Abstract
Five CD1 molecules are expressed in humans and it is unclear whether they have specialized or redundant functions. We found that sulfatide is a promiscuous CD1-binding ligand and have isolated T cell clones that are specific for sulfatide and restricted by distinct CD1 molecules. These clones have been used to compare the capacity of different CD1 to present the same glycolipid, to induce effector functions, and to form persistent immunogenic complexes. CD1a, CD1b, and CD1c molecules similarly load sulfatide on the cell surface without processing, and prime Th1 and Th2 responses. Stimulation by sulfatide-loaded CD1a persists much longer than that by CD1b and CD1c in living cells. Use of recombinant soluble CD1a confirmed the prolonged capacity to stimulate T cells. Moreover, other glycosphingolipids bind to all CD1, which suggests the presence of additional promiscuous ligands. Thus, group I CD1 molecules present an overlapping set of self-glycolipids, even though they are quite divergent from an evolutionary point of view.
Keywords: sulfatide, CD1, antigen presentation, αβ T cells, glycolipids
Introduction
T cells recognize foreign proteins as small peptides presented by MHC antigen-presenting molecules. In addition, some T cells recognize exogenous glycolipids associated with MHC-like CD1 molecules (1–3). Human APC may express five types of CD1 molecules that are grouped into two families. Group I includes CD1a, CD1b, CD1c, and CD1e, whereas group II is represented only by CD1d. CD1 molecules are predicted to share similar structures. All of them bind and present glycolipids to specific T cells.
In some aspects, the immunogenicity of glycolipids parallels that of proteins. Like exogenous proteins, certain glycolipids require internalization into APC to form complexes with CD1 molecules (4). Instead, other glycolipids associate with CD1 molecules on the cell surface (5–7), thus resembling small peptides that bind to MHC molecules without being internalized. Like proteins, glycolipids may also be subject to processing to give rise to immunogenic complexes with CD1 molecules. One example of a synthetic glycolipid containing a disaccharide that becomes stimulatory after endosomal processing has recently been described (8). However, this is not a general rule because glycolipids that bear large carbohydrates bind to CD1 and efficiently stimulate T cells without any processing (6). Therefore, the presence of large carbohydrates is compatible with CD1 binding and TCR interaction.
The formation of stable complexes between the antigen and the presenting molecule is an important shared property of MHC and CD1 systems. The capacity of MHC molecules to anchor the peptidic antigen is dependent on intimate interactions between pockets in the MHC structure and specific amino acids in the bound peptide. Similar direct interactions also occur between CD1 molecules and glycolipids. Within the CD1 groove there are two large hydrophobic pockets that anchor the lipid tails of glycolipids (9, 10). Although the peptide structural requirements for binding to MHC alleles have been thoroughly investigated, the lipid structural requirements for CD1 binding are only partially understood.
In contrast to MHC molecules, there is no evidence of the existence of functionally relevant CD1 alleles. It is also not known whether the five CD1 molecules represent a redundant system for presentation of glycolipids to T cells, or whether during evolution each CD1 isotype has acquired individual properties of unique physiological relevance. One important distinction between the different CD1 molecules is that they recycle and may load exogenous antigens in distinct intracellular compartments (7, 11, 12). This feature might specialize each CD1 molecule in surveillance of infectious agents confined within different cellular organelles (7).
A second difference is the tissue distribution of CD1 molecules. CD1a, CD1b, and CD1c molecules are expressed on cortical thymocytes, on dendritic cells (DC)* in both lymphoid and nonlymphoid organs, and are inducible by exposure to GM-CSF (13). However, CD1a is highly expressed by Langerhans cells in the dermis and epidermis, whereas CD1b is present in macrophages infiltrating chronic inflammatory areas, such as atheromatous plaques (14), nerve fibers in polyradiculoneuropathy (15), and demyelinating areas of brain in multiple sclerosis patients (16). Instead, CD1c is expressed on a subpopulation of circulating B cells, in the mantle zone of the spleen, and in tonsillar B cells (17). It is unclear whether the unequal tissue distribution reflects a nonredundant function of individual CD1 molecules.
So far it has been difficult to evaluate the possible redundancy in the CD1 system. One important issue is whether CD1 molecules have developed the capacity to bind different ligands, or whether they present the same glycolipids to T cells. If they present overlapping sets of glycolipids, T cells specific for the same antigen but restricted by different CD1 may be recruited in vivo to different anatomical sites, according to the distribution of CD1+ APC. This would increase the number of T cells recognizing that same antigen and have important implications for both antimicrobial and autoimmune responses. A second important issue is whether different CD1 molecules form complexes of equivalent or differing immunogenic potential. Light on this issue would clarify whether there is preferential stimulation of T cells by the different CD1 molecules. Differences in CD1 immunogenicity would have direct implications for the development of new vaccines based on CD1-binding glycolipids.
This study has addressed these two issues by comparing antigen presentation among CD1 molecules. The experimental model selected was T cell recognition of sulfatide. Sulfatide is made by a galactose sugar, modified by a sulfate ester in position 3, and connected with a β-glycosidic bond to a ceramide. Ceramides are amides of acyl groups with long chain dihydroxy or trihydroxy bases, the most common in animals being C18 sphingosine. The acyl group of ceramides is generally a long chain saturated or monounsaturated fatty acid. Sulfatide is formed from a galactosylceramide molecule by a sulfotransferase reaction. This enzyme is very active during the myelinization process. Interestingly, sulfatide is also found in tissues that are very active in sodium transport (kidney, salt glands, and gills). Here we show that sulfatide is a promiscuous ligand that binds group I CD1 molecules and is presented by CD1a, CD1b, and CD1c to specific T cells.
Materials and Methods
Glycolipid Antigens.
The following purified glycolipids were purchased from Fluka: sulfatide, galactosylceramide (GalCer), glucosylceramide (GlcCer), and sphingomyelin. Sulfatide, GM1, and lactosylceramide (LacCer) were purchased from Matreya. Semisynthetic sulfatide containing stearic acid or 14C-stearic acid as acyl moiety was purchased from Anawa. Purified ganglioside GM4 was kindly provided by S. Sonnino (University of Milano, Milano, Italy). All the glycolipids were 98–99% pure according to TLC analysis.
Desulfation of Sulfatide.
