Abstract
Some pathogens (e.g., Mycobacterium tuberculosis, Toxoplasma gondii, Leishmania spp) have been shown to persist in their host after clinical cure, establishing the risk of disease reactivation. We analyzed the conditions necessary for the long term maintenance of Leishmania major in genetically resistant C57BL/6 mice after spontaneous healing of their dermal lesions. Interleukin (IL)-10 was found to play an essential role in parasite persistence as sterile cure was achieved in IL-10–deficient and IL-4/IL-10 double-deficient mice. The requirement for IL-10 in establishing latency associated with natural infection was confirmed in IL-10–deficient mice challenged by bite of infected sand flies. The host-parasite equilibrium was maintained by CD4+ and CD8+ T cells which were each able to release IL-10 or interferon (IFN)-γ, and were found to accumulate in chronic sites of infection, including the skin and draining lymph node. A high frequency of the dermal CD4+ T cells released both IL-10 and IFN-γ. Wild-type mice treated transiently during the chronic phase with anti–IL-10 receptor antibodies achieved sterile cure, suggesting a novel therapeutic approach to eliminate latency, infection reservoirs, and the risk of reactivation disease.
Keywords: Leishmania major, IL-10, skin, chronic infection, CD8+ T cells
Introduction
The persistence of pathogens after clinical cure is a hallmark of certain viral, bacterial, and parasitic infections. With respect to the various clinical forms of Leishmaniasis, low numbers of viable organisms persist within lymphoid tissue and/or the site of the former skin lesion after self-cure or successful chemotherapy (1, 2). Such latent infections often give rise to severe forms of reactivation disease, including visceral leishmaniasis (VL) associated with, for example, HIV co-infection (3); the development of post-kala-azar dermal leishmaniasis (PKDL) after cure of VL (4); reactivation of former skin lesions, termed recidivans type (5); and development of mucosal leishmaniasis months or years after healing of a localized cutaneous ulcer (6). While L. major infection in genetically resistant mice has been typically studied to define the mechanisms involved in acquired resistance, the finding that sterile immunity is not achieved in these mice has established the model as useful for also defining the conditions favoring parasite persistence and reactivation (7). Immune pressure during the chronic phase is maintained by CD4+ T cells, IL-12, IFN-γ, and inducible nitric oxide synthase (iNOS),*as impairment of these responses during latency has been shown in each case to promote parasite growth and the reappearance of lesions (8–10). In contrast, the explanation as to why these control mechanisms fail to completely eliminate the parasite is not known; immunologic or genetic manipulations that can disrupt the host-parasite equilibrium in favor of the host have not been described. Nonetheless, the observations that dendritic cells (DCs) (11) and especially fibroblasts (12), harbor low numbers of amastigotes during latency have provided strong support for the concept of “safe targets”; i.e., that parasites persist in cells with intrinsic defects in immune-potentiated killing mechanisms.
In the present studies, the factors controlling L. major persistence and reactivation after clinical cure have been examined in a model of latency established either as a consequence of natural sand fly challenge or an infection model that combines two main aspects of natural challenge, low parasite dose (100 metacyclic promastigotes) and inoculation into skin (ear dermis) (13). The studies demonstrate that in addition to CD4+ T cells, CD8+ T cells are needed to maintain control of the parasite in the chronic site. More importantly, the results reveal a requirement for IL-10 in parasite persistence, and demonstrate a remarkable therapeutic effect of anti–IL-10 receptor Ab in eliminating chronic infection and the risk of reactivation disease.
Materials and Methods
Mice.
C57BL/6 mice, C57BL/10, C57BL/6 IL-4−/−, C57BL/10 IL-10−/−, and C57BL/6 IL-10/4−/− double cytokine-deficient mice (14) were obtained from Taconic Farms and were between 6 and 8 wk of age at the start of each study. For some experiments, C57BL/6 mice were purchased from the Division of Cancer Treatment, National Cancer Institute (Frederick, MD). All mice were maintained in the National Institute of Allergy and Infectious Diseases animal care facility under specific pathogen-free conditions.
Parasite Preparation, Intradermal Inoculation, and Estimation of Parasitic Load.
Leishmania major clone V1 (MHOM/IL/80/Friedlin) was cultured as described previously (15). Infective stage metacyclic promastigotes of L. major were isolated from stationary cultures (4–5 d old) by negative selection using peanut agglutinin. 100 metacyclic promastigotes were inoculated intradermally into the ear dermis (both ears) using a 27 1/2 G needle in a volume of ∼5 μl. The evolution of the lesion was monitored by measuring the diameter of the induration of the ear lesion with a direct reading vernier caliper (Thomas). Parasite titrations in biphasic media for estimation of parasite loads were performed as described previously (16). The plating efficiency of L. major in this limiting dilution assay ranges between 50 and 150% (17).
Ab Treatment of Healed C57Bl/6 Mice.
Clinically cured animals were injected intraperitoneally once a week for a period of 2 to 4 wk with 1 mg of monoclonal anti-CD4 (GK1.5), anti-CD8 (2.43), anti–IFN-γ (XMG-6), or isotype control (GL113, rat IgG1). For inhibition of IL-10, mice were inoculated intraperitoneally every 3 d with 0.5 mg monoclonal anti–IL-10 receptor mAb (DNAX [18]) or isotype control for a period of 2 wk. All of the mAbs were prepared by ammonium sulfate precipitation of ascites fluid and subsequent dialysis against PBS.
LN Cell Preparation and Culture.
The submandibular ear skin draining LNs were recovered and mechanically dissociated using a pellet pestle. The cell viability was assessed by trypan blue exclusion. For measurement of in vitro cytokine production, single-cell suspensions of LNs were pooled from four to five animals, diluted to 4 × 106 cells/ml, and dispensed into 96-well plates without antigen, or with soluble L. major antigen (SLA; 25 μg/ml) or Con A (2 μg/ml) in 100 μl of complete RPMI containing β-mercaptoethanol. Cultures were incubated at 37°C in 5% CO2. Supernatant fluids were harvested at 48–72 h and assayed by ELISA for IFN-γ as described previously (11) and for IL-10 using Endogen kit (Endogen) according to the manufacturer's instructions.
