Abstract
CD22 is a B cell–specific transmembrane protein of the Siglec family. It binds specifically to α2,6-linked sialic acid (Sia) residues, which are also present on glycoproteins on the B cell surface. CD22 acts as a negative regulator in B cell receptor–mediated signaling by recruitment of Src homology 2 domain–containing tyrosine phosphatase (SHP)-1 to its intracellular tail. To analyze how ligand-binding of CD22 influences its intracellular signaling domain, we designed synthetic sialosides as inhibitors for the lectin domain of CD22. One of these compounds inhibited binding of human CD22-Fc to target cells over 200-fold better than Sia and was highly selective for human CD22. When Daudi cells or primary B cells were stimulated with anti-immunoglobulin (Ig)M in presence of this sialoside inhibitor, a higher Ca2+ response was observed, similar to CD22-deficient B cells. Accordingly, a lower tyrosine-phosphorylation of CD22 and SHP-1 recruitment was demonstrated in presence of the sialoside. Thus, by interfering with ligand binding of CD22 on the B cell surface, we have shown for the first time that the lectin domain of CD22 has a direct, positive influence on its intracellular inhibitory domain. Also, we have developed a novel low molecular weight compound which can enhance the response of human B cells.
Keywords: B lymphocytes, CD22, Siglecs, sialic acid, Ca2+ flux
Introduction
CD22 is a B cell–specific transmembrane protein of the Ig superfamily with seven Ig-like domains. CD22 seems to have two distinct functions. First, it is associated with the B cell receptor (BCR) and inhibits the BCR signal, as has been demonstrated by characterization of CD22-deficient mice (1–4). B cells of these mice show an increased Ca2+ response when stimulated by anti-IgM. The inhibitory effect of CD22 is due to phosphorylation of three Ig-like tyrosine-based inhibitory motifs on its intracellular tail upon BCR stimulation. This results in recruitment and activation of Src homology 2 domain–containing tyrosine phosphatase (SHP)-1 (5), a tyrosine phosphatase which inhibits several signaling pathways, and binding of other intracellular proteins with unknown significance (for a review, references 6 and 7). Second, CD22 has properties of a lectin, belongs to a family of adhesion molecules, the Siglecs (sialic acid [Sia]–binding Ig-like lectins) and is also referred to as Siglec-2 (7, 8). CD22 has a high specificity for Sia in the α2,6-linkage (2,6Sia) (9, 10). When CD22 is transfected into heterologous cells, which do not display N-glycans with 2,6Sia, it can mediate Sia-dependent binding to various cell types (11, 12). In comparison, peripheral B cells usually display high levels of 2,6Sia on the cell surface, and CD22 is bound in cis on the majority of these cells (13, 14). Nevertheless, CD22 controls the homing of recirculating B cells back to the bone marrow by binding to ligands which are expressed on sinusoidal endothelium (15).
So far, it is not clear how these two apparently distinct functions of CD22 are linked. Binding to ligands on the B cell surface in cis may affect the subcellular localization and accessibility of the intracellular domain of CD22 and thereby control its inhibitory function. Solution of the 3-D structure of the first Ig domain of sialoadhesin (Sn, Siglec-1) in a cocrystal with 2,3 sialyllactose showed that all molecular contacts of conserved amino acids of the ligand-binding site are to the carbohydrate residues (16). Molecular modeling of a CD22 structure, as well as site-directed mutagenesis suggest a similar Sia binding site as in Sn (17). There is so far no evidence for protein epitopes contributing to CD22 ligand binding. Models trying to predict the influence of the ligand-binding domain on signaling have suggested that CD22 is sequestered away from the BCR by 2,6Sia carrying proteins, thereby releasing the BCR from the CD22 inhibition (18). Alternatively, Sia-binding could directly mediate CD22 interaction to the BCR (6) or the ligand-binding domain could have no influence on signaling at all.
We have addressed the role of the ligand-binding domain of CD22 by development of a new class of Sia analogs as artificial high affinity Siglec ligands. One of these sialosides could efficiently inhibit 2,6 Sia binding of human CD22 with a high specificity. The effect of this potent inhibitor sialoside on B cell signaling is described here.
