Abstract
The requirement of β7 integrins for lymphocyte migration was examined during an ongoing immune response in vivo. Transgenic mice (OT-I) expressing an ovalbumin-specific major histocompatibility complex class I–restricted T cell receptor for antigen were rendered deficient in expression of all β7 integrins or only the αEβ7 integrin. To quantitate the relative use of β7 integrins in migration in vivo, equal numbers of OT-I and OT-I-β7−/− or OT-I-αE−/− lymph node (LN) cells were adoptively transferred to normal mice. Although OT-I-β7−/− LN cells migrated to mesenteric LN and peripheral LN as well as wild-type cells, β7 integrins were required for naive CD8 T cell and B cell migration to Peyer's patch. After infection with a recombinant virus (vesicular stomatitis virus) encoding ovalbumin, β7 integrins became critical for migration of activated CD8 T cells to the mesenteric LN and Peyer's patch. Naive CD8 T cells did not enter the lamina propria or the intestinal epithelium, and the majority of migration of activated CD8 T cells to the small and large intestinal mucosa, including the epithelium, was β7 integrin–mediated. The αEβ7 integrin appeared to play no role in migration during a primary CD8 T cell immune response in vivo. Furthermore, despite dramatic upregulation of αEβ7 by CD8 T cells after entry into the epithelium, long-term retention of intestinal intraepithelial lymphocytes was also αEβ7 independent.
Keywords: migration, mucosa, integrins, activation, CD8
The dynamics of immune responses in vivo are controlled in part by the migratory patterns of lymphocytes and APC. Current hypotheses propose that antigen sampling by dendritic cells (DCs)1 occurs at external surfaces, such as the skin and mucosal sites, followed by DC migration to draining lymph nodes, where an immune response is initiated (1, 2). Naive lymphocytes traffic from the blood stream to lymph nodes via interactions with high endothelial venules, which subsequently allows antigen-specific interactions between naive lymphocytes and DC to occur in the paracortical regions of the lymph node (3, 4). Following activation, the migratory patterns of lymphocytes are altered such that activated T cells preferentially home to sites outside of secondary lymphoid tissue. This modification in trafficking patterns is accomplished via multiple mechanisms, including modulation of homing receptors on lymphocytes as well as modifications in endothelial cell chemokine and counter receptor expression, especially at sites of inflammation (3, 5–7).
Lymphocyte homing to the intestinal mucosa has been extensively studied (8–10). However, the intestinal mucosal immune system is composed of discrete inductive and effector sites and, therefore, the requirements for homing of activated and naive lymphocytes to the anatomically distinct areas may be different. Thus, the inductive sites, Peyer's patches (PPs) and mesenteric lymph nodes (MLNs), contain naive as well as activated/memory lymphocytes. In contrast, the effector sites, lamina propria (LP) and the intraepithelial lymphocyte (IEL) compartment, contain primarily, if not exclusively, activated or memory lymphocytes (11–14). The loosely organized tissue of the LP contains a mixture of CD4 and CD8 T cells, plasma cells, and memory B cells, whereas IELs are largely CD8+ T cells expressing either TCR-α/β or TCR-γ/δ, many of which express activation and/or memory phenotypes (15). To arrive in the epithelium, IELs presumably transmigrate across the basement membrane separating the LP and the overlying epithelium. As the LP lymphocyte and IEL populations are largely distinct with regard to subsets as well as TCR repertoire, there must exist a highly selective gate between the LP and IEL compartment, which is likely regulated at the level of adhesion receptors expressed by subsets of activated lymphocytes and ligands expressed by the basement membrane.
The α4β7 integrin and its ligand, the mucosal addressin MAdCAM-1 (mucosal addressin cell adhesion molecule 1), play an important role in entry of activated lymphocytes into PPs and LP (10). The other member of the β7 integrin family, αEβ7, is highly expressed by IEL (16–18), but whether this integrin is involved in migration of cells to the epithelium or in their retention following entry into the epithelium is not known (19, 20). Although much has been learned concerning lymphocyte migration to the mucosa using in vitro analyses and short-term in vivo adoptive transfer systems with labeled cells, visualization of trafficking during an ongoing immune response has been made possible only recently. Moreover, a comprehensive analysis of the role of integrins in lymphocyte trafficking during an immune response in vivo has not been performed. The adoptive transfer of TCR-transgenic T cells (21) or the detection of antigen-specific T cells using MHC tetramer reagents (22–24) has enabled the monitoring of antigen-specific T cells during immune responses in vivo. Using the system of transfer of TCR-transgenic T cells followed by immunization, we have shown that activation is required for entry of CD8 T cells into the LP and the intestinal epithelium (25, 26). In combination with mice lacking either all β7 integrins (27) or only the αEβ7 integrin, we have now used this system to analyze trafficking of CD8 T cells during an immune response in vivo.
Materials and Methods
Mice.
C57BL/6J (Ly5.1) mice were obtained from The Jackson Laboratory. C57BL/6-Ly5.2 mice were obtained from Charles River Labs. through the National Cancer Institute animal program. The OT-I mouse line (28) was maintained as a C57BL/6-Ly5.2 line or on a C57BL/6-RAG-1−/− background (The Jackson Laboratory). OT-I-β7−/− mice and OT-I-αE−/− mice were generated by intercross of the β7−/− (27) or αE−/− mouse lines with OT-I and screening for αE or β7 and transgenic TCR expression by flow cytometric analysis of peripheral blood. The αE−/− mouse line was produced by standard techniques and is described in detail elsewhere (29).
Recombinant Vesicular Stomatitis Virus Production and Infection.
Vesicular stomatitis virus encoding ovalbumin (VSV-ova) was produced by ligation of a XhoI-XbaI cDNA fragment containing the entire ova coding sequence into the pVSV-XN2 vector restricted by XhoI and NheI (30, 31). The ova gene–containing vector was transfected along with helper plasmids into BHK cells, and rVSV was recovered as previously described (30, 31). Ovalbumin production was assessed by Western blot analysis of detergent lysates and culture supernatants of infected BHK cells as previously described (26).
Adoptive Transfer.
This method was adopted from Kearney et al. (21). An equal mixture of 2.5 × 106 pooled LN cells (1.25 × 106 OT-I T cells from OT-I or OT-I-β7−/− or OT-I-αE−/− mice) were injected intravenously into C57BL/6J (Ly5.1), C57BL/6-Ly5.2, or C57BL/6-Ly5.1/5.2 mice, depending on the donor cell phenotype. 2 d later, mice were infected with 106 PFU wild-type Indiana serotype VSV (as control) or VSV-ova by intravenous injection. At the later times indicated, cells were isolated and analyzed for the presence of donor cells by flow cytometric analysis of Ly5.1/Ly5.2 expression.
Isolation of Lymphocyte Populations.
IEL and LP cells were isolated as described previously (32, 33). Superficial inguinal, axial, and brachial LNs (peripheral [P]LNs) or MLNs were removed and single cell suspensions were prepared using a tissue homogenizer. The resulting preparation was filtered through Nitex (Tetko Industries) and the filtrate centrifuged to pellet the cells.
Immunofluorescence Analysis.
