Abstract
Murine natural killer (NK) cells express inhibitory Ly49 receptors specific for major histocompatibility complex (MHC) class I molecules. We report that during interactions with cells in the environment, NK cells acquired MHC class I ligands from surrounding cells in a Ly49-specific fashion and displayed them at the cell surface. Ligand acquisition sometimes reached 20% of the MHC class I expression on surrounding cells, involved transfer of the entire MHC class I protein to the NK cell, and was independent of whether or not the NK cell expressed the MHC class I ligand itself. We also present indirect evidence for spontaneous MHC class I acquisition in vivo, as well as describe an in vitro coculture system with transfected cells in which the same phenomenon occurred. Functional studies in the latter model showed that uptake of H-2Dd by Ly49A+ NK cells was accompanied by a partial inactivation of cytotoxic activity in the NK cell, as tested against H-2Dd-negative target cells. In addition, ligand acquisition did not abrogate the ability of Ly49A+ NK cells to receive inhibitory signals from external H-2Dd molecules. This study is the first to describe ligand acquisition by NK cells, which parallels recently described phenomena in T and B cells.
Keywords: immunology, cellular immunity, natural immunity, cell communication, biological adaptation
Introduction
NK cells constitute the third major subset of lymphocytes and kill tumors and virus-infected cells without prior sensitization (1). In contrast to T and B cells, NK cells do not express rearranged receptors recognizing a single antigen on the target cell. Instead, the NK cells use several germ-line encoded receptors, with either activating or inhibitory function, whose activities are balanced to control self tolerance and reactivity (2). The “missing self” hypothesis proposes that NK cells kill other cells because they fail to express a complete set of host MHC class I molecules (3). Molecular support for this hypothesis was obtained when Karlhofer and colleagues identified an inhibitory receptor on NK cells that bound an MHC class I molecule (4). Murine NK cells express inhibitory receptors of the Ly49 family, which consists of at least 13 members expressed on overlapping subsets of NK cells. By contrast, human NK cells express killer Ig-like receptors (KIRs)* belonging to the Ig superfamily (2).
Recently, much interest in immunology has focused on posttranscriptional membrane-related events occurring when immune effector cells meet their target cells. Borrowing terminology from the neurosciences, the interphase between effector and target has been termed the immunological synapse (5). Synapse formation occurs for all lymphocyte subsets, including NK cells (6–10). In T cells, synapse formation appears to increase the density of relevant molecules in the target cell contact to ensure sufficient stimulation of the T cell receptor by the few relevant MHC/peptide complexes that are presented by the APCs (5–7).
An interesting consequence of the formation of the immunological synapse in T and B cells is that the effector cell acquire target cell membrane molecules and incorporate them in its own membrane (10–12). This uptake is dependent on specific receptors on the effector cells and may lead to functional effects such as fratricide in T cells and increased antigen-presenting capability of B cells (10, 11). The phenomenon is also known from neurobiology, in which a similar transfer of proteins between cells has been shown to be part of an intercellular communication system, with functional consequences for the cells involved (13).
We previously developed an adoptive transfer model to study regulation of Ly49 receptors in vivo (14). In those studies, we were able to show that mature transferred NK cells rapidly interacted with host cells and downregulated their expression of Ly49A in the presence of the H-2Dd ligand. In this study, we show that transferred Ly49A+ NK cells rapidly acquired H-2Dd molecules from H-2Dd–expressing bystander cells in parallel with downregulation of cell surface Ly49A expression. We also show that this phenomenon is not unique to Ly49A, but occurred for several other inhibitory Ly49 receptors. Interestingly, the one activating Ly49 receptor we tested did not acquire MHC class I from surrounding cells, suggesting that inhibitory and activating MHC class I receptors may behave differently in this respect. In addition, evidence for ligand acquisition was found in normal unmanipulated mice, suggesting that ligand acquisition may be a spontaneously occurring event. Furthermore, we demonstrate that when the rat NK cell line RNK-16 transfected with the Ly49A gene was cocultured with surrounding cells expressing the H-2Dd ligand, a similarly rapid acquisition of H-2Dd into the cell membrane of the NK cells occurred. This acquisition was blocked by antibodies against either Ly49A or H-2Dd, but not by antibodies against other MHC class I molecules expressed by the surrounding cells. Our analysis of the functional consequences of the H-2Dd-acquisition in the latter system led us to two conclusions: (i) the ability of the Ly49A receptors to receive inhibitory signals from H-2Dd–expressing target cells was not abrogated after ligand acquisition; and (ii) ligand acquisition was paralleled by a downregulation of the general cytolytic capacity in the NK cells. From our data, we speculate that MHC class I acquisition may be part of a regulatory system, in which NK cell function is continuously modulated in vivo by interactions with surrounding cells expressing ligands for inhibitory Ly49 receptors.
Materials and Methods
Mice.
All mice were kept and bred at the Microbiology and Tumor Biology Center, Karolinska Institute, Stockholm, Sweden. C56BL/6 (B6) mice expressing Ly49A under a modified CD2-promoter, B6VA49A, have been described previously (15). Mosaic DL6 mice and control DL1 mice, which are B6 mice expressing a transgene consisting of the α1/α2 domains from H-2Dd and the α3 from H-2Ld, have been described previously (16). TAP1−/β2m− mice that lack MHC class I expression and express high levels of all known Ly49 receptors have also been described previously (17). All animal experiments were approved by the department as well as by the Committee for Animal Ethics in Stockholm, Sweden.
Cell Lines and Media.
The RNK-16 and RNK.Ly49A rat NK leukemia cell lines have been described previously (18). The cells were cultured in RPMI Glutamax (GIBCO BRL) supplemented with 50 μM 2-ME, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FCS in 5% CO2 at 37°C. The transfectants were maintained in 1 mg/ml G418 for selection purposes. All cells were mycoplasma free. EL-4 is a lymphoma of B6 origin. EL-4Dd cells were created by stable transfection of EL-4 with a genomic construct encoding the H-2Dd molecule (19). EL-4Dd cells were cultured in RPMI 1640 medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, and 10% FCS in 10% CO2.
Antibodies.
The mAbs 3–25.4 (anti-α1/α2 domains of H-2Dd; FITC-conjugated), 34–2-12S (anti-α3 domain of H-2Dd; FITC-conjugated), AF6–88.5 (anti-H-2Kb; biotinylated), SF1–1.1 (anti-H-2Kd; biotinylated), PK136 (anti-NK1.1; PE-conjugated), 4E5 (anti-Ly49D; FITC conjugated), and A1 (anti-Ly49A; biotinylated) were purchased from BD PharMingen. Goat anti–mouse IgG (H+L)-PE–conjugated, goat anti–mouse IgG+IgM (H+L)-FITC–conjugated, and goat anti–mouse F(ab)2-biotinylated antisera were purchased from Caltag, streptavidin-RED670 from Life Technologies, and streptavidin-PE from Molecular Probes. mAb 34–5-8S (anti-H-2Dd, α1/α2), 34–4-21S (anti-H-2Dd, α1/α2), 30–5-7 (anti–H-2Ld), 4D11 (anti-Ly49G2), A1 (anti-Ly49A), and 2.4.G2 (anti-FcRγ) were produced from their respective hybridoma lines. Purified 30–5-7 and 34–4-21S was biotinylated using Sulfo-NHS-LC Biotin (Pierce Chemical Co.) according to the manufacturer's instructions. The mAb 4LO3311 (anti-Ly49C) was a gift from Susanne Lemieux, INRS, Quebec, Canada.
Adoptive Transfer.
Nylon wool nonadherent (NWNA) spleen cells (2–5 × 107 cells) were inoculated intravenously into 4 Gy irradiated recipient mice. Spleens were recovered from the recipient mice 20 min–18 h later and separated over a nylon-wool column. NWNA splenic NK cells were either analyzed for Ly49 and MHC class I expression in FACS® analysis or sorted on a FACSvantage™ (Becton Dickinson). In Fig. 1 C, spleens were recovered after 18 h and cells were cultured in 1,000 U rIL-2/ml at 37°C. Cells were analyzed by FACS® at indicated time points.
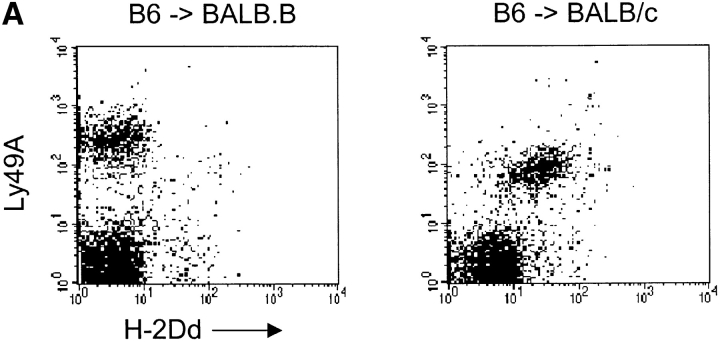
Figure 1.