Materials and Methods
Fc-Chimaera Inhibition Assay with Sia Analogs
Fc-Chimeras.
Fc-chimeras containing the NH2-terminal three domains of human CD22 (hCD22d1–3-Fc) (19), murine CD22 (mCD22d1–3-Fc), or murine Sn (Snd1–3-Fc) were produced in COS cells as described previously (9).
Synthetic Sialosides.
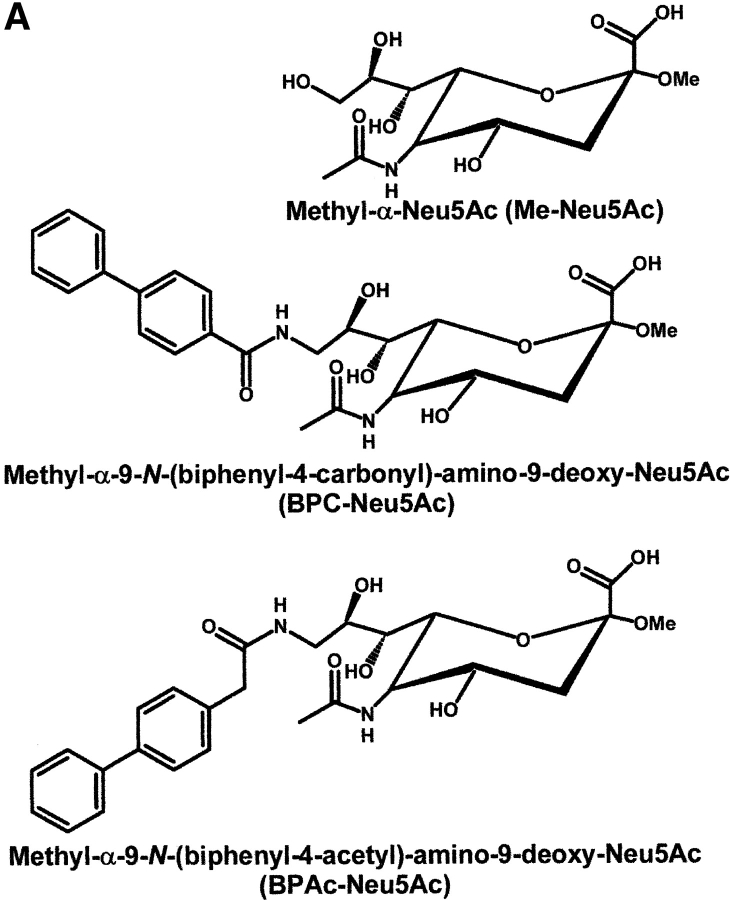
The synthesis of Methyl-α2-Neu5Ac (Me-Neu5Ac) has been described previously (20). Methyl-a-9-N-(biphenyl-4-carbonyl)-amino-9-deoxy-Neu5Ac (BPC-Neu5Ac) and Methyl-a-9-N-(biphenyl-4-acetyl)-amino-9-deoxy-Neu5Ac (BPAc-Neu5Ac) were prepared by acylation of methyl-α-9-amino-9-deoxy-Neu5Ac, obtained by a Mitsunobu reaction-based synthesis, using activated BPC and BPAc, as will be described in detail elsewhere. All these compounds were fully characterized by elemental analyses, MS and 1H-NMR spectroscopy.
Binding Assay.
Purified Fc-chimeras were labeled with carrier free Na125I (Amersham Pharmacia Biotech) and complexed with equimolar concentrations of anti–human IgG (Biodesign) to be used in binding assays with glutardialdehyde-fixed murine myeloma cells AG8 or human erythrocytes as described previously (9). For inhibition assays 10 μl radio-iodinated Fc-chimeras were mixed with an equal volume of the sialoside, pH 7.5, and incubated for 1 h at 4°C before 10 μl cells were added. After an overnight incubation at 4°C, the cells were washed and the bound radioactivity was quantified. The 50% inhibition concentrations (IC50) and the relative inhibitory potencies (rIP) were calculated as described previously (21).
