Abstract
Platelet endothelial cell adhesion molecule (PECAM)-1 is a 130-kD transmembrane glycoprotein having six Ig homology domains within its extracellular domain and an immunoreceptor tyrosine–based inhibitory motif within its cytoplasmic domain. Previous studies have shown that addition of bivalent anti–PECAM-1 mAbs to the surface of T cells, natural killer cells, neutrophils, or platelets result in increased cell adhesion to immobilized integrin ligands. However, the mechanism by which this occurs is not clear, and it is possible that anti–PECAM-1 mAbs elicit this effect by simply sequestering PECAM-1, via antibody-induced patching and capping, away from stimulatory receptors that it normally regulates. To determine whether dimerization or oligomerization of PECAM-1 directly initiates signal transduction pathways that affect integrin function in an antibody-independent manner, stable human embryonic kidney-293 cell lines were produced that expressed chimeric PECAM-1 cDNAs containing one or two FK506-binding protein (FKBP) domains at their COOH terminus. Controlled dimerization initiated by addition of the bivalent, membrane-permeable FKBP dimerizer, AP1510, nearly doubled homophilic binding capacity, whereas AP1510-induced oligomers favored cis PECAM-1/PECAM-1 associations within the plane of the plasma membrane at the expense of trans homophilic adhesion. Importantly, AP1510-induced oligomerization resulted in a marked increase in both adherence and spreading of PECAM/FKBP-2–transfected cells on immobilized fibronectin, a reaction that was mediated by the integrin α5β1. These data demonstrate that signals required for integrin activation can be elicited by clustering of PECAM-1 from inside the cell, and suggest that a dynamic equilibrium between PECAM-1 monomers, dimers, and oligomers may control cellular activation signals that influence the adhesive properties of vascular cells that express this novel member of the immunoreceptor tyrosine–based inhibitory motif family of regulatory receptors.
Keywords: PECAM-1, integrins, FKBP, dimerization, signal transduction
Introduction
Platelet endothelial cell adhesion molecule (PECAM)1-1 is a 130-kD member of the Ig superfamily and a newly recognized member of the immunoreceptor tyrosine–based inhibitory motif (ITIM) family (Newman et al. 1990; Newman 1997, Newman 1999). PECAM-1 is expressed on a variety of vascular and blood cells, including endothelial cells (Newman 1994), monocytes, neutrophils (Stockinger et al. 1990), certain subpopulations of T lymphocytes (Stockinger et al. 1992), and platelets (Newman 1994). There is compelling evidence that PECAM-1 is a multifunctional molecule, as it has been implicated in transendothelial migration of leukocytes (Schimmenti et al. 1992; Bird et al. 1993; Muller et al. 1993; Vaporciyan et al. 1993; Liao et al. 1995; Christofidou-Solomidou et al. 1997), T cell activation (Zehnder et al. 1995), and angiogenesis (DeLisser et al. 1997; Zhou et al. 1999).
Although expressed only diffusely on the surface of individual cells, PECAM-1 becomes enriched at sites of cell–cell contact (Albelda et al. 1991), an effect that appears to be controlled largely by the extracellular, rather than the cytoplasmic, domain of the molecule (Sun et al. 2000) However, during the process of cell migration (Schimmenti et al. 1992), as well as under certain inflammatory conditions (Ioffreda et al. 1993; Romer et al. 1995), PECAM-1 leaves cell junctions and becomes diffusely localized over the cell surface. Studies using full-length PECAM-1 incorporated into proteoliposomes and a bivalent PECAM-1/IgG chimeric protein (PECAM-1/IgG) have shown that PECAM-1 performs its adhesive function via PECAM-1/PECAM-1 homophilic interactions, which are mediated primarily by NH2-terminal Ig homology domains 1 and 2 (Sun et al. 1996a,Sun et al. 1996b; Newton et al. 1997). This region of the extracellular domain appears to be functionally important, as mAbs specific for Ig domains 1 or 2 inhibit homophilic binding (Yan et al. 1995), leukocyte extravasation (Liao et al. 1995; Nakada et al. 2000), and tumor angiogenesis (Sheibani et al. 1997; Zhou et al. 1999).
Like many other cell adhesion receptors, PECAM-1 is able to mediate both outside-in and inside-out signal transduction (Newman 1997). In particular, there have been many studies demonstrating that addition of anti–PECAM-1 mAbs to the cell surface results in upregulation of β1 (Tanaka et al. 1992; Leavesley et al. 1994), β2 (Piali et al. 1993; Berman and Muller 1995; Berman et al. 1996), and β3 (Varon et al. 1998; Chiba et al. 1999) integrin function. Whether these effects are due to PECAM-1–mediated changes in integrin affinity versus avidity is not well understood, however, Varon et al. 1998 have recently reported that selected anti–PECAM-1 F(ab′)2 fragments result in the conformational changes in the integrin αIIbβ3 that augment platelet adhesion and aggregation. Since monovalent Fab fragments have been, in general, ineffective integrin activators, it has been hypothesized that PECAM-1 dimerization or oligomerization may be necessary to transduce signals into the cell that modulate integrin function.
The mechanism by which antibody-induced dimerization of PECAM-1 regulates cell surface integrin activation is not clear, and might simply be due to sequestering PECAM-1, via antibody-induced patching and capping, away from stimulatory receptors that it normally regulates. To better understand the potential role of PECAM-1 in integrin activation, we undertook a study designed to examine (a) whether PECAM-1 might exist in dimeric or oligomeric form within the plane of the plasma membrane; (b) the consequences of such lateral PECAM-1/PECAM-1 associations on its adhesive and signaling properties; and (c) the ability of PECAM-1 dimers and oligomers to modulate integrin function. Using an antibody-independent approach that permits precise control of the degree of receptor interactions, we found that PECAM-1 homophilic adhesion is maximally supported by PECAM-1 dimers, but that integrin activation requires oligomerization of PECAM-1. These data suggest that a dynamic equilibrium between PECAM-1 monomers, dimers, and oligomers may control cellular activation signals that influence the adhesive properties of vascular cells that express this novel member of the ITIM-containing inhibitory receptor family.
Materials and Methods
Materials
PECAM-1.3, a murine anti–human PECAM-1 mAb specific for Ig homology domain 1 (Sun et al. 1996b), was fluorescently labeled with Alexa 488 using a protein labeling kit (Molecular Probes). A mouse anti–human mAb, specific for FKBP-12, MAR4 (mouse anti–human α5 integrin), and IIA1 (mouse anti–human β1 integrin) were purchased from PharMingen. P1D6 (mouse anti–human α5 integrin) and P4C10 (mouse anti–human β1 integrin) were purchased from GIBCO BRL. AP1510 and the pCF1E vector encoding FKBP cDNA were provided by ARIAD Pharmaceuticals, Inc. (Cambridge, MA). PMSF was purchased from Sigma-Aldrich. Chemical cross-linking agents, bis(sulfosuccinikidyl) suberate (BS3), and dithiobis(sulfosuccinimidylpropionate) (DTSSP) were purchased from Pierce Chemical Co. DTSSP is thiol cleavable, whereas BS3 is noncleavable.