The following mAbs were used in this study: 53-6.7, anti-CD8α (34); anti-Ly5.1 and anti-Ly5.2 (35); and 2E7, anti-αE integrin (18). mAbs specific for Vα2, Vβ5, and β7 integrin were obtained from PharMingen as fluorochrome or biotinylated conjugates. For staining, lymphocytes were resuspended in 0.2% PBS, 0.1% BSA, NaN3 (PBS/BSA/ NaN3) at a concentration of 106–107 cells/ml, followed by incubation at 4°C for 20 min with 100 μl properly diluted mAb. The mAbs were either directly labeled with FITC, PE, Cy5 (Amersham Life Science), or were biotinylated. For the latter, avidin– PE–Cy7 (Caltag Labs.) was used as a secondary reagent for detection. After staining, the cells were washed twice with PBS/BSA/ NaN3 and fixed in 3% paraformaldehyde buffer. Four-color analysis of relative fluorescence intensities was then performed with a FACSCalibur ™ (Becton Dickinson). Data were analyzed using LYSYS II™ (Becton Dickinson) or WinMDI software.
Results
Analysis of β7 Expression on β7- and αE-deficient TCR-transgenic T Cells.
To develop a system for analyzing the role of β7 integrins in trafficking of naive and activated CD8 T cells, we introduced TCR transgenes into mouse lines with disruption of the β7 integrin gene or the αE integrin gene. T cells of the OT-I mouse line express the transgene-encoded Vα2 and Vβ5 chains, are predominantly CD8, and recognize the ova peptide, SIINFEKL, in the context of MHC class I H-2Kb (28). LN cells from OT-I, OT-I-αE−/−, and OT-I-β7−/− mice were analyzed for TCR usage and CD8 and β7 integrin expression (Fig. 1). CD8 cells from the LN of all three strains of mice expressed Vα2 and Vβ5 (representative example shown in top panel). OT-I CD8 LN cells (either peripheral or mesenteric) expressed heterogenous levels of the β7 integrin, with a population of β7high cells evident. This population was absent in OT-I-αE−/− LN cells, indicating that the high-level expression was due to expression of the αE integrin chain coupled to the β7 chain. This was confirmed by simultaneous staining for αE and β7 integrins (data not shown). These patterns of β7 staining are analogous to what has been observed for nontransgenic naive T cell populations (36), indicating that despite homogenous TCR transgene expression, tissue-specific and cell type–specific heterogeneity in β7 integrin expression remained.
Figure 1.
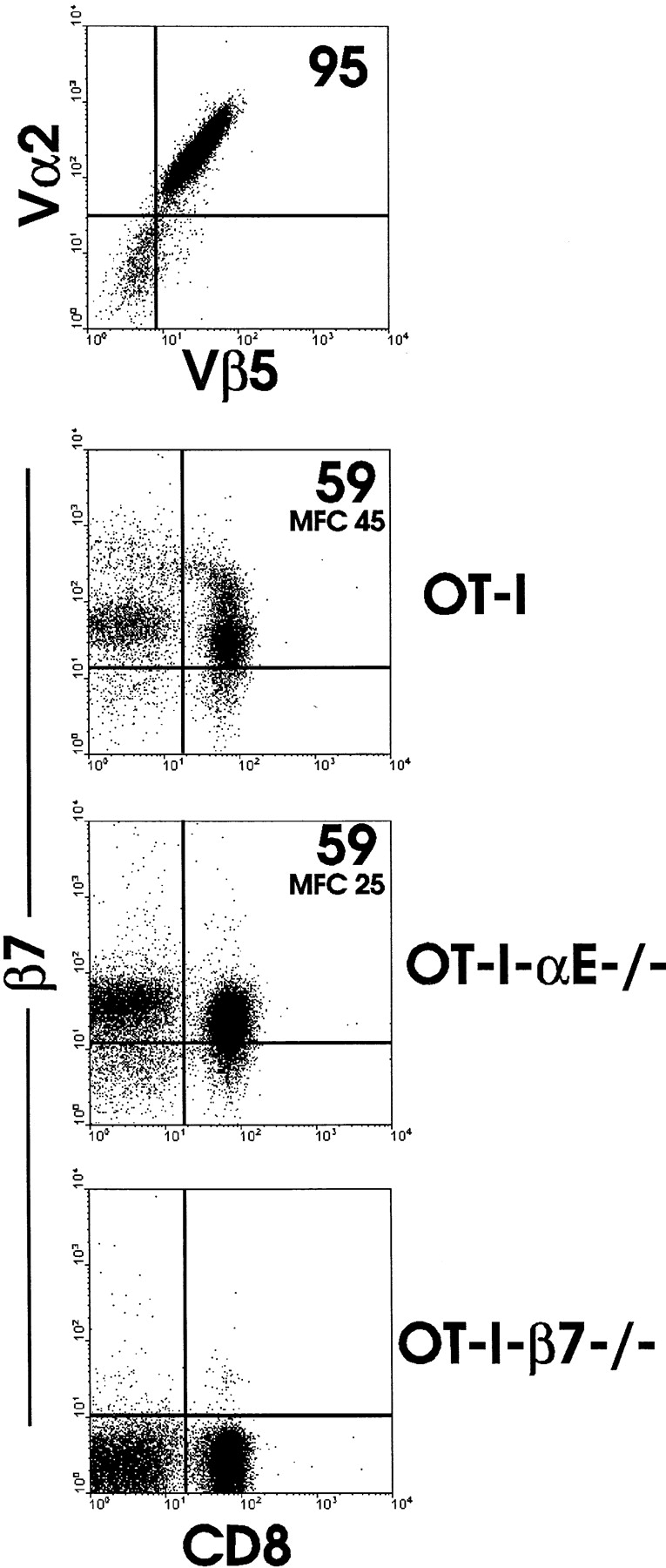
Analysis of β7 integrin expression in OT-I T cells lacking either β7 or αE chains. Lymphocytes from MLN of OT-I, OT-I-αE−/−, or OT-I-β7−/− mice were examined for expression of Vα2 and Vβ5 or for CD8 and β7 by fluorescence flow cytometry. Similar staining was observed with PLN cells (data not shown). Top panel, analysis of Vα2 and Vβ5 expression of gated CD8+ LN cells from OT-I mice. Similar results were obtained from analysis of CD8+ cells from all three mouse strains. MFC, mean fluorescence channel.
Adoptive Transfer System to Determine Requirements for β7 Integrins in Trafficking to Peripheral and Mucosal Inductive Sites.
To allow a direct comparison of migration of normal and integrin-deficient OT-I cells, we developed a system in which normal and mutant OT-I cells were mixed in equal proportions and then transferred to normal mice. By virtue of Ly5.1/Ly5.2 expression, transferred as well as host populations could be distinguished by flow cytometry (Fig. 2). In unimmunized mice, OT-I-β7−/− and normal OT-I cells migrated to PLN (data not shown) and MLN (Fig. 2, top panel) equally well. 3 d after immunization with rVSV-ova (26), a substantial increase in OT-I-β7−/− and OT-I cells had occurred in PLN. This result indicated that β7 integrins were not involved in primary activation of OT-I CD8 T cells and showed that β7 integrins were not necessary for migration of naive or activated CD8 T cells to PLN. In contrast, despite similar numbers of normal and mutant cells in the starting MLN population, 4.5-fold fewer OT-I-β7−/− cells were present in MLN after infection. All OT-I cells were activated, as determined by an increase in cell size and upregulation of CD44 (reference 26; data not shown). The population of OT-I-β7−/− cells present in MLN after infection was likely the result of expansion of those cells present before immunization. Thus, the difference between this value and the number of control cells may be used as an indicator of the degree of migration into MLN from the peripheral CD8 T cell pool. When PP lymphocytes were examined after infection, a barely detectable population of OT-I-β7−/− cells was found, indicating a near absolute requirement for β7 integrins in migration of activated CD8 T cells to PP.
