in humans. Two models exist to account for the dominance of Igκ expression in the mouse. From the observations that murine Igλ-producing myelomas have rearranged κ L chain genes, whereas Igκ-producing cells have the λ L chain locus in germline configuration, it was proposed initially that κ rearrangement must occur before λ rearrangement can begin (4, 5). Although the same observation applies for human B cells, the proportions of κ- and λ-producing cells are similar (4), suggesting that other factors are involved. The second proposal is that κ and λ loci are equally available for rearrangement at the same time, but the mouse κ locus is more efficient at engaging the rearrangement process (for review see reference 6). The occasional finding of cells with rearranged λ and the κ locus in germline configuration may support this (5, 7, 8). Any influence of antigen selection on the biased κ/λ ratio is discounted by the finding that the ratio is similar in fetal liver and in cells that have not encountered antigen (9–13).
L chain V-J rearrangement occurs at the transition from pre-B-II to immature B cells, where the surrogate L chain associated with membrane Igμ is replaced by κ or λ (14). Although the timing of L chain rearrangement is essentially defined, the processes that activate L chain locus rearrangement are not fully understood. From locus-silencing experiments, it is apparent that κ rearrangement is not a prerequisite for λ recombination (15), but instead that κ and λ rearrangements are independent events (16), the activation of which may be affected by differences in the strength of the respective enhancers. Targeted deletion of the κ 3′ enhancer in transgenic mice showed that this region is not essential for κ locus rearrangement or expression but is required for establishing the κ/λ ratio (17). A region that may regulate the accessibility of the human λ locus has been identified ∼10 kb downstream of Cλ7 (18, 19). Functional analysis using reporter gene assays identified a core enhancer region flanked by elements that can drastically reduce enhancer activity in pre-B cells (18). Although transfection studies showed that the κ and λ 3′ enhancer regions appear to be functionally equivalent, the core enhancer motifs are flanked by functional sequences that are remarkably dissimilar. The human Igλ locus on chromosome 22q11.2 is 1.1 Mb in size and typically contains 70 Vλ genes and 7 Jλ-Cλ gene segments (20, 21). Approximately half of the Vλ genes and Jλ-Cλ1, 2, 3, and 7 are regarded as functional. The Vλ genes are organized in three clusters, with the members of a particular V gene family contained within the same cluster. There are 10 Vλ gene families, with the largest, VλIII, having 23 members. In human peripheral blood lymphocytes, V gene segments of families I, II, and III from the J-C proximal cluster A are preferentially rearranged, with the contribution of the 2a2 Vλ segment (2–14 using a position-based nomenclature; reference 22) being unusually high (23). All λ gene segments have the same polarity that allows deletional rearrangement (24). The diversity of the Igλ repertoire is provided mainly by Vλ-Jλ combination. Additional CDR3 diversity due to N (nonencoded)1 or P (palindromic) nucleotide additions at the V to J junction is present in human sequences, although not as extensively as in IgH rearrangement, but is absent in sequences from mice (25–28), where the TdT (terminal deoxyribonucleotide transferase) activity is downregulated at the time of L chain rearrangement.
Here we have introduced a 410-kb yeast artificial chromosome (YAC), which contains most of the Vλ genes of cluster A and all the Jλ-Cλ segments in germline configuration, into mice that have one or both endogenous Igκ alleles disrupted. The translocus shows high expression in both backgrounds, and is able to compete equally with the endogenous mouse κ locus.
Materials and Methods
The HuIgλYAC, Introduction into Embryonic Stem Cells, and Derivation of Transgenic Mice.
The 410-kb HuIgλYAC, accommodating a 380-kb region (Vλ-JCλ) of the human λ L chain locus with V, J, and C genes in germline configuration, was constructed as previously described (29). To allow selection, two copies of the neomycin resistance gene (NEO r) were site-specifically integrated into the ampicillin gene on the left (centromeric) YAC arm. YAC-containing yeast cells were fused with HM-1 embryonic stem (ES) cells (a gift from D. Melton, Department of Pathology, University Medical School, Edinburgh, UK), as previously described (30), and G418-resistant colonies were picked and analyzed 2–3 wk after protoplast fusion. ES cells containing a complete HuIgλYAC copy, confirmed by Southern hybridization, were used for blastocyst injection to produce chimeric animals (31). Breeding of chimeric animals with BALB/c mice resulted in germline transmission. Further breeding with κ−/− mice (32) established the lines for analysis.
Southern Blot Analysis.
Conventional DNA was obtained (33) or high molecular weight DNA was prepared in agarose blocks (34). For the preparation of testis DNA, tissues were homogenized and passed through 70 μM nylon mesh. Pulsed-field gel electrophoresis (PFGE) conditions to separate in the 50–900 kb range were 1% agarose, 180 V, 70 s switch time, and 30 h running time at 3.5°C. Hybridization probes were Cλ2+3 and the left YAC arm probe (LA) comprising LYS2 (29).
Hybridoma Production and ELISA Assay.
Hybridomas were obtained from 3-mo-old HuIgλYAC/κ+/− animals by fusion of splenocytes with NS0 myeloma cells (35). After fusion, cells were plated on 96-well plates such as to obtain single clones. Human and mouse antibody production was determined in sandwich ELISA assays (36) on MaxiSorp plates (Nalge Nunc, Denmark). For the detection of human or mouse Igλ, coating reagents were a 1:500 dilution of anti–human λ L chain mAb HP-6054 (L 6522; Sigma Chemical Co.) or a 1:500 dilution of the 2.3 mg/ml rat anti–mouse λ mAB (L 2280; Sigma Chemical Co.), respectively. Respective binding was detected with biotinylated antibodies: polyclonal anti–human λ (B 0900; Sigma Chemical Co.), a 1:1,000 dilution of polyclonal anti–mouse λ (RPN 1178; Amersham International) or rat anti–mouse Igλ (No. 021172D; PharMingen) followed by streptavidin-conjugated horseradish peroxidase (Amersham International). Mouse IgG2aλ myeloma protein from HOPC1 (M 6034; Sigma Chemical Co.) and human serum IgGλ (I 4014; Sigma Chemical Co.) were used to standardize the assays. To determine mouse κ L chain levels, plates were coated with a 1:1,000 dilution of rat anti–mouse κ, clone EM34.1 (K 2132; Sigma Chemical Co.), and bound Ig was detected using biotinylated rat mAb anti–mouse Igκ (Cat. No. 04-6640; Zymed). Mouse myeloma proteins IgG2aκ and IgG1κ (UPC10, M 9144, and MOPC21, M 9269; Sigma Chemical Co.) were used as standards. For detection of mouse IgM, plates were coated with polyclonal anti–mouse μ (The Binding Site, UK) and bound Ig was detected with biotinylated goat anti–mouse μ (RPN1176; Amersham International) followed by streptavidin-conjugated horseradish peroxidase. Mouse plasmacytoma TEPC183, IgMκ (M 3795; Sigma Chemical Co.) was used as a standard.
