Abstract
Antisocial personality disorder (APD) is associated with increased problem severity in treatment seeking opioid dependent patients. Treatment studies have reported mixed results but generally show that APD patients make progress that is often comparable to drug dependent patients without the personality disorder. Much of this work is based on secondary analyses of studies evaluating responses to a variety of drug abuse treatment interventions. The present study reports on a randomized prospective trial evaluating a behavioral approach for managing opioid dependent patients with APD. Subjects (N=100) met DSM criteria for opioid dependence and APD using a structured clinical interview, and were randomly assigned to the experimental condition (n = 51) that used a highly structured contingency management intervention, or control condition (n = 49) that reflected standard methadone treatment. Subjects in the experimental group had significantly better counseling attendance and some indication of lower psychosocial impairment compared to the control group. The experimental intervention increased attendance in subjects with low, as well as high levels of psychopathy, and with and without other psychiatric comorbidity. These findings support the development of interventions more tailored to APD drug-dependent patients.
Keywords: Antisocial personality disorder, drug dependence, treatment outcome, contingency management
INTRODUCTION
Antisocial personality disorder (APD) is a debilitating long-term disorder that is strongly associated with substance use problems and other risks of harm to self and others (Black, Baumgard, Bell, 1995; Cleckley, 1941; Eronen, Hakola, & Tiihonen, 1996; Lewis, Rice, & Helzer, 1983; Schuckit, Klein, Twitchell, & Smith, 1994; Vaillant, 1975). Prevalence rates of APD in the general population range between 2% and 4% (Kessler et al., 1994), which are considerably lower than the 25% to 50% rates reported in treatment-seeking drug-dependent patients (Brooner, King, Kidorf, Schmidt, & Bigelow, 1997; Compton et al., 2000, Compton, Conway, Stinson, Colliver, & Grant, 2005; Khantzian & Treece, 1985; Kosten, Rounsaville, & Kleber, 1982). The overlap of antisocial personality to drug abuse has stimulated considerable research on the clinical characterization and management of patients with both disorders.
Studies have shown that drug dependent patients with versus without antisocial personality have more severe patterns and frequencies of drug use and HIV risk behavior and infection (e.g., Brooner, Bigelow, Strain, & Schmidt, 1990; Brooner, Greenfield, Schmidt, & Bigelow, 1993, Brooner, Herbst, Schmidt, Bigelow, & Costa, 1993a; Brooner et al., 1997; Compton, Cottler, Shillington, & Price, 1995; Dinwiddie, Reich, & Cloninger, 1992; Gill, Nolimal, & Crowley, 1992; Kelley & Petry, 2000; King, Kidorf, Stoller, Carter, & Brooner, 2001; Woody, McLellan, Luborsky, & O’Brien, 1985). APD in drug-dependent samples has also been associated with greater family and psychosocial problems, more psychiatric instability and suicide, and increased crime and violence (Bell, Mattick, Hay, Chan, & Hall, 1997; Bovasso, Alterman, Cacciola, & Rutherford, 2002; Brooner, Schmidt, Felch, & Bigelow, 1992; Cacciola, Rutherford, Alterman, & Snider, 1994; Cottler, Campbell, Krishna, Cunningham-Williams, & Abdallah, 2005; Cottler, Price, Compton, & Mager, 1995; Kosten, Kosten, &, Rounsaville, 1989; Moeller, Dougherty, & Rustin, 1997; Rousar, Brooner, Regier, & Bigelow, 1994; Rutherford, Cacciola, & Alterman, 1994). Prior work has also shown that about 40% of drug dependent patients with APD also meet criterion for other psychiatric diagnoses (Alterman, Rutherford, Cacciola, McKay, & Woody, 1996; Brooner et al., 1997; Compton et al., 2005; Goodwin & Hamilton, 2003; King et al., 2001; Woody et al., 1985), and that the added comorbidity is often associated with distinct clinical and drug use profiles and treatment response (Alterman, Cacciola & Rutherford, 1993; Brooner, Herbst, Schmidt, Bigelow, & Costa, 1993b; Brooner et al., 1997; King et al., 2001; Rousar et al., 1994; Woody et al., 1985). For example, opioid-dependent patients with only APD appear to favor heroin and cocaine, whereas those with APD and other psychiatric diagnoses appear more likely to use sedatives (King et al., 2001).
This work has led to a growing interest in the treatment of APD drug users. Early studies showed that APD was an indicator of poor treatment response, particularly in opioid-dependent samples (Alterman & Cacciola, 1991; Cacciola, Alterman, Rutherford, & Snider, 1995; Rounsaville, Dolinsky, Babor, & Meyer, 1987; Rounsaville, Kosten, Weissman, & Kleber, 1986; Woody et al., 1985). Later reports are mixed in their findings. Several studies have reported higher rates of cocaine and heroin use in opioid dependent patients with versus without APD (Alterman et al., 1996; King et al., 2001;), whereas others have reported few or no differences in outcome (e.g., Cacciola et al., 1995; Darke, Finlay-Jones, Kaye, & Blatt, 1996; Gill et al., 1992), and at least one study (Carroll & Rounsaville, 1993) reported better outcome in those with APD.
Some of the variability in outcome studies may be related to differences in outcome measures and the clinical characteristics of the samples. For example, while APD is associated with increased illegal activities and poorer psychosocial function during and following treatment (Bell et al., 1997; Kosten et al., 1989), a recent review of several studies show that patients with and without APD have comparable rates of treatment retention (Havens & Strathdee, 2005). Similarly, difference in measures of drug use might lead to different findings. For example, studies relying on self-reports of drug use might produce findings that vary from those relying on urinalysis drug testing (e.g., Alterman, Rutherford, Cacciola, McKay, & Boardman, 1998). It has also been shown that a substantial proportion of both APD and non-APD drug users meet diagnostic criterion for other psychiatric diagnoses, including ones that also convey a poor treatment prognosis (Alterman, et al., 1996; Brooner et al., 1997, Cacciola, Rutherford, Alterman, McKay, & Snider, 1996; King et al., 2001; Nace, Davis, & Gaspari 1991). For example, studies have reported that opioid dependent subjects with APD as the sole comorbid diagnosis respond poorer to drug abuse treatment than subjects with APD and other psychiatric problems (e.g., King et al., 2001, Woody et al., 1985). Collapsing these subgroups of APD drug users into one and comparing them to non-APD samples that include at least some cases of other psychiatric comorbidity might obscure meaningful subgroup differences. Some studies have also shown that APD drug users often improve as much as non-APD subjects but appear to have poorer outcomes because of greater baseline problems. Compton, Cottler, Spitznagel, Ben-Abdallah, and Gallagher (1998) found that APD injection drug users had higher rates of HIV risk behavior at baseline and follow-up compared to non-APD subjects, but noted that both groups reported comparable decreases in risk behavior over time.
Much of the prior work on the treatment response of APD dependent patients is either retrospective or post-hoc evaluations of response to routine drug abuse treatments (Brooner, Kidorf, King, & Stoller, 1998). Less is known about the response of these patients to interventions that target both unique and shared symptoms of both disorders (Alterman & Cacciola, 1991; Brooner et al., 1992; Gerstley, Alterman, McLellan, & Woody, 1990; Longabaugh et al., 1994). Specialized interventions that emphasize behavioral reinforcement may be a promising approach (e.g., Alterman & Cacciola, 1991; Arndt, McLellan, Dorozynsky, Woody, & O’Brien, 1994; Gerstley et al., 1990; Rounsaville et al., 1986; Vaillant, 1975; Woody et al., 1985). Vaillant (1975), for example, suggested that interventions for APD should be highly structured, provide consistent limits and frequent monitoring, and offer clear incentives for improvement. Opioid agonist programs are ideal settings for this type of work. They offer a highly structured environment with frequent monitoring and considerable opportunity for contingency management (Kidorf, Stitzer, Brooner, & Goldberg, 1994; Stitzer & Higgins, 1995). For example, making the delivery of some aspects of routine methadone treatment (e.g., take-home doses, dose changes) contingent on reduced drug use has often produced higher rates of drug-free urine specimens in opioid dependent samples (Iguchi, Stitzer, Bigelow, & Liebson, 1988; Magura, Casriel, & Goldsmith, 1988; Stitzer et al., 1977; Stitzer, Bickel, Bigelow, & Liebson, 1986; Stitzer, Iguchi, & Felch, 1992). A study by Brooner et al. (1998) reported preliminary findings from a two-group randomized trial evaluating the efficacy of a highly structured contingency contracting intervention for opioid dependent patients with APD. Data from the first 40 subjects showed good outcomes across treatment conditions but only modest evidence of greater improvement in those assigned to experimental condition.
