Abstract
Δ9-Tetrahydrocannabinol (Δ9-THC), the major psychoactive ingredient in preparations of Cannabis sativa (marijuana, hashish), elicits central nervous system (CNS) responses, including cognitive alterations and euphoria. These responses account for the abuse potential of cannabis, while other effects such as analgesia suggest potential medicinal applications. To study the role of the major known target of cannabinoids in the CNS, the CB1 cannabinoid receptor, we have produced a mouse strain with a disrupted CB1 gene. CB1 knockout mice appeared healthy and fertile, but they had a significantly increased mortality rate. They also displayed reduced locomotor activity, increased ring catalepsy, and hypoalgesia in hotplate and formalin tests. Δ9-THC-induced ring-catalepsy, hypomobility, and hypothermia were completely absent in CB1 mutant mice. In contrast, we still found Δ9-THC-induced analgesia in the tail-flick test and other behavioral (licking of the abdomen) and physiological (diarrhea) responses after Δ9-THC administration. Thus, most, but not all, CNS effects of Δ9-THC are mediated by the CB1 receptor.
Preparations of Cannabis sativa, such as marijuana and hashish, have been used for medicinal and recreational purposes for at least 4,000 years. Today, cannabis preparations are still among the most commonly used illegal drugs in the United States (1). Recently, cannabinoids have received renewed interest for their potential medicinal applications, which include analgesia (2, 3), attenuation of nausea (4), and appetite stimulation (5). The major psychoactive ingredient in C. sativa, Δ9-tetrahydrocannabinol (Δ9-THC), was isolated in 1964 by Gaoni and Mechoulam (6). Since then, a number of natural and synthetic compounds with cannabimimetic activity have been identified. These compounds have been highly useful in cannabinoid research (7–9).
Cannabinoids bind and activate G-protein coupled receptors (10). The CB1 cannabinoid receptor was first cloned from rat (11, 12) and subsequently from human (13) and mouse (14, 15). A second cannabinoid receptor, CB2, subsequently was cloned (16, 17) and found to have a low (≈45%) overall homology to the CB1 receptor. It is thought that the central nervous system (CNS) effects of cannabinoids are mediated by the CB1 receptor, which is highly expressed in the CNS (12, 18). CB2 expression is largely restricted to cells of the immune system and not found in the brain. The expression pattern of the CB1 receptor in the brain correlates well with cannabinoid binding sites that have been identified with radioligand binding studies (18–20).
We have begun to use a genetic approach to examine the role of the cannabinoid system. As a first step, we have generated mice with a targeted mutation in the CB1 receptor gene. These mice show an increased mortality rate and altered nociceptive and motor behaviors, and they are resistant to many effects of cannabinoid drugs.
MATERIALS AND METHODS
Animals.
The CB1 gene was mutated in MPI2 embryonic stem cells by using standard techniques (21–23). Homozygous CB1−/− animals and CB1+/+ controls were bred by back-crossing of chimeric and heterozygous animals to C57BL/6J mice and interbreeding of heterozygous animals. All animals (9–20 weeks old) were housed in a temperature- and humidity-controlled vivarium with a 12-hr dark-light cycle (19:00 to 07:00). Food and water were available ad libitum.
[3H]CP55,940 Binding.
Mice (CB1+/+, CB1+/−, and CB1−/−) were killed by decapitation. Brains and spleens were dissected, frozen (−40°C), and stored (−70°C) until sectioning. Sagittal and coronal sections (12 μm thick) were cryostat cut, mounted on gelatin-coated slides, and stored at −35°C. The distribution of binding sites labeled by [3H]CP55,940, a ligand with equal affinity for CB1 and CB2 (24), was examined in brain and spleen tissues. As described (18), binding was performed in cytomailers (3 hr at 37°C) in 50 mM Tris⋅HCl (pH 7.4) containing 5% BSA and 2.5 nM [3H]CP55,940 (specific activity 126 Ci/mmol, NEN). Nonspecific binding was determined in the presence of 10 μM CP55,244. Slides were washed (4 hr at 0°C) in the same buffer containing 1% BSA, fixed in 0.5% formalin in 50 mM Tris⋅HCl (pH 7.4 at 25°C), and blown dry. Sections were apposed to 3H-sensitive film (Hyperfilm, Amersham Pharmacia) together with 3H standards (3H microscales, Amersham Pharmacia) for 2 weeks. Percent specific binding was 92% and 56%, in brain and spleen, respectively. Image analysis was performed on a Macintosh computer-based densitometry system by using a solid-state video camera and image software (Wayne Rasband, National Institute of Mental Health, Bethesda, MD).
Drugs.
