Abstract
Interleukin (IL)-18 is a recently discovered cytokine that modulates both T helper type 1 (Th1) and Th2 responses. IL-18 is elevated during acute graft-versus-host disease (GVHD). We investigated the role of IL-18 in this disorder using a well characterized murine bone marrow transplantation (BMT) model (B6 → B6D2F1). Surprisingly, blockade of IL-18 accelerated acute GVHD-related mortality. In contrast, administration of IL-18 reduced serum tumor necrosis factor (TNF)-α and lipopolysaccharide (LPS) levels, decreased intestinal histopathology, and resulted in significantly improved survival (75 vs. 15%, P < 0.001). Administration of IL-18 attenuated early donor T cell expansion and was associated with increased Fas expression and greater apoptosis of donor T cells. The administration of IL-18 no longer protected BMT recipients from GVHD when Fas deficient (lpr) mice were used as donors. IL-18 also lost its ability to protect against acute GVHD when interferon (IFN)-γ knockout mice were used as donors. Together, these results demonstrate that IL-18 regulates acute GVHD by inducing enhanced Fas-mediated apoptosis of donor T cells early after BMT, and donor IFN-γ is critical for this protective effect.
Keywords: bone marrow transplantation, Th1/Th2 cytokines, IFN-γ, LPS, TNF-α
Introduction
IL-18 is a new member of the IL-1 family that was originally discovered as a factor that induces IFN-γ production from T cells in the presence of IL-12 (1, 2). IL-18 is produced by a wide variety of cells such as macrophages (including Kupffer cells, splenic, and alveolar macrophages), microglia, human peripheral blood mononuclear cells, dendritic cells, keratinocytes, intestinal and airway epithelium, osteoblasts, and adrenal corticocytes (3). IL-18 drives the production of IFN-γ, particularly in concert with IL-12, from a number of cells in the immune system such as Th1 cells, nonpolarized T cells, NK cells, B cells, and dendritic cells. However, IL-18 can also induce IL-4 and IL-13 production in T cells, NK cells, mast cells, and basophils. Thus IL-18 has the unique capacity to stimulate innate immunity and both Th1- and Th2-mediated responses (3, 4). As discussed in a recent review by Nakanishi et al. (3), IL-18 has been shown to play a protective role in host defense against a variety of intracellular microbes such as Mycobacterium avium, Leishmania major, and Cryptococcus neoformans and also in the clearance of certain viral infections such as HSV and influenza A. Furthermore, IL-18 may play a pathological role in certain autoimmune diseases such as diabetes, rheumatoid arthritis, Crohn's disease, and multiple sclerosis (3).
Acute GVHD, the major toxicity of allogeneic bone marrow transplantation (BMT),* is a complex process involving dysregulation of inflammatory cytokine cascades and distorted responses of donor cellular effectors, including T cells, to host alloantigens (5). The Th1/Th2 polarization of T helper cell subsets may play an important role in the development of acute GVHD (6). In some experimental models, a “cytokine storm” amplified by the Th1 phenotype correlates with the development of acute GVHD while a shift to Th2 polarization of donor cells inhibits acute GVHD (7). The Th1/Th2 dichotomy as it relates to GVHD, however, is not crisp; early administration of Th1 inducing cytokines, including IL-12, IFN-γ, and IL-2 have shown paradoxical ability to reduce the severity of acute GVHD (8–10). Some studies have failed to demonstrate beneficial effects of direct in vivo administration of Th2 cytokines in preventing or treating acute GVHD (11, 12). Furthermore, recent studies using donor mice deficient in IFN-γ, IL-4, or their molecular mediators (signal transducer and activator of transcription [STAT]4 or STAT6, respectively) showed that despite the absence of these in donor cells, acute GVHD can still occur (13–15).
IL-18 has been shown to prevent murine chronic GVHD (16), but its role in acute GVHD in not known. Serum concentrations of IL-18 are elevated in both clinical and experimental acute GVHD (17, 18). For this reason, and the fact that IL-18 can regulate the Th1/Th2 balance in different ways depending on the context, we investigated the role of IL-18 in modulating acute GVHD in a well-characterized murine BMT model.
Materials and Methods
Mice
Female C57BL/6 (B6, H-2b, CD45.2+), B6D2F1 (H-2b/d, CD45.2+), B6.129S7-IFN-γ tm1Ts(GKO, H-2b, CD45.2+), B6.MRL-TNF-rs6 lpr (lpr, H-2b) mice were purchased from The Jackson Laboratory. B6 CD45.1 (H-2b, CD45.1+) mice were purchased from Frederick Cancer Research Facility. The age of mice used for experiments ranged between 8 and 12 wk. Mice were housed in sterilized microisolator cages and received filtered water and normal chow or autoclaved hyper-chlorinated drinking water for the first 3 wk post-BMT.
BMT
Mice were transplanted according to a standard protocol described previously (19). Briefly, recipients received 13 cGy total body irradiation (TBI; 137Cs source), split into two doses separated by 3 h to minimize gastrointestinal (GI) toxicity. Bone marrow cells (5 × 106) plus 2 × 106 nylon wool–purified splenic T cells from respective allogeneic or syngeneic donors were resuspended (in 0.25 ml of Leibovitz's L-15 media; GIBCO BRL) and injected intravenously into recipients on day 0. For engraftment experiments, CD45.1 (H-2b, CD45.1+ CD45.1) animals were used as donors. Survival was monitored daily and recipient's body weights and GVHD clinical scores were measured weekly. Donor cell numbers were determined by examining the percentage of CD45.2+ cells in the recipient spleens at different time points.
Assessment of Acute GVHD
The degree of systemic acute GVHD was assessed by a scoring system that incorporates five clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity, and that is more accurate than weight loss alone as described previously (20). At the time of analysis, mice from coded cages were evaluated and graded from 0 to 2 for each criterion. A clinical index was subsequently generated by summation of the five criteria scores (maximum index = 10). Transplanted mice were ear-punched and individual scores were obtained and recorded on day 0 and weekly thereafter.
