Abstract
In previous work, we used a permeabilized cell assay that reconstitutes nuclear export of protein kinase inhibitor (PKI) to show that cytosol contains an export activity that is distinct from Crm1 (Holaska, J.M., and B.M. Paschal. 1995. Proc. Natl. Acad. Sci. USA. 95: 14739–14744). Here, we describe the purification and characterization of the activity as calreticulin (CRT), a protein previously ascribed to functions in the lumen of the ER. We show that cells contain both ER and cytosolic pools of CRT. The mechanism of CRT-dependent export of PKI requires a functional nuclear export signal (NES) in PKI and involves formation of an export complex that contains RanGTP. Previous studies linking CRT to downregulation of steroid hormone receptor function led us to examine its potential role in nuclear export of the glucocorticoid receptor (GR). We found that CRT mediates nuclear export of GR in permeabilized cell, microinjection, and transfection assays. GR export is insensitive to the Crm1 inhibitor leptomycin B in vivo, and it does not rely on a leucine-rich NES. Rather, GR export is facilitated by its DNA-binding domain, which is shown to function as an NES when transplanted to a green fluorescent protein reporter. CRT defines a new export pathway that may regulate the transcriptional activity of steroid hormone receptors.
Keywords: nucleocytoplasmic transport, Ran GTPase, nuclear pore complex, protein export, hormone receptor
Introduction
Transport between the nuclear and cytoplasmic compartments occurs through nuclear pore complexes (NPCs), elaborate channels that perforate the double membrane system of the nuclear envelope. The large size (125 mD), complex composition (>100 polypeptides), and architectural design of NPCs relates to the central role of these structures in both diffusion-based transport of small molecules and facilitated transport of macromolecules (Doye and Hurt 1997; Ohno et al. 1998). The facilitated transport pathways are initiated through the binding of soluble receptors to protein or ribonucleoprotein (RNP) cargo endowed with either nuclear localization signals (NLSs) or nuclear export signals (NESs). The receptor–cargo complex then undergoes a series of physical interactions with the NPC that culminate in the delivery of cargo to the appropriate compartment (Görlich and Kutay 1999; Nakielny and Dreyfuss 1999).
In the past five years, there has been substantial progress in characterizing the nuclear transport receptors and analyzing how their interactions with cargo are regulated. The nuclear transport receptors described to date comprise a superfamily of proteins sharing ∼20% amino acid identity, usually referred to as β-importins or β-karyopherins in vertebrates and Kaps in yeast (Görlich et al. 1997). For example, the budding yeast Saccharomyces cerevisiae encodes a total of fourteen β-importin superfamily members, many of whose transport functions have been characterized at the molecular level. Nine are import receptors, including the β-importin family member Kap95p, a receptor that requires the adapter Kap60p to specify the cargo-binding function of the complex. Adapter proteins are used extensively in higher eukaryotes to mediate cargo binding to β-importin. The adapters identified in vertebrates include α-importin, snurportin, XRIPα, importin7, and RanBP8 (Görlich and Kutay 1999). Notably, five distinct α-importins are expressed in human cells, several of which preferentially bind to certain NLS substrates (Kohler et al. 1999). Thus, adapters provide a form of combinatorial flexibility that allows a variety of NLS-containing cargo to be imported via a single β-importin.
The four export receptors identified thus far are exportin-t, Msn5p, CAS, and Crm1. Exportin-t (Los1p in S. cerevisiae) is the export receptor for tRNA (Arts et al. 1998a; Kutay et al. 1998). Msn5p is the export receptor for several transcription factors in yeast, including the phosphorylated form of Pho4p (Kaffman et al. 1998). CAS (Cse1p in S. cerevisiae) is a dedicated export receptor for α-importin, emphasizing the critical nature of recycling pathways for transport factors (Kutay et al. 1997; Hood and Silver 1998). Crm1 (Xpo1p in S. cerevisiae) is the nuclear export receptor that has attracted the most attention because it mediates export of proteins that contain the leucine-rich NES, which was originally characterized in Rev and protein kinase inhibitor (PKI) (Fischer et al. 1995; Wen et al. 1995; Fornerod et al. 1997; Stade et al. 1997). These results, together with functional identification of the leucine-rich NES in diverse proteins, has led to the view that Crm1 is a general receptor for nuclear protein export.
Crm1 also functions as an export receptor for RNA. Crm1 mediates nuclear export of partially spliced and unspliced transcripts encoded by HIV-1 (Cullen 2000), and it mediates nuclear export of certain uridine-rich snRNAs (Ohno et al. 2000; Ossareh-Nazari et al. 2000). In both cases, Crm1-dependent export requires one or more NES-containing adapter proteins that bridge its interaction with RNA cargo. Rev fulfills its adapter function through direct binding to a structured element within the RNA transcript. The adapters involved in Crm1-dependent export of uridine-rich snRNAs include several proteins that assemble into a cap-binding complex on these RNPs (Izaurralde et al. 1995). Crm1 has also been implicated in bulk mRNA export (Stade et al. 1997), however, this issue is controversial (Neville and Rosbash 1999). Indeed, establishing the identity of export receptors directly responsible for mRNA transport remains an area of active investigation. The best candidates for mRNA export receptors include TAP/Mex67p, Gle1p/Rss1p, Gle2p/Rae1p, and Dbp5p/Rat8p (Murphy et al. 1996; Murphy and Wente 1996; Gruter et al. 1998; Braun et al. 1999; Hodge et al. 1999; Pritchard et al. 1999). It is noteworthy that aside from Crm1, none of the putative mRNA export receptors are related to the β-importin superfamily at the sequence level. This can be interpreted as evidence for the existence of β-importin–independent pathways for transport through the NPC.
The export function of Crm1 requires participation of the Ran GTPase. In the nucleus, direct binding of RanGTP to Crm1 stabilizes the association of the receptor with NES cargo (Görlich et al. 1997; Askjaer et al. 1998); the resulting trimeric export complex can then undergo translocation through the NPC. When the export complex reaches the cytoplasmic side of the NPC, RanGAP-catalyzed hydrolysis of RanGTP to RanGDP is thought to trigger disassembly of the complex as part of the terminal step of the pathway (Askjaer et al. 1999; Kehlenbach et al. 1999). Crm1 and RanGDP are then reimported for the next round of export. The RanGTP-dependent formation of a trimeric complex in the nucleoplasm, and the disassembly of the complex by RanGAP in the cytoplasm, are reactions that are predicted to occur with the export receptors exportin-t, Msn5p, as well as CAS.
We recently developed a permeabilized cell assay that reconstitutes nuclear export of PKI in a cytosol-dependent manner, and showed that purified Crm1 and Ran are sufficient to drive nuclear export in this system (Holaska and Paschal 1998). In the course of these studies, we made the surprising observation that cytosol depleted of Crm1 still supports nuclear export of PKI. Since this implies that cytosol contains factors that can functionally substitute for Crm1 in our assay system, we hypothesized that additional export receptors are present in mammalian cells (Holaska and Paschal 1998). Here, we describe the purification and characterization of a cytosolic factor that can mediate nuclear export of PKI by a pathway that operates independent of Crm1. The cytosolic factor is calreticulin (CRT), a calcium-binding protein implicated in protein folding in the ER lumen (Krause and Michalak 1997). However, we found that cells contain both ER and cytosolic pools of CRT. We also demonstrated that CRT-dependent export is fundamentally similar to Crm1-dependent export, as both pathways involve formation of trimeric complexes with NES substrate and RanGTP. One striking difference between these pathways is that CRT, but not Crm1, mediates nuclear export of the glucocorticoid receptor (GR). CRT interacts with the DNA-binding domain (DBD) of GR, which we show is functional as an NES. These protein–protein interactions underlie a nuclear export–based mechanism for antagonizing the transcriptional activation potential of GR, and may represent an important regulatory step for other nuclear receptors as well.
Materials and Methods
Purification of the Cytosolic Export Activity
The export receptor (CRT) was purified from HeLa cell cytosol using PKI export as the assay during all fractionation steps (Holaska and Paschal 1998). Cytosol (20 ml, at a concentration of 10 mg/ml) was mixed end over end with phenyl-Sepharose beads at a final ratio of 3:1 (vol cytosol/vol beads) for 15 min at 4°C to deplete Crm1. A 55–70% ammonium sulfate precipitation of the Crm1-depleted cytosol was collected by centrifugation and resuspended in transport buffer (TB: 20 mM Hepes, pH 7.4, 110 mM potassium acetate, 2 mM magnesium acetate, 1 mM EGTA) containing 2 mM DTT and a protease inhibitor cocktail. The resuspended material was then diluted with saturated ammonium sulfate to a final concentration of 40%, clarified by centrifugation (40,000 g for 30 min), and bound to phenyl-Sepharose beads. A step gradient of decreasing ionic strength was used for batch elution of the beads, resulting in recovery of the export activity in 20% ammonium sulfate. The eluate was chromatographed on a Superose 6 column (24-ml bed volume) at a flow rate of 0.25 ml/min in 2× TB. The active fractions were pooled, diluted to 5 ml with 50 mM Tris-HCl buffer, pH 8.0, and chromatographed on a MonoQ column (1-ml bed volume) using a linear gradient of 0–1 M NaCl for elution. The export activity that eluted at ∼350–500 mM NaCl corresponded to an ∼60-kD polypeptide by SDS-PAGE. Mass spectrometry performed on tryptic fragments derived from the 60-kD band revealed the mass and sequence of 11 different peptides contained within the sequence of human CRT (sequence data available from GenBank/EMBL/DDBJ under accession no. A37057). The peptides and the corresponding residues in human CRT are EQFLDGDAWTNR (25–36), HKSDFGK (42–48), FYGDQEKDK (56–64), DKGLQTSQDAR (63–73), FYALSAR (74–80), HEQNIDCGGGYVK (99–111), KVHVIFNYK (143–151), IKDPDAAKPEDWDER (208–222), GEWKPR (273–278), QIDNPDYK (279–286), and KDQDEEQR (359–366).
PKI Export Assay
The PKI export assay was performed in suspension-culture HeLa cells (Holaska and Paschal 1998). The assay involves nuclear loading of fluorescently labeled streptavidin–NLS (FITC–STV–NLS), addition of biotinylated PKI (bPKI), and cytosolic factor–dependent export of the bPKI/FITC–STV–NLS complex. The readout is the reduction of nuclear fluorescence that results from export stimulated by the addition of fractionated cytosol or recombinant proteins. For quantitation of nuclear export, images of nuclei (∼50) were selected in the DAPI (4′6-diamidino-2-phenylindole) channel and captured in the FITC channel using a Nikon Microphot-SA microscope (40× objective, NA = 0.95) equipped with a Hamamatsu C-4742-95 CCD camera. Image acquisition and analysis was carried out using Openlab 2.06 software, and data figures were constructed using Adobe Photoshop® 5.02 and FreeHand 8.0. The error bars in each figure represent the SD of mean nuclear fluorescence within the sample. The concentrations of recombinant proteins used in individual PKI export experiments are stated in the figure legends. Full-length CRT fused to glutathione-S-transferase (GST) was generally used at a concentration of 50 μg/ml. We also expressed and characterized a GST–CRT fusion protein lacking the first 17 amino acids that comprise the ER signal sequence. The activity of recombinant CRT lacking the signal sequence was indistinguishable from full-length CRT in our assays. Recombinant Ran was not routinely added to permeabilized cell assays. The level of Ran that remains associated with permeabilized cells is apparently sufficient for CRT-dependent export. However, Ran does become rate-limiting at low concentrations of CRT, which was the basis for Fig. 3 C. Human Crm1 used in the PKI export assay was purified from HeLa cytosol by column chromatography. The effect of leptomycin B (LMB) on PKI export in permeabilized cells was examined by incubating the soluble factors with the compound (500 nM) for 15 min at room temperature before performing the export reactions.
