Abstract
To understand the relationship between the affinity of the B cell antigen receptor (BCR) and the immune response to antigen, two lines of immunoglobulin H chain transgenic (Tg) mice were created. H50Gμa and T1(V23)μa mice express μ H chain transgenes that associate with the λ1 L chains to bind the (4-hydroxy-3-nitrophenyl)acetyl hapten with association constants (K as) of only 1.2 × 105 M−1 and 3 × 104 M−1, respectively. Both lines mounted substantial antibody-forming cell (AFC) and germinal center (GC) responses. H50Gμa Tg mice also generated memory B cells. T1(V23)μa B cells formed AFC and GCs, but were largely replaced in late GCs by antigen-specific cells that express endogenous BCRs. Thus, B lymphocytes carrying BCRs with affinities previously thought to be irrelevant in specific immune responses are in fact capable of complete T cell–dependent immune responses when relieved of substantial competition from other B cells. The failure to observe such B cells normally in late primary responses and in memory B cell populations is the result of competition, rather than an intrinsic inability of low affinity B cells.
Keywords: mutation, antibody affinity, plasma cell, variable region gene, λ1 immunoglobulin
Introduction
Early humoral responses are heterogeneous and mainly low affinity (1–4). As the response progresses, Ab diversity is reduced and affinity increases (5–7). It is possible that early low affinity Ab is biologically irrelevant and the result of nonspecific or bystander stimulation (8, 9). Alternatively, very low affinity B cells may respond specifically but are lost as the response progresses. For example, intrinsic affinity requirements could limit cell participation in humoral responses. Survival in germinal centers (GCs) or memory differentiation may require signaling above a threshold (10–12). Alternatively, the loss of low affinity B cells from immune responses could reflect competition among antigen-reactive B cells (13).
To distinguish these models for the antigen-driven selection of B cells, we generated the following two lines of transgenic (Tg) mice expressing Ig H chain Tgs that encode extremely low affinity anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies when associated with the λ1 L chain: 1.2 × 105 M−1 for H50Gμa and ∼3.0 × 104 M−1 for T1(V23)μa.
Materials and Methods
Mice.
6–8-wk-old C.B-17 and C.B-17-SCID mice were purchased from Taconic Farms, Inc. All mice were maintained in sterile microisolator cages.
Creation of H50Gma and T1(V23)ma Tg Mice.
The VH186.2/DFL16.1 rearrangement, containing a single mutation at codon 50, designated H50G and V23 analogue VH rearrangement from an unmutated VDJ rearrangement of the V23 gene taken from a C57BL/6 mouse 10 d after immunization with NP, were subcloned into a vector that contains the 11.6-kb Cμ fragment that includes the membrane and secretory exons (14). The H50Gμa lineage had two copies of the Tg, whereas two separate T1(V23)μa lineages with similar phenotypes were used with two to three copies or a single copy of the Tg. Tg mice were backcrossed with C.B-17 mice for five or more generations.
Flow Cytometry.
The following antibodies were used: phycoerythrin-conjugated anti-B220 (CD45RA-PE; BD PharMingen); fluorescein-conjugated anti–mouse IgMa (RS3.1-FITC); biotinylated AF6-7.8 (anti-IgMb); fluorescein-conjugated or biotinylated Ls136 (anti-λ1); and biotinylated goat anti–mouse κ L chain–specific and biotinylated goat anti–mouse Ig (Southern Biotechnology Associates, Inc.). Red 670–conjugated streptavidin (GIBCO BRL) was used to reveal biotin-coupled Ab staining.
Antigens and Immunizations.
Mice were immunized intraperitoneally with 50 μg of NP16-chicken γ-globulin (CG), (4-hydroxy-5-iodo-3-nitrophenyl)acetyl (NIP)12-CG, or CG precipitated in alum. For secondary/memory responses and challenge of repopulated SCID mice, 20 μg of soluble NP16-CG in PBS was administered intravenously on the indicated days.
Immunohistology.
Immunohistology was performed as previously described (3, 15). GCs were enumerated as peanut agglutinin (PNA)+ areas within lymphoid follicles (± SD from three separate sections). Endogenous Ig–expressing B cells in the GC of H50Gμa or T1(V23)μa mice were enumerated using both AF6-7.8-biotin and biotinylated goat anti–mouse IgG1, followed by streptavidin-alkaline phosphatase. To detect apoptotic cells, TUNEL assays were performed using in situ apoptosis detection kits (Oncor).