IL-18 Treatment
Recombinant murine IL-18 was purchased from RD Inc. and reconstituted in PBS. Mice were injected intraperitoneally with IL-18 (1 μg/day/mouse) on days −2, −1, 0, 1, and 2 (five injections total). Mice from the control groups received only the diluent in a similar schedule. In the IL-18 blockade experiments rat anti–mouse IL-18 monoclonal antibody (R&D Systems) was administered intraperitoneally (10 μg/day/mouse), after reconstitution in PBS, on days –1, 0, 1, 2, and 3. The control groups received rat IgG reconstituted and injected in a similar fashion.
Carboxy Fluorescein Diacetate Succinimidyl Ester Labeling and Analysis of In Vivo Expansion of Donor T Cells
Fluorescent labeling of splenocytes was achieved as described (21). Briefly, spleens from the donor mice were harvested and T cells were isolated by nylon wool purification. Erythrocytes were lysed by hypotonic shock, and T cells were washed and resuspended at a density of 106 cells/ml in PBS. An equal volume of 2 μM carboxy-fluorescein diacetate succinimidyl ester (CFDASE; Molecular Probes, Inc.) in PBS was added, and the cells were gently mixed and incubated at 37°C for 15 min. Cells were then washed, centrifuged, and the supernatant was removed. Unbound CFDASE, or the deacetylated form of carboxy fluorescein diacetate succinimidyl ester (CFSE), was quenched by the addition of an equal volume of 10% FCS and incubated at 37°C for 30 min. Analysis of cells immediately following CFSE labeling indicated a labeling efficiency that exceeded 99%. These CFSE labeled cells were then resuspended in Leibovitz's L-15 media and infused into the recipient mice via the tail vein. After 3 d, the recipient mice were killed and the spleens were harvested. Splenocytes from three mice per group were pooled together and were harvested from the interface after density centrifugation of the spleen cells on Ficoll-Paque (Amersham Pharmacia Biotech). Single cell suspensions were thus prepared for cell surface staining and FACS® analysis.
FACS® Analysis
FITC-conjugated mAbs to mouse CD45.1 and PE-conjugated mAbs to Fas, CD4+, CD8+, and allophycocyanin (APC)-conjugated mAbs CD3+ antigens were purchased from BD PharMingen. For determining the extent of donor T cell number and engraftment (anti-Ly 5.2 mAb) was used as donor cell specific marker. The procedure was performed as described previously (19). Briefly, cells were first incubated with mAb 2.4G2 for 15 min at 4°C and then with the relevant FITC-conjugated mAb for 30 min at 4°C. Finally, cells were washed twice with PBS/0.2% bovine serum albumin and fixed with PBS/1% paraformaldehyde. Three-color flow cytometry was performed by using EPICS Elite ESP cell sorter (Beckman Coulter) and on FACSVantage™ SE cell sorter (Becton Dickinson).
Analysis of Donor Cell Apoptosis
Spleens from recipient mice in some of the experiments were harvested 4 d after transplantation and stained with PE-conjugated CD45.1 and then washed with 1× PBS and then stained with FITC-conjugated annexin (R&D Systems) in the dark for 15 min at room temperature in labeling buffer. Donor cell apoptosis was identified based on double staining for CD45.1 and annexin.
ELISA
Antibodies were purchased from BD PharMingen and assays were performed according to the manufacturer's protocol. Briefly, samples were diluted 1:2 to 1:5 and TNF-α or IFN-γ was captured by the specific primary mAb and detected by horseradish peroxidase (TNF-) or biotin-labeled (IL-1) secondary mAbs. Plates were read at 450 nm using a microplate reader (Model 3550; Bio-Rad Laboratories). Recombinant mTNF-α and mIFN-γ (BD PharMingen) were used as standards for ELISAs. Samples and standards were run in duplicate and the sensitivity of the assays was 16 to 20 pg/ml for both cytokines, depending on sample dilution.
Serum LPS Estimation
The Limulus Amebocyte Lysate (LAL) assay (Bio Whittaker) was performed according to the manufacturer's protocol to determine the endotoxin (LPS) concentration in serum. Briefly, serum samples were collected and analyzed using pyrogen-free materials, diluted 10% (vol/vol) in LAL reagent water, and heated to 70°C for 5 min to minimize nonspecific inhibition. Samples were then incubated with equal volumes of LAL for 10 min at 37°C and developed with equal volumes of substrate solution for 6 min. The absorbance of the assay plate was read at 405 nm using the same microplate reader used in cytokine assays. Samples and standards were run in duplicate and the lower limit of detection was 0.15 U/ml. All units expressed are relative to the US reference standard EC-6.
Histology
Formalin-preserved liver and small and large bowel were embedded in paraffin, cut into 5-μm thick sections, and stained with haematoxylin and eosin for histologic examination. Slides were coded without reference to prior treatment and examined in a blinded fashion by a pathologist (C. Liu). A semiquantitative scoring system was used to assess the following abnormalities known to be associated with GVHD (22): small intestine: villous blunting, crypt regeneration, loss of enterocyte brush border, luminal sloughing of cellular debri, crypt cell apoptosis, crypt destruction, and lamina propria lymphocytic infiltrate; colon: crypt regeneration, surface coloncytes, colonocyte vacuolization, surface colonocyte attenuation, crypt cell apoptosis, crypt destruction, and lamina propria lymphocytic infiltrate. The scoring system denoted 0 as normal, 0.5 as focal and rare, 1.0 as focal and mild, 2.0 as diffuse and mild, 3.0 as diffuse and moderate, and 4.0 as diffuse and severe. Scores were added to provide a total score for each specimen. After scoring the codes were broken and data compiled.
Statistical Analysis
The Mann-Whitney U test was used for the statistical analysis of cytokine data, LPS levels, clinical scores, weight loss, and histology, whereas the Wilcoxon rank test was used to analyze survival data. P < 0.05 was considered statistically significant.