Figure 3.
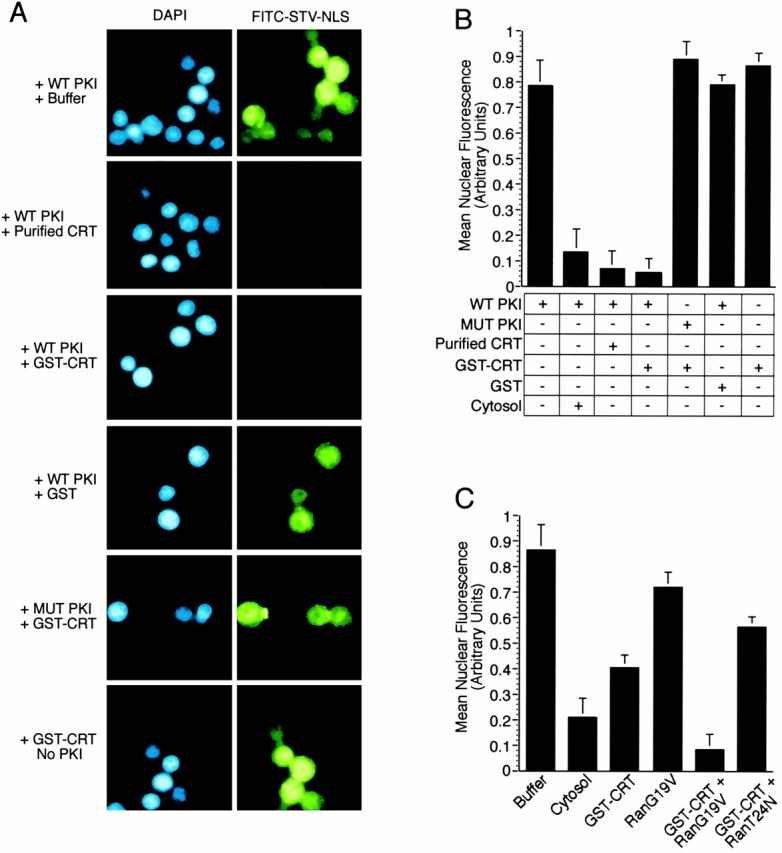
CRT-dependent export of PKI in digitonin permeabilized cells. (A) Nuclear export of PKI can be reconstituted by recombinant CRT, and requires a functional NES within PKI. HeLa cell nuclei were loaded with fluorescently labeled STV–NLS (FITC–STV–NLS), washed with buffer, and incubated with bPKI and soluble export factors. After the export reaction, cells were washed, pipetted onto glass slides, and viewed by fluorescence microscopy. Nuclear export of PKI/FITC–STV–NLS, visualized as the loss of nuclear fluorescence, was observed if the reaction was supplemented with HeLa cell–derived CRT (50 μg/ml) or recombinant GST–CRT (50 μg/ml). Nuclear export was not observed when the reaction was supplemented with GST (50 μg/ml), or if the NES mutant of PKI (L41,44A; MUT PKI) was used instead of WT PKI, or if PKI was omitted from the assay. Cells were stained with DAPI to show the location of the nuclei in each field. (B) Quantitation of the relative levels of nuclear export from the experiments shown in A. The level of nuclear export promoted by the addition of HeLa cell cytosol is shown for comparison. (C) CRT and Ran-dependent export of PKI. Export reactions were performed at a subsaturating concentration of CRT (5 μg/ml) and the Ran mutants G19V and T24N (each at 10 μg/ml). Export was promoted by the Ran G19V mutant and inhibited by the Ran T24N mutant. This indicates that CRT-dependent export requires the GTP form of Ran.
GR–GFP and RevM10–DBD–GFP Export Assays
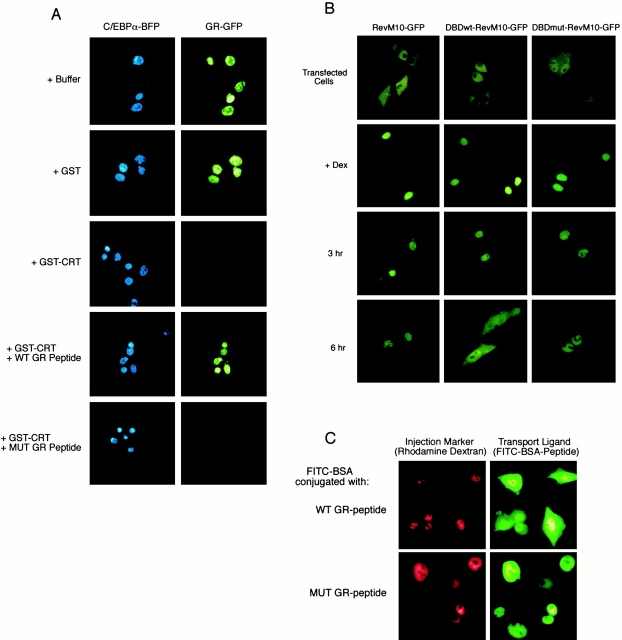
A plasmid encoding rat GR fused to green fluorescent protein (GR–GFP) was transfected into BHK cells grown on glass coverslips, using the calcium phosphate method. In certain experiments, a plasmid encoding CCAAT/enhancer-binding protein α fused to blue fluorescent protein (C/EBPα–BFP) was included as a transfection marker. After transfection, the cells were maintained in culture for 48 h, and then treated with 1 μM agonist (corticosteroid or dexamethasone) to induce nuclear accumulation of GR–GFP. The coverslips were washed, permeabilized with digitonin, and used in export assays. We assayed whether the DBD of the GR is involved in its CRT-dependent export by testing whether a segment within the domain can competitively inhibit transport on this pathway. GST–CRT was preincubated with wild-type (WT) GR peptide (CGGGKVFFKRAVEGQHNLY) and mutant GR peptide (CGGGKVAAKRAVEGQHNLY) for 15 min on ice. The GST–CRT/GR peptide mixture was combined with permeabilized cells expressing GR–GFP, and the effect on export was examined by fluorescence microscopy. Nuclear export of GR–GFP in vivo was studied by first inducing its nuclear import with corticosteroid; the cells were then washed six times with culture medium containing charcoal-stripped serum. The cells were incubated in the same medium in the absence and presence of 200 nM LMB, and images were recorded by fluorescence microscopy over a 26-h time course. crt −/− and crt +/+ cell lines were derived from mouse embryo fibroblasts and immortalized by SV40 large T antigen.
Plasmids encoding either RevM10–GFP (derived from pXM10) (Love et al. 1998), DBD–RevM10–GFP, or DBDmut–RevM10–DBD were constructed in pCDNA3, and used to transfect BHK cells grown on glass coverslips. 48 h after transfection, the GFP fusion proteins were induced to accumulate in the nucleus with 1 μM dexamethasone for 1 h. The coverslips were then placed into culture medium containing charcoal-stripped serum, and examined by fluorescence microscopy at 3 h intervals to score nuclear export.
Plasmids and Recombinant Proteins
The open reading frame of mouse CRT (sequence data available from GenBank/EMBL/DDBJ under accession no. AA243918) was amplified by PCR using the oligonucleotide primers 5′-GGCTCGAGATGCTCCTTTCGGTGCCGCTC-3′ and 5′-CCCCTCGAGCTACAGCTCATCCTTGGCTTG-3′ and cloned into the XhoI site of pGEX4T3. The GST–CRT fusion protein was expressed by induction with IPTG and purified on glutathione-Sepharose resin by established methods. Nucleotide-binding mutants of Ran (T24N and G19V) were also expressed as GST fusions, using plasmids provided by Dr. Ian Macara (University of Virginia, Charlottesville, VA) (Carey et al. 1996). His-tagged Ran, expressed from a plasmid provided by Dr. Dirk Görlich (University of Heidelburg, Heidelburg, Germany), was purified by sequential metal chelation and gel filtration chromatography. FITC–STV–NLS, WT, and NES-mutant forms of PKI were expressed and purified, as described previously (Holaska and Paschal 1998). All protein stocks were prepared as single-use aliquots, frozen in liquid nitrogen, and stored at −70°C.
Export Complex Assembly Reactions
The affinity of CRT for the NES of PKI in the absence and presence of RanGTP was measured using the Fisons Biosensor (Fisons). All manipulations were carried out at ambient temperature. The NES surface was assembled by first adding 50 μl neutravidin (1 mg/ml in PBS) (Pierce Chemical Co.) to cuvettes precoated with biotin for 15 min. After washing with PBS, 100 μl biotin-modified forms of WT NES peptide (bGSNDLALKLAGLDINKTGGC) or mutant NES peptide (bGSNDLALKAAGADINKTGGC) (each 0.5 mg/ml) was then added to the cuvette and incubated for 15 min. After washing with PBS, the cuvette was used for binding analysis. Recombinant GST–CRT, GST, and Ran (preloaded with GMP-PNP) were used alone or in combination, the concentrations of which are stated in the legend. The increase in refractive index was recorded as a function of time to measure association rate, and after a wash step, the decrease in refractive index was recorded as a function of time to measure dissociation rate. The FASTfit program (Fisons) was used to calculate the k a, k d, and k D using the relationship k D = k d/k a. A complete description of the methods used with the Fisons biosensor have been published (Rubio et al. 1997). The solid-phase assay used to measure RanGTP incorporation into CRT and Crm1 complexes was performed essentially as described previously (Black et al. 1999).