Δ9-THC was obtained from the National Institute on Drug Abuse. 3-(1,1-Dimethylheptyl)-(-)-11-hydroxy-Δ8-tetrahydrocannabinol (HU210) was obtained from Tocris Cookson (Ballwin, MO). All drugs were dispersed in drug/emulphor/saline solution (emulphor: 5%–20% Alkamuls EL-620, North American Chemicals, Cranbury, NJ), 76%–89% of 0.9% NaCl). Drugs were injected i.p. at various doses in a volume of 10 μl/g.
Animal Testing.
Mortality rates were evaluated for CB1−/− (n = 223), CB1+/− (n = 474), and CB1+/+ (n = 285) mice. Animals were ear-marked at the time of weaning (21–24 days after birth), and tail biopsies were used for genotyping. The rate of natural deaths was calculated between 4 and 24 weeks of age.
The behavioral and physiological effects of cannabinoid drugs in rodents commonly are evaluated by measuring body temperature, ring catalepsy, activity in the open field, and analgesia (9). Body temperature was measured by using a rectal probe 50 min after drug injection (model BAT-12, Physitemp Instruments, Clifton, NJ). Immobility in the ring-catalepsy test was measured 60 min after injection essentially as described (25). Animals were placed on a vertical tube (5.5 cm diameter). The immobility index (determined from videotaped recordings) was calculated as a percentage of time that the animal spent motionless during the 4-min test session on the ring. If an animal fell down or jumped off the ring, it was placed immediately on the ring again. After a maximum of five such escapes, the test was terminated. Immobility index = (time motionless ×100/duration of the test session). Tail-flick analgesia was measured 50 min after injection by using an automated tail-flick apparatus (Columbus Instruments, Columbus, OH) by using standard procedures. The cutoff latency was 12 sec. Baseline latencies were measured 15 min before the drug injection. Analgesia was determined 50 min after drug injection. Hotplate analgesia was measured with a hotplate analgesia meter (Columbus Instruments) heated to 52°C (−0,1°C) as described (23, 26). The latency until mice showed first signs of discomfort (licking or flinching of the hindpaws, jumping) was recorded. The cutoff time was 60 sec. In the formalin test, 20 μl of a 5% formalin solution were injected s.c. under the dorsal surface of the right hindpaw. The animals’ responses were recorded between 1–6 min and 15–20 min after the injection as described (26).
Data Analysis.
Genotype and drug effects were determined by two-way ANOVA followed by post hoc tests.
RESULTS
Targeted Disruption of the CB1 Gene.
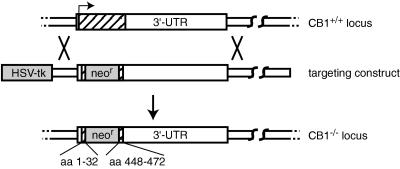
The CB1 receptor coding sequence is contained within a single large exon. To generate mice lacking the CB1 receptor we replaced most of the CB1 coding sequence with PGK-neor through homologous recombination in embryonic stem cells (Fig. 1) (21). Homozygous mutant CB1neor/CB1neor mice (henceforth referred to as CB1−/−) and wild-type control animals (CB1+/+) were obtained from matings of heterozygous (CB1+/−) mice.
Figure 1.
Targeting the CB1 gene. The CB1 gene was mutated by replacing the coding region, which is confined to a single exon (hatched box), between amino acids 32 and 448 with PGK-neo.
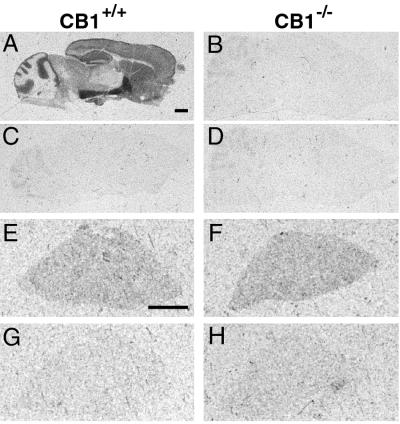
To confirm the absence of the CB1 receptor in homozygous mutant animals, we performed receptor binding studies by using a radiolabeled high affinity CB1 and CB2 ligand CP55,940. Abundant CP55,940 binding sites in the central nervous system of wild-type control mice were found in a pattern similar to that described previously (18) (Fig. 2A). In contrast, no specific CP55,940 binding was detected in brain sections of mutant CB1−/− animals (Fig. 2B), confirming that we have generated a CB1 null allele. In the spleen, where CB2 receptors are abundant, similar CP55,940 binding was observed in knockout mice and wild-type control littermates (Fig. 2 E and F), indicating that CB2 expression is not affected by the mutation.
Figure 2.