Results
Administration of Anti–Mouse IL-18 mAb Exacerbates Acute GVHD-related Mortality
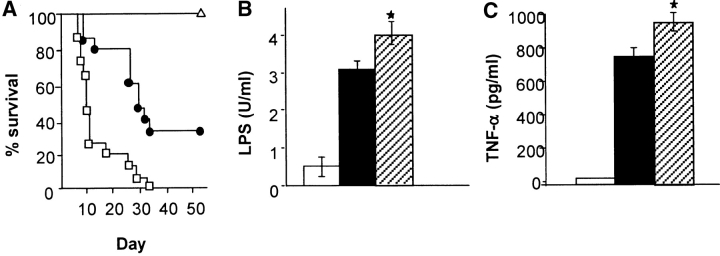
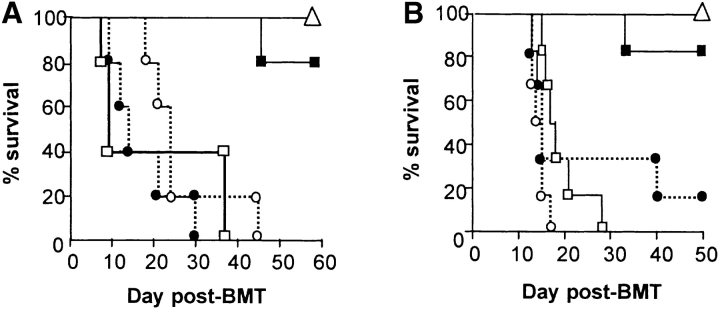
IL-18, a proinflammatory cytokine, is elevated in acute GVHD in both murine and human studies (17, 18). We first examined the effect of neutralizing IL-18 in vivo after allogeneic transplantation using a C57BL/6 (B6, H2b) Æ C57BL/6 × DBA2 (B6D2F1, H-2b/d) BMT model of acute GVHD. Mice were transplanted as described in Materials and Methods. Groups of syngeneic and allogeneic recipients received either 10 μg/mouse/day of rat anti–mouse IL-18 mAb (R&D Systems) or the control rat anti–mouse IgG antibody on days −1, 0, 1, 2, and 3. Antibody was administered beginning on day −1 in order to obtain adequate systemic levels at the time of transplant 24 h later, as in similar experiments that neutralized IL-12 (23). Anti–IL-18 mAb was given until 3 d after BMT because serum IFN-γ is increased at that time and IL-18 expression is known to correlate with IFN-γ secretion (18). Surprisingly, allogeneic BMT recipients injected IL-18 mAb exhibited mortality more rapidly than controls with 100% of animals dying by day 30, while the control allogeneic group mice exhibited 35% survival at the end of 50 d observation period, as shown in Fig. 1 A (P < 0.05). All allogeneic BMT recipients showed clinical features of acute GVHD at the time of death. Mice receiving syngeneic BMT (F1 → F1) and anti–IL-18 mAb showed 100% survival, thereby ruling out any nonspecific toxicity of the therapy.
Figure 1.
IL-18 blockade exacerbates acute GVHD mortality and increases serum LPS and TNF-α B6D2F1 mice were transplanted as described in Materials and Methods with 5 × 106 BM cells and 2 × 106 NWP T cells from B6 allogeneic donors after 1,300 cGy TBI and were injected intraperitoneally with 10 μg/mouse/day of anti–mouse IL-18 mAb (□, n = 15) or the control, rat IgG, intraperitoneally (•; n = 15) for 5 d (day −1 to day +3). Recipients of the syngeneic B6D2F1 cells (Δ, n = 10) were treated with the same dose and schedule of anti–mouse IL-18 mAb. One of two similar experiments is shown. (A) IL-18 blockade exacerbated acute GVHD mortality. P = 0.04, • vs. □, by Wilcoxon rank test. Syngeneic mice exhibited 100% survival during the 50-d observation period. (B) Mice were transplanted as in panel A and serum was obtained by performing retro-orbital venous puncture on day 7 post-BMT. Syngeneic plus anti–IL-18 mAb (white bar), allogeneic plus control IgG (black bar), and allogeneic plus anti–IL-18 mAb (dotted bar). *P = 0.04, dotted bar vs. solid bar. Data represent the mean ± SE (n = 4/group). One of three representative experiments is shown. (C) Mice were transplanted as in panel A and serum was obtained as in B. Syn plus anti–IL-18 mAb (white bar), allo plus control IgG (black bar), and allo plus anti–IL-18 mAb (dotted bar). *P < 0.05, dotted bar vs. black bar. Data represent the mean ± SE (n = 4/group). One of three representative experiments is shown.
The increase in acute GVHD in the anti IL-18 mAb group was associated with greater GI damage as measured by increased systemic translocation of LPS and higher levels of serum TNF-α (Fig. 1, B and C). Damage to the GI tract during acute GVHD from cytokines such as TNF-α cause increased leakage of inflammatory stimuli (including LPS) into the systemic circulation which then triggers additional TNF-α production, making the GI tract a pivotal target organ in the pathophysiology of this disorder (5). Greater levels of LPS and TNF-α were also associated with higher histopathologic scores of GI pathology for acute GVHD (data not shown). The administration of anti–IL-18 mAb in syngeneic BMT recipients did not increase the serum levels of either LPS or TNF-α concentrations, ruling out nonspecific effects of IL-18 neutralization. Furthermore, the mice that received anti–IL-18 mAb demonstrated diminished levels of serum IFN-γ compared with the control group (930 ± 55 pg/ml vs. 2,555 ± 294 pg/ml, P < 0.03), demonstrating the in vivo efficacy of anti–IL-18 mAb in regulating the secretion of IFN-γ after allogeneic BMT. However allogeneic recipients that received anti–IL-18 mAb had significantly greater number of total donor T and CD4 cells in their spleens during the first week when compared with the control group (Table I), suggesting that neutralization of IL-18 amplified the donor T cell response to host alloantigens.
Table I.