Microdissection of Cells, DNA Amplification, and Sequencing.
Microdissection of cells, DNA amplification, and sequencing was performed as previously described (14, 16). Cellular material (constituting 10–20 cells) was microdissected from individual GCs (3, 15, 17). In H50Gμa mice, GCs sampled were also IgMa+ and devoid of endogenous Ig–expressing (IgMb+ or IgG1 +) cells. DNA was amplified by Pfu polymerase (Stratagene) using nested primers specific for Vλ1 and Jλ1 elements (14).
Quantitation of Serum Ab.
Ab titers were determined by endpoint dilution in ELISA using plates coated with NP21-BSA, NIP19-BSA, or CG (7). The endpoint was the last serial dilution that demonstrated a signal greater than twofold above the background. NP-specific ELISAs using differentially haptenated substrates were used to quantitate high and low affinity Ab (3).
Reduction of Serum Ab by 2-Mercaptoethanol (2-ME).
Serum was assessed for NP reactivity by ELISA after treatment with 2-ME (3). For calculations of the percent of the IgMa titer resistant to 2-ME treatment, mock and 2-ME ELISA results were converted to relative Ab concentrations by comparison to a standard purified H50Gμa/λ1 transfectoma Ab.
Adoptive Transfers of Splenocytes.
5 × 106 splenocytes recovered from H50Gμa and C.B-17 mice 30 d after immunization with 50 μg of NP12-CG precipitated in alum (18) were injected intravenously into recipient SCID mice. Recipients were challenged with 20 μg of NP12-CG in PBS or PBS alone 24 h later. 9 d after immunization the recipient sera were collected.
Online Supplemental Material.
The supplementary material shows original Vλ sequence data as well as summary tables that analyze the distribution of mutations in the sequences. Fig. S1 shows all codons that contained mutations in Vλ1 sequences derived from either C.B-17 or H50Gμa mice, as described in the Materials and Methods and Results sections. Table S1 is a comparison of the two datasets, showing the frequency of overall mutations as well as replacement and silent mutations. Table S2 shows the ratios of replacement and silent ratios in each subregion of Vλ. Online supplemental material available at http://www.jem.org/cgi/content/full/jem.20011550/CD1.
Results
Characterization of H50Gμa and T1(V23)μa Tg Mice.
Splenic B cells from naive H50Gμa and T1(V23)μa mice demonstrated efficient allelic exclusion, with ≤2% expressing endogenous IgMb protein (unpublished data). The ratio of λ1:κ L chain–bearing B cells were comparable in both Tg and control mice, and all Tg lines displayed total surface Ig densities comparable to normal, naive B cells (unpublished data).
Humoral Immune Responses in H50Gμa Tg Mice.
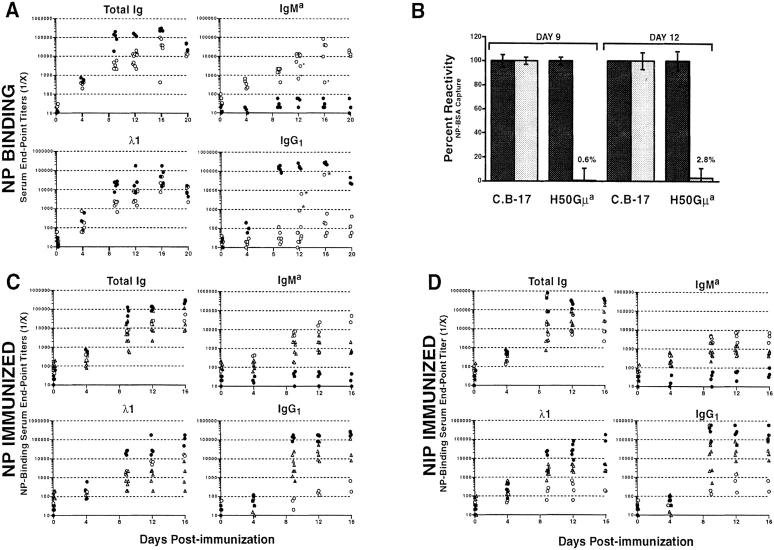
Naive H50Gμa Tg mice had low titers (≤1:60) of NP-specific serum Ab that sharply increased after immunization with NP-CG (Fig. 1 A). At day 12, the titers of NP-binding Tg (IgMa) Ab increased 1,000-fold in H50Gμa mice. Only in 3 out of 31 H50Gμa mice were substantial levels of IgG1 anti-NP detected (Fig. 1 A), indicating that typically most anti-NP was Tg derived. Levels for anti-NP λ1 and total Ig at day four were similar for Tg and control animals. However, C.B-17 mice later achieved 5–10-fold higher titers. To confirm that H50Gμa mice expressed only low affinity Tg-encoded antibodies in response to immunization, sera of H50Gμa and C.B-17 mice were mildly reduced with 2-ME (3). The binding of very low affinity IgM Ab is avidity dependent and lost upon 2-ME reduction (5), whereas higher affinity IgM or IgG Abs are unaffected. The reduction of Tg sera led to a near complete loss (≥97%) of NP reactivity (Fig. 1 B). This loss was comparable to that observed for purified H50Gμa/λ1 transfectoma Ab (3). In contrast, C.B-17 serum titers were unaffected.