The present study tests whether an intensive contingency management intervention will improve treatment outcomes among subjects with opioid dependence and APD and extends the earlier study by Brooner et al. (1998) by reporting findings from the full sample over 6 months of randomized care. Subjects were opioid dependent patients receiving methadone who were randomly assigned to the experimental intensive contingency management intervention or the control condition. Subjects assigned to the experimental condition were expected to have higher rates of attendance to scheduled counseling sessions, higher rates of drug-negative urine specimens, and fewer psychosocial and drug related problems compared to the control group.
METHODS
2.1 Study participants
Study subjects were recruited from the Addiction Treatment Services program between October 1993 and May 1998. Patients were eligible for enrollment if they met DSM-III-R criteria for both opioid dependence and APD. Exclusion conditions included pregnancy, bipolar disorder, and schizophrenia. One hundred twenty-eight patients were eligible and provided written informed consent to participate. The Institutional Review Board of the Johns Hopkins School of Medicine reviewed and approved the study. A total of 28 (22%) enrollees were withdrawn from the study prior to random assignment to treatment condition: 11 failed to complete baseline assessments, 8 withdrew consent, and 9 were removed because of new or escalating health problems that required intensive clinical management. Of the 100 subjects that were both consented and randomized, 75 were new admissions and 25 were already in treatment and responding poorly to routine care; these poor responders to routine care were stratified across study conditions.
2.2 Clinical assessment measures
The Structured Clinical Interview for DSM-III-R (SCID I & II: Spitzer, Williams, Gibbon, & First, 1992; Williams et al., 1992), a semi-structured interview that uses a decision-tree for assigning past and current Axis I and II psychiatric disorders, was used to diagnose substance use, personality, and other psychiatric disorders. The APD module of the SCID was administered to patients who screened positive for the disorder. The remainder of the SCID was completed approximately 3 weeks following study enrollment to help reduce the influence of acute situational crises and both drug intoxication and withdrawal states on psychiatric symptom reporting. The senior investigator (RKB) completed a clinical reappraisal of all subjects to confirm the diagnosis of APD prior to random assignment to treatment condition.
The revised Psychopathy Checklist (PCL-R) was administered at baseline as a dimensional measure of the severity of antisocial impairment (Hare, 1991). The PCL-R is a 20 item semi-structured interview that produces three summary scores (interpersonal factor, instability factor, and total score). The interpersonal factor includes items that assess selfishness, manipulativeness, absence of remorse, and the superficial charm characteristic of psychopathic personality traits described by Cleckley (1941). The instability factor includes items related to specific antisocial behaviors and activities (Rutherford, Alterman, Cacciola, & McKay, 1998). The PCL-R total score has the strongest retest reliability and predictive validity in opioid and other substance dependent samples (McDermott et al., 2000; Rutherford, Cacciola, Alterman, McKay, & Cook, 1999). For the present study, subjects were dichotomized into high (25 or greater total score) and low psychopathy groups (less than 25) as described in the Rutherford et al. report (1998).
The Addiction Severity Index (ASI 5th Edition:) is a semi-structured interview designed to dimensionally assess and quantify problem severity in seven areas commonly affected by substance use disorder (alcohol use, drug use, medical, legal, employment, family/social, and psychiatric problems). Composite scores are produced for each of these domains and range from 0.0 (no problems) to 1.0 (high severity), and are based on problems reported in the prior 30-days. The ASI has been shown to have good reliability and validity (e.g., McLellan et al., 1992; McLellan, Cacciola, Alterman, Rikoon, & Carise, 2006; Kosten, Rounsaville, & Kleber, 1983). It was administered at the end of the 4-week baseline evaluation and again at months 1, 2, 3, and 6 of randomized treatment.
Attendance to counseling sessions was tracked by a clinical research monitor who met individually with counseling staff on a weekly basis to confirm adherence to the study protocol and document patient attendance to scheduled sessions. Weekly urine specimens were collected under direct staff observation on a quasi-random weekly schedule (i.e., weekly collection was done on Monday, Wednesday, or Friday). The temperature of specimens was monitored to decrease the possibility of falsification. Urine tests employed enzyme multiplied immunoassay technique (EMIT) and thin layer chromatography (TLC) for the presence of opiates, cocaine, sedatives and alcohol.
2.3 Interview training
Study interviewers completed a three-step training procedure for the SCID, PCL-R, and ASI that included: 1) extensive didactic and practice interviewing sessions, 2) co-rating subjects interviews completed by an expert (R. Brooner, K. Kindbom), and 3) conducting subject interviews co-rated by an expert interviewer. Interviewers were trained to 100% agreement with expert ratings (i.e., diagnosis for SCID, individual items for the PCL-R and ASI) utilizing a procedure detailed in an earlier report (Brooner et al., 1997). Weekly booster sessions were used throughout the present study to reduce “interviewer drift” over time from initial training standards. This approach is associated with good to excellent rater reliability on the interview measures employed in the study.
2.4. Study procedures
Subjects completed all baseline study evaluations and were titrated to a daily methadone dose of at least 55 mg over the first 4 weeks (baseline period) of participation in the study. Subjects were stratified on race, gender, baseline urine results, presence of other psychiatric diagnoses, and therapist assignment, and randomized to one of two treatment conditions for a period of 6-months.
2.4.1 Experimental condition
The experimental intervention was a highly structured, contingency-based, adaptive treatment protocol designed to reinforce: 1) abstinence from monitored illicit drugs, and 2) adherence to scheduled counseling sessions. Subjects received a fact sheet that displayed the treatment steps and the requirements for the bi-directional movement between steps. The protocol incorporated nine steps of care designed to provide rapid delivery of predictable and increasingly positive consequences for attendance to scheduled counseling sessions and abstinence from drug use (steps +1 to +4), and increasingly negative consequences for missed counseling sessions and ongoing drug use (steps −1 to −4).
Multiple aspects of routine methadone agonist treatment versus monetary-based vouchers were utilized as incentives in order to improve the feasibility of the treatment in community-based treatment settings (methadone dose levels, dispensing times, number of weekly take-home doses and counseling sessions). All of these incentives had been rated as “very important” by APD patients on a prior clinic-wide survey evaluating the reinforcing levels of varying aspects of routine care (see Brooner et al., 1998) and were offered in combination to increase the magnitude of reinforcement at each step of care. An overall aspect of this treatment condition was that it systematically determined the extent of subject versus staff selection of major aspects of treatment (e.g., dose of methadone and counseling, medication dispensing times, take-home medication) in both a stepwise and highly predictable manner. Movement across steps was contingent on weekly rates of counseling attendance and drug use as determined by urinalysis testing. This procedure also created a potential avoidance schedule in which subjects might be inclined to attend counseling sessions and reduce drug use to prevent movement to steps conveying perceived negative consequences (increased counseling sessions, less convenient medication dispensing times, fewer take-home medication doses). These features were selected to help address specific vulnerabilities of patients with APD, including impulsivity, poor planning, and reward-dependence.