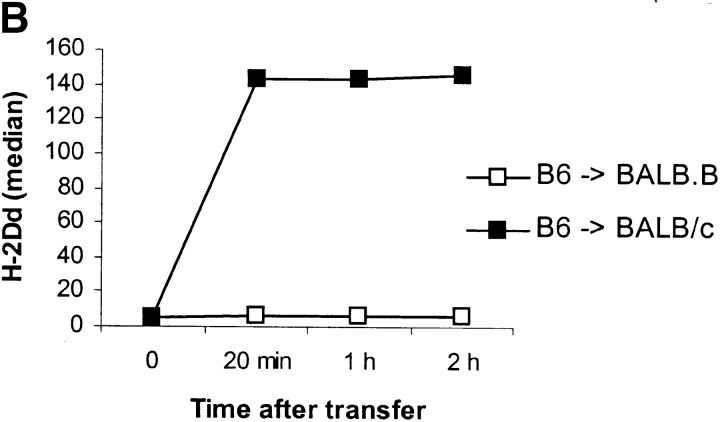
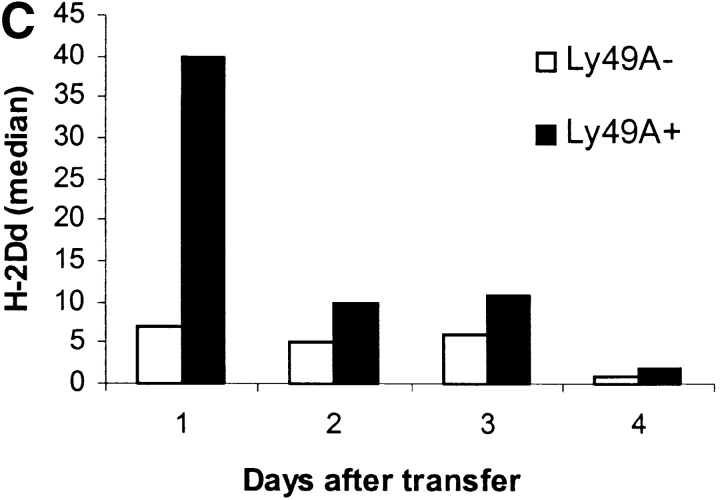
(A) Ly49A+ cells acquire H-2Dd after transfer into BALB/c mice. 20–50 × 106 NWNA splenocytes were inoculated into BALB.B and BALB/c, respectively. After 16–18 h, spleens were recovered and NK1.1+ cells were analyzed for their expression of Ly49A and H-2Dd. The density plots show cells gated on the NK1.1+ population. The figure shows one representative experiment out of 45. (B) H-2Dd is acquired at high levels 20 min after transfer of B6 cells into BALB/c. Spleens were recovered after transfer at indicated time points and H-2Dd expression on NK1.1+Ly49A+ B6 cells after transfer to BALB/c and BALB.B is shown. (C) Ly49A+ NK1.1+ cells lose H-2Dd expression after one night in culture. B6 cells were transferred into BALB/c mice and spleens were recovered after 18 h. Cells were cultured in rIL-2 and analyzed for H-2Dd expression at indicated time points.
Flow Cytometry Analysis after In Vivo Transfer.
106 cells per well were plated in V-bottomed 96-well plates. In all experiments cells were first incubated with anti-FcRγ Ab 20 min on ice to block Fc-binding receptors. Cells were then washed with PBS 1% FCS and incubated with various antibodies for 30 min–1 h, followed by washes in PBS 1% FCS. When secondary anti-IgG or anti-IgG plus IgM antibodies were used, this step was followed by a blocking step with normal mouse serum, used in a 1:25 dilution, before the next antibodies were added. After the final incubation, cells were washed and analyzed using a FACScan™ or FACSort™ (Becton Dickinson) with associated CELLQuest™ software.
Labeling of Cells with CFSE.
CFSE was purchased from Molecular Probes. Cells were diluted to 107 cells per milliliter in PBS with 1 μM CFSE, followed by 10-min incubation in 37°C and washed. Cells were then either inoculated intravenously or cocultured in vitro.
Cell Lysates and Immunoprecipitations.
NWNA B6VA49A, BALB/c cells, and sorted CFSE-labeled B6VA49A cells were surface biotinylated, using 0.5 mg/ml of EZ-Link™ Sulfo-NHS-LC-Biotin (Pierce Chemical Co.) in 50 μl PBS/0.5 × 106 cells. Cells were incubated on ice for 20 min and washed in PBS followed by a second biotinylation where fresh biotin was added. After the final biotinylation, the cells were washed three times with PBS supplemented with 50 mM NH4Cl, to remove unbound biotin. Cell lysates were made by incubating the cells with lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1mM EDTA, 10% glycerol, 1% NP-40, 1mM PMSF, 2 μg/ml leupeptin, aprotinin, and pepstatin) 20 min on ice, with occasional agitation. The lysates were cleared of debris by centrifugation at 14,000 rpm for 15 min. Precleared supernatant was immunoprecipitated with 5 μg anti–H-2Dd (34–5-8S) mAb conjugated to protein A-sepharose for 2 h at 4°C followed by 4–5 washes in washing buffer (50 mM Tris, 150 mM NaCl, 5 mM NaEDTA) with proteins (0.1% BSA) followed by 3–4 washes in washing buffer without proteins.
SDS-PAGE and Western Blot Analysis.
The samples were boiled at 95°C for 5 min in nonreducing sample buffer and run on a 10% SDS-PAGE. Proteins were then transferred to protran BA 85 cellulosenitrat(e) filter (Schleicher and Schuell). After the transfer, filter was blocked with 5% nonfat dry milk followed by 1-h incubation with 2 μg/ml streptavidin conjugated with HRP and washed extensively for 1 h in 1×TTBS solution (20 mM Tris, pH 7.5, 150 mM NaCl, 0.05% Tween 20). The membrane was developed using ECL Western blotting detection reagents (Amersham Pharmacia Biotech), following the instructions of the manufacturer.
Coculture Conditions, Antibody Stainings, and Blocking Experiments with RNK Cells.
To distinguish target cells from RNK effector cells in flow cytometry or in microscopy analysis, either cell type was first labeled with CFSE and used in subsequent NK/target cell coculture experiments. RNK-16 and RNK.Ly49A cells (1–2.5 × 105) were cocultured with EL4 or EL-4Dd cells at 1:1 ratio in flat-bottomed 24-well plates (Costar) for indicated time periods. In some experiments, an indirect staining procedure was employed. In this case, cells were harvested and labeled with the anti–H-2Dd antibody 34–5-8S followed by goat anti–mouse F(ab)2 biotin, and in a third step streptavidin-PE for flow cytometry and streptavidin-Texas Red for confocal microscopy (see below). All stainings and washing steps were done at 4°C. To block acquisition of H-2Dd, antibodies were added to the cocultures at the start of the culture. The following antibodies were used in the experiment shown in this paper: YE1/48 (anti–Ly49A, purified, 2 μg/well), 34–5-8S (anti-H-2Dd purified, 9 μg/well), B22 (anti-H-2Db, supernatant, 50 μl/well), Y3 (anti-H-2Kb, 9 μg/well). In other experiments, supernatant of 34–5-8S (anti-H-2Dd) was tested. It had the same blocking effect as the purified antibody (data not shown). In addition, a completely irrelevant antibody, 30–5-7 (anti-H-2Ld), was found to be without blocking effect (data not shown). In the blocking experiments, stainings to analyze acquisition of H-2Dd was done in one step using a FITC conjugated form of the antibody 34–2-12S, which recognizes the α3 domain of H-2Dd.
Confocal Microscopy.
Effector/target cocultures were prepared and stained as described previously. Cells were then allowed to settle onto poly-L-lysine coated coverslips, fixed in 2% paraformaldehyde for 15 min at 4°C, and finally mounted in Prolong™ antifade reagent (Molecular Probes). The slides were searched under an immunofluorescent microscope and cell morphology was checked by phase contrast. Digital images were acquired using a laser scanning confocal microscope ([LSCM] Multiprobe 2001; Molecular Dynamics). Images were captured with a z-step of 1 μm, and analyzed with accompanying software. A krypton/argon laser was used and images were collected at 488 nm (CFSE) and 568 nm (Texas-Red). The numerical aperture was 1.40 on an original magnification: 100× oil objective.
Cytotoxicity Assays.
RNK.Ly49A cocultures with EL4, EL4-Dd, or medium alone were analyzed by flow cytometry and RNK.Ly49A effector cells were isolated from the cell mixtures using FACSort™ technology gating on CFSE+ cells. To determine cytotoxic activity of the cocultured RNKLy49A cells, 51Cr release assays were performed as follows: YB2/0 and YB2/0-Dd target cells were labeled with 100 μCi Na2 51CrO4 (Nycomed Amersham PLC) for 1 h and washed. Sorted RNKLy49A effector cells were added in triplicate wells of U-bottomed 96-well microtiter plates followed by addition of 51Cr-labeled target cells. After 4 h of incubation at 37°C, 100 μl of the supernatant was harvested and its radioactive content was assayed in a γ-irradiation counter. The mean percentage of specific lysis of triplicate wells was calculated using the following formula: percentage of specific lysis = ((experimental release − spontaneous release)/(maximum release − spontaneous release)) × 100.
Results
Ly49A+ NK Cells from B6 Mice Acquire Host H-2Dd Molecules after Transfer to BALB/c Mice.
To study the influence of MHC class I molecules on NK cell Ly49-receptor expression, we previously developed an in vivo adoptive transfer protocol (14). To distinguish donor and host cells in this system, we used NK1.1-expressing B6 mice as donors, and mice negative for the NK1.1 marker, such as BALB/c and BALB.B, as recipients. Our analysis revealed that transferred NK cells rapidly downregulated their Ly49A expression when the H-2Dd ligand was present (14). We simultaneously observed that Ly49A+, but not Ly49A−, NK cells from B6 (H-2b) mice stained positive for H-2Dd at the cell surface after transfer into BALB/c mice (H-2d; Fig. 1 A). When B6 cells were transferred into BALB.B (H-2b), no H-2Dd staining was observed on the Ly49A+ cells, showing that the positive staining with the H-2Dd-specific mAb 3.25–4 was not caused by the transfer protocol itself or by insufficient electronic compensation of the flow cytometer (Fig. 1 A). The H-2Dd-staining on the B6 Ly49+ cells was not only seen with the 3.25–4 antibody, but also with H-2Dd-specific mAbs 34–2-12S, 34–4-21S and 34–5-8S (data not shown). Thus, the acquired H-2Dd molecules were seen using antibodies recognizing different epitopes, including three antibodies specific for the α1/α2 domains and one specific for the α3 domain (34–2-12S).
A kinetic analysis demonstrated that Ly49A+NK1.1+ B6 cells had acquired high levels of H-2Dd already 20 min after transfer (Fig. 1 B). To study how long time the acquired H-2Dd molecules stayed on the cell surface, recovered spleen cells from recipient mice were cultured at 37°C in the presence of in IL-2 and analyzed for H-2Dd expression at different time points. After 1 d of culture almost all H-2Dd molecules had disappeared from the cell surface of the Ly49A+NK1.1+ B6 cells (Fig. 1 C), showing that when surrounding H-2Dd–expressing cells are removed, the acquired molecules are quickly turned over and lost from the cells.