Effects of IL-18 Administration or Neutralization on Donor T Cell Expansion
| Donor splenocytes (× 106)
|
||||||
|---|---|---|---|---|---|---|
| Day +5
|
Day +7
|
|||||
| Treatment | CD3 | CD4 | CD8 | CD3 | CD4 | CD8 |
| Control | 6.4 ± 1.2 | 3.12 ± 0.7 | 2.3 ± 0.1 | 16.2 ± 3.2 | 6.8 ± 1.1 | 7.1 ± 2.0 |
| Anti–IL-18 mAb | 9.8 ± 0.6a | 5.4 ± 1a | 2.9 ± 1.2 | 26.2 ± 4.1a | 15.6 ± 3.8a | 10.8 ± 2.9 |
| IL-18 | 3.5 ± 1.0b | 1.3 ± 0.4b | 1.9 ± 0.05 | 8.8 ± 1.4b | 3.4 ± 0.9b | 3.9 ± 1.3 |
B6D2F1 mice were irradiated, transplanted with BM and T cells from B6 Ly5.2 donors and treated with anti–IL-18 mAb or the control Ab (as in Fig. 1) or IL-18 (as in Fig. 2). Splenocytes from three mice per group were pooled and analyzed for donor T cell expansion on days +5 and +7 post-BMT by two-color flow cytometry for expression of donor marker (CD45.1+) and CD3+, CD4+, and CD8+ cells. Data represent average of three mice per group mean ± SE from two or three experiments.
P < 0.05 vs. control.
P < 0.04 vs. control.
Administration of IL-18 Early after BMT Reduces Acute GVHD Mortality, Morbidity, and Histopathology
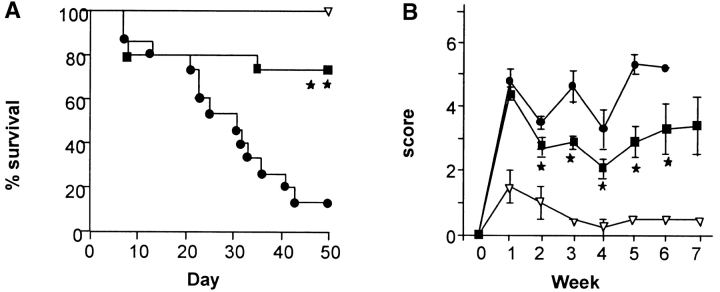
In light of these unexpected effects of IL-18 neutralization, we next studied the effect of exogenous administration of IL-18 itself on the severity of acute GVHD. Recombinant murine IL-18 (IL-18; RD Inc.) was administered to BMT recipients intraperitoneally for 5 d from day −2 to +2; control mice received identical injections of PBS. This dose of rmIL-18 was chosen because it was effective at modulating IFN-γ secretion (24). Preliminary experiments using different dosing schedules (day 0 only, day 0 to +2, day –2 to 0) that were successful for other cytokine modulators of GVHD (25, 26), had no effect on GVHD mortality (data not shown). As shown in Fig. 2 A, animals receiving IL-18 from day –2 to +2 showed significant improvement in survival at day 50 (75 vs. 15%, P < 0.001). The IL-18–treated animals also displayed significantly less clinical acute GVHD than allogeneic controls (Fig. 2 B, P < 0.05). All mice in both groups displayed complete donor hematopoietic chimerism as determined by FACS® analysis (data not shown) ruling out mixed chimerism as a cause for reduced GVHD.
Figure 2.
Exogenous administration of IL-18 reduces acute GVHD mortality and morbidity. B6D2F1 mice were given 1,300 cGy of TBI and transplanted with 5 × 106 BM cells and 2 × 106 NWP T cells from B6 donors and injected intraperitoneally with 1 μg/mouse/day of IL-18 (▪; n = 20) or diluent (•; n = 20) on days –2 to +2. Recipients of the syngeneic B6D2F1 cells (▿, n = 6) received the same dose and schedule of IL-18. Data from two similar experiments are combined. (A) Percent survival after BMT. ▪ vs. •, P =0.0008 by Wilcoxon rank test. (B) Animals were scored for clinical GVHD as described in Methods. ▪ vs. •, P < 0.05 by Mann-Whitney U test from day 14 to 42.
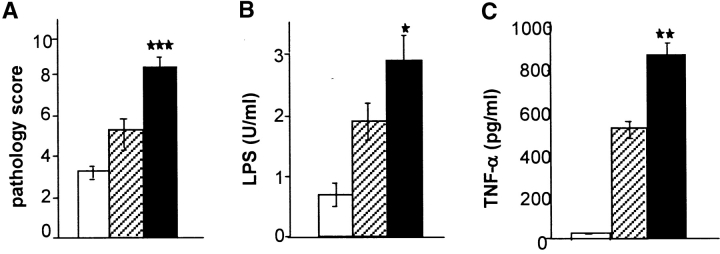
We next examined the bowel for histologic changes of acute GVHD using a semiquantitative index of GI damage (see the Materials and Methods). Allogeneic BMT recipients treated with IL-18 demonstrated significantly less GI tract pathology and reduced levels of serum LPS and TNF-α (Fig. 3, A–C). These results are consistent with our previous observations that GI tract damage of acute GVHD is associated with increased translocation of LPS into the systemic circulation and also with significantly greater serum levels of TNF-α (5). Thus early administration of IL-18 significantly prevented acute GVHD by all clinical, pathologic, and biochemical indices examined.
Figure 3.
IL-18 treatment reduces the pathological and biochemical indices of acute GVHD of the GI tract. B6D2F1 mice were transplanted as in Fig. 2, animals were killed, and GI tract and serum were obtained for analysis as described in Materials and Methods. (A) Coded slides were scored for pathological damage, as described in Materials and Methods, on day 7 post-BMT. Recipients of allogeneic BMT plus diluent (black bar, n = 4) or IL-18 (dotted bar, n = 4) and recipients of syngeneic plus IL-18 (white bar, n = 4) are shown. *P = 0.03 vs. IL-18 allo group. Data (mean ± SE) from one of two similar experiments are shown. (B) Serum LPS levels are reduced after IL-18 treatment. Controls (black bar) vs. IL-18–treated recipients (dotted bar), *P < 0.05. Data are from one of two similar experiments. (C) Serum TNF-α levels. Controls (black bar) vs. IL-18–treated recipients (dotted bar), P = 0.04.
