Abstract
Synaptotagmins (Syts) I and II are believed to act as Ca2+ sensors in the control of neurotransmission. Here we demonstrate that mast cells express Syt II in their lysosomal fraction. We further show that activation of mast cells by either aggregation of FcεRI or by Ca2+ ionophores results in exocytosis of lysosomes, in addition to the well documented exocytosis of their secretory granules. Syt II directly regulates lysosomal exocytosis, whereby overexpression of Syt II inhibited Ca2+-triggered release of the lysosomal processed form of cathepsin D, whereas suppression of Syt II expression markedly potentiated this release. These findings provide evidence for a novel function of Syt II in negatively regulating Ca2+-triggered exocytosis of lysosomes, and suggest that Syt II–regulated secretion from lysosomes may play an important role in mast cell biology.
Keywords: mast cells, lysosomes, calcium binding proteins, exocytosis, immunoglobulin E
Mast cells are specialized secretory cells that belong to the immune system. Through triggered exocytosis of their secretory granules (SGs),1 mast cells release biologically active substances, including vasoactive amines, proteases, and preformed cytokines. In addition, after their activation, mast cells produce and release arachidonic acid metabolites such as leukotrienes, prostaglandins, and multifunctional cytokines. Together, these mediators play central roles in both the immediate and late phase inflammatory reactions (1, 2). Although their physiological role in the body is less clear, mast cells importantly contribute to host defense against bacterial and parasite infections (3–8) as well as to cellular immune responses through their ability to present antigens and trigger antigen-specific T cell proliferation (9, 10).
Previous studies of exocytosis in mast cells indicate that the final trigger to exocytosis involves a late acting GTP-binding protein (11, 12) and Ca2+ (13, 14). The molecular identity of the mast cell exocytic Ca2+ sensor remains obscure. In the synapse, this role has been ascribed to synaptotagmins (Syts) I and II, abundant Ca2+ and phospholipid binding proteins localized on synaptic vesicles (SVs) (15– 20). Binding of Ca2+ to Syt results in a conformational (21) or electrostatic (22) change that, by an as yet unresolved mechanism, allows exocytosis to occur. The finding that Syt I and II belong to a larger family of ubiquitously expressed proteins suggests that Syt isoforms may function as general Ca2+ sensors (23, 24). This hypothesis is supported by the recent demonstration of a role for a Syt isoform in controlling insulin secretion (25, 26).
We have recently reported that expression of Syt I in RBL-2H3 cells (a mucosal mast cell line) resulted in prominent potentiation and acceleration of Ca2+-dependent exocytosis (27). Therefore, in this study we decided to identify the Syt isoform which is endogenously expressed in RBL cells, and explore its role in controlling exocytosis. We found that rat basophilic leukemia (RBL) cells endogenously express the Syt isoforms II, III, and V. The role of Syt II, the most abundant isoform in RBL cells, was investigated.
Materials and Methods
Antibodies.
Antibodies used included rabbit polyclonal serum directed against the cytoplasmic domain of Syt I (a gift from Dr. T.C. Sudhof, Howard Hughes Medical Institute, University of Texas Southwestern Medical School, Dallas, TX), mAbs directed against the NH2-terminal region of Syt II (a gift from Dr. M. Takahashi, Mitsubishi-Kasei Institute of Life Sciences, Tokyo, Japan), and polyclonal antibodies against cathepsin D (Calbiochem).
Isolation and Growth of Mast Cells.
Bone marrow-derived mast cells (BMMCs) were obtained as previously described (28). In brief, femoral bone marrow cells from 6-wk-old BALB/c mice were cultured in 50% WEHI-3 cells conditioned medium. Culture medium was changed weekly, and nonadhering cells were used for further growth. After 3 wk, at least 99% of the cells were identified as mast cells by toluidine blue staining. Rat peritoneal mast cells (RPMCs) were obtained from Wistar rats by peritoneal lavage, and purified as previously described (29). In brief, a suspension of washed peritoneal cells was layered over a cushion of 30% Ficoll 400 (Pharmacia Biotech Inc.) in buffered saline and 0.1% BSA and centrifuged at 150 g for 15 min. The purity of mast cells recovered from the bottom of the tube was >90%, as assessed by toluidine blue staining. RBL-2H3 cells (hereafter termed RBL cells) were maintained in adherent cultures in DMEM supplemented with 10% FCS in a humidified atmosphere of 6% CO2 at 37°C.
Reverse Transcription and PCR Amplification of Syt cDNA Fragments.
RNA was isolated from trypsinized RBL cells collected by centrifugation at 400 g for 5 min, and from brains that were rapidly excised from 150–200-g Sprague-Dawley rats killed by CO2 suffocation and then exsanguinated. Total RNA was isolated on a guanidine thiocyanate/CsCl gradient, extracted twice with phenol/chloroform, and then ethanol precipitated. The RNA was dissolved in 0.1% diethyl pyrocarbonate–treated water, quantified by measuring absorbance at 260 nm, evaluated for degradation by agarose-formaldehyde gel electrophoresis, and frozen until used. The mRNA was isolated from total RNA by oligo-dT cellulose chromatography [Poly(A)Pure; Ambion], and 2 μg was reverse transcribed by 125 U of Moloney's murine leukemia virus–reverse transcriptase (New England BioLabs) in a 50 μl reaction containing 2.5 μg each of (dT)18 and random octamers, 1 mM of each dNTP, and 40 U of RNAsin (Promega) at 37°C for 30 min, 42°C for 30 min, and 50°C for 15 min. The first round of nested PCR was performed with 1 μl of AmpliTaq (Perkin-Elmer Cetus) in 100 μl reaction buffer supplemented with 1.5 mM MgCl2, 10% (vol/vol) DMSO, 1 μM of each primer, 50 μM of each dNTP, and 1 μl of the reverse transcription reaction as template. The four primers correspond to RNA sequences encoding portions of Syt proteins schematized in Fig. 1 A, and their sequences were: A, TCWGACCCYTAYGTCAARRTCT; B, AGACCCARGTGCACMGGAAGAC; C, SYCYTTSACRTAGGGRTCTGA; D, GGGGTTSAGSGTGTTCTTCTT. For the first round of PCR, six cycles of 94°C for 1 min ramping to 49°C in 3 min, 49°C for 1 min, 72°C for 1 min were followed by 24 cycles of 94°C for 1 min, 65°C for 1 min, 72°C for 1 min, with a final extension at 72°C for 6 min. 1 μl of the PCR product obtained with RBL cell cDNA and the A and D primers was used for template in a second round of PCR identical to the first except that the initial six cycles with low annealing temperature were not included. The product of the second reaction using the B and C primers was purified by agarose gel electrophoresis, then ligated into the pCR-II vector (Invitrogen). DH5α cells were transformed with the ligation mixture and colonies were selected for sequencing.