Figure 1.
Humoral immune response in low affinity H50Gμa and T1(V23)μa Tg mice. (A) H50Gμa (○) and C.B-17 controls (•) were injected intraperitoneally with NP-CG in alum. Serial dilutions of sera were assayed for NP binding of total Ig, Tg IgMa, anti-NP characteristic λ1, and IgG1. *, H50Gμa mice in which significant anti-NP IgG1 titers may have affected the measurement of IgMa Tg levels. (B) Sera from H50Gμa and control C.B-17 mice after immunization with NP-CG were assayed for their ability to bind NP-BSA after 2-ME treatment (shaded bars) or mock treatment (solid bars). The change in the relative NP binding of treated samples is shown as a percentage change from mock-treated samples. Values represent the mean values (± SD) obtained from three independent experiments. T1(V23)μa Tg mice (▴) were compared with H50Gμa (○) and C.B-17 (•) mice in response to (C) NP-CG or (D) NIP-CG in alum. Sera were assayed for NP-binding components. Each symbol represents the average endpoint dilution value of serum from an individual mouse measured in triplicate.
Immune Response of Very Low Affinity B Cells.
To directly test whether B cells with very low affinities were capable of initiating and sustaining specific humoral responses, H50Gμa Tg mice were immunized with NIP-CG and T1(V23)μa Tg mice were challenged with NP-GC and NIP-CG. H50Gμa/λ1 Ab has a lower affinity for NIP (6.3 × 104 M−1) than for NP (3). T1(V23)μa/λ1 Ab has a K a= 5.0 × 104 M−1 for NIP and an affinity for NP that is too low for reliable measurement by fluorescence quenching (5).
T1(V23)μa mice had 8–10-fold increases in hapten-specific IgMa Ab between 4 and 16 d after immunization with either immunogen, with some mice showing 20–30-fold increases in Ab titer (Fig. 1, C and D; ▴, ▵). IgMa responses by T1(V23)μa mice to NP-CG or NIP-CG were lower (5–10-fold) than those observed in H50Gμa mice for the same immunogen (Fig. 1, C and D; ▴, ▵ vs. ○). IgG1 responses, presumably encoded by the endogenous Igh loci, were higher in T1(V23)μa mice compared with H50Gμa mice. The higher levels of endogenously derived anti-NP antibodies in T1(V23)μa mice may reflect the extraordinarily poor affinity of these Tg B cells, which confer little advantage in competition with rare B cells that express endogenous VH genes. B cells expressing endogenously encoded VH genes comprise 1–3% of the initial repertoire, of which only a small fraction of B cells would have NP/NIP specificity. Thus, the emergence of these B cells in response to NP/NIP reflects the competition between rare, presumably higher affinity B cells and the much more common low affinity Tg B cells.
At the height of the response, H50Gμa mice challenged with NIP had a 100-fold increase in hapten-binding IgMa Ab titers compared with controls (Fig. 1 D), which is 2–10-fold lower than those after challenge with NP-CG (Fig. 1, C and D). This observation establishes a relationship between B cell antigen receptor (BCR) affinity/avidity and the magnitude of the Ab response in a single Tg mouse line.
Low Affinity Tg B Cells Can Initiate Primary GCs.