Figure 2.
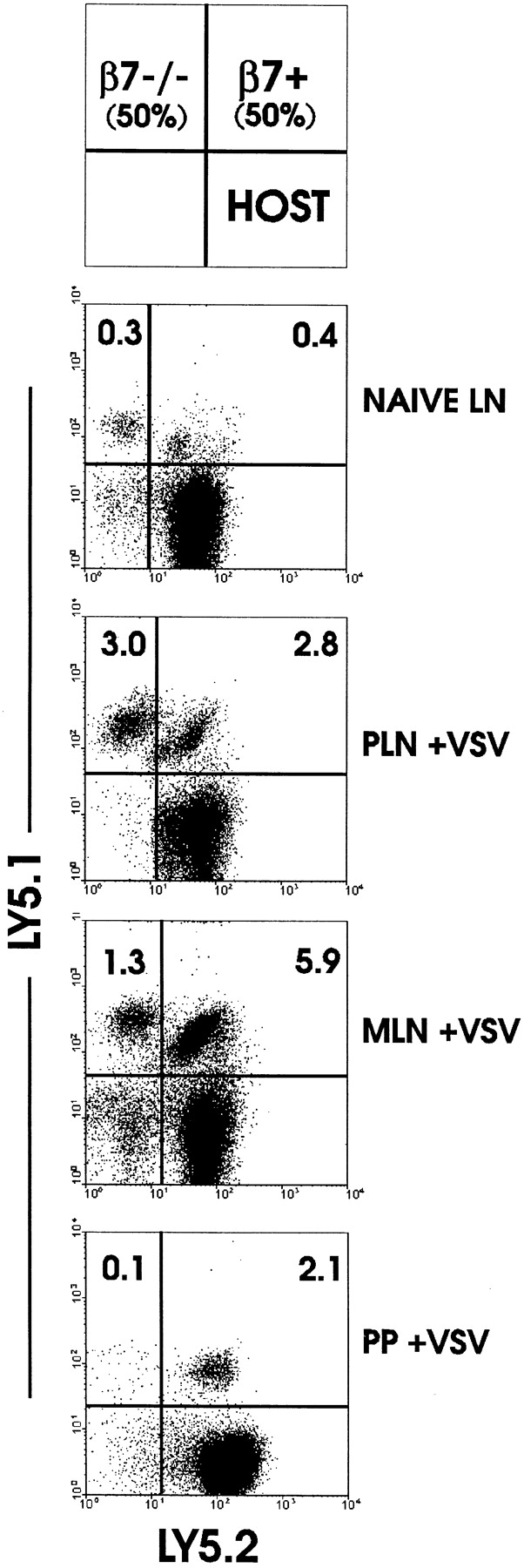
Tracking in secondary lymphoid organs of cotransferred OT-I and OT-I-β7−/− cells before and after virus infection. Equal numbers of LN cells from OT-I (Ly5.1+5.2+) and OT-I-β7−/−(Ly5.1+5.2−) mice were transferred to normal C57BL/6-Ly5.2 mice. 48 h later, mice were infected with VSV-ova (+VSV) or not (for naive LN, MLN cells are shown; PLN cells were similar). 3 d later, MLN and PLN cells were isolated and analyzed for the presence of transferred cells by flow cytometric detection of Ly5.1 and Ly5.2 expression.
Essential Requirement for β7 Integrins in Migration of Naive Lymphocytes to PP.
The finding that migration to MLN was apparently different than that to PP (based on a substantial number of OT-I-β7−/− cells in MLN of naive and immunized mice) suggested that migration of naive OT-I-β7−/− cells to PP may be distinct as compared with MLN. We tested this possibility by examination of PP 2 d after transfer of a mixture of naive LN cells from OT-I or OT-I-β7−/− mice (Fig. 3). This population contains primarily CD8 T cells and B cells. Analysis of total PP CD8 T cells indicated that β7 integrins were in fact required for naive OT-I T cells to enter PP, as a distinct population of normal OT-I but not OT-I-β7−/− cells was detected. Interestingly, examination of transferred B cells also demonstrated a requirement for β7 integrins in their migration to PP. These results indicated that migration to PP was much more stringent than migration to MLN, particularly for naive lymphocytes.
Figure 3.
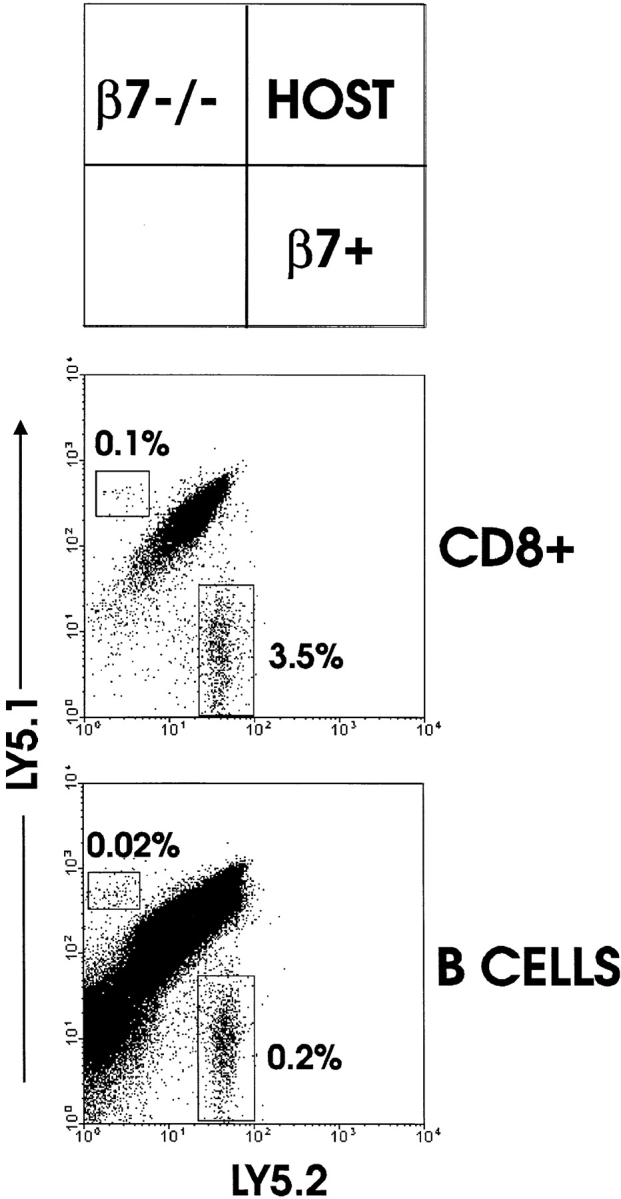
β7 integrins are required for trafficking of naive lymphocytes to PP. Equal numbers of LN cells (∼60% CD8 T cells and 40% B cells) from OT-I (Ly5.1−5.2+) and OT-I-β7−/− (Ly5.1+5.2−) mice were transferred to normal C57BL/6-Ly5.1+5.2+ mice. 2 d later, cells from PP were isolated and analyzed for presence of donor populations by detection of CD8, Ly5.1, and Ly5.2 by flow cytometry. Cells were positively gated for CD8+ cell analysis and negatively gated (CD8−) for B cell analysis. Separate analysis revealed that the CD8− donor cells were >95% B cells (data not shown).