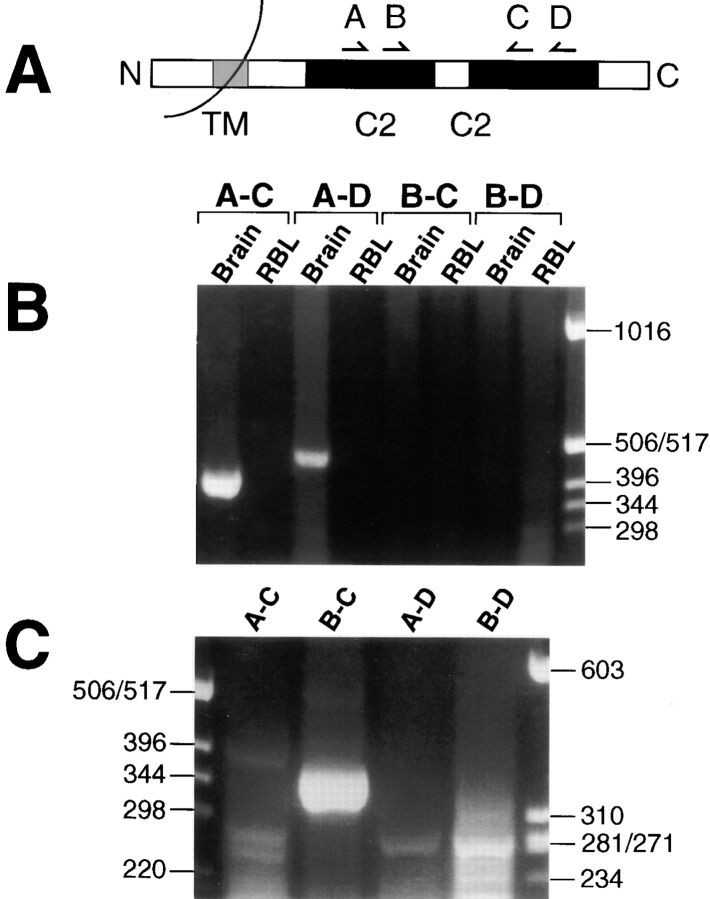
Figure 1.
PCR amplification of Syt isoforms. (A) Schematic portrayal of a representative Syt protein and the four primers for the PCR reactions. (B) An agarose gel of electrophoretically separated products of an initial round of PCR performed as described in Materials and Methods, then stained with ethidium bromide. Primer pairs and the source of cDNA for each reaction are shown above the gel, and size markers are shown on the right side. (C) An agarose gel of the products of a second round of PCR, using for a template of the PCR product of RBL cell cDNA with primers A and D. Primer pairs for the second-round reactions are shown above the gel, and size markers are shown on both sides.
Ribonuclease Protection Assay.
Vectors containing the PCR-cloned Syt fragments were linearized with NotI for SP6-directed synthesis of riboprobes or with BamHI for T7-directed cRNA synthesis. cRNA hybridization controls were generated in a 50 μl reaction containing 3 μg of template DNA, 1.6 U/μl RNAsin, 10 mM dithiothreitol (DTT), 0.1 mg/ml BSA, 1 mM of each NTP, 2.5 μM [3H]UTP (45 Ci/mmol; Amersham), and 100 U T7 RNA polymerase (New England Biolabs) in the manufacturer's buffer. Reactions were incubated for 4 h at 37°C, 10 U DNAse I was added, and the incubation was continued for another 20 min, and then cRNA was purified on a Nick-Spin column (Ambion). Riboprobes were transcribed in a 20 μl reaction using 1 μg of template DNA, 25 μM α-[32P]CTP (800 Ci/mmol; Amersham), 2 U/μl RNasin, 10 mM DTT, 0.1 mg/ml BSA, 0.5 mM each of other NTPs, and 10 U SP6 RNA polymerase (New England Biolabs) in the manufacturer's buffer for 1 h at 40°C. After treatment with 5 U of DNAse I, full-length transcripts were isolated by gel purification in 5% acrylamide/8 M urea gels. RNase protection assays were performed using the RPA II kit (Ambion). Cognate cRNA (0.1 pmol) and yeast RNA were used as positive and negative controls. Each experiment contained 1 pmol riboprobe and varying amounts of RBL cell RNA supplemented with yeast RNA to complete a total of 40 μg RNA. Hybridization was carried out overnight at 45°C. Protected probes were electrophoresed through 5% acrylamide/8 M urea gels and visualized by autoradiography.
Preparation of Mast Cell and Brain Lysates.
Mast cells (106) derived from different sources (RPMCs, BMMCs, and RBL-2H3) were washed in PBS and resuspended in 30 μl of lysis buffer (50 mM Hepes, pH 7.4, 150 mM NaCl, 10 mM EDTA, 2 mM EGTA, 1% Triton X-100, 0.1% SDS, 50 mM NaF, 10 mM NaPPi, 2 mM NaVO4, 1 mM PMSF, and 10 μg/ml leupeptin) and centrifuged at 12,000 g for 15 min at 4°C. The cleared supernatants were mixed with 5× Laemmli sample buffer to a final concentration of 1×, boiled for 5 min, and subjected to SDS-PAGE and immunoblotting. For the preparation of brain homogenate, whole brain from a Wistar rat was homogenized in PBS at 4°C using a Polytron (Kinematica, GmbH, Switzerland; 20 s, setting 7). Aliquots (5–10 μg protein) were mixed with 5× Laemmli sample buffer, boiled for 5 min, and subjected to SDS-PAGE and immunoblotting.
Subcellular Fractionation of RBL Cells.
RBL cells (7 × 107) were washed with PBS and suspended in homogenization buffer (0.25 M sucrose, 1 mM MgCl2, 800 U/ml DNase I [Sigma Chemical Co.], 10 mM Hepes, pH 7.4, 1 mM PMSF, and a cocktail of protease inhibitors [Boehringer Mannheim, Germany]). Cells were then disrupted by 3 cycles of freezing and thawing followed by 20 passages through a 21-gauge needle. Unbroken cells and nuclei were removed by sequential filtering through 5- and 2-μm filters (Poretics Co.). The final filtrate was then centrifuged for 10 min at 500 g and the supernatant loaded onto a continuous, 0.45–2.0 M sucrose gradient (10 ml), which was layered over a 0.3 ml cushion of 70% (wt/wt) sucrose and centrifuged for 18 h at 100,000 g. Histamine was assayed fluorimetrically after condensation in alkaline medium with o-phthalaldehyde (30). LDH activity was assayed using LDH reagent according to the manufacturer's instructions (Merck Diagnostica, Germany).
Secretion from RBL Cells.