PNA+ GCs in H50Gμa mice 12 d after immunization (Fig. 2 A) were observed in numbers (∼70–80 per section) equivalent to normal mice (6, 15). 65 ± 5.8% of the GCs in H50Gμa mice were stained with both anti-IgMa and anti-λ1 Ab, indicating that they contained NP-specific B cells expressing the H50Gμa Tg (Fig. 2, A and C). The percentage of λ1+ GCs was only slightly higher than that seen in non-Tg C.B-17 mice for the same day, which was 54 ± 3.4%. Many B cells in the splenic red pulp exhibited strong cytoplasmic staining with λ1- and IgMa-specific Ab (unpublished data), the characteristic phenotype of plasmablasts and antibody-forming cell (AFC) (18).
Figure 2.
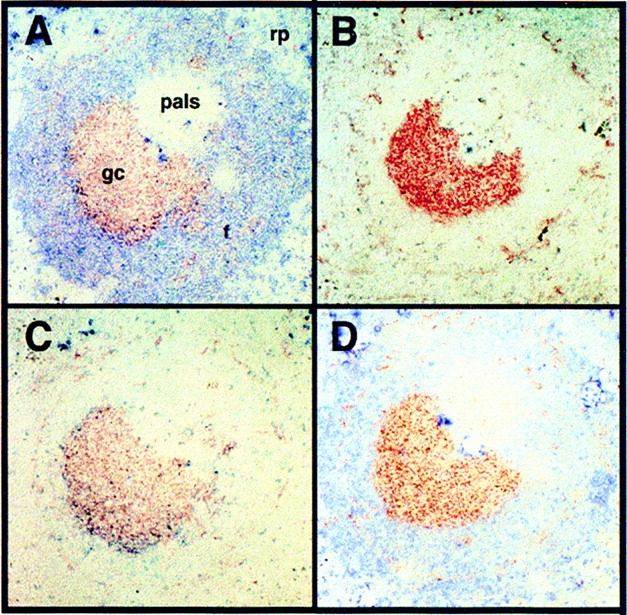
GC formation in H50Gμa mice 12 d after immunization with NP-CG. Splenic serial sections reveal PNA+ GC B cells (red) that were labeled in tandem (blue) for: (A) Tg IgMa+; (B) endogenous IgMb+; (C) λ1 L chain; and (D) κ L chain. rp, red pulp; pals, periarteriolar lymphoid sheath; gc, germinal center. Note that surface Ig staining is weak in GCs due to marked surface IgM down-regulation in GCs. However, the difference between the (A) IgMa and (B) IgMb staining of the same GC can be appreciated by how the IgMa staining modifies the color of the PNA-stained GC cells. A similar picture is seen comparing (C) λ1 and (D) κ staining. ×100.
H50Gμa Tg B cells also formed Tg+ GCs when their BCR affinity was less than 105 M−1, i.e., after immunization with NIP. Such mice had PNA+ and IgMa GCs in numbers equivalent to C.B-17 controls, although many of these GCs were smaller than those in control animals (unpublished data). We also investigated the capacity of very low affinity B cells to form GC in T1(V23)μa mice. Surprisingly, there was no difference in GC number and size in T1(V23)μa mice challenged with NP or NIP compared with C.B-17 controls. However, ∼80% of GCs in T1(V23)μa mice contained both Tg IgMa– and endogenous Ig–expressing B cells (unpublished data). When present, endogenous Ig–expressing B cells (demonstrated with anti-IgMb Ab and anti-IgG1 Ab) constituted as much as 50% of the PNA+ cells in each GC. Presumably these endogenously derived B cells reflect the competitive success of rare, higher affinity B cells. To confirm this, we microdissected and sequenced several clusters of λ1+ B cells costaining for IgMb/IgG1 and sequenced their VH regions. In fact, >75% of recovered sequences were VH186.2, the canonical VH of anti-NP responses, which established that the endogenously derived B cells are higher affinity anti-NP B cells. It is likely that the population of B cells expressing endogenous VDJ rearrangements accounts for the normal size and frequencies of GCs in immunized T1(V23)μa animals.
Late GCs of H50Gμa Mice Show Evidence of Increased Apoptosis.
12 d after immunization with NP-CG, B cells bearing BCRs encoded by the endogenous Igh b loci are infrequent in the GC of H50Gμa Tg mice (Fig. 3 A). However, at day 16 of the primary response, endogenous Ig–expressing B cells (IgMb+ or IgG1 +) begin to appear in the GCs, increasing to 5–10% of all PNA+ GC cells by day 20 (Fig. 3, B and C). Late GCs (16–20 d after immunization) of H50Gμa mice had two- to threefold more apoptotic B cells compared with C.B-17 controls, as indicated by TUNEL labeling (Fig. 3, D and E). GCs (n = 30) sampled from six H50Gμa mice at 16 or 20 d after immunization had an average 32% (±10%) TUNEL+/PNA+/IgD− cells. This was in contrast to GCs (n = 20) from four C.B-17 mice at similar days, which contained only 12% (±7%) TUNEL+ GC cells.