Analysis of Lymphocytes in the Skin.
To characterize leukocytes present in the inoculation site, the ears were collected and the ventral and dorsal dermal sheets separated and incubated 1 h at 37°C, dermal side down on RPMI 1640, NaHCO3, penicillin/streptomycin/gentamycin, containing 0.28 Wunsch units/ml of liberase blendzyme CI (Roche). The dermal sheets from three to five animals were pooled, and processed in the presence of 0.05% DNase (Sigma-Aldrich) using Medimachine (BD PharMingen) according to the manufacturer's instructions. After processing, cell viability was assessed by trypan blue exclusion and the cells were filtered trough a 50-μm filter and washed before activation and/or immunolabeling.
In Vitro Restimulation, Immunolabeling, and Flow Cytometry.
Unfractionated LN cells or dermal cells were incubated for 4 h in the presence of 10 μg/ml of anti-CD28 (37.51; BD PharMingen), 5 ng/ml IL-2 (Endogen), and 25 μg/ml of SLA or alternatively with fetal skin–derived dendritic cells (FSDDCs), prepared from C57BL/6 mice and infected as described previously (19) followed by a 2-h incubation with Brefeldin A (Sigma-Aldrich). Prior to staining, LN or dermal cells were incubated with an anti-FcγIII/II receptor (BD PharMingen) mAb and 10% normal mouse serum (NMS) in PBS containing 0.1% BSA, 0.01% NaN3. The lymphocytes were identified by characteristic size (forward scatter [FSC]) and granularity (side scatter [SSC]), in combination with anti-TCR β chain (H57–597, FITC conjugated; BD PharMingen) and anti-CD4 (H129.19) or CD8 (53–6.7) (CyChrome or allophycocyanin [APC] conjugated; BD PharMingen) surface staining followed or not by a permeabilization step and staining with anti–IFN-γ (XNG1.2; BD PharMingen) or/and anti–IL-10 (JES5–16E3). The isotype controls used were rat IgG2b (A95–1; BD PharMingen), rat IgG2a (R35–95; BD PharMingen), and hamster IgG, group2 (Ha4/8; BD PharMingen). For each sample, between 200,000 and 400,000 cells were analyzed. The data were collected and analyzed using CELLQuest™ software and a FACSCalibur™ flow cytometer (Becton Dickinson).
Natural Sand Fly Challenge.
2–4-d-old Phlebotomus papatasi females were obtained from a colony initiated from field specimens collected in Saudi Arabia. Flies were infected by artificial feeding on a chick membrane at 37°C as described previously (20). 15 d after the blood meal, 10 infected flies per ear were allowed to feed for 2–3 h in the dark, after which time each fly was examined for blood. The ability of infected ears to provide a source of parasites for sand fly infections was investigated using 2–4-d-old uninfected flies that were allowed to feed on the ears of chronically infected mice. Blood fed flies were separated, provided a 50% sucrose solution and water, and their midguts were dissected 48 h later and examined microscopically for the presence of promastigotes.
Results
Persistence of L. major in the Skin after Healing in Resistant Mice.
After inoculation of 100 metacyclic promastigotes of L. major into the ear dermis of C57BL/6 mice, the parasite number increased in the absence of overt pathology for a period of 4–5 wk (Fig. 1 A). Killing and/or clearance of dermal parasites occurred after the 5th week, and was associated with the development of a small lesion that healed spontaneously 8–10 wk after infection. Resolution of the dermal lesion was associated with >99% reduction in the number of parasites in the skin. Nonetheless, sterile immunity was not achieved in the inoculation site (6,000 parasites/ear 24 wk after challenge; Fig. 1 A) or in the draining LN (460 ± 320 parasites/node, data not shown). This low level of infection persisted for the life of the animal (>1 yr). When ears harboring latent infections (6 mo) were exposed to the bites of a natural vector, Phlebotomus papatasi, 75% (6 of 8 ears) transmitted parasites to these flies (data not shown). Thus the persistence of even low numbers of parasites in the skin after healing was formally shown to maintain the host as a long term reservoir of infection.
Figure 1.
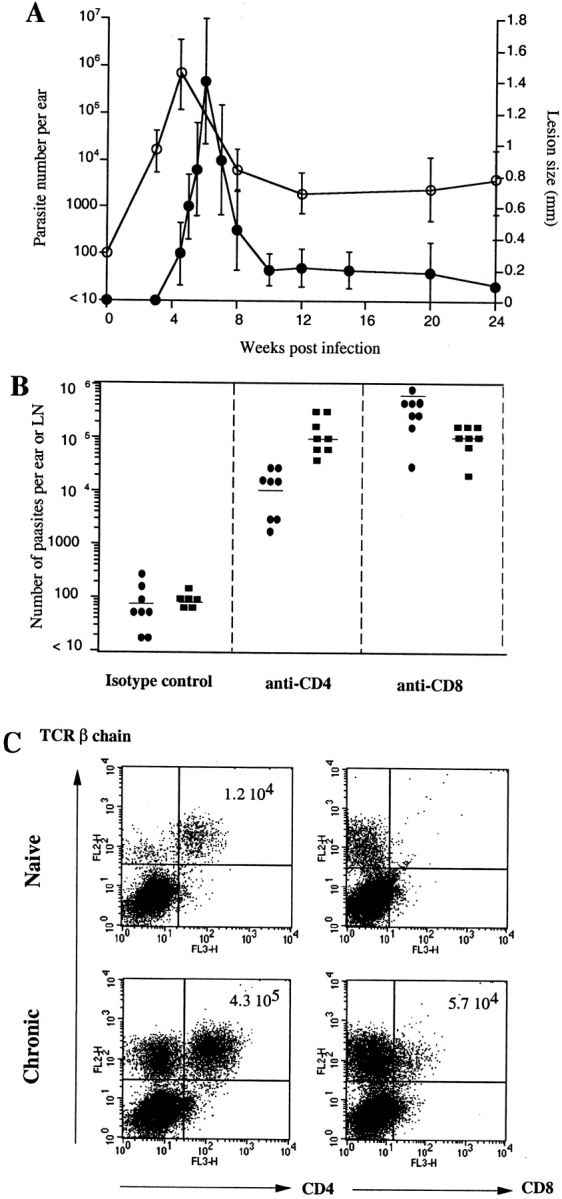
Chronic phase L. major infection in C57BL/6 mice. (A) Number of parasites per ear (○) and diameter of induration (•) after intradermal inoculation of 100 L. major metacyclics in the ear of C57BL/6 mice. Values represent mean induration in mm ± SD, 3–5 mice per group, and geometric mean parasite number per ear ± SD, 5 mice and 10 ears per group. (B) Number of parasites in the ear 5 mo after intradermal inoculation of 100 L.major metacyclics and treated for 2.5 (•) or 4.5 (▪) wk with anti-CD4, anti-CD8, or isotype control before titration. Bars represent geometric mean parasite number per ear, four mice and eight ears per group. The experiment is representative of three separate experiments. (C) Dot plots of TCRβ1CD4+ and TCRβ1CD8+ cells present in the ear dermis 6 mo after challenge. The dermal sheets of four mice were pooled. The data shown are from a single experiment, representative of three separate experiments.
