Abstract
HIV-1 pathogenicity factor Nef has been shown to modulate calcium signaling in host cells, but the underlying molecular mechanisms have remained unclear. Here we show that calcium/calcineurin-dependent activation of nuclear factor of activated T cells (NFAT) by Nef in Jurkat T cells requires the endoplasmic reticulum-resident inositol trisphosphate receptor (IP3R), but yet does not involve increase in phospholipase-Cγ1 (PLCγ1)-catalyzed production of IP3 or depletion of IP3-regulated intracellular calcium stores. Nef could be coprecipitated with endogenous IP3R type-1 (IP3R1) from Nef-transfected Jurkat T cells as well as from HIV-infected primary human peripheral mononuclear cells. Thus, the Nef/IP3R1-interaction defines a novel T cell receptor–independent mechanism by which Nef can promote T cell activation, and appears to involve atypical IP3R-triggered activation of plasma membrane calcium influx channels in a manner that is uncoupled from depletion of intracellular calcium stores.
Keywords: accessory protein, capacitative calcium entry, IL-2, NFAT, T cell activation
Introduction
Nef is a 25–34 kD myristoylated protein of primate immunodeficiency viruses (HIV-1, HIV-2, simian immunodeficiency viruses [SIVs]), which plays an important role in the pathogenesis of AIDS. The molecular mechanisms underlying the pathogenic effect of Nef are still incompletely understood, but several lines of evidence indicate involvement of Nef-induced changes in the activity of cellular signaling pathways. Nef has no enzymatic activity, and instead functions as an adaptor bringing together different host cells proteins. A large number of proteins, mainly protein kinases and components of endocytic trafficking machinery, have been reported to bind to Nef (1–3).
The ability of HIV to replicate in T cells is strongly influenced by T cell activation, which regulates both pre- and postintegration steps in HIV life cycle (4, 5). Significant experimental attention has therefore been directed to address a possible role of Nef in T cell activation. Indeed, T lymphocytes from transgenic mice show hyperresponsive behavior suggesting that Nef can promote T cell activation (6, 7).
Up-regulation of IL-2 expression and subsequent autocrine stimulation by this cytokine are hallmarks of T cell activation. The transcription factor nuclear factor of activated T cells (NFAT)* is a critical mediator of increased IL-2 gene expression during T cell activation (8, 9). NFAT is composed of a cytoplasmic NFAT core factor that translocates to the nucleus upon dephosphorylation by the calcium-regulated phosphatase calcineurin, and a preexisting nuclear component (typically consisting of members of the activator protein-1 [AP-1] family of transcription factors) that is activated by the mitogen-activated protein kinase (MAPK) cascade (8, 10). Binding of TCR to its ligand, provided by an antigen-presenting cell or experimental activation by TCR–cross-linking antibodies, results in activation of the MAPK-cascade and the Ca2+/calcineurin pathway. Thus, TCR engagement is sufficient to trigger both of the major T cell signaling pathways required for activation of NFAT-mediated gene expression (8).
Stimulation of T cells via TCR initiates sequential activation of TCR-associated protein- and lipid kinases, which leads to membrane localization and tyrosine phosphorylation of phospholipase-Cγ1 (PLCγ1) (11). This results in increased catalytic activity of PLCγ1, and thereby production of diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). IP3 binds to and activates inositol 1,4,5-trisphosphate receptor (IP3R), which functions as a calcium channel controlling the release of calcium from intracellular stores in the endoplasmic reticulum (ER) (12, 13). This results in a transient increase in free cytoplasmic calcium, which in itself is insufficient to promote transcriptional activation in T cells (14, 15). Instead, the depleted calcium stores in the ER serve as a trigger that results in opening of calcium channels in the plasma membrane, and thereby calcium influx from outside of the cell (12, 13). This process is known as capacitative calcium entry (CCE) or store-operated calcium entry (SOCE) (16, 17).
The identity of store-operated channels (SOCs) on the plasma membrane has remained unclear. A recent report (18) has provided substantial evidence suggesting that CaT1, a Ca2+ channel previously isolated from intestinal cells (19), would be a SOC. However, other evidence suggests that mammalian homologues of Drosophila Trp (transient receptor potential) may represent components of these channels at least in some cell types (17). The SOC found in T cells is called Ca2+ release-activated calcium channel (CRAC) (12, 13). A major open question in this field concerns the nature of the signal generated by depleted ER calcium stores to cause opening of SOCs/CRACs. While some studies have suggested a diffusible signaling factor (“calcium influx factor” hypothesis), a number of recent findings favor an alternative hypothesis known as “conformational coupling model,” which involves direct physical communication between IP3R and SOC/CRAC (12, 17).
The role of calcium metabolism in Ca2+/calmodulin-regulated, calcineurin-mediated activation of NFAT has been elucidated in detail. These studies have established that a sustained elevation in cytoplasmic free calcium, which in nonexcitable cells such as T lymphocytes, is dependent on calcium influx via CRACs, is necessary to keep NFAT in the nucleus (15, 20). Engagement of the TCR normally triggers a series of oscillating Ca2+ spikes (12, 13). Interestingly, the frequency of these spikes seems to be important for optimal NFAT activation, thus providing specificity to calcium-regulated signaling processes (21, 22). Because of the oscillating nature of these changes, NFAT can be efficiently activated by only a small net increase in cytoplasmic calcium levels (21).
Recently, Simmons et al. demonstrated by gene expression profiling that expression of Nef in Jurkat T cells can trigger a transcriptional program that is nearly identical to that induced by anti-CD3 antibodies (23). Moreover, two other studies have observed potentiation of TCR-triggered activation of T cells by Nef, as measured by increased NFAT-dependent gene expression and IL-2 secretion (24, 25). Because of the observed accumulation of Nef in lipid rafts enriched in TCR-associated signaling proteins, it was suggested that Nef could mediate this positive effect by facilitating interactions among these signaling proteins (25). However, other studies have concluded that expression of Nef either inhibits or has no effects on TCR-mediated activation (26–31). The ability of Nef to cause downmodulation of cell surface expression of important components of the TCR complex, such as TCRζ chain and CD28, could be one factor accounting for the differing results of these studies (32, 33).
On the other hand, we have recently described an additional TCR-independent mechanism by which Nef can contribute to T cell activation (34, 35). We found that activation of the MAPK-cascade alone was sufficient to cause a robust activation of NFAT-dependent gene expression in T cells expressing high levels of Nef. Studies in Lck-negative cells and experiments using dominant-negative expression constructs and inhibitory drugs indicated that this potential of Nef to activate calcium signaling did not depend on the TCR-associated signaling complex. In this study we have examined the molecular mechanism underlying TCR-independent activation of calcium signaling by Nef. We found that this effect of Nef was strictly dependent on cellular IP3R function, although it did not involve increased IP3 production by PLCγ1, nor depletion of the IP3-sensitive intracellular calcium stores. These observations together with the ability of Nef to coprecipitate with IP3R1 suggested that Nef, by interacting with IP3R1, triggers an aberrant activation of CCE independently of the filling state of intracellular calcium stores.
Materials and Methods
Plasmids and Antibodies.