RBL-2H3 cells were seeded in 24-well plates at 2 × 105 cells per well and incubated overnight in a humidified incubator at 37°C. The cells were then washed three times in Tyrode's buffer (10 mM Hepes, pH 7.4, 130 mM NaCl, 5 mM KCl, 1.4 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 0.1% BSA) and stimulated in the same buffer with the indicated concentrations of the calcium ionophore A23187 and the phorbol ester 12-O-tetradecanoyl-13-acetate (TPA; Calbiochem). Secretion was allowed to proceed for 30 min at 37°C. Aliquots from the supernatants were taken for measurements of released β-hexosaminidase activity. Cells in control wells were lysed by addition of 0.1% Triton X-100 to determine the total enzyme content. For FcεRI induced secretion, cells were passively sensitized by overnight incubation with DNP specific monoclonal IgE (SPE7, a gift of Dr. Z. Eshhar, the Weizmann Institute of Science, Rehovot, Israel), washed three times in Tyrode's buffer, and then stimulated with the indicated concentrations of the antigen, DNP-BSA. Activity of the released β-hexosaminidase was determined by incubating aliquots (20 μl) of supernatants and cell lysates for 90 min at 37°C with 50 μl of the substrate solution consisting of 1.3 mg/ml p-nitrophenyl-N-acetyl-β-d-glucosaminide (Sigma Chemical Co.) in 0.1 M citrate pH 4.5. The reaction was stopped by the addition of 150 μl of 0.2 M glycine, pH 10.7. OD was read at 405 nm, in an ELISA reader. Results were expressed as percentage of total β-hexosaminidase activity present in the cells. To assay the release of cathepsin D, supernatants of cells, stimulated as above, were concentrated in VivaSpin concentrators with a 10 kD cut-off (VivaScience, UK). The concentrates were mixed with 5× Laemmli sample buffer, boiled for 5 min, and subjected to SDS-PAGE and immunoblotting with anti–cathepsin D antibodies. For measurement of serotonin release, cells were incubated overnight with 2 μCi of [3H]5-hydroxytryptamine (NEN), washed, and stimulated as above. Aliquots from the supernatants were taken for measurement of radioactivity.
SDS-PAGE and Immunoblotting.
Samples (normalized according to protein content or number of cells) were separated by SDS-PAGE using 10 or 12% polyacrylamide gels. They were then electrophoretically transferred to nitrocellulose filters. Blots were blocked for 3 h in TBST (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 0.05% Tween 20) containing 5% skim milk followed by overnight incubation at 4°C with the indicated primary antibodies. Blots were washed three times and incubated for 1 h at room temperature with the secondary antibody (horseradish peroxidase–conjugated goat anti–rabbit or anti–mouse IgG; Jackson Research Labs.). Immunoreactive bands were visualized by the enhanced chemiluminescence method according to standard procedures.
Cell Transfection.
A full-length rat Syt II cDNA (provided by Dr. T.C. Sudhof) was subcloned into the EcoRI site of the pcDNA3 expression vector (Invitrogen) both in the sense and antisense orientations. RBL-2H3 cells (8 × 106) were transfected with 20 μg DNA of pcDNA3-Syt II or pcDNA3 alone, by electroporation (0.25 V, 960 μF). Cells were immediately replated in tissue culture dishes containing growth medium (supplemented DMEM). G418 (1 mg/ml) was added 24 h after transfection and stable transfectants were selected within 14 d.
Results
Expression Analysis of Endogenous Syt Isoforms.
Primers were chosen from the conserved C2 domains (Fig. 1 A) that averaged 91% identity with known Syt isoforms. An initial round of PCR with four different primer pairs did not yield any visible product in reactions containing RBL cell cDNA, even though abundant product was obtained from brain cDNA using two different primer pairs (Fig. 1 B). When the PCR product of RBL cell cDNA with primers A and D was then used as template in a second round of PCR, the nested reaction with primers B and C yielded abundant product of the predicted size of 365 bp (Fig. 1 C). Sequencing the inserts of 21 colonies of subcloned PCR product yielded 10 colonies encoding a fragment of Syt II, 9 colonies encoding Syt III, and 2 colonies encoding Syt V. These findings were supported by the results of restriction digestions of the PCR product with multiple frequent cutting enzymes (data not shown) that were consistent with the presence of these three isoforms and did not indicate the presence of additional isoforms based on the known sequences of Syt isoforms.
RNase protection assays were then performed to quantitatively assess the expression of Syt isoforms in RBL cells. Syt II was the most abundant (Fig. 2), and serial dilution of mRNA used to protect the Syt II probe indicated that this isoform was approximately fivefold more concentrated in RBL cells than in Syt III. The Syt V isoform was not protected even when mRNA was present at a level at least 10-fold higher than that which measurably protected the Syt II probe (Fig. 2), suggesting that Syt V mRNA is present in RBL cells at a concentration less than one-tenth the level of Syt II.
Figure 2.
RNase protection assay of RBL cell transcripts. Autoradiogram of the products of an RNase protection assay after PAGE. The riboprobes used for hybridization are listed in the top row, the amount of mRNA loaded in each lane is listed in the second row, and a size marker is shown on the left side. This figure is a representative example of an experiment that was repeated four times.
Because Syt II, which shares the highest homology with the predominant neural isoform Syt I (31), was the most abundant isoform, we chose to focus this study on Syt II. We next examined the expression of the Syt II protein using specific antibodies (mAb 8G2B, directed against the NH2 terminus of Syt II). A single immunoreactive protein was detected in RBL cells (Fig. 3, lane 2). Immunoreactivity in RBL cells (Mr ∼80 kD) had less mobility on SDS-PAGE than immunoreactivity in the brain (Fig. 3, lane 1). Nevertheless, an 80-kD Syt II–immunoreactive protein was also detected in lysates from fully differentiated, connective tissue–type, RPMCs (Fig. 3, lane 3) and primary murine BMMCs (Fig. 3, lane 4). These size differences in Syt II may thus arise from tissue-specific posttranslational modifications.
Figure 3.

Expression of Syt II protein in mast cells. Whole lysates (106 cell equivalents) derived from RBL-2H3 cells (lane 2), RPMCs (lane 3), BMMCs (lane 4), and a crude brain homogenate (lane 1, 10 μg protein), were resolved by SDS-PAGE and immunoblotted using the mAb 8G2B directed against the NH2 terminus of Syt II.
Effect of Syt II Overexpression on Ca2+-induced Exocytosis.
To study the functional role of Syt II, we stably transfected RBL cells with neural Syt II cDNA and selected clones with increased levels (approximately twofold) of Syt II expression (Syt II+; Fig. 4, lanes 1–3) for further studies. Notably, transfection with neural Syt II cDNA resulted in overexpression of the same 80-kD Syt II–immunoreactive protein, strengthening the concept that the increased apparent Mr of RBL-Syt II was due to tissue-specific posttranslational modifications.