Both CD4+ and CD8+ T Cells Are Required to Maintain Immunity in the Site.
Reactivation of latent L. major infection has been previously shown to occur after in vivo treatment using Abs against CD4, IFN-γ, or IL-12, as well as iNOS inhibitors (9, 10, 21, 22). The effect of in vivo depletion of CD8+ T cells has not been reported, although the maintenance of iNOS activity was found to be CD4 dependent and CD8 independent (9). 3 mo after healing of their dermal lesions, C57Bl/6 mice were treated with anti-CD4 or anti-CD8 Abs. Control-treated mice maintained between 10 and 1,000 parasites in the primary site of the infection (Fig. 1 B). At 2.5 wk after CD8 depletion, the number of parasites increased dramatically (10,000-fold), whereas the increase in the CD4-depleted mice was more modest at this time (100-fold). At 4.5 wk, both groups harbored a 3–4 log fold increase in the number of parasites in the chronic site compared with control treated mice. Both groups of mice reactivated their dermal pathology, as early as 1 wk after treatment for the anti-CD8 and at 2 wk for the anti-CD4–treated mice (data not shown). The requirement for both T cell subsets in maintaining immune pressure on the parasite in the chronic site was associated with a high number of CD4+ T cells (4.3 × 105 cells/ear) and CD8+ T cells (5.7 × 104 cells/ear) recovered from the infected dermis compared with steady-state ears (1.2 × 104 CD4+ T cells and no CD8+ T cells) (Fig. 1 C).
IL-10–deficient Mice Achieve Sterile Cure.
Both IL-4 and IL-10 have been shown to play a role in promoting nonhealing L. major infections in BALB/c mice (23–25). The role of these cytokines in favoring the long term persistence of the parasite after healing in resistant mice was investigated by comparing acute and chronic stage infections in C57BL/6 and C57BL/10 wild-type and in IL-10, IL-4, and IL-10/4 knockout (KO) mice. The immunodeficient mice developed self-healing cutaneous lesions comparable to their respective wild-type controls (Fig. 2 A). The IL-10/4 KO mice required 3–4 wk longer for lesion resolution, likely due to the impaired ability of these mice to downregulate type 1–mediated inflammatory reactions (14). While all of the IL-10 and IL-10/4 KO mice showed clear evidence of established infections at 6 wk after challenge, they had a 100–500-fold reduction in the number of parasites in the skin and LN compared with their wild-type controls (Fig. 2 B). At 9.5 wk after infection, when low level, chronic stage infections had been established in the wild-type mice, the IL-4–deficient mice maintained an even higher number of parasites in the site. In contrast, both the IL-10 and IL-10/4 KO mice completely cleared the parasite from the primary site of the infection and from the draining LN (Fig. 2 C). The complete clearance achieved in the IL-10–deficient groups was not related to the lower levels of peak parasitemias established in these mice, since in a separate experiment, when parasites loads were determined at 4 wk after infection before the onset of immunity, the numbers in the IL-10 KO and C57BL/10 wild-type mice were comparable (1.9 × 105 ± 2.3 × 104 versus 3.0 × 105 ± 1.9 × 104 parasites/ear).
Figure 2.
IL-10 and IL-10/4 KO mice achieve sterile immunity. (A) Diameter of induration after intradermal inoculation of 100 L.major metacyclics in C57BL/6 (•), C57BL10 (▿), IL-10−/− (▴), IL-4−/− (□), and IL-10/4−/− (○) mice. Values represent mean induration in mm ± SD, 12–9 mice and 24–18 ears per group. (B) Number of parasites 6 wk, and (C) and 9.5 wk after challenge in the dermal site (▪) or in the LN (♦), three mice and six ears per group. The experiment is representative of four separate experiments. WT, wild-type. (D) Number of parasites in the dermis or (E) in the draining LN 6 mo after challenge and treated with anti–IFN-γ or isotype control for 2.5 wk before parasite titration; three mice, six ears or LNs per group. The experiment is representative of three separate experiments.
To substantiate that acquired immunity in the IL-10 or IL-10/4 KO mice resulted in sterile cure, the healed mice were treated with anti–IFN-γ in order to reveal the presence of residual organisms. 3 wk after IFN-γ neutralization, the number of dermal and LN parasites increased by 20-fold and 100-fold in the wild-type and IL-4 KO mice, respectively (Fig. 2, D and E). In the IL-10 and IL-10/4 KO mice, the anti–IFN-γ treatment failed to reveal the presence of parasites in 11 of 12 lesions (Fig. 2 D). All of the draining LNs remained negative for parasites (Fig. 2 E).
The persistence of L. major in the skin after healing of dermal lesions resulting from sand fly challenge has recently been demonstrated (20). To confirm the role of IL-10 in this natural infection model, the ears of C57BL/10 and IL-10 KO mice were exposed on each ear to the bites of 10 P. papatasi sand flies harboring mature L. major infections. 12/14 ears in the wild-type mice and 11/12 ears in the IL-10 KO mice developed lesions. The lesions appeared earlier and progressed more rapidly in the IL-10 KO mice, but resolved rapidly by 4 wk in both groups of mice (Fig. 3 A). At 9 wk after infection, the healed ears from all of the wild-type mice harbored between 102 and 2 × 106 parasites per ear (Fig. 3 B). The two ears that never developed lesions were negative for parasites. In addition, the pooled draining nodes from each of the wild-type mice harbored an average of 5 × 103 parasites per node. In the IL-10 KO mice, 4 of 6 mice and 8 of 12 ears were negative for L. major, and the 4 positive ears harbored only low numbers of parasites (<200). The ear draining nodes, pooled for each mouse, were negative for parasites in 4 of 6 mice (Fig. 3 B).