The sulfate group of sulfatide was hydrolyzed essentially as described previously (18). In brief, 1 mg sulfatide was incubated in 1 ml of methanol with 50 mM of HCl at room temperature. After 16 h, 3 ml of chloroform, 0.5 ml of methanol, and 1.1 ml of 0.2% Na2CO3 were added. The lower phase was washed with 2 ml of chloroform:methanol:0.1 M KCl (3:48:47, vol/vol) and then with 2 ml of chloroform:methanol:H2O (3:48:47, vol/vol). Efficiency of sulfatide desulfation was tested by TLC on Silica gel high performance thin layer chromatography plates (Merck) using chloroform:methanol:0.25% KCl (50:40:10, vol/vol) as run-in solvent. Glycolipids were visualized by orcinol staining.
T Cell Clones and APCs.
Sulfatide-specific T cell lines and clones were derived as described previously (19). DC were isolated from peripheral blood mononuclear cells by culturing in the presence of IL-4 and recombinant GM-CSF, as described previously (13). Each preparation of DC was tested for the expression of CD1 molecules using mAbs specific for CD1a (OKT6, ATCC CRL8019; American Type Culture Collection), CD1b (WM-25; Immunokontakt), and CD1c (L161; Instrumentation Laboratory). CD1a-, CD1b-, CD1c-, CD1d-, or mock-transfected C1R lymphoblastoma cells (provided by S. Porcelli, Albert Einstein College of Medicine, New York, NY) were used as APC in some experiments.
Antigen Presentation Assays.
DC (5 × 104/well) or CD1-transfected C1R cells (5 × 104/well) in RPMI-1640 medium containing 10% FCS were preincubated for 2 h at 37°C with sonicated antigen (0.01–20 μM) before the addition of T cells (6 × 104/well in triplicate). Supernatants were harvested after 36 h and released cytokines were measured by ELISA. TNF-α and IFN-γ were detected using sandwich ELISA kits according to manufacturer's instruction (Instrumentation Laboratory). IL-4 was detected using anti–IL-4 mAbs (BD PharMingen). Data are expressed as mean pg/ml ± SD of triplicates. All experiments were repeated at least two times.
For antibody blocking experiments, DC were preincubated with 3 μM of sulfatide and then with OKT6, WM25, or L161 mAbs, or with Fab fragments of TR66 mAbs (anti-CD3ε) for 20 min before the addition of T cells. Anti-TCRVγ9 mAbs were used as the isotype-matched control.
Fixation of DC.
DC were washed and suspended in PBS (2–3 × 106/ml) containing 0.05% glutaraldehyde for 30 s at 37°C. Additional fixation was blocked with 0.2 M of lysine. The efficiency of fixation was controlled by the [3H]thymidine incorporation of fixed cells and also by the stimulation of an MHC class II–restricted purified protein derivative (PPD)-specific T cell clone. In some experiments, APC were first fixed and then washed and pulsed with 10 μM of sulfatide for 1 h before the addition of T cells.
To investigate the requirements for antigen internalization, DC (106) were preincubated for 1 h at 4°C, or at 37°C, in the presence or absence of 80 μM of chloroquine, or 20 μM of monensin, and then pulsed with 10 μM of sulfatide for an additional 1 h. After washing, DC were fixed and used to stimulate T cells.
Intracellular Staining for IL-4 and IFN-γ.
T cell lines established for 12 d were used in these assays. Although the number of sulfatide-specific T cells was small, this time point was chosen to reduce in vitro bias for cytokine production. Intracellular staining was performed on T cells activated by CD1a-, CD1b-, CD1c-, or mock-transfected C1R cells pulsed with 10 μM of sulfatide. After 3 h, Brefeldin A (5 μg/ml; Sigma-Aldrich) was added and incubated for an additional 12 h. After washing, the cells were fixed with 2% of paraformaldehyde and permeabilized with 0.1% of saponin. Four color immunofluorescence analysis was performed using anti–CD3-ECD (Immunotech), anti–CD25-FITC, anti–IL-4–PE, and anti–IFN-γ–allophycocyanin mAbs (all from BD PharMingen). Cells were analyzed on a FACSVantage® SE (Becton Dickinson), gated according to forward scatter and side scatter, and then on the CD3+CD25+ double-positive population.
Displacement of Bound Sulfatide.
Displacement studies were performed by using fixed DC pulsed with 60 μM of sulfatide for 1 h at 37°C, and then extensively washed and plated (3 × 104/well) with various doses of other glycolipids (6–60 μM) for 1 h before the addition of T cells (5 × 104/well). Isopentenyl-pyrophosphate–specific Vγ9Vδ2 T cell clone G2B9 was used as the negative control.
Persistence of CD1–Sulfatide Complexes.
6 × 106 of DC were pulsed with 60 μM of sulfatide at 37°C for 2 h, and then washed three times and plated (4 × 105 cells/well) in triplicates. At different time points, DC were again washed twice before the addition of T cells (5 × 105 cells/well). At each time point in control wells, 10 μM of sulfatide was added to assess the maximal presentation capacity of DC. Results are expressed as a percentage of control at each time point. As another control, CD1a, CD1b, and CD1c expression was tested by immunofluorescence, and median fluorescence values did not change during the culture periods.
Generation of Soluble CD1a (sCD1a).
Recombinant sCD1a and β2 microglobulin (β2m) were obtained as previously described for soluble CD1b (6). In vitro refolding of CD1a β2m was done by dilution at pH 7.2 in the presence of sulfatide. After concentration on Amicon 10K, the refolded sCD1a–sulfatide complexes were purified from aggregated proteins on a HiLoad 16/60 Superdex 200 column (Amersham Pharmacia Biotech). The purity of soluble protein was >90% as assessed by Coomassie staining.
Ligand Binding to sCD1a and T Cell Stimulation Assay.
To test the stability of sCD1a–sulfatide complexes, refolded sCD1a was loaded with 2 μM of [14C]sulfatide at room temperature for 2 h. The excess [14C]sulfatide was removed by size-exclusion chromatography using Superdex 200 10/30 column (Amersham Pharmacia Biotech), and purified sCD1a–sulfatide complexes were incubated for 1, 4, and 7 d at 18°C. At each time point, dissociated [14C]sulfatide was separated by size-exclusion chromatography, and radioactivity associated with sCD1a was measured by liquid scintillation. To assess proper refolding, sCD1a was tested in a sandwich ELISA using anti-β2m and anti-CD1a mAbs.
For activation assays, 30 μg/ml of sCD1a was preincubated with 10 μM of sulfatide and immobilized on 96-well plates. After washing away the excess protein and sulfatide, T cells were plated (1 × 105/well) with 1 ng/ml of PMA. Released cytokines were measured after 24 h of incubation.