Subcellular Fractionation of CRT
Digitonin treatment of HeLa cells was carried out under conditions that selectively permeabilize only the plasma membrane, resulting in the release of cytosolic proteins. Suspension-culture HeLa cells (109) were collected by centrifugation, washed in TB, diluted to 5 × 106 cells/ml in TB, and treated with 0.005% digitonin for 5 min on ice. These cells were then diluted to 2 × 106 cells/ml with TB, collected by centrifugation, and the supernatant and pellet were recovered. Cell equivalents of intact cells, digitonin-permeabilized cells, and the digitonin-released fraction were analyzed by SDS-PAGE and immunoblotting using primary antibodies to CRT, Grp94, ERp72, Hsp70, PDI (all from StressGen), Crm1 (Holaska and Paschal 1998), NTF2 (Steggerda et al. 2000), and peroxidase-labeled secondary antibodies (Pierce Chemical Co.), and detected by enhanced chemiluminescence. The relative amounts of CRT and marker proteins in the whole cell, permeabilized cell, and released fractions were determined by scanning densitometry of films using ImageQuaNT 4.2. For subcellular fractionation, 109 suspension HeLa cells were mechanically lysed in 0.25 M sucrose using a 21-gauge needle attached to a 10-ml syringe. The lysate was clarified twice at 1,000 g, and the postnuclear supernatant was fractionated by ultracentrifugation over 0.5 M sucrose at 160,000 g for 2 h. Gradient fractions were recovered from the bottom of the tube and analyzed by SDS-PAGE and immunoblotting.
Microinjection Analysis
BHK cells grown on gridded coverslips were microinjected using femtotips mounted on a Micromanipulator and Transjector (Eppendorf). We addressed whether the sequence from the DNA-binding domain of the GR can function as an NES using fluorescent conjugates of the WT and mutant GR peptides used in the competition experiment (see above). The peptides were coupled to FITC-labeled BSA using the heterobifunctional cross-linker sulfo-SMCC (Pierce Chemical Co.) essentially as described for preparation of FITC–BSA–NLS conjugates (Paschal and Gerace 1995). Each GR peptide conjugate (1 mg/ml) was coinjected with rhodamine-dextran (0.5 mg/ml) into the nuclei of BHK cells and incubated for 30 min at 37°C. The cells were then processed for fluorescence microscopy. The ability of cytosolic CRT to stimulate nuclear export of the GR in vivo was examined using a microinjection assay. Transfected BHK cells were treated with 1 μM dexamethasone to induce nuclear accumulation of GR–GFP. GST–CRT (0.5 mg/ml) or GST (0.5 mg/ml) was coinjected with rhodamine-dextran (0.5 mg/ml) into the cytoplasm of BHK cells and incubated for 45 min at 37°C. The cells were then processed for fluorescence microscopy.
Results
Purification of a Protein That Mediates PKI Export
In a previous study, we described a permeabilized cell assay that reconstitutes nuclear export of bPKI complexed with fluorescent STV (Holaska and Paschal 1998). Nuclear export in this system requires a functional NES within PKI and its recognition by one or more factors present in HeLa cell cytosol. We also found that cytosol depleted of Crm1, or treated with N-ethylmaleimide to inactivate Crm1, promoted nuclear export of PKI to nearly the same extent as control cytosol (Holaska and Paschal 1998). Moreover, we found that cytosol depleted of Crm1 was insensitive to LMB, a potent inhibitor of Crm1 (Holaska, J.M., and B.M. Paschal, unpublished observations). These observations suggested that cytosol contains an export factor, biochemically distinct from Crm1, that recognizes the NES within PKI and mediates its export from the nucleus.
We used the PKI export assay and cytosol depleted of Crm1 (Holaska and Paschal 1998) for purification of the export factor. We found that the export factor could be recovered in a 55–70% ammonium sulfate precipitation, which provided significant enrichment since most cytosolic proteins were precipitated with 55% ammonium sulfate (data not shown). We ultimately designed a purification scheme that involved ammonium sulfate precipitation followed by chromatography on hydrophobic interaction, gel filtration, and ion-exchange resins (see Materials and Methods). The final step of the purification involved binding the export factor to a MonoQ column and eluting with a linear salt gradient. We observed that maximal PKI export activity eluted from the MonoQ column at a salt concentration of ∼400 mM NaCl, corresponding to fractions 44 and 45 in the chromatographic profile (Fig. 1 B). The same fractions were analyzed by gel electrophoresis and silver staining and found to contain an ∼60-kD polypeptide (Fig. 1 A). Since the relative abundance of the 60-kD polypeptide in fractions 44 and 45 correlates with the levels of PKI export activity promoted by these fractions, we conclude that it represents the export factor.
Figure 1.
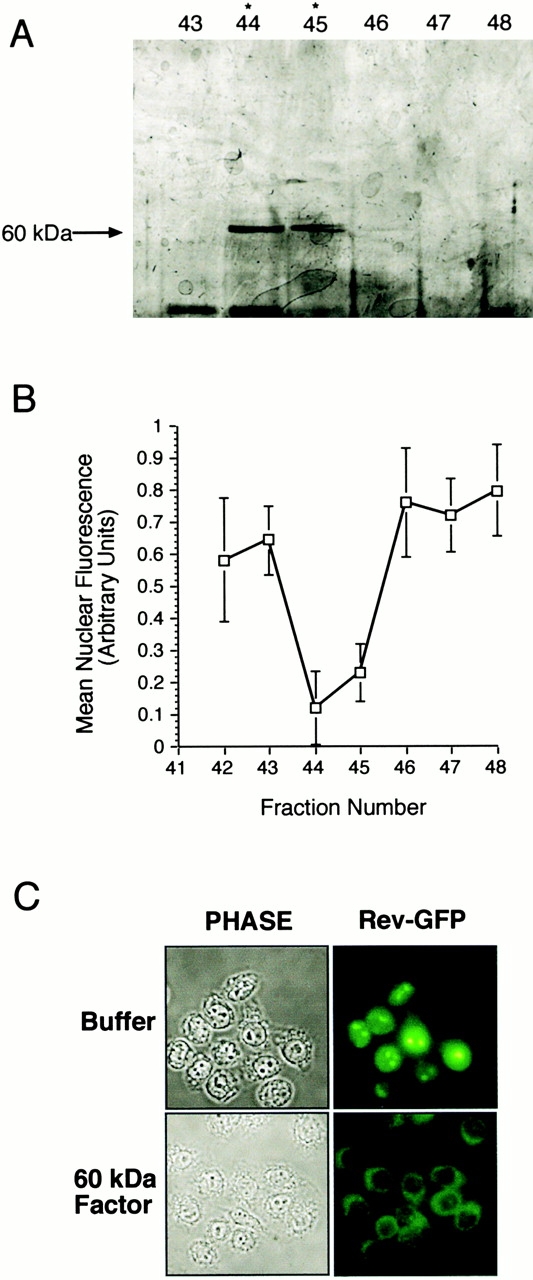
Identification of a 60-kD export factor as CRT. (A and B) Purification of the export activity. Crm1-depleted cytosol was fractionated by ammonium sulfate precipitation, hydrophobic interaction, gel filtration, and MonoQ chromatography. Each fraction was tested for activity in the PKI export assay (Holaska and Paschal 1998). The final step of the purification was MonoQ chromatography. The export activity profile is plotted as the decrease in mean nuclear fluorescence, and the protein profile is shown by SDS-PAGE (7% gel, silver-stained). The maximum export activity in fractions 44 and 45 corresponds to the abundance of a 60-kD polypeptide. Mass spectrometry of tryptic fragments from the 60-kD factor revealed its identity as CRT. (C) The 60-kD factor (CRT) mediates nuclear export of Rev. A cell line expressing Rev–GFP (Love et al. 1998) was grown on coverslips, permeabilized with digitonin, and incubated with buffer or purified CRT. Nuclear export, as visualized in the fluorescence microscope, was observed in samples incubated with CRT, but not with buffer alone.
We examined whether the 60-kD export factor mediates nuclear export of Rev, which, like PKI, contains a hydrophobic NES. A cell line expressing a Rev–GFP fusion protein (Love et al. 1998) was permeabilized with digitonin and incubated with either buffer or the 60-kD factor. Nuclear export of Rev–GFP was observed in the presence of the 60-kD factor (Fig. 1 C), and was blocked in the presence of wheat germ agglutinin (data not shown), the latter experiment confirming that export occurs through the NPC.
The 60-kD Nuclear Export Factor Is Cytosolic CRT
To determine the molecular identity of the 60-kD export factor, we used the purified protein to generate tryptic peptides, and analyzed their composition by mass spectrometry. The predicted masses of 11 different peptides (see Materials and Methods) containing a total of 116 amino acids allowed us to identify the 60-kD factor as CRT, an abundant calcium-binding protein. CRT is involved in calcium homeostasis, and it is also thought to interact transiently with partially folded proteins as part of a quality control mechanism that operates in the lumen of the ER (Krause and Michalak 1997). However, several laboratories have reported that CRT may reside in the nucleus and cytoplasm (Jethmalani et al. 1997; Roderick et al. 1997), suggesting that cells contain both ER lumenal and cytosolic forms of the protein.
Since we purified CRT from a highly clarified (150,000 g) extract free of membranous organelles, it seemed very likely that the CRT identified in our purification scheme is a cytosolic protein. To demonstrate that cultured cells contain a cytosolic form of CRT, we incubated HeLa cells with a concentration of digitonin (0.005%) that selectively permeabilizes the plasma membrane, thereby releasing only cytosolic proteins. Immunoblotting of pre- and postpermeabilization fractions revealed that approximately one third of total cellular CRT is released from cells by treatment with digitonin (Fig. 2 A). In contrast, the lumenal proteins Grp94, ERp72, and PDI were retained within digitonin-permeabilized cells, confirming that the integrity of the ER was not compromised during the experiment. The CRT released from cells by digitonin is not contained within vesicles, based on the sensitivity of the protein to proteinase K digestion (Fig. 2 B).
Figure 2.