Cellular Inhibition Assays with Sia Analogs
Daudi cells (human B cell line) or mouse splenic B cells were treated with sialidase (from A. ureafaciens; Roche Laboratories) at 37°C. These cells or untreated controls were preincubated with sialosides on ice for 10 min. Then cells were stained with NeuGc2,6-PAA-bio (Neu5Gcα2–6Galβ1–4GlcNAc-O(CH2)2-PAA) (unpublished results). This was followed by streptavidin-PE staining (plus B220-FITC for mouse cells). Cells were analyzed by flow cytometry.
Measurement of Intracellular Ca2+ Mobilization
Daudi cells were loaded with 4.5 μM Indo-1 plus 0.003% pluronic F-127 (both Molecular Probes) in RPMI 1640 with 1% FCS for 45 min at 37°C. Primary human lymphocytes were prepared from blood by Ficoll-Paque (Amersham Pharmacia Biotech) purification of mononuclear cells. These were Indo1-loaded similar to Daudi and then stained on ice with anti–CD20-FITC. Both types of cells were washed and preincubated for 5 min on ice with or without Sia analogs. The baseline Ca2+ concentration (proportional to the FL5 to FL4 ratio) was recorded at 37°C and then 2 μg/ml anti-IgM (clone BU.1; The Binding Site) was added. For primary lymphocytes, B cells were gated as CD20-positive and Ca2+ responses were analyzed in this gate. The staining antibody had no effect on Ca2+, as controlled by unstained cells. Increases in intracellular free Ca2+ were recorded in real time with the use of a FACSvantage™ (Becton Dickinson).
Immunoprecipitation of CD22
6 × 106 Daudi cells per immunoprecipitation were preincubated in 70 μl on ice for 5 min with the indicated Sia analogue. Cells were stimulated after filling up with prewarmed RPMI 1640 medium to 1 ml at 37°C for the indicated time points by adding 2 μg/ml anti-IgM antibody (BU.1). Cells were lysed in ice-cold NP-40 lysis-buffer containing protease inhibitors. Anti-CD22 (Dako) and anti-Vav (Santa Cruz Biotechnology, Inc.) were added together for immunoprecipitation. Precipitates were resolved by SDS-PAGE and analyzed by immunoblotting with anti–phospho-tyrosine (clone 4G10), anti–SHP-1, and anti-Vav (all UBI). Reblotting was done with anti-CD22 (Santa Cruz Biotechnology, Inc.).
Results
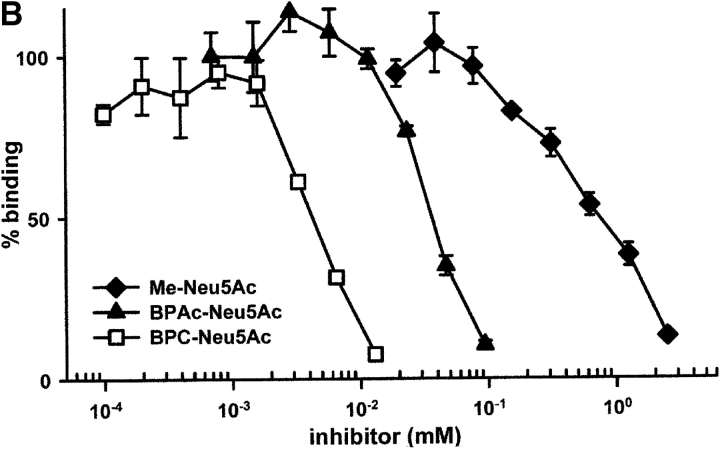
Siglecs bind their ligand Sia with low affinity (8). Based on the crystal structure of Sn, complexed with 2,3 sialyllactose, synthetic sialosides were prepared with different substituents at the C9 of Me-Neu5Ac. These compounds were designed to allow additional interactions mainly with hydrophobic areas observed in the crystal structure of Sn to increase the affinity. About 50 such compounds were synthesized and used in hapten inhibition assays to determine their rIP (unpublished data). One of these compounds, BPC-Neu5Ac, had an >200-fold rIP for human CD22 when Me-Neu5Ac was used as a reference (Fig. 1) , whereas several compounds with very similar structures, such as BPAc-Neu5Ac, bound much more weakly. Interestingly, this high rIP of BPC-Neu5Ac was not observed with murine CD22 (mCD22) or Sn. In contrast, BPAc-Neu5Ac was a more potent inhibitor for mCD22 than BPC-Neu5Ac (Table I).