The Nef, pLacZ, and NFAT-luciferase plasmids used have been described earlier (34). PLCγ1-H335F and hemagglutinin (HA)-apoaequorin (Molecular Probes) cDNAs were cloned into eF1α-promoter-driven vector (referred to as pEBB; reference 34). pEGFP-NFATc1 was obtained from Päivi Koskinen (University of Turku, Turku, Finland), pCMVI-9-IP3R1 from Gregory A. Mignery (Loyola University of Chicago, Chicago, IL) and pEGFP-N2/human muscarinic receptor 1 (hM1) from Karl Åkerman (University of Uppsala, Uppsala, Sweden). The cDNA encoding for a truncated CD8 molecule (extracellular and transmembrane domains of human CD8 (amino acids 1–207) followed by two residues [I, D]) was generated by PCR and cloned into pEBB. The HIV-1 NL4–3-derived proviral Nef(+) and Nef(−) plasmids have been described previously (36). SRE-luciferase reporter construct containing three copies of consensus serum response factor/ternary complex factor binding sites in front of a minimal promoter was obtained from Roya Khosravi-Far (Harvard Medical School, Boston, MA). Anti-CD3 (HIT3a) antibody was purchased from BD PharMingen, anti-IP3R1-antibody was from A.G. Scientific Inc., anti-CD8 (LT8)-antibody was from Serotec, biotinylated goat anti–mouse IgG was from DAKO, polyclonal Nef-antisera was provided by Mark Harris (Leeds University, Leeds, UK), and monoclonal Nef-antibodies by FIT Biotech.
Cell Lines, Transfections, and Luciferase Assays.
Jurkat (JE-6; from American Type Culture Collection) and the J.IP3R1AS (provided by Andrew Marks, Columbia University, New York, NY) cells were maintained in complete RPMI 1640 (Hyclone) supplied with 10% fetal calf serum (BioWhittaker). Every 2 to 3 mo, J.IP3R1AS cells were grown in the presence of 2 mg/ml of Hygromycin B (Calbiochem) for 10 d. Human embryonic kidney 293T cells (from American Type Culture Collection) were maintained in complete DMEM (Hyclone; 10% serum) under standard conditions. HEK293T cells were transfected with a modified CaPO4-method as described previously (37). Jurkat and J.IP3R1AS cells were transfected with either Fugene (Roche Molecular Biochemicals) as described previously (34) or with DMRIE-C reagent (GIBCO BRL/Life Technologies) according to manufacturer's instructions. In brief, DMRIE-C reagent (2/3 ratio μl DMRIE-C/μg DNA) was incubated in 500 μl of OPTIMEM (GIBCO BRL/Life Technologies) for 45 min at room temperature (RT). Exponentially growing cells were washed once with OPTIMEM and were then resuspended into OPTIMEM (15 × 106 cells/ml). 100 μl of cell suspension was pipetted onto DMRIE-C/DNA mixture and incubated for 4 h at +37°C (5% CO2). 1 ml of complete RPMI-1640 containing 15% FCS was added and incubation was continued overnight. 20 h after transfection some of the cultures were stimulated for 4–5 h with 100 ng/ml PMA (Sigma-Aldrich), 50 ng/ml HIT3a, or 30 nM thapsigargin (Alexis Biochemicals). When indicated, 500 μM EGTA (Sigma-Aldrich), 200 nm cyclosporin A (CsA; Sigma-Aldrich), 30 μM SKF-96365 (Alexis Biochemicals), or 75 nM 2-aminoethoxydiphenyl borate (2-APB; Calbiochem) were added to the cells 30 min before their stimulation. Luciferase activities in these cells were determined and normalized to β-galactosidase activities measured from the same lysates as described previously (34).
Confocal Microscopy of Green Fluorescent Protein–expressing Cells.
20 h after transfection 106 Jurkat cells were resuspended into 30 μl of RPMI-1640. An equal volume of prewarmed (+40°C) PBS containing 1% (wt/vol) low melting point agarose (BDH Laboratory Supplies) was added, and a sample of this mixture was pipetted between a glass slide and a coverslip resting on thin plastic spacers. Fluorescence microscopy images were taken with an Ultraview confocal imaging system (PerkinElmer) using an Olympus X70 microscope.
Flow Cytometric Analysis of Intracellular Calcium.
Petri dishes were coated with anti–mouse IgG (10 μg/ml in 1 ml of 0.05 M Tris-HCl pH 9.5) for 1 h at RT and washed three times with PBS containing 5% FCS. 20 h after transfection of 3 × 106 Jurkat cells with pEBB-CD8 (1–207; 1 μg) and Nef or a control vector (3 μg), cells were incubated with 0.5 μg/ml of anti-CD8 for 30 min at RT, washed twice with PBS plus 5% FCS and pipetted onto coated Petri dishes in 1 ml of PBS plus 5% FCS. Cells were incubated for 45 min at RT and gently washed three times with PBS plus 1% FCS. Bound cells (75–90% of which were transfection-positive as determined by parallel green fluorescent protein (GFP)/CD8 transfection controls) were collected by pipetting, washed twice with 800 μl of RPMI-1640 (without phenol red; Hyclone), and loaded with 3 μM Fluo-3- and 5 μM Fura-Red-acetomethylesters in the presence of 0.02% Pluronic F-127 (all three from Molecular Probes) for 20 min at RT. Cells were washed twice with RPMI-1640 (1% serum, without phenol red) and suspended into 1 ml of this medium followed by an incubation at +37°C, 5% CO2 for 20 min. The measurements were performed in a Becton Dickinson FACScan™ cytometer (excitation 488 nm).
Virus Production and Infections.
HEK293T cells were transfected with 15 μg of either HIV-1 NL4–3-derived Nef(+) or Nef(−) proviral plasmids by using a modified CaPO4 method (37). 48 h after transfection, the virus-containing supernatants were passed through 0.45-μm filters (Schleicher & Schuell) to remove cellular debris. Supernatants were either used immediately or were frozen in aliquots at −80°C. The supernatant p24 concentrations were determined by a p24 ELISA assay. Human PBMCs were separated from buffy coats of healthy donors (Finnish Red Cross blood transfusion service, Tampere) through Ficoll-Hypaque (Amersham Pharmacia Biotech) gradient centrifugation. PBMCs were grown in complete RPMI-1640 supplemented with 2 U/ml of human IL-2 (R&D Systems). Equal quantities (1,000 ng of p24) of Nef(+)HIV or Nef(−)HIV virions were used to infect 20 × 106 PBMCs, which were lysed 5 d after the infection.
Detection of Nef and IP3R1.
20 h after transfection, 10 × 106 Jurkat or J.IP3R1-AS cells transfected with 15 μg of Nef or an empty control vector were washed with PBS and lysed in cell-lysis buffer (50 mM HEPES pH 7.4, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM EGTA, 1.5 mM MgCl2, 1 mM PMSF, 10 μg/ml approtinin). One fifth of the lysate was immunoprecipitated with polyclonal Nef-antisera and the rest with IP3R1 antibody. After three washes with cell-lysis buffer these immunocomplexes were analyzed by Western blotting using a mixture of monoclonal Nef antibodies as described previously (37). 5 d after infection of 20 × 106 PBMCs with either Nef(+)HIV or Nef(−)HIV or 24 h after transfection of HEK293T cells with 5 μg of pEBB-Nef or an empty pEBB-vector together with 5 μg of IP3R1 or a control vector, these cells were washed once with PBS and lysed in 1 ml of cell-lysis buffer. The lysates were split in two, the other half was immunoprecipitated with polyclonal Nef antisera and the other with IP3R1 antibody followed by Western blotting with anti-Nef or anti-IP3R1 antibodies as described above.