Flow Cytometry Analysis.
Cell suspensions were obtained from bone marrow, spleen, and Peyer's patches (PPs). Multicolor staining was then carried out with the following reagents in combinations (illustrated in Fig. 4): FITC-conjugated anti–human λ (F 5266; Sigma Chemical Co.), PE-conjugated anti–mouse c-kit (CD117) receptor (clone ACK45, cat. No. 09995B; PharMingen), PE-conjugated anti–mouse CD25 (IL-2 receptor) (clone 3C7, P 3317; Sigma Chemical Co.), biotin-conjugated anti– human κ (clone G20-193, cat. No. 08172D, PharMingen), biotin-conjugated anti-mouse CD19 (clone 1D3, cat. No. 09654D; PharMingen), followed by Streptavidin-Quantum red (S 2899; Sigma Chemical Co.) or Streptavidin-PerCP (cat. No. 340130; Becton Dickinson) and rat monoclonal anti–mouse κ L chain (clone MRC-OX-20, cat. MCA152; Serotec, UK) coupled according to the manufacturer's recommendations with allophycocyanin (APC) (PJ25C; ProZyme). Data were collected from 106 stained cells on a FACScalibur® flow cytometer (Becton Dickinson) as previously described (32). Cells were first gated on forward and side scatter to exclude dead cells. To obtain accurate percentage distribution for comparison, cells from normal mice were stained in parallel. In addition, human peripheral blood lymphocytes were purified on Ficoll gradients (1.077 g/ml) and stained with PE-conjugated anti–human CD19 antibody (P 7437, clone SJ25-C1; Sigma Chemical Co.), biotinylated anti–human κ followed by Streptavidin-Quantum red, and FITC-conjugated anti–human λ antibodies as above.
Figure 4.
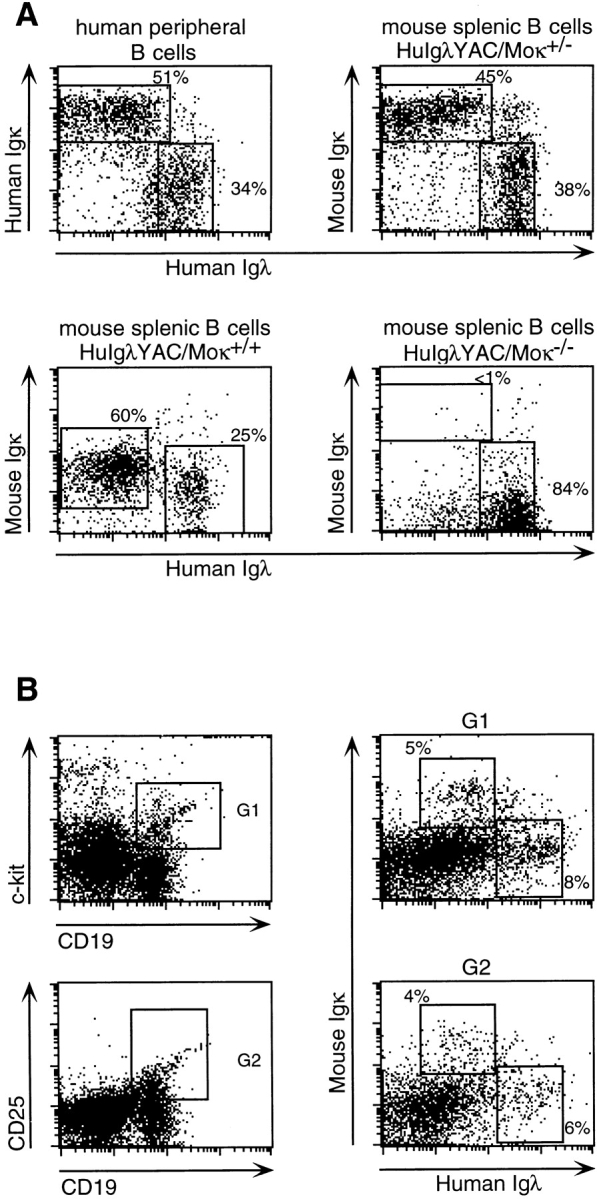
Flow cytometric analysis of L chain expression during B cell development. (A) Distribution of κ and λ L chain expression of CD19+ human peripheral lymphocytes and B220+ mouse spleen cells from HuIgλ YAC/Moκ+/−, HuIgλYAC/Moκ+/−, and HuIgλYAC/Moκ−/− mice. (B) Mouse Igκ and human Igλ L chain expression in CD19+/c-kit+ (top) and CD19+/CD25+ (bottom) HuIgλYAC/Moκ+/− bone marrow cells.
For reverse transcriptase (RT)-PCR cloning of Vλ genes, PP cells were stained with FITC-conjugated peanut agglutinin (PNA) (L 7381; Sigma Chemical Co.) and PE-conjugated anti–mouse B220 antibodies (P 3567; Sigma Chemical Co.). Double-positive cells were sorted on the FACStarPlus flow cytometer (Becton Dickinson) as previously described (32) and 5 × 103 cells were lysed in denaturing solution (37). 5′RACE was carried out as described below with one modification: 2 μg of carrier RNA was added to the cell lysates before RNA extraction and precipitation.
Cloning and Sequencing of 5′RACE Products.