Results
CD1a, CD1b, and CD1c Molecules Present Sulfatide to Specific T Cells.
A panel of CD1-restricted T cell lines specific for sulfatide was established from peripheral blood of multiple sclerosis patients and normal donors. T cell clones were obtained by limiting dilution and scored for antigen specificity and CD1 restriction using CD1-transfected APC. A total of 58 sulfatide-specific T cell clones were isolated from two different lines. Reverse transcription-PCR analysis of the TCR BV genes of 26 clones showed that 9 out of 16 CD1a-restricted clones and 8 out of 10 CD1b-restricted clones present different rearrangements (unpublished data). It is reasonable to conceive that ∼50% of the 58 isolated clones are different cells. Most of the T cell clones only reacted to sulfatide-pulsed APC, whereas one CD1a-restricted clone was also weakly activated by DC in the absence of exogenous sulfatide (unpublished data). These latter findings could be attributed to pulsing with sulfatide present in the serum or produced by the APC themselves. Interestingly, only DC, and not B cells transfected with the CD1a gene, activated this clone.
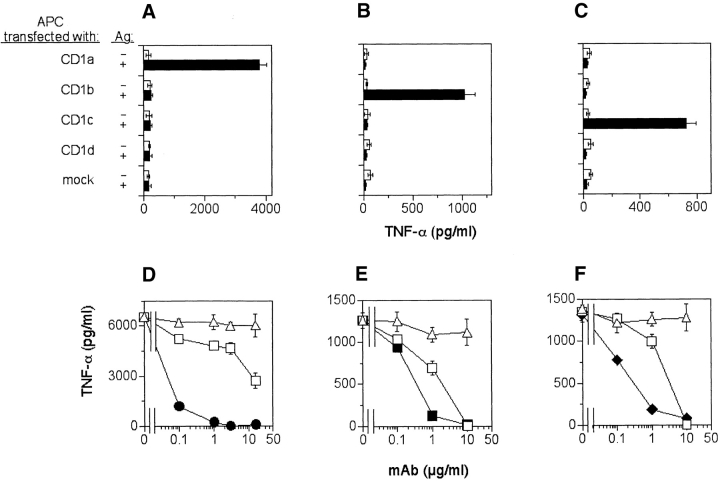
Experiments conducted with CD1 transfectants showed that 19 clones were restricted by CD1a, 38 by CD1b, and 1 by CD1c. Fig. 1, A–C shows three examples of clones, each restricted by an individual CD1 molecule. The restriction by different CD1 molecules was confirmed by inhibition with specific relevant anti-CD1 mAbs, whereas control irrelevant mAbs had no effects (Fig. 1, D and F). Inhibition was also observed with anti-CD3ε mAb Fab fragments (Fig. 1, D and F), implying TCR-mediated activation. Most of the clones were CD4+, and only four were CD8+. All clones were TCR αβ+ and did not express the invariant Vα24/Vβ11 TCR present on NK T cells (unpublished data).
Figure 1.
Presentation of sulfatide by CD1a, CD1b, and CD1c antigen-presenting molecules. (A–C) CD1a-, CD1b-, CD1c-, CD1d-, or mock-transfected C1R cells were pulsed with 10 μM of sulfatide and used to stimulate the following T cell clones: K34B9.1 (A and D, CD1a restricted), DS1C9b (B and E, CD1b restricted) and DS1B9c (C and F, CD1c restricted). Stimulation in the presence (solid bars) or the absence (open bars) of sulfatide is shown. (D–F) Presentation of sulfatide by DC to the specific T cell clones was blocked by anti-CD1a (•), anti-CD1b (▪), anti-CD1c (♦), or anti-TCR Fab (□) fragments, but not by isotype-matched control mAbs (▵).
Fine Antigen Specificity of Sulfatide-reactive T Cells.
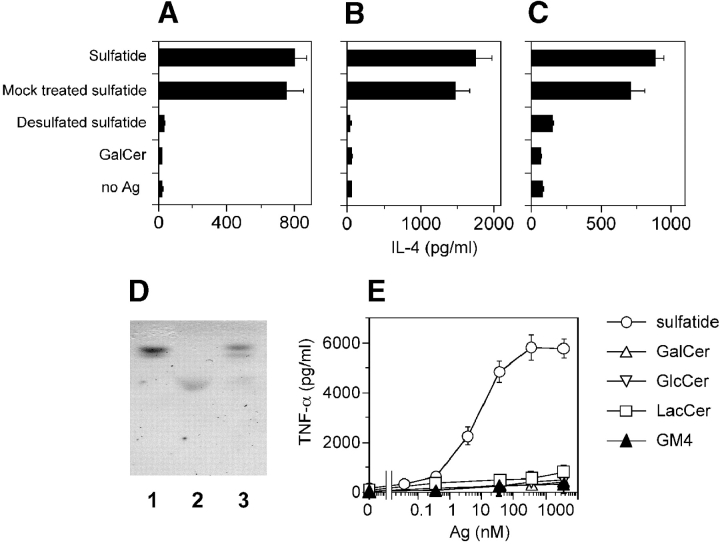
The above results show that sulfatide is a promiscuous ligand capable of forming immunogenic complexes with all group I CD1 molecules. As the bulk cell lines were established using a highly pure preparation of sulfatide from bovine brain, it was important to confirm that the stimulatory ligand is indeed sulfatide and not minor contaminating products. The following experiments confirmed that sulfatide is the active ligand. First, when sulfatide was desulfated (Fig. 2 D) it was no longer active (Fig. 2, A–C), thus confirming the importance of the sulfate group. Second, the purity of sulfatide was confirmed to be >98% by gas chromatography-mass spectrometry and its structure was validated by 1H-nuclear magnetic resonance (unpublished data). Third, dose response studies showed that most of the clones were reactive even to doses of 1–10 nM (Fig. 2 E), thus making it unlikely that minor contaminants are the stimulatory ligands. The fine antigen specificity of T cell clones was also tested using a panel of highly pure glycolipids. Only four clones weakly cross reacted with β GalCer, which lacks the sulfate group present in sulfatide. None of the clones reacted to either GlcCer, LacCer, GM4 (Fig. 2 E), sphingomyelin, or GM1 ganglioside (unpublished data), all of which share the ceramide tail with sulfatide. These findings confirm the sulfatide specificity of the isolated clones and demonstrate that their recognition is highly sensitive to changes in the carbohydrate moiety of sulfatide.
Figure 2.