Figure 3.
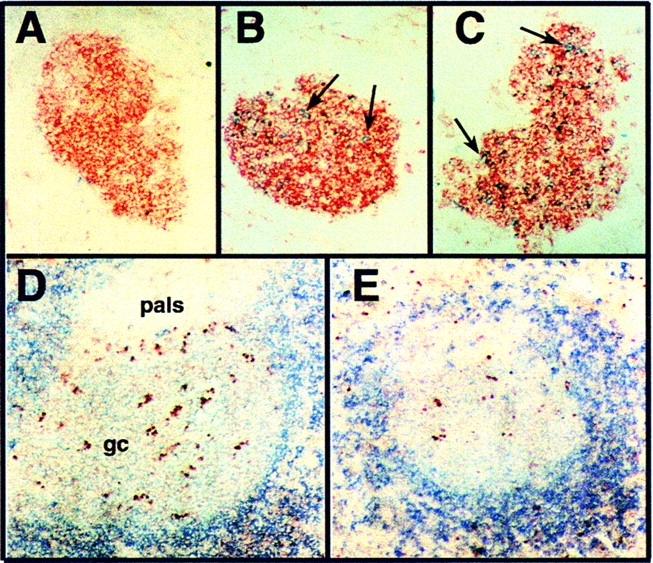
GCs in H50Gμa mice have increased frequencies of endogenous BCRs and enhanced apoptosis. PNA+ GCs (red) from H50Gμa mice (A) 12, (B) 16, (C) and 20 d after immunization with NP-CG were stained for the presence of endogenous Ig (blue, IgMb or IgG1). For detecting apoptotic GC cells, (D) H50Gμa and (E) C.B-17 sections of spleen from mice at day 16 of the primary immune response were labeled by TUNEL+ (red) and with anti-IgM (blue). GCs appear as faint blue areas within darker blue follicles due to surface Ig downmodulation on GC B cells.
Somatic Mutation in H50Gμa Mice.
Somatic hypermutation was assayed in the endogenous λ1 L chain gene of H50Gμa mice, as ectopic heavy chain Tg generally does not mutate. IgMa+/λ1+, GC cells from three H50Gμa mice, and three C.B-17 mice were microdissected and their Vλ regions amplified and sequenced (Fig. S1 and Tables S1 and S2). Of 28 H50Gμa Vλ1/Jλ1 rearrangements examined, 22 were mutated with an average of 5.1 (±3.7) mutations per rearrangement.
The distribution and type of somatic mutations observed in Vλ1 genes present in Tg and control GC B cells is summarized in Tables SI and SII. 57 of 68 H50Gμa Vλ1 mutations encoded amino acid replacements. The 68 unique mutations were a striking contrast to the 11 unique mutations found in C.B-17 GCs. Mutations in the H50Gμa Vλ1/Jλ1 rearrangements had an overall replacement/silent (R/S) ratio three times greater than C.B-17 rearrangements. In addition, the R/S ratio of mutations in CDR1 and CDR2 of H50Gμa Vλ1 genes was 10- and 6-fold higher, respectively, than the predicted ratios based on codon usage. These data demonstrate enhanced mutation and selection in the endogenous Vλ1 gene rearrangements of H50Gμa GC B cells.
Low Affinity Primary and Memory Responses in H50Gμa Mice.