Spleen RNA was prepared as previously described (37) and for cDNA preparation 2–3 μg of RNA was ethanol precipitated and air dried. For rapid amplification of 5′ cDNA ends (5′RACE) (38) first strand cDNA was primed with oligo(dT)22, and 100 U of Super Script II reverse transcriptase (GIBCO BRL) was used at 46°C according to the manufacturer's instructions with 20 U of placental RNAse inhibitor (Promega). The DNA/RNA duplex was passed through 1 ml G-50 equilibrated with TE (10 mM Tris-HCl, pH 7.8, and 1 mM EDTA) in a hypodermic syringe to remove excess oligo(dT). For G-tailing, 20 U of TdT (Cambio, UK) was used according to standard protocols (39). Double-stranded cDNA was obtained from G-tailed single-stranded cDNA by addition of oligonucleotide Pr1 (see below), 100 μM dNTP, and 2.5 U of Klenow fragment (Cambio), followed by incubation for 10 min at 40°C. After heating the reaction for 1 min at 94°C and extraction with phenol-chloroform, the double-stranded cDNA was passed through G-50 to remove primer Pr1. PCR amplifications, 35 cycles, were carried out in the RoboCycler Gradient 96 Thermal Cycler (Stratagene) using oligonucleotides Pr2 and Pr3. For PCR of PP cDNA 50 cycles were used: 40 cycles in the first amplification and 10 cycles in additional amplifications. Pfu Thermostable Polymerase (Stratagene) was used instead of Taq polymerase to reduce PCR error rates. The amplification products were purified using a GENECLEAN II kit (BIO 101) and reamplified for five cycles with primers Pr2 and Pr4 to allow cloning into EcoRI sites. Oligonucleotide for 5′RACE of Vλ genes were: Pr1, 5′-AATTCTAAAACTACAAACTGCCCCCCCCA/T/G-3′; Pr2, 5′-AATTCTAAAACTACAAACTGC-3′ (sense); Pr3, 5′-CTCCCGGGTAGAAGTCAC-3′ (reverse); and Pr4, 5'-AATTCGTGTGGCCTTGTTGGCT-3′ (reverse nested).
Vλ PCR products of ∼500 bp were cut out from agarose gels and purified on GENECLEAN II. The DNA was incubated in 50 mM Tris-HCl, pH 7.4, and 10 mM MgCl2, with 100 μM dGTP/dCTP and 1 U of Klenow fragment for 10 min at room temperature. Under these conditions the Klenow fragment removed the 3′ ends of the PCR products (AATT) leaving ligatable EcoRI overhangs. DNA was ligated with EcoRI-restricted pUC19, transformed into competent E. coli XL1Blue, and colonies were selected on X-Gal/IPTG/amp plates. Plasmid DNA prepared from white colonies was used for sequencing. Sequencing of both strands was done on the ABI 373 automated sequencer (Applied Biosystems, Inc.) in the Babraham Institute Microchemical Facility.
Results
The Transgenic Human Igλ Locus.
The human Igλ translocus (Fig. 1) was assembled as a YAC by recombining one YAC containing about half of the human Vλ gene segments with three overlapping cosmids containing Vλ and Jλ-Cλ gene segments and the 3′ enhancer (29). This produced a 410-kb YAC accommodating a 380-kb region of the human λ L chain locus containing 15 Vλ genes regarded as functional, 3 Vλs with open reading frames not found to be expressed, and 13 Vλ pseudogenes (40). This HuIgλYAC was introduced into ES cells by protoplast fusion (30) and chimeric mice were produced by blastocyst injection (31). The ES cell clone used for blastocyst injection showed a 450-kb NotI fragment corresponding to HuIgλYAC, as identified by PFGE and Southern hybridization with probes to the 3′ end of the construct, identifying the Cλ2+3 regions, and to the left centromeric YAC arm at the 5′ end, identifying the LYS2 gene (data not shown). Germline transmission was obtained, and PFGE analysis of testis DNA from one animal is illustrated in Fig. 2. A NotI fragment larger than 380 kb is necessary to accommodate this region of the HuIgλYAC, and the 450-kb band obtained indicates random integration involving the single NotI site 3′ of Jλ-Cλ and a NotI site in the mouse chromosome. Digests with EcoRI/HindIII and hybridization with the Cλ2+3 probe further confirmed the integrity of the transferred HuIgλYAC (Fig. 2). The results indicated that one complete copy of the HuIgλYAC was integrated in the mouse genome.
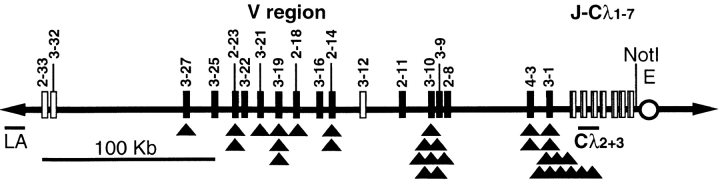
Figure 1.
The HuIgλYAC accommodates a 380-kb region of the human λ L chain locus in authentic configuration with all Vλ genes of cluster A (21, 22, 40), the Jλ-Cλ segments, and the 3′ enhancer (17). Black boxes represent functional Vλ genes (3-27, 3-25, 2-23, 3-22, 3-21, 3-19, 2-18, 3-16, 2-14, 2-11, 3-10, 3-9, 2-8, 4-3, and 3-1) and white boxes show Vλ genes with open reading frames (2-33, 3-32, and 3-12) that have not been identified in productive rearrangements of human lymphocytes (40). Pseudogenes are not shown. Black triangles (▴) indicate V gene use in functionally Igλ rearrangements (mutated [see Fig. 5] and unmutated) found by RT-PCR in spleen and sorted PP cells from HuIgλ mice. Rearrangement to Jλ1 was found in 5 sequences, Jλ2 in 18, and Jλ3 in 8. The unique NotI restriction site is indicated. Probes to assess the integrity of the HuIgλYAC, LA (left arm) and Cλ2+3 are indicated.
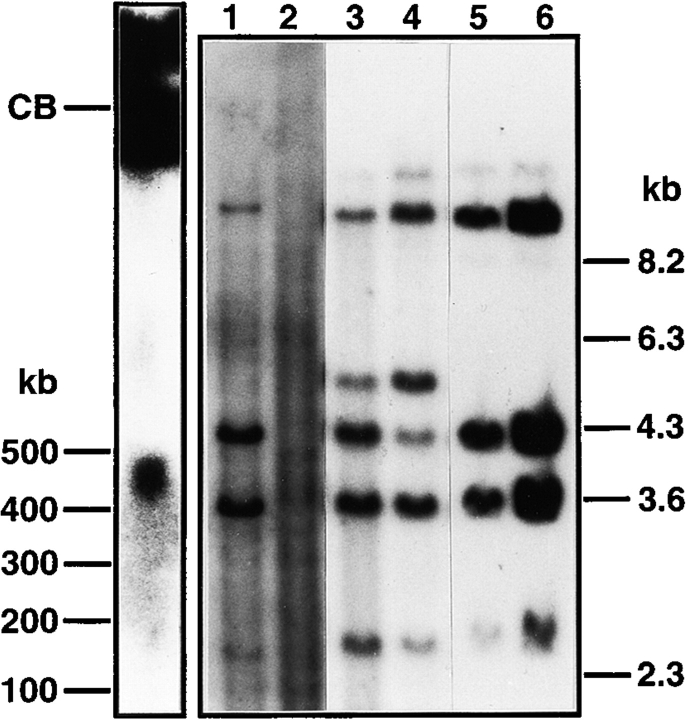
Figure 2.