Figure 4.
Overexpression of Syt II in RBL cells. Whole lysates derived from G418-resistant RBL clones (1.5 × 106 cell equivalents), transfected with either the pcDNA3-Syt II recombinant vector (Syt II+, lanes 1–3) or with the empty pcDNA3 vector (control, lanes 4–6) were resolved by SDS-PAGE and immunoblotted using monoclonal 8G2B anti–Syt II antibodies.
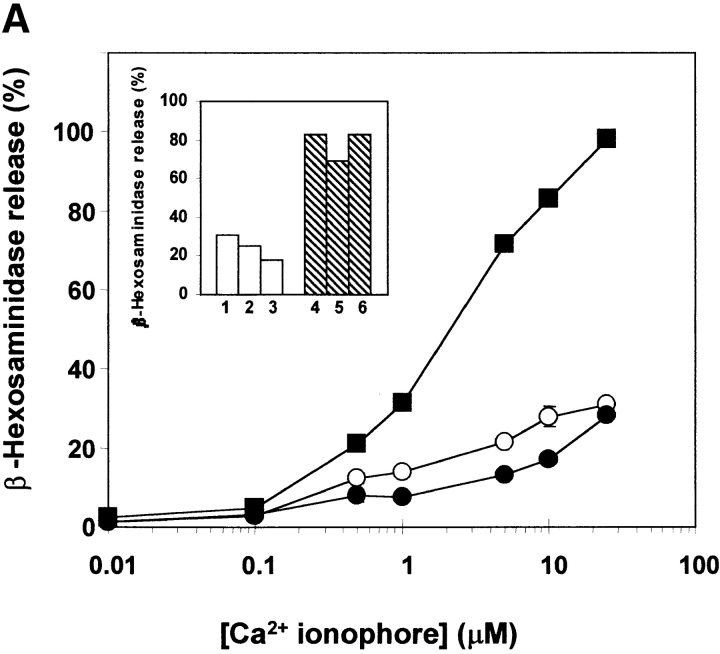
Overexpression of Syt II had no effect on the spontaneous release of the SG-associated enzyme, β-hexosaminidase (32). In the absence of any stimulus, both control cells (empty vector-transfected) and cells overexpressing Syt II released up to 5% of their total β-hexosaminidase (Fig. 5 A). However, in contrast to transfection with Syt I (27), overexpression of Syt II failed to potentiate Ca2+-dependent exocytosis evoked by a Ca2+ ionophore alone (Fig. 5 A), or in the presence of phorbol ester (Fig. 5 B). Instead, a mild inhibition could be observed when the cells were triggered with low (<10 μM) concentrations of the Ca2+ ionophore.
Figure 5.
Modulation of exocytosis by Syt II. Control (○), Syt II+ (•), and Syt II− (▪) cells were incubated for 30 min at 37°C with the indicated concentrations of the Ca2+ ionophore A23187, alone (A) or together with 50 nM TPA (B). The extent of release is presented as percentage of total β-hexosaminidase activity. The results presented in A are from one experiment, which included single clones stably transfected with the empty pcDNA3 vector, the pcDNA3–Syt II recombinant vector, or the pcDNA3–Syt II recombinant vector in the antisense orientation. Similar results were obtained on five occasions and using two additional clones. The data points presented in B are means ± SEM of four determinations and include two independent clones stably transfected with the empty pcDNA3 vector, one clone stably transfected with the pcDNA3-Syt II recombinant vector, and two independent clones stably transfected with pcDNA3-Syt II in the antisense orientation. Similar results were obtained on five occasions. Inset: β-hexosaminidase release of individual clones (1–3 stably transfected with the empty pcDNA3 vector and 4–6 stably transfected with pcDNA3-Syt II in the antisense orientation) at a representative concentration of agonist: A, 10 μM A23187; B, 1 μM A23187 and 50 nM TPA.
Effect of the Suppression of Syt II Expression on Ca2+-induced Exocytosis.
The fact that Syt II, unlike Syt I, is endogenously expressed in RBL cells enabled us to extend these results by investigating the effect of reducing the level of Syt II expression on exocytosis. To this end, cells were stably transfected with Syt II cDNA subcloned in the antisense orientation, resulting in substantially reduced levels of Syt II expression (15, 6, 47, and 6% of control levels) (Fig. 6). Clones expressing the lowest levels (6–15%) were chosen for further analyses. In these cells (Syt II−), the basal, spontaneous release of β-hexosaminidase was not affected, revealing that Syt II was not acting as a limiting fusion clamp. However, β-hexosaminidase release triggered by a Ca2+ ionophore alone (Fig. 5 A) or with phorbol ester (Fig. 5 B) was markedly (by up to fivefold) potentiated.
Figure 6.
Suppression of Syt II expression. Whole lysates derived from G418-resistant RBL clones (1.5 × 106 cell equivalents), transfected with either the pcDNA3-Syt II antisense orientation (Syt II−, lanes 1–4) or with the empty pcDNA3 vector (control, lanes 5–7) were resolved by SDS-PAGE and immunoblotted as in Fig. 3.
The Role of Syt II in FcεRI-induced Exocytosis.
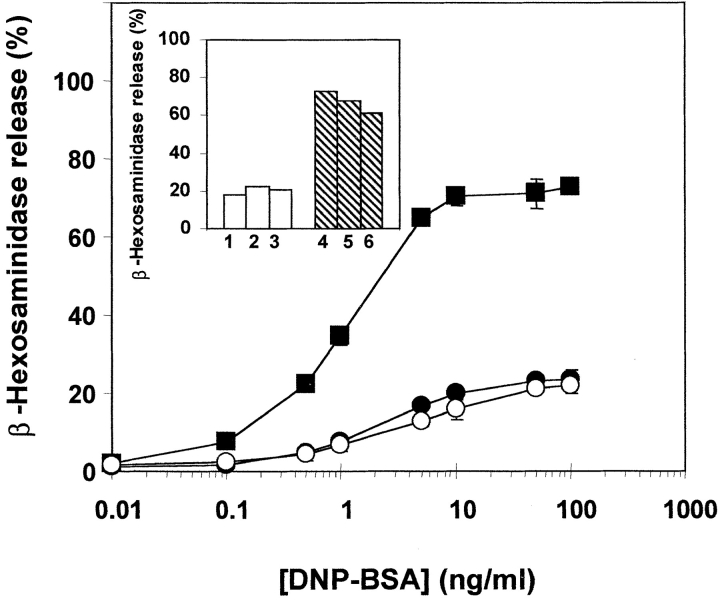
Physiologically, exocytosis in mast cells can be triggered by antigen- induced aggregation of the high-affinity receptors (FcεRI) for IgE (33). To investigate the involvement of Syt II in controlling FcεRI-mediated exocytosis, antigen-induced secretion was studied in the Syt II− and Syt II+ cells. Secretion of β-hexosaminidase was unaffected in the Syt II+ cells but was significantly elevated (by fourfold) in the Syt II− cells (Fig. 7). Taken together, these results suggest that Syt II negatively regulates release of β-hexosaminidase, whether triggered by Ca2+ ionophore or by an immunological trigger.