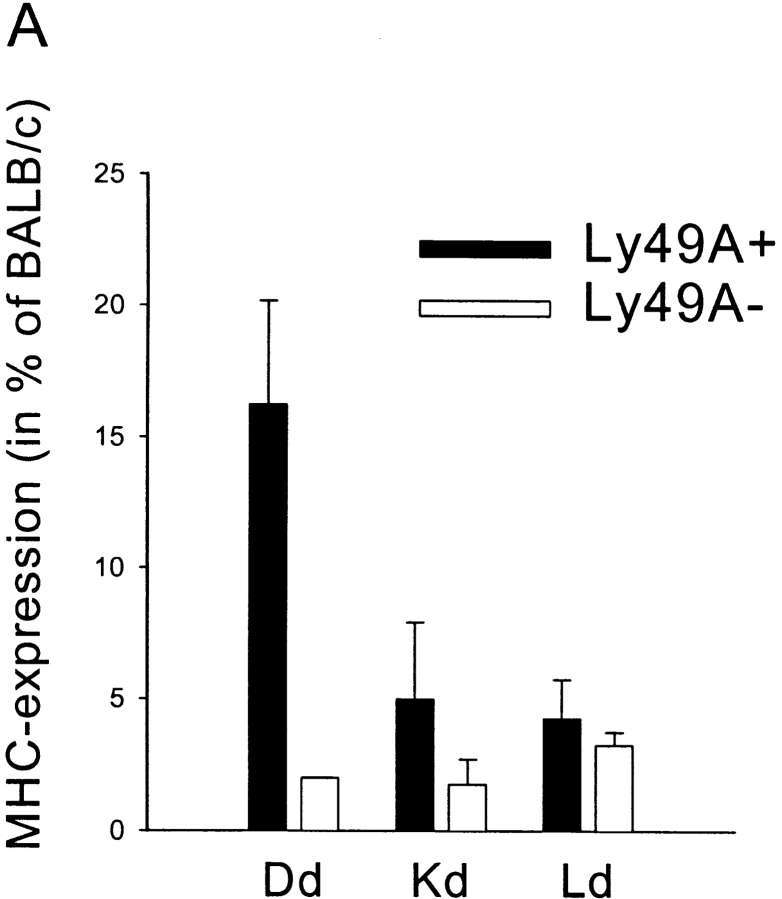
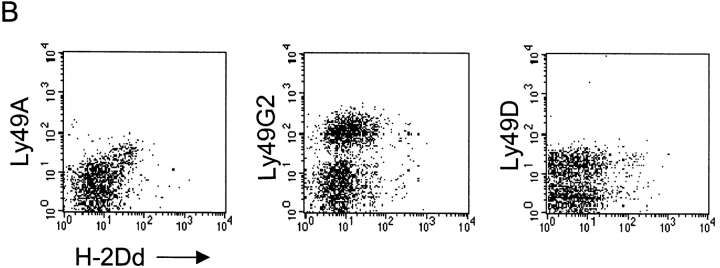
To investigate whether Ly49A+ cells could acquire any MHC class I molecule expressed in BALB/c mice or whether H-2Dd was unique, we looked at expression of all MHC class Ia molecules in the H-2d haplotype on Ly49A+ cells after transfer. H-2Dd was efficiently acquired by Ly49A+ NK cells (Fig. 2 A), resulting in a cell surface level on the NK cells which, as a mean, reached 16% of the H-2Dd expression on BALB/c splenocytes (100%). Acquisition of H-2Kd was much less pronounced (5% of BALB/c) but was still statistically significant, and that of H-2Ld even smaller (Fig. 2 A). Thus, H-2Dd was the main target for Ly49A+ NK cells, which correlates well with the fact that H-2Dd is a strong ligand for Ly49A (20). Conversely, we also wanted to see whether Ly49A was the only H-2Dd–binding Ly49 receptor that could acquire H-2Dd from surrounding cells after transfer. To study this, we analyzed two other receptors in the B6 haplotype that bind H-2Dd–Ly49G2 and Ly49D (21, 22). Like Ly49A, Ly49G2 is an inhibitory receptor, while the Ly49D receptor is activating. Our results showed a small shift in H-2Dd staining in the Ly49G2+ population compared with the Ly49G2−, at least in a subpopulation of the cells (Fig. 2 B). In contrast, Ly49D+ cells did not seem to differ at all from Ly49D- cells (Fig. 2 B).
Figure 2.
(A) Expression of H-2Dd, H-2Kd, and H-2Ld on NK1.1+ B6 cells after transfer to BALB/c mice. MHC class I expression is indicated as percentage of the respective MHC class I expression in BALB/c (100%). Each bar represent the mean value from four individual mice from two experiments and SDs are indicated as bars. Paired Student's t tests were used to compare the relative fluorescence intensities between Ly49A+ and Ly49A− cell populations after they had been normalized against BALB/c. P < 0.01 for H-2Dd, P < 0.05 for H-2Kd, and nonsignificant results for H-2Ld. (B) Comparison of H-2Dd acquisition among three different H-2Dd–binding Ly49 receptors. Density plots showing H-2Dd expression on Ly49A, G2, and D populations after B6->BALB/c transfer. All cells are gated on NK1.1.
The Acquired H-2Dd Protein Is of Similar Size as Endogenously Encoded H-2Dd Molecules from BALB/c.
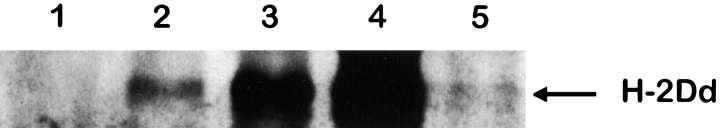
To determine the size of the transferred H-2Dd protein, we immunoprecipitated H-2Dd from transferred Ly49A+ cells. To obtain sufficient amounts of Ly49A+ cells with acquired H-2Dd molecules, we used B6 cells transgenic for Ly49A (B6VA49A) as donors. To distinguish the inoculated cells without staining them with antibodies, and thereby possibly interfering with the subsequent immunoprecipitation protocol, we labeled B6VA49A donor cells with the intracellular dye CFSE before inoculating them to BALB/c mice. 18 h after transfer, CFSE+ cells were sorted and surface biotinylated. We then lysed the cells and used an anti–H-2Dd antibody to immunoprecipitate the transferred protein. The proteins were separated on an SDS-PAGE gel followed by Western blot analysis, using streptavidin linked to HRP to detect the bands. We observed a band that migrated around 41 kD, corresponding to the size of the entire H-2Dd protein (Fig. 3, lane 2). A band of similar size was observed after immunoprecipitation from a BALB/c lysate, which corresponds to the entire H-2Dd protein (Fig. 3, lanes 3 and 4). To exclude that the immunoprecipitated molecules were derived from contaminating BALB/c cells, we included a control, in which B6VA49A cells were deliberately contaminated with 2% BALB/c cells (corresponding to the observed contamination of transferred B6VA49A cells after FACS® sorting in the experiment shown in Fig. 3) before lysis and immunoprecipitation. Although a band was seen in the contamination control (Fig. 3, lane 5), it was much weaker than the band in the lane containing the transferred cells. No band was observed in the sample with B6VA49A cells alone. These results indicate that we could immunoprecipitate acquired H-2Dd molecules on transferred B6VA49A cells, and that the acquired H-2Dd protein corresponded to a full-length molecule.
Figure 3.
Western blot analysis showing the H-2Dd protein from sorted B6VA49A cells after transfer to BALB/c. CFSE-labeled B6VA49A cells were transferred into BALB/c and sorted on a FACSVantage™. Immunoprecipitation with an anti–H-2Dd mAb was performed followed by Western blot analysis. 1, 1.5 × 106 untransferred B6VA49A cells; 2, 1.5 × 106 sorted B6VA49A cells after transfer to BALB/c; 3, 1.5 × 106 BALB/c cells; 4, 5.0 × 106 BALB/c cells; 5, mixture of 98% B6VA49A cells and 2% BALB/c cells (1.5 × 106 cells in total). The figure shows one experiment out of four.
MHC Class I Transfer and Receptor Downregulation also Occur for the Ly49C/H-2Kb Receptor–Ligand Pair.
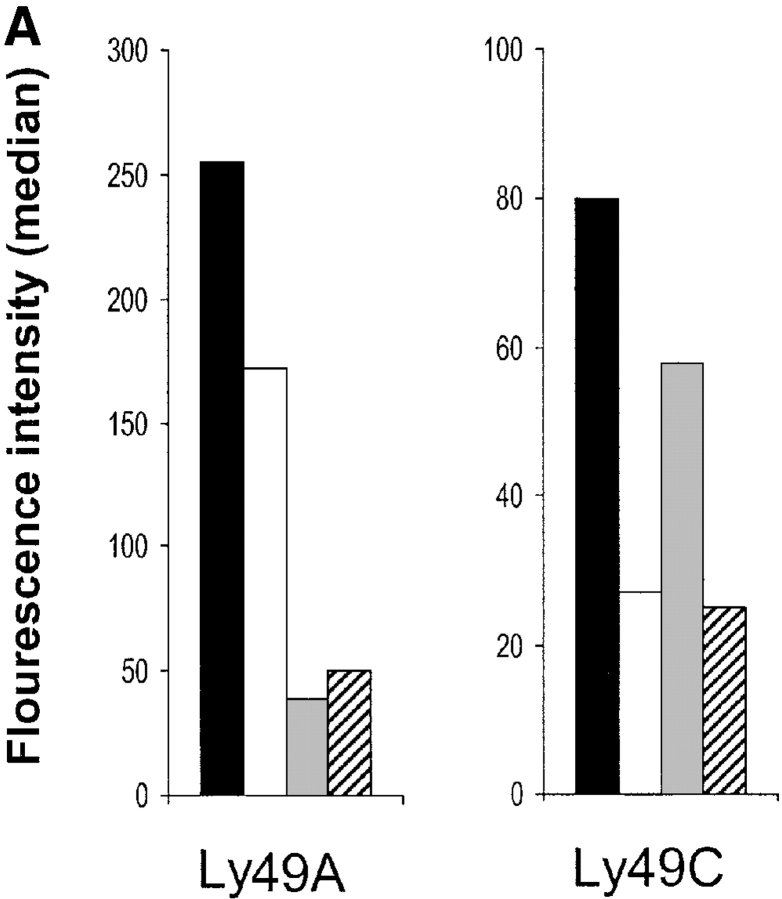
The result with H-2Dd–binding receptors did not conclusively demonstrate whether ligand acquisition was a general phenomenon for Ly49 receptors or unique to just a few. Therefore, we decided to extend our system further and look at Ly49C and its major ligand H-2Kb. For this purpose we used spleen cells from TAP1−/β2m− mice as donors, since they express high levels of Ly49C. After transfer of TAP1−/β2m− spleen cells to BALB.B and (BALB/c × BALB.B)F1 mice (both expressing H-2Kb), we found that the cell surface level of Ly49C was strongly downregulated on donor NK1.1+ cells (Fig. 4 A, right graph). A small downregulation was also seen in H-2Kb− BALB/c recipients. This downregulation was less pronounced but could result from interaction with a ligand for Ly49C on the H-2d background. In the same experiment, we confirmed our previous observation (14) that Ly49A expression was rapidly downregulated after inoculation to BALB/c recipients and also after inoculation to (BALB/c × BALB.B)F1 mice (Fig. 4 A, left graph). Downregulation of Ly49A in BALB.B was moderate but reproducible, possibly reflecting a weak interaction between Ly49A and an MHC class I molecule in the H-2b haplotype. Thus, Ly49A and Ly49C appears to be regulated similarly when they encounter neighboring cells expressing their specific ligands.