Fine antigen specificity of sulfatide-specific T cell clones. (A–C) Desulfation abolishes immunogenicity of sulfatide. DC were incubated with 10 μM of sulfatide, mock-treated sulfatide, desulfated sulfatide, or GalCer before the addition of the T cell clones K34B9.1 (A, CD1a restricted), DS1C9b (B, CD1b restricted), and DS1B9c (C, CD1c restricted). (D) Thin layer chromatographic analysis of GalCer (lane 1), sulfatide (lane 2), and sulfatide after desulfation (lane 3). (E) Dose response of a representative T cell clone (K34B9.1, CD1a restricted) stimulated with DC pulsed with sulfatide, GalCer, GlcCer, LacCer, and ganglioside GM4. Similar results were obtained with most of the clones and also with IL-4 ELISA (unpublished data). The results are representative of three independent experiments.
Group I CD1 Molecules Show Common Sulfatide Presentation Requirements.
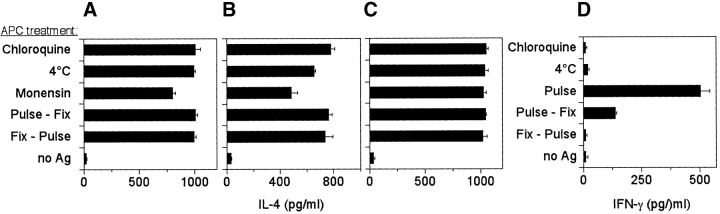
The generation of T cell clones restricted by different CD1 molecules and with the same antigen specificity offered for the first time the possibility of comparing the antigen presentation requirements of CD1a, CD1b, and CD1c. DC, which express all of the CD1 molecules, were pulsed with sulfatide at 4°C or in the presence of monensin (to inhibit CD1 recycling) or chloroquine (to block endosomal acidification). These treatments did not inhibit sulfatide presentation by CD1a, CD1b, or CD1c isoforms (Fig. 3, A–C) , whereas they completely inhibited presentation to MHC class II–restricted T cells (Fig. 3 D). When DC were first fixed and then pulsed with sulfatide, the T cell clones were also efficiently activated (Fig. 3, A–C). Thus, neither internalization of sulfatide, the presence of acidified endosomes, nor CD1 recycling are necessary for the generation of immunogenic complexes between sulfatide and each of the three group I CD1 molecules.
Figure 3.
CD1a, CD1b, and CD1c molecules do not require internalization and endosomal acidification for sulfatide presentation. DC were pretreated under different conditions (as described in Materials and Methods), and then pulsed with sulfatide and fixed before the addition of the T cell clones K34B9.1 (A, CD1a restricted), DS1C9b (B, CD1b restricted), and DS1B9c (C, CD1c restricted). (D) The same treatments completely abolished the response of the clone GP2.7, which is specific for PPD and HLA-DR restricted. Fix-Pulse stands for the fixation of APC before pulsing with the antigen. Results are representative of three independent experiments.
CD1 Restriction Does Not Influence the T Cell Functional Phenotype.
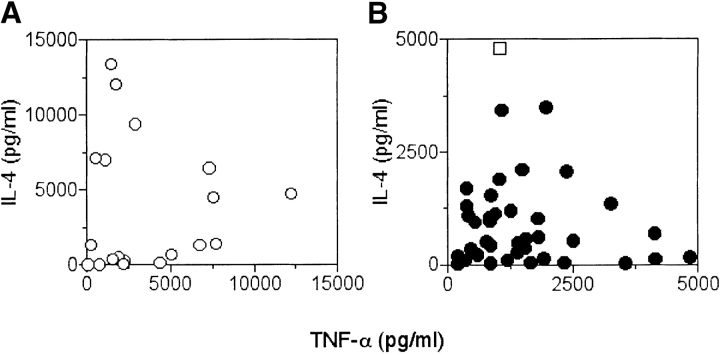
Next, we investigated whether restriction by different CD1 molecules may influence lymphokine release. Evaluation of IL-4 and TNF-α release by each T cell clone after stimulation with sulfatide-pulsed DC showed no bias by CD1 restriction. Clones showed a Th1, Th2, or Th0 phenotype (Fig. 4) . To estimate the extent of in vitro–induced functional maturation, intracellular staining was performed on two freshly established sulfatide-specific bulk lines. Each line was challenged with CD1a-, CD1b-, CD1c-, or mock-transfected APC and then intracellular cytokines were studied. Anti-CD25 and anti-CD3 mAbs were used to gate activated T cells, and anti–IL-4 and anti–IFN-γ mAbs to detect these two intracellular cytokines (Table I). A very small number of T cells (1%) up-regulated CD25 in the absence of sulfatide, whereas a larger fraction (3.5–11%) became CD25+ after stimulation with sulfatide (unpublished data). The number of T cells up-regulating CD25 in the presence of sulfatide was similar for the three groups of CD1 transfectants. The analysis of intracellular cytokines showed that most of the cells produce IL-4 or IFN-γ. Interestingly, only a few cells produced both lymphokines after sulfatide recognition.
Figure 4.
Functional phenotype of CD1-restricted and sulfatide-specific T cell clones. DC pulsed with 10 μM of sulfatide were used to stimulate (A) CD1a- (○), (B) CD1b- (•), or CD1c-restricted (□) T cell clones. After 36 h the released IL-4 and TNF-α were detected by ELISA. Results from 58 clones are shown.
Table I.
Cytokine Production of Freshly Established T Cell Lines
| Line | APC | IL-4+ | IFN-γ1 | IL-4+ and IFN-γ1 |
|---|---|---|---|---|
| 1 | Mock | 0.05 | 0.21 | 0.04 |
| CD1a | 2.98 | 2.56 | 0.14 | |
| CD1b | 1.62 | 2.34 | 0.05 | |
| CD1c | 1.23 | 4.12 | 0.12 | |
| 2 | Mock | 0.04 | 0.13 | 0.20 |
| CD1a | 0.18 | 4.52 | 0.10 | |
| CD1b | 1.53 | 7.56 | 0.12 | |
| CD1c | 1.16 | 1.42 | 0.50 |
Number represent percentage of CD3+ cells stained with cytokine-specific mAbs. Values represent the difference between groups stimulated in the presence or absence of sulfatide.
In conclusion, these experiments show that response to sulfatide can be either of a Th1 or Th2 type.
CD1–Sulfatide Complexes Are Displaced by Other Glycolipids.