Figure 3.
Natural infection of IL-10 KO mice. (A) The course of infection after transmission of L. major by bite of P. papatasi in C57BL/10 (•) and C57BL/10 IL-10−/− mice (○); 6 to 8 mice, 12 to 16 ears per time point. The value shown at each time point are the sum of the lesion's diameter ± 1 SD. (B) Parasite number in the ear (▪) and draining LN (♦), 9 wk after bite. The two draining LNs from individual mice were pooled. The bar represents geometric mean parasite number; 6–8 LNs and 12–16 ears per group.
Treatment Using Anti–IL-10 Receptor Ab Promotes Sterile Cure in C57Bl/6 Mice.
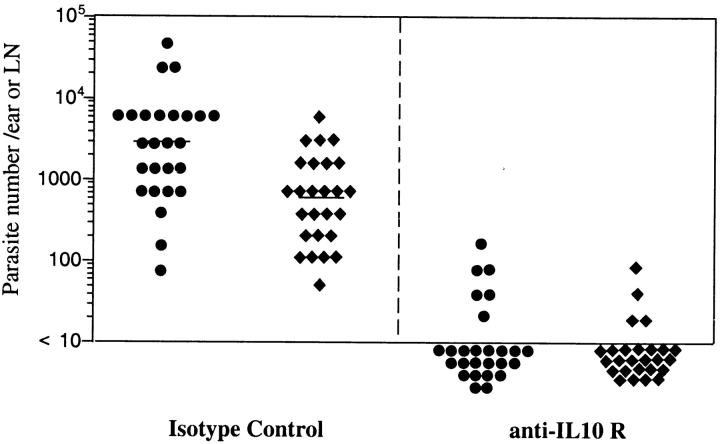
To confirm the role of IL-10 for the maintenance of L. major in the wild-type mice, and to investigate the effects of IL-10 neutralization initiated after latency has already been established, C57BL/6 mice were treated at 24 wk after infection with anti–IL-10R mAb. Control treated mice harbored an average of 3,100 parasites in the dermis and 520 parasites in the draining LN (Fig. 4). 2 wk after treatment using anti–IL-10R mAb, 10 of 13 mice (20 of 26 ears and 22 of 26 LNs) were negative for parasites.
Figure 4.
Anti–IL-10 receptor treatment of chronically infected mice. Number of parasites in the dermis (•) or LN (♦) of C57BL/6 mice treated beginning at 7 mo after infection with anti–IL-10R mAb or isotype control for 2 wk before titration. Bars represent the geometric mean of the parasite number per ear, 13 mice and 26 ears or draining LNs per group. The data represent a pool of three separate experiments.
Analysis of IFN-γ and IL-10 Production by T Cells within the Dermis and Draining LN.
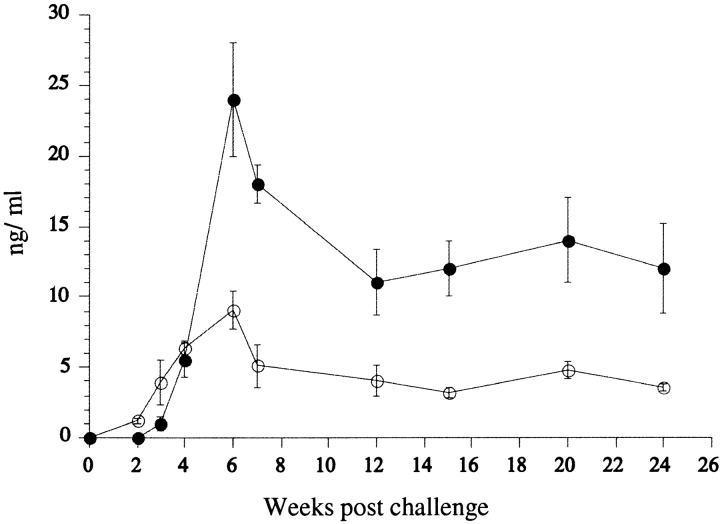
The IL-10 and IFN-γ response of LN cells to SLA was monitored after low dose inoculation into the skin for a period of 24 wk (Fig. 5). Low levels of IFN-γ were detectable by 3 wk and its production followed the evolution of the lesion, with a peak at 6–7 wk and its maintenance at high levels during the chronic stage (11–14 ng/ml). IL-10 was also detectable as early as 3 wk, peaked during the acute stage of the infection at 6 wk (9.1 ng/ml), and remained detectable throughout the chronic stage (3.6 ng/ml 24 wk after challenge). LN cells from IL-10 or IL-10/4 KO mice produced higher levels of IFN-γ in response to SLA during the acute stage of the infection compared with the wild-type mice or to IL-4 KO mice (Table I). 15 wk after challenge, when parasites could no longer be detected, neither the IL-10 nor 10/4 KO mice produced significant levels of IFN-γ, while its production remained relatively high in the wild-type and IL-4 KO mice.
Figure 5.
Antigen-specific cytokine release by LN cells. (A) LN cells from C57BL/6 mice were pooled at each time point from four mice and eight LNs, stimulated in vitro with SLA, and the supernatants were collected at 48 h for determination of IL-10 (○) and IFN-γ (•). Values represent the mean cytokine concentration of four separate experiments ± 1 SD.
Table I.
IFN-γ Production by LN Cells from Immunodeficient Mice
| C57BL/6 | C57BL/10 | IL-10−/− | IL-4−/− | IL-10/4−/− | |
|---|---|---|---|---|---|
| 6 wk | 21 ± 5.5a | 48 ± 6.2 | 81 ± 5.1 | 42 ± 2 | 79 ± 8.2 |
| 15 wk | 34 ± 2.1 | 26 ± 1.1 | 0 | 84 ± 4.5 | 3 ± 1.1 |
Mean cytokine concentration 6 + SD (ng/ml) produced by draining LN cells (four mice, eight LNs per group) assayed 72 h after stimulation with 25 μg/ml SLA.