Comparison of the specific binding of [3H]CP55,940 to cannabinoid receptors in CB1+/+ and CB1−/− mice. The distribution of [3H]CP55,940 binding sites in sagittal brain sections (A and B) and transverse spleen sections (E and F) are shown in CB1+/+ (Left) and CB1−/− (Right) mice. Nonspecific binding, determined in the presence of 10 μM CP55,244, is shown in brain (C and D) and spleen (G and H). [3H]CP55,940 binding in sagittal brain sections in CB1−/− mice did not differ from nonspecific binding in CB1+/+ mice. [3H]CP55,940 binding in spleen was comparable in CB1+/+ and CB1−/− mice. (Bar equals 1 mm.)
Increased Mortality in CB1 Knockout Mice.
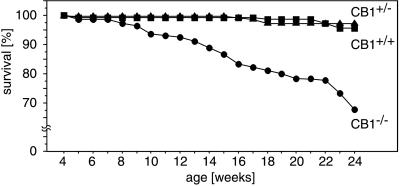
The distribution of genotypes among offspring of heterozygous (CB1+/− × CB1+/−) matings was close to the expected Mendelian frequency (CB1+/+, 29%; CB1+/−, 47.7%; CB1−/−, 23.3%; n = 1439) at the time of weaning (21–24 days). CB1−/− mice appeared healthy, were fertile, had normal body weight, and did not exhibit any obvious morphological abnormalities. However, over time it became obvious that the number of spontaneous deaths was higher among CB1−/− mice than CB1+/− or CB1+/+ animals. Analysis of the death rates among 982 offspring from heterozygous (CB1+/− × CB1+/−) matings revealed a significantly (P < 0.0001) increased mortality in knockout animals throughout the entire observation period (Fig. 3). CB1 knockout animals died suddenly without any obvious signs of disease, such as weight loss, dehydration, or abnormal posture. Pathological examination did not reveal any obvious cause of death.
Figure 3.
Mortality rate in offspring from heterozygous (CB1+/− × CB1+/−) matings. Note that more than 30% of all CB1 knockout mice under 24 weeks of age die of natural causes. In contrast, less than 5% of the heterozygous and wild-type littermates die during the same time span.
Physiological and Behavioral Alterations in CB1 Knockout Mice.
To determine the physiological and behavioral effects of the CB1 mutation we measured the body temperature, activity in the open field, catalepsy in the ring test, and nociceptive responses under drug-free conditions in CB1−/− and CB1+/+ mice. These parameters are commonly used to assess cannabinoid effects in rodents (9).
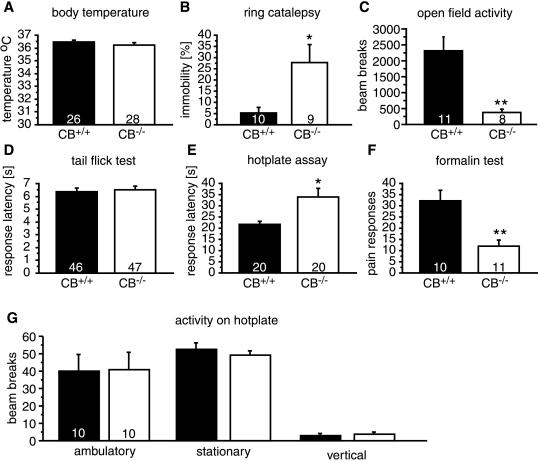
As shown in Fig. 4, the body temperature of mutant mice was not different from wild-type controls (P = 0.3301). In contrast, CB1 knockout mice spend significantly longer time motionless in the ring catalepsy test compared with wild-type mice (P = 0.0124). These animals were also hypoactive in the open-field test (P = 0.0023).
Figure 4.
Baseline physiological and behavioral responses in CB1+/+ and CB1−/− mice. (A) Body temperature was similar in both genotypes. CB1−/− mice showed (B) increased immobility in the ring catalepsy test, (C) decreased activity in the open field, (D) normal responses in the tail-flick test, and (E) hypoalgesia in the hotplate and (F) formalin tests. (G) Locomotor activity of knockout and wild-type animals was similar during the hotplate test. The number of animals studied in each test is shown at the bottom of each bar. ∗ denotes P < 0.05; ∗∗ denotes P < 0.005, n = 8–12 for each genotype.
We measured nociceptive responses to acute pain by using tail-flick and hotplate tests and to tonic pain by using the formalin test (Fig. 4 D–F). It generally is accepted that different neuronal systems mediate the behavioral responses in these tests and that they have different analgesic requirements. For example, the tail-flick response is considered to be a spinal reflex whereas the hotplate and formalin tests involve supraspinal processing (27). In the tail-flick test, baseline nociceptive responses were not different between wild-type and knockout mice (P = 0.7034), but CB1−/− mice showed significantly increased response latencies in the hotplate test (P = 0.0062) and a reduced number of pain responses in the early phase of the formalin test (P = 0.0019). Thus, CB1−/− mice appeared to be less pain sensitive in two tests of supraspinal pain responses.