Southern blot analysis of HuIgλYAC integration. (Left) NotI-digested testis DNA resolved on PFGE and hybridized with the Cλ2+3 probe. The same size band was obtained with the left arm probe (data not shown). The majority of the hybridization signal remains in the compression band (CB) presumably due to protection of the NotI site by methylation. (Right) EcoRI/HindIII digests hybridized with the Cλ2+3 probe. Lane 1, HuIgλYAC ES cell DNA from a protoplast fusion clone; lane 2, normal ES cell DNA; lane 3, human genomic DNA (XZ); lane 4, human KB carcinoma (53) DNA; lanes 5 and 6, tail DNA from two HuIgλYAC germline transmission mice. Note that the human DNA shows an additional 5.2-kb band that represents an allelic variation (54).
Human Igλ Expression Is Dominant in Mouse κ−/− Animals.
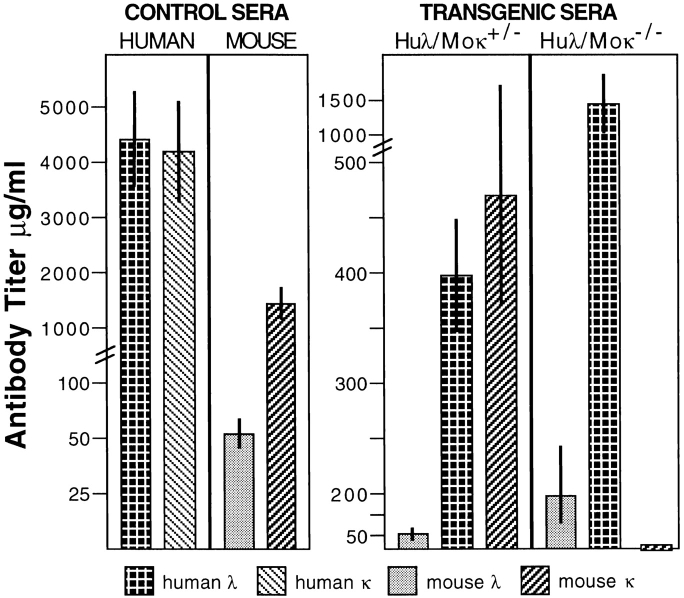
To assess the human λ L chain repertoire for the production of authentic human antibodies, the HuIgλYAC mice were bred with mice in which endogenous Igκ production was silenced by gene targeting (32). In these κ−/− mice, the mouse Igλ titers are elevated compared with κ+/+ strains (32, 41). Serum titrations (Fig. 3) showed that human Igλ antibody titers in HuIgλYAC/κ−/− mice are between 1 and 2 mg/ml, which is up to 10-fold higher than average mouse Igλ levels. Interestingly, in the HuIgλYAC/κ−/− mice, the mouse Igλ production returned to levels similar to that found in normal mice. High numbers of human Igλ+ cells were also identified in flow cytometric analysis of splenic B cells from HuIgλYAC/κ−/− mice (Fig. 4 A), with human λ expressed on the surface of ∼84% of the B cells and mouse Igλ+ expressed on <5% (data not shown).
Figure 3.
Human Igλ, mouse Igκ, and mouse Igλ serum titers for HuIgλYAC/Moκ+/− and HuIgλYAC/Moκ−/− mice (five to six mice per group kept in germfree conditions and five human sera). Antibody levels presented were obtained from 2–3-mo-old animals but the serum titers from older mice were similar. From the five HuIgλYAC/Moκ+/− mice tested, three animals had somewhat higher mouse Igκ titers than human Igλ, whereas two animals showed higher human Igλ levels. The controls show L chain distribution in human and normal mouse serum. Total Ig levels are in good agreement with the sum of individual titers (data not shown).
Human Igλ Expression Equals Mouse Igκ Production.
Assessment of human Igλ production in heterozygous HuIgλ YAC+/κ+/− mice allowed a detailed comparison of expression and activation of endogenous versus transgenic L chain loci present at equal functional numbers. Serum analysis (Fig. 3) of mice capable of expressing both human λ and mouse κ showed similar titers for human and mouse L chains. Human Igλ levels in HuIgλYAC/κ+/+ transgenic mice were similar to those in HuIgλYAC/κ+/− mice. Total Ig levels in HuIgλYAC+/κ+/− mice were 1–2 mg/ml, with an average contribution of ∼51% mouse Igκ, 43% human Igλ, and 6% mouse Igλ. As is also seen in human serum, the analysis of individual HuIgλYAC/κ+/− animals showed there were variations in the λ/κ ratios. Three of the HuIgλYAC/κ+/− mice produced somewhat higher κ levels, whereas in two mice the human λ levels were higher than the Igκ titers. In HuIgλYAC/κ+/− mice, high translocus expression was also found in B220+ B cells from different tissues, with 38% of spleen cells expressing human λ and 45% expressing mouse κ (Fig. 4 A). As illustrated, these values closely resemble the levels in human volunteers with 34% Igλ+ versus 51% Igκ+ in CD19+ peripheral blood lymphocytes. In HuIgλYAC/ κ+/+ mice, which carry a wild-type κ locus, the levels of Igλ are ∼25% and endogenous κ levels are ∼60% (Fig. 4 A). It is likely that these differences in expression levels are dependent on the number of active gene loci.
To assess the developmental stage at which the high contribution of the human λ translocus becomes established, we examined surface L chain expression by bone marrow cells of HuIgλYAC/κ+/− mice. For this, B cell lineage marker CD19 and specific antibodies to human λ and mouse κ were used in four-color staining with the early B cell markers c-kit (CD117) and CD25. Fig. 4 B shows that surface L chain expression (human λ or mouse κ) was detectable on a similar small proportion of B cells at each of these stages of development, which suggests that human and mouse L chain rearrangements are simultaneous. The specificity of the staining detecting mouse κ and human λ on small numbers of early B cells, which has been reported independently (42), was verified by the absence of similar positive cells in the analysis of bone marrow from control mice (data not shown).