IL-18 Alters Donor T Cell Expansion
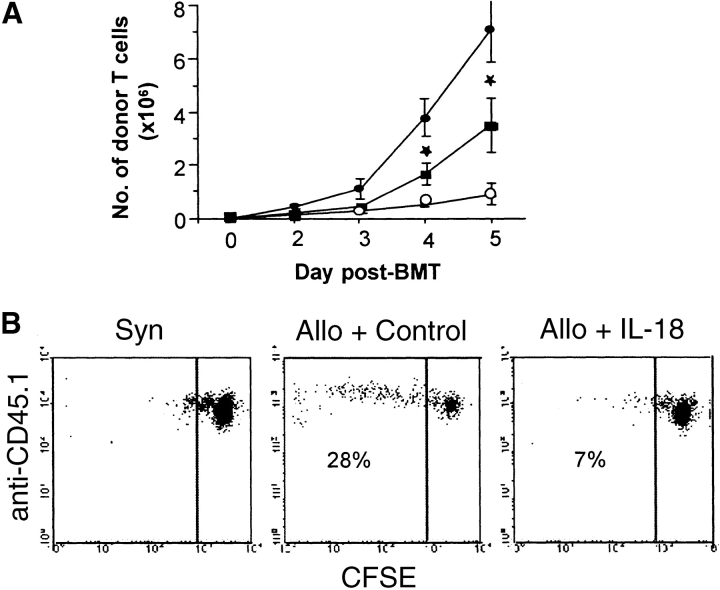
The expansion of donor T cells caused by anti–IL-18, combined with the clear effects of IL-18 in reducing GVHD, suggested that IL-18 might exert its protective effect through alteration of donor T cell response to host antigens. We first investigated whether IL-18 administration dampened the kinetics of donor T cell expansion in the recipient spleens in the first 5 d after BMT. Consistent with our hypothesis, IL-18 treatment markedly reduced the donor T cell expansion as shown in Fig. 4 A. Given that acute GVHD in this donor – recipient strain combination is predominantly driven by CD4+ donor T cells (14) we also investigated the kinetics of CD4 and CD8 expansion. IL-18 administration reduced the expansion primarily of the CD4 T cell subset (Table I). CFSE analysis confirmed this inhibition by IL-18 of splenic donor T cells undergoing more than two cell divisions (Fig. 4 B).
Figure 4.
IL-18 treatment reduces early donor T cell expansion. Recipient mice were transplanted as in Fig. 2. (A) Splenocytes were harvested from the recipients at each time point as shown (n = 4/per group) and labeled with anti-CD3 PE and anti-CD45.1 FITC. The number of donor T cells (CD45.1+ CD3+) were determined (Allo + control, • vs. Allo + IL-18, ▪) at various time points, from days 2 to 5 post-BMT. *P = 0.03. Recipients of syngeneic BMT (○) demonstrated lower numbers of donor T cells at all time points. Data from one of three similar experiments is shown. (B) Nylon wool purified B6Ly5.2 donor T cells were labeled with CFSE as described in Materials and Methods and injected into B6D2F1 recipients after 1,300 cGy TBI. Splenocytes were harvested and pooled (n = 3/group) on day 3 post-BMT, and labeled with anti-CD45.1 APC. The percent of donor cells (CFSE+ CD45.1+) undergoing cell expansion was determined. Fewer percentage of donor cells underwent beyond two cell divisions in IL-18–treated group than allogeneic controls, 7 vs. 28%. One of three representative experiments is shown.
IL-18 Causes Donor T Cell Apoptosis by Upregulating Fas
Reduced donor T cell expansion could be due to either lack of stimulation or increased apoptosis of activated cells (activation-induced cell death [AICD]). Fas, a member of TNF-R receptor family, is known to mediate AICD (27) and hence we next investigated whether IL-18 induced Fas expression on donor T cells. Spleens from the transplanted mice were harvested on day 4 and donor cells were analyzed by flow cytometry for annexin staining as a measure of apoptosis. As shown in Table II, IL-18–treated mice showed a greater percentage of donor cell apoptosis. Using three-color flow cytometry (FCM) analysis, we next determined that the apoptosis is associated with increased Fas expression on donor T cells (CD 45.1+/CD3+) in the IL-18–treated group compared with the control group (Table II).
Table II.
Donor T Cell Apoptosis and Fas Expression
| Percent Fas+ | Percent Annexin+ | |
|---|---|---|
| Syn plus IL-18 | 29 ± 4 | 9 ± 3 |
| Allo plus control | 48 ± 7 | 24 ± 4 |
| Allo plus IL-18 | 64 ± 5a | 44 ± 6a |
B6D2F1 mice were irradiated, transplanted, and treated with IL-18 or the diluent as in Fig. 2. Splenocytes were pooled from four mice per group and analyzed on day 4 post-BMT by three-color flow cytometry for expression of Fas, CD45.1+, and CD3+ cells. Gates were set for CD45.1+CD3+ (donor) cells and percentage of cells expressing Fas or annexin was determined. Data represent mean ± SE from three experiments.
P < 0.05 allo plus IL-18 versus allo plus control.
To determine the functional relevance of the increased Fas expression and AICD by donor T cells in IL-18–mediated protection from GVHD we tested whether IL-18 would protect animals if the donor cells lacked Fas. When Fas-deficient (lpr, H-2b) mice were used as donors, all recipient mice died whether or not they received IL-18, whereas IL-18 protected recipients of the wild-type donors, as seen previously (Fig. 5 A). Furthermore, donor T cell expansion was not suppressed by rmIL-18 administration when lpr mice were used as donors (3.9 ± 0.4 × 106 vs. 4.1 ± 0.8 × 106 on day +3). Thus, IL-18 protected mice against acute GVHD by increasing Fas-mediated donor T cell apoptosis early after BMT.
Figure 5.
Requirement of Fas expression and IFN-γ production by donor T cells for IL-18–mediated GVHD protection. (A) B6D2F1 mice were transplanted as in Fig. 2 with either B6D2F1 (syn) donors (▵, n = 6) or lpr (H2b; □, n = 8 and ○, n = 8) or wild-type B6 donors (•, n = 8 and ▪, n = 8). Transplanted mice received IL-18 (▪ and □) or the control diluent (• and ○) from day –2 to +2. Results from one of two similar experiments is shown. • vs. ▪ P = 0.0008; □ vs. ○, P = 0.1; ▪ vs. □, P = 0.001. (B) B6D2F1 mice were transplanted as in Fig. 2 with either B6D2F1 donors (▵, n = 6) or B6.129S7-IFN-g tm1Ts (H2b; □, n = 8 and O, n = 8) or wild-type B6 donors (•, n = 8 and ▪, n = 8). Transplanted mice received IL-18 (▪ and □) or the control diluent (• and ○) from day –2 to +2. • vs. ▪ P = 0.001; □ vs. O P = 0.07; ▪ vs. □ P = 0.0009.