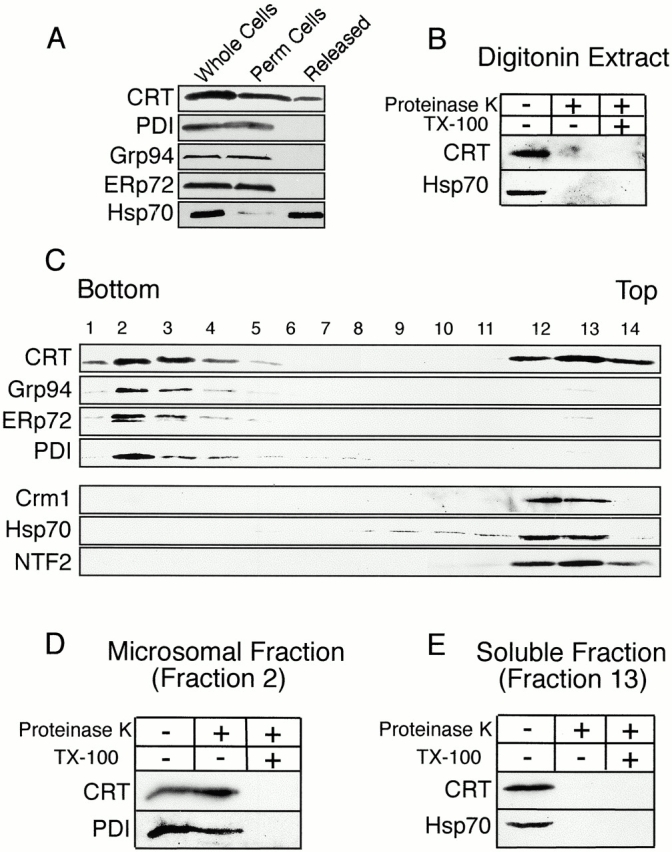
HeLa cells contain both microsomal and cytosolic pools of CRT. (A and B) CRT is released from HeLa cells by digitonin permeabilization. Suspension HeLa cells (whole cells) were permeabilized with digitonin, collected by centrifugation, and then the fractions were analyzed by immunoblotting. A pool of CRT, and most of the Hsp70, are released from cells (Released), whereas the ER lumenal proteins Grp94, ERp72, and PDI are retained (Perm cells). The CRT released by digitonin permeabilization is susceptible to proteinase K digestion, indicating it is not enclosed within membrane-bound vesicles. (C) CRT fractionates as both a microsomal and a cytosolic protein. A postnuclear supernatant from HeLa cells was fractionated over a sucrose gradient and analyzed by SDS PAGE and immunoblotting. CRT and the ER markers Grp94, Erp72, and PDI partition with microsomes (fractions 2–4). CRT also partitions with the soluble proteins Crm1, Hsp70, and NTF2 (fractions 12–14). (D) The CRT in the microsomal fraction is protected from protease digestion. Fraction 2 from the sucrose gradient was treated with proteinase K (80 μg/ml, 15 min at room temperature) in the absence and presence of detergent (1% Triton X-100); only the latter condition resulted in digestion of CRT and the ER marker protein PDI. (E) The CRT in the soluble fraction is susceptible to protease digestion. Fraction 13 from the sucrose gradient was treated with proteinase K as above. CRT and the soluble marker Hsp70 were degraded in the absence of detergent.
Additional evidence for cytosolic and ER forms of CRT was obtained by subcellular fractionation. Postnuclear supernatants from mechanically lysed HeLa cells were subjected to ultracentrifugation on sucrose gradients, and the fractions were collected and analyzed by immunoblotting. CRT and the ER marker proteins Grp94, ERp72, and PDI were detected in the microsomal fraction at the bottom of the gradient (Fig. 2 C, lanes 2–4). CRT was also detected in the cytosolic fraction at the top of the gradient, along with the soluble proteins Crm1, Hsp70, and NTF2 (Fig. 2 C, lanes 12–14). We extended this analysis to show that the CRT in the microsomal fraction is protected from proteinase K digestion, unless the membrane is solubilized by the detergent Triton X-100 (Fig. 2 D). In contrast, the CRT in the soluble fraction is susceptible to proteinase K digestion, even in the absence of detergent. Our data provides unequivocal evidence for two pools of CRT, the first contained within the lumen of the ER, and the second contained within cytosol.
The NES of PKI Is Required for CRT-dependent Export
A logical interpretation of our results showing that cytosolic CRT can stimulate nuclear export of PKI and Rev is that it functions as a nuclear export receptor. One property expected of a receptor is specific recognition of a signal within the macromolecule that undergoes nuclear export. For example, Crm1 can discriminate between a WT NES and a transport-defective NES in proteins such as Rev and PKI (Fischer et al. 1995; Wen et al. 1995), and exportin-t can discriminate between mature and incompletely processed tRNAs (Arts et al. 1998b). We addressed this issue by producing recombinant CRT, and examining whether it can discriminate between WT PKI that is functional for export, and a mutant (MUT) PKI that is nonfunctional for export (Wen et al. 1995). Using the permeabilized cell assay, we determined that GST–CRT (50 μg/ml) stimulates export of WT PKI to the same extent as CRT (50 μg/ml) purified from cells, confirming that CRT itself is the active component from HeLa cytosol (Fig. 3A and Fig. B). However, GST–CRT does not stimulate export of a mutant PKI containing the two well-characterized amino acid substitutions (L41,44A) that inactivate the leucine-rich NES (Wen et al. 1995). In addition, nuclear export was not observed if PKI was omitted from the assay (Fig. 3A and Fig. B). These results indicate that nuclear export mediated by CRT requires specific recognition of the NES within PKI. Our results also suggest that CRT, like Crm1, physically contacts hydrophobic amino acids within the NES of its export substrate.
We obtained evidence that CRT shares a second property with Crm1, namely, that its export function is regulated by the Ran GTPase system. In permeabilized cell assays containing a subsaturating concentration of GST–CRT, nuclear export of PKI could be reconstituted in the presence of WT Ran or a mutant Ran that mimics the GTP-bound form (G19V) of the GTPase, but not by a mutant Ran that mimics the nucleotide-free form (T24N) of the GTPase (Fig. 3 C). Taken together, our data indicate that CRT and Ran cooperate to mediate nuclear export of PKI, and that this pathway involves CRT recognition of the leucine-rich NES of PKI. These properties are consistent with CRT functioning as a nuclear export receptor for PKI.
CRT Assembles into a Trimeric Complex Containing NES and RanGTP
The functional interactions between CRT, PKI, and Ran measured in the permeabilized cell assay suggests these proteins may assemble into a trimeric export complex. For example, the export receptor Crm1 coassembles with NES-containing cargo and the GTP-bound form of Ran in vitro (Fornerod et al. 1997; Askjaer et al. 1998); this trimeric assembly of proteins is thought to represent the complex that is actively exported from the nucleus (Görlich and Kutay 1999; Nakielny and Dreyfuss 1999). Each of the export receptors of the β-importin superfamily is thought to assemble into trimeric complexes that contain their respective cargo and RanGTP.
We examined whether CRT can discriminate between a functional and mutant NES and the potential role of RanGTP using a biosensor assay that measures molecular interactions in real time. The principle involves monitoring dynamic changes in refractive index that occur when a protein in solution binds to a ligand immobilized on the sensor surface (Rubio et al. 1997). The ligand used for this analysis corresponded to the NES of PKI, synthesized as a peptide containing an NH2-terminal biotin. This facilitates orientation-specific capture of the NES peptide onto the neutravidin-coated surface of the sensor. Addition of recombinant CRT (50 μg/ml) to the surface containing the WT NES resulted in a negligible change in refractive index (Fig. 4 A, green tracing). Similarly, addition of recombinant Ran (50 μg/ml, preloaded with GMP-PNP) to the WT NES caused only a slight change in refractive index (dark blue tracing), most of which was lost upon infusion of buffer (arrow). In contrast to these results, simultaneous addition of CRT (50 μg/ml) and Ran (50 μg/ml) to the WT NES resulted in an increase in refractive index to ∼140 arc s (red tracing), one third of which was retained upon buffer infusion. Thus, CRT and Ran exhibit cooperative binding to the NES. The binding of CRT and Ran in this reaction is specific for a WT NES, since addition of these proteins to a surface containing a mutant NES did not cause a detectable increase in refractive index (light blue tracing).
Figure 4.
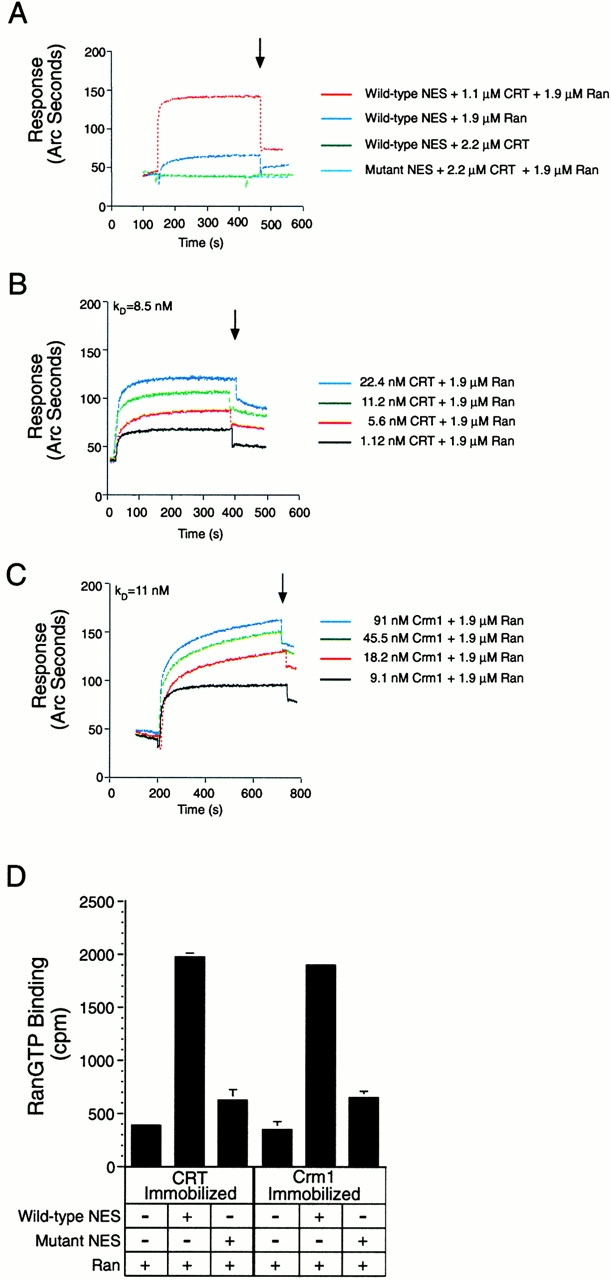
CRT forms a trimeric complex with the leucine-rich NES and RanGTP. A synthetic peptide containing the NES of PKI (WT or NES mutant) was immobilized on the surface of the biosensor cuvette through a biotin–STV linkage. The binding response of recombinant proteins to immobilized peptide was measured as the increase in refractive index as a function of time. (A) CRT binding to the WT NES requires the presence of RanGTP. GST–CRT (50 μg/ml) and Ran preloaded with GMP-PNP (50 μg/ml) were added alone, or in combination, to cuvettes containing immobilized WT NES or mutant NES (L41,44A). The arrow indicates the time at which buffer (PBS) was infused. Complex formation is observed in the presence of a WT NES, CRT, and RanGTP (red tracing). CRT and Ran do not form a complex on a mutant NES (light blue tracing), and only background levels of binding are observed if CRT or Ran is omitted from the assay (dark blue and green tracings, respectively). Note that the light blue and green tracings overlap at ∼40 arc s, which represents background binding. (B) Measurement of the affinity of CRT for NES in the presence of RanGTP. The association rate of CRT for NES in the presence of Ran was measured over a concentration range of CRT (1.12–22.4 nM). The dissociation rate was measured in a similar manner after infusion of buffer (beginning at 400 s), and the FASTfit program was used to calculate the affinity of CRT for NES in the presence of Ran (K D = 8.5 nM). (C) Measurement of the affinity of Crm1 for NES in the presence of RanGTP. The methods described above were used to measure the association and dissociation rates of the trimeric complex, and the FASTfit program was used to calculate the affinity (K D = 11 nM). (D) RanGTP binding to CRT and Crm1 is stimulated by the NES. His-tagged CRT and Crm1 were adsorbed to microtiter wells, and binding of Ran was measured in the absence and presence of the WT and mutant NES peptides. Since the recombinant Ran was preloaded with GTP radiolabeled on the terminal phosphate, the counts measured in the bound fraction specifically reflect the triphosphate form of Ran. Including WT NES in the binding reaction stabilizes the interaction of RanGTP with both CRT and Crm1.