Steps +1 to +4 were characterized by increasingly positive consequences; steps −1 to −4 were characterized by increasingly negative consequences. Subjects entered the study at Step 0 and received a methadone dose of 55 mg per day, two individual counseling sessions per week, and medication dispensing times beginning no earlier than noon each day. An opportunity for movement to higher steps (positive reinforcement) occurred every two weeks and was based upon the presence of drug-negative urine specimens for both weeks and attendance at all scheduled counseling sessions. Subjects who missed a counseling session or were drug-positive in each of the two weeks moved to the next lowest step (negative reinforcement). Lower steps resulted in more negative contingencies. Subjects who met criteria for movement in either direction for only 1 of the 2 weeks were kept at their current step of care.
2.4.2 Control condition
Subjects in the control condition received a starting methadone dose of 55 mg per day and two individual counseling sessions per week; the frequency of counseling sessions remained constant throughout the study. Changes to methadone dose were possible once every two weeks and were always clinic determined and based on rates of opioid use. The mean methadone dose of the control group was monitored monthly to ensure that it remained comparable to the mean dose of subjects in the experimental condition. While subjects in the control group were exposed to some positive and negative incentives, these were presented separately versus together, were solely determined by the clinical staff, and were offered only after relatively extended periods of time in treatment. For example, subjects in the control condition could earn a methadone take-home dose but only after producing 12 consecutive weeks of drug negative urine specimens and attendance to all scheduled counseling sessions. Subjects could not select the specific day of the week to receive take-home medication, and while some subjects were given access to more convenient clinic medication dispensing times, this occurred only in response to verified employment.
2.5 Individual counseling
Subjects in both treatment conditions were offered scheduled counseling sessions over the course of the study. Counseling sessions were 30 to 40 minutes in duration and focused on problem-solving techniques to help patients reduce drug use, understand and adhere to their treatment plan and encourage them to earn available positive incentives. Individual counseling sessions were delivered by protocol trained staff with a master’s degree in the behavioral sciences. The counseling schedule was determined by study protocol; content of the counseling sessions followed overall guidelines listed in Treatment of Opiate Addiction with Methadone: A Counselor Manual (McCann, Rawson, Obert, & Hasson, 1994) but was not specifically manually-guided. Counseling staff received weekly supervision by licensed clinical psychologists (M. Kidorf & R. Brooner) and the clinical research monitor to help maintain good fidelity with the study protocol. Counseling staff could not be blinded to treatment condition given the nature of the study; they were assigned an equal number of subjects randomized to the experimental and control conditions. Counselors reviewed the contingencies operating within study condition with subjects on a weekly basis.
2.6 Therapeutic transfer
A therapeutic transfer procedure was included in the study for partial and poor responders to either treatment condition. Subjects in either condition were transferred to routine care in the program that included up to six hours per week of individual and group counseling in response to ongoing drug use and poor attendance to scheduled services (Brooner & Kidorf, 2002; Brooner et al., 2004). Subjects assigned to both treatment conditions were informed that transfer to routine care would result if 50% or more of their weekly urine specimens tested positive for drugs and/or they attended less than 50% of scheduled counseling sessions over the first 90-days of participation. These transfer criteria (50% positive urine specimens or missed counseling sessions) exceed the limits used in routine care to intensify the treatment of partial and poor responders); the 90-day time frame was based on prior studies showing that a good response to methadone is typically observed within the first 3 months of care (Stitzer et al., 1986; Woody et al., 1985; Woody, McLellan, Luborsky, & O’Brien, 1987).
2.7 Data analyses
To evaluate the success of randomization, baseline demographic and clinical characteristics for subjects in each study condition were compared using t-tests for continuous data and chi-square tests for categorical variables. Generalized Estimating Equations (GEE) evaluated the effect of study condition on counseling attendance, urinalysis test results, study completion, and rates of therapeutic transfer to routine MSC care (Zeger, Liang, & Albert, 1988). Experimental and control subjects had comparable rates of missing urinalysis data (experimental: 31% missing; control: 33% missing; p = .524), indicating that missing data was equally distributed across study conditions. The effects of study condition on each of the outcome variables were presented as unadjusted odds ratios (OR) with 95% confidence intervals (CI) and corresponding p-values. Mixed regression models were used to evaluate ASI composite scores over time (baseline and months one, two, three and six). Terms in this model included treatment condition (experimental vs. control), time, and an interaction term (condition × time).
Because the two study conditions differed significantly at baseline on one ASI item that assessed days of poly-drug use (experimental: 5.3 days; control: 9.1 days; p < .05), this variable was used as a covariate for analyses of ASI scores. Analyses of counseling adherence and urinalysis test results were repeated with this variable as a covariate and the results remained unchanged and are not presented here. The two study conditions also differed on rates of lifetime alcohol use disorder (see Table 3). All analyses were repeated with this variable as a covariate; the results were similar and are not presented (these analyses are available on request: rkbrooner@aol.com).
Table 3.
Clinical Characteristics of Study Population
| Variables | Experimental (n = 51) | Control (n = 49) | TOTAL (n= 100) | p Value * |
|---|---|---|---|---|
| Substance Use Disorders (%) | ||||
| Includes Dependence and Abuse | ||||
| Opioid | ||||
| Lifetime | 100 | 100 | 100 | - |
| Current | 100 | 100 | 100 | |
| Cocaine | ||||
| Lifetime | 92 | 98 | 95 | 0.183 |
| Current | 45 | 53 | 49 | 0.426 |
| Alcohol | ||||
| Lifetime | 72 | 92 | 82 | 0.011 |
| Current | 20 | 16 | 18 | 0.636 |
| Sedative | ||||
| Lifetime | 57 | 59 | 58 | 0.814 |
| Current | 8 | 14 | 11 | 0.303 |
| Cannabis | ||||
| Lifetime | 69 | 80 | 74 | 0.211 |
| Current | 10 | 14 | 12 | 0.491 |
| Other Stimulant | ||||
| Lifetime | 35 | 47 | 41 | 0.237 |
| Current | 0 | 0 | 0 | - |
| Hallucinogen | ||||
| Lifetime | 31 | 45 | 38 | 0.164 |
| Current | 2 | 0 | 1 | 0.325 |
| Other Psychiatric Diagnoses (%) | ||||
| Other Axis I Diagnosis | ||||
| Lifetime | 33 | 38 | 35 | 0.665 |
| Current | 22 | 29 | 25 | 0.384 |
| Other Axis II Diagnosis | 29 | 27 | 28 | 0.748 |
| Any Axis I or Axis II | 43 | 49 | 46 | 0.558 |
Generated using chi square for dichotomous data
Finally, GEE was used to evaluate the impact of three baseline predictor variables on outcomes (counseling attendance; urinalysis results; study completion; rates of therapeutic transfer) within each study condition, and for the combined sample: 1) comorbid current Axis I disorder (yes vs. no), 2) PCL-R scores (high: ≥ 25 vs. low: < 25, and 3) number of APD symptoms (high: ≥12 vs. low: <12). PCL-R total scores of 25 or higher were used to operationally define high psychopathy (e.g., Rutherford et al., 1999); number of APD symptoms was dichotomized into low versus high using a median split. Effects of each variable on outcomes were calculated as unadjusted ORs with 95% CIs. These predictor variables were then included with treatment condition (experimental vs. control) to predict each of the study outcomes across both study conditions. Mixed regression models were used to evaluate the association of these variables with ASI scores.
RESULTS
3.1 Sample demographics and clinical characteristics
The sample had a mean age of 39 years; 77% were male, 60% were African-American, 12% were married, and 66% were unemployed. The sample had a mean of 11 years of education. No condition differences were observed on the demographic variables (Table 2).
Table 2.