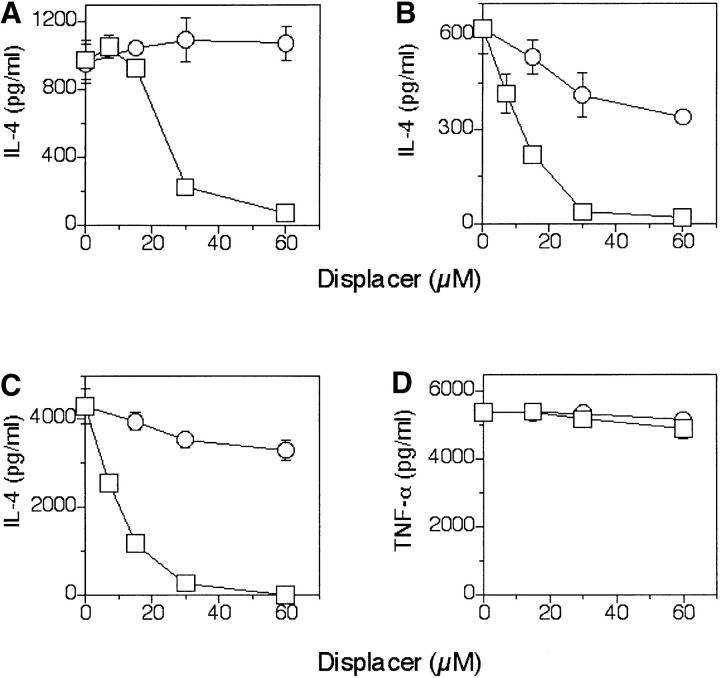
We have previously shown that self-glycosphingolipids bound to CD1b molecules are readily displaced by ligands that have an appropriate CD1b binding structure (6). Therefore, we asked whether sulfatide bound to each of the three CD1 molecules is displaceable. Fixed DC first pulsed with sulfatide and then incubated with increasing amounts of GM1 showed a reduced stimulation of all CD1-restricted T cells (Fig. 5, A–C) . The same concentration of GM1 did not influence the response of control TCR γδ T cells to their specific ligand (Fig. 5 D). Although GM1 at high doses almost completely eliminated T cell responsiveness, sphingomyelin was much less inhibitory even at high doses exceeding the critical micelle concentration (Fig. 5, A–C). Therefore, not all ligands containing a ceramide tail displace CD1-bound antigens.
Figure 5.
Sulfatide previously bound to CD1 is displaced by other glycolipids. (A–C) Fixed DC pulsed with high doses of sulfatide (60 μM) and then incubated with various doses of GM1 (□) or sphingomyelin (○) were used to stimulate the T cell clones K34B9.1 (A, CD1a restricted), DS1C9b (B, CD1b restricted), and DS1B9c (C, CD1c restricted). (D) To demonstrate the lack of toxicity, control Vγ9Vδ2 T cells activated by isopentenyl pyrophosphate were included. The data shown is representative of three independent experiments.
CD1a–Sulfatide Complexes Persist Longer than CD1b or CD1c Complexes.
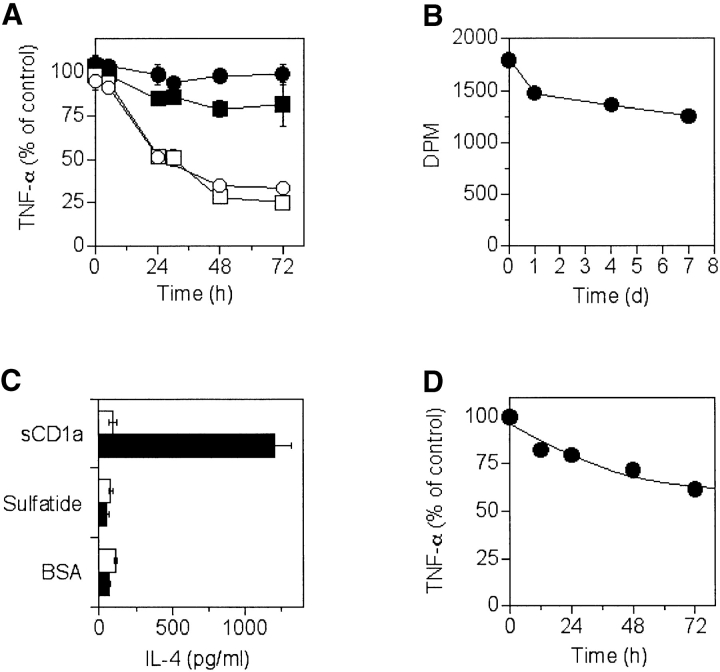
It is not known whether CD1 molecules differ in their capacity to form long-lived complexes with glycolipids. To address this issue, DC were pulsed with sulfatide, washed extensively, and then chased for different times before the addition of T cell clones restricted by different CD1 molecules. As positive controls, DC treated as described above were also incubated with fresh sulfatide to restore maximal presentation capacity before the addition of T cells. In these experiments a high dose of sulfatide was used to avoid a possible influence due to different antigen sensitivity of individual T cell clones. Stimulation by CD1b– and CD1c–sulfatide complexes decreased to 50% within 24 h and remained at ∼30% of the control levels after 72 h. Surprisingly, CD1a continued to present sulfatide with >80% efficiency after a 72-h chase (Fig. 6 A). This was confirmed using two different CD1a-restricted sulfatide-specific T cell clones. In some experiments DC were also washed before the addition of T cells to avoid a possible reuptake of free sulfatide, and again similar results were obtained (unpublished data). Furthermore, DC did not change surface expression of CD1 molecules over 72 h (unpublished data), thus excluding CD1 downmodulation as a limiting condition. These findings indicate that CD1a–sulfatide complexes generated in living cells are immunogenic longer than complexes formed with CD1b and CD1c.
Figure 6.
Persistence and stability of CD1–sulfatide complexes. (A) DC pulsed with 60 μM of sulfatide were used to stimulate the CD1a-restricted T cell clones K34B9.1 and DS1A16a (• and ▪, respectively), the CD1b-restricted clone DS1C9b (□), or the CD1c-restricted clone DS1B9c (○). Results are expressed as a percentage of controls (as described in Materials and Methods). (B) Soluble CD1a–[14C]sulfatide complexes were incubated for the indicated time at room temperature and the remaining radioactivity associated with sCD1a was measured after size exclusion chromatography (as described in Materials and Methods). (C) Soluble CD1a preincubated with sulfatide was immobilized and used to stimulate the CD1a-restricted sulfatide-specific T cell clone K34B9.1 (solid bars) or the MHC class II–restricted PPD-specific GP2.9 clone (open bars). Immobilized BSA or sulfatide were used as negative controls. (D) Immobilized sCD1a–sulfatide complexes were chased for the indicated time and used to stimulate the CD1a-restricted T cell clone K34B9.1 (as described in Materials and Methods).