We measured the on-rates for cooperative CRT and Ran binding to the WT NES over a 20-fold concentration range of CRT (Fig. 4 B), and, from this, we calculated an association rate constant k a = 7.41 × 105 M−1 s−1. The CRT/Ran/NES complex dissociated with a rate constant k d = 6.30 × 10−3 s−1. The ratio k d/k a reveals a K D = 8.5 nM. As a positive control for this analysis, we performed binding experiments using Crm1 purified from HeLa cells, and confirmed that under our reaction conditions Crm1 and Ran bind cooperatively to the NES (Fig. 4 C). The association and dissociation rate constants for the Crm1/Ran/NES complex are k a = 7.55 × 105 M−1 s−1 and k d = 8.4 × 10−3 s −1, respectively, revealing a K D = 11 nM. In summary, our data show that CRT, like Crm1, can selectively bind to the leucine-rich NES through a high-affinity interaction that requires the presence of RanGTP.
We used a complementary approach to verify that RanGTP coassembles into the complex containing CRT and NES peptides. Recombinant CRT was immobilized in microtiter wells, and [γ-32P]GTP-loaded Ran was added to wells in the absence or presence of WT or mutant NES peptides (500 nM each). As a positive control, recombinant Crm1 was immobilized and tested in parallel. In this assay, RanGTP binding to both CRT and Crm1 is stimulated approximately fourfold in the presence of WT NES peptide (Fig. 4 D). In contrast, RanGTP binding to both CRT and Crm1 in the presence of mutant NES peptide was similar to the level of binding in the absence of peptide. Our results indicate that the NES is necessary for efficient incorporation of RanGTP into the CRT export complex. The assembly of RanGTP into export complexes appears to be a conserved feature of both CRT- and Crm1-dependent export pathways.
Nuclear Export of GR Is Mediated by Its DBD
Our finding that CRT can function as a nuclear export receptor for NES-containing proteins was intriguing in light of reports that CRT may physically interact with nuclear hormone receptors that are known to undergo nuclear import and export. CRT was shown to block binding of the glucocorticoid, androgen, and vitamin D receptors to their respective DNA response elements in gel-shift assays (Burns et al. 1994; Dedhar et al. 1994; Wheeler et al. 1995). The interaction was proposed to involve the DNA recognition helix situated between the two zinc fingers in the DBD of the receptors. This was based on the observation that a DNA recognition helix peptide inhibits the ability of CRT to block nuclear receptor binding to DNA, presumably because the peptide acts as a competitive inhibitor for the binding reaction (Burns et al. 1994; Dedhar et al. 1994; Wheeler et al. 1995). In addition, overexpression of CRT was shown to antagonize nuclear receptor-dependent transcriptional activation. CRT could antagonize nuclear receptor-dependent transcriptional activation by interfering with the association of the receptor with its DNA response element, by mediating nuclear export of the steroid receptor (see below), or through a combination of interference- and export-based mechanisms.
We tested whether CRT can mediate nuclear export of GR in a permeabilized cell assay. GR was expressed in BHK cells as a GFP fusion protein (Carey et al. 1996), together with a BFP fusion of a nuclear protein (C/EBPα–BFP). After inducing nuclear import of GR–GFP with corticosteroid, the cells were washed, permeabilized with digitonin, and incubated with the indicated proteins. Upon addition of GST–CRT, the GR–GFP was exported from the nucleus, whereas no export was observed upon addition of GST (Fig. 5 A). In all samples, C/EBPα–BFP remained within the nucleus, demonstrating that the nuclear envelope remained intact during the experiment.
Figure 5.
CRT-dependent nuclear export of the GR is mediated by its DBD. (A) BHK cells were cotransfected with plasmids encoding C/EBPα–BFP and GR–GFP and grown for 48 h. The transfected cells were permeabilized with digitonin and export assays were performed in the presence of buffer alone, GST (50 μg/ml), or GST–CRT (50 μg/ml). Export assays were also performed with GST–CRT that was preincubated with a peptide (1 mg/ml) from within the DBD of the GR (WT GR peptide; residues 460–474: CGGGKVFFKRAVEGQHNLY). The control was a mutant peptide with two amino acid changes (MUT GR peptide; residues 460–474: CGGGKVAAKRAVEGQHNLY). CRT-dependent export of GR–GFP was blocked by preincubation with WT, but not mutant, peptide. This suggests that CRT recognition of the GR involves the DNA recognition helix within the DBD. (B) The DBD of GR is a functional NES. A plasmid encoding the NES mutant of Rev (RevM10) fused to the hormone-binding domain of GR and GFP was used to assay nuclear export. The GFP fusion undergoes agonist-dependent nuclear import (+Dex), but fails to undergo nuclear export because of the mutations (L78D,E79L) in the NES of Rev. Nuclear export is restored in a GFP fusion containing a WT DBD (DBDwt–RevM10–GFP), but not in a GFP fusion containing a mutant DBD (DBDmut–RevM10–GFP). The DBD in these constructs corresponds to residues 432–528 in human GR, and the mutations are F463,464A. (C) The DNA recognition helix in GR functions as an NES. The peptides used in the competition experiment were coupled to fluorescently labeled BSA, and analyzed by nuclear microinjection and microscopy. The WT GR-peptide, but not the MUT GR-peptide, promotes nuclear export of the fluorescent conjugate to the cytoplasm of BHK cells.
To address whether the DBD of GR can function as an NES, we designed a transfection-based assay that scores the distribution of a GFP reporter protein by fluorescence microscopy. The GFP reporter plasmid encodes an NES mutant of Rev and the hormone-binding domain of GR; the latter facilitates agonist-dependent nuclear import. Because of the mutation in the NES of Rev, the GFP reporter remains nuclear after agonist removal (Fig. 5 B) (Love et al. 1998). We found that fusion of the DBD of GR to the reporter restored its ability to undergo nuclear export, evident at the 6-h time point. In contrast, mutations (F463,464A) within the DNA recognition helix abolished the ability of the DBD to mediate nuclear export of the GFP reporter. These results identify the DBD of GR as a new type of NES, and implicate the DNA recognition helix as an integral part of the signal.
The capacity of the DNA recognition helix itself to function as an NES was analyzed by microinjection in cultured cells. Peptides (GR residues 460–474) containing WT and mutant (F463,464A) forms of the DNA recognition helix were synthesized and coupled to FITC-labeled BSA. After nuclear microinjection, the fluorescent conjugate containing the WT peptide (WT GR-peptide) was exported to the cytoplasm, whereas the fluorescent conjugate containing the mutant peptide (MUT GR-peptide) remained within the nucleus (Fig. 5 C). In both cases, the rhodamine-labeled dextran that was coinjected remained in the nucleus, indicating that the nuclear envelope remained intact during experimental manipulations. These data confirm and extend our results obtained by transfection (Fig. 5 B), showing that the DBD of GR is a functional NES.
Given our results showing that the DBD, and, more specifically, the DNA recognition helix, can function as an NES, we predicted that a peptide from the DBD might act as a competitive inhibitor for CRT-dependent export of GR. We tested this in the permeabilized cell assay by analyzing nuclear export of GR–GFP in the presence of the DNA recognition helix peptide. We found that CRT-dependent nuclear export of GR was blocked in the presence of WT peptide (+WT GR Peptide), but not in the presence of mutant peptide (+MUT GR peptide) containing two mutations that abrogate the export activity of the intact DBD or the helix (Fig. 5 A). Taken together, our results clearly demonstrate that CRT mediates nuclear export of GR through an interaction with the DBD, and that binding is specific for the DNA recognition helix.
LMB-insensitive Nuclear Protein Export In Vitro and In Vivo
LMB inhibits Crm1-dependent export pathways by modification of a critical cysteine necessary for NES binding (C529; Kudo et al. 1999). We examined whether CRT-dependent nuclear export is inhibited by LMB in the PKI assay, using Crm1 as a positive control. We found that the export activity of CRT is not inhibited by LMB under conditions where the compound blocks the activity of Crm1 (Fig. 6 A). This result, together with our data showing that CRT can mediate nuclear export of GR in permeabilized cells, led us to hypothesize that steroid receptor export would be insensitive to LMB. We tested this by monitoring nuclear export of GR–GFP in the absence and presence of LMB over a 26-h time course. We observed no differences in the apparent rate of GR–GFP nuclear export, or in the extent of its cytoplasmic accumulation (Fig. 6 B). We confirmed that the Crm1-dependent export pathway was inhibited in cells taken at the 6-h time point. This involved showing that a fluorescent conjugate of PKI is retained within nuclei after microinjection (data not shown). These results provide compelling pharmacological evidence that nuclear export of GR occurs independently of the Crm1 export pathway. Similar results have been obtained by immunofluorescence microscopy of the progesterone and GRs (Tyagi et al. 1998; Liu and DeFranco 2000). The LMB insensitivity of CRT and its ability to stimulate nuclear export of GR in permeabilized cells implies that it could mediate nuclear export of GR in living cells.
Figure 6.