Construction of cDNAs Encoding PECAM-1/FKBP-1 and PECAM-1/FKBP-2 Fusion Proteins
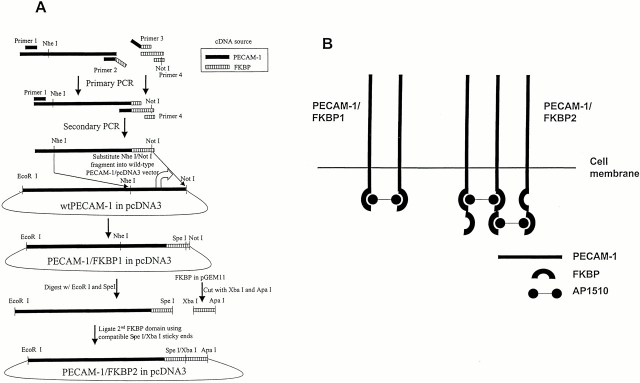
One or two copies of the FK506-binding protein (FKBP), FKBP-12, were fused, to the COOH terminus of the cytoplasmic domain of PECAM-1, using standard overlapping PCR techniques (Fig. 1). PECAM-1/FKBP-1, containing one FKBP motif, was produced by PCR. A segment of PECAM-1 was amplified from amino acid residue 375 to the COOH terminus of PECAM-1 using PECAM-1 primer 1 (5′-CCTGTCAAGTAAGGTGGTGGAGTCT-3′) and PECAM-1 primer 2 (5′-CACCTGCA-CGCCTCTAGAAGTTCCATCAAGGGAGCC-3′), and was joined to a full-length FKBP motif using FKBP primer 3 (5′-GGCTCCCTTGATGGAACTTCTAGAGGCGTGCAGGTG-3′) and FKBP primer 4 (5′-GTCCTGAATGCTCTTCCAGGCGGCCGCTTATGCGTAGTCTGGTAC-3′). The two segments were subsequently mixed together with primers 1 and 4, and secondary overlap PCR was performed. The final amplified product was digested with Nhe I and Not I and inserted into similarly cut wild-type PECAM-1 cDNA that had been cloned into the mammalian expression vector pcDNA3. PECAM-1/FKBP-2, containing two FKBP domains fused to the COOH terminus of PECAM-1, was constructed by addition of a second FKBP domain to PECAM-1/FKBP-1. The integrity and authenticity of both constructs were confirmed by nucleotide sequencing.
Figure 1.
Construction of PECAM-1/FKBP chimeric proteins. (A) Schematic diagram detailing the production of PECAM-1/FKBP-1 and PECAM-1/FKBP-2. cDNAs encoding PECAM-1 and FKBP were used as templates for standard overlap PCR to produce the resulting chimeric proteins, which contain either one or two FKBP dimerization domains. (B) Controlled dimerization and oligomerization of PECAM-1. PECAM-1/FKBP-1, containing one FKBP domain, forms self-limiting dimers upon exposure to the cell permeable synthetic dimerizer AP1510, whereas PECAM-1/FKBP-2 is capable of forming oligomers.
Development of Stable PECAM-1/FKBP-1– and PECAM-1/FKBP-2–expressing HEK-293 Cell Lines
HEK (human embryonic kidney)-293 cells and human erythroleukemia (HEL) cells were obtained from American Type Culture Collection and cultured in MEM and RPMI-1640 medium (Sigma-Aldrich), respectively, with 10% heat-inactivated fetal calf serum at 37°C in a humidified atmosphere of 5% CO2. HEK-293 cells were grown to 80–90% confluence in 100-mm dishes, incubated with 10 μg of pcDNA3.0 (Invitrogen) containing PECAM-1/FKBP-1 or PECAM-1/FKBP-2 in a lipofectamine mixture (GIBCO BRL) for 4 h in serum-free medium (Sigma-Aldrich). Cells were cultured for an additional 48 h in serum-containing MEM before adding 0.6 mg/ml G418 (geneticin; GIBCO BRL). G418-resistant cell lines were analyzed, using flow cytometry, for cell surface expression of PECAM-1/FKBP-1 and PECAM-1/FKBK2. Cell clones were obtained by cell sorting and limited dilution subcloning. The properties of at least three different clonal lines were examined so that the influence of clonal variation on the observed phenotype could be determined.
Western Blot and Immunoprecipitation Analysis
HEL and HEK-293 cells were lifted using 0.01% trypsin and 10 mM EDTA, washed in sterile HPBS, and resuspended at a final concentration of 106 cells/ml. Chemical cross-linking was initiated by addition of 2 mM BS3 or DTSSP for 1 h at 4°C, and then 50 mM Tris-HCl was added to stop the reaction. Cell lysates were prepared in lysis buffer (2% Triton X-100, 50 mM Tris-HCl, 2 mM PMSF), and the 15,000 g Triton-soluble fraction was analyzed under both reducing and nonreducing conditions on a 7% SDS–polyacrylamide gel. After transfer to an Immobilion-P membrane (Millipore), proteins were visualized using antibodies to PECAM-1 (PECAM-1.3) or FKBP-12 in conjunction with enhanced chemiluminescence immunodetection (Amersham Pharmacia Biotech) using peroxidase-conjugated goat anti–mouse IgG (Jackson ImmunoResearch Laboratories). For immunoprecipitation analysis, detergent cell lysates were prepared as above, and the Triton-soluble fractions were incubated with 10 μg/ml PECAM-1.3 IgG overnight at 4°C. Immune complexes were captured with 50 μl of 50% slurry of protein G–Sepharose for 1 h at 4°C, and then washed five times with immunoprecipitation wash buffer (50 mM Tris-HCl, pH 7.4, containing 150 mM NaCl, and 2% Triton X-100). Bound proteins were eluted from the beads by boiling for 10 min in 30 μl of SDS-reducing buffer and analyzed by SDS-PAGE, as described above.
Flow Cytometry
4 μg of PECAM-1.3 or PECAM-1/IgG in 100 μl of HPBS was added to 5 × 105 wild-type or transfected HEK-293 cells in 100 μl of HPBS and allowed to incubate for 60 min at room temperature in the presence or absence of various concentrations of AP1510. The cells were then washed by centrifugation with 1 ml of HPBS and then resuspended in 100 μl of HPBS containing 1 μg of FITC-labeled goat anti–human or goat anti–mouse IgG γ chain (Jackson ImmunoResearch Laboratories). After a 30 min incubation at 4°C, the cells were washed once more in HPBS, and then subjected to flow cytometric analysis.
Fluorescence Microscopy
Wild-type or stably transfected 293 cells were incubated in the presence or absence of 1 μM AP1510 for 30 min at room temperature, and then by 5 μg/ml Alexa 488–conjugated PECAM-1.3 for 30 min at 4°C. The cells were then washed, fixed with 1% formaldehyde, and mounted onto glass slides using a cytospin preparation. The cell surface distribution of PECAM-1 was analyzed using a Zeiss fluorescent microscope (ZEISS).
Quantitative Evaluation of the Binding of 293 Cells To Immobilized Fibronectin
Adherent 293 cells were lifted using 0.01% trypsin and 10 mM EDTA, washed in sterile HPBS, and resuspended at 106 cells/ml in prewarmed (37°C) serum-free medium. After incubation with 2 μg/ml calcein AM (Molecular Probes) for 30 min at 37°C, cells were washed and resuspended at 106 cells/ml in prewarmed serum-free medium, and then incubated again, this time with varying concentrations of AP1510 in the presence of selected antibodies for 30 min at 22°C. 200-μl cell aliquots were then added to wells of 96-well microtiter tissue culture plates (Dynatech Laboratories) that had been coated with either 10 μg/ml fibronectin (Sigma-Aldrich) or 10 mg/ml BSA (ICN Biomedicals). Antibody competition experiments were performed by preincubating cells with varying concentrations of selected integrin-specific mAbs for 30 min at 22°C, before addition of cells to the wells. The reaction was stopped by addition of 70 μl of 4% formaldehyde for 15 min at 37°C, and total well fluorescence was determined in a CytoFluor fluorescent plate reader (PerSeptive Biosystem). Loosely adherent and unbound cells were then removed by washing the wells with prewarmed serum-free medium, and fluorescence was measured once more to determine percent cell adhesion.