We considered the possibility that the hypoactivity of CB1−/− mice may have influenced the results of the hotplate test. We therefore studied the locomotor activity of CB1 knockouts during the hotplate test. As shown in Fig. 4G, there was no difference in ambulatory (P = 0.9698), stationary (P = 0.5708), or vertical (P = 0.7337) activity between wild-type and knockout mice on the hotplate.
HU210 Effects.
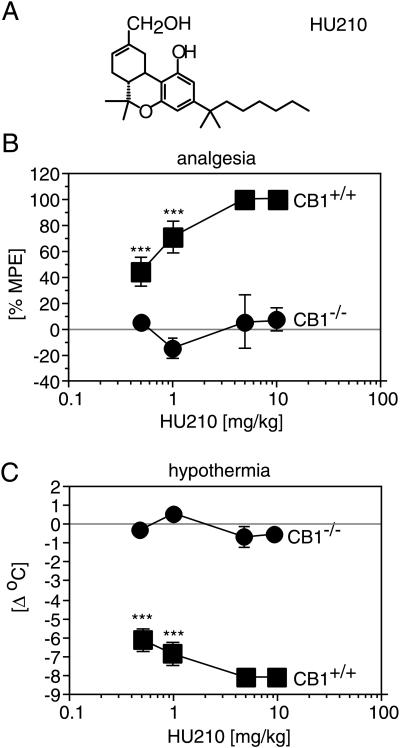
We first analyzed the behavioral and physiological effects of the highly potent synthetic cannabinoid agonist HU210 after i.p. injection into CB1 receptor knockout and wild-type mice. HU210 produced strong analgesia in the tail-flick test, hypothermia (Fig. 5), and immobility (data not shown) in wild-type animals, whereas it was completely ineffective in knockouts, even at high concentrations (10 mg/kg).
Figure 5.
HU210 effects. (A) Chemical structure of HU210. (B) Body temperature was measured by using a rectal probe immediately before and 50 min after the drug injection. HU210 produces a pronounced hypothermia in CB1+/+ mice, but not in CB1−/− mice. (C) HU210 produces a robust analgesia in the tail-flick test in CB1+/+ mice, but not in CB1−/− mice. Note that only the response of a single CB1+/+ animal is shown for comparison after injection of 5 mg/kg and 10 mg/kg HU210, respectively. ∗∗ denotes P < 0.005; ∗∗∗ denotes P < 0.0005; n = 10 for each genotype.
Δ9-THC Effects in CB1−/− Mice.
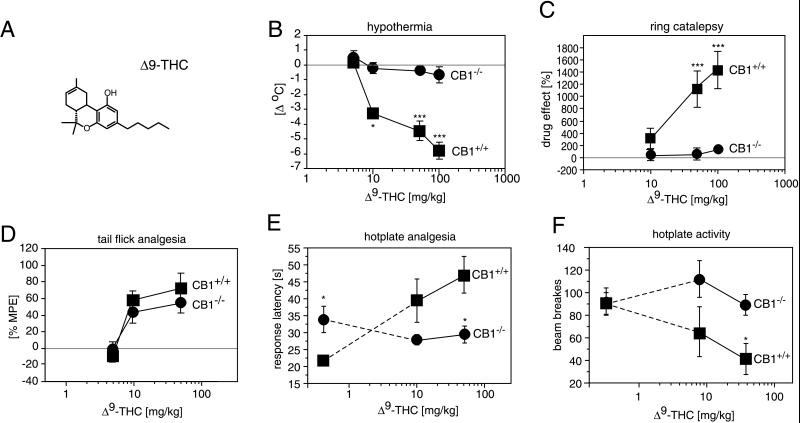
We next analyzed the effects of Δ9-THC, the major psychoactive compound in C. sativa preparations (6), in CB1 receptor knockout mice, which is at least 100 times less potent than HU210 at the CB1 receptor (9, 28, 29). Administration of Δ9-THC results in a profound, dose-dependent reduction of the body temperature (hypothermia) in normal mice (Fig. 6B; P < 0.0001). In contrast, the body temperature of CB1−/− mice did not change significantly at any of the drug doses tested (P = 0.2012).
Figure 6.