To define the contribution of CD4+ and CD8+ T lymphocytes to the production of IL-10 and IFN-γ, LN cells and cells extracted from the chronic dermal site were restimulated in vitro with SLA, anti-CD28, and IL-2, or alternatively with L. major infected FSDDCs, and analyzed by flow cytometry. While both conditions efficiently activated CD4+ T cells for cytokine production, intracellular staining of cytokines in CD8+ T was only observed after restimulation using infected FSDDCs. Under these conditions, 29% of the CD4+ T cells recovered from the skin produced IFN-γ and 7.1% produced IL-10, while 2.7% of the CD8+ T cells produced IFN-γ and 3.1% produced IL-10 (Fig. 6 A). The majority of the dermal CD4+ T cells that produced IL-10 also produced IFN-γ (Fig. 6 B). Using infected FSDDCs for in vitro restimulation of LN cells, 1.2% of the CD4+ T cells produced only IL-10, 1.1% produced only IFN-γ, and 0.9% produced both cytokines (Fig. 6 B). CD8+ T cells recovered from LN were also able to release IL-10 or IFN-γ (0.6% and 5% of the CD8+ T cells, respectively). LN and dermal cells from naive mice stimulated under the same conditions were negative for either cytokine (data not shown).
Figure 6.

Single cell analysis of cytokine production by dermal and LN T cells. (A) 7 mo after infection, dermal leukocytes were pooled from four mice (eight ears), restimulated with L. major infected FSDDCs, and stained for detection of surface markers and intracellular cytokines. Cells were gated on TCRβ1 cells. The numbers represent the percentage of CD4+ or CD8+ cells also positive for IL-10 or IFN-γ. (B) Dermal or LN cells from mice 7 mo after infection were gated on CD4+TCRβ1 or CD8+TCRβ1 cells. The numbers represent the percentage of gated cells positive for IL-10, IFN-γ, or both cytokines. The data shown in A and B are from a single experiment, representative of five separate experiment. (C) 6 mo after infection, C57Bl/6 mice were treated for 1 wk with anti–IL-10R mAb or isotype control and dermal cells from four mice (eight ears) per group were pooled, restimulated with anti-CD28, IL-2, and SLA, and stained. Cells were gated on TCRβ1 cells. Numbers shown are the absolute number of CD4+ or CD8+ cells per ear positive or negative for IL-10 or IFN-γ. The data are from a single experiment that is representative of two separate experiments.
The effect of IL-10 neutralization on cytokine production was analyzed after 1 and 2 wk of Ab treatment. 1 wk after treatment, the level of IFN-γ secreted by LN cells in response to SLA was modestly increased compared with control treated, chronically infected mice (18.2 ± 3.2 vs. 12 ± 2.3 ng/ml). 2 wk after treatment the level of IFN-γ was lower in the anti–IL-10R-treated mice compared with the chronic control mice (9.1 ng/ml ± 1.2 vs. 14.5 ± 3.1 ng/ml). 1 wk after anti–IL-10R treatment the numbers of CD4+ and CD8+ T cells recovered from the chronic site were dramatically reduced by 86 and 97%, respectively (Fig. 6 C). Of the CD4+ T cells remaining in the site, 5% still produced IFN-γ, whereas IL-10–producing cells were no longer detected.
Discussion
Many pathogens are capable of long term persistence in their host after clinical cure, creating the risk of disease reactivation when the host/parasite equilibrium is disturbed. In humans, most Leishmania species are known to establish latency, and their reactivation can produce severe clinical outcomes, including visceral, cutaneous, or mucocutaneous forms of disease. In the present studies, low dose, intradermal inoculation of L. major in resistant mice, as well as actual sand fly challenge, were used to investigate the factors favoring parasite persistence and reactivation in a model that more accurately reflects the conditions of latency associated with natural infection. The results confirm that the number of parasites in the skin during latency are sufficient to maintain the immune host as a long term reservoir of infection for vector sand flies. The results also extend prior observations regarding the immune mechanisms involved in maintaining host control over parasite replication in the skin, indicating that in addition to CD4+ T cells, CD8+ T cells are required. Most importantly, the results clearly demonstrate that IL-10 is required for L. major persistence, as evidenced by the finding that IL-10–deficient mice or wild-type mice treated during the chronic stage with anti–IL-10 receptor Ab, achieved sterile immunity in the skin and draining LNs, and were no longer at risk of disease reactivation.
A role for IL-10 in promoting susceptibility to L. major infections in the mouse remained unsupported (26, 27) until a recent report indicating that resistant mice expressing an IL-10 transgene under the control of the MHC II Ea promoter were more susceptible (24), and conversely, that BALB/c IL-10 KO mice were more resistant, though they still harbored high numbers of parasites (25). In each case, over production of IL-10 was concluded to promote nonhealing, progressive disease. The present model describes a role for IL-10 that functions in the context of a powerful Th1 response that promotes healing and effectively eliminates the majority of parasites from the host, and that may reflect a homeostatic mechanism to dampen the potentially harmful effects of this immune response on the host. It is important to note that while the high level of IL-4 induced by L. major is clearly a codeterminant of nonhealing infections in BALB/c mice (28), IL-4 appears to play no role in promoting parasite persistence in healed, resistant mice, since chronic infection became just as well established in the IL-4 KO as in the wild-type mice.