The Beneficial Effect of IL-18 on Acute GVHD Is Dependent on Donor-derived IFN-γ
IFN-γ has been shown to be important in regulating the death of activated T lymphocytes (28) and it is important in mediating the biological effects of IL-18 (2). When serum levels were analyzed after BMT, IL-18 treatment resulted in higher IFN-γ levels than controls on days 2 and 3; levels on day 4 were equivalent between the groups (data not shown). In a final set of experiments we evaluated the role of donor-derived IFN-γ in mediating the effect of IL-18 in our model of acute GVHD using IFN-γ knockout (GKO) mice as donors. All mice receiving GKO donor cells died irrespective of IL-18 treatment (Fig. 5 B). Analysis of T cell expansion (day +3) demonstrated equivalent expansion of GKO donor T cells in the recipient spleen irrespective of IL-18 treatment (4.9 ± 0.7 × 106 vs. 5.8 ± 0.2 × 106). Thus, the ability of IL-18 to cause donor T cell apoptosis and reduce the severity of acute GVHD depends, at least in part, upon donor-derived IFN-γ.
Discussion
To determine the role of IL-18 in acute GVHD we used a well-defined irradiated murine BMT model, B6 (H2b) → B6D2F1 (H-2b/d) in which the host and donor differ at both MHC class I, MHC class II, and multiple minor histocompatibility antigens. Administration of anti–IL-18 mAb increased acute GVHD. This was surprising because IL-18 is elevated during acute GVHD (17, 18) and is known to induce Th1 differentiation and cytotoxic T lymphocyte function (2), both of which have been implicated in the pathogenesis of acute GVHD. Blockade of endogenous IL-18 was associated with greater expansion of donor T cells and an increase in serum levels of TNF-α. More interestingly, injection of IL-18 early in BMT to the recipients reduced acute GVHD and caused a decrease in serum TNF-α and LPS levels, which correlate with the severity of acute GVHD in this model (19). The donor, recipient strain combination used in our experiments is characterized by “hybrid resistance” mediated by host NK cells and can result in graft rejection (29). IL-18 is known to enhance NK cell activity (30) and might therefore increase the risk of graft rejection. The protective effect of IL-18 treatment is not, however, due to graft rejection because IL-18 treated recipients showed complete donor hematopoietic chimerism.
IL-18 administration did inhibit early expansion of donor T cells, blunting and delaying the proliferative responses of donor T cells to host alloantigens. Interestingly, IL-18 caused a reduction in expansion of both CD4 and CD8 subsets, albeit the effect on CD8 T cells was not statistically significant (Table I). The effect of IL-18 on effector CD8 cells has been shown to be CD4 dependent (31), hence it remains to be determined whether IL-18 attenuates acute GVHD in a CD8-dependent murine BMT model. We further observed that IL-18 treatment caused greater apoptosis and increased Fas expression on donor T cells in recipient spleens. In addition to Fas, IL-18 also increased CD25 and CD69 expression on donor T cells early after BMT (data not shown) thus suggesting an increased activation of donor T cells with IL-18 treatment. The functional importance of Fas on donor T cells was confirmed when IL-18 did not protect recipients of Fas−/− (lpr) donors cells from acute GVHD. Thus, blocking Fas-mediated apoptosis by using lpr donors inhibited peripheral T cell deletion induced by administration of IL-18.
IFN-γ plays an important role in regulating the death of activated T lymphocytes (28) and is known to be critical in mediating the biological effects of IL-18 (2). When IFN-γ–deficient mice were used as donors, recipients were not protected by IL-18 from GVHD mortality. Thus IL-18–induced protection against GVHD requires IFN-γ production by allogeneic donor cells. Donor-derived IFN-γ also appears to play a critical role in the inhibition of early donor T cell proliferation by IL-18 as measured by CFSE staining (data not shown). Although our study cannot rule out the possibility that host-derived IFN-γ also contributes to AICD of donor T cells, our data indicates that host-derived IFN-γ alone cannot mediate the protective effects of IL-18. However, it is possible that the GKO and wild-type T cells may differ in their susceptibility to apoptosis and kinetics of expansion (32) and hence may induce GVHD via distinct mechanisms. Therefore, the differential effect of IL-18 on GVHD in the two groups may reflect the presence of distinct effector mechanisms in the presence and absence of donor derived IFN-γ.
Similar inhibition of GVHD has been noted however by administration of other Th1 cytokines such as IFN-γ, IL-2, and IL-12 (8–10). IL-18 administration appears to impart some of its beneficial effects in a manner similar to IL-12, e.g., perturbation of early donor T cell expansion and requirement of Fas expression and IFN-γ production by donor cells (8, 14, 33). IL-18 and IL-12 differ in several respects, however. Blockade of IL-18 increased GVHD related mortality unlike IL-12 blockade (23); IL-18 treatment caused a decrease in serum TNF-α but IL-12 treatment did not (33). Finally, the biphasic effect on serum IFN-γ levels reported with IL-12 treatment (8) was not observed with IL-18 treatment. It has also been demonstrated that IL-12 from both donor and recipient sources contribute to increased severity of acute GVHD (34). Although a recent study suggests that the elevated levels of serum IL-18 observed after allogeneic BMT is likely to be host derived (35), the study did not address its role in GVHD pathophysiology. Future experiments will determine if host- or donor-derived IL-18 is critical and whether there is a synergistic benefit by administering IL-12 and IL-18 early in BMT.