Results
PECAM-1 Exists in an Equilibrium between Monomeric, Dimeric, and Oligomeric Forms Within the Plasma Membrane
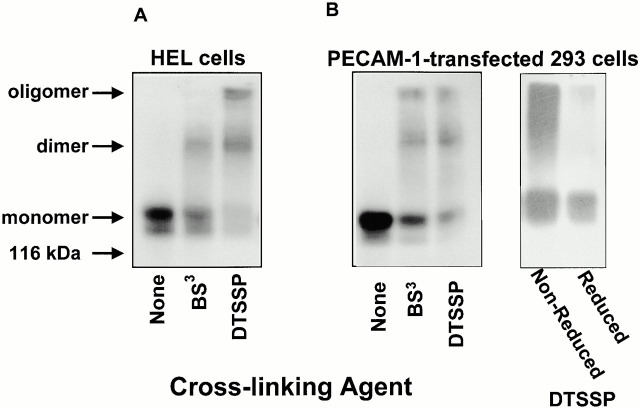
Previous studies have shown that antibody-mediated dimerization or oligomerization of PECAM-1 can lead to upregulation of integrin function in a variety of different cell types. Whether PECAM-1 actually forms lateral associations within the plane of the plasma membrane and whether these associations are able to influence the adhesive phenotype of the cell are not known. To determine the molecular organization of PECAM-1 on the cell surface, HEL cells, which constitutively express PECAM-1, and HEK-293 cells, which had been stably transfected with PECAM-1, were treated with the homobifunctional N-hydroxysuccimide–based chemical cross-linkers BS3 and DTSSP to stabilize preexisting dimers, oligomers, and other membrane proteins that might be associated on the cell surface in close proximity to PECAM-1. Since PECAM-1 is able to interact homophilically with itself (Sun et al. 1996a,Sun et al. 1996b), cross-linking reactions were performed at 4°C at low cell density to minimize trans interactions between PECAM-1 molecules on adjacent cells.
As shown in Fig. 2, exposure of cells to either BS3 or DTSSP resulted in the production of stable, high molecular weight complexes corresponding to dimeric and oligomeric forms of PECAM-1. When the high molecular weight complexes formed in the presence of DTSSP were exposed to reducing conditions, only a 130-kD PECAM-1 doublet remained. Although we cannot exclude the possibility that PECAM-1 is associated with a different 130-kD protein, these data strongly suggest that the high molecular weight species are comprised solely of PECAM-1, and that PECAM-1 is not associated with membrane proteins other than itself—at least in resting cells.
Figure 2.
Chemical cross-linking of cell surface PECAM-1 results in formation of stable high molecular weight complexes. (A) HEL cells were treated with chemical cross-linking agents BS3 or DTSSP, and detergent lysates were analyzed by SDS-PAGE using the anti–PECAM-1 mAb, PECAM-1.3. The prominent 130-kD doublet observed in untreated cells corresponds to variously glycosylated monomeric PECAM-1. Note the high molecular weight forms corresponding to PECAM-1 dimers and oligomers. (B) Immunoblot analysis of PECAM-1–transfected HEK-293 lysates that had been exposed to either BS3 (noncleavable) or DTSSP (cleavable). Note that the high molecular weight forms of PECAM-1 present under nonreducing conditions after cross-linking with DTSSP all revert upon reduction to a single band with a mobility identical to monomeric PECAM-1, suggesting the presence of homodimers and homooligomers.
Expression of PECAM-1/FKBP-1 and PECAM-1/FKBP-2 in HEK-293 Cells
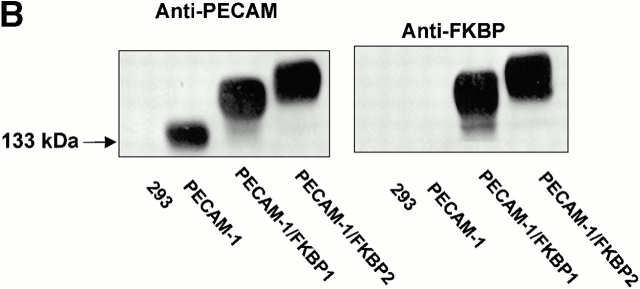
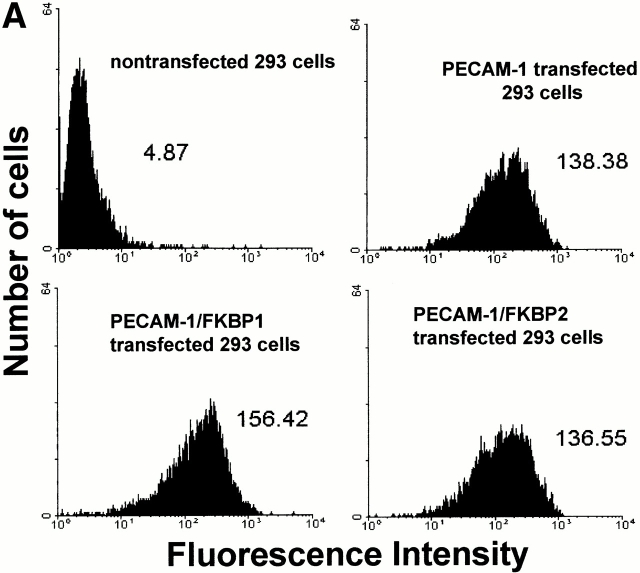
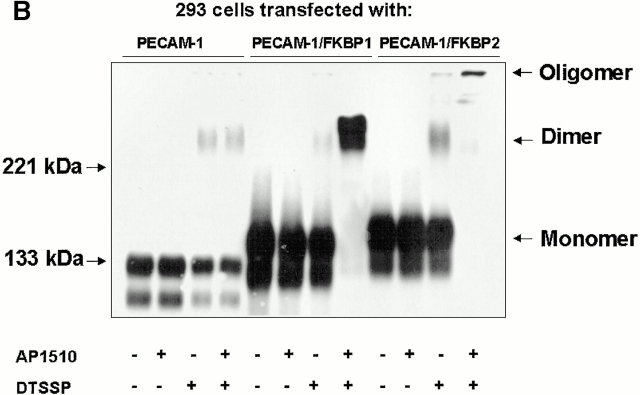
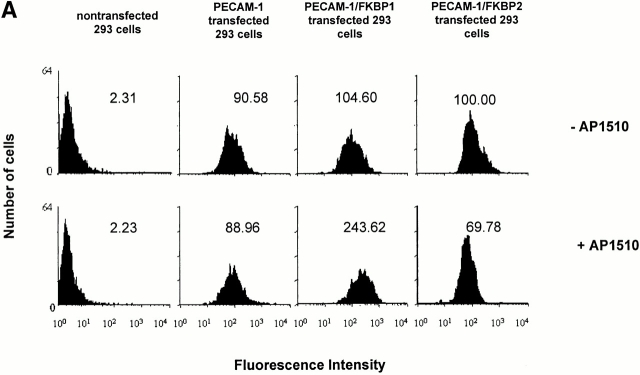
Recent studies have shown that antibody-mediated cross-linking of PECAM-1 elicits many subsequent downstream events, including integrin-mediated adhesion (Tanaka et al. 1992; Piali et al. 1993; Berman and Muller 1995; Berman et al. 1996) and phosphorylation of PECAM-1 itself (Varon et al. 1998; Newton-Nash and Newman 1999). However, it is possible that these effects may have been due, at least in part, to nonspecific antibody-induced patching and capping. To examine whether PECAM-1/PECAM-1 associations within the plane of the membrane can influence cell physiology in an antibody-independent manner, we employed an inducible, targeted dimerization and oligomerization system consisting of PECAM-1/FKBP chimeric proteins and the synthetic membrane-permeable “dimerizer,” AP1510 (Spencer et al. 1993). As shown in Fig. 3, HEK-293 cell lines transfected with cDNAs encoding wild-type PECAM-1, PECAM-1/FKBP-1, and PECAM-1/FKBP-2 expressed roughly equivalent levels of the recombinant proteins (Fig. 3 A), and the chimeric proteins were of the expected size and immunoreactivity (Fig. 3 B).