Measurement of Intracellular Calcium by Apoaequorin.
20 h after transfection of three million Jurkat cells with apoaequorin (3 μg) and Nef or a control vector (1 μg), and in some cases an hM1 vector (1 μg), these cells were incubated for 1–2 h in 500 μl of complete RPMI-1640 containing 2.5 μM coelenterazine-h (Molecular Probes), washed twice with 800 μl of RPMI-1640 (1% serum, w/o phenol red), and suspended into 300 μl of this medium. Calcium-dependent light emission was continuously monitored with a Luminova 1254 luminometer (Labsystems). During measurements, 0.5 mM EGTA (Sigma-Aldrich), 2 μM A23187 (Calbiochem), or 10 μM carbachol (Calbiochem) was added as indicated. At the end of each experiment the cells were lysed in 300 μl of hypotonic lysis buffer (10 mM Tris-HCl, pH 7.2, 0.1 mM EGTA) followed by addition of 30 mM CaCl2 to measure the remaining aequorin signal from each transfection. The total aequorin signal measured during the experiment was used to normalize for transfection efficiency (38).
Results
Nef Induces Nuclear Translocation of NFATc.
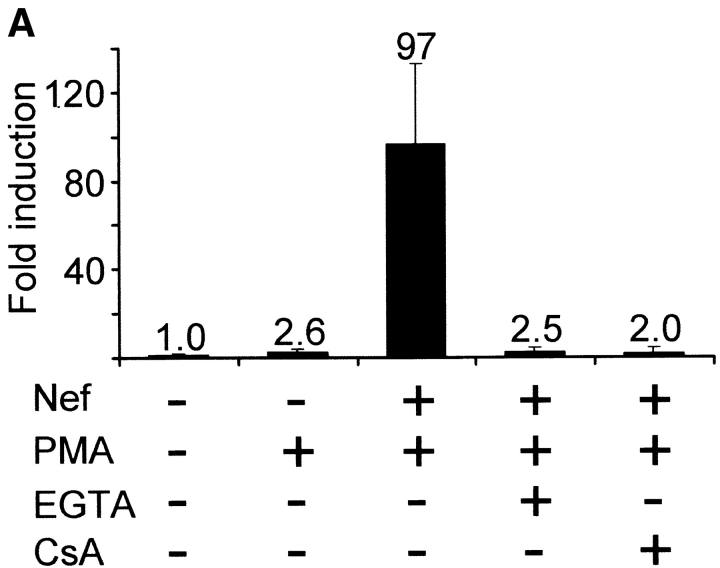
We have previously used a reporter gene construct driven by tandem copies of the antigen receptor response element of the IL-2 gene (ARRE2; reference 39) to reveal the potential of Nef to activate the transcription factor NFAT (34, 35). The effect of Nef on ARRE2-directed gene expression in Jurkat cells is demonstrated in Fig. 1 A. In agreement with our previous findings, a 4-h treatment with the MAPK cascade-activator phorbol 12-myristate 13-acetate (PMA) alone was not sufficient to induce significant expression of the transfected NFAT reporter gene in control vector-transfected Jurkat cells, whereas in Nef-expressing cells the same treatment resulted in efficient activation of luciferase expression. This effect was abolished if 500 μM EGTA or 200 nM CsA was added into these cultures before PMA, indicating that it involved influx of extracellular calcium and action of calcineurin (Fig. 1 A). Similarly, pretreatment of cultures with another calcineurin inhibitor, FK506, led up to 99% inhibition of Nef plus PMA-mediated activation of NFAT-driven transcription (data not shown).
Figure 1.
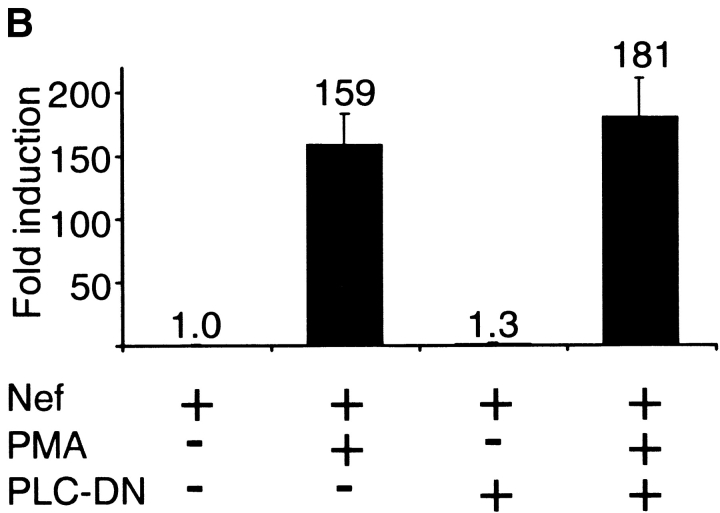
Calcium/calcineurin-dependent activation of NFAT by Nef. (A) Relative increase in NFAT-dependent luciferase expression in Jurkat cells cotransfected either with an empty control vector or Nef. 20 h later some of the cultures were pretreated with EGTA or CsA for 30 min before 4 h stimulation with PMA as indicated. Luciferase values normalized based on a cotransfected β-galactosidase vector are shown relative to the value from untreated control-transfected cells, which was set to one. The data are mean values from two independent transfection experiments performed in duplicate, and the standard error bars indicate variation among these four samples. (B) Fluorescence confocal microscopy of Jurkat cells transfected with a GFP-tagged NFATc1 together with an empty control vector or Nef. The percentages of cytoplasmic/nuclear localization of GFP-NFATc1 indicated represent data from three independent experiments in which a total of 70 GFP-positive cells from each sample were scored. (C) Flow cytometric analysis of intracellular calcium in control- or Nef-transfected Jurkat cells. Transfected Jurkat cells were enriched by panning and loaded with Ca2+-indicator dyes Fluo-3 and Fura-Red as described in Materials and Methods. Elevated Ca2+-levels enhance the Fluo-3 fluorescence but decrease the Fura-Red fluorescence. The data shown is representative of six independent transfection experiments.
To further validate the ARRE2-directed transcriptional study system as a read-out for Nef-induced changes in calcium signaling, and to exclude the possibility that also the activity of Nef itself might depend on PMA stimulation, we examined the ability of Nef to induce nuclear translocation of green fluorescent protein (GFP)-tagged NFATc1 (NFAT2) in the absence of MAPK activation. As shown in Fig. 1 B, in majority of Nef-expressing Jurkat cells NFATc1-GFP was found in the nucleus, whereas in control cells it was mainly cytoplasmic. Like NFAT-dependent gene expression (Fig. 1 A), Nef-induced nuclear translocation of NFATc1-GFP was abrogated by CsA-treatment (cytoplasmic localization in >95% of treated cells, data not shown). Moreover, chelating extracellular Ca2+ with EGTA or addition of 30 μM of SKF-96365, a widely used inhibitor of Ca2+-influx via store-operated and receptor-gated Ca2+ channels (40), also led to complete reversal of the Nef-induced nuclear NFATc1-GFP fluorescence (data not shown). These findings confirmed that the NFAT-activating function of Nef requires Ca2+ influx, and implicated CRAC channels in this process.