IL-18 has been shown to prevent murine chronic GVHD by inducing IFN-γ production from lymphocytes and thereby inhibiting IgG1 and IgE production (16, 36). But the increase in serum levels of IL-18 in acute GVHD (17, 18) initially suggested that perhaps this protein would amplify cytokine dysregulation that characterizes this disorder. Clearly, our data demonstrated that IL-18 does not play such a role and rather suggests that the increased production of IL-18 may be a regulatory response to an aggressive systemic alloreaction. The increase in apoptosis of donor T cells in the control recipient of allogeneic (compared with syngeneic) BMT suggests that an attempt at such regulation may indeed occur normally during a GVHD reaction, and that there is a natural contraction of the donor T cell response to host alloantigens that follows immediately upon its rapid activation. This contraction requires Fas expression and IFN-γ production by donor T cells leading to peripheral deletion by donor T cell “fratricide”. Peripheral deletion of allo-reactive T cells can lead to tolerance (37). Thus, IL-18 when given early in BMT enhances this peripheral deletion of donor T cells and reduces GVHD as can other Th1 inducing cytokines such as IL-12 and Th1 cytokines such as IFN-γ and IL-2 (8–10). Administration of Th1-inducing cytokines, such as IL-12, can accelerate acute GVHD when administered later during the cytokine cascade (38); it remains to be determined whether delayed administration of IL-18 behaves in a similar detrimental fashion in this model. Thus, Th1 cytokines can play a dual role in acute GVHD, as effector cytokines that can damage GVHD target tissues and amplify production of inflammatory cytokines (5, 7) and also as regulators of alloreactive T cells that leads to their deletion by apoptosis early after BMT. The timing of administration and the systemic production of any given cytokine may therefore be critical to the eventual outcome of acute GVHD, and improved understanding of interactions between cells and cytokines may offer new therapeutic opportunities to modulate this serious and complex clinical disorder.
Acknowledgments
This work is supported by National Institutes of Health grants CA39542 and HL55162 (J.L.M. Ferrara), and National Institutes of Health grant CA74886.
Footnotes
Abbreviations used in this paper: AICD, activation-induced cell death; BMT, bone marrow transplantation; CFSE, carboxy fluorescein diacetate succinimidyl ester; GI, gastrointestinal; LAL, Limulus Amebocyte Lysate; TBI, total body irradiation.
References
- 1.Okamura, H., H. Tsutsi, T. Komatsu, M. Yutsudo, A. Hakura, T. Tanimoto, K. Torigoe, T. Okura, Y. Nukada, K. Hattori, et al. 1995. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 378:88–91. [DOI] [PubMed] [Google Scholar]
- 2.Dinarello, C.A. 2000. Interleukin-18, a proinflammatory cytokine. Eur. Cytokine Netw. 11:483–486. [PubMed] [Google Scholar]
- 3.Nakanishi, K., T. Yoshimoto, H. Tsutsui, and H. Okamura. 2001. Interleukin-18 regulates both th1 and th2 responses. Annu. Rev. Immunol. 19:423–474. [DOI] [PubMed] [Google Scholar]
- 4.Hoshino, T., Y. Kawase, M. Okamoto, K. Yokota, K. Yoshino, K. Yamamura, J. Miyazaki, H.A. Young, and K. Oizumi. 2001. Cutting edge: IL-18-transgenic mice: in vivo evidence of a broad role for IL-18 in modulating immune function. J. Immunol. 166:7014–7018. [DOI] [PubMed] [Google Scholar]
- 5.Hill, G.R., and J.L. Ferrara. 2000. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 95:2754–2759. [PubMed] [Google Scholar]
- 6.Fowler, D.H., and R.E. Gress. 2000. Th2 and Tc2 cells in the regulation of GVHD, GVL, and graft rejection: considerations for the allogeneic transplantation therapy of leukemia and lymphoma. Leuk. Lymphoma. 38:221–234. [DOI] [PubMed] [Google Scholar]
- 7.Antin, J.H., and J.L.M. Ferrara. 1992. Cytokine dysregulation and acute graft-versus-host disease. Blood. 80:2964–2968. [PubMed] [Google Scholar]
- 8.Sykes, M., G.L. Szot, P.L. Nguyen, and D.A. Pearson. 1995. Interleukin-12 inhibits murine graft-versus-host disease. Blood. 86:2429–2438. [PubMed] [Google Scholar]
- 9.Brok, H.P.M., P.J. Heidt, P.H. van der Meide, C. Zurcher, and J.M. Vossen. 1993. Interferon-γ prevents graft-versus-host disease after allogeneic bone marrow transplantation in mice. J. Immunol. 151:6451–6459. [PubMed] [Google Scholar]
- 10.Sykes, M., M.L. Romick, and D.H. Sachs. 1990. Interleukin 2 prevents graft-versus-host disease while preserving the graft-versus-leukemia effect of allogeneic T cells. Proc. Natl. Acad. Sci. USA. 87:5633–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krenger, W., K. Snyder, S. Smith, and J.L.M. Ferrara. 1994. Effects of exogenous interleukin-10 in a murine model of graft-versus-host disease to minor histocompatibility antigens. Transplantation. 58:1251–1257. [PubMed] [Google Scholar]
- 12.Blazar, B.R., P.A. Taylor, S. Smith, and D.A. Vallera. 1995. Interleukin-10 administration decreases survival in murine recipients of major histocompatibility complex disparate donor bone marrow grafts. Blood. 85:842–851. [PubMed] [Google Scholar]
- 13.Murphy, W.J., L.A. Welniak, D.D. Taub, R.H. Wiltrout, P.A. Taylor, D.A. Vallera, M. Kopf, H. Young, D.L. Longo, and B.R. Blazar. 1998. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J. Clin. Invest. 102:1742–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang, Y.G., B.R. Dey, J.J. Sergio, D.A. Pearson, and M. Sykes. 1998. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J. Clin. Invest. 102:2126–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolic, B., S. Lee, R.T. Bronson, M.J. Grusby, and M. Sykes. 2000. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J. Clin. Invest. 105:1289–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamoto, I., K. Kohno, T. Tanimoto, K. Iwaki, T. Ishihara, S. Akamatsu, H. Ikegami, and M. Kurimoto. 2000. IL-18 prevents the development of chronic graft-versus-host disease in mice. J. Immunol. 164:6067–6074. [DOI] [PubMed] [Google Scholar]
- 17.Fujimori, Y., H. Takatsuka, Y. Takemoto, H. Hara, H. Okamura, K. Nakanishi, and E. Kakishita. 2000. Elevated interleukin (IL)-18 levels during acute graft-versus-host disease after allogeneic bone marrow transplantation. Br. J. Haematol. 109:652–657. [DOI] [PubMed] [Google Scholar]
- 18.Hu, H.Z., G.L. Li, Y.K. Lim, S.H. Chan, and E.H. Yap. 1999. Kinetics of interferon-gamma secretion and its regulatory factors in the early phase of acute graft-versus-host disease. Immunology. 98:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill, G.R., J.M. Crawford, K.J. Cooke, Y.S. Brinson, L. Pan, and J.L.M. Ferrara. 1997. Total body irradiation and acute graft versus host disease. The role of gastrointestinal damage and inflammatory cytokines. Blood. 90:3204–3213. [PubMed] [Google Scholar]
- 20.Cooke, K.R., L. Kobzik, T.R. Martin, J. Brewer, J. Delmonte, J.M. Crawford, and J.L.M. Ferrara. 1996. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation. I. The roles of minor H antigens and endotoxin. Blood. 88:3230–3239. [PubMed] [Google Scholar]
- 21.Wells, A.D., H. Gudmundsdottir, and L.A. Turka. 1997. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J. Clin. Invest. 100:3173–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mowat, A. 1997. Intestinal graft versus disease. Graft-versus-Host Disease. J.L.M. Ferrara, H.J. Deeg, and S.J. Burakoff, editors. Marcel Dekker, New York. 337–384.