Figure 3.
PECAM-1, PECAM-1/FKBP-1, and PECAM-1/FKBP-2 expression on 293 cell surface. (A) Flow cytometric analysis of cell surface expression. Note that untransfected 293 cells do not express PECAM-1, whereas the transfectants express nearly equivalent level of PECAM-1, PECAM-1/FKBP-1, and PECAM-1/FKBP-2, respectively. (B) PECAM-1.3 immunoblot analysis of untransfected 293 cells, or 293 cells transfected with wild-type PECAM-1, PECAM-1/FKBP-1, and PECAM-1/FKBP-2. Identical blots were probed with either PECAM-1.3 (left) or anti-FKBP (right). Note that addition of FKBP domains decreases the mobility of PECAM-1 (left), and also confers immunoreactivity to anti-FKBP-12 antibodies (right).
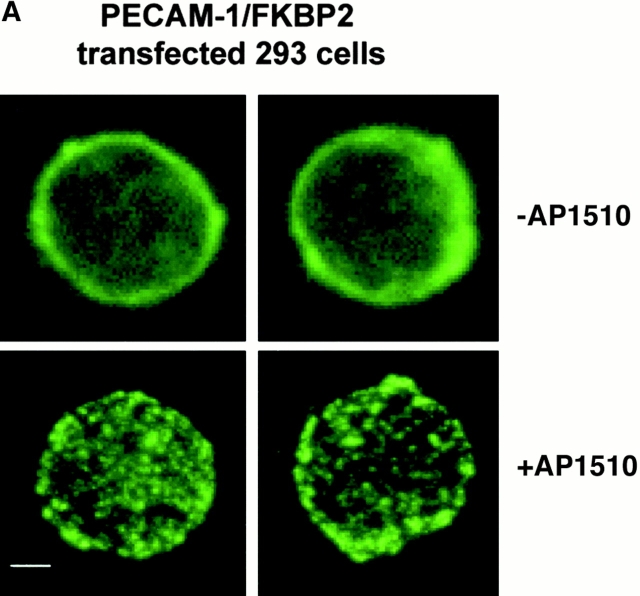
To determine whether AP1510-induced PECAM-1 dimerization or oligomerization could be visualized morphologically, 293-cell transfectants were incubated with AP1510 and stained with Alexa-conjugated PECAM-1.3. Cells expressing wild-type PECAM-1 showed an even distribution of PECAM-1 in the presence or absence of AP1510, as did cells expressing PECAM-1/FKBP-1 (not shown). However, as shown in Fig. 4 A, oligomerization of PECAM-1/FKBP-2 by AP1510 could be readily observed.
Figure 4.
AP1510-induced dimerization and oligomerization of PECAM-1. (A) 293 cells stably expressing PECAM-1/FKBP-2 were treated with AP1510, and the distribution PECAM-1 visualized with Alexa 488–conjugated mAb PECAM-1.3. Whereas PECAM-1 oligomers are readily observed, changes in cell surface distribution of PECAM-1/FKBP-1, upon treatment with AP1510, were not apparent (not shown), probably due to the inability of light microscopy to visualize simple dimers of the receptor. Bar, 4 μM. (B) 293 cells stably expressing wild-type PECAM-1, PECAM-1/FKBP-1, and PECAM-1/FKBP-2 were treated with the chemical cross-linking agent DTSSP in the presence or absence of AP1510, and subjected to immunoblot analysis using the anti–PECAM-1 mAb, PECAM-1.3. Note that nearly all the cell surface PECAM-1/FKBP-1 is forced into dimers by AP1510, whereas the majority of PECAM-1/FKBP-2 exists in high molecular weight oligomers after AP1510 treatment.
Dimerization and Oligomerization Affect the Adhesive Properties of PECAM-1
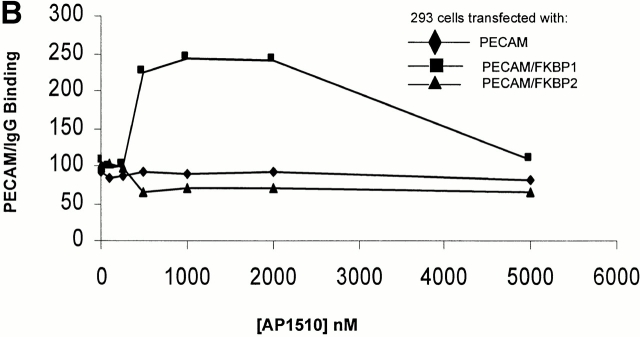
To determine whether PECAM-1 dimerization or oligomerization affects its ability to participate in trans homophilic interactions, PECAM-1/FKBP-1 and PECAM-1/FKBP-2 transfectants were incubated with soluble PECAM-1/IgG, a chimeric protein consisting of the extracellular domain of PECAM-1 fused with the Fc region of human IgG (Sun et al. 1996b), and binding was quantified by flow cytometry. As shown in the top graphs of Fig. 5 A, 293 cells expressing wild-type PECAM-1, PECAM-1/FKBP-1, and PECAM-1/FKBP-2 bound nearly equivalent amounts of PECAM-1/IgG in the absence of AP1510, indicating that the function of the extracellular domain is unaffected by the presence of FKBP at the COOH terminus. Addition of AP1510 had no effect on the ability of wild-type PECAM-1 to bind to PECAM-1/IgG, as expected. However, the AP1510-induced dimerization of PECAM-1/FKBP-1 more than doubled the binding of PECAM-1/IgG. In contrast, oligomerization of PECAM-1/FKBP-2 decreased the homophilic binding capacity by 30–40%. This was not due to changes in cell surface expression of either PECAM-1 chimeric protein, but rather to the inaccessibility of the homophilic binding site located in Ig domain 1 (data not shown). As shown in Fig. 5 B, the effect on homophilic binding of AP1510-induced dimerization was dose dependent, with peak activity occurring at about 1 μM. Together, these data indicate that cell surface dimers optimally support high avidity trans homophilic interactions (i.e., cell surface PECAM-1 binding to exogenously added PECAM-1/IgG, or PECAM-1 on an adjacent cell), whereas PECAM-1 oligomers favor increased cis (i.e., lateral) associations within the plane of the plasma membrane, at the expense of decreased trans homophilic binding capacity. A schematic model consistent with these findings is provided in Fig. 6.
Figure 5.
Effect of PECAM-1 dimerization and oligomerization on PECAM-1 homophilic adhesion. (A) PECAM-1/IgG was added to the indicated transfected 293 cell lines in either the absence (top) and presence (lower) of AP1510. After incubation with FITC-labeled mouse anti–human Fc, binding was quantified by flow cytometry. At 1 μM, AP1510 more than doubled the amount of PECAM-1/IgG that became associated with PECAM-1/FKBP-1–transfected cells, an effect that was dose dependent (B) and reversible at high concentrations of the drug, probably due to saturation of available FKBP sites and the resulting formation of PECAM-1 dimers and monomers. AP1510 had no effect on PECAM-1/IgG binding to cells expressing wild-type PECAM-1, as expected, however, oligomerization of PECAM-1/FKBP-2 results in decreased homophilic binding capacity, suggesting that PECAM-1/PECAM-1 cis interactions occur at the expense of its ability to support trans homophilic interactions.
Figure 6.