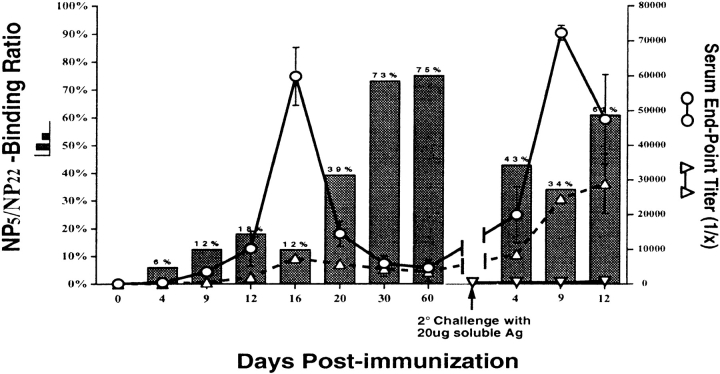
To determine the degree of affinity maturation in the primary and memory responses of H50Gμa mice, Tg and C.B-17 mice were immunized with NP-CG and 60 d later were rechallenged intravenously with soluble NP-CG. Fig. 4 shows the change in the NP-specific IgMa-Tg Ab titers. Upon secondary immunization, a prompt and significant increase in NP-specific IgMa Ab was observed. By day 9 of the secondary response, Tg-IgMa endpoint titers increased from 1:4,500 to 1:72,000 (Fig. 4, ○). No significant Ab response (>1 out of 200) was observed in unprimed H50Gμa mice given the same soluble antigen (Fig. 4, ▿).
Figure 4.
Affinity maturation of the humoral response in H50Gμa mice. Bars show the NP5/NP22 binding ratio for IgMa Ab. Endpoint titers for NP5- (▵) and NP22- (○) binding IgMa from H50Gμa mice are superimposed to demonstrate the actual Ab concentration shifts during the response. The response of naive H50Gμa mice to a primary intravenous immunization with soluble NP-CG is also shown (▿). Values represent the mean ± SD (error bars) of four or five mice for each time point.
Affinity maturation, presumably through the mutation of Vλ1, did enhance the avidity of H50Gμa Ab, as reflected by the increased portion that bound NP5-BSA (Fig. 4; ▵, bars). However, this affinity maturation was modest, as 85–90% of secondary Ab generated by H50Gμa mice remained sensitive to 2-ME treatment (unpublished data). Interestingly, the rechallenge of primed H50Gμa mice with soluble NP-CG elicited a rapid increase in low affinity Ab, as inferred from the precipitous decrease in the NP5/NP22 binding ratio of the serum Ab (Fig. 4, bars). Only 30% of the NP-specific IgMa titer at day 9 of the secondary response showed the ability to bind NP5-BSA. This is a contrast to the 70% observed in the late primary response (Fig. 4) and consistent with the reactivation of low affinity memory B cells and their differentiation into AFC (7).
To exclude that T cell priming alone was responsible for the memory response of H50Gμa mice, Tg and control mice were primed with the carrier protein CG, and 60 d later challenged intravenously with soluble NP-CG. Average NP-specific λ1+ Ab titers from CG-primed H50Gμa mice were 15-fold lower than cohorts primed with NP-CG (Table I; 1:2,200 vs. 1:33,400). On challenge with soluble antigen, NP-CG–primed H50Gμa mice exhibited a 38% absolute increase in the serum Ab titer capable of binding NP5-BSA, whereas there was no appreciable increase in the NP5-BSA–binding titer in CG-primed H50Gμa mice. Thus, T cell priming alone cannot account for the extent of low affinity Ab elicited in the memory responses of H50Gμa mice.
Table I.
Memory Response to Soluble Antigen from NP-CG Primed and Carrier Primed Micea
| Immunization Protocolb
|
Serum Responsec(end-point dilution)
|
|||
|---|---|---|---|---|
| Mice | 1°(i.p.) | 1°(i.v.) | α-NP22 λ1 titer (1/X) |
Percent NP5/NP22Ratio |
| CB-17 | NP-CG | NP-CG | 135,826 ± 69,607 | 53 |
| H50Gμa | 33,417 ± 10,919 | 38 | ||
| C.B-17 | CG | NP-CG | 4,797 ± 2,010 | 23 |
| H50Gμa | 2,218 ± 1,581 | 8 | ||
| C.B-17 | none | NP-CG | 130 ± 60 | 0 |
| H50Gμa | 60 ± 20 | 0 | ||
Serum data were obtained from mice 9 d after secondary challenge with soluble antigen.
Primary immunizations were by intraperitoneal administration of 50 μg NP-CG or CG alone in alum. Secondary/memory responses were elicited through the intravenous injection of 20 μg soluble NP-CG 60 d after primary challenge.
The NP-specific 1λ1 end-point serum titer was used as a measure of the secondary immune response to allow direct comparison between H50Gμa and C.B-17 mice.