Figure 4.
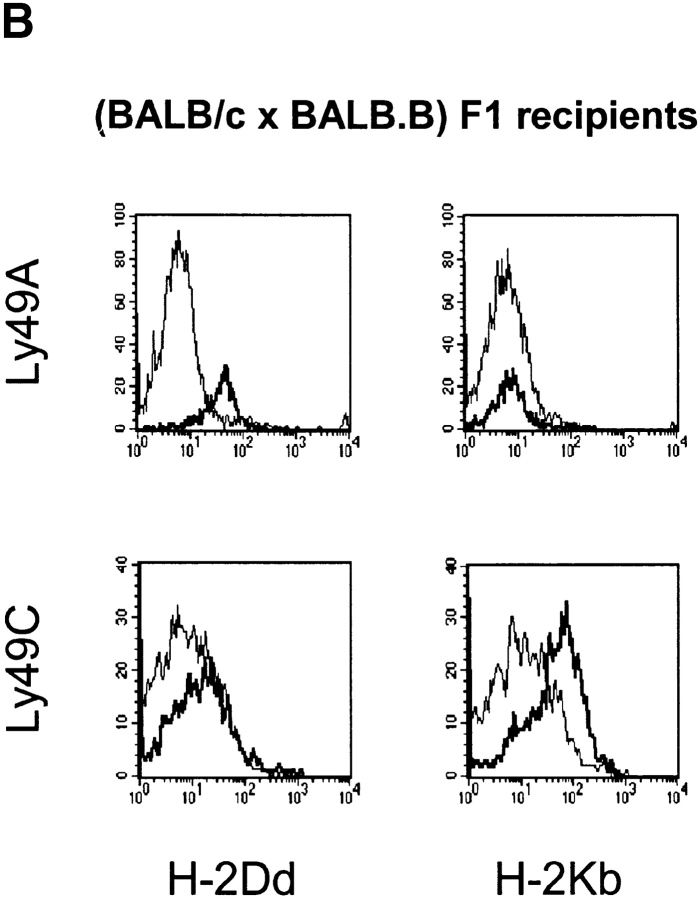
(A). Downregulation of Ly49A and Ly49C on TAP1−/β2m− NK cells transferred to BALB/c, BALB.B, and (BALB/c × BALB.B)F1 recipients. Black bars show median fluorescence intensity before transfer, white bars after transfer to BALB.b, gray bars after transfer to BALB/c and hatched bars after transfer to (BALB/c × BALB.B)F1. (B) TAP1−/β2m−/Ly49A+ cells acquire H-2Dd but not H-2Kb after transfer of NK cells to (BALB/c × BALB.B)F1 mice. In the same transfer, Ly49C+ NK cells acquire H-2Kb and some H-2Dd. Histograms show H-2Dd and H-2Kb expression on Ly49A+ (bold lines) and Ly49A− (thin lines) cells, and on Ly49C+ (bold lines) and Ly49C− (thin lines). All cells are gated on NK1.1. The figure shows one experiment out of four.
To investigate whether Ly49C+ NK cells would acquire H-2Kb from host cells and to analyze the specificity and selectivity of the protein uptake when two strong receptor/ligand pairs were compared, we inoculated TAP1−/β2m− spleen cells into (BALB/c × BALB.B)F1 recipients. As mentioned above, Ly49A and Ly49C receptors were both strongly downregulated (Fig. 4 A). This phenomenon was accompanied by an acquisition of host MHC class I molecules, which correlated with Ly49 receptor specificity. Thus, Ly49A+ NK cells stained positive for H-2Dd and Ly49C+ NK cells stained positive for H-2Kb (Fig. 4 B), while coacquisition of “irrelevant” MHC class I ligands was much less pronounced. For Ly49A+ NK cells, very little, if any, coacquisition of H-2Kb was seen compared with Ly49A− NK cells, while Ly49C+ cells did acquire an appreciable amount of H-2Dd compared with Ly49C− NK cells. Taken together, these results parallel receptor specificity as demonstrated in functional tests and in binding assays (4, 20, 23), and seem to correlate well with strong downregulation of the Ly49 receptor upon contact with the specific ligand on surrounding cells.
Ly49A+ NK Cells in Mosaic DL6 Mice Are Distributed Unequally between the H-2Dd/Ld-positive and H-2DdLd-negative Populations and Are Enriched in an H-2Dd/Ld Intermediate Population.
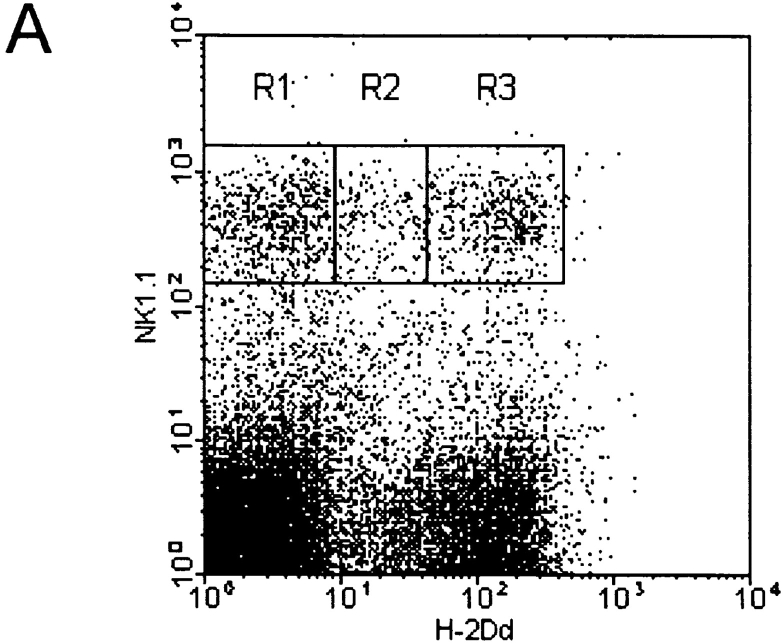
To investigate whether acquisition of exogenous H-2Dd molecules was a phenomenon seen only after transfer of splenocytes to irradiated recipients, or whether it could occur naturally in vivo, we took advantage of an MHC class I transgenic mouse that expresses an H-2Dd/Ld transgene in a mosaic fashion (16). The transgene in DL6 mice encodes a chimeric MHC class I molecule consisting of α1/α2 domains from H-2Dd and the α3 domain from Ld. The ratio of H-2Dd/Ld-positive to H-2Dd/Ld-negative cells vary between individual DL6 mice, but are constant in an individual mouse. Furthermore, the mosaic expression pattern is not the result of cell type–specific expression, but is seen in many different cell populations, probably reflecting a position variegation type of effect decided early in embryogenesis (24). Thus, the percentages of T and B cells in the H-2Dd/Ld-positive and H-2Dd/Ld-negative fractions in DL6 mice were similar to the percentages of T and B cells in the whole spleen. However, one exception to this was a small overrepresentation of NK cells in the H-2Dd/Ld-positive population compared with the H-2Dd/Ld-negative (16). In light of the present findings, we hypothesized that acquisition of exogenous H-2Dd/Ld molecules by H-2Dd/Ld-negative Ly49A+ NK cells could be the reason for this skewing. Indeed, Ly49A+ NK cells were more frequent within the H-2Dd/Ld-positive fraction compared with the H-2Dd/Ld-negative population (Fig. 5 A, gates R1 and R3). In addition, we had earlier discovered a population of H-2Dd/Ld intermediate cells, especially among NK1.1+ cells (Fig. 5 A, gate R2), which was difficult to interpret. Flow cytometric analysis revealed that Ly49A+ cells were even more overrepresented among NK1.1+ cells in the H-2Dd/Ld intermediate population, both compared with the H-2Dd/Ld-negative and to the H-2Dd/Ld-positive populations (Fig. 5 B). A mean of 48% of NK1.1+ cells in the intermediate fraction were Ly49A+, while the H-2Dd/Ld-negative and the H-2Dd/Ld-positive populations contained 18 and 30% Ly49A+ NK cells, respectively. Thus, relative to the H-2Dd/Ld-negative population, the increased percentage of cells in the H-2Dd/Ld intermediate population was almost threefold, and in the H-2Dd/Ld-positive population less than twofold. Although it is difficult to explain the accumulation of Ly49A+ NK cells in the H-2Dd/Ld-positive population, one way to interpret the strong accumulation of Ly49A+ NK cells in the intermediate population is to argue that Ly49A+ NK cells genetically determined to become H-2Dd/Ld-negative have acquired H-2Dd/Ld molecules from surrounding cells. Thus, apart from giving an explanation for the skewed distribution of NK cells between the H-2Dd/Ld-positive and H-2Dd/Ld-negative populations in DL6 mice (16), these results suggest that ligand acquisition may occur in vivo independent of an artificial cell transfer protocol, and may take place spontaneously when NK cells encounter surrounding cells expressing the ligand. As a control for the results on Ly49A, we analyzed the percentage of Ly49C+/I+ and Ly49G2+ NK cells in the same populations. Ly49C/I did not differ (44 compared with 43%), while Ly49G2 showed a minor increase (68 compared with 59%) in the H-2Dd/Ld intermediate population compared with the H-2Dd/Ld-negative (data not shown). Overall, this pattern correlates well with the observations on H-2Dd acquisition seen in the adoptive transfer model.