Recombinant sCD1a–Sulfatide Complexes Are Stable and Immunogenic.
To study the stability of CD1a–sulfatide complexes, recombinant sCD1a molecules were generated. Radioactive sulfatide remained significantly associated with sCD1a for 7 d (71% of control values at time 0), even when the samples were maintained at room temperature, which shows a very stable interaction (Fig. 6 B). In similar experiments soluble recombinant mouse CD1d-α–GalCer complexes lost T cell stimulatory capacity after a 24-h chase (20).
Next, we studied whether the T cell stimulatory capacity of recombinant CD1a–sulfatide complexes might also persist for a long period as observed with living cells. The sCD1a molecules appeared properly refolded, as demonstrated by sandwich ELISA (unpublished data), because they stimulated CD1a-restricted sulfatide-specific T cells, but not T cells specific for another antigen (Fig. 6 C). When sulfatide-loaded sCD1a molecules were immobilized to plastic and chased for different times, they continued to stimulate specific T cells with an efficiency of 60% even after a 72-h chase (Fig. 6 D). These results confirm the data obtained with living cells, and clearly show that CD1a–sulfatide complexes are very stable and maintain T cell stimulatory capacity for a long time.
Discussion
Our results demonstrate that sulfatide can bind to CD1a, CD1b, and CD1c molecules, and stimulate specific T cells. Sulfatide is thus a promiscuous ligand for group I CD1 molecules and, in this respect, resembles promiscuous peptides that bind to several MHC molecules and activate T cells in a specific manner (21–23). The availability of a promiscuous CD1 ligand facilitated a series of comparative studies that were previously not possible with other known self- or bacterial glycolipids. This study has addressed three issues relevant to the physiology of glycolipid recognition by T cells.
The first issue concerns antigen presentation similarities and differences among group I CD1 molecules. Our data show that CD1a, CD1b, and CD1c have several common properties. Sulfatide is efficiently presented by all of them in the absence of endosomal acidification, internalization, and intracellular and extracellular processing. It remains to be determined whether CD1 molecules share antigen loading and presentation requirements for glycolipids that have lipid tails distinct from ceramide.
Furthermore, all CD1–sulfatide complexes are displaced by other glycolipids, which suggests that the immunogenicity of CD1–glycolipid complexes can be conditioned by the presence of other CD1 ligands. The importance of this feature, which represents an original and flexible regulation of antigen presentation for group I CD1 molecules, has to be confirmed in vivo.
Our results also demonstrate that CD1 molecules are characterized by relevant differences that mainly concern the persistence of stimulatory complexes. CD1a–sulfatide complexes remain immunogenic at least 72 h after chase. CD1b and CD1c, in contrast, have much shorter persistence, similar to that of mouse CD1d-α–GalCer complexes (20). Differential persistence may have several explanations. The first is provided by the high stability of the CD1a–sulfatide complexes, as revealed by experiments with recombinant CD1a molecules. CD1a and sulfatide remain associated in vitro over a period of 7 d and retain the capacity to stimulate T cells with high efficiency after 3 d of chase, even when immobilized. A second explanation is that the CD1–sulfatide complexes may differ in their susceptibility to displacement by other glycolipids. This seems unlikely, because all CD1–sulfatide complexes are displaced by GM1. However, even if testing a larger variety of glycolipids, this possibility cannot be excluded. A third explanation, albeit not investigated in this study, is that the different intracellular recycling pathways of individual CD1 molecules (7, 11, 12) affect the persistence of CD1–sulfatide complexes. Recycling in compartments with different pH might directly influence the stability of CD1 molecules and the binding of glycolipids.
Whatever the mechanism, what is of physiological importance is that all types of CD1–sulfatide complexes persist long enough to recruit specific T cells in vivo, and all are potentially immunogenic. If prolonged persistence of CD1a complexes is also applicable to other glycolipids, antigen presentation by CD1a might be perceived as being more efficient than that by other CD1 molecules. This hypothesis deserves careful investigation and should be challenged using microbial ligands to evaluate its possible therapeutic implications.
The second issue concerns the relationship between the sulfatide structure and its promiscuous binding to CD1. Our results extend the notion of structural requirements for CD1 binding. Sulfatide is composed of a ceramide tail linked through a β-1 glycosidic bond to galactose modified by a sulfate group. This is a small structure compared with that of other glycosphingolipids such as GM1, GD1a, GT1b, and GQ1a gangliosides, which are made of complex carbohydrates with five to eight monosaccharides and also activate specific T cells (6). It is unlikely that the promiscuous binding of sulfatide to CD1 is due to its small glycosidic moiety, because GM1, which is composed of five sugars, displaces sulfatide bound to CD1a, CD1b, and CD1c (Fig. 5), is presented by CD1b to specific T cells (6), and associates with mouse (20, 24) and human CD1d (unpublished data). Because the lipid moiety is responsible for anchoring glycolipids to CD1 (9, 10), promiscuous binding can be attributed to the ceramide tail, common to both sulfatide and gangliosides. Thus, and in consideration of available data from the literature (24, 25), ceramide might be considered a universal anchor for all CD1 molecules.
A point to emphasize is that different CD1 molecules can present the same lipids, although from an evolutionary point of view they are quite divergent. This is not what the analysis of CD1 restriction of the mycobacterial dolichol phosphate ligand has implied (26). In that case the observed predominant CD1c restriction could have arisen from important differences in immunogenicity rather than in binding capacity to individual CD1 molecules. It is difficult to envisage whether there is a biased CD1 binding in the case of other mycobacterial antigens, such as mycolic acid (27), lipoarabinomannan (4), and glucose monomycolate (28), which are presented by CD1b. This is due to the small number of T cell clones with these antigen specificities that have been isolated and described, so far. If the presentation of an overlapping set of ligands is the common rule, a major rationale for maintaining different CD1 molecules would be the pattern of tissue expression or perhaps the patrol of different endosomal compartments (11) (not as relevant for sulfatide), rather than the ability to present different lipids.
A third issue concerns the significance of CD1 presentation of self-glycolipids and the functional role of specific T cells. Self-glycolipids might represent low avidity ligands with the function of selecting the CD1-restricted repertoire in the thymus. In the periphery of normal donors these ligands might instead facilitate the persistence of naive CD1-restricted T cells, as reported for self-peptides that allow the survival of naive MHC-restricted T cells (29–31).