Figure 1.
Inhibition of binding of CD22-Fc to target cells by synthetic sialosides. (A) Structures of sialosides and abbreviations used in this study. (B) Binding assays with human CD22. Binding of hCD22d1–3-Fc to murine AG8 cells was measured as described under Materials and Methods in the presence of sialosides at the concentrations indicated.
Table I.
Comparison of Inhibitory Potential of Synthetic Sialosides
| hCD22
|
mCD22
|
mSn
|
||||
|---|---|---|---|---|---|---|
| IC50μM | rIP | IC50μM | rIP | IC50μM | rIP | |
| Me-Neu5Ac | 1,400 | 1 | 4,689 | 1 | 884 | 1 |
| BPAc-Neu5Ac | 35 | 29 | 123 | 48 | 3,000 | 0.3 |
| BPC-Neu5Ac | 4 | 224 | 1,220 | 5 | 52 | 13 |
IC50-and rIP values of hCD22d1–3-Fc, mCD22d1–3-Fc, and mSnd1–3-Fc were determined from three to six independent experiments.
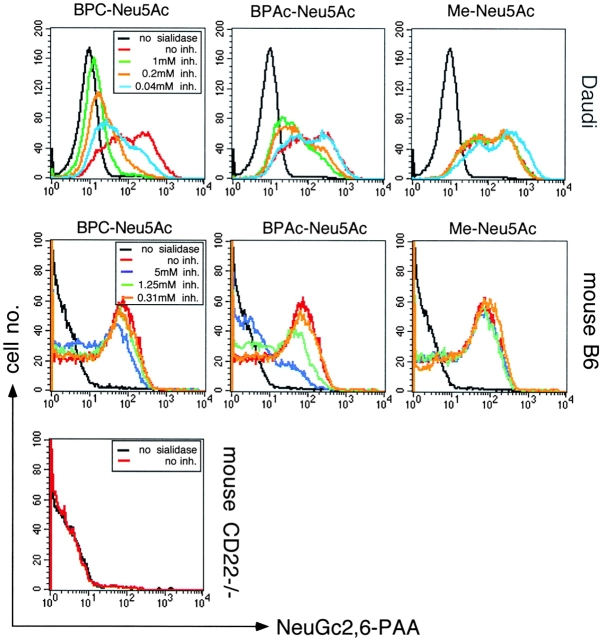
To test the specificity of the synthetic sialosides also for cellular CD22, the human B cell line Daudi was stained with a biotinylated polyacrylamide-based synthetic ligand for mCD22 and hCD22, NeuGc2,6-PAA. Daudi cells could only be stained with this probe after sialidase treatment which led to unmasking of CD22. Pretreatment with BPC-Neu5Ac was most potent in inhibiting the binding of the synthetic CD22 ligand, confirming the selective high affinity of this compound for hCD22 (Fig. 2) . When the same assay was performed on mouse B cells, BPAc-Neu5Ac was the more potent inhibitor, confirming the results of Fig. 1 and Table I. Sialidase treated mouse CD22−/− B cells could not be stained with NeuGc2,6-PAA, showing the CD22 specificity of this synthetic ligand (Fig. 2).
Figure 2.
Specificity of the synthetic sialosides for cellular CD22. Human Daudi cells, mouse C57BL/6 (B6), or mouse CD22−/− B cells were not pretreated (black curves) or sialidase pretreated (all colored curves), then incubated with no inhibitor (no inh., in red) or the indicated inhibitors (same conc. used for one type of cells) and afterwards stained with NeuGc2,6-PAA. The assay shows inhibition of binding of NeuGc2,6-PAA (a synthetic CD22 ligand) to hCD22 and mCD22 by the indicated sialosides. Mouse cells are gated as B220+.