Figure 5.
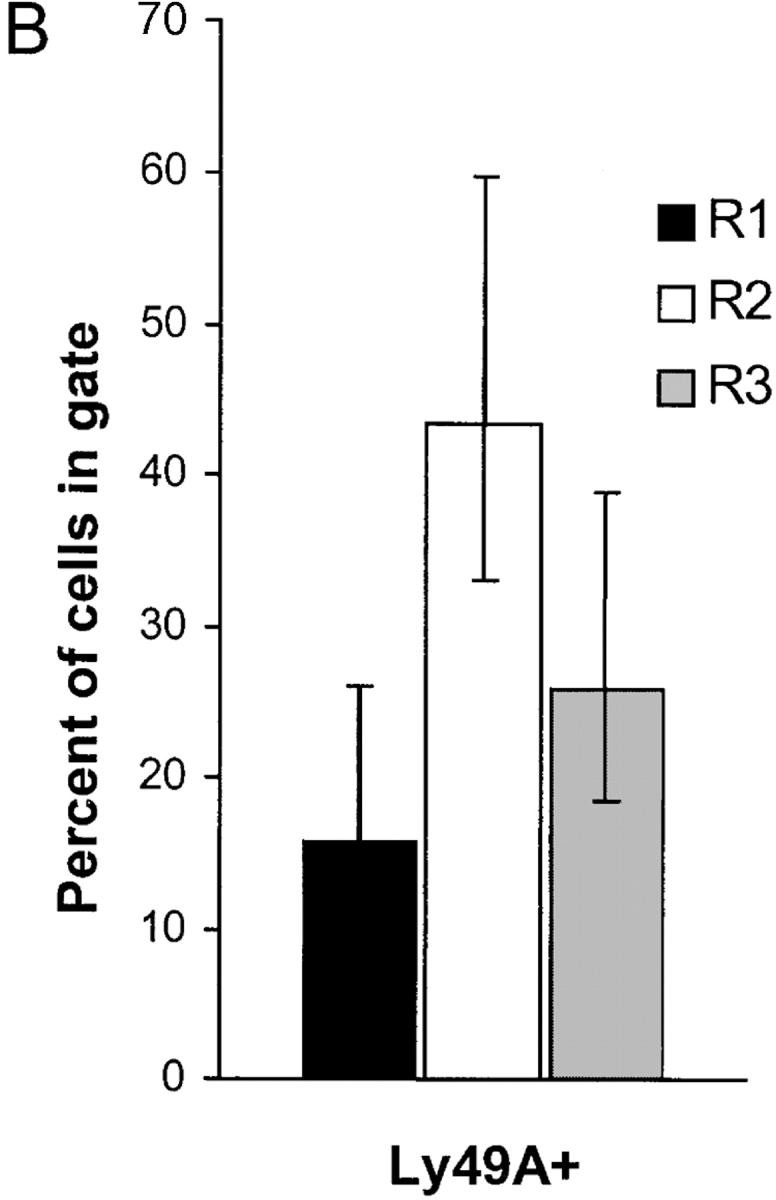
(A) Staining of mosaic DL6 mice with NK1.1 and H-2Dd reveals three populations with different H-2Dd levels; a low (R1), intermediate (R2), and a high (R3) population. (B) Percentage of Ly49A+ cells among NK1.1+ cells in the three populations. The graph shows a summary of 16 consecutive experiments including 16 individual mice. Error bars show SD. Paired Student's t tests were used to compare the amount of Ly49A-positive cells in the intermediate population R2 compared with the negative population R1 (P < 0.001) and between the intermediate population R2 compared with the positive population R3 (P < 0.001).
Ly49A+ Cells Expressing Endogenous H-2Dd Molecules Acquire External H-2Dd after Transfer to BALB/c Mice.
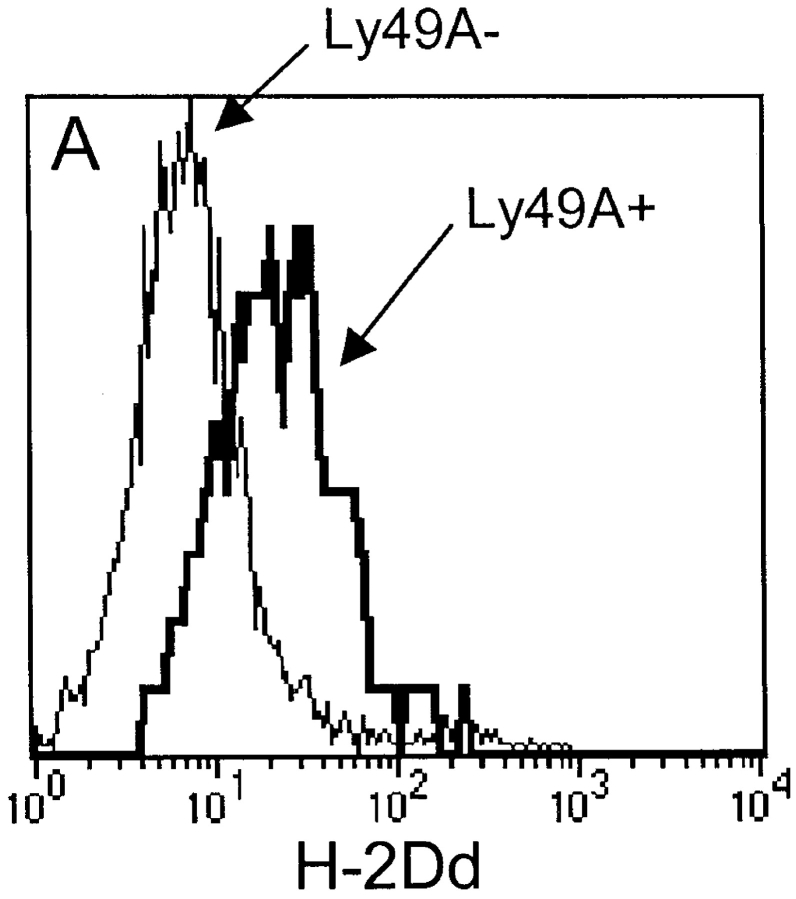
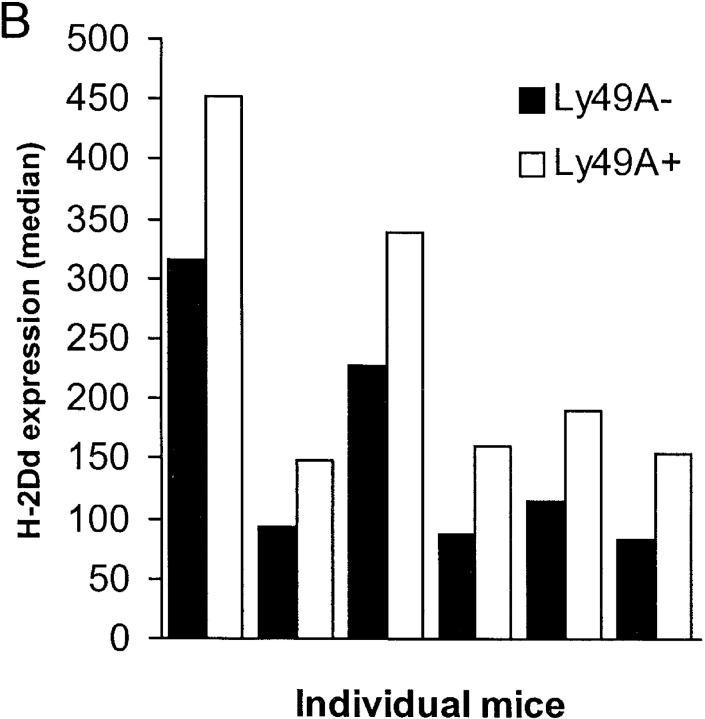
In the experiments discussed so far, we had investigated the H-2Dd uptake by H-2Dd- cells expressing Ly49A. This could represent a different situation compared with normal in vivo conditions, in which NK cells express the same MHC molecules as their surrounding cells. To test whether Ly49A+ NK cells expressing H-2Dd themselves were also able to acquire H-2Dd, we transferred spleen cells from DL1 transgenic mice into BALB/c recipients. DL1 mice express the same chimeric transgene as DL6 mice, and we could therefore use an antibody specific for the α3 region from H-2Dd to differentiate between recipient and donor H-2Dd molecules. The results showed that Ly49A+ DL1 cells were indeed able to acquire H-2Dd from the host, even though they expressed H-2Dd on their own cell surface (Fig. 6 A). If Ly49A+ NK cells were able to acquire H-2Dd even if they expressed H-2Dd themselves, we hypothesized that Ly49A+ NK cells in H-2Dd+ mice would have higher H-2Dd expression than would Ly49A− NK cells. In six individual mice analyzed, Ly49A+ NK cells expressed more H-2Dd than did Ly49A− NK cells (Fig. 6 B). This is consistent with, but does not prove, the idea that Ly49A+ NK cells acquire H-2Dd molecules from surrounding cells also in a normal mouse. However, other explanations are possible, i.e., a longer half life of the H-2Dd molecules as a result of binding to Ly49A at the cell surface.
Figure 6.
(A) Acquisition of H-2Dd by DL1 NK cells after transfer to BALB/c mice. Histogram overlays show H-2Dd expression on Ly49A+ (bold line) and Ly49A− (thin line) DL1 cells after transfer to BALB/c. H-2Dd expression on DL1 cells is measured with a mAb recognizing α3 of H-2Dd in order to distinguish H-2Dd derived from BALB/c since DL1 cells express a chimeric H-2Dd molecule containing α3 from H-2Ld. (B) H-2Dd expression on Ly49A+ and Ly49A− NK cells from H-2Dd transgenic D8 mice. The figure shows six individual mice. In both A and B, cells gated on NK1.1+ cells.
The Rat NK Tumor Cell Line RNK-16 Transfected with Ly49A Acquires H-2Dd after Coculture with an H-2Dd–expressing Tumor Cell Line In Vitro.