The function of IL-10 as a suppressive or deactivating cytokine is well described; in vitro, it has been shown to inhibit antigen presentation (29), antigen-specific T cell proliferation and type 1 cytokine production (30–32), and to render macrophages refractory to activation by IFN-γ for intracellular killing (25, 33, 34). The sterile immunity achieved in the IL-10 KO and IL-10/4 KO mice might be explained, at least in part, by the fact that they developed a more potent TH1 response during the acute stage, consistent with prior observations regarding IL-10 KO mice (35). Wild-type mice treated with anti IL-10 receptor mAb also showed a transient upregulation of IFN-γ, consistent with the use of this reagent as an adjuvant to prime TH1 cells in response to soluble antigen (36). While in this study the anti–IL-10 receptor mAb only worked if LPS was also present to activate the innate response, in the chronic infection model, the presence of L. major amastigotes (37) and/or activated T cells expressing CD40L (38) may have provided a sufficient stimulus, in conjunction with IL-10 neutralization, to appropriately activate DCs for TH1 upregulation. On the other hand, after 2 wk of treatment with anti–IL-10 receptor Ab, the amount of IFN-γ secreted by LN cells was lower than control treated mice and the number of T cells producing IFN-γ in the site was dramatically reduced. The level of IFN-γ produced by wild-type mice during the chronic stage remained in any case high, even when IL-10 was present. Thus, an enhanced TH1 response seems less a predictor of sterile cure than is the absence of IL-10 or IL-10 receptor signaling. This is consistent with the observations in the IL-10 transgenic B6 mice, which were converted to an L. major susceptible phenotype despite the fact that they were still able to mount a strong Leishmania-specific TH1 response (24). The data favor a role for IL-10 in conditioning the host cells so that they become poorly responsive to even high levels of IFN-γ for intracellular killing. Persistence does not appear to be explained by the “safe target” model (12), as regardless of the nature of the cells harboring the parasites, the absence of IL-10 revealed an ability of these cells to be activated for effective killing. These mechanisms are not mutually exclusive, however, as it is possible that were it not for the uptake of parasites by cells that are poorly responsive to IFN-γ and/or highly sensitive to deactivation by IL-10, then the infections might be cleared even in the presence of IL-10. In our own study, we found a high proportion of DC-like cells harboring Leishmania in the skin during the chronic phase. While the capacity of DC to release NO and trigger the killing of the parasite have differed depending on the source of DCs and the activation signals involved (9, 39), our data suggest that the equilibrium of the chronic site is maintained by infected DCs and macrophages that remain responsive to both IFN-γ and IL-10.
CD4+ T cells appear to be the main producers of IFN-γ and IL-10 in the dermis, although CD8+ T cells were also able to produce either cytokine when the in vitro restimulation conditions were altered to include infected FSDDCs. In the draining node, both subsets were able to produce IFN-γ or IL-10. Interestingly, the majority of the CD4+ T cells in the dermis and a smaller subset of CD4+ T cells in the draining node were able to produce both IL-10 and IFN-γ. CD4+ T cells with a similar cytokine profile, termed Tr-1 cells, have recently been shown to play an essential role in the maintenance of peripheral tolerance (40–43). Their role in chronic infection has not been demonstrated in experimental models, but is suggested by the presence of IFN-γ/IL-10 secreting T cells that are generated in response to persistent infections, including tuberculosis (44), malaria (45), and B. burgdorferi infection in humans (46). In the later example, the generation of these cells was IL-12 dependent, consistent with the ability of IL-12 to prime human CD4+ and CD8+ T cell clones for production of both IFN-γ and IL-10 (47). Non T cell sources of IL-10 need to be considered; Leishmania are known, for example, to efficiently prime host macrophages for IL-10 production in response to second activation signals involving IFN-γ or Fcγ receptor ligation (15, 25). The relative contribution of these various cells to the amount or localization of IL-10 required to maintain parasite survival is not apparent from our studies. Any role that the IL-10 producing CD4+ and/or CD8+ T cells play in promoting parasite survival that might have been discerned by depletion experiments was obscured by their essential role in maintaining host immunity. As reported previously (8) and confirmed here, anti-CD4 treatment during latency resulted in disease reactivation. Our finding that CD8+ cells are also necessary to maintain immune pressure in the chronic dermal site is consistent with prior observation in CBA mice with healed footpad lesions that were reactivated when the mice were treated with anti-CD8 Abs at the time of secondary high dose challenge in a different site (48). The requirement for both subsets is presumably related to the cumulative level and/or localization of IFN-γ that they release. Alternatively, as the high amount of IFN-γ produced by CD4+ T cells alone would seem sufficient, CD8+ cells might contribute additional effector activities to immune maintenance (e.g., cytotoxicity, chemokine release).
In humans, the involvement of IL-10 in Leishmania persistence and in a reactivation process is indicated by the finding that high levels of plasma IL-10 and the expression of IL-10 by keratinocytes was predictive of the development of PKDL (49). The clinical findings that are most relevant to the present studies are those indicating that even in cured cases of visceral or localized cutaneous disease, IL-10 continues to be produced along with IFN-γ (50–53), and may explain the failure of these individuals to achieve sterile cure. Our finding that healed mice, treated for a short period of time during latency with anti–IL-10 receptor Abs, achieved complete clearance of parasites from the skin and draining nodes, suggests a therapeutic approach that either alone or in conjunction with standard treatments might promote sterile immunity, thereby reducing the pool of infection reservoirs and eliminating the risk of reactivation disease.
Acknowledgments
We thank Sandra Cooper for help with the mouse care, and Govind Modi and Edgar Rowton for help with the sand fly rearing.
Footnotes
Abbreviations used in this paper: DC, dendritic cell; FSDDC, fetal skin–derived DC; iNOS, inducible nitric oxide synthase; KO, knockout; SLA, Leishmania major antigen.