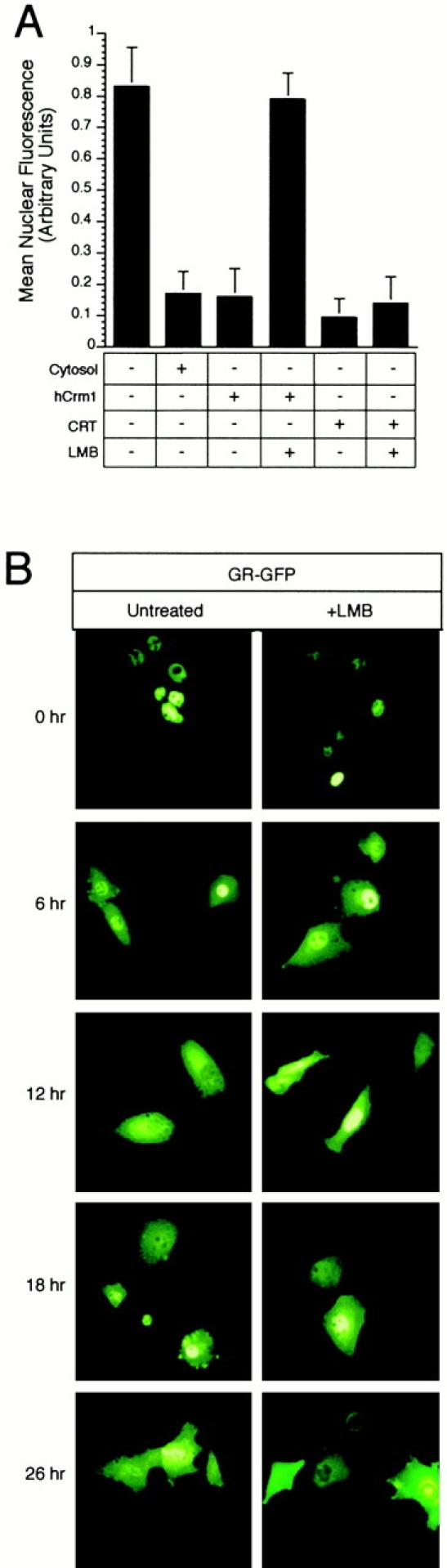
LMB-insensitive nuclear export analyzed in vitro and in vivo. (A) Nuclear export mediated by CRT is not inhibited by LMB under conditions where Crm1-dependent export is inhibited. PKI export was measured in digitonin-permeabilized cells using recombinant CRT (25 μg/ml) and purified Crm1 (50 μg/ml), pretreated with 500 nM LMB for 15 min at room temperature. The level of export promoted by HeLa cytosol is shown for comparison. (B) Nuclear export of the GR is not inhibited by LMB in living cells. BHK cells were transfected with full-length GR fused to GFP (GR–GFP). After nuclear import of GR–GFP was induced with 1 μM corticosteroid for 1 h, the cells were washed extensively with medium containing charcoal-stripped serum, and incubated at 37°C in the absence or presence of 200 nM LMB. At the indicated time points, coverslips were removed and the localization of GR–GFP recorded by fluorescence microscopy. Nuclear export of GR–GFP from the nucleus to the cytoplasm is observed in 6 h, and nuclear export is unaffected by the presence of LMB. Nuclear export mediated by Crm1 was blocked at the 6-h time point, as measured by nuclear microinjection of an NES-containing reporter protein (data not shown).
CRT Mediates Nuclear Export of GR In Vivo
The results of two experiments demonstrated that CRT mediates nuclear export of GR in the cell. In the first experiment, we injected GST–CRT into the cytoplasm of cells expressing nuclear GR–GFP. After microinjection into the cytoplasm, GST–CRT stimulated nuclear export of GR–GFP, whereas GST had no effect (Fig. 7 A). The rhodamine-dextran remained in the cytoplasmic compartment, showing that the nuclear envelope of injected cells remained intact. In the second experiment, we assayed nuclear export of GR–GFP in mouse embryo fibroblasts derived from crt −/− mice (Mesaeli et al. 1999). As controls, we assayed nuclear export of GR–GFP in WT cells (crt +/+) and in the knockout cells transfected with a plasmid encoding CRT (crt −/−[+CRT]). Immunoblotting of lysates from these cell lines confirmed the expression of CRT in WT (crt +/+) and transfected cells (crt −/−[+CRT]), and that the expression of Crm1 was similar in all three cell lines. (Fig. 7 B). GR–GFP was transfected into the cell lines and induced to undergo nuclear import with dexamethasone. After removal of dexamethasone, the cells were examined at 3-h time points to evaluate nuclear export of GR–GFP. We found that GR–GFP export in crt +/+ cells was visible at the 6-h time point and was virtually complete by the 9-h time point. In contrast, GR–GFP was restricted to the nucleus in the crt −/− cells, indicating that the reporter failed to undergo nuclear export. The crt −/− cells transfected with the CRT plasmid showed a slight enhancement of GR–GFP export at the 3-h time point, and with nearly quantitative export at the 9-h time point. These data demonstrate that CRT is necessary for nuclear export of GR in vivo, and provide compelling support for our hypothesis that CRT is the receptor for a distinct nuclear export pathway.
Figure 7.
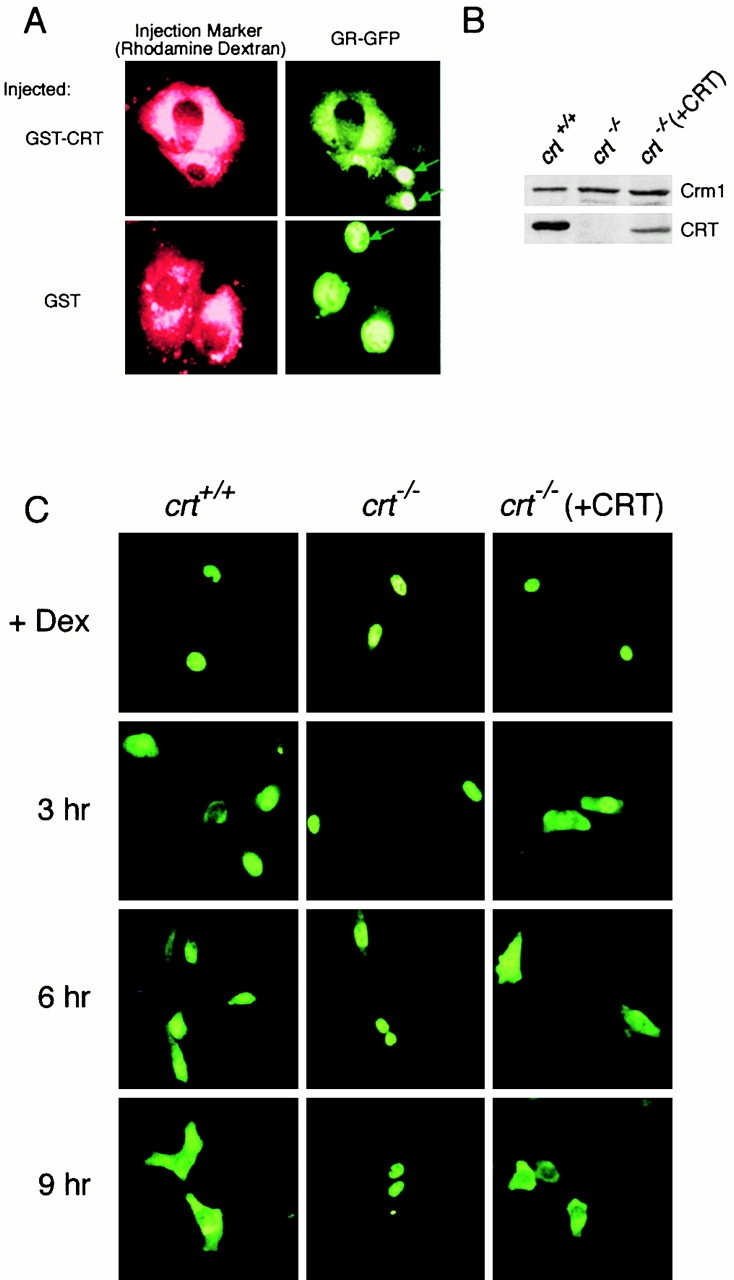
CRT mediates nuclear export of GR in vivo. (A) Cytoplasmic injection of recombinant CRT is sufficient to stimulate nuclear export of GR. BHK cells expressing GR–GFP were injected with GST–CRT or GST (0.5 mg/ml each), and the distribution of GR–GFP was examined by fluorescence microscopy after a 45 min incubation at 37°C. Uninjected cells are indicated with arrows. (B) Immunoblot showing CRT and Crm1 expression in immortalized mouse embryo fibroblasts derived from WT cells (crt +/+), CRT knockout cells (crt −/−) (Mesaeli et al. 1999), and CRT-knockout cells transfected with full-length CRT (crt −/− [+CRT]). (C) Nuclear export of GR is impaired in the absence of CRT, and is restored by CRT expression. A plasmid encoding GR–GFP was transfected into the indicated cell lines, and nuclear accumulation of the reporter was induced with dexamethasone (+Dex). After agonist removal, the cells were examined at 3 h intervals to monitor nuclear export. Nuclear export of the GR–GFP reporter was observed in WT (crt +/+) and CRT-transfected (crt −/− [+CRT]) cells, but not in CRT-deficient (crt −/−) cells.
Discussion
Here, we have identified cytosolic CRT as a receptor for nuclear protein export. The approach involved fractionation of HeLa cell cytosol by column chromatography, and analysis of these fractions using the PKI export assay (Holaska and Paschal 1998). An important element of our strategy for identifying new export activities was the use of cytosol that was incubated with phenyl-Sepharose, a pretreatment resulting in quantitative depletion of Crm1. Since total PKI export activity in the pretreated cytosol is reduced by ∼10% compared with neat cytosol (Holaska and Paschal 1998), it was logical to infer the presence of additional rate-limiting export factors. Evidence that the export factor would correspond to a receptor, as opposed to a regulator, was obtained by showing its specific depletion by an NES conjugate (Holaska and Paschal 1998). These biochemical criteria led us to suggest that mammalian cells utilize at least one additional receptor for nuclear transport of proteins with hydrophobic export signals, a hypothesis that we have now validated with the purification and characterization of CRT.
The primary structure of CRT is unrelated to the β-importin superfamily of nuclear transport receptors, including the export receptors Crm1, CAS, exportin-t, and Msn5p. However, CRT does share important functional properties with Crm1 that underlie its function as an export receptor. Both CRT and Crm1 bind directly to transport substrates through interactions that involve bulky hydrophobic residues within the NES of PKI. The formation of these export receptor–substrate complexes requires the presence of RanGTP, indicating the assembly reaction is cooperative (Fornerod et al. 1997; Askjaer et al. 1998) and that it is likely to occur in the nucleoplasm (Richards et al. 1997). Similar mechanisms could also be used to promote the disassembly of CRT and Crm1 export complexes. In the case of Crm1, RanGAP-mediated hydrolysis of RanGTP to RanGDP on the cytoplasmic side of the NPC is important for disassembly of the Crm1 export complex in the terminal step of export (Askjaer et al. 1999).
An important finding that emerged from our study is that CRT and Crm1 have overlapping, but nonidentical, specificity for export substrates. We purified CRT based on its ability to mediate nuclear export of PKI, and we further showed that it binds directly to the NES of PKI in the presence of RanGTP with a K D = 8.5 nM. In the same assay, we found that Crm1 binds to the NES of PKI in the presence of RanGTP with a K D = 11 nM. CRT was also found to mediate nuclear export of Rev. Since PKI and Rev are also exported by Crm1, these represent overlapping export substrates for the two receptors. Point mutations in the PKI NES that disrupt CRT binding also reduce Crm1 binding (Wen et al. 1995), indicating that similar molecular determinants within the NES in PKI are recognized by both export receptors.
Using two different receptors for the same export substrates may provide redundancy for this essential process. CRT could also define a more specialized protein export pathway that is responsive to the physiological state of the cell. Total CRT expression increases in response to heat shock (Conway et al. 1995), an effect that has been interpreted with regard to its role in protein folding (Trombetta and Helenius 1998). However, it is tempting to speculate that cellular stress may also increase the cytosolic pool of CRT, which could mediate nuclear export of proteins and RNAs that are important for the heat shock response. CRT can bind to certain RNPs in vitro through both protein and RNA contacts (Nakhasi et al. 1994; Cheng et al. 1996). Though the significance of these interactions is unknown, they may warrant an investigation of a potential role for CRT in RNA export.