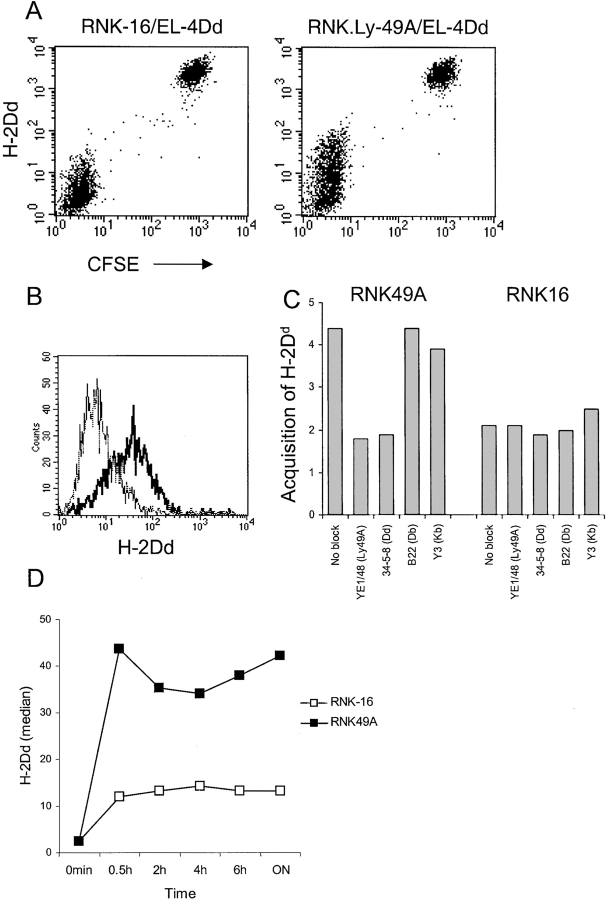
All previous results were generated in vivo and we were interested to see whether we could observe this phenomenon in an independent and more defined in vitro system. The rat NK cell line RNK-16, either untransfected or transfected to express Ly49A (RNK.Ly49A) was cocultured with EL-4 cells transfected with H-2Dd (EL-4Dd). EL-4Dd cells were stained with CFSE to make the different cell types easily distinguishable by flow cytometry. Our results showed that RNK.Ly49A cells acquired H-2Dd from EL-4Dd cells, while nontransfected RNK-16 cells without Ly49A did not (Fig. 7 A). To verify that the H-2Dd staining on RNK.Ly49A cells was not due to an abnormal property of RNK.Ly49A cells to passively adsorb the H-2Dd antibody, we compared RNK.Ly49A cells cultured with EL-4Dd with RNK.Ly49A cells cultured alone or with nontransfected EL-4 cells. H-2Dd staining was only seen when RNK.Ly49A cells were cocultured with EL-4Dd (Fig. 7 B), showing that H-2Dd staining on RNK.Ly49A was a consequence of coculture with cells positive for H-2Dd.
Figure 7.
(A) Acquisition of H-2Dd on RNK.Ly49A cells. EL-4Dd cells were labeled with CFSE and cocultured overnight with RNK.Ly49A or RNK-16 effector cells and analyzed by flow cytometry using anti–H-2Dd mAb plus goat anti–mouse biotin antiserum plus streptavidin-PE, as described previously. Panels show H-2Dd stainings of cell mixtures containing EL-4Dd cells cocultured with RNK-16 cells (left) or RNK.Ly49A (right), respectively. One experiment of five. (B) H-2Dd staining of RNK.Ly49A cells cultured overnight alone (dotted line), together with EL-4 (thin solid line) or together with EL-4Dd (thick solid line). (C) Relative acquisition of H-2Dd by RNK.Ly49A and RNK-16 cells after cocultures with EL-4Dd in the presence of antibodies against Ly49A or either one of the classical MHC class I molecules expressed on EL4-Dd: H-2Kb, H-2Db, and H-2Dd. Acquisition of H-2Dd by RNK.Ly49A was only prevented when antibodies against either Ly49A or H-2Dd was added to the culture. H-2Dd staining is plotted relative (fold-increase) to the staining of RNK.Ly49A or RNK-16 cells cultured alone in the same experiment. (D) Kinetics of H-2Dd uptake. Median values of H-2Dd staining on gated RNK-16 or RNK.Ly49A cells after coculture with EL-4Dd cells for various time periods. One experiment of four with similar results.
To further study if the acquisition of H-2Dd was dependent on the interaction between Ly49A and H-2Dd, we added antibodies against H-2Dd and Ly49A to the coculture. Acquisition of H-2Dd was completely abrogated when antibodies to H-2Dd or Ly49A were added to the in vitro cultures (Fig. 7 C), while antibodies against the other MHC class I molecules expressed by EL-4Dd, i.e. H-2Db and H-2Kb, had no effect on the acquisition of H-2Dd (Fig. 7 C). This results shows that the interaction between Ly49A and H-2Dd was necessary for the acquisition of H-2Dd to occur. Kinetic analysis showed that H-2Dd was detected on RNK.Ly49A cells already after 30 min of coculture with EL-4Dd, and that it remained higher than on Ly49A− RNK-16 control cells throughout the experiment (Fig. 7 D). The kinetic data obtained with RNK.Ly49A cells thus resembles the kinetics seen in the adoptive transfer model (Fig. 1 B) and emphasizes that the acquisition is a rapid event.
We reproducibly observed a minor shift in staining intensity on nontransfected RNK-16 cells after coculture with EL-4Dd (Fig. 7 D, white squares), although the intensity of this staining was much lower compared with the staining on RNK.Ly49A cells cultured under similar conditions. One explanation for this could be that a small amount of H-2Dd molecules are indeed transferred from EL-4Dd to RNK-16 cells without the help of a specific receptor. Alternatively, RNK-16 cells may express a rat receptor with specificity for H-2Dd which would be responsible for the uptake. In any case, the low background staining on nontransfected RNK-16 cells does not affect the interpretation of Ly49A as a receptor acquiring H-2Dd from surrounding cells also in vitro.
The Acquired H-2Dd Molecules Are Distributed in a Patch-like Pattern on the Cell Surface.
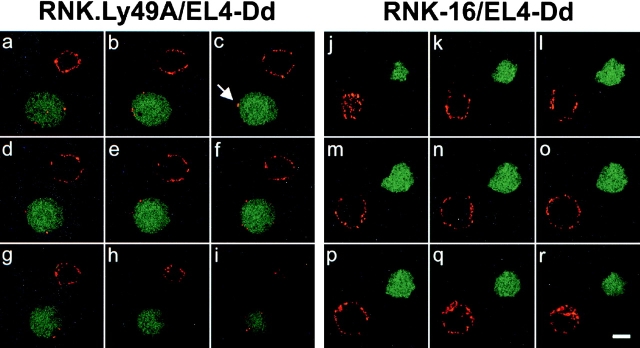
To visualize the H-2Dd acquisition and to analyze the distribution on the cell surface, i.e. to see whether the H-2Dd molecules were localized to a singular cap or evenly distributed over the membrane surface, we analyzed the RNK.Ly49A cells cocultured with EL-4Dd in a LSCM. To distinguish the two cell types in the confocal microscope we labeled the RNK.Ly49A with CFSE (green) before coculture, and then stained all cells for H-2Dd (red). RNK cells could then be identified as completely green cells, whereas the EL-4Dd cells obtained a red surface staining. We found that RNK.Ly49A cells acquired H-2Dd-molecules in a patch-like pattern on the cell surface, as shown by the red H-2Dd-dots on the green RNK.Ly49A cells (Fig. 8 a-i). In contrast, RNK-16 cells did not acquire the red H-2Dd-dots on their cell surface (Fig. 8 j-r), demonstrating that the Ly49A receptor had to be present in order for the H-2Dd acquisition to occur. When only biotinylated secondary and Texas Red-conjugated tertiary antibodies were used or when an irrelevant IgG2a-antibody (PK136) was used as the primary antibody, no red staining was observed on either RNK.Ly49A, RNK-16, or EL-4Dd (data not shown). The cells were analyzed by collecting 1-μm thick serial cross sections, with the digital images representing one section each. Some weak red dots were sometimes observed on a fraction of the RNK-16 cells when analyzed by fluorescence microscopy, an observation that correlated with the weak background staining discussed in the previous section. These dots were never bright enough to be detected by the LSCM. By counting the number of H-2Dd dots on RNK.Ly49A cells under UV-light, we found that 77% of the cells (100 cells scored) displayed five bright dots or more, while only 5% of the RNK-16 cells had more than five dots and, which were weaker in intensity.
Figure 8.
RNK.Ly49A cells acquire H-2Dd-molecules in a patch-like pattern on the cell surface. CFSE (green) labeled RNK.Ly49A (a-i) and RNK-16 (j-r) cells were cocultured with unlabeled EL-4Dd cells. All cells were stained with α-H-2Dd (red) mAb. The cells were analyzed on a LSCM and displayed as a series of sequential cross-sections. Each image represents one RNK and one EL-4Dd cell. Acquired H-2Dd-molecules are observed as red dots on the green RNK.Ly49A cells, as indicated by the arrow in c. Scale bar is 5 μm.
RNK.Ly49A Cells that Have Acquired H-2Dd Molecules Showed Reduced Killing against YB2/O Tumor Cells but Were Still Inhibited by H-2Dd Molecules Expressed by Target Cells.
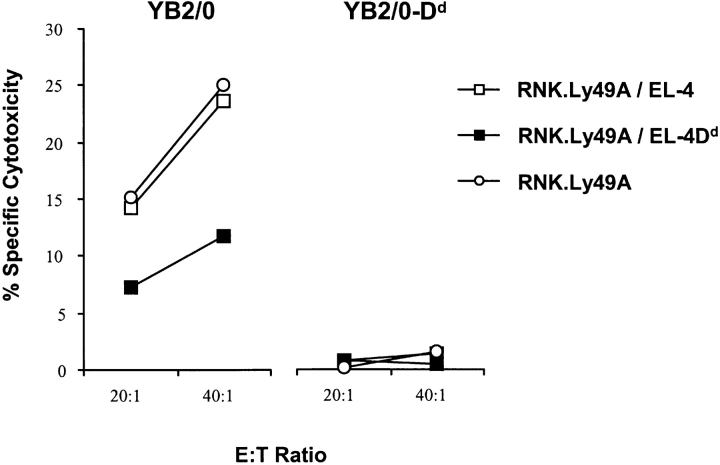
RNK.Ly49A cells were cultured with EL-4, EL-4Dd, or in media alone and were subsequently sorted by FACS® and used as effector cells in cytotoxicity assays. Surprisingly, RNK.Ly49A cells cultured with EL-4Dd killed the NK sensitive YB2/0 tumor cell line less well than did RNK.Ly49A cells that were cultured with EL-4 or with media alone (Fig. 9). However, some killing remained unless the target cell also expressed H-2Dd. In this case, complete inhibition of killing was seen, showing that RNK.Ly49A cells with acquired H-2Dd molecules on the cell surface were still able to receive inhibitory signals by H-2Dd on target cells (Fig. 9).