DNA Rearrangement and Diversification of a Highly Active Human λ Translocus.
To further clarify the potential of the L chain translocus to contribute to the antibody repertoire, we analyzed human λ and mouse κ L chain production using individual hybridoma clones from HuIgλYAC/ κ+/− mice. Results from two fusions suggest that human λ and mouse κ L chain–producing cells were present in the spleen of HuIgλYAC/κ−/+ mice at similar frequencies. Furthermore, in the hybridomas the amounts of human Igλ (2–20 μg/ml) or mouse Igκ (4–25 μg/ml) were very similar. To determine whether Igκ rearrangement precedes Igλ, as found in mice and humans (4, 5), the configuration of the endogenous Igκ and the human λ translocus were analyzed in these hybridomas. Southern blot hybridization of randomly picked hybridoma clones showed that in 11 human Igλ expressers, 7 had the mouse κ locus in germline configuration, 1 clone had mouse Igκ rearranged, and 3 clones had the mouse κ locus deleted, whereas in 19 mouse Igκ expressers, all but 2 had the human Igλ locus in germline configuration. This result suggests that there is no locus activation bias and further emphasizes that the human λ translocus performs with similar efficiency as the endogenous κ locus.
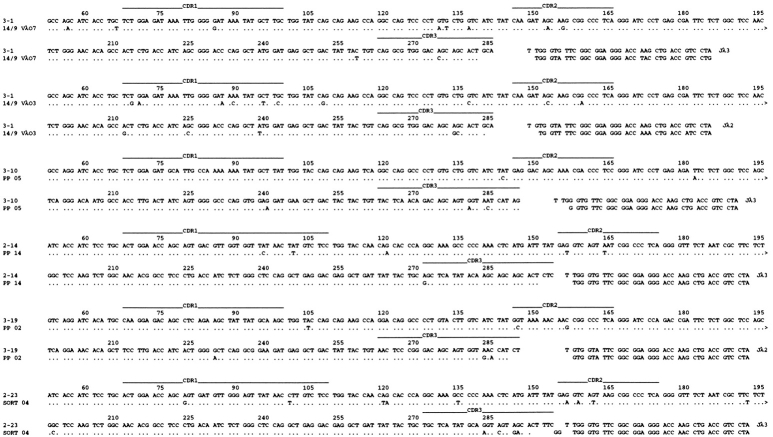
The capacity of the human λ locus to produce a diverse antibody repertoire is further documented by the V-J rearrangement. Sequences were isolated from spleen and PP cells by 5′RACE PCR amplification to avoid bias from specific V gene primers. The use of individual Vλ genes is indicated by the triangles in Fig. 1, and shows that a substantial proportion of the Vλ genes on the translocus are being used in productive rearrangements, with Vλ3-1 and Vλ3-10 being most frequently expressed. In Vλ-Jλ rearrangements, Jλ2 was used preferentially and Jλ3 and 1 were used less frequently, whereas, as expected, Jλ4, 5, and 6 were not used as they are adjacent to ψCs. Extensive variability due to N or P sequence additions, which is found in human but not mouse L chain sequences (25, 27, 28), was not observed. Sequences obtained by RT-PCR from FACS®-sorted PP germinal center B cells (B220+/PNA+) revealed that somatic hypermutation is operative in HuIgλ YAC mice (Fig. 5). We identified 11 unique Vλ-Jλ rearrangements with two or more changes in the V region, excluding the CDR3, which may be affected by Vλ-Jλ recombination. The majority of mutations lead to amino acid replacements, but there was no preferential distribution in CDR1 and CDR2.
Figure 5.
Hypermutated human Vλ sequences from sorted B220+ and PNA+ PP B cells from HuIgλ+YAC/κ+/− mice. The sequences are a representative selection of the functional Vλ-Jλ rearrangements (indicated by the triangles in Fig. 1) isolated from RT-PCR.
Discussion
The ratio of λ to κ L chain expression varies considerably between different species (1–3, 43, 44), and in mice the low λ L chain levels are believed to be a result of inefficient activation of the mouse λ locus during B cell differentiation (for review see reference 6). The Igλ (∼40%) to Igκ (∼60%) ratio in humans is more balanced and suggests that both λ and κ play an equally important role in immune responses. This is supported by the finding that the mouse Vλ genes are most similar to the less frequently used distal human Vλ gene families, whereas no genes comparable to the major contributors to the human Vλ repertoire are present in the mouse locus (40). With the HuIgλYAC, these Vλ genes are available, and are able to make a significant contribution to the antibody repertoire. The 410-kb HuIgλYAC translocus accommodates V gene region cluster A containing at least 15 functional Vλ genes (see Fig. 1). In humans, cluster A is the main contributor to the λ antibody repertoire, with Vλ 2-14 (2a2) expressed most frequently at 27% in blood lymphocytes (23). We also find expression of Vλ 2-14 in the translocus mice, but the main contributors to the λ L chain repertoire were 3-1 (the Vλ gene most proximal to the J-C region) and 3-10, both of which are expressed at ∼3% in humans. Although the validity of conclusions about the contribution of different genes is dependent on the numbers examined, the overexpression of Vλ3-1 (11 sequences) and Vλ3-10 (8 sequences) in the 31 sequences obtained may imply that rearrangement or selection preferences are different in mice and humans. Analysis of recombination signal sequences (RSS) in mouse L chains showed that κ and λ RSS differ significantly, and that those genes with the highest similarity to consensus RSS rearrange most frequently (45). The RSS of Vλ3-1 and Vλ3-10 show a 100% match with the mouse consensus sequence, which may explain their frequent expression in the translocus mice. In addition, most human Vλ RSS match the established consensus sequence significantly better than mouse Vλs (21, 45).
We found extensive somatic hypermutation of many rearranged human Igλ sequences, indicating that they are able to participate in normal immune responses. The levels of mutation in B220+/PNA+ PP cells from HuIgλYAC translocus mice were similar to what has been reported for mouse L chains (46). Rather unexpected was the pattern of somatic hypermutation with similar numbers of silent and replacement point mutations found in the complementarity-determining and framework regions. Somatic hypermutation is usually associated with a higher level of replacement mutations in CDRs and more silent mutations in the framework regions, and the distribution observed here may argue against efficient antigen selection having taken place. Interestingly, however, λ L chain sequences obtained from human peripheral blood lymphocytes also showed high numbers of mutations in framework 2 (23). Part of framework 2 lies at the interface of the VL and VH domains and it has been suggested that this region may be important for optimal H and λ L chain interaction and, in particular, interaction of the human λ L chain and the endogenous mouse H chain (26).