CRT was identified about 25 years ago as an abundant calcium-binding protein of the ER (Ostwald and MacLennan 1974), and it has been ascribed two different, but potentially overlapping, functions. The first function involves calcium homeostasis, a logical role given the presence of calcium-binding sites in the protein, and its ability to regulate the calcium ATPase SERCA2 through protein–protein interactions (Camacho and Lechleiter 1995). The second function involves a role in the folding and oligomerization of glycoproteins (Vassilakos et al. 1998), and protection of nonglycosylated proteins from thermal denaturation (Saito et al. 1999). How these chaperone-like properties of CRT may relate to its function as a nuclear export receptor is unknown, however, it should be noted that CRT binding to export substrates is highly specific and displays nanomolar affinity in the presence of RanGTP. This affinity is 400-fold greater than the affinity of CRT for folding intermediates of hemagglutinin (Peterson and Helenius 1999).
Cytosolic CRT could be regulated through intracellular signaling pathways involving calcium binding or phosphorylation (Krause and Michalak 1997). However, we note that these modes of regulation do not appear to be necessary for the export function of CRT in our assays based on two observations. First, the permeabilized cell assays were performed in the presence of EGTA, and supplementing the buffer with excess calcium had no effect on the level of PKI export stimulated by CRT (Holaska, J.M., and B.M. Paschal, unpublished observations). Second, the cooperative assembly of CRT, NES, and RanGTP was achieved using recombinant proteins produced in Escherichia coli, indicating that posttranslational modifications are not required for formation of the trimeric export complex. However, it remains possible that recombinant CRT undergoes posttranslational modification upon addition to the permeabilized cell assay.
CRT contains a hydrophobic signal sequence to specify targeting to the ER. Nonetheless, digitonin permeabilization of HeLa cells releases approximately one third of total CRT under conditions where the ER remains intact, as assessed by immunoblotting for the lumenal proteins Grp94, ERp72, and PDI. Furthermore, the subcellular fractionation of HeLa cells clearly demonstrates the presence of a significant cytosolic pool of CRT. Since genomic analysis, Northern blotting, and EST sequencing have not provided evidence for a CRT isoform that lacks a signal sequence, the molecular mechanism for generating cytosolic CRT is obscure. CRT could be produced posttranslationally by retrotranslocation from the lumenal to cytoplasmic compartments (Johnson and van Waes 1999). Alternatively, cytosolic CRT could be translated on ribosomes that are not engaged with the ER. The synthesis of signal sequence–containing proteins on free ribosomes is known to occur in budding yeast (Matlack et al. 1998).
Recombinant CRT stimulates nuclear export of GR in the permeabilized cell assay. In contrast, purified Crm1 does not stimulate nuclear export of GR (Holaska, J.M., and B.M. Paschal, unpublished observations), presumably because Crm1 fails to bind to the export signal in GR. This is consistent with our finding that nuclear export of the GR in vivo proceeds on a pathway that is independent of Crm1, based on its insensitivity to LMB. Significant evidence that CRT is, in fact, required for nuclear export of GR was obtained by using cells lacking CRT. We showed that GR export is deficient in crt −/− cells, and that GR export can be restored by reintroduction of CRT into the cells. We have also found that GR export can be restored by expression of CRT that lacks its NH2-terminal signal sequence (Holaska, J.M., and B.M. Paschal, unpublished observations). This indicates that the cytosolic, nonER form of CRT is sufficient to promote nuclear export of GR in vivo. These observations, taken together, are consistent with our proposal that CRT defines a Crm1-independent mechanism that is used for nuclear export of nuclear receptors such as GR.
CRT appears to mediate nuclear export of GR through a direct protein–protein interaction. In vitro, CRT can bind to the sequence KGFFKR, which is situated between the two zinc fingers in the DNA recognition helix of GR (Burns et al. 1994). Notably, it has been shown using purified components that CRT can prevent binding of GR to its glucocorticoid response element (GRE) in mobility shift assays, and overexpression of CRT inhibits GR-dependent transcriptional activation from a GRE-dependent reporter gene in vivo (Burns et al. 1994). These results showing that CRT can antagonize GR function together with our finding that CRT mediates nuclear export of GR led us to hypothesize that the DBD within GR functions as the NES. Three results indicate this hypothesis is correct. First, the DBD of GR can function as an NES in the context of a GFP fusion protein. Second, a peptide containing the DNA recognition helix from the DBD is sufficient to specify nuclear export after microinjection into the nucleus. Third, the DNA recognition helix peptide blocks CRT-dependent export of GR in permeabilized cells. Results showing that CRT also inhibits the activities of the androgen and vitamin D receptors (Dedhar et al. 1994; Wheeler et al. 1995), together with the sequence similarity of the DBD in these and other hormone receptors, indicates that our study has revealed both the receptor and the signal for a major export pathway
The data we have presented, together with previous studies linking nuclear receptors and CRT, suggest a simple two-step model for regulating the function of these transcriptional activators. The first step involves a direct interaction between CRT and the DBD of nuclear receptors, an event that is sufficient to block receptor association with DNA in vitro. The second step involves nuclear export of the nuclear receptor/CRT/RanGTP complex to the cytoplasm, an event that would terminate transcriptional activation until reimport occurs. The balance of ligand-induced import and CRT-dependent export provides the cell with a nuclear transport–based mechanism that may play an important role in regulating the activity of a broad spectrum of nuclear receptors.
Acknowledgments
We thank Drs. David Wotton, David Castle, and Lucy Pemberton for helpful comments on the manuscript, and members of the Paschal lab for discussions throughout the course of this work. We thank Dr. David Brautigan for his enthusiastic support of this work, Drs. Ian Macara, Dirk Görlich, and Richard Day for providing plasmids used in this study, and Dr. Marek Michalak for providing the CRT knockout cells. The leptomycin B was a gift from Ana Suter (Novartis Pharmaceuticals Corporation, East Hanover, NJ).
These studies were supported by the National Institutes of Health (GM58639-01 to B.M. Paschal) and by the Lucille P. Markey Charitable Trust.
Footnotes
Abbreviations used in this paper: BFP, blue fluorescent protein; bPKI, biotinylated PKI; CRT, calreticulin; DBD, DNA-binding domain; GFP, green fluorescent protein; GR, glucocorticoid receptor; GST, glutathione-S-transferase; LMB, leptomycin B; MUT, mutant; NES, nuclear export signal; NLS, nuclear localization signal; NPC, nuclear pore complex; PKI, protein kinase inhibitor; RNP, ribonucleoprotein; STV, streptavidin; TB, transport buffer; WT, wild-type.
References
- Arts G.J., Fornerod M., Mattaj I.W. Identification of a nuclear export receptor for tRNA Curr. Biol. 8 1998. 305 314a [DOI] [PubMed] [Google Scholar]
- Arts G.J., Kuersten S., Romby P., Ehresmann B., Mattaj I.W. The role of exportin-t in selective nuclear export of mature tRNAs EMBO (Eur. Mol. Biol. Organ.) J. 17 1998. 7430 7441b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askjaer P., Jensen T.H., Nilsson J., Englmeier L., Kjems J. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J. Biol. Chem. 1998;273:33414–33422. doi: 10.1074/jbc.273.50.33414. [DOI] [PubMed] [Google Scholar]
- Askjaer P., Bachi A., Wilm M., Bischoff F.R., Weeks D.L., Ogniewski V., Ohno M., Niehrs C., Kjems J., Mattaj I.W., Fornerod M. RanGTP-regulated interactions of CRM1 with nucleoporins and a shuttling DEAD-box helicase. Mol. Cell. Biol. 1999;19:6276–6285. doi: 10.1128/mcb.19.9.6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black B.E., Lévesque L., Holaska J.M., Wood T.C., Paschal B.M. Identification of an NTF2-related factor that binds Ran-GTP and regulates nuclear protein export. Mol. Cell. Biol. 1999;19:8616–8624. doi: 10.1128/mcb.19.12.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun I.C., Rohrbach E., Schmitt C., Izaurralde E. TAP binds to the constitutive transport element (CTE) through a novel RNA-binding motif that is sufficient to promote CTE-dependent RNA export from the nucleus. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:1953–1965. doi: 10.1093/emboj/18.7.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns K., Duggan B., Atkinson E.A., Famulski K.S., Nemer M., Bleackley R.C., Michalak M. Modulation of gene expression by calreticulin binding to the glucocorticoid receptor. Nature. 1994;367:476–480. doi: 10.1038/367476a0. [DOI] [PubMed] [Google Scholar]
- Camacho P., Lechleiter J.D. Calreticulin inhibits repetitive intracellular Ca2+ waves. Cell. 1995;82:765–771. doi: 10.1016/0092-8674(95)90473-5. [DOI] [PubMed] [Google Scholar]
- Carey K.L., Richards S.A., Lounsbury K.M., Macara I.G. Evidence using a green fluorescent protein–glucocorticoid receptor chimera that the Ran/TC4 GTPase mediates an essential function independent of nuclear protein import. J. Cell Biol. 1996;133:985–996. doi: 10.1083/jcb.133.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S.T., Nguyen T.Q., Yang Y.S., Capra J.D., Sontheimer R.D. Calreticulin binds hYRNA and the 52-kDa polypeptide component of the Ro/SS-A ribonucleoprotein autoantigen. J. Immunol. 1996;156:4484–4491. [PubMed] [Google Scholar]
- Conway E.M., Liu L., Nowakowski B., Steiner-Mosonyi M., Ribeiro S.P., Michalak M. Heat shock-sensitive expression of calreticulin. In vitro and in vivo up-regulation. J. Biol. Chem. 1995;270:17011–17016. doi: 10.1074/jbc.270.28.17011. [DOI] [PubMed] [Google Scholar]