Figure 9.
Reduced cytotoxic activity by sorted RNK.Ly49A cells after acquisition of H-2Dd. Lysis of 51Cr-labeled YB2/0 cells and YB2/0-Dd cells by sorted RNK.Ly49A cells cocultured overnight with EL-4 (white squares), EL-4Dd (black squares), and no cells (white circles). The figure shows one representative experiment of four.
Discussion
Circumstantial evidence for transfer of MHC molecules between cells of the immune system was reported already 30 y ago (25, 26). More recent data have conclusively shown that effector T cells acquire both MHC class I and II molecules from APC (11, 12, 27). Also, B cells acquire membrane antigens from surrounding cells, a phenomenon which is followed by a very efficient presentation of these antigens to T cells (10). We show here for the first time that receptor-mediated ligand acquisition occurs also for NK cells, both in vitro and in vivo. Inhibitory Ly49 receptors on NK cells specifically acquired MHC class I molecules from surrounding cells, both when normal NK cells were transferred into a host expressing the ligand for the Ly49 molecule analyzed, and also in vitro when cells transfected with Ly49A were cocultured with cells transfected with H-2Dd. Ligand acquisition was a very rapid event, occurring within 30 min in both systems. The amount of acquired molecules remained high as long as the surrounding cells were present. However, after separation from surrounding cells the NK cells rapidly lost the acquired molecules, suggesting that in order to maintain high levels of acquired molecules, continuous interactions with cells in the environment were necessary. In addition, we present results consistent with the idea that Ly49A+ NK cells spontaneously acquire H-2Dd from surrounding cells in vivo in mice that have not been experimentally manipulated (Figs. 5 and 6). This is important, since all studies on ligand acquisition by B and T cells published so far are based on in vitro experimentation.
MHC class I acquisition in NK cells was related to the specific interaction between the Ly49 receptor and its MHC class I ligand, with some exceptions. For example, Ly49A+ NK cells acquired mainly H-2Dd, but in some cases, we observed a small and parallel acquisition of H-2Kd. A similar coacquisition of H-2Kb and H-2Dd was seen for Ly49C+ NK cells after transfer to (BALB/c × BALB.B)F1 mice. Coacquisition of several MHC class I molecules could be due to specific interactions between the receptor under study and all the acquired ligands. Alternatively, other Ly49 receptors on the NK cell could bind to and acquire additional MHC class I molecules. Coacquisition could also be the result of an unspecific membrane transfer, additional MHC class I molecules being absorbed as a bystander effect. We do not favor this explanation for the following reasons: if acquisition in our transfer experiments resulted from membrane fusions involving large chunks of membrane (27), then all MHC class I molecules would be equally cotransferred; however, for Ly49A+ NK cells, we did not find evidence for uptake of the third MHC class Ia molecule in the H-2d haplotype, H-2Ld, despite strong acquisition of H-2Dd and weak acquisition of H-2Kd in the same experiment. In addition, Ly49A+ NK cells did not coacquire H-2Kb when TAP1−/β2m− donor cells were transferred to (BALB/c × BALB.B)F1 mice and RNK.Ly49A did not acquire Kb or Db expressed on EL-4Dd (unpublished data). Finally, experiments in which surrounding H-2Dd+ cells were stained with the membrane dye DiI and cocultured with Ly49A+ NK cells did not reveal transfer of dye to the NK cell despite strong acquisition of H-2Dd from the DiI+ cells (unpublished data). Therefore, we favor the idea that all acquisitions are receptor specific, either resulting from broad specificity of the Ly49 receptor under study or from coexpression of additional receptors on the NK cell.
The amount of MHC class I ligand that was acquired by Ly49A was substantial, sometimes reaching 20% of the MHC class I expression on surrounding cells. When several receptors were analyzed, we found that the amount of acquired molecules varied between different receptors. Ly49A+ cells always picked up a large amount of H-2Dd, Ly49C+ cells a large amount of H-2Kb, Ly49G2+ NK cells much less H-2Dd, and Ly49D+ NK cells no H-2Dd at all. The difference between Ly49G2 and Ly49A could reflect differences in affinities for H-2Dd. Hanke et al. have demonstrated that H-2Dd tetramers stained NK cells expressing Ly49A+ stronger than they stained Ly49G2+ NK cells, supporting the idea of differences in the strength of binding between H-2Dd and these two receptors (20). It is not known how differences in Ly49 receptor affinity affect NK cell function, but our previous and present data suggest that difference in binding strength is associated with reduced amount, but not absence, of acquired MHC class I ligand after in vivo transfer (this study), and a small but reproducible receptor downregulation in the presence of H-2Dd (28).
Huard et al. recently showed that an inhibitory KIR was downregulated on human NK cells after coculture with surrounding MHC class I+ cells, while an activating form of the same KIR receptor was not downregulated (29). Similarly, we found no downregulation and no H-2Dd acquisition of the activating Ly49D receptor in this study. An interesting possibility inferred from these results is that downregulation and ligand acquisition by MHC class I–binding receptors on NK cells may depend on the presence of an immunoreceptor tyrosine-based inhibitory motif in the intracellular part of the receptor.
To directly analyze the effects of ligand acquisition on NK cell function, we sorted RNK.Ly49A cells from the cocultures and tested their killing capacity against tumor targets. We found, surprisingly, that RNK.Ly49A NK cells that had acquired H-2Dd displayed reduced killing against H-2Dd-negative YB2/0 cells. One explanation for these results is that acquired H-2Dd molecules somehow associate with Ly49A molecules at the NK cell surface and trigger Ly49A signaling, with a paradoxical Ly49A-mediated inhibition of lysis against target cells lacking H-2Dd as a result. MHC class I molecules on the NK cell itself has previously been suggested to modulate Ly49A expression (14), and a cis interaction between Ly49A and H-2Dd is also inferred from structural studies (30). However, an equally likely hypothesis is that the reduced killing against YB2/0 targets could result from codownmodulation of activating receptors involved in NK cell triggering. Such a mechanism is not without precedent, since it was recently shown to be responsible for decreased NK cell killing after coculturing human KIR-expressing NK cells with tumor cells expressing specific MHC class I ligands (29).
RNK.Ly49A NK cells do not themselves express H-2Dd, which is an advantage in that it allows an isolated analysis of the interactions between Ly49A and external H-2Dd molecules. However, the lack of H-2Dd on RNK.Ly49A cells is also a point of concern since it represents a different situation compared with physiological in vivo situations, in which NK cells with useful Ly49 receptor will always express ligands for those receptors themselves. It has previously been suggested that Ly49A and H-2Dd interact in cis on the NK cell itself (14, 30). It could be argued that such cis interactions, either with endogenously synthesized H-2Dd molecules or with H-2Dd molecules acquired from other cells, would occupy Ly49A receptors and change the rules for interactions with external H-2Dd molecules. Our experimental models have not allowed us to critically test this possibility. However, our data demonstrate that normal H-2Dd+Ly49A+ NK cells were able to acquire external H-2Dd molecules in vivo (Fig. 6). Such NK cells are also efficiently inhibited by target cells expressing H-2Dd (31) as are RNK.Ly49A NK cells with acquired H-2Dd molecules (Fig. 9). Thus, cell surface expression of H-2Dd by the NK cell itself is still compatible with Ly49A/H-2Dd interactions in trans. We believe that cis and trans interactions between Ly49A and H-2Dd follow different and independent rules and that these two possible types of interactions are not mutually exclusive.
Irrespective of the mechanism involved in the reduced killing of YB2/0 targets by RNK.Ly49A cells with acquired H-2Dd molecules, our results need to be reconciled with data suggesting that MHC class I molecules on surrounding cells do not inhibit NK cells in trans, i.e. in cold target inhibition experiments (32, 33). In this respect, it may be important that we assay the lytic capacity of the cocultured NK cells after an overnight incubation with MHC class I+ targets, while classical cold target inhibition experiments are usually performed in assays ≤4 h. In addition, hot and cold targets are usually added simultaneously at the start of the latter assay (32). Thus, in a classical cold target inhibition experiment, it may be that the time interval between interactions with cold and hot targets is too short in order for a mechanism similar to the one operating in our system to develop.
Confocal microscopy showed that acquired MHC class I molecules aggregated in small dots in the NK cell membrane. Our experimental setup did not allow us to conclude whether each effector/target interaction resulted in only one dot, or whether several aggregates were transferred in each interaction. The latter seems to be the case in 2C T cells during acquisition of H-2Ld molecules from APC (11). Why are the acquired MHC class I molecules aggregating in dots at the cell surface? An interesting possibility is that acquired MHC class I molecules, as reported for MHC class II molecules (34), are concentrated in lipid rafts in the NK cell membrane. Their recruitment to subsequent target cell interaction sites where they may modulate activating signals by interfering with Ly49 receptor signaling, as discussed previously, may thus be facilitated (9). Interestingly, H-2Dd appeared in patches, albeit larger, also on EL-4Dd cells, indicating that H-2Dd molecules were unevenly distributed at the cell surface also on those cells. Similar findings have been reported previously (35, 36).
Finally, it should be noted that MHC class I acquisition may have other consequences for the NK cell. For T cells, it was shown that MHC class I uptake can render the T cells sensitive to killing by surrounding T cells with the same specificity, i.e. fratricide (11). Perhaps in a similar way, the transfer of MHC class I molecules to NK cells at the site of an infection could serve to protect them from neighboring NK cells attacking infected cells. Another possibility is that NK cells may act to spread processed antigen by picking up MHC class I/peptide complexes from other cells and either delivering them at a distant site or acting as APC themselves. NK cell Ly49 receptors are expressed by 15–60% of NK cells, they are not peptide-specific and they are constantly interacting with MHC complexes on surrounding cells (14). Thus, NK cells can potentially contribute to the spread of a large variety of MHC/peptide complexes, at least under normal conditions and in the early phase of the immune responses.