- 23.Williamson, E., P. Garside, J.A. Bradley, I.A.R. More, and A.M. Mowat. 1997. Neutralizing IL-12 during induction of murine acute graft-versus-host disease polarizes the cytokine profile toward a Th2-type alloimmune response and confers long term protection from disease. J. Immunol. 159:1208–1215. [PubMed] [Google Scholar]
- 24.Carson, W.E., J.E. Dierksheide, S. Jabbour, M. Anghelina, P. Bouchard, G. Ku, H. Yu, H. Baumann, M.H. Shah, M.A. Cooper, et al. 2000. Coadministration of interleukin-18 and interleukin-12 induces a fatal inflammatory response in mice: critical role of natural killer cell interferon-gamma production and STAT-mediated signal transduction. Blood. 96:1465–1473. [PubMed] [Google Scholar]
- 25.Sykes, M., D.A. Pearson, P.A. Taylor, G.L. Szot, S.J. Goldman, and B.R. Blazar. 1999. Dose and timing of interleukin (IL)-12 and timing and type of total-body irradiation: effects on graft-vs.-host disease inhibition and toxicity of exogenous IL-12 in murine bone marrow transplant recipients. Biol. Blood Marrow Transplant. 5:277–284. [DOI] [PubMed] [Google Scholar]
- 26.Hill, G.R., K.R. Cooke, T. Teshima, J.M. Crawford, J.C. Keith, Y.S. Brinson, D. Bungard, and J.L.M. Ferrara. 1998. Interleukin-11 promotes T cell polarization and prevents acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Clin. Invest. 102:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel, R.M., F.K. Chan, H.J. Chun, and M.J. Lenardo. 2000. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat. Immunol. 1:469–474. [DOI] [PubMed] [Google Scholar]
- 28.Liu, Y., and C.A. Janeway, Jr. 1990. Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J. Exp. Med. 172:1735–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy, W.J., V. Kumar, and M. Bennett. 1987. Acute rejection of murine bone marrow allografts by natural killer cells and T cells. Differences in kinetics and target antigens recognized. J. Exp. Med. 166:1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda, K., H. Tsutsui, T. Yoshimoto, O. Adachi, N. Yoshida, T. Kishimoto, H. Okamura, K. Nakanishi, and S. Akira. 1998. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 8:383–390. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto, I., K. Kohno, T. Tanimoto, H. Ikegami, and M. Kurimoto. 1999. Development of CD8+ effector T cells is differentially regulated by IL-18 and IL-12. J. Immunol. 162:3202–3211. [PubMed] [Google Scholar]
- 32.Konieczny, B.T., Z. Dai, E.T. Elwood, S. Saleem, P.S. Linsley, F.K. Baddoura, C.P. Larsen, T.C. Pearson, and F.G. Lakkis. 1998. IFN-gamma is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J. Immunol. 160:2059–2064. [PubMed] [Google Scholar]
- 33.Dey, B.R., Y.G. Yang, G.L. Szot, D.A. Pearson, and M. Sykes. 1998. Interleukin-12 inhibits graft-versus-host disease through an Fas-mediated mechanism associated with alterations in donor T-cell activation and expansion. Blood. 91:3315–3322. [PubMed] [Google Scholar]
- 34.Welniak, L.A., B.R. Blazar, R.H. Wiltrout, M.R. Anver, and W.J. Murphy. 2001. Role of interleukin-12 in acute graft-versus-host disease(1). Transplant Proc. 33:1752–1753. [DOI] [PubMed] [Google Scholar]
- 35.Itoi, H., Y. Fujimori, H. Tsutsui, K. Matsui, S. Futatsugi, H. Okamura, H. Hara, T. Hada, E. Kakishita, and K. Nakanishi. 2001. Fas ligand-induced caspase-1-dependent accumulation of interleukin-18 in mice with acute graft-versus-host disease. Blood. 98:235–237. [DOI] [PubMed] [Google Scholar]
- 36.Lauwerys, B.R., J.C. Renauld, and F.A. Houssiau. 1998. Inhibition of in vitro immunoglobulin production by IL-12 in murine chronic graft-vs.-host disease: synergism with IL-18. Eur. J. Immunol. 28:2017–2024. [DOI] [PubMed] [Google Scholar]
- 37.Li, X.C., T.B. Strom, L.A. Turka, and A.D. Wells. 2001. T cell death and transplantation tolerance. Immunity. 14:407–416. [DOI] [PubMed] [Google Scholar]
- 38.Williamson, E., P. Garside, J.A. Bradley, and A.M. Mowat. 1996. IL-12 is a central mediator of acute graft-versus-host disease in mice. J. Immunol. 157:689–699. [PubMed] [Google Scholar]