Our experimental findings show that the function of self-glycolipid–reactive T cells is not biased by the CD1 restriction and that both Th1 and Th2 cells are primed by all group I CD1 molecules. Thus, T cells reactive with self-glycosphingolipids may function either as proinflammatory or helper cells. It is not clear whether these are related to disease, homeostasis, or both. We have found an increased frequency of self-glycolipid–reactive T cells in patients with multiple sclerosis. However, the same type of antigen specificities were also detected in normal donors (6, 19), thus leaving open each possibility. Because these autoreactive lymphocytes are not always associated with disease, they might exert important regulatory functions whenever alterations of glycolipid production or presentation occur. This raises questions as to whether T cell tolerance to self-glycolipids exists and how such tolerance is broken. Sulfatide is one of the most abundant glycolipids in brain tissue, an important constituent of almost all membranes, and found in the serum at concentrations of 0.5–2.0 μM (32). Anti-sulfatide antibodies are present in normal donors and have been observed in patients with peripheral neuropathies associated with IgM paraproteinaemia, or after HIV infection (33–35). In all of these instances, mainly IgM anti-sulfatide antibodies are found. In contrast, prediabetic and newly diagnosed type 1 diabetic patients, and those with dilatative cardiomyopathy and developing Chagas' disease after Trypanosoma cruzi infection, have exceptionally high amounts of IgG anti-sulfatide antibodies (36, 37). In these patients, the priming and expansion of pathogenic sulfatide-specific Th cells could explain the presence of IgG autoantibodies. In the case of Chagas' disease, the priming of T cells could be provided by trypanosomal glycolipids, known to structurally resemble sulfatide (38). Thus, mimicry of microbial ligands with self-glycolipids might represent a potential mechanism to prime autoreactive T cells and break self-tolerance to glycolipids.
In conclusion, like peptides, some glycolipids behave like promiscuous ligands by binding to different antigen-presenting molecules and stimulating specific T cells. It remains to be investigated how general this phenomenon is. Promiscuous CD1 presentation of sulfatide adheres to common rules including the stable binding to group I CD1 molecules, and is regulated by displacement with other ligands. However, the CD1 complexes do not always behave identically and differ in their in vivo persistence, which may bias antigen sampling and presentation. These findings provide a framework to further dissect the cellular and structural requirements for the formation, stability, and immunogenicity of CD1–glycolipid complexes.
Acknowledgments
We are grateful to S. Porcelli for providing us with CD1 transfectants and to S. Sonnino for GM4. We thank P. Dellabona, M. Kronenberg, R. MacDonald, and T. Resink for critical reading of the manuscript, and colleagues in our laboratory for discussions. We also thank N. Prokazova for performing gas chromatography-mass spectrometry and 1H-nuclear magnetic resonance analysis of sulfatide.
This work was supported by the Swiss National Foundation (grant NF 3100-055698.98), the Human Frontier Science Program (grant RG0168/2000-M), and The Swiss Multiple Sclerosis Society.
A. Shamshiev and H.-J. Gober contributed equally to this work.
Footnotes
Abbreviations used in this paper: β2m, β2 microglobulin; DC, dendritic cells; GalCer, galactosylceramide; GlcCer, glucosylceramide; LacCer, lactosylceramide; PPD, purified protein derivative; sCD1a, soluble CD1a.
References
- 1.Porcelli, S.A., and R.L. Modlin. 1999. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 17:297–329. [DOI] [PubMed] [Google Scholar]
- 2.Park, S.H., and A. Bendelac. 2000. CD1-restricted T-cell responses and microbial infection. Nature. 406:788–792. [DOI] [PubMed] [Google Scholar]
- 3.Matsuda, J.L., and M. Kronenberg. 2001. Presentation of self and microbial lipids by CD1 molecules. Curr. Opin. Immunol. 13:19–25. [DOI] [PubMed] [Google Scholar]
- 4.Sieling, P.A., D. Chatterjee, S.A. Porcelli, T.I. Prigozy, R.J. Mazzaccaro, T. Soriano, B.R. Bloom, M.B. Brenner, M. Kronenberg, P.J. Brennan, et al. 1995. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 269:227–230. [DOI] [PubMed] [Google Scholar]
- 5.Burdin, N., L. Brossay, Y. Koezuka, S.T. Smiley, M.J. Grusby, M. Gui, M. Taniguchi, K. Hayakawa, and M. Kronenberg. 1998. Selective ability of mouse CD1 to present glycolipids: alpha-galactosylceramide specifically stimulates V alpha 14+ NK T lymphocytes. J. Immunol. 161:3271–3281. [PubMed] [Google Scholar]
- 6.Shamshiev, A., A. Donda, T.I. Prigozy, L. Mori, V. Chigorno, C.A. Benedict, L. Kappos, S. Sonnino, M. Kronenberg, and G. De Libero. 2000. The alphabeta T cell response to self-glycolipids shows a novel mechanism of CD1b loading and a requirement for complex oligosaccharides. Immunity. 13:255–264. [DOI] [PubMed] [Google Scholar]
- 7.Briken, V., R.M. Jackman, G.F. Watts, R.A. Rogers, and S.A. Porcelli. 2000. Human CD1b and CD1c isoforms survey different intracellular compartments for the presentation of microbial lipid antigens. J. Exp. Med. 192:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prigozy, T.I., O. Naidenko, P. Qasba, D. Elewaut, L. Brossay, A. Khurana, T. Natori, Y. Koezuka, A. Kulkarni, and M. Kronenberg. 2001. Glycolipid antigen processing for presentation by CD1d molecules. Science. 291:664–667. [DOI] [PubMed] [Google Scholar]
- 9.Brenner, M., and S. Porcelli. 1997. Antigen presentation: a balanced diet. Science. 277:332. [DOI] [PubMed] [Google Scholar]
- 10.Zeng, Z., A.R. Castano, B.W. Segelke, E.A. Stura, P.A. Peterson, and I.A. Wilson. 1997. Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science. 277:339–345. [DOI] [PubMed] [Google Scholar]