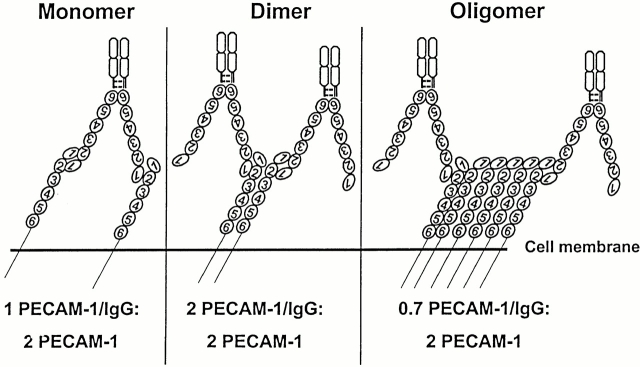
Model of PECAM-1/IgG binding to monomers, dimers, and oligomers of PECAM-1 on the cell surface. Note that the number of PECAM-1/IgG molecules bound is limited by the availability of free Ig domain 1. The predicted stoichiometry of PECAM-1/IgG to cell surface PECAM-1 correlates with the flow cytometric data shown in Fig. 5i.e., cell surface PECAM-1 dimers maximally support trans homophilic adhesion, whereas PECAM-1 oligomers are involved in cis interactions.
Integrin Activation by Regulated Oligomerization of PECAM-1
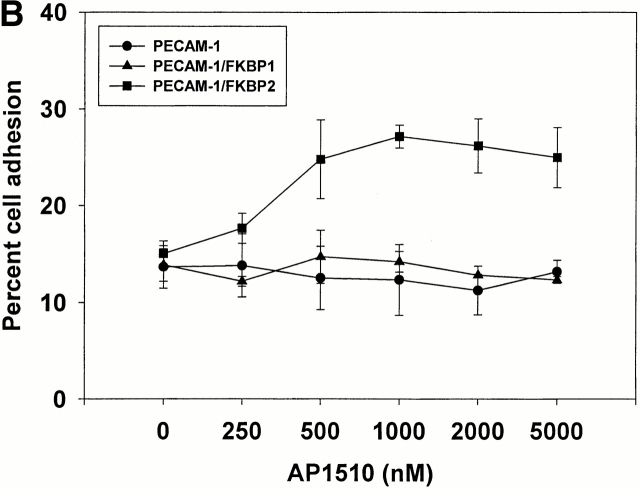
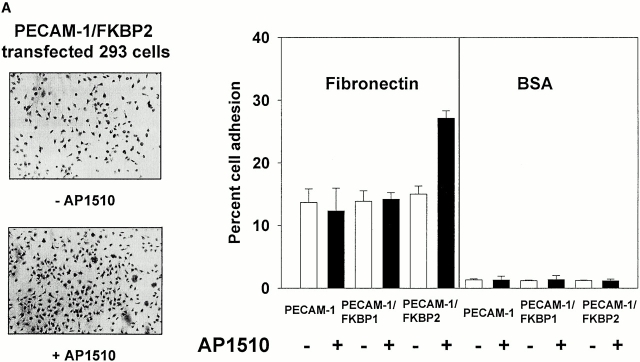
To determine whether controlled dimerization or oligomerization of PECAM-1 from within the cell might be able to initiate signal transduction pathways that affect integrin function, 293 cells expressing PECAM-1, PECAM-1/FKBP-1, and PECAM-1/FKBP-2 were labeled with calcein-AM, and their ability to bind fibronectin-coated microtiter wells were assessed in the presence or absence of AP1510. As shown in Fig. 7, adhesion of wild-type– or PECAM-1/FKBP-1–transfected 293 cells to immobilized fibronectin was unaffected by addition of AP1510. In contrast, binding and spreading of 293 cells expressing PECAM-1/FKBP-2 increased markedly upon addition of AP1510—an effect that was dose dependent (Fig. 7 B). These data suggest that oligomerization of PECAM-1 is required for integrin activation, and that PECAM dimerization is insufficient.
Figure 7.
PECAM-1 oligomerization is required to effect integrin activation. (A) 2 × 105 cells in 200 μl of serum-free cell culture media were added to microtiter wells containing immobilized fibronectin or BSA in the presence or absence of 1 μM AP1510, and cell adhesion was visualized using light microscopy (left) or quantitated using a fluorescent plate reader (right). Note that oligomerization of PECAM-1 conferred increased adhesion to fibronectin only for PECAM-1/FKBP-2–transfected cells. (B) The degree of PECAM-1 oligomerization correlated in a dose-dependent manner with cell adhesion to fibronectin, with optimal adhesion occurring at 1 μM AP1510. At higher doses of AP1510, the drug is likely to saturate available FKBP sites, leading to the formation of dimers and monomers.
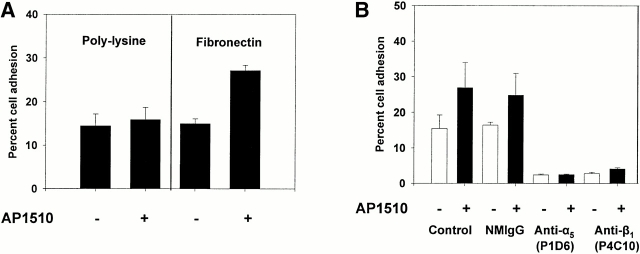
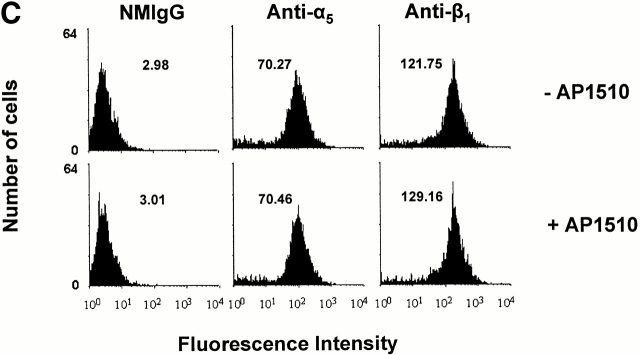
Cell adhesion to fibronectin is mediated by many different integrins, including α5β1 (Piotrowicz et al. 1988). To determine whether the AP1510-induced increase in cell adhesion was being mediated specifically by this integrin receptor, we allowed PECAM-1/FKBP-2 cells to adhere to poly-l-lysine–coated microtiter wells in the presence and absence of AP1510. As shown in Fig. 8 A, no increase in cell adhesion to poly-l-lysine was observed upon oligomerization of PECAM-1/FKBP-2, suggesting that the AP1510-induced increase in cell binding to immobilized fibronectin is integrin specific. To determine which integrins were involved, we examined the effects of integrin subunit–specific inhibitory antibodies on adhesion to fibronectin. Antibodies to β3, α2, and αv were without effect (not shown), however mAbs to α5 and β1 blocked adhesion to baseline levels (Fig. 8 B), implicating α5β1 as the integrin upregulated by PECAM-1 oligomerization in this system. PECAM-1–mediated adhesion to fibronectin was not due to increased expression of α5β1 on the cell surface (Fig. 8 C), suggesting that the signaling pathway from PECAM-1 to α5β1 modulated either the affinity or avidity of this integrin complex.
Figure 8.
PECAM-1 is a specific integrin activator. (A) Calcein AM–labeled 293 cells expressing PECAM-1/FKBP-2 were added to microtiter wells containing immobilized poly-l-lysine or fibronectin in the presence or absence of AP1510. Note that the increase in cell adhesion induced by oligomerization of PECAM-1 is specific for integrin substrates, and is mediated by the integrin α5β1, as antibodies to either of these two integrin subunits reduced cell adhesion to near background levels (B). The AP1510-induced increase in cell adhesion appears to operate via either affinity or avidity regulation, as oligomerization of PECAM-1 does not increase the level of cell surface expression of integrin α5β1 (C).