Figure 7.
Modulation of FcεRI-dependent release by Syt II. Passively sensitized control (○), Syt II+ (•), and Syt II− (▪) cells were incubated for 30 min at 37°C with the indicated concentrations of the corresponding antigen, DNP-BSA. The extent of release is presented as percentage of total β-hexosaminidase activity. The results presented are of a representative experiment, that included three independent clones, stably transfected with the empty pcDNA3 vector, three independent clones stably transfected with the pcDNA3-Syt II recombinant vector and three independent clones stably transfected with pcDNA3-Syt II in the antisense orientation. The data points are means ± SEM of six determinations. Similar results were obtained on five occasions. Inset: β-hexosaminidase release of individual clones (1–3 stably transfected with the empty pcDNA3 vector and 4–6 stably transfected with pcDNA3-Syt II in the antisense orientation) at a representative concentration of antigen (10 ng/ml DNP-BSA).
Subcellular Distribution of Syt II in RBL Cells.
To understand the opposite effects exerted by the transfected Syt I and Syt II proteins, we investigated their distribution in RBL cells using a continuous sucrose gradient. All of the Syt II immunoreactivity present in either the control or the Syt II– transfected cells comigrated with 60% of the β-hexosaminidase activity, present in fractions 6–13 at ∼0.75 M sucrose (Fig. 8, A, B, E, and F). These fractions did not include the histamine-containing SGs, which migrated at fractions 16–23 at 1.3 M sucrose and included the remaining β-hexosaminidase activity (Fig. 8, E and F). Histamine was also found at the top of the gradient (Fig. 8 E), but this probably reflected the contents of SGs that were released during cell disruption. Therefore, these results indicate that, unlike transfected Syt I (Fig. 8 C), the endogenous and transfected Syt II proteins were not targeted to the histamine-containing SGs, but to a different intracellular compartment.
Figure 8.

Subcellular fractionation of control and Syt II–transfected RBL cell lysates. Fractions from a continuous sucrose gradient were collected from the top, and assayed for: A, Syt II immunoreactivity in control cells; B, Syt II immunoreactivity in Syt II+ cells; C, Syt I immunoreactivity in Syt I+ cells; D, pro-cathepsin D immunoreactivity; E, β-hexosaminidase activity (presented as OD read at 405 nm) (▪); histamine content (□) and LDH activity (•); (F) protein (•) and sucrose density (○). The data presented in panels E and F is the average of three sucrose gradients performed on control, Syt II+, and Syt I+ cells.
The presence of β-hexosaminidase in fractions 6–13 of the sucrose gradient suggested the presence of a lysosomal organelle distinct from the histamine-containing SGs. Indeed, fractions 6–13 also contained procathepsin D, Mr 53 kD, the precursor of the lysosomal protease cathepsin D (Fig. 8 D).
Effects of Syt II on the Release of Cathepsin D.
Lysosomes were recently shown to behave as Ca2+-regulated exocytic vesicles (34). Since β-hexosaminidase is distributed between histamine-containing SGs and procathepsin D–containing lysosomes in RBL cells, it was important to determine whether secretion of the content of the latter compartment is negatively regulated by Syt II. To address this question, we examined whether Syt II could modulate release of the lysosomally processed form of cathepsin D (mature cathepsin D, Mr ∼43 kD) (35). Concentrating the cell supernatants by 20-fold allowed the detection of cathepsin D in supernatants from Ca2+ ionophore- or antigen-triggered cells (Fig. 9 A, lanes 1–3). The amount of secreted mature cathepsin D was significantly inhibited or increased in the Syt II+ or Syt II− cells, respectively (Fig. 9). Ca2+ ionophore was more effective than the immunological stimulus in the Syt II+ cells, but did not differ significantly in Syt II− cells. The precursor form of cathepsin D (53 kD) was detected in supernatants of both triggered and nontriggered cells (data not shown), reflecting the constitutive release of unprocessed cathepsin D (34). These results demonstrate that mast cell activation triggers exocytosis of a lysosomal fraction distinct from histamine-containing SGs, and that mobilization of this compartment depends substantially on the expression level of Syt II.
Figure 9.
Release of Cathepsin D. (A) Control RBL cells (lanes 1–3), Syt II+ cells (lanes 4–6), and Syt II− cells (lanes 7–9) were incubated for 30 min at 37°C with buffer (lanes 1, 4, and 7), 50 ng/ml of the DNP-BSA antigen (lanes 2, 5, and 8), or 10 μM of the Ca2+ ionophore A23187 (lanes 3, 6, and 9). The concentrated cell supernatants were resolved by SDS-PAGE and immunoblotted using anti–cathepsin D (Cat D) antibodies. (B) The intensity of the band corresponding to mature cathepsin D was quantitated by densitometry (using a B.I.S. 202D densitometer, Dinco & Rhenium, Israel) and is presented as fold stimulation of the level in control, nonstimulated cells.
Effects of Syt II on the Release of Serotonin.
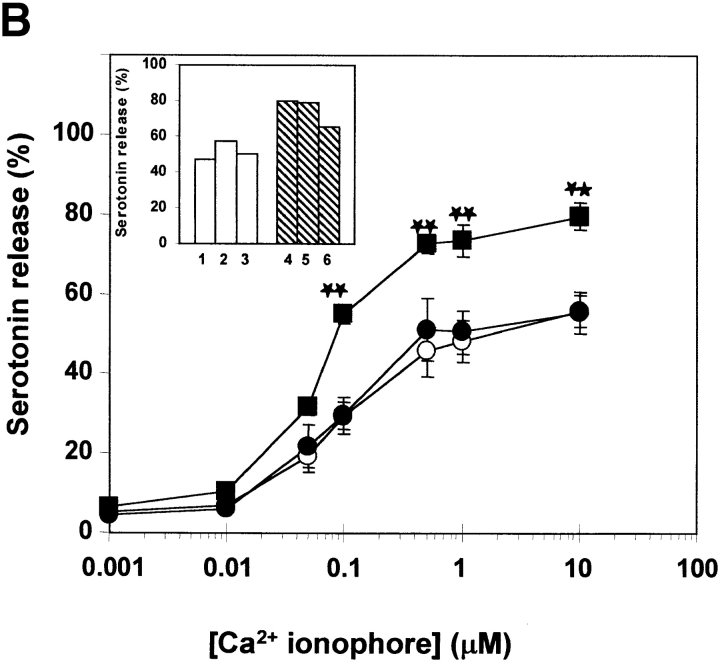
We have also evaluated the effects of Syt II on the triggered release of serotonin, to exclusively monitor exocytosis of SGs (36). Overexpression of Syt II had no significant effect on serotonin release triggered by either secretagogue (Fig. 10, A–C). However, reducing its level of expression in the Syt II− cells had a small but significant stimulatory effect (Fig. 10, A–C).