Role of β7 Integrins in Migration of CD8 T Cells to Mucosal Effector Sites.
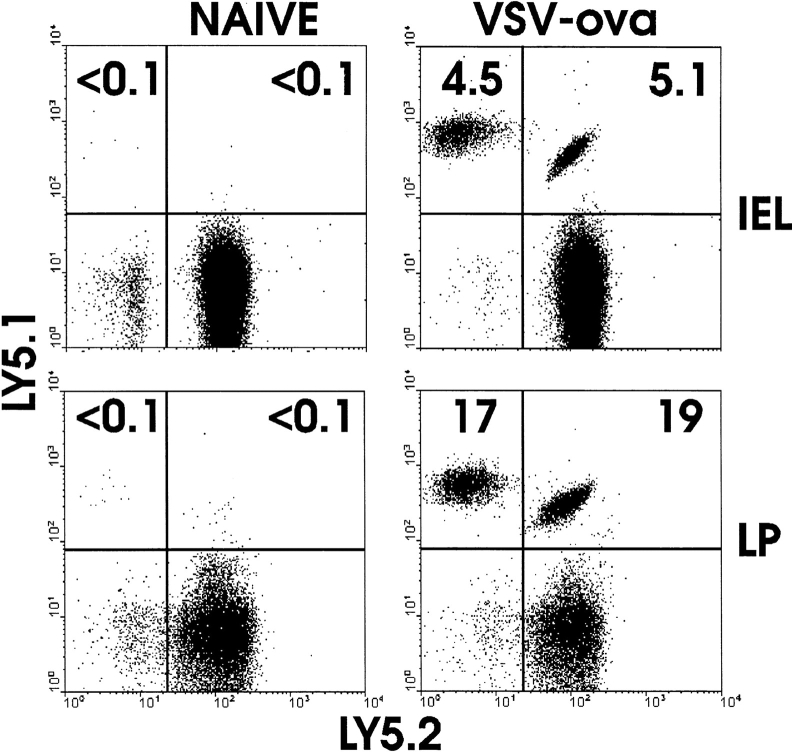
The requirement for β7 integrins in homing to the LP and IEL compartment of small and large intestine was examined using the adoptive transfer system. After transfer of naive OT-I cells to normal mice, few if any transgenic T cells could be detected in LP or IEL (Fig. 4). After immunization with VSV-ova, a large population of normal OT-I CD8 cells were present in IEL and LP. However, 7–10-fold fewer OT-I-β7−/− cells were present in either site, indicating a stringent but not absolute requirement for β7 integrins in migration of activated CD8 T cells to small intestine effector sites. Similarly, migration of activated CD8 T cells to large intestine IEL was also dependent on β7 integrins (Fig. 4). These results agree well with analysis of β7-deficient mice in which LP and IEL populations are reduced (27) and suggest that many mucosal effector cells are derived from cells activated outside of the LP and IEL compartments.
Figure 4.
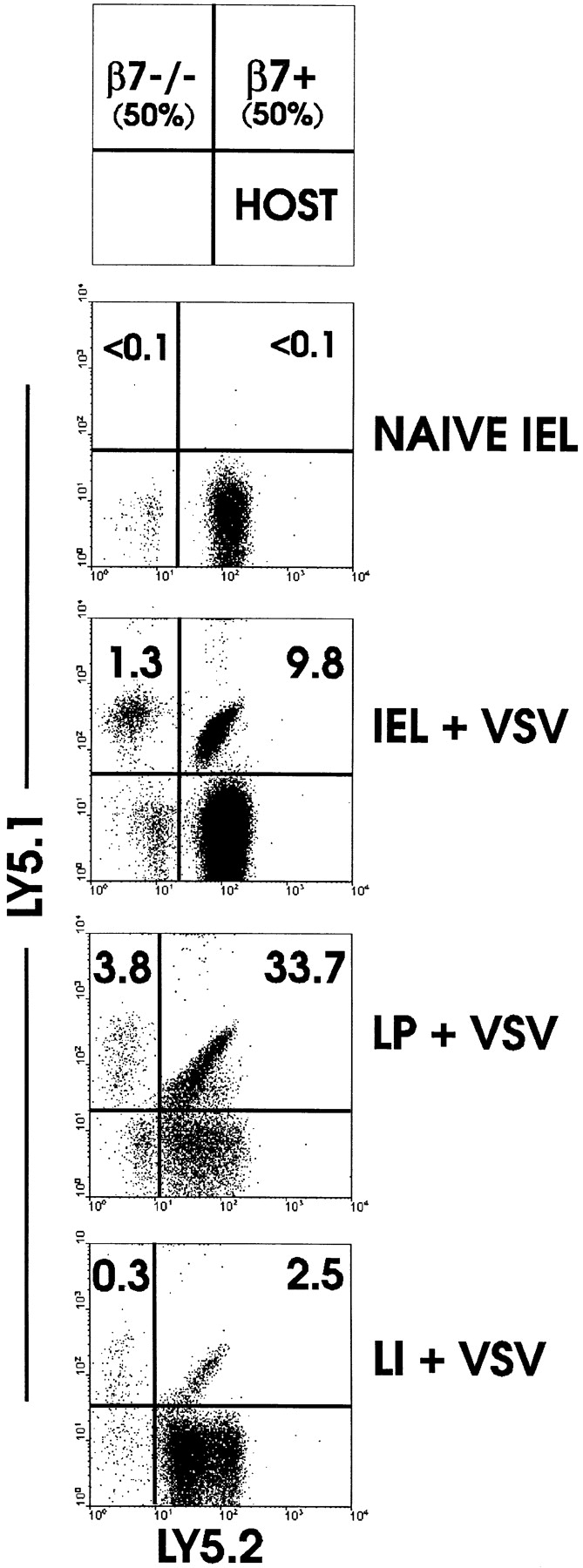
Migration of activated CD8 T cells to intestinal mucosa is dependent on β7 integrins. Equal numbers of LN cells from OT-I (Ly5.1+5.2+) and OT-I-β7−/− (Ly5.1+5.2−) mice were transferred to normal C57BL/6-Ly5.1−5.2+ mice. 48 h later, mice were infected with VSV-ova or not. 3 d after infection, mice were killed and lymphocyte populations were isolated and examined for the presence of donor populations by detection of Ly5.1/Ly5.2 expression by fluorescence flow cytometry.
Regulation of β7 Integrin Expression on Antigen-specific T Cells After In Vivo Immunization.