Discussion
Like members of the integrin family of adhesion receptors, there is growing evidence that PECAM-1 is able to participate in bidirectional signal transduction, i.e., PECAM-1 both initiates and responds to changes in cellular physiology. Evidence that engagement of PECAM-1 initiates specific signal transduction pathways was first provided by Tanaka et al. 1992. They showed that antibody-induced dimerization of PECAM-1 on the surface of T cells resulted in their increased adherence to the β1 integrin substrates VCAM-1 (via α4β1) and fibronectin (via α5β1). Monovalent Fab fragments of PECAM-1 mAbs were ineffective, suggesting that dimerization or oligomerization of PECAM-1 on the cell surface might be responsible for the observed upregulation of β1 integrin function. Since that time, affinity modulation of β1 integrins induced by cross-linking PECAM-1 has been reported in CD34+ hematopoietic progenitor cells (Leavesley et al. 1994), neutrophils (Pellegatta et al. 1998), and eosinophils (Chiba et al. 1999). Furthermore, β2 integrin function has been shown to be modulated by PECAM-1 dimerization in lymphokine-activated killer cells (Piali et al. 1993), monocytes and neutrophils (Berman and Muller 1995), and natural killer cells (Berman et al. 1996). The ability of PECAM-1 to function as an integrin activator has recently been extended to the β3 integrin family by the studies of Varon et al. 1998. They showed that the binding of anti–PECAM-1 F(ab′)2 fragments caused conformational changes in the platelet integrin αIIbβ3. Chiba et al. 1999 have also shown that mAb-induced cross-linking of PECAM-1 results in activation of the endothelial cell αvβ3, both enhancing adhesion to immobilized RGD peptide and enabling its heterophilic interaction with leukocyte PECAM-1. Interestingly, these authors found that αvβ3/PECAM-1 interactions, rather than supporting cell–cell adhesion (as was reported by two other groups [Piali et al. 1995; Buckley et al. 1996]), instead, constituted an amplification loop that resulted in PECAM-1–dependent activation of leukocyte α4β1. Taken together with previous findings, these studies provide compelling evidence that antibody-induced lateral associations of PECAM-1 are able to influence integrin function in a wide variety of vascular cells.
Here, we attempted to address several unresolved questions related to the ability of PECAM-1 to serve as an integrin activator. First, we employed a series of heterobifunctional cross-linking agents to determine whether PECAM-1 might actually exist in other than a monomeric form on the surface of resting cells. We found evidence for abundant levels of PECAM-1 cis homodimers and homooligomers (Fig. 2). These findings are consistent with those of Newton et al. 1999, who found that soluble recombinant forms of PECAM-1 exist in an equilibrium between monomeric and dimeric states, having a K d of ∼12 μM. Importantly, these investigators also found that PECAM-1 was able to form dimers at 4°C on the surface of U937 monocytic cells, as well as PECAM-1–transfected Cos cells. By using a combination of nonreducible and disulfide bond–containing cross-linking reagents, we were able to examine whether nonPECAM-1 components might be associated with PECAM-1 complexes, but detected none—at least in resting cells. Whether cellular activation results in novel associations of other membrane or cytosolic proteins with PECAM-1 remains an intriguing area for future investigation.
Previous studies, including our own, on the ability of PECAM-1 to serve as an amplifier of integrin function relied on the use of bivalent or monovalent mAbs, often in conjunction with additional bivalent secondary antibodies. Thus, it has been impossible to determine whether dimerization versus oligomerization of PECAM-1 is sufficient to effect integrin activation. In addition, these studies all suffer the limitation that addition of large, bulky antibody molecules to the cell surface can (a) result in antibody-induced patching, capping, and subsequent “surface-cleansing” and internalization of antibody–antigen complexes and (b) lead to nonphysiologic changes in the organization of the underlying membrane skeleton and associated signal transduction machinery. Moreover, many investigations, especially early on, employed intact antibodies, and the observed “PECAM-1–induced” cellular activation events were undoubtedly due to unintentional activation of Fc receptors. Finally, changes in cellular activation after addition of anti–PECAM-1 mAbs might be explained by antibody-mediated sequestration of this ITIM-containing inhibitory receptor away from agonist receptors that it normally regulates, resulting in a cell with increased adhesive properties. Such an explanation has been proposed for the observed stimulatory activity of anti-CD22 mAbs, which activate B cells even though CD22 is now known to be an ITIM-bearing negative regulator of B cell function (Neel 1997).
To avoid these potentially artifactual effects, we engineered two different PECAM-1 chimeric proteins expressing either one (PECAM-1/FKBP-1) or two (PECAM-1/FKBP-2) FK506-binding protein domains at their COOH terminus. When expressed in HEK-293 cells, these PECAM-1 chimeras, in conjunction with the membrane-permeable dimerizer AP1510, could be (a) induced to form controlled, self-limiting dimers or oligomers, respectively, that remained stably expressed on the cell surface and (b) examined for their ability to initiate outside-in PECAM-1–mediated signal transduction. Dimerization of cell surface receptors is commonly used by cells to transmit extracellular signals into the cell interior and regulate cellular responses (Weiss and Schlessinger 1998; Remy et al. 1999), and this FKBP-controlled dimerization system, originally developed by Spencer et al. 1993, has been used to imitate Escherichia coli aspartate receptor signaling (Cochran and Kim 1996), dissect elements of integrin regulation (Hato et al. 1998), and regulate gene transcription (Amara et al. 1997). We found that controlled dimerization of PECAM-1, initiated by addition of AP1510, had very different effects on the adhesive properties of PECAM-1 compared with oligomerization. Thus, whereas dimerization nearly doubled homophilic (trans) interactions with soluble PECAM-1, oligomerization of PECAM-1, instead, increased lateral cis interactions, at the expense of decreased capacity to support trans homophilic binding of PECAM-1/IgG (Fig. 5). Physiologically, it is interesting to note that the basal rate of interaction of leukocytes with the vessel wall is normally quite low, despite the fact that monocytes and neutrophils express abundant levels of cell surface PECAM-1, and that endothelial cells contain up to 106 PECAM-1 molecules/cell, most of which is concentrated at cell–cell borders (Newman 1994). It is possible that the high concentration of PECAM-1 at endothelial cell junctions results in lateral cis PECAM-1/PECAM-1 associations that preclude trans homophilic interactions between PECAM-1 molecules on adjacent cells—a situation that we appear to have mimicked with AP1510-induced oligomerization of PECAM-1/FKBP-2. Notably, upon exposure to tumor necrosis factor-α and γ-interferon, PECAM-1 leaves endothelial cell junctions and redistributes diffusely onto the cell surface (Romer et al. 1995). Since leukocyte trafficking occurs under similar inflammatory conditions, it is tempting to speculate that the redistributed cell surface PECAM-1 exists in monomeric and dimeric form, rather than junctional oligomers, and that these species may be involved in leukocyte capture and subsequent transendothelial migration.