Demographic characteristics of sample
| Variables | Experimental (n = 51) | Control (n = 49) | TOTAL (n= 100) | p Value * |
|---|---|---|---|---|
| Percent Male (n) | 73 (38) | 82 (40) | 77 (77) | 0.281 |
| Mean Age in yrs (s.d.) | 38 (7.6) | 39 (6.6) | 39 (7.1) | 0.410 |
| Percent Caucasian (n) | 38 (19) | 43 (21) | 40 (40) | 0.568 |
| Percent Married (n) | 8 (4) | 16 (8) | 12 (12) | 0.192 |
| Education in years (s.d.) | 10.8 (2.1) | 10.6 (2.1) | 10.7 (2.1) | 0.790 |
| Percent Employed (n) | 29 (15) | 39 (19) | 34 (34) | 0.323 |
| Percent Reporting Income< $500/month (n) | 76 (39) | 67(33) | 72 (72) | 0.389 |
Generated using chi square for dichotomous or t test for continuous data
Clinical characteristics for the sample are described in Table 3. All subjects met criteria for current opioid dependence and approximately half met criteria for a current cocaine use disorder (dependence or abuse). Current alcohol (18%), sedative (11%), and cannabis (12%) use disorders were also common. Prevalence of each class of substance use disorder was similar across study conditions, with the exception that control subjects had a higher rate of lifetime alcohol use disorder. Study conditions also did not differ in prevalence of a lifetime or current comorbid Axis I or II disorder. Overall, almost half of this antisocial sample met diagnostic criterion for at least one additional Axis I or II psychiatric disorder.
3.2 Treatment outcomes by study condition
3.2.1 Adherence to scheduled counseling sessions
As shown in Table 4, subjects in the experimental group attended a significantly higher percentage of scheduled counseling sessions than control subjects (83% vs. 53%; unadjusted OR = 4.00; CI: 2.39–6.70, p < .0001).
Table 4.
Treatment Outcomes by Study Condition
| Treatment Outcome | Experimental (n = 51) | Control (n = 49) | OR (95% CI) 1 | p Value | ||
|---|---|---|---|---|---|---|
| Adherence to Counseling Sessions | Number Scheduled | Percent Attended | Number Scheduled | Percent Attended | ||
| Weeks 1–26 | 1545 | 83.2 | 1679 | 53.4 | 4.00 (2.39–6.70) | < .0001 |
| Urine Test Results | ||||||
| Weeks 1–26 Results | ||||||
| % Negative for Opioids | 80.5 | 73.7 | 1.31 (0.71–2.42) | .393 | ||
| % Negative for Cocaine | 77.3 | 66.7 | 1.59 (0.86–2.96) | .139 | ||
| % Negative for Sedatives | 96.2 | 90.8 | 1.82 (0.75–4.42) | .184 | ||
| % Negative for Any Drugs | 68.7 | 54.2 | 1.70 (0.94–3.07) | .081 | ||
Unadjusted odds ratios for attendance data and urine testing calculated with GEE
3.2.2 Urinalysis test results
No statistically significant condition effects were found for urinalysis results.
3.2.3 Psychosocial and other drug-related problem severity
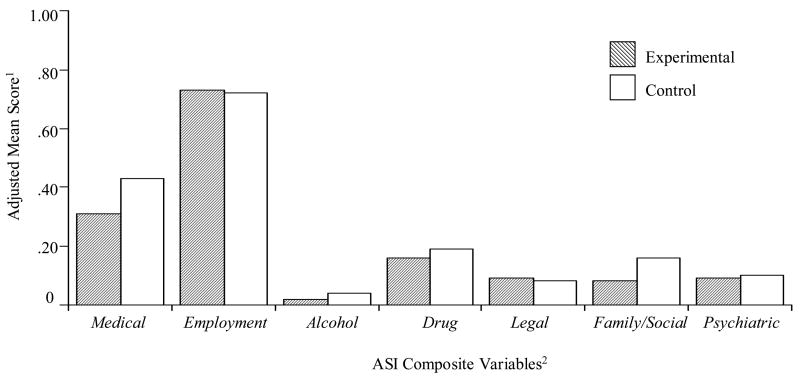
As shown in Figure 1, a condition main effect was observed for ASI family/social composite scores, such that experimental versus control subjects reported lower family/social severity (0.08 vs. 0.16; F(1, 81) = 8.32, p < 0.01). No other main effects or interactions were found.
Figure 1.
Adjusted Mean Addiction Severity Index Composite Scores by Study Condition: Months 1–6
1. Adjusted mean composite score were generated from ASI’s done at 1, 2, 3, & 6 months using hierarchical regression models. Terms included treatment condition, time, and interaction term (time × condition) and ASI89 (poly drug use) at baseline.
2. N=86; data not available on 14 participants (9 experimental and 5 control missing)
3.3 Associations between comorbidity, antisocial severity, and treatment response
3.3.1 Counseling attendance
Experimental Condition
Comorbid psychiatric disorder and severity of antisocial problems (PCL-R score, APD symptom count) were not associated with counseling attendance.
Control Condition
Subjects with versus without a comorbid psychiatric disorder attended a higher proportion of counseling sessions (unadjusted OR = 2.11; CI: 1.00–4.43, p < .05). PCL-R scores and number of APD symptoms were not associated with counseling attendance.
Combined Sample
The association between comorbid psychiatric disorder and counseling attendance observed in the control condition was no longer significant after adjusting for treatment condition, PCL-R scores, and number of APD symptoms (adjusted OR = 1.37; CI: 0.77–2.43; ns), although attendance to scheduled sessions remained strongly associated with treatment condition (adjusted OR = 3.93; CI: 0.29–6.61; p < .001).
3.3.2 Urinalysis results
Experimental Condition
Subjects with lower vs. higher PCL-R scores submitted a higher proportion of drug-negative urine samples (74% vs. 52%; unadjusted OR = 2.65; CI: 1.10–6.37; p < .05). Similarly, subjects with low versus high APD symptom counts submitted a higher proportion of drug negative urine specimens (74% vs. 59%; unadjusted OR 2.43; CI: 1.05–5.63; p < .05). Comorbid psychiatric disorder was not associated with urinalysis results.
Control Condition
Comorbid psychiatric disorder and severity of antisocial problems (PCL-R scores, APD symptom count) were not associated with urinalysis results.
Combined Sample
The association between PCL-R scores and urinalysis results observed in the experimental condition was weaker after adjusting for treatment condition, comorbid psychiatric disorder, and APD symptom count (adjusted OR = 1.89; CI: 0.98–3.64; p = .058), while the association between APD symptom count and urinalysis results was no longer significant after controlling for the other predictor variables (adjusted OR = 1.17; CI: 0.61–2.27; ns).
3.3.3 ASI Composite Scores
Psychiatric comorbidity, PCL-R scores, and number of APD symptoms were not associated with ASI composite scores in either study condition or comparing across the combined sample.
3.4 Rates of therapeutic transfer and study completion
Fewer experimental versus control subjects were therapeutically transferred to routine care because of partial and poor treatment response (20% vs. 37%, unadjusted OR = 2.38, CI: 0.97–5.87; p = 0.05); both treatment conditions had comparable rates of study completion (experimental: 55% vs. control: 43%; unadjusted OR = 1.62; CI: 0.74–3.58; ns). Psychiatric comorbidity, PCL-R score, and number of APD symptoms were not associated with rates of either therapeutic transfer to routine care or study completion.