To directly visualize the increased intracellular calcium level that promotes nuclear localization of NFAT in Nef-expressing cells, we enriched the Jurkat cells transfected with Nef or the control vector by a panning strategy based on a cotransfected extracellular tag, and double-loaded this subpopulation of cells with calcium fluorophores Fluo-3 and Fura-Red. As shown in Fig. 1 C, a modest but obvious shift (toward the lower right quadrant) indicative of higher intracellular calcium levels was indeed observed in Nef-transfected cells compared with identically processed cells transfected with the control vector. Due to the greater sensitivity of Fluo-3 as compared with Fura-Red this shift was more apparent in the x- than the y-axis. The fact that this relatively small apparent increase in intracellular calcium is able to efficiently activate NFAT is in good agreement with previous studies that have established NFAT as a sensitive marker for small and oscillating increases in cytoplasmic free Ca2+ levels (21, 22).
PLCγ1 Is Not Involved in Regulation of Calcium Signaling by Nef.
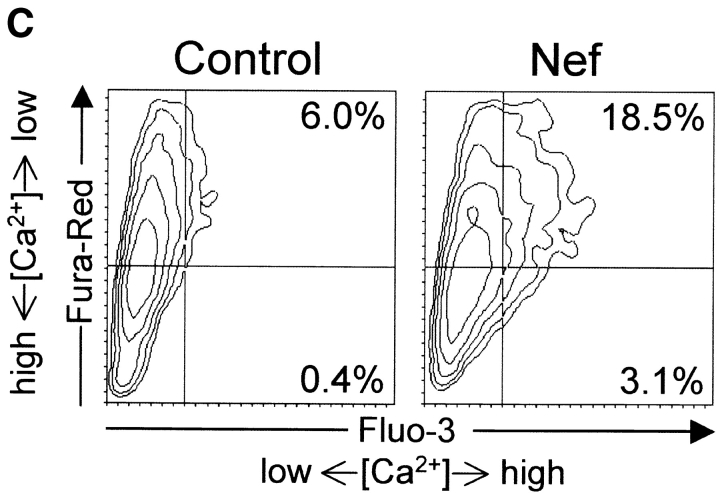
During normal T cell activation the elevated levels of IP3 that trigger calcium influx via CCE are caused by increased activity of PLCγ1 (12, 13). To examine if the effect of Nef on calcium signaling was mediated via PLCγ1-catalyzed IP3 production, we constructed a vector for overexpression of a dominant negative mutant of PLCγ1 (PLCγ1-H335F, dubbed as PLC-DN). A similar mutant has previously been successfully used to examine the role of the catalytic activity of PLCγ1 in serum-induced DNA synthesis (41) and in TCR-mediated signaling (42). Cotransfection of PLC-DN with an NFAT-dependent reporter plasmid into Jurkat cells efficiently inhibited TCR-mediated activation of NFAT triggered by an anti-CD3 antibody (Fig. 2 A). This result was in agreement with recent data on Jurkat cells lacking PLCγ1 expression (42) and on B cells of PLCγ2-deficient mice (43), and confirmed the feasibility of this approach for inhibition of PLCγ1-catalyzed IP3 production in transiently transfected Jurkat cells. As expected, the inhibition of NFAT activation by PLC-DN could be bypassed by costimulation of cells with PMA and the calcium ionophore A23187 (data not shown), which activate MAPK- and calcium signaling downstream of PLCγ1. As shown in Fig. 2 B, the effect of Nef was also found to be downstream of PLCγ1, because cotransfection of PLC-DN had no inhibitory effect on Nef plus PMA-induced NFAT activation. Thus, we concluded that activation of calcium signaling by Nef was not due to increased IP3 production caused by action of Nef on PLCγ1 or its upstream regulators.
Figure 2.
Effect of overexpression of a dominant-negative PLCγ1 mutant on NFAT activation by TCR-stimulation or Nef expression. (A) Jurkat cells were cotransfected with the NFAT reporter plasmid together with an empty control vector or a PLCγ1-H335F-expression plasmid (PLC-DN). 20 h after transfection cells were either left untreated or stimulated for 5 h with an anti-CD3 antibody (anti-CD3). Luciferase activities relative to the untreated control-transfected cells are expressed as in Fig. 1 A. (B) Relative NFAT activities in untreated or PMA-stimulated Jurkat cells transfected with Nef. An empty control vector or PLC-DN was cotransfected as indicated. These data represent mean values ± SE from five independent experiments.
Nef-induced Calcium Signaling Depends on IP3R1 Function.
The observed IP3 independence of NFAT activation by Nef suggested that Nef might act directly on the cellular machinery involved in calcium influx via CCE. To characterize the target of Nef in this system we used a recently described cell-permeable IP3R-inhibitor 2-APB (44, 45). IP3R is centrally positioned in the CCE apparatus because, in addition to binding to IP3, it serves as a calcium release channel in ER and may also be physically involved in the coupling step between calcium release and influx (45–47). As expected, 2-APB efficiently blocked NFAT activation triggered by TCR stimulation (Fig. 3 A). Strikingly, 2-APB was also a potent inhibitor of Nef-induced NFAT activation (Fig. 3 A). To exclude the possibility that this inhibition might have involved nonspecific effects by 2-APB, such as toxicity or interference with PMA-induced MAPK activation, we examined the effect of 2-APB on PMA-stimulated luciferase expression in Jurkat cells transfected with a serum response element (SRE)-driven reporter construct. In contrast to the complete inhibition of the anti-CD3- and Nef plus PMA-induced NFAT activation (96 and 98%, respectively), PMA-stimulated SRE-dependent luciferase expression was only modestly (34%) reduced by 2-APB treatment (data not shown). Moreover, because regulation of SRE is complex, it is possible that also this small inhibition was caused, at least in part, by specific action of 2-APB on IP3R.
Figure 3.
Dependence of NFAT activation by Nef on IP3R function. (A) Relative NFAT-dependent luciferase expression in Jurkat cells transfected with a control vector or Nef and stimulated with anti-CD3 antibodies or PMA. When indicated, 2-APB was added to the cultures 30 min before their stimulation. (B) Relative NFAT-dependent luciferase expression in IP3R1-deficient J.IP3R1AS cells transfected with a control vector or Nef and treated with the indicated activators (anti-CD3, PMA, and thapsigargin [TG]) as described in Materials and Methods. The data sets shown are representative of four (A) and seven (B) independent transfection experiments with similar results.
Recent data have indicated that in addition to its effect on IP3R, however, 2-APB may also directly inhibit the function of CRACs at the plasma membrane (48, 49). To further explore the role of IP3R as an effector of Nef we used a Jurkat-derived cell line, generated and characterized by Marks and coworkers, which stably expresses an antisense-RNA for IP3R1 (50) (referred to as J.IP3R1AS cells in the following). Although the three different types of IP3 receptors (IP3R1, IP3R2, and IP3R3) may have redundant functions (51), J.IP3R1AS cells, which express only low levels of IP3R2 and IP3R3, have been demonstrated to be unable to mobilize calcium and to produce IL-2 after TCR stimulation (50). By contrast, IP3R-independent depletion of intracellular Ca2+ stores using the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) inhibitor thapsigargin (52) does result in calcium influx also in J.IP3R1AS cells, indicating that the rest of the machinery involved in CRAC opening is functional in these cells (50).