Δ9-THC effects. (A) Chemical structure of Δ9-THC. (B) Wild-type CB1+/+ animals displayed significant dose-dependent hypothermia. In contrast, CB1−/− mice showed no alteration in body temperature. (C) CB1+/+ mice, but not CB1−/− mice, displayed a profound Δ9-THC-induced catalepsy in the Pertwee ring test (25). As the baseline responses differed somewhat between the two genotypes (Fig. 3B), the drug-induced scores are expressed relative to values of vehicle injected mice. (D) Δ9-THC-analgesia determined with the tail-flick test. Note that the analgesic effects of Δ9-THC are similar in CB1+/+ and CB1−/− mice at doses up to 50 mg/kg. A dose of 100 mg/kg did not increase tail-flick latencies, but produced other behavioral and physiological responses (see text for details). In the hotplate test, Δ9-THC induced (E) analgesia and (F) hypoactivity in CB1+/+ but not CB1−/− mice. ∗ denotes P < 0.05; ∗∗ denotes P < 0.005; ∗∗∗ denotes P < 0.0005; n = 8–12 for each genotype.
Δ9-THC has deleterious effects on psychomotor performance in humans and animals (30). In normal mice, Δ9-THC administration reduces locomotor activity and increases the immobility index in the ring-catalepsy test. We did not measure drug effects on locomotor activity in the open field, because CB1−/− mice already were extremely hypoactive under baseline conditions. Rather, we evaluated Δ9-THC effects on locomotor activity during the hotplate test, where CB1−/− and CB1+/+ mice displayed very similar baseline activities. As shown in Fig. 6F, Δ9-THC significantly reduced the activity of wild-type animals during the hotplate test (P = 0.0369), but was without effect in CB1 knockouts (P = 0.9638). In addition, Δ9-THC-induced ring immobility was observed in CB1+/+ animals (P = 0.0015), but not in CB1−/− mice (P = 0.3233).
Δ9-THC-induced analgesia was measured in tail-flick and hotplate tests (Fig. 6 D and E). Interestingly, Δ9-THC doses up to 50 mg/kg were similarly effective in the tail-flick test in knockout and wild-type mice. In the hotplate test, we also found Δ9-THC-induced analgesia in CB1+/+ mice (P < 0.0001), but not in CB1−/− animals (P = 0.4656).
In addition to tail-flick analgesia, Δ9-THC produced other behavioral and physiological responses in CB1−/− mice. CB1−/− animals frequently displayed a characteristic head bobbing and assumed a hunched position after injection of high Δ9-THC doses (50–100 mg/kg), a posture associated with discomfort. They also frequently licked their abdomens in the vicinity of their genitalia. In contrast, CB1+/+ mice showed a pronounced hypoactivity at these drug doses, and all CB1+/+ animals displayed muscular prostration. Most strikingly, all CB1−/− animals suffered from strong diarrhea within 15–30 min after injection of 100 mg/kg Δ9-THC. We never observed diarrhea in wild-type control animals after Δ9-THC injection.
DISCUSSION
This study examines the phenotypes of mice with a mutation in the central cannabinoid receptor CB1. The CB1 receptor does not appear to play a critical role during embryonic development because CB1−/− animals display no gross anatomical defects. On the other hand, we found that CB1−/− and CB1+/− offspring from heterozygous matings were underrepresented (CB1−/− 23.3% vs. 29% expected; CB1+/− 47.7% vs. 58% expected), indicating that there may be a loss of CB1−/− and CB1+/− mice during embryonic development or during early postnatal life. Such a loss would be consistent with a presumed role of the cannabinoid system in embryo implantation (31, 32).
Despite the lack of any apparent health problems or physiological abnormalities, CB1−/− mice displayed a significant increase in mortality compared with wild-type or heterozygous littermates. The reason for this increased mortality is currently unknown and needs to be determined in future experiments. As cannabinoids can cause pronounced hypotension and bradycardia (33, 34), probably through activation of the CB1 receptor (35, 36), it is conceivable that CB1 knockout mice may succumb to latent cardiovascular problems. Alternatively, CB1−/− mice have an increased risk of developing neurological problems such as seizures. It is unlikely that the mechanism causing later deaths was the same as that causing the non-Mendelian distribution of the genotypes at the time of weaning, because heterozygous mutant mice were underrepresented, yet their mortality was similar to wild-type animals.
The disruption of the CB1 cannabinoid receptor gene resulted in alterations in locomotor activity and in nociceptive behaviors. CB1−/− mice displayed hypoactivity in the open field, increased immobility in the ring catalepsy test, and hypoalgesia in the hotplate and formalin tests. These results confirm a postulated role of the endogenous cannabinoid system in the modulation of nociceptive responses and motor activity. Alterations in nociceptive behaviors were observed only in the hotplate and formalin tests, but not in the tail-flick test. One of the major differences between these tests is the level of information processing (27). The tail-flick test is a spinal reflex, whereas nociceptive responses on the hotplate and after formalin administration require higher brain functions. Thus, the CB1 mutation seems to affect specifically the supraspinal processing of nociceptive information processing.