In the mouse, unlike in humans, L chain diversification due to untemplated nucleotide addition is essentially absent, because TdT expression has been downregulated by the stage at which L chain rearrangement takes place (28, 47). This concept is challenged by the observation that mouse L chain rearrangement can occur at the same time as VH to DJH rearrangements (48) or even earlier (42). Our results also show L chain rearrangement at the pre-B cell stage with a similar number of human λ- or mouse κ-expressing B cells also expressing c-kit+ or CD25+ (see Fig. 4). Although the cell numbers are small, the results suggest that there is no preferential activation of either the human λ translocus or the endogenous κ locus. However, despite this early activation, there is no accumulation of N or P nucleotide diversity in the rearranged human λ L chains, unlike rearranged λ L chains from human peripheral B cells (27). The small number (<1%) of human Igλ+/mouse Igκ+ double positive spleen and bone marrow cells may indicate that haplotype exclusion at the L chain level is less strictly controlled than is IgH exclusion (49).
In transgenic mice carrying Ig regions in germline configuration on minigene constructs, efficient DNA rearrangement and high antibody expression levels are rarely achieved. Competition with the endogenous locus can be eliminated using Ig knockout strains, in which transgene expression is usually improved (50). Poor transloci expression levels could be a result of the failure of human sequences to work efficiently in the mouse background or, alternatively, of the absence of locus-specific control regions that are more likely to be included on larger transgenic regions (51–53). Recently we addressed this question in transgenic mice by the introduction of different sized minigene- and YAC-based human κ L chain loci (53). The result showed that neither the size of the V gene cluster nor the V gene numbers present were relevant to achieving high translocus expression levels. The YAC-based loci contained downstream regions of the human κ locus, and it is possible that the presence of an undefined region with cis-controlled regulatory sequences may have been crucial in determining expressibility and subsequently L chain choice. The HuIgλYAC contains equivalent regions from the human Igλ locus, which may promote the use of the translocus in the L chain repertoire. Hybridomas from HuIgλYAC+/ κ+/− mice show no evidence for a bias in L chain locus selection during development, as demonstrated by the absence of rearrangement of the nonexpressed locus. This is in contrast with what is seen in Ig expressing mouse and human B cell clones (4, 5), and supports the model that λ and κ rearrangements are indeed independent (15, 54) and that poor Igλ expression levels in mice may be the result of inefficient signals acting during recombination (16). A possible signal that initiates L chain recombination has been identified through gene targeting experiments where the 3′ κ enhancer was deleted (17). In these mice, the κ/λ ratio was reduced from 20:1 in normal mice down to 1:1, and the κ locus was largely in germline configuration in λ-expressing cells, as we also see in the HuIgλYAC+/κ+/− hybridoma clones. The high level of human Igλ expression in the HuIgλYAC+/κ+/− mice could be due to the strength of the downstream enhancer of the human λ locus. An analysis of human L chain enhancer activities identified three synergistic modules at the 3′ end of the λ locus which constitute a powerful pre-B cell specific enhancer that appears to be stronger than the corresponding κ enhancer (55). Analysis of the mouse λ 3′ enhancer suggests the biased κ/λ ratio in mice may be a direct result of the differences in locus specific regulation provided by the respective enhancers (19, 56). The results suggest that strength and ability of the human 3′ λ enhancer to function in the mouse background may be the reason that λ and κ loci can compete equally at the pre-B cell stage to initiate L chain rearrangement, resulting in the similar levels of human Igλ and mouse Igκ seen in the HuIgλYAC+/κ+/− mice.
Acknowledgments
We thank Drs. I. Tomlinson, G. Winter, and O. Ignatovich for access to their database of human Vλ sequences and helpful discussions. We are grateful to Drs. D. Melton for provision of the HM-1 ES cells, E. Corps for hybridoma production, B. Goyenechea for help with the Southern hybridization, and N. Miller for helping with the flow cytometry.
This work was supported by the Biotechnology and Biological Sciences Research Council and the Babraham Institute.
Abbreviations used in this paper
- ES
embryonic stem
- Hu
human
- N
nonencoded
- P
palindromic
- PFGE
pulsed-field gel electrophoresis
- PNA
peanut agglutinin
- PP
Peyer's patch
- RACE
rapid amplification of cDNA ends
- RSS
recombination signal sequence(s)
- RT
reverse transcriptase
- TdT
terminal deoxyribonucleotide transferase
- YAC
yeast artificial chromosome
Footnotes
A.V. Popov and X. Zou contributed equally to this work.
References
- 1.Hood L, Gray WR, Sanders BG, Dreyer WY. Light chain evolution: antibodies. Cold Spring Harbor Symp Quant Biol. 1967;32:133–146. [Google Scholar]
- 2.McIntire KR, Rouse AM. Mouse immunoglobulin light chains: alterations of κ:λ ratio. Fed Proc. 1970;19:704. [Google Scholar]
- 3.Arun SS, Breuer W, Hermanns W. Immunohistochemical examination of light-chain expression (lambda/ kappa ratio) in canine, feline, equine, bovine and porcine plasma cells. Zentralbl Veterinarmed A. 1996;43:573–576. doi: 10.1111/j.1439-0442.1996.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 4.Hieter PA, Korsmeyer SJ, Waldmann TA, Leder P. Human immunoglobulin κ light-chain genes are deleted or rearranged in λ-producing B cells. Nature. 1981;290:368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- 5.Coleclough C, Perry RP, Karjalainen K, Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981;290:372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- 6.Selsing, E., and L.E. Daitch. 1995. Immunoglobulin λ genes. In Immunoglobulin Genes. Second Edition. T. Honjo and F.W. Alt, editors. Academic Press, London, UK. 193–203.