Acknowledgments
We thank Margareta Hagelin and Maj-Britt Alter for expert assistance with in vivo experiments and all the staff in the animal house for taking care of the mice. We would also like to thank Laszlo Szekely and Agneta Richter-Dahlfors for help with microscopy, Birgitta Wester for help with FACS® sorting, James Ryan for providing the RNK-16 cells, and all members of the Höglund and Kärre laboratories for fruitful discussions.
This work was supported by grants to A. Sjöström from the Swedish Society for Medical Research, and to P. Höglund from the Karolinska Institute, the Swedish Cancer Society, Cancer Research Institute (USA), the Wenner-Gren Foundations, and the Åke Wiberg Foundation.
Footnotes
Abbreviations used in this paper: APC, allophycocyanin; NWNA, nylon wool nonadherent; LSCM, laser scanning confocal microscope; KIR, killer inhibitory receptor.
References
- 1.Yokoyama, W.M. 1999. Natural Killer Cells. 4th edition. Fundamental Immunology. P. Ewe, editor. Lippincott-Raven, Philadelphia. 575–603 pp.
- 2.Lanier, L.L. 1998. NK cell receptors. Annu. Rev. Immunol. 16:359–393. [DOI] [PubMed] [Google Scholar]
- 3.Ljunggren, H.G., and K. Kärre. 1990. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today. 11:237–244. [DOI] [PubMed] [Google Scholar]
- 4.Karlhofer, F.M., R.K. Ribaudo, and W.M. Yokoyama. 1992. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 358:66–70. [DOI] [PubMed] [Google Scholar]
- 5.Grakoui, A., S.K. Bromley, C. Sumen, M.M. Davis, A.S. Shaw, P.M. Allen, and M.L. Dustin. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science. 285:221–227. [DOI] [PubMed] [Google Scholar]
- 6.Viola, A., S. Schroeder, Y. Sakakibara, and A. Lanzavecchia. 1999. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 283:680–682. [DOI] [PubMed] [Google Scholar]
- 7.Monks, C.R., B.A. Freiberg, H. Kupfer, N. Sciaky, and A. Kupfer. 1998. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 395:82–86. [DOI] [PubMed] [Google Scholar]
- 8.Davis, D.M., I. Chiu, M. Fassett, G.B. Cohen, O. Mandelboim, and J.L. Strominger. 1999. The human natural killer cell immune synapse. Proc. Natl. Acad. Sci. USA. 96:15062–15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lou, Z., D. Jevremovic, D.D. Billadeau, and P.J. Leibson. 2000. A balance between positive and negative signals in cytotoxic lymphocytes regulates the polarization of lipid rafts during the development of cell-mediated killing. J. Exp. Med. 191:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batista, F.D., D. Iber, and M.S. Neuberger. 2001. B cells acquire antigen from target cells after synapse formation. Nature. 411:489–494. [DOI] [PubMed] [Google Scholar]
- 11.Huang, J.F., Y. Yang, H. Sepulveda, W. Shi, I. Hwang, P.A. Peterson, M.R. Jackson, J. Sprent, and Z. Cai. 1999. TCR-mediated internalization of peptide-MHC complexes acquired by T cells. Science. 286:952–954. [DOI] [PubMed] [Google Scholar]
- 12.Hwang, I., J.F. Huang, H. Kishimoto, A. Brunmark, P.A. Peterson, M.R. Jackson, C.D. Surh, Z. Cai, and J. Sprent. 2000. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J. Exp. Med. 191:1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cagan, R.L., H. Kramer, A.C. Hart, and S.L. Zipursky. 1992. The bride of sevenless and sevenless interaction: internalization of a transmembrane ligand. Cell. 69:393–399. [DOI] [PubMed] [Google Scholar]
- 14.Käse, A., M.H. Johansson, M.Y. Olsson-Alheim, K. Kärre, and P. Höglund. 1998. External and internal calibration of the MHC class I-specific receptor Ly49A on murine natural killer cells. J. Immunol. 161:6133–6138. [PubMed] [Google Scholar]
- 15.Fahlen, L., L. Öberg, T. Brännstrom, N.K. Khoo, U. Lendahl, and C.L. Sentman. 2000. Ly49A expression on T cells alters T cell selection. Int. Immunol. 12:215–222. [DOI] [PubMed] [Google Scholar]
- 16.Johansson, M.H., C. Bieberich, G. Jay, K. Kärre, and P. Höglund. 1997. Natural killer cell tolerance in mice with mosaic expression of major histocompatibility complex class I transgene. J. Exp. Med. 186:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ljunggren, H.G., L. Van Kaer, M.S. Sabatine, H. Auchincloss, Jr., S. Tonegawa, and H.L. Ploegh. 1995. MHC class I expression and CD8+ T cell development in TAP1/β2-microglobulin double mutant mice. Int. Immunol. 7:975–984. [DOI] [PubMed] [Google Scholar]
- 18.Sundbäck, J., M.C. Nakamura, M. Waldenström, E.C. Niemi, W.E. Seaman, J.C. Ryan, and K. Kärre. 1998. The α2 domain of H-2Dd restricts the allelic specificity of the murine NK cell inhibitory receptor Ly-49A. J. Immunol. 160:5971–5978. [PubMed] [Google Scholar]
- 19.Glas, R., C. Öhlen, P. Höglund, and K. Kärre. 1994. The CD8+ T cell repertoire in β2-microglobulin-deficient mice is biased towards reactivity against self-major histocompatibility class I. J. Exp. Med. 179:661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanke, T., H. Takizawa, C.W. McMahon, D.H. Busch, E.G. Pamer, J.D. Miller, J.D. Altman, Y. Liu, D. Cado, F.A. Lemonnier, et al. 1999. Direct assessment of MHC class I binding by seven Ly49 inhibitory NK cell receptors. Immunity. 11:67–77. [DOI] [PubMed] [Google Scholar]
- 21.Mason, L.H., J.R. Ortaldo, H.A. Young, V. Kumar, M. Bennett, and S.K. Anderson. 1995. Cloning and functional characteristics of murine large granular lymphocyte-1: a member of the Ly-49 gene family (Ly-49G2). J. Exp. Med. 182:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura, M.C., P.A. Linnemeyer, E.C. Niemi, L.H. Mason, J.R. Ortaldo, J.C. Ryan, and W.E. Seaman. 1999. Mouse Ly-49D recognizes H-2Dd and activates natural killer cell cytotoxicity. J. Exp. Med. 189:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George, T., Y.Y. Yu, J. Liu, C. Davenport, S. Lemieux, E. Stoneman, P.A. Mathew, V. Kumar, and M. Bennett. 1997. Allorecognition by murine natural killer cells: lysis of T-lymphoblasts and rejection of bone-marrow grafts. Immunol. Rev. 155:29–40. [DOI] [PubMed] [Google Scholar]
- 24.Henikoff, S. 1992. Position effect and related phenomena. Curr. Opin. Genet. Dev. 2:907–912. [DOI] [PubMed] [Google Scholar]
- 25.Sharrow, S.O., B.J. Mathieson, and A. Singer. 1981. Cell surface appearance of unexpected host MHC determinants on thymocytes from radiation bone marrow chimeras. J. Immunol. 126:1327–1335. [PubMed] [Google Scholar]
- 26.Hudson, L., J. Sprent, J.F. Miller, and J.H. Playfair. 1974. B cell-derived immunoglobulin on activated mouse T lymphocytes. Nature. 251:60–62. [DOI] [PubMed] [Google Scholar]
- 27.Patel, D.M., P.Y. Arnold, G.A. White, J.P. Nardella, and M.D. Mannie. 1999. Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition. J. Immunol. 163:5201–5210. [PubMed] [Google Scholar]
- 28.Johansson, M.H., E. Höglund, M.C. Nakamura, J.C. Ryan, and P. Höglund. 1998. α1/α2 domains of H-2Dd, but not H-2Ld, induce “missing self” reactivity in vivo—no effect of H-2Ld on protection against NK cells expressing the inhibitory receptor Ly49G2. Eur. J. Immunol. 28:4198–4206. [DOI] [PubMed] [Google Scholar]
- 29.Huard, B., L. Karlsson, and F. Triebel. 2001. KIR down-regulation on NK cells is associated with down-regulation of activating receptors and NK cell inactivation. Eur. J. Immunol. 31:1728–1735. [DOI] [PubMed] [Google Scholar]
- 30.Tormo, J., K. Natarajan, D.H. Margulies, and R.A. Mariuzza. 1999. Crystal structure of a lectin-like natural killer cell receptor bound to its MHC class I ligand. Nature. 402:623–631. [DOI] [PubMed] [Google Scholar]
- 31.Olsson, M.Y., K. Kärre, and C.L. Sentman. 1995. Altered phenotype and function of natural killer cells expressing the major histocompatibility complex receptor Ly-49 in mice transgenic for its ligand. Proc. Natl. Acad. Sci. USA. 92:1649–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ljunggren, H.G., C. Öhlen, P. Höglund, T. Yamasaki, G. Klein, and K. Kärre. 1988. Afferent and efferent cellular interactions in natural resistance directed against MHC class I deficient tumor grafts. J. Immunol. 140:671–678. [PubMed] [Google Scholar]
- 33.Eriksson, M., G. Leitz, E. Fällman, O. Axner, J.C. Ryan, M.C. Nakamura, and C.L. Sentman. 1999. Inhibitory receptors alter natural killer cell interactions with target cells yet allow simultaneous killing of susceptible targets. J. Exp. Med. 190:1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson, H.A., E.M. Hiltbold, and P.A. Roche. 2000. Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nat. Immunol. 1:156–162. [DOI] [PubMed] [Google Scholar]
- 35.Cariappa, A., D.C. Flyer, C.T. Rollins, D.C. Roopenian, R.A. Flavell, D. Brown, and G.L. Waneck. 1996. Glycosylphosphatidylinositol-anchored H-2Db molecules are defective in antigen processing and presentation to cytotoxic T lymphocytes. Eur. J. Immunol. 26:2215–2224. [DOI] [PubMed] [Google Scholar]
- 36.Chiu, I., D.M. Davis, and J.L. Strominger. 1999. Trafficking of spontaneously endocytosed MHC proteins. Proc. Natl. Acad. Sci. USA. 96:13944–13949. [DOI] [PMC free article] [PubMed] [Google Scholar]