Because the β7 integrin requirements for migration to peripheral and mucosal lymphoid tissue were distinct, we analyzed β7 integrin expression of adoptively transferred normal OT-I cells before and after immunization with VSV-ova. After transfer, naive OT-I cells in PLN and MLN retained low levels of β7 integrin expression (Fig. 5). After immunization of mice with VSV-ova, OT-I cells in PLN were comprised of two major populations based on β7 integrin expression. One subset expressed β7 levels slightly higher than those of naive cells, and the other population (approximately half the cells) had high levels of β7 integrins. The majority of this expression was due to α4β7 expression, as determined by two-color flow cytometry and analysis of αE−/− OT-I cells (data not shown). In contrast to activated OT-I cells in PLN, OT-I cells in MLN and IEL expressed homogeneously high levels of β7 integrins. In these populations, the αEβ7 integrin contributed more significantly to overall β7 integrin expression than it did in OT-I cells in PLN (25; data not shown). These results indicated that activated, but not naive, CD8 cells expressing high levels of β7 integrins preferentially home to MLN and IEL, a finding which correlates with our demonstrated requirement for β7 integrins in migration of activated CD8 T cells to MLN and PP.
Figure 5.
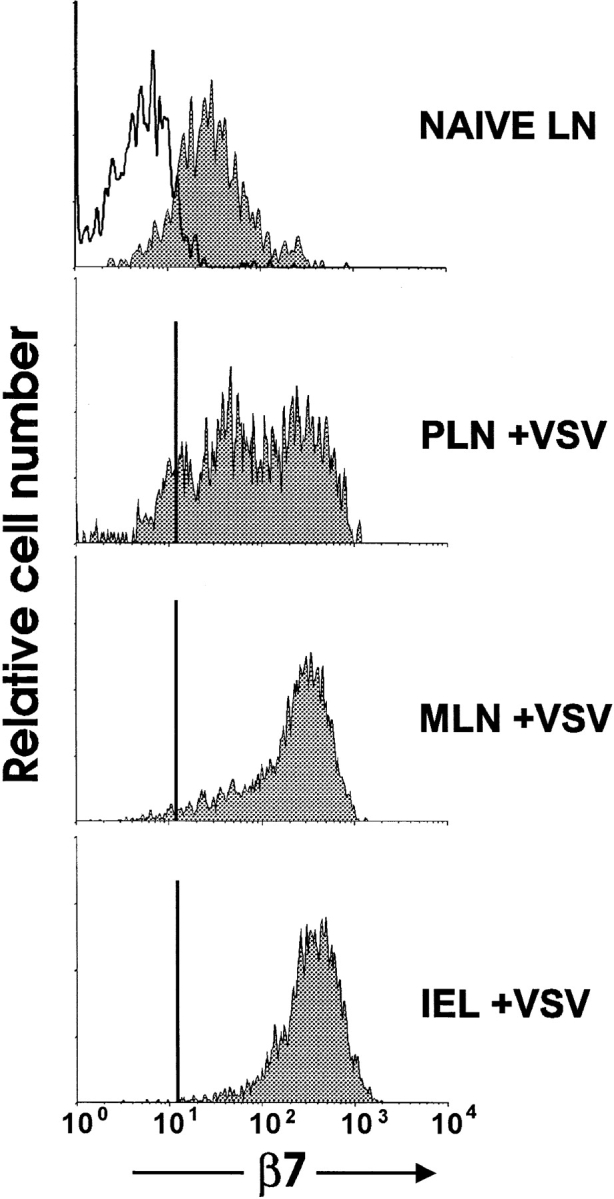
Upregulation of β7 integrins on antigen-specific CD8 T cells after immunization with VSV-ova. Equal numbers of LN cells from OT-I (Ly5.1+5.2+) and OT-I-β7−/− (Ly5.1+5.2−) mice were transferred to normal C57BL/6-Ly5.1−5.2+ mice. 2 d after transfer, mice were infected with VSV-ova. 3 d later, lymphocytes from the indicated tissues were analyzed for expression of CD8, Ly5.1, Ly5.2, and β7 integrin by four-color flow cytometry. Analysis shown is of donor CD8+ cells. Open histogram, OT-I-β7−/− cells; filled histograms, OT-I cells. Top, naive MLN cells; naive PLN cells had a similar expression pattern.
Analysis of the Role of αEβ7 Integrin in Migration of CD8 T Cells During a Primary Immune Response In Vivo.
The data thus far using β7 integrin–deficient OT-I cells does not allow us to distinguish the relative roles of α4β7 integrin and αEβ7 integrin in migration. Although the available literature shows a clear role for α4β7 in migration of lymphocytes to the mucosa, the role of αEβ7 in trafficking of lymphocytes remains unclear. Therefore, as described above for OT-I-β7−/− cells, we transferred a mixture of naive normal OT-I and OT-I-αE−/− cells to normal mice and analyzed distribution of naive and activated populations. Trafficking of naive OT-I cells to PLN, MLN (Fig. 6), or PP (data not shown) did not require αEβ7 expression. Similarly, after infection with VSV-ova, OT-I and OT-I-αE−/− cells were equally distributed in PLN and MLN (Fig. 6), indicating that the requirement for β7 integrins in homing to MLN (Fig. 2) was solely attributable to α4β7. As with normal naive OT-I cells, naive OT-I-αE−/− cells did not migrate to LP or IEL (Fig. 7). Moreover, activation via VSV-ova infection resulted in equivalent migration of OT-I-αE−/− and normal OT-I cells into LP and the IEL compartment. This result indicated that the α4β7 integrin was the primary β7 integrin participating in homing of activated CD8 T cells to mucosal effector sites.
Figure 6.