- 11.Sugita, M., E.P. Grant, E. van Donselaar, V.W. Hsu, R.A. Rogers, P.J. Peters, and M.B. Brenner. 1999. Separate pathways for antigen presentation by CD1 molecules. Immunity. 11:743–752. [DOI] [PubMed] [Google Scholar]
- 12.Sugita, M., N. van Der Wel, R.A. Rogers, P.J. Peters, and M.B. Brenner. 2000. CD1c molecules broadly survey the endocytic system. Proc. Natl. Acad. Sci. USA. 97:8445–8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porcelli, S., C.T. Morita, and M.B. Brenner. 1992. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. 360:593–597. [DOI] [PubMed] [Google Scholar]
- 14.Melian, A., Y.J. Geng, G.K. Sukhova, P. Libby, and S.A. Porcelli. 1999. CD1 expression in human atherosclerosis. A potential mechanism for T cell activation by foam cells. Am. J. Pathol. 155:775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalili-Shirazi, A., N.A. Gregson, M. Londei, L. Summers, and R.A. Hughes. 1998. The distribution of CD1 molecules in inflammatory neuropathy. J. Neurol. Sci. 158:154–163. [DOI] [PubMed] [Google Scholar]
- 16.Battistini, L., F.R. Fischer, C.S. Raine, and C.F. Brosnan. 1996. CD1b is expressed in multiple sclerosis lesions. J. Neuroimmunol. 67:145–151. [DOI] [PubMed] [Google Scholar]
- 17.Porcelli, S.A. 1995. The CD1 family: a third lineage of antigen-presenting molecules. Adv. Immunol. 59:1–98. [DOI] [PubMed] [Google Scholar]
- 18.Natowicz, M.R., E.M. Prence, P. Chaturvedi, and D.S. Newburg. 1996. Urine sulfatides and the diagnosis of metachromatic leukodystrophy. Clin. Chem. 42:232–238. [PubMed] [Google Scholar]
- 19.Shamshiev, A., A. Donda, I. Carena, L. Mori, L. Kappos, and G. De Libero. 1999. Self glycolipids as T-cell autoantigens. Eur. J. Immunol. 29:1667–1675. [DOI] [PubMed] [Google Scholar]
- 20.Benlagha, K., A. Weiss, A. Beavis, L. Teyton, and A. Bendelac. 2000. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191:1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinigaglia, F., M. Guttinger, J. Kilgus, D.M. Doran, H. Matile, H. Etlinger, A. Trzeciak, D. Gillessen, and J.R. Pink. 1988. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 336:778–780. [DOI] [PubMed] [Google Scholar]
- 22.Panina-Bordignon, P., A. Tan, A. Termijtelen, S. Demotz, G. Corradin, and A. Lanzavecchia. 1989. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur. J. Immunol. 19:2237–2242. [DOI] [PubMed] [Google Scholar]
- 23.Hammer, J., P. Valsasnini, K. Tolba, D. Bolin, J. Higelin, B. Takacs, and F. Sinigaglia. 1993. Promiscuous and allele-specific anchors in HLA-DR-binding peptides. Cell. 74:197–203. [DOI] [PubMed] [Google Scholar]
- 24.Naidenko, O.V., J.K. Maher, W.A. Ernst, T. Sakai, R.L. Modlin, and M. Kronenberg. 1999. Binding and antigen presentation of ceramide-containing glycolipids by soluble mouse and human CD1d molecules. J. Exp. Med. 190:1069–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, et al. 1997. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 278:1626–1629. [DOI] [PubMed] [Google Scholar]
- 26.Moody, D.B., T. Ulrichs, W. Muhlecker, D.C. Young, S.S. Gurcha, E. Grant, J.P. Rosat, M.B. Brenner, C.E. Costello, G.S. Besra, and S.A. Porcelli. 2000. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 404:884–888. [DOI] [PubMed] [Google Scholar]
- 27.Beckman, E.M., S.A. Porcelli, C.T. Morita, S.M. Behar, S.T. Furlong, and M.B. Brenner. 1994. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 372:691–694. [DOI] [PubMed] [Google Scholar]
- 28.Moody, D.B., B.B. Reinhold, M.R. Guy, E.M. Beckman, D.E. Frederique, S.T. Furlong, S. Ye, V.N. Reinhold, P.A. Sieling, R.L. Modlin, G.S. Besra, and S.A. Porcelli. 1997. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 278:283–286. [DOI] [PubMed] [Google Scholar]
- 29.Hogquist, K.A., S.C. Jameson, and M.J. Bevan. 1995. Strong agonist ligands for the T cell receptor do not mediate positive selection of functional CD8+ T cells. Immunity. 3:79–86. [DOI] [PubMed] [Google Scholar]
- 30.Goldrath, A.W., and M.J. Bevan. 1999. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 11:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barton, G.M., and A.Y. Rudensky. 1999. Requirement for diverse, low-abundance peptides in positive selection of T cells. Science. 283:67–70. [DOI] [PubMed] [Google Scholar]
- 32.Sugiyama, E., A. Hara, and K. Uemura. 1999. A quantitative analysis of serum sulfatide by matrix-assisted laser desorption ionization time-of-flight mass spectrometry with delayed ion extraction. Anal. Biochem. 274:90–97. [DOI] [PubMed] [Google Scholar]
- 33.Petratos, S., V.J. Turnbull, R. Papadopoulos, M. Ayers, and M.F. Gonzales. 2000. High-titre IgM anti-sulfatide antibodies in individuals with IgM paraproteinaemia and associated peripheral neuropathy. Immunol. Cell Biol. 78:124–132. [DOI] [PubMed] [Google Scholar]
- 34.Isoardo, G., B. Ferrero, P. Barbero, A. Cucci, A. Oggero, A. Pipieri, A. Ricci, E. Verdun, B. Bergamasco, and L. Durelli. 2001. Anti-GM1 and anti-sulfatide antibodies in polyneuropathies. Threshold titers and accuracy. Acta Neurol. Scand. 103:180–187. [DOI] [PubMed] [Google Scholar]
- 35.Petratos, S., and M.E. Gonzales. 2000. Can antiglycolipid antibodies present in HIV-infected individuals induce immune demyelination? Neuropathology. 20:257–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buschard, K., K. Josefsen, T. Horn, and P. Fredman. 1993. Sulphatide and sulphatide antibodies in insulin-dependent diabetes mellitus. Lancet. 342:840. [DOI] [PubMed] [Google Scholar]
- 37.Avila, J.L., M. Rojas, and H. Carrasco. 1993. Elevated levels of antibodies against sulphatide are present in all chronic chagasic and dilated cardiomyopathy sera. Clin. Exp. Immunol. 92:460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petry, K., E. Nudelman, H. Eisen, and S. Hakomori. 1988. Sulfated lipids represent common antigens on the surface of Trypanosoma cruzi and mammalian tissues. Mol. Biochem. Parasitol. 30:113–121. [DOI] [PubMed] [Google Scholar]