- 7.Berg J, McDowell M, Jäck HM, Wabl M. Immunoglobulin λ gene rearrangement can precede κ gene rearrangement. Dev Immunol. 1990;1:53–57. doi: 10.1155/1990/56014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abken H, Bützler C. Re-organization of the immunoglobulin kappa gene on both alleles is not an obligatory prerequisite for Ig lambda gene expression in human cells. Immunology. 1991;74:709–713. [PMC free article] [PubMed] [Google Scholar]
- 9.Takemori T, Rajewsky K. Lambda chain expression at different stages of ontogeny in C57BL/6, BALB/c and SJL mice. Eur J Immunol. 1981;11:618–625. doi: 10.1002/eji.1830110806. [DOI] [PubMed] [Google Scholar]
- 10.McGuire KL, Vitetta ES. κ/λ shifts do not occur during maturation of murine B cells. J Immunol. 1981;127:1670–1673. [PubMed] [Google Scholar]
- 11.Kessler S, Kim KJ, Scher I. Surface membrane κ and λ light chain expression on spleen cells of neonatal and maturing normal and immune-defective CBA/NB mice: the ratio is constant. J Immunol. 1981;127:1674–1678. [PubMed] [Google Scholar]
- 12.Lejeune JM, Briles DE, Lawton AR, Kearney JF. Estimate of the light chain repertoire size of fetal and adult BALB/cJ and CBA/J mice. J Immunol. 1982;129:673–677. [PubMed] [Google Scholar]
- 13.Rolink A, Streb M, Melchers F. The κ/λ ratio in surface immunoglobulin molecules on B lymphocytes differentiating from DHJH -rearranged murine pre-B cell clones in vitro. . Eur J Immunol. 1991;21:2895–2898. doi: 10.1002/eji.1830211137. [DOI] [PubMed] [Google Scholar]
- 14.Osmond DJ, Rolink A, Melchers F. Murine B lymphopoiesis: towards a unified model. Immunol Today. 1998;19:65–68. doi: 10.1016/s0167-5699(97)01203-6. [DOI] [PubMed] [Google Scholar]
- 15.Zou YR, Takeda S, Rajewsky K. Gene targeting in the Igκ locus: efficient generation of λ chain-expressing B cells, independent of gene rearrangements in Igκ. EMBO (Eur Mol Biol Organ) J. 1993;12:811–820. doi: 10.1002/j.1460-2075.1993.tb05721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arakawa H, Shimizu T, Takeda S. Re-evaluation of the probabilities for productive rearrangements on the κ and λ loci. Int Immunol. 1996;8:91–99. doi: 10.1093/intimm/8.1.91. [DOI] [PubMed] [Google Scholar]
- 17.Gorman JR, van der Stoep N, Monroe R, Cogne M, Davidson L, Alt FW. The Igκ 3′ enhancer influences the ratio of Igκ versus Igλ B lymphocytes. Immunity. 1996;5:241–252. doi: 10.1016/s1074-7613(00)80319-2. [DOI] [PubMed] [Google Scholar]
- 18.Glozak M, Blomberg BB. The human immunoglobulin enhancer is controlled by both positive elements and developmentally regulated negative elements. Mol Immunol. 1996;33:427–438. doi: 10.1016/0161-5890(95)00146-8. [DOI] [PubMed] [Google Scholar]
- 19.Asenbauer H, Klobeck HG. Tissue-specific deoxyribonuclease I-hypersensitive sites in the vicinity of the immunoglobulin C lambda cluster of man. Eur J Immunol. 1996;26:142–150. doi: 10.1002/eji.1830260122. [DOI] [PubMed] [Google Scholar]
- 20.Frippiat JP, Williams SC, Tomlinson IM, Cook GP, Cherif D, Le Paslier D, Collins JE, Dunham I, Winter G, Lefranc MP. Organization of the human immunoglobulin lambda light-chain locus on chromosome 22q11.2. Hum Mol Genet. 1995;4:983–991. doi: 10.1093/hmg/4.6.983. [DOI] [PubMed] [Google Scholar]
- 21.Kawasaki K, Minoshima S, Nakato E, Shibuya K, Shintani A, Schmeits JL, Wang J, Shimizu N. One-megabase sequence analysis of the human immunoglobulin λ gene locus. Genome Res. 1997;7:260–261. doi: 10.1101/gr.7.3.250. [DOI] [PubMed] [Google Scholar]
- 22.Giudicelli V, Chaume D, Bodmer J, Muller W, Busin C, Marsh S, Bontrop R, Marc L, Malik A, Lefranc M-P. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 1997;25:206–211. doi: 10.1093/nar/25.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ignatovich O, Tomlinson IM, Jones PT, Winter G. The creation of diversity in the human immunoglobulin V(lambda) repertoire. J Mol Biol. 1997;268:69–77. doi: 10.1006/jmbi.1997.0956. [DOI] [PubMed] [Google Scholar]
- 24.Combriato G, Klobeck H-G. Vλ and Jλ-Cλ gene segments of the human immunoglobulin λ light chain locus are separated by 14 kb and rearrange by a deletion mechanism. Eur J Immunol. 1991;21:1513–1522. doi: 10.1002/eji.1830210627. [DOI] [PubMed] [Google Scholar]
- 25.Foster SJ, Brezinschek H-P, Brezinschek RI, Lipsky PE. Molecular mechanisms and selective influences that shape the κ gene repertoire of IgM+B cells. J Clin Invest. 1997;99:1614–1627. doi: 10.1172/JCI119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ignatovich, O. 1998. The creation of diversity in the human immunoglobulin Vλ repertoire. Ph.D. thesis. University of Cambridge, Cambridge, UK.
- 27.Bridges SL, Lee SK, Johnson ML, Lavelle JC, Fowler PG, Koopman WJ, Schroeder HW. Somatic mutation and CDR3 length of immunoglobulin κ light chains expressed in patients with rheumatoid arthritis and in normal individuals. J Clin Invest. 1995;96:831–841. doi: 10.1172/JCI118129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Victor KD, Vu K, Feeney AJ. Limited junctional diversity in κ light chains. J Immunol. 1994;152:3467–3475. [PubMed] [Google Scholar]
- 29.Popov AV, Bützler C, Frippiat J-P, Lefranc M-P, Brüggemann M. Assembly and extension of yeast artificial chromosomes to build up a large locus. Gene. 1996;177:195–201. doi: 10.1016/0378-1119(96)00301-0. [DOI] [PubMed] [Google Scholar]
- 30.Davies, N.P., A.V. Popov, X. Zou, and M. Brüggemann. 1996. Human antibody repertoires in transgenic mice: manipulation and transfer of YACs. In Antibody Engineering: A Practical Approach. J. McCafferty, H.R. Hoogenboom, and D.J. Chiswell, editors. IRL Press, Oxford. 59–76.