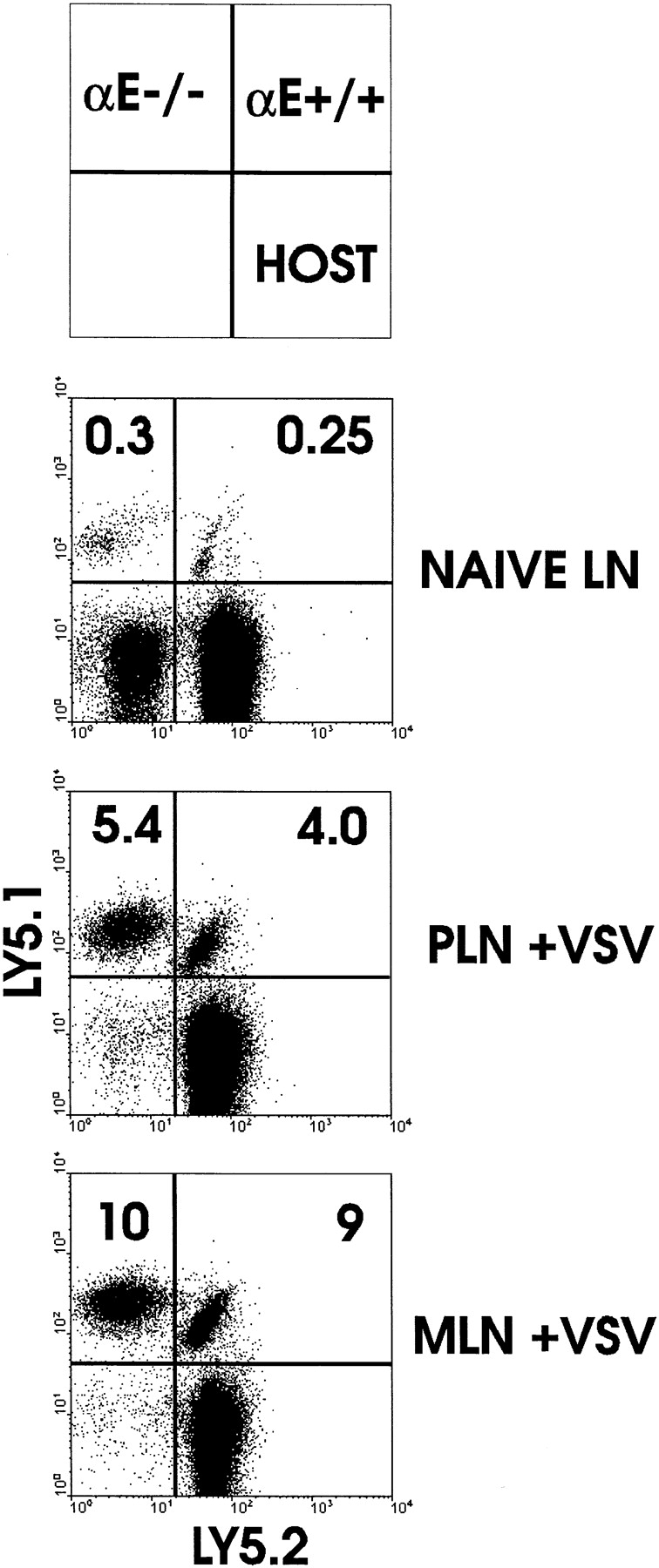
Analysis of migration in LNs of CD8 T cells lacking αEβ7. Equal numbers of LN cells from OT-I (Ly5.1+5.2+) and OT-I-αE−/− (Ly5.1+5.2−) mice were transferred to normal C57BL/6-Ly5.1−5.2+ mice. 48 h later, mice were infected with VSV-ova (+VSV) or not (naive LN). 3 d later, MLN and PLN cells were isolated and analyzed for the presence of transferred cells by flow cytometric detection of Ly5.1 and Ly5.2 expression.
Figure 7.
αEβ7 integrin is not required for entry of activated CD8 T cells into the intestinal mucosa. Equal numbers of LN cells from OT-I (Ly5.1+5.2+) and OT-I-αE−/− (Ly5.1+5.2−) mice were transferred to normal C57BL/6-Ly5.1−5.2+ mice. 48 h later, mice were infected with VSV-ova or not (naive). 3 d later, IEL and LP cells were isolated and analyzed for the presence of transferred cells by flow cytometric detection of Ly5.1 and Ly5.2 expression.
Long-Term Retention of CD8 Cells in the Intestinal Epithelium Does Not Require αEβ7.
Normal IELs express high levels of αEβ7 and low levels of α4β7 (36), supporting the concept that αEβ7 may be involved in tethering of IELs in the epithelium. We tested whether OT-I cells modulated their β7 integrin expression following entry into the epithelium by analyzing long-lived mucosal OT-I cells (Fig. 8). 3 wk after immunization, a small population of OT-I cells was detected in the epithelium (Fig. 8) as well as in peripheral lymphoid organs (data not shown). Analysis of the long-lived transferred IEL revealed that normal OT-I cells had high levels of β7 integrins, and this was nearly all attributable to αEβ7 expression identical to that of host IEL. The latter finding was supported by the lack of appreciable β7 integrin expression by long-lived OT-I-αE−/− IEL. These results indicated that as a consequence of entry into the epithelium, α4β7 was downregulated and αEβ7 was highly upregulated. This phenomenon was not observed with long-lived nonmucosal OT-I cells (data not shown). A comparison of the long-term retention of OT-I cells with or without expression of αEβ7 revealed no significant differences between the populations. In the experiment shown, OT-I-αE−/− IEL were reduced by half as compared with normal OT-I cells (Fig. 8). However, this difference was also evident in PLN and MLN and was not consistently observed. Thus, at least within the time frame analyzed, αEβ7 and perhaps α4β7 were not required for retention of CD8 T cells in the intestinal epithelium.
Figure 8.
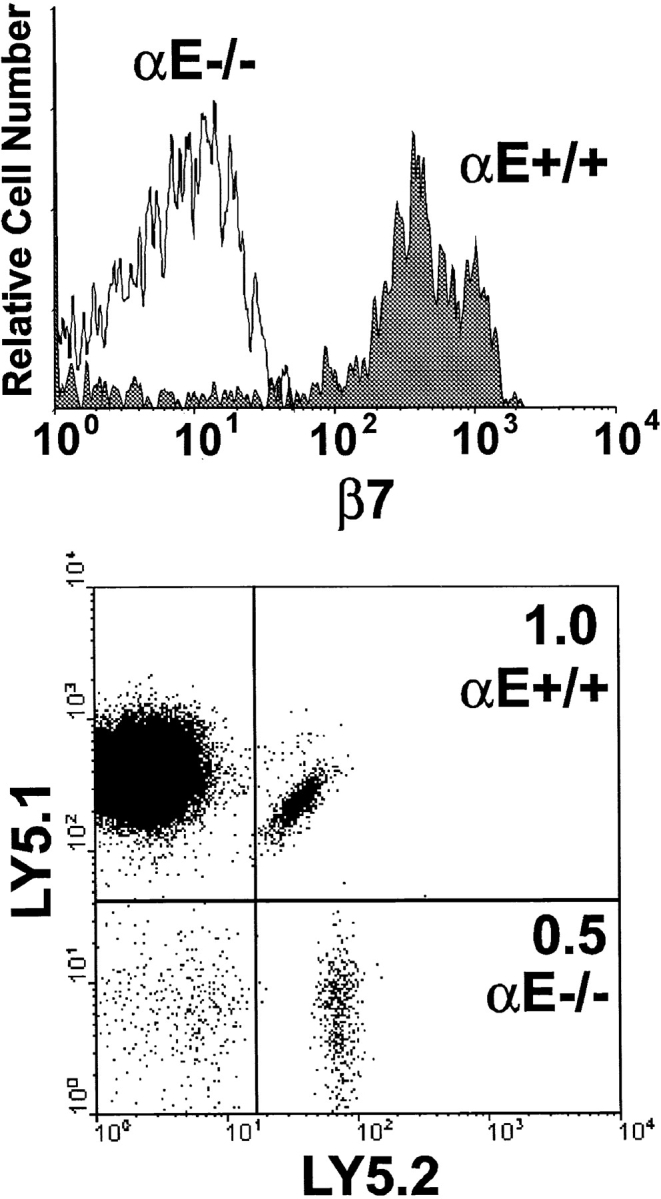
Long-term retention of IEL is not dependent on αEβ7 integrin. Equal numbers of LN cells from OT-I (Ly5.1−5.2+) and OT-I-αE−/− (Ly5.1+5.2+) mice were transferred to normal C57BL/6-Ly5.1+5.2− mice. 48 h later, mice were infected with VSV-ova or not (naive). 3 wk later, IELs were isolated and analyzed for expression of CD8, Ly5.1, Ly5.2, and β7 integrin by four-color flow cytometry. Top panel, β7 integrin expression of CD8+OT-I-αE−/− IEL (open histogram) and CD8+OT-I-αE+/+ IEL (filled histogram).