In agreement with the previous data from Marks and coworkers, we found that TCR-mediated activation of NFAT was abolished in J.IP3R1AS cells, whereas thapsigargin-induced CCE in combination with MAPK activation (by PMA) elicited a robust increase in NFAT-dependent luciferase expression (Fig. 3 B). More important to the present study, however, we found that expression of Nef in PMA-treated J.IP3R1AS cells failed to activate NFAT (Fig. 3 B). Thus, apart from the question whether the effect of 2-APB also involved direct inhibition of CRACs, these results clearly implicated IP3R1 as an effector molecule of Nef in activation of calcium signaling.
Nef Associates with IP3R1 in Cells.
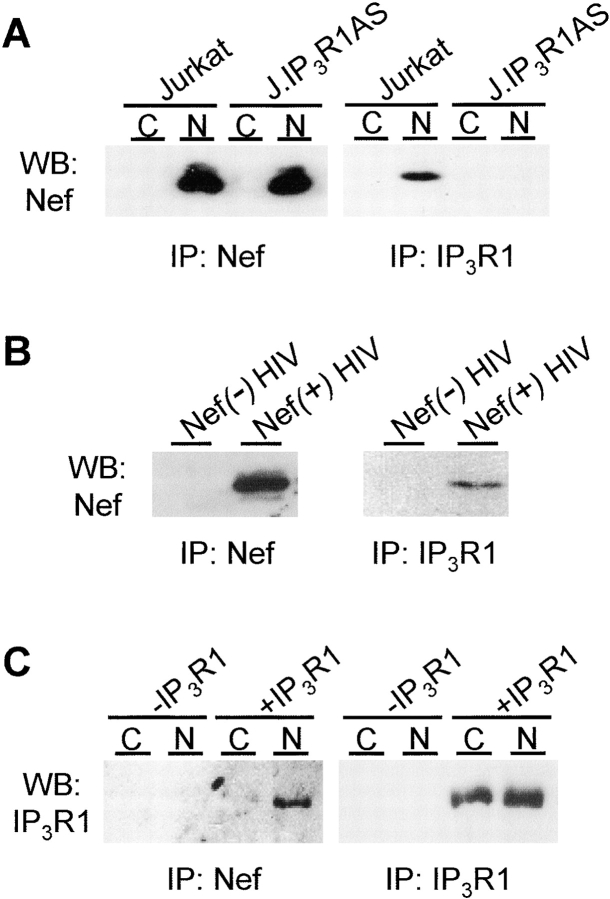
To examine if the IP3R1-dependent activation of calcium influx by Nef would involve a physical interaction between these two proteins, we examined the presence of Nef in anti-IP3R1 immunoprecipitations from lysates of Jurkat cells and their IP3R1-deficient derivatives. As shown in Fig. 4 A, readily detectable amounts of Nef coprecipitated with endogenous IP3R1 from Nef-transfected but not from control-transfected Jurkat cells. By contrast, despite equal Nef-expression in J.IP3R1AS cells, no Nef protein could be immunoprecipitated from these cells using anti-IP3R1 antibodies confirming that Nef was not precipitated nonspecifically or because of cross-reactivity of the IP3R1 antiserum. Moreover, Nef/IP3R1 association could be confirmed in a more physiological experimental system as Nef could also be coprecipitated by IP3R1 antibodies from lysates of HIV-infected human peripheral blood mononuclear cells (Fig. 4 B). In addition, IP3R1 and Nef could be coprecipitated when they were overexpressed together in HEK293 cells, indicating that this interaction was not restricted to T cells (Fig. 4 C). Thus, these data show that regulation of IP3R1 function by Nef involves an intracellular protein complex containing both of these proteins, but of course does not prove a direct contact between Nef and IP3R1.
Figure 4.
Coprecipitation of IP3R1 and Nef. (A) Control (C) and Nef-transfected (N) Jurkat and J.IP3R1AS cells were lysed and subjected to immunoprecipitations using antibodies for Nef (left panel) or IP3R1 (right panel) followed by analysis of the precipitated material by anti-Nef Western blotting. (B) PBMCs were infected with Nef-negative (Nef(−) HIV) or wild-type (Nef(−) HIV) HIV particles and subjected to anti-Nef (left panel) or anti-IP3R1 (right panel) immunoprecipitations as described above. The precipitated material was analyzed by anti-Nef Western blotting. (C) HEK293T fibroblasts were cotransfected with a control vector (C) or Nef (N) together with IP3R1 (+IP3R1) or an empty control plasmid (−IP3R1) as indicated. Cells were subjected to immunoprecipitations as described in 4A and the precipitated material was analyzed by anti-IP3R1 Western blotting.
Nef-induced Calcium Influx Does Not Involve Depletion of Cellular Calcium Stores.
To examine if binding of Nef to IP3R1 might mimic the effect of its natural ligand IP3, and thereby trigger CCE by activating the calcium release channel function of IP3R1, we compared the filling state of total ionophore-sensitive as well as IP3-sensitive intracellular calcium stores in control- and in Nef-transfected Jurkat cells. In these studies we used a strategy based on cotransfection of an apoaequorin expression vector, which makes it possible to examine free Ca2+ levels only in the positively transfected subpopulation of cells (38). When the cell-permeable luminophore coelenterazine is provided it associates with the transfected apoaequorin to form an aequorin complex whose calcium-dependent light emission can be measured with a luminometer.
The baseline aequorin-luminescence was similar in control- and in Nef-transfected cells (Fig. 5) , which could be expected because of the relatively lower sensitivity of this method than the fluorescent method used in experiments shown in Fig. 1 C. When apoaequorin-expressing cells were treated with ionophore in nominally calcium-free medium (in the presence of EGTA), similar amounts of calcium were released from intracellular stores in control- and in Nef-transfected cells (Fig. 5 A). However, because calcium ionophore might release calcium from intracellular compartments with a larger capacity than the IP3-sensitive stores relevant for T cell activation, we also specifically measured calcium released from the IP3-gated stores. For this purpose an expression vector encoding hM1 was cotransfected with apoaequorin in order to trigger a robust PLCβ1-catalyzed production of IP3 by stimulating these cells with the hM1 agonist carbachol. As expected, the IP3-sensitive calcium store contents released by carbachol stimulation were smaller (∼25%) than those released by ionophore treatment (Fig. 5). Importantly, Nef did not cause depletion of the IP3-sensitive calcium stores because the amount of calcium released upon carbachol treatment was not reduced, but in fact slightly higher in Nef-expressing cells than in the control-transfected cells (Fig. 5 B).
Figure 5.