References
- 1.Ramirez, J.L., and P. Guevara. 1997. Persistent infections by Leishmania (Viannia) braziliensis. Mem. Inst. Oswaldo Cruz. 92:333–338. [DOI] [PubMed] [Google Scholar]
- 2.Schubach, A., F. Haddad, M.P. Oliveira-Neto, W. Degrave, C. Pirmez, G. Grimaldi, Jr., and O. Fernandes. 1998. Detection of Leishmania DNA by polymerase chain reaction in scars of treated human patients. J. Infect. Dis. 178:911–914. [DOI] [PubMed] [Google Scholar]
- 3.Alvar, J., C. Canavate, B. Gutierrez-Solar, M. Jimenez, F. Laguna, R. Lopez-Velez, R. Molina, and J. Moreno. 1997. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin. Microbiol. Rev. 10:298–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.el Hassan, A.M., H.W. Ghalib, E.E. Zijlstra, I.A. Eltoum, M. Satti, M.S. Ali, and H.M. Ali. 1992. Post kala-azar dermal leishmaniasis in the Sudan: clinical features, pathology and treatment. Trans. R. Soc. Trop. Med. Hyg. 86:245–248. [DOI] [PubMed] [Google Scholar]
- 5.Momeni, A.Z., and M. Aminjavaheri. 1994. Clinical picture of cutaneous leishmaniasis in Isfahan, Iran. Int. J. Dermatol. 33:260–265. [DOI] [PubMed] [Google Scholar]
- 6.Saravia, N.G., A.F. Holguin, D. McMahon-Pratt, and A. D'Alessandro. 1985. Mucocutaneous leishmaniasis in Colombia: Leishmania braziliensis subspecies diversity. Am. J. Trop. Med. Hyg. 34:714–720. [DOI] [PubMed] [Google Scholar]
- 7.Aebischer, T., S.F. Moody, and E. Handman. 1993. Persistence of virulent Leishmania major in murine cutaneous leishmaniasis: a possible hazard for the host. Infect. Immun. 61:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller, I., and J.A. Louis. 1989. Immunity to experimental infection with Leishmania major: generation of protective L3T4+ T cell clones recognizing antigen(s) associated with live parasites. Eur. J. Immunol. 19:865–871. [DOI] [PubMed] [Google Scholar]
- 9.Stenger, S., N. Donhauser, H. Thuring, M. Rollinghoff, and C. Bogdan. 1996. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J. Exp. Med. 183:1501–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stobie, L., S. Gurunathan, C. Prussin, D.L. Sacks, N. Glaichenhaus, C.Y. Wu, and R.A. Seder. 2000. The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge. Proc. Natl. Acad. Sci. USA. 97:8427–8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moll, H., S. Flohe, and C. Blank. 1995. Dendritic cells seclude Leishmania parasites that persist in cured mice—a role in the maintenance of T-cell memory? Adv. Exp. Med. Biol. 378:507–509. [DOI] [PubMed] [Google Scholar]
- 12.Bogdan, C., N. Donhauser, R. Doring, M. Rollinghoff, A. Diefenbach, and M.G. Rittig. 2000. Fibroblasts as host cells in latent leishmaniosis. J. Exp. Med. 191:2121–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969–977. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann, K.F., S.L. James, A.W. Cheever, and T.A. Wynn. 1999. Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J. Immunol. 163:927–938. [PubMed] [Google Scholar]
- 15.Carrera, L., R.T. Gazzinelli, R. Badolato, S. Hieny, W. Muller, R. Kuhn, and D.L. Sacks. 1996. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J. Exp. Med. 183:515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belkaid, Y., S. Kamhawi, G. Modi, J. Valenzuela, N. Noben-Trauth, E. Rowton, J. Ribeiro, and D.L. Sacks. 1998. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188:1941–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Titus, R.G., M. Marchand, T. Boon, and J.A. Louis. 1985. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 7:545–555. [DOI] [PubMed] [Google Scholar]
- 18.O'Farrell, A.M., Y. Liu, K.W. Moore, and A.L. Mui. 1998. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 17:1006–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakob, T., A. Saitoh, and M.C. Udey. 1997. E-cadherin-mediated adhesion involving Langerhans cell-like dendritic cells expanded from murine fetal skin. J. Immunol. 159:2693–2701. [PubMed] [Google Scholar]
- 20.Kamhawi, S., Y. Belkaid, G. Modi, E. Rowton, and D. Sacks. 2000. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science. 290:1351–1354. [DOI] [PubMed] [Google Scholar]
- 21.Muller, I., J.A. Garcia-Sanz, R. Titus, R. Behin, and J. Louis. 1989. Analysis of the cellular parameters of the immune responses contributing to resistance and susceptibility of mice to infection with the intracellular parasite, Leishmania major. Immunol. Rev. 112:95–113. [DOI] [PubMed] [Google Scholar]
- 22.Park, A.Y., B.D. Hondowicz, and P. Scott. 2000. IL-12 is required to maintain a Th1 response during Leishmania major infection. J. Immunol. 165:896–902. [DOI] [PubMed] [Google Scholar]
- 23.Lezama-Davila, C.M., D.M. Williams, G. Gallagher, and J. Alexander. 1992. Cytokine control of Leishmania infection in the BALB/c mouse: enhancement and inhibition of parasite growth by local administration of IL-2 or IL-4 is species and time dependent. Parasite Immunol. 14:37–48. [DOI] [PubMed] [Google Scholar]
- 24.Groux, H., F. Cottrez, M. Rouleau, S. Mauze, S. Antonenko, S. Hurst, T. McNeil, M. Bigler, M.G. Roncarolo, and R.L. Coffman. 1999. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J. Immunol. 162:1723–1729. [PubMed] [Google Scholar]
- 25.Kane, M.M., and D.M. Mosser. 2001. The role of IL-10 in promoting disease progression in Leishmaniasis. J. Immunol. 166:1141–1147. [DOI] [PubMed] [Google Scholar]
- 26.Hagenbaugh, A., S. Sharma, S.M. Dubinett, S.H. Wei, R. Aranda, H. Cheroutre, D.J. Fowell, S. Binder, B. Tsao, R.M. Locksley, K.W. Moore, and M. Kronenberg. 1997. Altered immune responses in interleukin 10 transgenic mice. J. Exp. Med. 185:2101–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatelain, R., S. Mauze, and R.L. Coffman. 1999. Experimental Leishmania major infection in mice: role of IL-10. Parasite Immunol. 21:211–218. [DOI] [PubMed] [Google Scholar]