DISCUSSION
Prior work has shown that APD drug dependent patients often respond as well to routine drug abuse treatment as non-APD patients, though the response was sometimes poor across groups and many of the studies were retrospective or post-hoc evaluations (Alterman et al., 1998; Darke, Hall, & Swife, 1994; Gill et al., 1992). The present study prospectively evaluated an intensive and adaptive behavioral intervention that targeted some clinical aspects shared by both APD and drug use disorders. This approach has been suggested over many years (e.g., Vaillant, 1975) and is supported by a few prospective studies conducted in alcohol dependent samples (Longabaugh et al., 1994; Kadden, Cooney, Getter, & Litt, 1991). While the intensive behavioral intervention in the present study was associated with a few areas of improved outcome, subjects in the controlled condition responded remarkably well and most treatment outcomes were comparable. Several of these results are discussed below, including findings on attendance and drug use, and the influence of severity of antisocial problems and other psychiatric comorbidity on treatment response.
4.1 Attendance to scheduled counseling sessions
One significant difference between treatment conditions was the greater attendance to scheduled counseling sessions in the experimental versus control group. Poor adherence to prescribed treatments is a serious problem in health care delivery and often reduces the benefits of even highly efficacious therapies. This problem was documented in the treatment of substance use disorder over 30 years ago (Sellers, Cappell, & Marshman, 1977) and it remains extremely problematic (Alterman, Rutherford, Cacciola, McKay, & Boardman, 1998; Brooner et al., 2004; Kidorf, King, & Brooner, 2006; Martinez-Raga, Marshall, Keaney, Ball, & Strang, 2002; Ross, Dermatis, Levounis, & Galanter, 2003). While the present study could not determine if antisocial personality increases non-adherence because a non-APD comparison group was not included in the design; the results do show that the experimental intervention was associated with a four-fold increase in the likelihood of attending scheduled counseling sessions. The high rate of counseling attendance (80%) observed in the experimental group is considerably better than the average attendance rate (about 50%) reported across other treatment outcome studies of opioid-dependent patients receiving methadone (Fiorentine & Anglin, 1997; Kidorf, King, & Brooner, 2006; McLellan, Arndt, Metzger, Woody, & O’Brien, 1993). The high rate of attendance in the present study is also consistent with earlier reports showing that behavioral incentives can be used contingently to motivate good patient attendance to a variety of types and intensities of routine and specialized counseling and therapy in drug-dependent patients (Brooner et al., 2004; Kidorf et al., 2006).
The fact that attendance was affected by other psychiatric diagnoses in the control but not the experimental group was also interesting. One possibility is that the behavioral contingencies were able to overcome the otherwise significant effects of other psychiatric problems on attendance behavior. The significant association between other psychiatric disorders in the control group and improved counseling attendance is also interesting and consistent with prior work and speculation that subjective distress may motivate treatment-seeking behavior (Alterman et al., 1996; Brooner, Schmidt, & Herbst, 1994; King et al., 2001; Woody et al., 1985). Attendance was also unaffected by the level of psychopathy in this sample of APD subjects.
4.2 Drug use and other psychosocial problems
While the experimental group had higher rates of drug-negative urine specimens compared to the control group, these differences were not statistically significant. This was partly related to the good response to standard treatment in the control condition (50% of specimens negative for any drug use). The low rates of drug use observed in the study are particularly notable given the high rates of use in subjects entering the study. While the low rates of use may be partially explained by subjects trying to avoid therapeutic transfer to routine care, this remains speculative. At the very least, the findings on drug use provide additional support to the growing view that APD patients can respond well to drug abuse treatment. In fact, the rates of drug-negative urine specimens in the control group of antisocial subjects was better than those reported in many treatment outcome studies with largely non-antisocial samples of opioid dependent patients (Alterman et al., 1996; Brooner et al., 2004; Woody et al., 1985). Alterman and colleagues (1996), for example, reported rates of opioids (47%), cocaine (43%) and sedative (19%) positive urine specimens in opioid-dependent patients without APD, all of which are substantially worse than the corresponding rates of drug use in the present study for the control and the experimental groups both separately, and combined.
While subjects with high versus low psychopathy scores (PCL: 25+) had higher rates of drug use, this association was weakened after controlling for treatment condition, number of DSM APD symptoms, and presence of other psychiatric diagnoses. The association between high psychopathy and drug use observed in the study is generally consistent with other reports (e.g., Alterman et al., 1998). The fact that the association was weakened by treatment condition and other psychiatric comorbidity is also important. It appears that the negative influence of high psychopathy on drug use can be modified by at least some types of treatment and by the presence of other psychiatric diagnoses.
4.3 Generalizabilty of findings
The low rates of drug use across treatment conditions compared to other reports of opioid dependent patients do not seem related to differences in baseline drug use problems or less overall severity of symptoms. For example, the ASI baseline drug composite severity scores in the present study are comparable to those reported in other studies of opioid dependent patients with APD (Alterman et al., 1998). The same is true for baseline PCL-R psychopathy scores. The mean total psychopathy score (M = 21.8) in the present study is very similar to mean scores reported in other samples of opioid dependent patients (e.g., Alterman et al., 1993), and only slightly lower than scores reported in prison populations with APD (M = 23.6; Hare, Hart, & Harpur, 1991). The findings observed in the present study may therefore generalize to samples in other community-based methadone treatment settings.
4.4 Study limitations
This study has several important limitations. Perhaps the biggest problem is the therapeutic transfer procedure. This “rescue” intervention appears to have created an avoidance schedule that may have reduced rates of drug use in this study, particularly in the control group that had a higher rate of therapeutic transfer because of poor treatment response (i.e., high rates of continued drug use). Subjects may have reduced drug use in efforts to avoid transfer to the intensive (3 to 9 hours of weekly counseling) services associated with the routine MSC treatment condition. The combination of the transfer effects and a drop-out rate of 23% reduced power to detect differences between the groups, although the drop-out rate is comparable to other studies of opioid-dependent subjects (Joe, Simpson, & Broome, 1999). Evaluation of this treatment intervention, unaffected by the “rescue” procedure employed in the present study, would be very instructive.
The study design also did not include a non-APD comparison group. The research grant supporting this study was reviewed and funded at a time when it was widely thought that APD was uniformly associated with poor drug treatment response. Within this context, it was considered more important to develop interventions to improve the treatment response of opioid-dependent patients with APD, than to highlight their poorer outcomes compared to non-APD patients. It is now clear that the present report would have been strengthened by the addition of a non-APD comparison group.
Another limitation of the study was the inability to blind staff to treatment assignment. To offset this weakness, a comparable number of subjects in the experimental and control conditions were randomly assigned to each of the counseling staff. Counselors were supervised on a weekly basis to help maintain good fidelity to the treatment protocol, although some cross contamination of treatment conditions may have occurred, and may have biased the findings toward the null hypothesis.
4.5 Summary
Emil Kraepelin commented on the futility of psychiatric treatments for patients suffering from “moral insanity” almost 100 years ago (Lectures on Clinical Psychiatry, reprinted, 1988), a clinical syndrome that today is often referred to as antisocial personality. Considerable progress has been made over the last century and much of it occurred in the last few decades. The present findings support this work by showing that opioid dependent patients with APD are very responsive to treatment. The behavioral incentives employed in the study also have the advantage of being routine clinic-based treatment variables and therefore more feasible to employ than monetary-based voucher incentives for treatment programs that use methadone. While notable clinical improvement may not render this an “easy-to-manage” group of patients because of ongoing symptoms of antisocial personality, the clinical gains they can achieve provide a strong basis for therapeutic optimism.
Table 1.
Treatment Conditions in Steps of Behavioral Intervention for Experimental Group
| Treatment Steps1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Variables | − 4 | − 3 | − 2 | − 1 | 0 | +1 | +2 | +3 | +4 |
| Number of Scheduled Counseling Sessions/Week | 3 | 3 | 3 | 2 | 2 | 2 | Patient Selects 1, 2, or 3 Sessions/Week | ||
| Number of Methadone Take-Home Doses/Week2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 3 |
| Methadone Dosing Hours At Clinic3 | Split Dose; Attends Clinic Twice/Day | Clinic Staff Selects Hours; Attends Once/Day | Patient Selects Hours; Attends Once/Day | ||||||
| Medication Dose4 | 20% Decrease | No Change | 20% Decrease | Clinic Staff Selects Dose | Patient Selects Dose | ||||
Patients start at step 0 with a methadone dose of 55 mg. Each step is two weeks in duration and movement up or down depends on results of urine testing and adherence to scheduled counseling.