To minimize the influence of preformed Ab on secondary responses, splenocytes from naive or NP-CG–primed (day 30) H50Gμa and C.B-17 mice were adoptively transferred into SCID recipients, which were then challenged intravenously with soluble NP-CG. SCID mice that received primed H50Gμa splenocytes generated Ab titers that were more than 100-fold higher than SCIDs that were repopulated with naive H50Gμa splenocytes. The magnitude of the increase was similar in control mice receiving C.B-17 donor cells (unpublished data). These differences in serum Ab were not due to the transfer of residual AFC, as SCID mice that received primed H50Gμa splenocytes but were not challenged with antigen had no appreciable anti-NP serum Ab (titer of 1:60).
Discussion
Normally, very low affinity cells are rare in late GCs, in memory cell populations, or as long-lived AFC (19, 20). By fixing the B cell repertoire at low affinity using a nonmutating ectopic Tg, we show that the virtual absence of low affinity cells in these compartments is due to competition from higher affinity cells. When clonal competition is reduced, very low affinity B cells fill GCs and contribute to memory responses.
Although competition is reduced, low numbers of B cells expressing endogenous VH gene segments provide detectable interclonal competition. Endogenously derived cells are rare in H50Gμa Tg mice, especially early in the response. However, many B cells expressing non-Tg BCR emerge in immunized T1(V23)μa mice, and to a lesser extent H50Gμa mice, as demonstrated by the presence of non-Tg Ab (Fig. 1) and histology (Fig. 3). Presumably, the extremely low affinity of T1(V23)μa B cells for the NP and NIP haptens allows them to be overwhelmed by rare B lymphocytes that escape allelic exclusion and respond to NP/NIP, as indicated by their frequent expression of VH186.2, a hallmark of high affinity NP Ab. Thus, higher affinity B cells expressing endogenous genes outcompete a numerous but very low affinity Tg population.
As V(D)J hypermutation creates L chain diversity in GCs (Figure S1), intraclonal competition also exists. R/S ratios in Vλ CDR1 and Vλ CDR2 suggest strong selection for GC B cell mutants. Although Vλ mutations can improve BCR affinity in H50Gμa mice, the low affinity of secondary Ab in these mice (Fig. 4) suggests that this pathway for affinity maturation is limited. Nonetheless, the high frequency (1.4%) of point mutations in the Vλ1 rearrangements of H50Gμa GC B cells is almost twice that found in a similar mouse with a germline affinity, VH 186.2 Tg (14), and much higher than wild-type mice. The high frequency and intense selection of Vλ1 mutations in H50Gμa mice could reflect the difficulty in improving BCR affinity through L chain mutations alone. However, mutation rates may be intrinsically higher in low affinity B cells.
Our data do not rule out the role(s) for differential signaling governed by affinity/avidity in determining B cell fates (11, 12). Aside from memory development, other aspects of B cell differentiation like isotype switching could be governed by BCR signal strength.
The ability of very low affinity B cells to establish GCs and humoral memory has implications for understanding natural B cell responses. Low affinity BCRs can acquire higher affinities with a single point mutation (19, 21), and the many low affinity B cells that reach GCs are the potential precursors of effective memory B cells. This view is also consistent with direct estimates of clonal diversity in nascent GCs (6) and computer models, which suggest that GCs are seeded by 40–50 precursors (22). Finally, the latent genetic potential of low affinity clones could underlie “clonal shifts” in secondary responses whereby dominant, primary clonotypes are replaced by mutated, dissimilar clones (2, 23).
Acknowledgments
This work was supported by National Institutes of Health grants AI24335, AG10207, and AG13789 to G. Kelsoe, and AI43603 to M.J. Shlomchik. A.M. Haberman was the recipient of a Donaghue Foundation postdoctoral fellowship.
The online version of this article contains supplemental material.