- 31.Hogan, B., R. Beddington, F. Costantini, and E. Lacy. 1994. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 497 pp.
- 32.Zou X, Xian J, Popov AV, Rosewell IR, Müller M, Brüggemann M. Subtle differences in antibody responses and hypermutation of λ light chains in mice with a disrupted κ constant region. Eur J Immunol. 1995;25:2154–2162. doi: 10.1002/eji.1830250806. [DOI] [PubMed] [Google Scholar]
- 33.Wurst, W., and A.L. Joyner. 1993. Production of targeted embryonic stem cell DNA. In Gene Targeting. A.L. Joyner, editor. IRL Press, Oxford. 33–61.
- 34.Herrmann BG, Barlow DP, Lehrach H. A large inverted duplication allows homologous recombination between chromosomes heterozygous for the proximal t complex inversion. Cell. 1987;48:813–825. doi: 10.1016/0092-8674(87)90078-x. [DOI] [PubMed] [Google Scholar]
- 35.Galfré G, Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73:3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- 36.Tijssen, P. 1985. Practice and theory of enzyme immunoassays. In Laboratory Techniques in Biochemistry and Molecular Biology. Volume 15. R.H. Burdon and P.H. Knippenberg, editors. Elsevier, Amsterdam.
- 37.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 38.Frohman MA, Dush MK, Martin GR. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, K. Struhl, and J.A. Smith, editors. 1995. Current Protocols In Molecular Biology. Massachusetts General Hospital, Boston, MA; Harvard Medical School, Boston, MA; University of Alabama, Birmingham, AL; Wiley & Sons, New York.
- 40.Williams SC, Frippiat J-P, Tomlinson IM, Ignatovich O, Lefranc M-P, Winter G. Sequence and evolution of the human germline Vλ repertoire. J Mol Biol. 1996;264:220–232. doi: 10.1006/jmbi.1996.0636. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Trounstine M, Kurahara C, Young F, Kuo C-C, Xu Y, Loring JF, Alt FW, Huszar D. B cell development in mice that lack one or both immunoglobulin κ light chain genes. EMBO (Eur Mol Biol Organ) J. 1993;12:821–830. doi: 10.1002/j.1460-2075.1993.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novobrantseva TI, Martin VM, Pelanda RM, Muller W, Rajewsky K, Ehlich A. Rearrangement and expression of immunoglobulin light chain genes can precede heavy chain expression during normal B cell development in mice. J Exp Med. 1999;189:75–88. doi: 10.1084/jem.189.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saitta M, Iavarone A, Cappello N, Bergami MR, Fiorucci GC, Aguzzi F. Reference values for immunoglobulin kappa and lambda light chains and the kappa/ lambda ratio in children's serum. Clin Chem. 1992;38:2454–2457. [PubMed] [Google Scholar]
- 44.Hood L, Gray WR, Dreyer WY. On the mechanism of antibody synthesis: a species comparison of L-chains. Proc Natl Acad Sci USA. 1966;55:826–835. doi: 10.1073/pnas.55.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramsden DA, Wu GE. Mouse κ light-chain recombination signal sequence mediate recombination more frequently than do those of λ light chain. Proc Natl Acad Sci USA. 1991;88:10721–10725. doi: 10.1073/pnas.88.23.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez-Fernandez A, Gupta SK, Pannell R, Neuberger MS, Milstein C. Somatic mutation of immunoglobulin lambda chains: a segment of the major intron hypermutates as much as the complementarity-determining regions. Proc Natl Acad Sci USA. 1994;91:12614–12618. doi: 10.1073/pnas.91.26.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y-S, Hayakawa K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harada K, Yamagishi H. Lack of feedback inhibition of Vκ gene rearrangement by productively rearranged alleles. J Exp Med. 1991;173:409–415. doi: 10.1084/jem.173.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brüggemann M, Neuberger MS. Strategies for expressing human antibody repertoires in transgenic mice. Immunol Today. 1996;17:391–397. doi: 10.1016/0167-5699(96)10025-6. [DOI] [PubMed] [Google Scholar]
- 51.Green LL, Jakobovits A. Regulation of B cell development by variable gene complexity in mice reconstituted with human immunoglobulin yeast artificial chromosomes. J Exp Med. 1998;188:483–495. doi: 10.1084/jem.188.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zou X, Xian J, Davies NP, Popov AV, Brüggemann M. Dominant expression of a 1.3 Mb human Igκ locus replacing mouse light chain production. FASEB J. 1996;10:1227–1232. doi: 10.1096/fasebj.10.10.8751726. [DOI] [PubMed] [Google Scholar]
- 53.Xian J, Zou X, Popov AV, Mundt CA, Miller N, Williams GT, Davies SL, Neuberger MS, Brüggemann M. Comparison of the performance of a plasmid-based human Igκ minilocus and YAC-based human Igκ transloci for the production of a human antibody repertoire in transgenic mice. Transgenics. 1998;2:333–343. [Google Scholar]
- 54.Nadel B, Cazenave P-A, Sanchez P. Murine lambda gene rearrangements: the stochastic model prevails over the ordered model. EMBO (Eur Mol Biol Organ) J. 1990;9:435–440. doi: 10.1002/j.1460-2075.1990.tb08128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asenbauer H, Combriato G, Klobeck H-G. The immunoglobulin lambda light chain enhancer consists of three modules which synergize in activation of transcription. Eur J Immunol. 1999;29:713–724. doi: 10.1002/(SICI)1521-4141(199902)29:02<713::AID-IMMU713>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 56.Hagman J, Rudin CM, Haasch C, Chaplin D, Storb U. A novel enhancer in the immunoglobulin lambda locus is duplicated and functionally independent of NF kappa B. Genes Dev. 1990;4:978–992. doi: 10.1101/gad.4.6.978. [DOI] [PubMed] [Google Scholar]
- 57.Eagle H. Propagation in a fluid medium of a human epidermoid carcinoma strain KB. Proc Soc Exp Biol Med. 1955;89:362–364. doi: 10.3181/00379727-89-21811. [DOI] [PubMed] [Google Scholar]
- 58.Taub RA, Hollis GF, Hieter PA, Korsmeyer S, Waldmann TA, Leder P. Variable amplification of immunoglobulin λ light chain genes in human populations. Nature. 1983;304:172–174. doi: 10.1038/304172a0. [DOI] [PubMed] [Google Scholar]