The direction of the phenotypic changes was surprising, because CB1 ligands can produce similar behavioral alterations (hypoalgesia, hypolocomotion, catalepsy) in normal mice. Moreover, blockade of the CB1 receptor with the antagonist SR141716A produces hyperalgesia (37, 38) and stimulates locomotor activity (39). The differences between the pharmacological results and this genetic study may be accounted for by the inverse agonist properties of SR141716A (40), or by differences in acute vs. chronic inactivation of the CB1 receptor. The latter hypothesis is supported by demonstration of hyperalgesia after CB1 antisense treatment (37).
A receptor-mediated mechanism for the action of cannabinoid drugs first was suggested by the demonstration of stereoselectivity of enantiomers of cannabinoid agonists (18, 41), in particular HU210 and HU211 (42–44), as well as by the specific binding of cannabinoid agonists to rat membranes (45, 46). CB1 knockout mice display no binding sites for the cannabinoid agonist CP55,940 in the brain. Thus, the CB1 receptor accounts for most, if not all, CP55,940 binding sites in the central nervous system. Accordingly, most of the physiological effects of cannabinoid drugs were abolished by the mutation in the CB1 receptor gene. CB1-deficient mice showed no hypothermia, no hypoactivity, and no catalepsy after administration of Δ9-THC or HU210. In addition, we found no Δ9-THC-induced analgesia in the hotplate test. On the other hand, CB1−/− mice displayed robust Δ9-THC-induced analgesia in the tail-flick test. In fact, CB1−/− and CB1+/+ mice showed similar dose-response curves with Δ9-THC doses between 5 mg/kg and 50 mg/kg. The absence of HU210 analgesia in the tail-flick tests suggests that Δ9-THC is less specific for the CB1 receptor than HU210 and it may produce neuronal effects that are not mediated by the CB1 receptor.
There is now overwhelming evidence that the antinociceptive effects of cannabinoids represent true analgesia (suppression of pain processing) and are not an artifact of other drug effects (hypothermia, motor impairment) (47–50). Nevertheless, we cannot exclude the possibility that such other drug effects may influence nociceptive behaviors in knockout and wild-type animals differently. For example, it has been shown that the skin temperature strongly influences tail-flick latencies, with high temperatures causing shorter latencies and vice versa (51). Because Δ9-THC treatment had no effect on the body temperature of knockout mice, in contrast to the very pronounced dose-dependent decrease in wild-type animals, the analgesic effects of Δ9-THC actually may be greater in CB1−/− than in CB1+/+ mice.
A high dose of 100 mg/kg Δ9-THC produced obvious discomfort in CB1 knockout mice. CB1−/− animals assumed a hunched position, frequently licked their abdomens, and suffered from diarrhea, in contrast to CB1+/+ mice who showed none of these symptoms. It is unlikely that these drug effects are mediated by the CB2 receptor, because they also were observed in CB1/CB2 double knockout mice that were generated in our lab (N. Buckley and A.Z., unpublished data). An involvement of the cannabinoid system in the regulation of intestinal motility previously was suggested by the finding that cannabinoid drugs can reduce intestinal transit and gastric emptying (52). It is thus possible that the cannabinoid system plays a dual role in the regulation of intestinal functions: reducing gut motility through activation of the CB1 receptor and increasing motility through a CB1-independent mechanism.
The cloning of cannabinoid receptors and now the genetic deletion of the neuronal receptor CB1 show clearly that most of the effects of cannabinoid drugs on the brain are indeed mediated by the CB1 receptor. However, it also has been shown that cannabinoid drugs stimulate both receptor-mediated and receptor-independent signaling pathways (24). The molecular basis for the analgesic and other effects of Δ9-THC in CB1−/− mice remains to be determined, but may involve drug-membrane interactions and drug effects on other proteins such as ion channels, or it may involve a yet unknown neuronal receptor. In any case, the absence of CP55,940 binding sites and the absence of physiological responses to HU210 in CB1 knockout mice indicate that CB1-mediated and CB1-independent effects of cannabinoid drugs can be pharmacologically separated.
ABBREVIATION
- Δ9-THC
Δ9-tetrahydrocannabinol
Footnotes
A Commentary on this article begins on page 5338.