Comparison of ionophore- and IP3-sensitive intracellular Ca2+-stores in control and in Nef-expressing cells. (A) Calcium released from ionophore-sensitive intracellular stores in Jurkat cells cotransfected with an apoaequorin vector together with a control vector (open squares) or Nef (black triangles) were measured based on luminescence by Ca2+/aequorin/coelenterazine complexes in these cells. A similar measurement of coelenterazine-loaded cells that were not transfected with apoaequorin is shown as a negative control (dotted line). EGTA and ionophore (Iono) were added to the cells during the course of the recordings as indicated. (B) Calcium released from IP3-sensitive stores in Jurkat cells transfected as in panel A, except that a vector for human muscarinic receptor-1 (hM1) was also included. EGTA and carbachol (CCh) were added to the cells during the course of the recordings as indicated. The mean values of quadruplicate samples are shown. These measurements were performed 5 (A) and 10 (B) times with similar results.
In conclusion, no deficits in ionophore- or IP3-sensitive Ca2+ stores were observed in Nef-expressing cells, suggesting that the induction of IP3R1-dependent calcium signaling by Nef did not involve activation of the calcium release channel function of IP3R. This suggested that Nef acts by promoting communication between IP3R1 and CRACs in a manner that leads to increased calcium influx independently of the filling state of intracellular calcium stores.
Discussion
In this study we have characterized a novel IP3R-dependent but TCR-independent molecular mechanism by which Nef may contribute to T cell activation and HIV replication. It is important to note that this mode of action is not mutually exclusive with an additional function of Nef via modulation of TCR signaling previously described by others (23–25, 53). In fact, such dual level of regulation of calcium signaling by Nef could be envisioned to be important in order to ensure continued T cell activation after downmodulation of TCR function in Nef-expressing cells (32, 33). Besides facilitating HIV replication in the infected cell, Nef-induced increase in the activity of NFAT and other calcium-regulated cellular processes could also render the neighboring cells more susceptible to HIV infection via paracrine effects of IL-2 and other NFAT-regulated cytokines.
TCR-independent activation of T cell calcium signaling by Nef was found to involve a physical association between Nef and IP3R1. Because of the undiminished intracellular IP3-gated calcium stores in Nef-expressing cells, we concluded that the effect of Nef on IP3R1 did not mimic binding of IP3, which would lead to CCE via depletion of these stores. Although it would be difficult to formally exclude the possibility of depletion of some small and specialized calcium stores, which despite their insignificant overall capacity would play a critical role in regulating CCE, our data suggest that Nef/IP3R1 interaction does not activate calcium release function of IP3R, but rather promotes a later step in the chain of events that lead to calcium influx via CRACs.
The strict dependence of Nef on IP3R function clearly indicated that Nef did not act by directly modulating the conductance of CRAC or other plasma membrane calcium channels. In theory, binding of Nef could induce an alternative conformation of IP3R, which would otherwise be associated with low calcium levels in ER, and thereby trigger the yet unknown mechanism that normally mediates coupling of ER calcium store depletion to calcium influx. Alternatively, Nef could play a more direct role in promoting such communication between IP3R and the plasma membrane CRAC channels. Interestingly, a number of recent studies have suggested that IP3R itself would be physically involved in this coupling by directly contacting the SOC/CRAC channels (45–47) (conformational coupling model). Nef being a multifunctional adaptor known to modify interactions between host cell signal transducing proteins (1–3), it is tempting to consider our data as further support for the conformational coupling hypothesis, and propose that Nef triggers calcium influx by promoting the assembly of a complex where protein–protein interactions would locally connect ER and the plasma membrane and thereby physically couple IP3R to the relevant CRAC channel(s). As an attempt to address this possibility we have examined if overexpression of human Trp3, a potential IP3R-interacting component of SOCs/CRACs (46, 47, 54), would increase Nef/IP3R-complex formation. The results of these studies were negative (unpublished data), however, arguing against a simple model where Nef would stabilize the formation of an IP3R/Trp3 complex. Moreover, it should also be noted here that not all available data support the conformational coupling model of CCE (12, 49, 55).
Our previous studies have indicated that Src-homology 3 domain (SH3) domain-binding capacity of Nef is required for its ability to activate NFAT-dependent gene expression (35). On the other hand, in the course of the present studies we have noticed that Nef mutants unable to bind SH3 still retained the capacity to associate with IP3R1 (data not shown). As IP3R1 does not contain an SH3 domain, it seems likely that cellular partners of Nef other than IP3R1 are also recruited to this protein complex and are involved in mediating the effect of Nef on calcium signaling. Interestingly, Src-kinases, some of which can bind Nef via their SH3 domains (36), have also been reported to interact with and regulate the function of IP3R (56–58). Therefore, we have also considered the possibility that a Src kinase capable of binding both Nef and IP3R1, such as Hck, could be involved in the Nef/IP3R-mediated activation of calcium signaling. Based on our previous pharmacological studies, however, catalytic activity of a Src kinase does not seem to be involved, because we found that doses of the Src inhibitor PP1 that fully suppressed TCR-triggered Jurkat cell activation failed to prevent Nef-mediated NFAT activation (34). On the other hand, we have observed no increase (or decrease) in coprecipitation of Nef and IP3R1 from cells transfected with vectors overexpressing Hck or Fyn (unpublished data). These observations are in agreement with those by Foti and coworkers, who have examined the cellular interactions of Hck with Nef and with IP3R1 (58). Although these authors did not consider the possibility that Nef and IP3R1 might associate with each other in a trimolecular or multiprotein complex containing Hck, their data clearly show that transfection of Nef had no effect on the association of Hck and IP3R1. Thus, while common intracellular protein complexes containing Nef, IP3R1, and a Src kinase might exist, together the data discussed above do not support the idea that a Src kinase would play a critical role in bridging Nef and IP3R1, or that the effect of Nef on calcium signaling would be caused by targeting Src tyrosine kinase activity to IP3R1.
Because of toxicity of long-term exposure to elevated levels of cytoplasmic calcium, activation of calcium influx by Nef might also explain the difficulty of generating T cell lines that would stably express Nef (e.g., see reference 53). In line with this hypothesis, we have noticed that stably Nef-expressing clones of J.IP3R1AS cells cotransfected with Nef and an eukaryotic selection marker plasmid can be routinely obtained, whereas it is very difficult to generate Nef-expressing clones of parental Jurkat cells using the same approach (unpublished data). It is also of interest to note that abnormalities in calcium homeostasis, namely increased accumulation of calcium into intracellular stores (58, 59), have been described in cell lines successfully engineered to stably express Nef, which in the light of our data could be readily explained as an adaptive response to chronic Nef expression.
Elucidation of the molecular mechanism that couples IP3R and SOC/CRAC functions is under active scrutiny in many laboratories, and represents a major challenge in understanding of TCR-triggered activation of lymphocytes and similar calcium-mediated signaling processes in other nonexcitable cells. Progress in this area of research can also be expected to provide us with novel molecular tools for further dissection of the IP3R-dependent mechanism of T cell activation by Nef. Such information could have valuable therapeutic implications, as the mechanistic differences observed between Nef-mediated and TCR-triggered activation of T cell calcium signaling suggest that targeting of this function of Nef could be a promising approach for development of novel strategies to interfere with the pathogenesis of HIV infection. On the other hand, involvement of Nef in the heart of the apparatus that controls CCE could also be exploited to gain more insight into this important process itself.
Acknowledgments
We thank A. Marks for reagents and help, P. Koskinen, G.A. Mignery, and K. Åkerman for plasmids, and Kristina Lehtinen for expert technical assistance.