Patients can earn up to 3 take- home doses per week and can choose which day to use them.
If patient selects medication timing, hours to choose from becomes wider as the subject progresses in the steps (i.e. 9:00 to 19:30 in Step + 4). If clinic selected, hours are more restricted and less convenient. If split, patient must come to the clinic two times each day for full methadone dose.
Patient selected methadone doses allow the patient to increase 5 to 10 mg over the next two weeks, up to a total dose of 80 mg; clinic selected doses changes may or may not be made in Step −1 and fixed reduction occur in Steps −2 and −4.
Table 5.
Association of Psychopathy Severity with Treatment Outcome
| Treatment Outcome | Less Psychopathy (Total PCL Score < 25) n = 64 | More Psychopathy (Total PCL Score ≥ 25) n = 36 | OR (95% CI)1 | p Value | ||
|---|---|---|---|---|---|---|
| Adherence to Counseling Sessions | Number Scheduled | Percent Attended | Number Scheduled | Percent Attended | ||
| Weeks 1–26 | 1958 | 70.5 | 1268 | 63.3 | 1.66 (0.96–2.85) | .070 |
| Urine Test Results | ||||||
| Weeks 1–26 | ||||||
| % Negative for Opioids | 81.2 | 69.8 | 1.70 (0.92–3.17) | .092 | ||
| % Negative for Cocaine | 76.5 | 64.1 | 1.59 (0.86–2.95) | .137 | ||
| % Negative for Sedatives | 95.7 | 89.7 | 2.05 (0.80–5.22) | .133 | ||
| % Negative for All Drugs | 68.4 | 49.2 | 2.11 (1.17–3.80) | .014 | ||
Unadjusted odds ratios for attendance data and urine testing calculated with GEE
Acknowledgments
This work was supported by NIH-NIDA grant R01DA05569 (PI: RK Brooner). The authors also acknowledge and greatly thank Kori Kindbom, Samantha DiBastiani, and Rachel Burns for their contributions to the conduct of this work, the Baltimore Substance Abuse System, Inc. for their support of the Addiction Treatment Services program, and the patients who participated in this evaluation.
Grant Support: NIH-NIDA RO1 DA05569 (PI: RK Brooner)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alterman AI, Cacciola JS. The antisocial personality disorder diagnosis in substance abusers: Problems and issues. Journal of Nervous and Mental Diseases. 1991;179:401–409. doi: 10.1097/00005053-199107000-00003. [DOI] [PubMed] [Google Scholar]
- Alterman AI, Cacciola JS, Rutherford MJ. Reliability of the revised psychopathy checklist in substance abuse patients. Psychological Assessment. 1993;5:442–448. [Google Scholar]
- Alterman AI, Rutherford MJ, Cacciola JS, McKay JR, Woody GE. Response to methadone maintenance and counseling in antisocial patients with and without major depression. Journal of Nervous and Mental Diseases. 1996;184:695–702. doi: 10.1097/00005053-199611000-00007. [DOI] [PubMed] [Google Scholar]
- Alterman AI, Rutherford MJ, Cacciola JS, McKay JR, Boardman CR. Prediction of 7 months methadone maintenance treatment response by four measures of antisociality. Drug and Alcohol Dependence. 1998;49:217–23. doi: 10.1016/s0376-8716(98)00015-5. [DOI] [PubMed] [Google Scholar]
- Arndt IO, McLellan AT, Dorozynsky L, Woody GE, O’Brien CP. Desipramine treatment for cocaine dependence. Role of antisocial personality disorder. Journal of Nervous and Mental Diseases. 1994;182:151–156. doi: 10.1097/00005053-199403000-00004. [DOI] [PubMed] [Google Scholar]
- Bell J, Mattick R, Hay A, Chan J, Hall W. Methadone maintenance and drug-related crimes. Journal of Substance Abuse. 1997;9:15–25. doi: 10.1016/s0899-3289(97)90003-1. [DOI] [PubMed] [Google Scholar]
- Black DW, Baumgard CH, Bell SE. A 16- to 45-year follow-up of 71 men with antisocial personality disorder. Comprehensive Psychiatry. 1995;36:130–140. doi: 10.1016/s0010-440x(95)90108-6. [DOI] [PubMed] [Google Scholar]
- Bovasso GB, Alterman AI, Cacciola JS, Rutherford MJ. The prediction of violent and nonviolent criminal behavior in a methadone maintenance population. Journal of Personality Disorders. 2002;16:360–73. doi: 10.1521/pedi.16.4.360.24124. [DOI] [PubMed] [Google Scholar]
- Brooner RK, Bigelow GE, Strain E, Schmidt CW. Intravenous drug abusers with antisocial personality disorder: increased HIV risk behavior. Drug and Alcohol Dependence. 1990;26:39–44. doi: 10.1016/0376-8716(90)90081-o. [DOI] [PubMed] [Google Scholar]
- Brooner RK, Greenfield L, Schmidt CW, Bigelow GE. Antisocial personality disorder and HIV infection among intravenous drug abusers. American Journal of Psychiatry. 1993a;150:53–58. doi: 10.1176/ajp.150.1.53. [DOI] [PubMed] [Google Scholar]
- Brooner RK, Herbst JH, Schmidt CW, Bigelow GE, Costa PT. Antisocial personality disorder among drug abusers: relations to other personality diagnoses and the five-factor model of personality. Journal of Nervous and Mental Diseases. 1993b;181:313–319. [PubMed] [Google Scholar]
- Brooner RK, Kidorf MS. Using behavioral reinforcement to improve methadone treatment participation. Science and Practice Perspectives. 2002;1:38–46. doi: 10.1151/spp021138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooner RK, Kidorf M, King VL, Stoller K. Preliminary evidence of good treatment response in antisocial drug abusers. Drug and Alcohol Dependence. 1998;49:249–260. doi: 10.1016/s0376-8716(98)00018-0. [DOI] [PubMed] [Google Scholar]
- Brooner RK, Kidorf MS, King VL, Stoller KB, Peirce JM, Bigelow GE. Behavioral contingencies improve counseling attendance in an adaptive treatment model. Journal of Substance Abuse Treatment. 2004;27:223–232. doi: 10.1016/j.jsat.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Brooner RK, King VL, Kidorf M, Schmidt CW, Jr, Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Archives of General Psychiatry. 1997;54:71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- Brooner RK, Schmidt CW, Felch LJ, Bigelow GE. Antisocial behavior of intravenous drug abusers: Implications for diagnosis of antisocial personality disorder. American Journal of Psychiatry. 1992;149:482–7. doi: 10.1176/ajp.149.4.482. [DOI] [PubMed] [Google Scholar]
- Brooner RK, Schmidt CW, Herbst JH. Personality disorders among opioid abusers and their NEO-PI personality profiles. In: Costa P, Widiger T, editors. Personality Disorders and the Five-Factor Model of Personality. Washington D.C.: American Psychological Association; 1994. pp. 131–148. [Google Scholar]
- Cacciola JS, Alterman AI, Rutherford MJ, Snider EC. Treatment response of antisocial substance abusers. Journal of Nervous and Mental Diseases. 1995;183:166–71. doi: 10.1097/00005053-199503000-00007. [DOI] [PubMed] [Google Scholar]
- Cacciola JS, Rutherford MJ, Alterman AI, McKay JR, Snider EC. Personality disorders and treatment outcome in methadone maintenance patients. Journal of Nervous and Mental Disease. 1996;184:234–239. doi: 10.1097/00005053-199604000-00006. [DOI] [PubMed] [Google Scholar]
- Cacciola JS, Rutherford MJ, Alterman AI, Snider EC. An examination of the diagnostic criteria for antisocial personality disorder in substance abusers. Journal of Nervous and Mental Diseases. 1994;182:517–523. doi: 10.1097/00005053-199409000-00007. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ. History and significance of childhood attention deficit disorder in treatment-seeking cocaine abusers. Comprehensive Psychiatry. 1993;34:75–82. doi: 10.1016/0010-440x(93)90050-e. [DOI] [PubMed] [Google Scholar]