References
- 1.Adams I B, Martin B R. Addiction. 1996;91:1585–1614. [PubMed] [Google Scholar]
- 2.Noyes R, Jr, Brunk S F, Baram D A, Canter A. J Clin Pharmacol. 1975;15:139–143. doi: 10.1002/j.1552-4604.1975.tb02348.x. [DOI] [PubMed] [Google Scholar]
- 3.Holdcroft A, Smith M, Jacklin A, Hodgson H, Smith B, Newton M, Evans F. Anaesthesia. 1997;52:483–486. doi: 10.1111/j.1365-2044.1997.139-az0132.x. [DOI] [PubMed] [Google Scholar]
- 4.Sallan S E, Zinberg N E, Frei E d. N Engl J Med. 1975;293:795–797. doi: 10.1056/NEJM197510162931603. [DOI] [PubMed] [Google Scholar]
- 5.Nelson K, Walsh D, Deeter P, Sheehan F. J Palliat Care. 1994;10:14–18. [PubMed] [Google Scholar]
- 6.Gaoni Y, Mechoulam R. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- 7.Mechoulam R, Breuer A, Feigenbaum J J, Devane W A. Farmaco. 1991;46:267–276. [PubMed] [Google Scholar]
- 8.Mechoulam R, Feigenbaum J J. Prog Med Chem. 1987;24:159–207. doi: 10.1016/s0079-6468(08)70422-3. [DOI] [PubMed] [Google Scholar]
- 9.Martin B R, Compton D R, Thomas B F, Prescott W R, Little P J, Razdan R K, Johnson M R, Melvin L S, Mechoulam R, Ward S J. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- 10.Felder C C, Glass M. Annu Rev Pharmacol Toxicol. 1998;38:179–200. doi: 10.1146/annurev.pharmtox.38.1.179. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda L A, Lolait S J, Brownstein M J, Young A C, Bonner T I. Nature (London) 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda L A, Bonner T I, Lolait S J. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- 13.Gerard C M, Mollereau C, Vassart G, Parmentier M. Biochem J. 1991;279:129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakrabarti A, Onaivi E S, Chaudhuri G. DNA Sequence. 1995;5:385–388. doi: 10.3109/10425179509020870. [DOI] [PubMed] [Google Scholar]
- 15.Abood M E, Ditto K E, Noel M A, Showalter V M, Tao Q. Biochem Pharmacol. 1997;53:207–214. doi: 10.1016/s0006-2952(96)00727-7. [DOI] [PubMed] [Google Scholar]
- 16.Munro S, Thomas K L, Abu-Shaar M. Nature (London) 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 17.Shire D, Calandra B, Rinaldi-Carmona M, Oustric D, Pessegue B, Bonnin-Cabanne O, Le Fur G, Caput D, Ferrara P. Biochim Biophys Acta. 1996;1307:132–136. doi: 10.1016/0167-4781(96)00047-4. [DOI] [PubMed] [Google Scholar]
- 18.Herkenham M, Lynn A B, Little M D, Johnson M R, Melvin L S, de Costa B R, Rice K C. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris L S, Carchman R A, Martin B R. Life Sci. 1978;22:1131–1137. doi: 10.1016/0024-3205(78)90082-6. [DOI] [PubMed] [Google Scholar]
- 20.Devane W A, Dysarz F A d, Johnson M R, Melvin L S, Howlett A C. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 21.Zimmer A. Annu Rev Neurosci. 1992;15:115–137. doi: 10.1146/annurev.ne.15.030192.000555. [DOI] [PubMed] [Google Scholar]
- 22.Wojnowski L, Zimmer A M, Beck T W, Hahn H, Bernal R, Rapp U R, Zimmer A. Nat Genet. 1997;16:293–297. doi: 10.1038/ng0797-293. [DOI] [PubMed] [Google Scholar]
- 23.Zimmer A, Zimmer A M, Baffi J, Usdin T, Reynolds K, König M, Palkovits M, Mezey E. Proc Natl Acad Sci USA. 1998;95:2630–2635. doi: 10.1073/pnas.95.5.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felder C C, Veluz J S, Williams H L, Briley E M, Matsuda L A. Mol Pharmacol. 1992;42:838–845. [PubMed] [Google Scholar]
- 25.Pertwee R G. Br J Pharmacol. 1972;46:753–763. doi: 10.1111/j.1476-5381.1972.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.König M, Zimmer A M, Steiner H, Holmes P V, Crawley J N, Brownstein M J, Zimmer A. Nature (London) 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- 27.Franklin K B J, Abbott F V. In: Techniques for Assessing the Effects of Drugs on Nociceptive Responses. Boulton A A, Baker G B, Greenshaw A J, editors. Vol. 13. Clifton, NJ: Humana; 1989. pp. 145–216. [Google Scholar]