This study was supported by the Academy of Finland, the medical research fund of Tampere University Hospital, and the EU-funded project QLK2-CT-2000-01630.
Footnotes
Abbreviations used in this paper: 2-APB, 2-aminoethoxydiphenyl borate; ARRE, antigen receptor response element; CCE, capacitative calcium entry; CRAC, calcium release-activated calcium channel; CsA, cyclosporin A; ER, endoplasmic reticulum; GFP, green fluorescent protein; hM1, human muscarinic receptor 1; IP3, inositol 1,4,5-trisphosphate; MAPK, mitogen-activated protein kinase; NFAT, nuclear factor of activated T cells; PLC, phospholipase; RT, room temperature; SH3, Src-homology 3 domain; SOC, store-operated calcium channel; SRE, serum response element; Trp, transient receptor potential.
References
- 1.Piguet, V., and D. Trono. 1999. The Nef protein of primate lentiviruses. Rev. Med. Virol. 9:111–120. [DOI] [PubMed] [Google Scholar]
- 2.Skowronski, J., M.E. Greenberg, M. Lock, R. Mariani, S. Salghetti, T. Swigut, and A.J. Iafrate. 1999. HIV and SIV Nef modulate signal transduction and protein sorting in T cells. Cold Spring Harb. Symp. Quant. Biol. 64:453–463. [DOI] [PubMed] [Google Scholar]
- 3.Renkema, G.H., and K. Saksela. 2000. Interactions of HIV-1 Nef with cellular signal transducing proteins. Front. Biosci. 5:D268–D283. [DOI] [PubMed] [Google Scholar]
- 4.Nabel, G., and D. Baltimore. 1987. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 326:711–713. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson, M., T.L. Stanwick, M.P. Dempsey, and C.A. Lamonica. 1990. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 9:1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skowronski, J., D. Parks, and R. Mariani. 1993. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 12:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna, Z., D.G. Kay, N. Rebai, A. Guimond, S. Jothy, and P. Jolicoeur. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 95:163–175. [DOI] [PubMed] [Google Scholar]
- 8.Crabtree, G.R. 1999. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell. 96:611–614. [DOI] [PubMed] [Google Scholar]
- 9.Chow, C.W., M. Rincon, and R.J. Davis. 1999. Requirement for transcription factor NFAT in interleukin-2 expression. Mol. Cell. Biol. 19:2300–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao, A., C. Luo, and P.G. Hogan. 1997. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15:707–747. [DOI] [PubMed] [Google Scholar]
- 11.Rhee, S.G., and Y.S. Bae. 1997. Regulation of phosphoinositide-specific phospholipase C isozymes. J. Biol. Chem. 272:15045–15048. [DOI] [PubMed] [Google Scholar]
- 12.Lewis, R.S. 2001. Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 19:497–521. [DOI] [PubMed] [Google Scholar]
- 13.Guse, A.H. 1998. Ca2+ signaling in T-lymphocytes. Crit. Rev. Immunol. 18:419–448. [DOI] [PubMed] [Google Scholar]
- 14.Negulescu, P.A., N. Shastri, and M.D. Cahalan. 1994. Intracellular calcium dependence of gene expression in single T lymphocytes. Proc. Natl. Acad. Sci. USA. 91:2873–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timmerman, L.A., N.A. Clipstone, S.N. Ho, J.P. Northrop, and G.R. Crabtree. 1996. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature. 383:837–840. [DOI] [PubMed] [Google Scholar]
- 16.Berridge, M.J. 1995. Capacitative calcium entry. Biochem. J. 312:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putney, J.W. 1999. “Kissin' cousins”: intimate plasma membrane-ER interactions underlie capacitative calcium entry. Cell. 99:5–8. [DOI] [PubMed] [Google Scholar]
- 18.Yue, L., J.B. Peng, M.A. Hediger, and D.E. Clapham. 2001. CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature. 410:705–709. [DOI] [PubMed] [Google Scholar]
- 19.Peng, J.B., X.Z. Chen, U.V. Berger, P.M. Vassilev, H. Tsukaguchi, E.M. Brown, and M.A. Hediger. 1999. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J. Biol. Chem. 274:22739–22746. [DOI] [PubMed] [Google Scholar]
- 20.Dolmetsch, R.E., R.S. Lewis, C.C. Goodnow, and J.I. Healy. 1997. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 386:855–858. [DOI] [PubMed] [Google Scholar]
- 21.Dolmetsch, R.E., K. Xu, and R.S. Lewis. 1998. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 392:933–936. [DOI] [PubMed] [Google Scholar]
- 22.Li, W., J. Llopis, M. Whitney, G. Zlokarnik, and R.Y. Tsien. 1998. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 392:936–941. [DOI] [PubMed] [Google Scholar]
- 23.Simmons, A., V. Aluvihare, and A. McMichael. 2001. Nef triggers a transcriptional program in T cells imitating single- signal T cell activation and inducing HIV virulence mediators. Immunity. 14:763–777. [DOI] [PubMed] [Google Scholar]
- 24.Schrager, J.A., and J.W. Marsh. 1999. HIV-1 Nef increases T cell activation in a stimulus-dependent manner. Proc. Natl. Acad. Sci. USA. 96:8167–8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, J.K., E. Kiyokawa, E. Verdin, and D. Trono. 2000. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc. Natl. Acad. Sci. USA. 97:394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz, O., F. Arenzana-Seisdedos, J.M. Heard, and O. Danos. 1992. Activation pathways and human immunodeficiency virus type 1 replication are not altered in CD4+ T cells expressing the nef protein. AIDS Res. Hum. Retroviruses. 8:545–551. [DOI] [PubMed] [Google Scholar]
- 27.Bandres, J.C., and L. Ratner. 1994. Human immunodeficiency virus type 1 Nef protein down-regulates transcription factors NF-kappa B and AP-1 in human T cells in vitro after T-cell receptor stimulation. J. Virol. 68:3243–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carreer, R., H. Groux, J.C. Ameisen, and A. Capron. 1994. Role of HIV-1 Nef expression in activation pathways in CD4+ T cells. AIDS Res. Hum. Retroviruses. 10:523–527. [DOI] [PubMed] [Google Scholar]
- 29.Collette, Y., H. Dutartre, A. Benziane, M. Ramos, R. Benarous, M. Harris, and D. Olive. 1996. Physical and functional interaction of Nef with Lck. HIV-1 Nef-induced T-cell signaling defects. J. Biol. Chem. 271:6333–6341. [DOI] [PubMed] [Google Scholar]
- 30.Iafrate, A.J., S. Bronson, and J. Skowronski. 1997. Separable functions of Nef disrupt two aspects of T cell receptor machinery: CD4 expression and CD3 signaling. EMBO J. 16:673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page, K.A., W.C. van Schooten, and M.B. Feinberg. 1997. Human immunodeficiency virus type 1 Nef does not alter T-cell sensitivity to antigen-specific stimulation. J. Virol. 71:3776–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell, I., C. Ashman, J. Maughan, E. Hooker, F. Cook, and T.A. Reinhart. 1998. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR down-modulation. J. Gen. Virol. 79:2717–2727. [DOI] [PubMed] [Google Scholar]