- Cleckley HM. The mask of sanity. An attempt to reinterpret the so-called psychopathic personality. St. Louis: C. V. Mosby Co; 1941. [Google Scholar]
- Compton WM, Conway KP, Stinson FS, Colliver JD, Grant BF. Prevalence, correlates, and comorbidity of DSM-IV antisocial personality syndromes and alcohol and specific drug use disorders in the United States: Results from the national epidemiologic survey on alcohol and related conditions. The Journal of Clinical Psychiatry. 2005;66:677–685. doi: 10.4088/jcp.v66n0602. [DOI] [PubMed] [Google Scholar]
- Compton WM, Cottler LB, Ben Abdallah A, Phelps DL, Spitznagel EL, Horton JC. Substance dependence and other psychiatric disorders among drug dependent subjects: Race and gender correlates. The American Journal on Addictions/American Academy of Psychiatrists in Alcoholism and Addictions. 2000;9:113–125. doi: 10.1080/10550490050173181. [DOI] [PubMed] [Google Scholar]
- Compton WM, Cottler LB, Shillington AM, Price RK. Is antisocial personality disorder associated with increased HIV risk behaviors in cocaine users? Drug and Alcohol Dependence. 1995;37:37–44. doi: 10.1016/0376-8716(94)01056-q. [DOI] [PubMed] [Google Scholar]
- Compton WM, Cottler LB, Spitznagel EL, Ben-Abdallah A, Gallagher T. Cocaine users with antisocial personality improve HIV risk behaviors as much as those without antisocial personality. Drug and Alcohol Dependence. 1998;49:239–247. doi: 10.1016/s0376-8716(98)00017-9. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Campbell W, Krishna VA, Cunningham-Williams RM, Abdallah AB. Predictors of high rates of suicidal ideation among drug users. Journal of Nervous and Mental Disorders. 2005;193:431–437. doi: 10.1097/01.nmd.0000168245.56563.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottler LB, Price RK, Compton WM, Mager DE. Subtypes of adult antisocial personality behavior among drug abusers. Journal of Nervous and Mental Diseases. 1995;183:154–161. doi: 10.1097/00005053-199503000-00005. [DOI] [PubMed] [Google Scholar]
- Darke S, Finlay-Jones R, Kaye S, Blatt T. Anti-social personality disorder and response to methadone maintenance treatment. Drug and Alcohol Review. 1996;15:271–276. doi: 10.1080/09595239600186011. [DOI] [PubMed] [Google Scholar]
- Darke S, Hall W, Swife W. Prevalence, symptoms and correlates of antisocial personality disorder among methadone maintenance clients. Drug and Alcohol Dependence. 1994;34:253–257. doi: 10.1016/0376-8716(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Dinwiddie SH, Reich T, Cloninger CR. Psychiatric comorbidity and suicidality among intravenous drug users. Journal of Clinical Psychiatry. 1992;53:364–369. [PubMed] [Google Scholar]
- Eronen M, Hakola P, Tiihonen J. Mental disorders and homicidal behavior in Finland. Archives of General Psychiatry. 1996;53:497–501. doi: 10.1001/archpsyc.1996.01830060039005. [DOI] [PubMed] [Google Scholar]
- Fiorentine R, Anglin MD. Does increasing the opportunity for counseling increase the effectiveness of outpatient drug treatment? American Journal of Drug and Alcohol Abuse. 1997;23:369–382. doi: 10.3109/00952999709016883. [DOI] [PubMed] [Google Scholar]
- Gerstley LJ, Alterman AI, McLellan AT, Woody GE. Antisocial personality disorder in patients with substance abuse disorders: a problematic diagnosis? American Journal of Psychiatry. 1990;147:173–8. doi: 10.1176/ajp.147.2.173. [DOI] [PubMed] [Google Scholar]
- Gill K, Nolimal D, Crowley TJ. Antisocial personality disorder, HIV risk behavior and retention in methadone maintenance therapy. Drug and Alcohol Dependence. 1992;30:247–252. doi: 10.1016/0376-8716(92)90059-l. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Hamilton SP. Lifetime comorbidity of antisocial personality disorder and anxiety disorders among adults in the community. Psychiatry Research. 2003;117:159–166. doi: 10.1016/s0165-1781(02)00320-7. [DOI] [PubMed] [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist-Revised. Toronto, ON: Multi-Health Systems; 1991. [Google Scholar]
- Hare RD, Hart SD, Harpur TJ. Psychopathy and the DSM-IV criteria for antisocial personality disorder. Journal of Abnormal Psychology. 1991;100:391–398. doi: 10.1037//0021-843x.100.3.391. [DOI] [PubMed] [Google Scholar]
- Havens JR, Strathdee SA. Antisocial personality disorder and opioid treatment outcomes: a review. Addictive Disorders and their Treatment. 2005;4:85–97. [Google Scholar]
- Iguchi MY, Stitzer ML, Bigelow GE, Liebson IA. Contingency management in methadone maintenance: Effects of reinforcing and aversive consequences on illicit polydrug use. Drug Alcohol Depend. 1988;22:1–7. doi: 10.1016/0376-8716(88)90030-0. [DOI] [PubMed] [Google Scholar]
- Joe GW, Simpson DD, Broome KM. Retention and patient engagement models for different treatment modalities in DATOS. Drug and Alcohol Dependence. 1999;57:113–125. doi: 10.1016/s0376-8716(99)00088-5. [DOI] [PubMed] [Google Scholar]
- Kadden RM, Cooney NL, Getter H, Litt MD. Matching alcoholics to coping skills or interactional therapies: Two-year follow-up results. Journal of Consulting Clinical Psychology. 1991;59:598–601. doi: 10.1037//0022-006x.59.4.598. [DOI] [PubMed] [Google Scholar]
- Kelley JL, Petry NM. HIV risk behaviors in male substance abusers with and without antisocial personality disorder. Journal of Substance Abuse Treatment. 2000;19:59–66. doi: 10.1016/s0740-5472(99)00100-2. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ, Treece C. DSM-III psychiatric diagnosis of narcotics addicts: recent findings. Archives of General Psychiatry. 1985;42:1067–1071. doi: 10.1001/archpsyc.1985.01790340045007. [DOI] [PubMed] [Google Scholar]
- Kidorf M, King V, Brooner RK. Counseling and psychosocial services. In: Stitzer M, Strain E, editors. The treatment of opioid dependence. Baltimore: Johns Hopkins University Press; 2006. pp. 119–150. [Google Scholar]
- Kidorf M, Stitzer ML, Brooner RK, Goldberg J. Contingent methadone take-home doses reinforce adjunct therapy attendance of methadone maintenance patients. Drug Alcohol Dependence. 1994;36:221–226. doi: 10.1016/0376-8716(94)90148-1. [DOI] [PubMed] [Google Scholar]
- King VL, Kidorf MS, Stoller KB, Carter JA, Brooner RK. Influence of antisocial personality subtypes on drug abuse treatment response. The Journal of Nervous and Mental Disease. 2001;189:593–601. doi: 10.1097/00005053-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Kosten TR, Rounsaville BJ. Personality disorders in opiate addicts show prognostic specificity. Journal of Substance Abuse Treatments. 1989;6:163–168. doi: 10.1016/0740-5472(89)90003-2. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. DSM-III personality disorders in opiate addicts. Comprehensive Psychiatry. 1982;23:572–581. doi: 10.1016/0010-440x(82)90050-5. [DOI] [PubMed] [Google Scholar]
- Kosten T, Rounsaville B, Kleber H. Concurrent validity of the addiction severity index. The Journal of Nervous and Mental Disease. 1983;171:606–610. doi: 10.1097/00005053-198310000-00003. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. The Classics of Psychiatry & Behavioral Sciences Library. Birmingham, Alabama: 1988. Lecture: Morbid criminals and vagabonds. A Special Reprint: Lectures on Clinical Psychiatry; pp. 310–325. [Google Scholar]