- Cullen B.R. Connections between the processing and nuclear export of mRNAevidence for an export license? Proc. Natl. Acad. Sci. USA. 2000;97:4–6. doi: 10.1073/pnas.97.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S., Rennie P.S., Shago M., Hagesteijn C.Y., Yang H., Filmus J., Hawley R.G., Bruchovsky N., Cheng H., Matusik R.J. Inhibition of nuclear hormone receptor activity by calreticulin. Nature. 1994;367:480–483. doi: 10.1038/367480a0. [DOI] [PubMed] [Google Scholar]
- Doye V., Hurt E. From nucleoporins to nuclear pore complexes. Curr. Opin. Cell Biol. 1997;9:401–411. doi: 10.1016/s0955-0674(97)80014-2. [DOI] [PubMed] [Google Scholar]
- Fischer U., Huber J., Boelens W.C., Mattaj I.W., Luhrmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- Fornerod M., Ohno M., Yoshida M., Mattaj I.W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Görlich D., Kutay U. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- Görlich D., Dabrowski M., Bischoff F.R., Kutay U., Bork P., Hartmann E., Prehn S., Izaurralde E. A novel class of RanGTP binding proteins. J. Cell Biol. 1997;138:65–80. doi: 10.1083/jcb.138.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruter P., Tabernero C., von Kobbe C., Schmitt C., Saavedra C., Bachi A., Wilm M., Felber B.K., Izaurralde E. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol. Cell. 1998;1:649–659. doi: 10.1016/s1097-2765(00)80065-9. [DOI] [PubMed] [Google Scholar]
- Hodge C.A., Colot H.V., Stafford P., Cole C.N. Rat8p/Dbp5p is a shuttling transport factor that interacts with Rat7p/Nup159p and Gle1p and suppresses the mRNA export defect of xpo1-1 cells. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:5778–5788. doi: 10.1093/emboj/18.20.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaska J.M., Paschal B.M. A cytosolic activity distinct from crm1 mediates nuclear export of protein kinase inhibitor in permeabilized cells. Proc. Natl. Acad. Sci. USA. 1998;95:14739–14744. doi: 10.1073/pnas.95.25.14739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood J., Silver P. Cse1p is required for export of Srp1p/importin-alpha from the nucleus in Saccharomyces cerevisiae . J. Biol. Chem. 1998;273:35142–35146. doi: 10.1074/jbc.273.52.35142. [DOI] [PubMed] [Google Scholar]
- Izaurralde E., Lewis J., Gamberi C., Jarmolowski A., McGuigan C., Mattaj I.W. A cap-binding protein complex mediating U snRNA export. Nature. 1995;376:709–712. doi: 10.1038/376709a0. [DOI] [PubMed] [Google Scholar]
- Jethmalani S.M., Henle K.J., Gazitt Y., Walker P.D., Wang S.Y. Intracellular distribution of heat-induced stress glycoproteins. J. Cell. Biochem. 1997;66:98–111. [PubMed] [Google Scholar]
- Johnson A.E., van Waes M.A. The translocona dynamic gateway at the ER membrane. Annu. Rev. Cell Dev. Biol. 1999;15:799–842. doi: 10.1146/annurev.cellbio.15.1.799. [DOI] [PubMed] [Google Scholar]
- Kaffman A., Rank N.M., O'Neill E.M., Huang L.S., O'Shea E.K. The receptor Msn5 exports the phosphorylated transcription factor Pho4 out of the nucleus. Nature. 1998;396:482–486. doi: 10.1038/24898. [DOI] [PubMed] [Google Scholar]
- Kehlenbach R.H., Dickmanns A., Kehlenbach A., Guan T., Gerace L. A role for RanBP1 in the release of CRM1 from the nuclear pore complex in a terminal step of nuclear export. J. Cell Biol. 1999;145:645–657. doi: 10.1083/jcb.145.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M., Speck C., Christiansen M., Bischoff F.R., Prehn S., Haller H., Görlich D., Hartmann E. Evidence for distinct substrate specificities of importin β family members in nuclear protein import. Mol. Cell. Biol. 1999;19:7782–7791. doi: 10.1128/mcb.19.11.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K.H., Michalak M. Calreticulin. Cell. 1997;88:439–443. doi: 10.1016/s0092-8674(00)81884-x. [DOI] [PubMed] [Google Scholar]
- Kudo N., Matsumori N., Taoka H., Fujiwara D., Schreiner E.P., Wolff B., Yoshida M., Horinouchi S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA. 1999;96:9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U., Bischoff F.R., Kostka S., Kraft R., Görlich D. Export of importin alpha from the nucleus is mediated by a specific nuclear transport factor. Cell. 1997;90:1061–1071. doi: 10.1016/s0092-8674(00)80372-4. [DOI] [PubMed] [Google Scholar]
- Kutay U., Lipowsky G., Izaurralde E., Bischoff F.R., Schwarzmaier P., Hartmann E., Görlich D. Identification of a tRNA-specific nuclear export receptor. Mol. Cell. 1998;1:359–369. doi: 10.1016/s1097-2765(00)80036-2. [DOI] [PubMed] [Google Scholar]
- Liu J., DeFranco D. Protracted nuclear export of the glucocorticoid receptor limits its turnover and does not require the exportin1/CRM1-directed nuclear export pathway. Mol. Endocrinol. 2000;14:40–51. doi: 10.1210/mend.14.1.0398. [DOI] [PubMed] [Google Scholar]
- Love D.C., Sweitzer T.D., Hanover J.A. Reconstitution of HIV-1 rev nuclear exportindependent requirements for nuclear import and export. Proc. Natl. Acad. Sci. USA. 1998;95:10608–10613. doi: 10.1073/pnas.95.18.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlack K.E., Mothes W., Rapoport T.A. Protein translocationtunnel vision. Cell. 1998;92:381–390. doi: 10.1016/s0092-8674(00)80930-7. [DOI] [PubMed] [Google Scholar]
- Mesaeli N., Nakamura K., Zvaritch E., Dickie P., Dziak E., Krause K.H., Opas M., MacLennan D.H., Michalak M. Calreticulin is essential for cardiac development. J. Cell Biol. 1999;144:857–868. doi: 10.1083/jcb.144.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R., Wente S.R. An RNA-export mediator with an essential nuclear export signal. Nature. 1996;383:357–360. doi: 10.1038/383357a0. [DOI] [PubMed] [Google Scholar]
- Murphy R., Watkins J.L., Wente S.R. GLE2, a Saccharomyces cerevisiae homologue of the Schizosaccharomyces pombe export factor RAE1, is required for nuclear pore complex structure and function. Mol. Biol. Cell. 1996;7:1921–1937. doi: 10.1091/mbc.7.12.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhasi H.L., Singh N.K., Pogue G.P., Cao X.Q., Rouault T.A. Identification and characterization of host factor interactions with cis-acting elements of rubella virus RNA. Arch. Virol. (Suppl.). 1994;9:255–267. doi: 10.1007/978-3-7091-9326-6_26. [DOI] [PubMed] [Google Scholar]
- Nakielny S., Dreyfuss G. Transport of proteins and RNAs in and out of the nucleus. Cell. 1999;99:677–690. doi: 10.1016/s0092-8674(00)81666-9. [DOI] [PubMed] [Google Scholar]
- Neville M., Rosbash M. The NES-Crm1p export pathway is not a major mRNA export route in Saccharomyces cerevisiae . EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:3746–3756. doi: 10.1093/emboj/18.13.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M., Fornerod M., Mattaj I.W. Nucleocytoplasmic transportthe last 200 nanometers. Cell. 1998;92:327–336. doi: 10.1016/s0092-8674(00)80926-5. [DOI] [PubMed] [Google Scholar]
- Ohno M., Segref A., Bachi A., Wilm M., Mattaj I.W. PHAX, a mediator of U snRNA nuclear export whose activity is regulated by phosphorylation. Cell. 2000;101:187–198. doi: 10.1016/S0092-8674(00)80829-6. [DOI] [PubMed] [Google Scholar]
- Ossareh-Nazari B., Maison C., Black B.E., Levesque L., Paschal B.M., Dargemont C. The RanGTP-binding protein NXT1 facilitates nuclear export of different classes of RNA in vitro. Mol. Cell. Biol. 2000;20:4562–4571. doi: 10.1128/mcb.20.13.4562-4571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostwald T.J., MacLennan D.H. Isolation of a high affinity calcium-binding protein from sarcoplasmic reticulum. J. Biol. Chem. 1974;249:974–979. [PubMed] [Google Scholar]
- Paschal B.M., Gerace L. Identification of NTF2, a cytosolic factor for nuclear import that interacts with nuclear pore complex protein p62. J. Cell Biol. 1995;129:925–937. doi: 10.1083/jcb.129.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J.R., Helenius A. In vitro reconstitution of calreticulin-substrate interactions. J. Cell Sci. 1999;112:2775–2784. doi: 10.1242/jcs.112.16.2775. [DOI] [PubMed] [Google Scholar]
- Pritchard C.E., Fornerod M., Kasper L.H., van Deursen J.M. RAE1 is a shuttling mRNA export factor that binds to a GLEBS-like NUP98 motif at the nuclear pore complex through multiple domains. J. Cell Biol. 1999;145:237–254. doi: 10.1083/jcb.145.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S.A., Carey K.L., Macara I.G. Requirement of guanosine triphosphate-bound ran for signal-mediated nuclear protein export. Science. 1997;276:1842–1844. doi: 10.1126/science.276.5320.1842. [DOI] [PubMed] [Google Scholar]
- Roderick H.L., Campbell A.K., Llewellyn D.H. Nuclear localisation of calreticulin in vivo is enhanced by its interaction with glucocorticoid receptors. FEBS Lett. 1997;405:181–185. doi: 10.1016/s0014-5793(97)00183-x. [DOI] [PubMed] [Google Scholar]
- Rubio I., Buckle P., Trutnau H., Wetzker R. Real-time assay of the interaction of a GST fusion protein with a protein ligate using resonant mirror technique. Biotechniques. 1997;22:269–271. doi: 10.2144/97222bm15. [DOI] [PubMed] [Google Scholar]
- Saito Y., Ihara Y., Leach M.R., Cohen-Doyle M.F., Williams D.B. Calreticulin functions in vitro as a molecular chaperone for both glycosylated and non-glycosylated proteins. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:6718–6729. doi: 10.1093/emboj/18.23.6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stade K., Ford C.S., Guthrie C., Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Steggerda S.M., Black B.E., Paschal B.M. Monoclonal antibodies to NTF2 inhibit nuclear protein import by preventing nuclear translocation of the GTPase Ran. Mol. Biol. Cell. 2000;11:703–719. doi: 10.1091/mbc.11.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta E.S., Helenius A. Lectins as chaperones in glycoprotein folding. Curr. Opin. Struct. Biol. 1998;8:587–592. doi: 10.1016/s0959-440x(98)80148-6. [DOI] [PubMed] [Google Scholar]
- Tyagi R.K., Amazit L., Lescop P., Milgrom E., Guiochon-Mantel A. Mechanisms of progesterone receptor export from nucleirole of nuclear localization signal, nuclear export signal, and ran guanosine triphosphate. Mol. Endocrinol. 1998;12:1684–1695. doi: 10.1210/mend.12.11.0197. [DOI] [PubMed] [Google Scholar]
- Vassilakos A., Michalak M., Lehrman M.A., Williams D.B. Oligosaccharide binding characteristics of the molecular chaperones calnexin and calreticulin. Biochemistry. 1998;37:3480–3490. doi: 10.1021/bi972465g. [DOI] [PubMed] [Google Scholar]
- Wen W., Meinkoth J.L., Tsien R.Y., Taylor S.S. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- Wheeler D.G., Horsford J., Michalak M., White J.H., Hendy G.N. Calreticulin inhibits vitamin D3 signal transduction. Nucleic Acids Res. 1995;23:3268–3274. doi: 10.1093/nar/23.16.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]