- 28.Felder C C, Joyce K E, Briley E M, Mansouri J, Mackie K, Blond O, Lai Y, Ma A L, Mitchell R L. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- 29.Rhee M H, Vogel Z, Barg J, Bayewitch M, Levy R, Hanus L, Breuer A, Mechoulam R. J Med Chem. 1997;40:3228–3233. doi: 10.1021/jm970126f. [DOI] [PubMed] [Google Scholar]
- 30.Dewey W L. Pharmacol Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- 31.Buckley N E, Hansson S, Harta G, Mezey E. Neuroscience. 1998;82:1131–1149. doi: 10.1016/s0306-4522(97)00348-5. [DOI] [PubMed] [Google Scholar]
- 32.Schmid P C, Paria B C, Krebsbach R J, Schmid H H, Dey S K. Proc Natl Acad Sci USA. 1997;94:4188–4192. doi: 10.1073/pnas.94.8.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dewey W L, Harris L S, Howes J F, Kennedy J S, Granchelli F E, Pars H G, Razdan R K. Nature (London) 1970;226:1265–1267. doi: 10.1038/2261265a0. [DOI] [PubMed] [Google Scholar]
- 34.Vollmer R R, Cavero I, Ertel R J, Solomon T A, Buckley J P. J Pharm Pharmacol. 1974;26:186–192. doi: 10.1111/j.2042-7158.1974.tb09252.x. [DOI] [PubMed] [Google Scholar]
- 35.Varga K, Lake K, Martin B R, Kunos G. Eur J Pharmacol. 1995;278:279–283. doi: 10.1016/0014-2999(95)00181-j. [DOI] [PubMed] [Google Scholar]
- 36.Lake K D, Compton D R, Varga K, Martin B R, Kunos G. J Pharmacol Exp Ther. 1997;281:1030–1037. [PubMed] [Google Scholar]
- 37.Richardson J D, Aanonsen L, Hargreaves K M. J Neurosci. 1998;18:451–457. doi: 10.1523/JNEUROSCI.18-01-00451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calignano A, La Rana G, Giuffrida A, Piomelli D. Nature (London) 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 39.Compton D R, Aceto M D, Lowe J, Martin B R. J Pharmacol Exp Ther. 1996;277:586–594. [PubMed] [Google Scholar]
- 40.Landsman R S, Burkey T H, Consroe P, Roeske W R, Yamamura H I. Eur J Pharmacol. 1997;334:R1–R2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- 41.Dewey W L, Martin B R, May E L. In: Cannabinoid Steroisomers: Pharmacological Effects. Smith D F, editor. Boca Raton, Fl: CRC; 1984. pp. 317–326. [Google Scholar]
- 42.Howlett A C, Champion T M, Wilken G H, Mechoulam R. Neuropharmacology. 1990;29:161–165. doi: 10.1016/0028-3908(90)90056-w. [DOI] [PubMed] [Google Scholar]
- 43.Little P J, Compton D R, Mechoulam R, Martin B R. Pharmacol Biochem Behav. 1989;32:661–666. doi: 10.1016/0091-3057(89)90014-2. [DOI] [PubMed] [Google Scholar]
- 44.Jarbe T U, Hiltunen A J, Mechoulam R. J Pharmacol Exp Ther. 1989;250:1000–1005. [PubMed] [Google Scholar]
- 45.Devane W A, Dysarz F A, Johnson M R, Melvin L S, Howlett A C. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 46.Howlett A C, Bidaut-Russell M, Devane W A, Melvin L S, Johnson M R, Herkenham M. Trends Neurosci. 1990;13:420–423. doi: 10.1016/0166-2236(90)90124-s. [DOI] [PubMed] [Google Scholar]
- 47.Walker, B. M., Hohmann, A. G., Martin, W. J., Strangman, N. M., Huang, S. M. & Tsou, K. (1999) Life Sci., in press. [DOI] [PubMed]
- 48.Hohmann A G, Tsou K, Walker B M. Neurosci Lett. 1998;257:119–122. doi: 10.1016/s0304-3940(98)00802-7. [DOI] [PubMed] [Google Scholar]
- 49.Hohmann A G, Tsou K, Walker B M. J Neurophysiol. 1999;81:575–583. doi: 10.1152/jn.1999.81.2.575. [DOI] [PubMed] [Google Scholar]
- 50.Martin W J, Hohmann A G, Walker J M. J Neurosci. 1996;16:6601–6611. doi: 10.1523/JNEUROSCI.16-20-06601.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hole K, Tjolsen A. Pain. 1993;53:247–254. doi: 10.1016/0304-3959(93)90220-J. [DOI] [PubMed] [Google Scholar]
- 52.Shook J E, Burks T F. J Pharmacol Exp Ther. 1989;249:444–449. [PubMed] [Google Scholar]