- Lewis CE, Rice J, Helzer JE. Diagnostic interactions: Alcoholism and antisocial personality. Journal of Nervous and Mental Disease. 1983;171:105–113. doi: 10.1097/00005053-198302000-00007. [DOI] [PubMed] [Google Scholar]
- Longabaugh R, Rubin A, Malloy P, Beattie M, Clifford PR, Noel N. Drinking outcomes of alcohol abusers diagnosed as antisocial personality disorder. Alcoholism, Clinical and Experimental Research. 1994;18:778–785. doi: 10.1111/j.1530-0277.1994.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Magura S, Casriel C, Goldsmith DS. Contingency contracting with polydrug-abusing methadone patients. Addictive Behaviors. 1988;13:113–118. doi: 10.1016/0306-4603(88)90035-4. [DOI] [PubMed] [Google Scholar]
- Martinez-Raga J, Marshall EJ, Keaney F, Ball D, Strang J. Unplanned versus planned discharges from in-patient alcohol detoxification: retrospective analysis of 470 first-episode admissions. Alcohol and Alcoholism. 2002;37:277–81. doi: 10.1093/alcalc/37.3.277. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Rawson RA, Obert JL, Hasson AJ. Treatment of Opiate Addiction with Methadone: A Counselor Manual. U.S. Department of Health and Human Services; Rockville, MD: 1994. [Google Scholar]
- McDermott PA, Alterman AI, Cacciola JS, Rutherford MJ, Newman JP, Mulholland EM. Generality of psychopathy checklist-revised factors over prisoners and substance-dependent patients. Journal of Consulting and Clinical Psychology. 2000;68:181–186. [PubMed] [Google Scholar]
- McLellan AT, Arndt IO, Metzger DS, Woody GE, O’Brien CP. The effects of psychosocial services in substance abuse treatment. Journal of the American Medical Association. 1993;269:1953–1959. [PubMed] [Google Scholar]
- McLellan T, Cacciola J, Alterman A, Rikoon S, Carise D. The addiction severity index at 25: Origins, contributions and transitions. American Journal of Addictions. 2006;15:113–124. doi: 10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the addiction severity index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Rustin T. Antisocial personality disorder and aggression in recently abstinent cocaine dependent subjects. Drug and Alcohol Dependence. 1997;44:175–182. doi: 10.1016/s0376-8716(96)01335-x. [DOI] [PubMed] [Google Scholar]
- Nace EP, Davis CW, Gaspari JP. Axis II comorbidity in substance abusers. American Journal of Psychiatry. 1991;148:118–120. doi: 10.1176/ajp.148.1.118. [DOI] [PubMed] [Google Scholar]
- Ross S, Dermatis H, Levounis P, Galanter M. A comparison between dually diagnosed inpatients with and without Axis II comorbidity and the relationship to treatment outcome. American Journal of Drug and Alcohol Abuse. 2003;29:263–79. doi: 10.1081/ada-120020511. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Dolinsky ZS, Babor TF, Meyer RE. Psychopathology as a predictor of treatment outcome in alcoholics. Archives of General Psychiatry. 1987;44:505–513. doi: 10.1001/archpsyc.1987.01800180015002. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Kosten TR, Weissman MM, Kleber HD. Prognostic significance of psychopathology in treated opiate addicts. A 2.5-year follow-up study. Archives of General Psychiatry. 1986;43:739–745. doi: 10.1001/archpsyc.1986.01800080025004. [DOI] [PubMed] [Google Scholar]
- Rousar E, Brooner RK, Regier MW, Bigelow GE. Psychiatric distress in antisocial drug abusers: relation to other personality disorders. Drug and Alcohol Dependence. 1994;34:149–154. doi: 10.1016/0376-8716(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Rutherford MJ, Alterman AI, Cacciola JS, McKay JR. Gender differences in the relationship of antisocial personality disorder criteria to psychopathy checklist-revised scores. Journal of Personality Disorders. 1998;12:69–76. doi: 10.1521/pedi.1998.12.1.69. [DOI] [PubMed] [Google Scholar]
- Rutherford MJ, Cacciola JS, Alterman AI. Relationships of personality disorders with problem severity in methadone patients. Drug and Alcohol Dependence. 1994;35:69–76. doi: 10.1016/0376-8716(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Rutherford M, Cacciola JS, Alterman AI, McKay JR, Cook TG. The 2-year test-retest reliability of the psychopathy checklist-revised in methadone patients. Assessment. 1999;6:285–292. doi: 10.1177/107319119900600308. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS Version 8. Cary, NC: SAS Institute Inc; 2004. [Google Scholar]
- Schuckit MA, Klein J, Twitchell G, Smith T. Personality test scores as predictors of alcoholism almost a decade later. American Journal of Psychiatry. 1994;151:1038–1042. doi: 10.1176/ajp.151.7.1038. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Cappell HD, Marshman JA. Compliance in the control of alcohol abuse. In: Haynes RB, Taylor DW, Sackett DL, editors. Compliance in Health Care. Baltimore: The Johns Hopkins University Press; 1977. pp. 223–243. [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The structured clinical interview for DSM-III-R (SCID). I: History, rationale, and description. Archives of General Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bickel WK, Bigelow GE, Liebson IA. Effect of methadone dose contingencies on urinalysis test results of polydrug-abusing methadone-maintenance patients. Drug and Alcohol Dependence. 1986;18:341–348. doi: 10.1016/0376-8716(86)90097-9. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE, Lawrence C, Cohen J, D’Lugoff B, Hawthorne J. Medication take-home as a reinforcer in a methadone maintenance program. Addictive Behaviors. 1977;2:9–14. doi: 10.1016/0306-4603(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Higgins ST. Behavioral treatment of drug and alcohol abuse. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. [Google Scholar]
- Stitzer M, Iguchi MY, Felch LJ. Contingent take-home incentive: effects on drug use of methadone maintenance patients. Journal of Consulting Clinical Psychology. 1992;60:927–934. doi: 10.1037//0022-006x.60.6.927. [DOI] [PubMed] [Google Scholar]
- Vaillant GE. Sociopathy as a human process. A viewpoint. Archives of General Psychiatry. 1975;32:178–183. doi: 10.1001/archpsyc.1975.01760200042003. [DOI] [PubMed] [Google Scholar]
- Williams JB, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, et al. The structured clinical interview for DSM-III-R (SCID). II. multisite test-retest reliability. Archives of General Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- Woody GE, McLellan AT, Luborsky L, O’Brien CP. Sociopathy and psychotherapy outcome. Archives of General Psychiatry. 1985;42:1081–1086. doi: 10.1001/archpsyc.1985.01790340059009. [DOI] [PubMed] [Google Scholar]
- Woody GE, McLellan AT, Luborsky L, O’Brien CP. Twelve-month follow-up of psychotherapy for opiate dependence. The American Journal of Psychiatry. 1987;144:590–596. doi: 10.1176/ajp.144.5.590. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: A generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]