A major finding of our present work was the observation that AP1510-induced oligomerization, but not dimerization, of PECAM-1 results in a marked increase in both cell adhesion and spreading on immobilized fibronectin (Fig. 7), a reaction that is (a) mediated by the integrin α5β1 (Fig. 8 B); (b) not due to changes in the expression level of this cell surface integrin (Fig. 8 C); and (c) antibody independent, and, therefore, free of any of the potential artifacts associated with cellular responses to bound immunoglobulin (discussed above). Having demonstrated that signals required for integrin activation can be elicited by clustering of PECAM-1 from within the cell, many important questions remain to be addressed. First, we were surprised to find that whereas PECAM-1 dimers appear to be especially suited to participate in high affinity trans homophilic interactions, dimerization of PECAM-1 alone does not appear to be sufficient to effect integrin activation. Might PECAM-1 oligomers uniquely associate with other, still-to-be-identified molecules within the plane of the plasma membrane, or with cytosolic signaling molecules or cytoskeletal components, to exert their effects? Second, whereas PECAM-1 monomers, dimers, and oligomers exist in equilibrium on the cell surface (Fig. 2), the cell physiological processes that control the dimerization/oligomerization state of PECAM-1 have not yet begun to be examined, and the regions within PECAM-1 that mediate lateral cis associations remain to be identified. Finally, whereas selected members of the β1, β2, and β3 integrin family have been shown to be capable of PECAM-1–mediated activation, the full range of integrins whose function can be modulated by PECAM-1 remains to be determined. Answers to these and related questions are the subject of active, ongoing investigations in various laboratories, and should provide significant insight into the ability of this molecule to influence the adhesive phenotype of the vascular cells that express it.
In summary, the production of PECAM-1/FKBP chimeric proteins, in conjunction with the use of the membrane-permeable dimerizer, AP1510, has allowed us to determine specific functional consequences of PECAM-1 homodimerization and oligomerization. We found that PECAM-1 dimers, but not oligomers, efficiently support trans PECAM-1 homophilic interactions, and provided a model that predicts that such species, when present on the surface of adjacent cells that contact each other, may facilitate cellular interactions that lead to leukocyte transendothelial migration. In contrast, the ability of PECAM-1 to serve as a positive modulator of integrin function requires supermolecular associations within the plane of the plasma membrane. Taken together, these data suggest that a dynamic equilibrium between PECAM-1 monomers, dimers, and oligomers may control cellular activation signals that influence the adhesive properties of vascular cells that express this novel member of the ITIM-containing inhibitory receptor family. Further studies aimed at characterizing the components of the bidirectional signaling pathway that enable communication between integrins and PECAM-1 are likely to improve our understanding of the role of PECAM-1 in inflammation, angiogenesis, and the immune response.
Acknowledgments
We are grateful to Dr. Sanford Shattil (The Scripps Research Institute, La Jolla, CA) for helpful advice and comments, and to Cathy Paddock and Dr. Qi-Hong Sun for providing technical advice during the early stages of this investigation.
This work was supported by grant HL-40126 (to P.J. Newman) from the National Institutes of Health and by a Predoctoral Fellowship Award 9910136Z (to T. Zhao) from the American Heart Association.
Footnotes
Abbreviations used in this paper: BS3, bis(sulfosuccinikidyl) suberate; DTSSP, dithiobis(sulfosuccinimidylpropionate); FKBP, FK506-binding protein; HEK, human embryonic kidney; HEL, human erythroleukemia; ITIM, immunoreceptor tyrosine–based inhibitory motif; PECAM-1, platelet endothelial cell adhesion molecule-1.
References
- Albelda S.M., Muller W.A., Buck C.A., Newman P.J. Molecular and cellular properties of PECAM-1 (endoCAM/CD31)a novel vascular cell–cell adhesion molecule. J. Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara J.F., Clackson T., Rivera V.M., Guo T., Keenan T., Natesan S., Pollock R., Yang W., Courage N.L., Holt D.A., Gilman M. A versatile synthetic dimerizer for the regulation of protein–protein interactions. Proc. Natl. Acad. Sci. USA. 1997;94:10618–10623. doi: 10.1073/pnas.94.20.10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M.E., Muller W.A. Ligation of platelet/endothelial cell adhesion molecule 1 (PECAM-1/CD31) on monocytes and neutrophils increases binding capacity of leukocyte CR3 (CD11b/CD18) J. Immunol. 1995;154:299–307. [PubMed] [Google Scholar]
- Berman M.E., Xie Y., Muller W.A. Roles of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in natural killer cell transendothelial migration and β2 integrin activation. J. Immunol. 1996;156:1515–1524. [PubMed] [Google Scholar]
- Bird I.N., Spragg J.H., Ager A., Matthews N. Studies of lymphocyte transendothelial migrationanalysis of migrated cell phenotypes with regard to CD31 (PECAM-1), CD45RA and CD45RO. Immunology. 1993;80:553–560. [PMC free article] [PubMed] [Google Scholar]
- Buckley C.D., Doyonnas R., Newton J.P., Blystone S.D., Brown E.J., Watt S.M., Simmons D.L. Identification of αvβ3 as a heterotypic ligand for CD31/PECAM-1. J. Cell Sci. 1996;109:437–445. doi: 10.1242/jcs.109.2.437. [DOI] [PubMed] [Google Scholar]
- Chiba R., Nakagawa N., Kurasawa K., Tanaka Y., Saito Y., Iwamoto I. Ligation of CD31 (PECAM-1) on endothelial cells increases adhesive function of alphavbeta3 integrin and enhances beta1 integrin-mediated adhesion of eosinophils to endothelial cells. Blood. 1999;94:1319–1329. [PubMed] [Google Scholar]
- Christofidou-Solomidou M., Nakada M.T., Williams J., Muller W.A., DeLisser H.M. Neutrophil platelet endothelial cell adhesion molecule-1 participates in neutrophil recruitment at inflammatory sites and is down-regulated after leukocyte extravasation. J. Immunol. 1997;158:4872–4878. [PubMed] [Google Scholar]
- Cochran A.G., Kim P.S. Imitation of Escherichia coli aspartate receptor signaling in engineered dimers of the cytoplasmic domain. Science. 1996;271:1113–1116. doi: 10.1126/science.271.5252.1113. [DOI] [PubMed] [Google Scholar]
- DeLisser H.M., Christofidou-Solomidou M., Strieter R.M., Burdick M.D., Robinson C.S., Wexler R.S., Kerr J.S., Garlanda C., Merwin J.R., Madri J.A., Albelda S.M. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am. J. Pathol. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
- Hato T., Pampori N., Shattil S.J. Complementary roles for receptor clustering and conformational change in the adhesive and signaling functions of integrin alphaIIb beta3. J. Cell Biol. 1998;141:1685–1695. doi: 10.1083/jcb.141.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioffreda M.D., Albelda S.M., Elder D.E., Radu A., Leventhal L.C., Zweiman B., Murphy G.F. TNFα induces E-selectin expression and PECAM-1 (CD31) redistribution in extracutaneous tissues. Endothelium. 1993;1:47–54. [Google Scholar]
- Leavesley D.I., Oliver J.M., Swart B.W., Berndt M.C., Haylock D.N., Simmons P.J. Signals from platelet/endothelial cell adhesion molecule enhance the adhesive activity of the very late antigen-4 integrin of human CD34+ hemopoietic progenitor cells. J. Immunol. 1994;153:4673–4683. [PubMed] [Google Scholar]