- 33.Swigut, T., N. Shohdy, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 20:1593–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manninen, A., G.H. Renkema, and K. Saksela. 2000. Synergistic activation of NFAT by HIV-1 Nef and the Ras/MAPK pathway. J. Biol. Chem. 275:16513–16517. [DOI] [PubMed] [Google Scholar]
- 35.Manninen, A., P. Huotari, M. Hiipakka, G.H. Renkema, and K. Saksela. 2001. Activation of NFAT-dependent gene expression by Nef: conservation among divergent Nef alleles, dependence on SH3 binding and membrane association, and cooperation with protein kinase C-θ. J. Virol. 75:3034–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saksela, K., G. Cheng, and D. Baltimore. 1995. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 14:484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manninen, A., M. Hiipakka, M. Vihinen, W. Lu, B.J. Mayer, and K. Saksela. 1998. SH3-domain binding function of HIV-1 Nef is required for association with a PAK-related kinase. Virology. 250:273–282. [DOI] [PubMed] [Google Scholar]
- 38.Brini, M., R. Marsault, C. Bastianutto, J. Alvarez, T. Pozzan, and R. Rizzuto. 1995. Transfected aequorin in the measurement of cytosolic Ca2+ concentration ([Ca2+]c). A critical evaluation. J. Biol. Chem. 270:9896–9903. [DOI] [PubMed] [Google Scholar]
- 39.Northrop, J.P., K.S. Ullman, and G.R. Crabtree. 1993. Characterization of the nuclear and cytoplasmic components of the lymphoid-specific nuclear factor of activated T cells (NF-AT) complex. J. Biol. Chem. 268:2917–2923. [PubMed] [Google Scholar]
- 40.Chung, S.C., T.V. McDonald, and P. Gardner. 1994. Inhibition by SK&F 96365 of Ca2+ current, IL-2 production and activation in T lymphocytes. Br. J. Pharmacol. 113:861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, M.R., Y.L. Liu, N.T. Matthews, S.G. Rhee, W.K. Sung, and H.F. Kung. 1994. Phospholipase C-gamma 1 can induce DNA synthesis by a mechanism independent of its lipase activity. Proc. Natl. Acad. Sci. USA. 91:6554–6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irvin, B.J., B.L. Williams, A.E. Nilson, H.O. Maynor, and R.T. Abraham. 2000. Pleiotropic contributions of phospholipase C-gamma1 (PLC-gamma1) to T-cell antigen receptor-mediated signaling: reconstitution studies of a PLC-gamma1-deficient Jurkat T-cell line. Mol. Cell. Biol. 20:9149–9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, D., J. Feng, R. Wen, J.C. Marine, M.Y. Sangster, E. Parganas, A. Hoffmeyer, C.W. Jackson, J.L. Cleveland, P.J. Murray, and J.N. Ihle. 2000. Phospholipase Cgamma2 is essential in the functions of B cell and several Fc receptors. Immunity. 13:25–35. [DOI] [PubMed] [Google Scholar]
- 44.Maruyama, T., T. Kanaji, S. Nakade, T. Kanno, and K. Mikoshiba. 1997. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. 122:498–505. [DOI] [PubMed] [Google Scholar]
- 45.Ma, H.T., R.L. Patterson, D.B. van Rossum, L. Birnbaumer, K. Mikoshiba, and D.L. Gill. 2000. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science. 287:1647–1651. [DOI] [PubMed] [Google Scholar]
- 46.Kiselyov, K., X. Xu, G. Mozhayeva, T. Kuo, I. Pessah, G. Mignery, X. Zhu, L. Birnbaumer, and S. Muallem. 1998. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 396:478–482. [DOI] [PubMed] [Google Scholar]
- 47.Boulay, G., D.M. Brown, N. Qin, M. Jiang, A. Dietrich, M.X. Zhu, Z. Chen, M. Birnbaumer, K. Mikoshiba, and L. Birnbaumer. 1999. Modulation of Ca(2+) entry by polypeptides of the inositol 1,4, 5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca(2+) entry. Proc. Natl. Acad. Sci. USA. 96:14955–14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broad, L.M., F.J. Braun, J.P. Lievremont, G.S. Bird, T. Kurosaki, and J.W. Putney, Jr. 2001. Role of the phospholipase C - inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J. Biol. Chem. 276:15945–15952. [DOI] [PubMed] [Google Scholar]
- 49.Braun, F.J., L.M. Broad, D.L. Armstrong, and J.W. Putney, Jr. 2001. Stable activation of single Ca2+ release-activated Ca2+ channels in divalent cation-free solutions. J. Biol. Chem. 276:1063–1070. [DOI] [PubMed] [Google Scholar]
- 50.Jayaraman, T., E. Ondriasova, K. Ondrias, D.J. Harnick, and A.R. Marks. 1995. The inositol 1,4,5-trisphosphate receptor is essential for T-cell receptor signaling. Proc. Natl. Acad. Sci. USA. 92:6007–6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirota, J., M. Baba, M. Matsumoto, T. Furuichi, K. Takatsu, and K. Mikoshiba. 1998. T-cell-receptor signalling in inositol 1,4,5-trisphosphate receptor (IP3R) type-1-deficient mice: is IP3R type 1 essential for T-cell-receptor signalling? Biochem. J. 333:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bian, J.H., T.K. Ghosh, J.C. Wang, and D.L. Gill. 1991. Identification of intracellular calcium pools. Selective modification by thapsigargin. J. Biol. Chem. 266:8801–8806. [PubMed] [Google Scholar]
- 53.Baur, A.S., E.T. Sawai, P. Dazin, W.J. Fantl, C. Cheng-Mayer, and B.M. Peterlin. 1994. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1:373–384. [DOI] [PubMed] [Google Scholar]
- 54.Zhu, X., M. Jiang, M. Peyton, G. Boulay, R. Hurst, E. Stefani, and L. Birnbaumer. 1996. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 85:661–671. [DOI] [PubMed] [Google Scholar]
- 55.Sugawara, H., M. Kurosaki, M. Takata, and T. Kurosaki. 1997. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 16:3078–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jayaraman, T., K. Ondrias, E. Ondriasova, and A.R. Marks. 1996. Regulation of the inositol 1,4,5-trisphosphate receptor by tyrosine phosphorylation. Science. 272:1492–1494. [DOI] [PubMed] [Google Scholar]
- 57.Yokoyama, K., I. Su Ih, T. Tezuka, T. Yasuda, K. Mikoshiba, A. Tarakhovsky, and T. Yamamoto. 2002. BANK regulates BCR-induced calcium mobilization by promoting tyrosine phosphorylation of IP(3) receptor. EMBO J. 21:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foti, M., L. Cartier, V. Piguet, D.P. Lew, J.L. Carpentier, D. Trono, and K.H. Krause. 1999. The HIV Nef protein alters Ca(2+) signaling in myelomonocytic cells through SH3-mediated protein-protein interactions. J. Biol. Chem. 274:34765–34772. [DOI] [PubMed] [Google Scholar]
- 59.Zegarra-Moran, O., A. Rasola, M. Rugolo, A.M. Porcelli, B. Rossi, and L.J. Galietta. 1999. HIV-1 nef expression inhibits the activity of a Ca2+-dependent K+ channel involved in the control of the resting potential in CEM lymphocytes. J. Immunol. 162:5359–5366. [PubMed] [Google Scholar]