Figure 10.
Release of serotonin. Control (○), Syt II+ (•) and Syt II− (▪) cells, loaded with [3H]5-hydroxytryptamine (serotonin), were stimulated for 30 min at 37°C with the indicated concentrations of the Ca2+ ionophore A23187 alone (A), together with 50 nM TPA (B), or with the antigen DNP-BSA (C). The extent of serotonin release is presented as percentage of the total radioactivity in the cells. The data points presented are means ± SEM of 8–12 determinations and include three independent clones stably transfected with the empty pcDNA3 vector, three independent clones stably transfected with the pcDNA3-Syt II recombinant vector, and three independent clones stably transfected with pcDNA3-Syt II in the antisense orientation. Statistical analysis was performed using two-tailed student's t test. *P < 0.05; **P < 0.01. Inset: serotonin release of individual clones (1–3 stably transfected with the empty pcDNA3 vector and 4–6 stably transfected with pcDNA3-Syt II in the antisense orientation) at a representative concentration of agonist: A, 10 μM A23187; B, 1 μM A23187 and 50 nM TPA; and C, 10 ng/ml DNP-BSA.
Discussion
Previous studies have already alluded to the possibility that Syt isoforms may serve the role of general Ca2+ sensors, controlling regulated exocytosis also in nonneuronal secretory cells (23, 25, 26). We and others have previously shown that mast cells express Syt and SNAREs that probably function to control mediators released from these cells (27, 37). Here, we demonstrate that RBL cells endogenously express at least three distinct isoforms of Syt, including Syt II, III, and V. Syt II was identified both by RNAase protection assays (Fig. 2) and at the protein level, on the basis of its immunoreactivity with specific antibodies (Fig. 3). However, in contrast to its location on SVs or SGs in neurons or endocrine cells, in the RBL cells, Syt II cofractionates with the lysosomal fraction rather than with the histamine-containing SGs (Fig. 8). Furthermore, transfection of the RBL cells with neural Syt II cDNA resulted in overexpression of Syt II (Fig. 4) and its targeting to the same fraction (Fig. 8).
Mast cells belong to immune cells of the hemopoietic lineage, where an intimate connection exists between lysosomes and SGs (38). The SGs of mast cells include, in addition to their secretory cargo of vasoactive amines (e.g., histamine and serotonin), lysosomal enzymes such as β-hexosaminidase, β-glucuronidase, arylsulfatase, and carboxypeptidases (32), as well as lysosomal integral membrane proteins (LIMPs) (39). Therefore, mast cell SGs can be defined as secretory lysosomes. Nevertheless, in consistence with previous data (40, 41), our data indicate that in addition to the lysosomal, amine-containing SGs, mast cells also contain lysosomes, which lack biogenic amines and with which Syt II is associated (Fig. 8). Such amine-free lysosomes were previously reported to resist cell triggering by immunologic or Ca2+ ionophore stimulation (40, 41). Whether the two populations of granules are sequentially formed and by what mechanism selective retention of the nonsecretory lysosomes is achieved, remained unknown. We now demonstrate that mast cells can, to some extent, release also their lysosomal pool of hydrolases, upon both an immunologic and a Ca2+ ionophore trigger. In this process both lysosomal enzymes, which are distributed between both SGs and lysosomes, such as β-hexosaminidase, as well as hydrolases localized exclusively to the amine-free lysosomal fraction, such as cathepsin D (Fig. 9), are released. However, this release is inhibited by overexpression of Syt II and markedly potentiated by reducing the level of Syt II expression (Fig. 9). Recently, three types of granules were ultrastructurally distinguished in IFN-γ–treated mast cells (Table I). Type I and type II granules were both labeled by a fluid phase endocytic marker and both contained MHC class II as well as lysosomal markers (42). These results have therefore suggested their position in the endocytic pathway, similarly to lysosomal compartments (42). Serotonin was localized to type II and type III granules, of which the latter type did not internalize the fluid phase endocytic marker, nor did it contain MHC class II (42). Based on these results, it was suggested that a fusion event between type I (amine-free lysosomes) and III (e.g., SGs) granules may account for the formation of type II granules (42). Our results are compatible with this model and define Syt II as the molecular entity, which may control this fusion event and effect selective retention of the nonsecretory lysosomes during cell activation (see model shown in Fig. 11). Furthermore, this model predicts that downregulation of Syt II should also indirectly affect SG exocytosis by facilitating the fusion event between the amine-free lysosomes and SGs. Indeed, we found that suppression of Syt II level of expression also moderately potentiates serotonin release (Fig. 10).
Table I.
Characterization of Mast Cell Granules
| Granule type | MHC class II | β-hexosaminidase | Serotonin | |||
|---|---|---|---|---|---|---|
| I | + | + | − | |||
| II | + | + | + | |||
| III | − | + | + |
Based on Raposo et al. (42).
Figure 11.
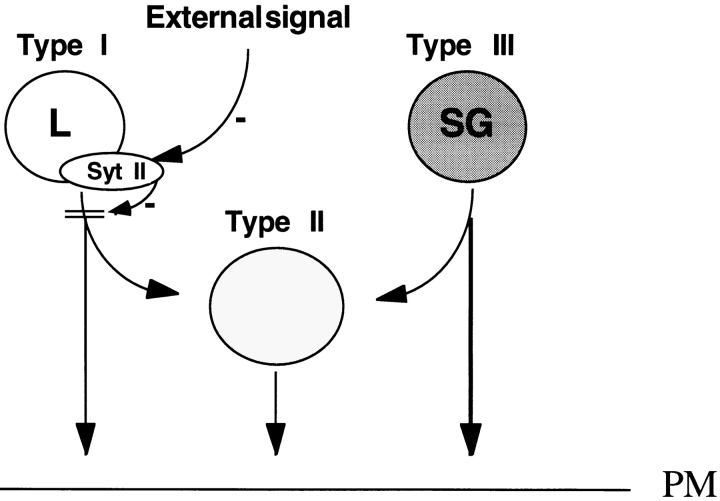
Model of regulation of lysosomal exocytosis by Syt II. According to this model, Syt II is localized to lysosomes (L), where it acts to inhibit fusion with SGs and the plasma membrane, at Ca2+ concentrations that already support SG exocytosis. External signals are predicted to downregulate Syt II and thereby remove this inhibition and facilitate lysosomal exocytosis as well as fusion with SGs.