- Liao F., Huynh H.K., Eiroa A., Greene T., Polizzi E., Muller W.A. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J. Exp. Med. 1995;182:1337–1343. doi: 10.1084/jem.182.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W.A., Weigl S.A., Deng X., Phillips D.M. PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada M.T., Amin K., Christofidou-Solomidou M., O'Brien C.D., Sun J., Gurubhagavatula I., Heavner G.A., Taylor A.H., Paddock C., Sun Q.H. Antibodies against the first Ig-like domain of human platelet endothelial cell adhesion molecule-1 (PECAM-1) that inhibit PECAM-1-dependent homophilic adhesion block in vivo neutrophil recruitment. J. Immunol. 2000;164:452–462. doi: 10.4049/jimmunol.164.1.452. [DOI] [PubMed] [Google Scholar]
- Neel B.G. Role of phosphatases in lymphocyte activation. Curr. Opin. Immunol. 1997;9:405–420. doi: 10.1016/s0952-7915(97)80088-x. [DOI] [PubMed] [Google Scholar]
- Newman P.J. The role of PECAM-1 in vascular cell biology. In: Fitzgerald G.A., Jennings L.K., Patrono C., editors. Platelet-Dependent Vascular Occlusion. New York Academy of Sciences; New York: 1994. pp. 165–174. [DOI] [PubMed] [Google Scholar]
- Newman P.J. The biology of PECAM-1. J. Clin. Invest. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P.J. Switched at birtha new family for PECAM-1. J. Clin. Invest. 1999;103:5–9. doi: 10.1172/JCI5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P.J., Berndt M.C., Gorski J., White G.C., Lyman S., Paddock C., Muller W.A. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Newton-Nash D.K., Newman P.J. A new role for platelet-endothelial cell adhesion molecule-1 (CD31)inhibition of TCR-mediated signal transduction. J. Immunol. 1999;163:682–688. [PubMed] [Google Scholar]
- Newton J.P., Buckley C.D., Jones E.Y., Simmons D.L. Residues on both faces of the first immunoglobulin fold contribute to homophilic binding sites of PECAM-1/CD31. J. Biol. Chem. 1997;272:20555–20563. doi: 10.1074/jbc.272.33.20555. [DOI] [PubMed] [Google Scholar]
- Newton J.P., Hunter A.P., Simmons D.L., Buckley C.D., Harvey D.J. CD31 (PECAM-1) exists as a dimer and is heavily N-glycosylated. Biochem. Biophys. Res. Commun. 1999;261:283–291. doi: 10.1006/bbrc.1999.1018. [DOI] [PubMed] [Google Scholar]
- Pellegatta F., Chierchia S.L., Zocchi M.R. Functional association of platelet endothelial cell adhesion molecule-1 and phosphoinositide 3-kinase in human neutrophils. J. Biol. Chem. 1998;273:27768–27771. doi: 10.1074/jbc.273.43.27768. [DOI] [PubMed] [Google Scholar]
- Piali L., Albelda S.M., Baldwin H.S., Hammel P., Gisler R.H., Imhof B.A. Murine platelet endothelial cell adhesion molecule (PECAM-1/CD31) modulates β2 integrins on lymphokine-activated killer cells. Eur. J. Immunol. 1993;23:2464–2471. doi: 10.1002/eji.1830231013. [DOI] [PubMed] [Google Scholar]
- Piali L., Hammel P., Uherek C., Bachmann F., Gisler R.H., Dunon D., Imhof B.A. CD31/PECAM-1 is a ligand for αvβ3 integrin involved in adhesion of leukocytes to endothelium. J. Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowicz R.S., Orchekowski R.P., Nugent D.J., Yamada K.Y., Kunicki T.J. Glycoprotein Ic-IIa functions as an activation-independent fibronectin receptor on human platelets. J. Cell Biol. 1988;106:1359–1364. doi: 10.1083/jcb.106.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy I., Wilson I.A., Michnick S.W. Erythropoietin receptor activation by a ligand-induced conformation change. Science. 1999;283:990–993. doi: 10.1126/science.283.5404.990. [DOI] [PubMed] [Google Scholar]
- Romer L.H., McLean N.V., Yan H.C., Daise M., Sun J., DeLisser H.M. IFN-gamma and TNF-alpha induce redistribution of PECAM-1 (CD31) on human endothelial cells. J. Immunol. 1995;154:6582–6592. [PubMed] [Google Scholar]
- Schimmenti L.A., Yan H.-C., Madri J.A., Albelda S.M. Platelet endothelial cell adhesion molecule, PECAM-1, modulates cell migration. J. Cell. Physiol. 1992;153:417–428. doi: 10.1002/jcp.1041530222. [DOI] [PubMed] [Google Scholar]
- Sheibani N., Newman P.J., Frazier W.A. Thrombospondin-1, a natural inhibitor of angiogenesis, regulates platelet-endothelial cell adhesion molecule-1 expression and endothelial cell morphogenesis. Mol. Biol. Cell. 1997;8:1329–1341. doi: 10.1091/mbc.8.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D.M., Wandless T.J., Schreiber S.L., Crabtree G.R. Controlling signal transduction with synthetic ligands. Science. 1993;262:1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- Stockinger H., Gadd S.J., Eher R., Majdic O., Schreiber W., Kasinrerk W., Strass B., Schnabl E., Knapp W. Molecular characterization and functional analysis of the leukocyte surface protein CD31. J. Immunol. 1990;145:3889–3897. [PubMed] [Google Scholar]
- Stockinger H., Schreiber W., Majdic O., Holter W., Maurer D., Knapp W. Phenotype of human T cells expressing CD31, a molecule of the immunoglobulin supergene family. Immunology. 1992;75:53–58. [PMC free article] [PubMed] [Google Scholar]
- Sun J., Williams J., Yan H.-C., Amin K.M., Albelda S.M., DeLisser H.M. Platelet endothelial cell adhesion molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression J. Biol. Chem. 271 1996. 18561 18570a [DOI] [PubMed] [Google Scholar]
- Sun Q.-H., DeLisser H.M., Zukowski M.M., Paddock C., Albelda S.M., Newman P.J. Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity J. Biol. Chem. 271 1996. 11090 11098b [DOI] [PubMed] [Google Scholar]
- Sun J., Paddock C., Shubert J., Zhang H., Amin K., Newman P.J., Albelda S.M. Contributions of the extracellular and cytoplasmic domains of platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) in regulating cell–cell localization. J. Cell Sci. 2000;113:1459–1469. doi: 10.1242/jcs.113.8.1459. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Albelda S.M., Horgan K.J., Van Seventer G.A., Shimizu Y., Newman W., Hallam J., Newman P.J., Buck C.A., Shaw S. CD31 expressed on distinctive T cell subsets is a preferential amplifier of β1 integrin-mediated adhesion. J. Exp. Med. 1992;176:245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaporciyan A.A., DeLisser H.M., Yan H.-C., Mendiguren I.I., Thom S.R., Jones M.L., Ward P.A., Albelda S.M. Involvement of platelet endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science. 1993;262:1580–1582. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- Varon D., Jackson D.E., Shenkman B., Dardik R., Tamarin I., Savion N., Newman P.J. Platelet/endothelial cell adhesion molecule-1 serves as a co-stimulatory agonist receptor that modulates integrin-dependent adhesion and aggregation of human platelets. Blood. 1998;91:500–507. [PubMed] [Google Scholar]
- Weiss A., Schlessinger J. Switching signals on or off by receptor dimerization. Cell. 1998;94:277–280. doi: 10.1016/s0092-8674(00)81469-5. [DOI] [PubMed] [Google Scholar]
- Yan H.-C., Pilewski J.M., Zhang Q., DeLisser H.M., Romer L., Albelda S.M. Localization of multiple functional domains on human PECAM-1 (CD31) by monoclonal antibody epitope mapping. Cell Adhes. Commun. 1995;3:45–66. doi: 10.3109/15419069509081277. [DOI] [PubMed] [Google Scholar]
- Zehnder J.L., Shatsky M., Leung L.L.K., Butcher E.C., McGregor J.L., Levitt L.J. Involvement of CD31 in lymphocyte-mediated immune responsesimportance of the membrane-proximal immunoglobulin domain and identification of an inhibiting CD31 peptide. Blood. 1995;85:1282–1288. [PubMed] [Google Scholar]
- Zhou Z., Christofidou-Solomidou M., Garlanda C., DeLisser H.M. Antibody against murine PECAM-1 inhibits tumor angiogenesis in mice. Angiogenesis. 1999;3:181–188. doi: 10.1023/a:1009092107382. [DOI] [PubMed] [Google Scholar]