G. Kelsoe and M.J. Shlomchik contributed equally to this work.
References
- 1.Chua, M.M., S.H. Goodgal, and F. Karush. 1987. Germ-line affinity and germ-line variable-region genes in the B cell response. J. Immunol. 138:1281–1288. [PubMed] [Google Scholar]
- 2.Clarke, S.H., L.M. Staudt, J. Kavaler, D. Schwartz, W.U. Gerhard, and M.G. Weigert. 1990. V region gene usage and somatic mutation in the primary and secondary responses to influenza virus hemagglutinin. J. Immunol. 144:2795–2801. [PubMed] [Google Scholar]
- 3.Dal Porto, J.M., A.M. Haberman, M.J. Shlomchik, and G. Kelsoe. 1998. Antigen drives very low affinity B cells to become plasmacytes and enter germinal centers. J. Immunol. 161:5373–5381. [PubMed] [Google Scholar]
- 4.Eisen, H.N., and G.W. Siskind. 1964. Variations in affinity of antibodies during the immune response. Biochemistry. 3:996–999. [DOI] [PubMed] [Google Scholar]
- 5.Berek, C., and M. Ziegner. 1993. The maturation of the immune response. Immunol. Today. 14:400–404. [DOI] [PubMed] [Google Scholar]
- 6.Jacob, J., J. Przylepa, C. Miller, and G. Kelsoe. 1993. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J. Exp. Med. 178:1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi, Y., P.R. Dutta, D.M. Cerasoli, and G. Kelsoe. 1998. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J. Exp. Med. 187:885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conger, J.D., B.L. Pike, and G.J. Nossal. 1988. Analysis of the B lymphocyte repertoire by polyclonal activation. Hindrance by clones yielding antibodies which bind promiscuously to plastic. J. Immunol. Methods. 106:181–189. [DOI] [PubMed] [Google Scholar]
- 9.Urbain-Vansanten, G. 1970. Concomitant synthesis, in separate cells, of non-reactive immunoglobulins and specific antibodies after immunization with tobacco mosaic virus. Immunology. 19:783–797. [PMC free article] [PubMed] [Google Scholar]
- 10.Madrenas, J., R.L. Wange, J.L. Wang, N. Isakov, L.E. Samelson, and R.N. Germain. 1995. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 267:515–518. [DOI] [PubMed] [Google Scholar]
- 11.Kouskoff, V., S. Famiglietti, G. Lacaud, P. Lang, J.E. Rider, B.K. Kay, J.C. Cambier, and D. Nemazee. 1998. Antigens varying in affinity for the B cell receptor induce differential B lymphocyte responses. J. Exp. Med. 188:1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benschop, R.J., D. Melamed, D. Nemazee, and J.C. Cambier. 1999. Distinct signal thresholds for the unique antigen receptor–linked gene expression programs in mature and immature B cells. J. Exp. Med. 190:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siskind, G.W., and B. Benacerraf. 1969. Cell selection by antigen in the immune response. Advanced Immunology. F.J. Dixon and H.G. Kunkel, editors. Academic Press, New York. 1–50. [DOI] [PubMed]
- 14.Hannum, L.G., D. Ni, A.M. Haberman, M.G. Weigert, and M.J. Shlomchik. 1996. A disease-related rheumatoid factor autoantibody is not tolerized in a normal mouse: implications for the origins of autoantibodies in autoimmune disease. J. Exp. Med. 184:1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob, J., R. Kassir, and G. Kelsoe. 1991. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 173:1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacob, J., G. Kelsoe, K. Rajewsky, and U. Weiss. 1991. Intraclonal generation of antibody mutants in germinal centres. Nature. 354:389–392. [DOI] [PubMed] [Google Scholar]
- 17.Jacob, J., and G. Kelsoe. 1992. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J. Exp. Med. 176:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith, K.G., T.D. Hewitson, G.J. Nossal, and D.M. Tarlinton. 1996. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur. J. Immunol. 26:444–448. [DOI] [PubMed] [Google Scholar]
- 19.Weiss, U., and K. Rajewsky. 1990. The repertoire of somatic antibody mutants accumulating in the memory compartment after primary immunization is restricted through affinity maturation and mirrors that expressed in the secondary response. J. Exp. Med. 172:1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziegner, M., G. Steinhauser, and C. Berek. 1994. Development of antibody diversity in single germinal centers: selective expansion of high-affinity variants. Eur. J. Immunol. 24:2393–2400. [DOI] [PubMed] [Google Scholar]
- 21.Bruggemann, M., A. Radbruch, and K. Rajewsky. 1982. Immunoglobulin V region variants in hybridoma cells. I. Isolation of a variant with altered idiotypic and antigen binding specificity. EMBO J. 1:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shlomchik, M.J., P. Watts, M.G. Weigert, and S. Litwin. 1998. Clone: a Monte-Carlo computer simulation of B cell clonal expansion, somatic mutation and antigen-driven selection. Curr. Top. Microbiol. Immunol. 229:173–197. [DOI] [PubMed] [Google Scholar]
- 23.Wysocki, L., T. Manser, and M.L. Gefter. 1986. Somatic evolution of variable region structures during an immune response. Proc. Natl. Acad. Sci. USA. 83:1847–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]