The molecular mechanism by which Syt II inhibits lysosome exocytosis is currently unknown. Syts fall into three distinct classes that for syntaxin binding require either high Ca2+ concentrations (200 μM) (class A) or low Ca2+ concentrations (<1 μM) (class B), or do not bind syntaxin in a Ca2+-dependent manner (class C) (20). Syt II, the major RBL isoform, and Syt V, the least abundant isoform, are class A proteins. However, although Ca2+ concentrations measured in neurons during an action potential are high enough to support Ca2+-dependent interaction of class A Syts with syntaxins, the rise of intracellular Ca2+ concentration in mast cells induced by FcεRI cross-linking rarely exceeds 1 μM and would not be predicted to support such interaction (43). Calcium-dependent Syts negatively regulate neuronal exocytosis at basal Ca2+ concentrations (44), whereas positive effects on exocytosis are observed only at elevated Ca2+ concentrations and are thought to depend on interaction with syntaxin (20). In the mast cells Syt II seems to increase the Ca2+ requirements for lysosomal exocytosis, since Ca2+ ionophore is far more effective than immunologic stimulation in triggering cathepsin D release from control cells, but both are equally potent in Syt II− cells (Fig. 9). It is of great interest that Syt II appears to be used in mast cells as a negative regulator of Ca2+-dependent exocytosis and of a subclass of secretory vesicles, and is the first example to our knowledge. Syt II inhibitory function appears to be linked to its lysosomal association since Syt I, although highly homologous to Syt II, potentiated Ca2+-dependent exocytosis of SG when transfected into the RBL cells, alongside its SG targeting (27). The reasons for this differential targeting of Syt I and Syt II remain unknown.
Although not proven here, the remaining Syt isoform expressed in RBL cells, Syt III, which is a class B protein, would be an adequate candidate to serve as the positive regulator of SG exocytosis, whose action is mimicked by transfected Syt I.
In conclusion, our findings provide unequivocal evidence for an active role of Syt II in negatively controlling Ca2+-regulated lysosomal exocytosis. This observation extends the function of Syt II to regulation of exocytosis of secretory organelles exclusive to SVs or SGs. Specifically, in mast cells regulation by Syt II may have important implications on their function as APCs in host defense mechanisms, as this process requires uptake and lysosomal processing of antigens, followed by presentation of MHC class II–peptide complexes on the mast cell surface (10, 45). Our model predicts that the cellular level of Syt II could be up- or downregulated to determine the balance of mast cell effector function between the secretion of inflammatory mediators from SG exocytosis and the presentation of antigen by externalization of MHC II–containing lysosomes. Syt II may thus play a central role in controlling the physiological functions of mast cells.
Acknowledgments
We thank Drs. Y. Zick and D. Neumann for helpful discussions and a critical reading of this manuscript; and Drs. T.C. Sudhof, M. Takahashi, and Z. Eshhar for their generous gifts of cDNA and antibodies.
This work was supported by grants from the Israel Science Foundation, founded by the Israel Academy for Sciences and Humanities, and by the Thyssen Stiftung (to R. Sagi-Eisenberg).
Abbreviations used in this paper
- BMMC
bone marrow-derived mast cell
- RBL
rat basophilic leukemia
- RPMC
rat peritoneal mast cell
- SG
secretory granule
- SV
synaptic vesicle
- Syt
synaptotagmin
- TPA
12-O-tetradecanoylphorbol-13-acetate
References
- 1.Stevens RL, Austen KF. Recent advance in the cellular and molecular biology of mast cells. Immunol Today. 1989;10:381–385. doi: 10.1016/0167-5699(89)90272-7. [DOI] [PubMed] [Google Scholar]
- 2.Galli SJ, Gordon JR, Wershil BK. Cytokine production by mast cells and basophils. Curr Opin Immunol. 1991;3:865–872. doi: 10.1016/s0952-7915(05)80005-6. [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe DD, Kaliner M, Donlon MA. The mast cell. Crit Rev Immunol. 1981;3:23–74. [PubMed] [Google Scholar]
- 4.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 5.Prodeus AP, Zhou X, Maurer M, Galli SJ, Carroll MC. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature. 1997;390:172–175. doi: 10.1038/36586. [DOI] [PubMed] [Google Scholar]
- 6.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 7.Abraham SN, Malaviya R. Mast cells in infection and immunity. Infect Immun. 1997;65:3501–3508. doi: 10.1128/iai.65.9.3501-3508.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galli SJ. The Paul Kallos memorial lecture. The mast cell: a versatile effector cell for a challenging world. Int Arch Allergy Immunol. 1997;113:14–22. doi: 10.1159/000237497. [DOI] [PubMed] [Google Scholar]
- 9.Malaviya R, Twesten NJ, Ross EA, Abraham SN, Pfeifer JD. Mast cells process bacterial Ags through a phagocytic route for class I MHC presentation to T cells. J Immunol. 1996;156:1490–1496. [PubMed] [Google Scholar]
- 10.Frandji P, Oskeritzian C, Cacaraci F, Lapeyre J, Peronet R, David B, Guillet JG, Mecheri S. Antigen-dependent stimulation by bone marrow-derived mast cells of MHC class II-restricted T cell hybridoma. J Immunol. 1993;151:6318–6328. [PubMed] [Google Scholar]
- 11.Gomperts BD. Ge: a GTP-binding protein mediating exocytosis. Annu Rev Physiol. 1990;52:591–606. doi: 10.1146/annurev.ph.52.030190.003111. [DOI] [PubMed] [Google Scholar]
- 12.Aridor M, Rajmilevich G, Beaven MA, Sagi-Eisenberg R. Activation of exocytosis by the heterotrimeric G protein Gi3. Science. 1993;262:1569–1572. doi: 10.1126/science.7504324. [DOI] [PubMed] [Google Scholar]
- 13.Foreman JC, Mongar JL, Gomperts BD. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973;245:249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- 14.Howell TW, Cockcroft S, Gomperts BD. Essential synergy between calcium and guanine nucleotides in exocytotic secretion from permeabilized rat mast cells. J Cell Biol. 1987;105:191–198. doi: 10.1083/jcb.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bommert K, Charlton MP, DeBello WM, Chin GJ, Betz H, Augustine GJ. Inhibition of neurotransmitter release by C2-domain peptides implicates synaptotagmin in exocytosis. Nature. 1993;363:163–165. doi: 10.1038/363163a0. [DOI] [PubMed] [Google Scholar]