Discussion
The modified adoptive transfer system described here allowed, for the first time, visualization of the role of β7 integrins during an ongoing antiviral immune response in vivo. By transferring equal numbers of trackable naive normal and β7-deficient antigen-specific CD8 T cells, a direct comparison of the relative requirement for β7 integrins in lymphocyte migration before and after in vivo activation could be made. The system showed clearly that naive CD8 LN cells do not enter the effector sites of the intestinal mucosa, the LP and IEL compartments, whereas naive cells entered the inductive sites, the MLN and PP. However, an interesting finding was that the integrin requirements for trafficking of naive lymphocytes to MLN and PP were distinct. Thus, whereas the α4β7 integrin was not required for migration of naive LN cells to MLN or PLN, there was a near absolute requirement for entry of naive CD8 T cells and B cells into PP. The results from our and other studies (37, 38) indicate that lymphocyte entry into MLN can be mediated by l-selectin in combination with α4β7 or LFA-1, and entry into PP requires l-selectin in combination with α4β7 or α4β7 alone, whereas the combination of l-selectin/LFA-1 is not sufficient for lymphocyte entry into PP. These findings suggest that a functional distinction exists even within inductive sites of the mucosal immune system, in that MLN and PP exhibit distinct requirements for lymphocyte entry.
In contrast to the lack of a requirement for β7 integrins for naive lymphocytes to enter MLN, activated CD8 T cells relied heavily on this integrin to migrate to MLN. In this case, our system allowed an estimation of the degree of CD8 T cell expansion in situ versus the degree of migration to the MLN. That all OT-I-β7−/− cells in MLN after immunization were activated suggested that these cells originated from the population of OT-I-β7−/− cells present in MLN before immunization and underwent in situ expansion, which is not dependent on β7 integrins. If in situ expansion was dependent on β7 integrins, then a significant proportion of nonactivated OT-I-β7−/− cells should have been detected in the MLN after immunization. There was also a clear early preference for activated CD8 T cell migration to MLN versus PLN, because after immunization, the proportion of antigen-specific CD8 T cells was greater in MLN versus PLN. Immunization resulted in a relative ∼4-fold increase in OT-I-β7−/− cells in MLN versus an ∼20-fold increase in normal OT-I cells in the same MLN. If migration accounts for the majority of this difference, then ∼80% of the increase in CD8 T cells in the MLN was the result of migration rather than in situ expansion. These results delineated a dichotomy in β7 integrin requirements for entry into MLN, because activated but not naive CD8 cells required α4β7 for this movement. The α4β7 integrin was also essential for migration of activated CD8 T cells to PP. This effect was dramatic, as few naive OT-I-β7−/− cells were present in PP before immunization. In short-term homing assays (90 min), little migration of β7−/− whole spleen cells to PP was detected (27), in agreement with the results shown here. In the case of MLN, migration was reduced by approximately half in the absence of β7−/− (27). As naive β7−/− and β7+/+ CD8 cells homed equally well to MLN in the present system, these results in short-term migration may indicate supplantation of β7 by other molecules in the longer term (37) or could be due to decreased binding of non-CD8+ cells or poor binding of memory lymphocytes contained in the spleen populations. Nevertheless, these results indicated a near absolute requirement for α4β7 to direct migration of naive and activated lymphocytes to PP, whereas the requirements for entry of naive and activated cells to MLN were different.
Although migration of CD8 T cells to PP was totally dependent on β7 integrins, some β7-independent migration of activated CD8 T cells to intestinal mucosal effector sites was noted. Naive OT-I cells did not enter the LP or IEL compartments, whether they expressed β7 integrins or not. After immunization, however, large numbers of activated CD8 T cells entered the LP and the epithelium. This migration begins ∼48 h after immunization (25, 26; data not shown). Only ∼85–90% of this migration was β7 integrin dependent, as determined by comparison of migration of normal and β7−/− OT-I cells. This was true in small intestinal LP and the IEL compartments of small and large intestine. This was a conservative estimate, as the adoptive transfer system may overestimate the significance of β7 integrins due to competition between normal and mutant cells. That migration to the IEL compartment required, for the most part, β7 integrins suggested that either α4β7 interaction with basement membrane allows CD8 cells to enter the epithelium from the LP or the inhibition of entry to the LP resulted in decreased availability of cells for entry into the IEL population. Further analysis of other adhesion molecules will be necessary to answer this question. The demonstration of some β7-independent migration indicated that other adhesion molecules are capable of directing migration to the LP and the epithelium. Although α4β7 is thought to be the major participant in contact, rolling, arrest, and diapedesis of lymphocytes homing to LP (3), we previously demonstrated that β2 integrins and intracellular adhesion molecule 1 are important for the establishment of the CD8αβ LP and IEL populations (39). These findings suggest that the β2 integrins, perhaps LFA-1, may be able to direct some level of homing to intestinal effector sites. Perhaps other adhesion molecules, such as l-selectin, are also involved in β7-independent homing to mucosa.
In contrast to the well-described functions of the α4β7 integrin, the function of the αEβ7 integrin in vivo remains unknown. The only known ligand for αEβ7 is E-cadherin, which is highly expressed by intestinal and other epithelial cells (19, 40). Because IEL half-life is much less than that of epithelial cells, this finding supported the hypothesis that IEL are retained in the epithelium via an αEβ7–E-cadherin interaction. As α4 integrin–deficient mice have normal IEL numbers (41), whereas β7 integrin–deficient mice have reduced IEL numbers (27), these results also support the possibility that αEβ7 is involved in some aspect of IEL migration or retention. However, the data shown here do not support this concept. The absence of the αE integrin chain on OT-I cells had no effect on their ability to migrate to the LP or the epithelium after primary activation, indicating that all β7-mediated migration was α4β7 dependent. Moreover, OT-I cells with or without αE expression were retained equally well in the epithelium for up to 3 wk. Indeed, the long-lived cells expressed low levels of β7 integrins, suggesting that α4β7 was also not involved in long-term retention of IEL in the epithelium. Therefore, other adhesive molecules must be involved in allowing long-lived IEL to be maintained in the epithelium, perhaps via interactions with the basement membrane rather than the epithelial cells. Given the in vitro data, it seems likely that the αEβ7 integrin plays an adhesive role not measured by our system. What remains unknown is the resulting functional outcome of such an interaction.
Acknowledgments
This work was supported by National Institutes of Health grants AI41576 and DK45260 and an American Cancer Society Faculty Research Award to L. Lefrançois and by a grant from the Deutsche Forschungsgemeinschaft (WA 1127/1-1) to W. Muller and N. Wagner.