- 28.Sadick, M.D., F.P. Heinzel, B.J. Holaday, R.T. Pu, R.S. Dawkins, and R.M. Locksley. 1990. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell–dependent, interferon gamma–independent mechanism. J. Exp. Med. 171:115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitra, R.S., T.A. Judge, F.O. Nestle, L.A. Turka, and B.J. Nickoloff. 1995. Psoriatic skin-derived dendritic cell function is inhibited by exogenous IL-10. Differential modulation of B7-1 (CD80) and B7-2 (CD86) expression. J. Immunol. 154:2668–2677. [PubMed] [Google Scholar]
- 30.Mosmann, T.R., and K.W. Moore. 1991. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol. Today. 12:A49–A53. [DOI] [PubMed] [Google Scholar]
- 31.Fiorentino, D.F., A. Zlotnik, T.R. Mosmann, M. Howard, and A. O'Garra. 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147:3815–3822. [PubMed] [Google Scholar]
- 32.Taga, K., and G. Tosato. 1992. IL-10 inhibits human T cell proliferation and IL-2 production. J. Immunol. 148:1143–1148. [PubMed] [Google Scholar]
- 33.Gazzinelli, R.T., I.P. Oswald, S.L. James, and A. Sher. 1992. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J. Immunol. 148:1792–1796. [PubMed] [Google Scholar]
- 34.Vieth, M., A. Will, K. Schroppel, M. Rollinghoff, and A. Gessner. 1994. Interleukin-10 inhibits antimicrobial activity against Leishmania major in murine macrophages. Scand. J. Immunol. 40:403–409. [DOI] [PubMed] [Google Scholar]
- 35.Davidson, N.J., M.M. Fort, W. Muller, M.W. Leach, and D.M. Rennick. 2000. Chronic colitis in IL-10-/- mice: insufficient counter regulation of a Th1 response. Int. Rev. Immunol. 19:91–121. [DOI] [PubMed] [Google Scholar]
- 36.Castro, A.G., M. Neighbors, S.D. Hurst, F. Zonin, R.A. Silva, E. Murphy, Y.J. Liu, and A. O'Garra. 2000. Anti-interleukin 10 receptor monoclonal antibody is an adjuvant for T helper cell type 1 responses to soluble antigen only in the presence of lipopolysaccharide. J. Exp. Med. 192:1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Stebut, E., Y. Belkaid, T. Jakob, D.L. Sacks, and M.C. Udey. 1998. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J. Exp. Med. 188:1547–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marovich, M.A., M.A. McDowell, E.K. Thomas, and T.B. Nutman. 2000. IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand-dependent process. J. Immunol. 164:5858–5865. [DOI] [PubMed] [Google Scholar]
- 39.Blank, C., C. Bogdan, C. Bauer, K. Erb, and H. Moll. 1996. Murine epidermal Langerhans cells do not express inducible nitric oxide synthase. Eur. J. Immunol. 26:792–796. [DOI] [PubMed] [Google Scholar]
- 40.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J.E. de Vries, and M.G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742. [DOI] [PubMed] [Google Scholar]
- 41.Groux, H., and F. Powrie. 1999. Regulatory T cells and inflammatory bowel disease. Immunol. Today. 20:442–445. [DOI] [PubMed] [Google Scholar]
- 42.Jonuleit, H., E. Schmitt, G. Schuler, J. Knop, and A.H. Enk. 2000. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarz, A., S. Beissert, K. Grosse-Heitmeyer, M. Gunzer, J.A. Bluestone, S. Grabbe, and T. Schwarz. 2000. Evidence for functional relevance of CTLA-4 in ultraviolet-radiation-induced tolerance. J. Immunol. 165:1824–1831. [DOI] [PubMed] [Google Scholar]
- 44.Gerosa, F., C. Nisii, S. Righetti, R. Micciolo, M. Marchesini, A. Cazzadori, and G. Trinchieri. 1999. CD4(+) T cell clones producing both interferon-gamma and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin. Immunol. 92:224–234. [DOI] [PubMed] [Google Scholar]
- 45.Plebanski, M., K.L. Flanagan, E.A. Lee, W.H. Reece, K. Hart, C. Gelder, G. Gillespie, M. Pinder, and A.V. Hill. 1999. Interleukin 10-mediated immunosuppression by a variant CD4 T cell epitope of Plasmodium falciparum. Immunity. 10:651–660. [DOI] [PubMed] [Google Scholar]
- 46.Pohl-Koppe, A., K.E. Balashov, A.C. Steere, E.L. Logigian, and D.A. Hafler. 1998. Identification of a T cell subset capable of both IFN-gamma and IL-10 secretion in patients with chronic Borrelia burgdorferi infection. J. Immunol. 160:1804–1810. [PubMed] [Google Scholar]
- 47.Gerosa, F., C. Paganin, D. Peritt, F. Paiola, M.T. Scupoli, M. Aste-Amezaga, I. Frank, and G. Trinchieri. 1996. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-gamma and interleukin-10. J. Exp. Med. 183:2559–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muller, I. 1992. Role of T cell subsets during the recall of immunologic memory to Leishmania major. Eur. J. Immunol. 22:3063–3069. [DOI] [PubMed] [Google Scholar]
- 49.Gasim, S., A.M. Elhassan, E.A. Khalil, A. Ismail, A.M. Kadaru, A. Kharazmi, and T.G. Theander. 1998. High levels of plasma IL-10 and expression of IL-10 by keratinocytes during visceral leishmaniasis predict subsequent development of post- kala-azar dermal leishmaniasis. Clin. Exp. Immunol. 111:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melby, P.C., F.J. Andrade-Narvaez, B.J. Darnell, G. Valencia-Pacheco, V.V. Tryon, and A. Palomo-Cetina. 1994. Increased expression of proinflammatory cytokines in chronic lesions of human cutaneous leishmaniasis. Infect. Immun. 62:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Louzir, H., P.C. Melby, A. Ben Salah, H. Marrakchi, K. Aoun, R. Ben Ismail, and K. Dellagi. 1998. Immunologic determinants of disease evolution in localized cutaneous leishmaniasis due to Leishmania major. J. Infect. Dis. 177:1687–1695. [DOI] [PubMed] [Google Scholar]
- 52.Kemp, K., T.G. Theander, L. Hviid, A. Garfar, A. Kharazmi, and M. Kemp. 1999. Interferon-gamma- and tumour necrosis factor-alpha-producing cells in humans who are immune to cutaneous leishmaniasis. Scand. J. Immunol. 49:655–659. [DOI] [PubMed] [Google Scholar]
- 53.Bosque, F., N.G. Saravia, L. Valderrama, and G. Milon. 2000. Distinct innate and acquired immune responses to Leishmania in putative susceptible and resistant human populations endemically exposed to L. (Viannia) panamensis infection. Scand. J. Immunol. 51:533–541. [DOI] [PubMed] [Google Scholar]