- 16.Brose N, Petrenko AG, Sudhof TC, Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- 17.DeBello WM, Betz H, Augustine GJ. Synaptotagmin and neurotransmitter release. Cell. 1993;74:947–950. doi: 10.1016/0092-8674(93)90716-4. [DOI] [PubMed] [Google Scholar]
- 18.Elfernik LA, Peterson MR, Scheller RH. A role for synaptotagmin (p65) in regulated exocytosis. Cell. 1993;72:153–159. doi: 10.1016/0092-8674(93)90059-y. [DOI] [PubMed] [Google Scholar]
- 19.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major Ca2+sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 20.Sudhof TC, Rizo J. Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron. 1996;17:379–388. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- 21.Chapman ER, Hanson PI, An S, Jahn R. Ca2+regulates the interaction between synaptotagmin and syntaxin 1. J Biol Chem. 1995;270:23667–23671. doi: 10.1074/jbc.270.40.23667. [DOI] [PubMed] [Google Scholar]
- 22.Shao X, Li C, Fernandez I, Zhang X, Sudhof TC, Rizo J. Synaptotagmin-syntaxin interaction: the C2 domain as a Ca2+-dependent electrostatic switch. Neuron. 1997;18:133–142. doi: 10.1016/s0896-6273(01)80052-0. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Ullrich B, Zhang JZ, Anderson RG, Brose N, Sudhof TC. Ca2+-dependent and -independent activities of neural and non-neural synaptotagmins. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 24.von Poser C, Ichtchenko K, Shao X, Rizo J, Sudhof TC. The evolutionary pressure to inactivate. A subclass of synaptotagmins with an amino acid substitution that abolishes Ca2+binding. J Biol Chem. 1997;272:14314–14319. doi: 10.1074/jbc.272.22.14314. [DOI] [PubMed] [Google Scholar]
- 25.Lang J, Fukuda M, Zhang H, Mikoshiba K, Wollheim CB. The first C2 domain of synaptotagmin is required for exocytosis of insulin from pancreatic beta-cells: action of synaptotagmin at low micromolar calcium. EMBO (Eur Mol Biol Organ) J. 1997;16:5837–5846. doi: 10.1093/emboj/16.19.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizuta M, Kurose T, Miki T, Shoji KY, Takahashi M, Seino S, Matsukura S. Localization and functional role of synaptotagmin III in insulin secretory vesicles in pancreatic beta-cells. Diabetes. 1997;46:2002–2006. doi: 10.2337/diab.46.12.2002. [DOI] [PubMed] [Google Scholar]
- 27.Baram D, Linial M, Mekori YA, Sagi-Eisenberg R. Ca2+-dependent exocytosis in mast cells is stimulated by the Ca2+sensor, synaptotagmin I. J Immunol. 1998;161:5120–5123. [PubMed] [Google Scholar]
- 28.Katz HR, Dayton ET, Levi SF, Benson AC, Austen KF, Stevens RL. Coculture of mouse IL-3-dependent mast cells with 3T3 fibroblasts stimulates synthesis of globopentaosylceramide (Forssman glycolipid) by fibroblasts and surface expression on both populations. J Immunol. 1988;140:3090–3097. [PubMed] [Google Scholar]
- 29.Aridor M, Traub LM, Sagi-Eisenberg R. Exocytosis in mast cells by basic secretagogues: evidence for direct activation of GTP-binding proteins. J Cell Biol. 1990;111:909–917. doi: 10.1083/jcb.111.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shore PA, Burkhalter A, Cohn VH. A method for the fluorimetric assay of histamine in tissues. J Pharmacol Exp Ther. 1959;127:182–185. [PubMed] [Google Scholar]
- 31.Geppert M, Archer BT, III, Sudhof TC. Synaptotagmin II. A novel differentially distributed form of synaptotagmin. J Biol Chem. 1991;266:13548–13552. [PubMed] [Google Scholar]
- 32.Schwartz LB, Austen KF. Enzymes of the mast cell granule. J Invest Dermatol. 1980;74:349–353. doi: 10.1111/1523-1747.ep12543620. [DOI] [PubMed] [Google Scholar]
- 33.Segal DM, Taurog J, Metzger H. Dimeric immunoglobulin E serves as a unit signal for mast cell degranulation. Proc Natl Acad Sci USA. 1977;74:2993–2997. doi: 10.1073/pnas.74.7.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez A, Webster P, Ortego J, Andrews NW. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J Cell Biol. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishimura Y, Kato K, Furuno K, Himeno M. Biosynthesis and processing of lysosomal cathepsin D in primary cultures of rat hepatocytes. Biol Pharmacol Bull. 1995;18:825–828. doi: 10.1248/bpb.18.825. [DOI] [PubMed] [Google Scholar]
- 36.Mazingue C, Dessaint JP, Capron A. [3H]serotonin release: an improved method to measure mast cell degranulation. J Immunol Methods. 1978;21:65–77. doi: 10.1016/0022-1759(78)90224-7. [DOI] [PubMed] [Google Scholar]
- 37.Guo Z, Turner C, Castle D. Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell. 1998;94:537–548. doi: 10.1016/s0092-8674(00)81594-9. [DOI] [PubMed] [Google Scholar]
- 38.Griffiths GM. Secretory lysosomes-a special mechanism of regulated secretion in haemopoietic cells. Trends Cell Biol. 1996;6:329–332. doi: 10.1016/0962-8924(96)20031-5. [DOI] [PubMed] [Google Scholar]
- 39.Suarez QC. The distribution of four lysosomal integral membrane proteins (LIMPs) in rat basophilic leukemia cells. Tissue Cell. 1987;19:495–504. doi: 10.1016/0040-8166(87)90043-7. [DOI] [PubMed] [Google Scholar]
- 40.Sannes PL, Spicer SS. The heterophagic granules of mast cells: dipeptidyl aminopeptidase II activity and resistance to exocytosis. Am J Pathol. 1979;94:447–457. [PMC free article] [PubMed] [Google Scholar]
- 41.Jamur MC, Vugman I, Hand AR. Ultrastructural and cytochemical studies of acid phosphatase and trimetaphosphatase in rat peritoneal mast cells developing in vivo. Cell Tissue Res. 1986;244:557–563. doi: 10.1007/BF00212533. [DOI] [PubMed] [Google Scholar]
- 42.Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell. 1997;8:2631–2645. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim TD, Eddlestone GT, Mahmoud SF, Kuchtey J, Fewtrell C. Correlating Ca2+responses and secretion in individual RBL-2H3 mucosal mast cells. J Biol Chem. 1997;272:31225–31229. doi: 10.1074/jbc.272.50.31225. [DOI] [PubMed] [Google Scholar]
- 44.Littleton JT, Stern M, Perin M, Bellen HJ. Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin mutants. Proc Natl Acad Sci USA. 1994;91:10888–10892. doi: 10.1073/pnas.91.23.10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fox CC, Jewell SD, Whitacre CC. Rat peritoneal mast cells present antigen to a PPD-specific T cell line. Cell Immunol. 1994;158:253–264. doi: 10.1006/cimm.1994.1272. [DOI] [PubMed] [Google Scholar]