Abbreviations used in this paper
- DCs
dendritic cells
- IEL
intraepithelial lymphocyte
- LP
lamina propria
- MLNs
mesenteric lymph nodes
- PLN
peripheral lymph node
- PPs
Peyer's patches
- VSV-ova
vesicular stomatitis virus encoding ovalbumin
References
- 1.McWilliam AS, Napoli S, Marsh AM, Pemper FL, Nelson DJ, Pimm CL, Stumbles PA, Wells TN, Holt PG. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med. 1996;184:2429–2432. doi: 10.1084/jem.184.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 4.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 5.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 6.Ebnet K, Kaldjian EP, Anderson AO, Shaw S. Orchestrated information transfer underlying leukocyte endothelial interactions. Annu Rev Immunol. 1996;14:155–177. doi: 10.1146/annurev.immunol.14.1.155. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 8.Bargatze RF, Jutila MA, Butcher EC. Distinct roles of l-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 9.Abitorabi MA, Mackay CR, Jerome EH, Osorio O, Butcher EC, Erle DJ. Differential expression of homing molecules on recirculating lymphocytes from sheep gut, peripheral, and lung lymph. J Immunol. 1996;156:3111–3117. [PubMed] [Google Scholar]
- 10.Williams MB, Butcher EC. Homing of naive and memory T lymphocyte subsets to Peyer's patches, lymph nodes, and spleen. J Immunol. 1997;159:1746–1752. [PubMed] [Google Scholar]
- 11.Lefrançois L, Goodman T. In vivo modulation of cytolytic activity and Thy-1 expression in TCR-γ+intraepithelial lymphocytes. Science. 1989;243:1716–1718. doi: 10.1126/science.2564701. [DOI] [PubMed] [Google Scholar]
- 12.James SP, Kwan WC, Sneller MC. T cells in inductive and effector compartments of the intestinal mucosal immune system of nonhuman primates differ in lymphokine mRNA expression, lymphokine utilization, and regulatory function. J Immunol. 1990;144:1251–1256. [PubMed] [Google Scholar]
- 13.Lundqvist C, Melgar S, Yeung MM-W, Hammarstrom S, Hammarstrom M-L. Intraepithelial lymphocytes in human gut have lytic potential and cytokine profile that suggest T helper 1 and cytotoxic functions. J Immunol. 1996;157:1926–1934. [PubMed] [Google Scholar]
- 14.Huleatt JW, Lefrançois L. Antigen-driven induction of CD11c on intestinal intraepithelial lymphocytes and CD8(+) T cells in vivo. J Immunol. 1995;154:5684–5693. [PubMed] [Google Scholar]
- 15.Goodman T, Lefrançois L. Intraepithelial lymphocytes. Anatomical site, not T cell receptor form, dictates phenotype and function. J Exp Med. 1989;170:1569–1581. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerf-Bensussan N, Begue B, Gagnon J, Meo T. The human intraepithelial lymphocyte marker HML-1 is an integrin consisting of a β7 subunit associated with a distinctive α chain. Eur J Immunol. 1992;22:273–277. doi: 10.1002/eji.1830220140. [DOI] [PubMed] [Google Scholar]
- 17.Roberts K, Kilshaw PJ. The mucosal T-cell integrin alpha (M290) beta-7 recognizes a ligand on mucosal epithelial cell lines. Eur J Immunol. 1993;23:1630–1635. doi: 10.1002/eji.1830230735. [DOI] [PubMed] [Google Scholar]
- 18.Lefrançois L, Barrett TA, Havran WL, Puddington L. Developmental expression of the αIELβ7integrin on T cell receptor γδ and T cell receptor αβ T cells. Eur J Immunol. 1994;24:635–640. doi: 10.1002/eji.1830240322. [DOI] [PubMed] [Google Scholar]
- 19.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and an integrin, αEβ7 . Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 20.Austrup F, Rebstock S, Kilshaw PJ, Hamann A. Transforming growth factor-beta(1)-induced expression of the mucosa-related integrin alpha(e) on lymphocytes is not associated with mucosa-specific homing. Eur J Immunol. 1995;25:1487–1491. doi: 10.1002/eji.1830250602. [DOI] [PubMed] [Google Scholar]
- 21.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 22.Altman JD, Moss PAH, Goulder PJR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 23.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 24.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 25.Kim S-K, Reed DS, Heath WR, Carbone F, Lefrançois L. Activation and migration of CD8 T cells in the intestinal mucosa. J Immunol. 1997;159:4295–4306. [PubMed] [Google Scholar]
- 26.Kim SK, Reed DS, Olson S, Schnell MJ, Rose JK, Morton PA, Lefrançois L. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc Natl Acad Sci USA. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner N, Lohler J, Kunkel EJ, Ley K, Leung E, Krissansen G, Rajewsky K, Muller W. Critical role for β7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 28.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonistic peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 29.Schon, M.P., A. Arya, E.A. Murphy, C.M. Adams, U.G. Strauch, W.W. Agace, J. Marsal, J.P. Donohue, H. Her, D.R. Beier, et al. 1999. Mucosal T lymphocyte numbers are selectively reduced in integrin αE (CD103) deficient mice. J. Immunol. In press. [PubMed]
- 30.Lawson ND, Stillman EA, Whitt MA, Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci USA. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnell MJ, Buonocore L, Whitt MA, Rose JK. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J Virol. 1996;70:2318–2323. doi: 10.1128/jvi.70.4.2318-2323.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodman T, Lefrançois L. Expression of the γ-δ T-cell receptor on intestinal CD8+intraepithelial lymphocytes. Nature. 1988;333:855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- 33.Laky K, Lefrançois L, Puddington L. Age- dependent intestinal lymphoproliferative disorder due to stem cell factor receptor deficiency. J Immunol. 1997;158:1417–1427. [PubMed] [Google Scholar]
- 34.Sarmiento M, Glasebrook AL, Fitch FW. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt-2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 35.Shen, F.W. 1981. Monoclonal antibodies to mouse lymphocyte differentiation alloantigens. In Monoclonal Antibodies and T-cell Hybridomas. Perspectives and Technical Advances. G.J. Hammerling, U. Hammerling, and J.F. Kearney, editors. Elsevier/North-Holland, Inc., Amsterdam. 25–31.
- 36.Andrew DP, Rott LS, Kilshaw PJ, Butcher EC. Distribution of α4β7 and αEβ7 integrins on thymocytes, intestinal epithelial lymphocytes and peripheral lymphocytes. Eur J Immunol. 1996;26:897–905. doi: 10.1002/eji.1830260427. [DOI] [PubMed] [Google Scholar]
- 37.Wagner N, Lohler J, Tedder TF, Rajewsky K, Muller W, Steeber DA. l-selectin and β7 integrin synergistically mediate lymphocyte migration to mesenteric lymph nodes. Eur J Immunol. 1998;28:3832–3839. doi: 10.1002/(SICI)1521-4141(199811)28:11<3832::AID-IMMU3832>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 38.Andrew DP, Spellberg JP, Takimoto H, Schmits R, Mak TW, Zukowski MM. Transendothelial migration and trafficking of leukocytes in LFA-1-deficient mice. Eur J Immunol. 1998;28:1959–1969. doi: 10.1002/(SICI)1521-4141(199806)28:06<1959::AID-IMMU1959>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Huleatt J, Lefrançois L. β2 integrins and ICAM-1 are involved in establishment of the intestinal mucosal T cell compartment. Immunity. 1996;5:263–273. doi: 10.1016/s1074-7613(00)80321-0. [DOI] [PubMed] [Google Scholar]
- 40.Karecla PI, Bowden SJ, Green SJ, Kilshaw PJ. Recognition of E-cadherin on epithelial cells by the mucosal T cell integrin alpha M290 beta 7 (alpha E beta 7) Eur J Immunol. 1995;25:852–856. doi: 10.1002/eji.1830250333. [DOI] [PubMed] [Google Scholar]
- 41.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for α4 integrins during fetal and adult hematopoiesis. Cell. 1996;85:997–1008. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]