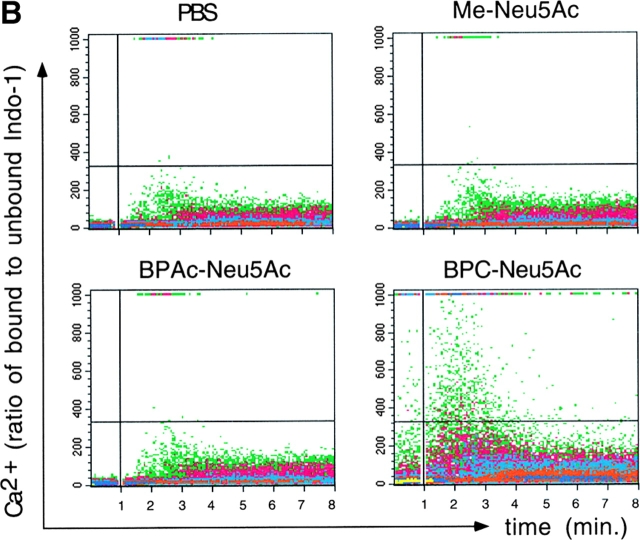
The potent and selective sialoside inhibitor BPC-Neu5Ac allowed us to test directly in cellular signaling assays whether the Sia binding activity of CD22 is important for its biological function. Since CD22 inhibits the BCR-triggered Ca2+ response, this response was analyzed in Daudi cells. While stimulating Daudi cells with anti-IgM in the presence of Me-Neu5Ac had no effect on the triggered Ca2+ response, pretreatment with BPC-Neu5Ac led to an increase in the transient Ca2+ peak (Fig. 3 A). Treatment with this sialoside led to a clearly increased Ca2+ response also in anti-IgM stimulated primary human blood B lymphocytes (Fig. 3 B). The structurally highly similar compound, but weaker inhibitor BPAc-Neu5Ac had no effect on the Ca2+ response in primary cells (Fig. 3 B) or in the Daudi cell line (data not shown). This demonstrates that the increased Ca2+ flux is specific for BPC-Neu5Ac and that a certain threshold affinity of the inhibitor for CD22 is needed to observe the effect.
Figure 3.
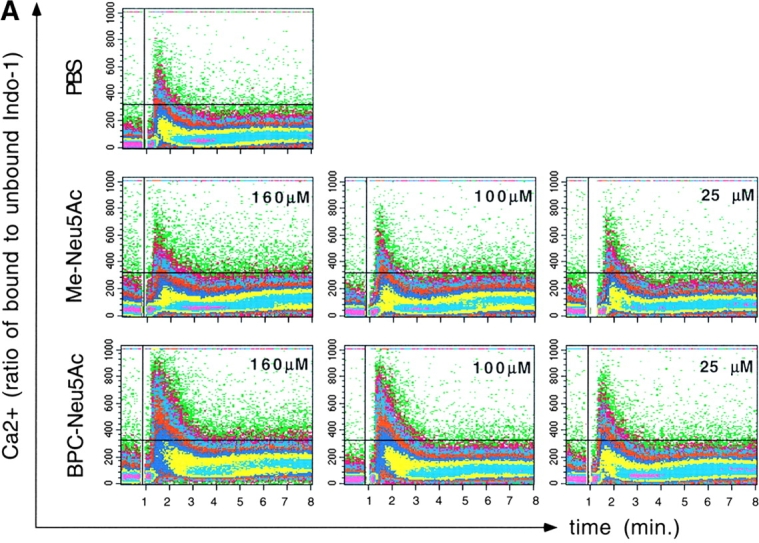
Presence of the sialoside BPC-Neu5Ac leads to increased BCR-triggered Ca2+ responses. (A) Daudi B cells were stimulated with anti-IgM in presence of either PBS, Me-Neu5Ac, or BPC-Neu5Ac at the given concentrations. (B) Human blood lymphocytes were stimulated with anti-IgM in presence of either PBS, Me-Neu5Ac, BPAc-Neu5Ac, or BPC-Neu5Ac (250 μM each). CD20+ gated cells are shown. Addition of antibody: at the vertical lines. Horizontal lines are drawn for quantitative comparison. One typical experiment, out of five experiments (for A) or two experiments (for B) is shown.
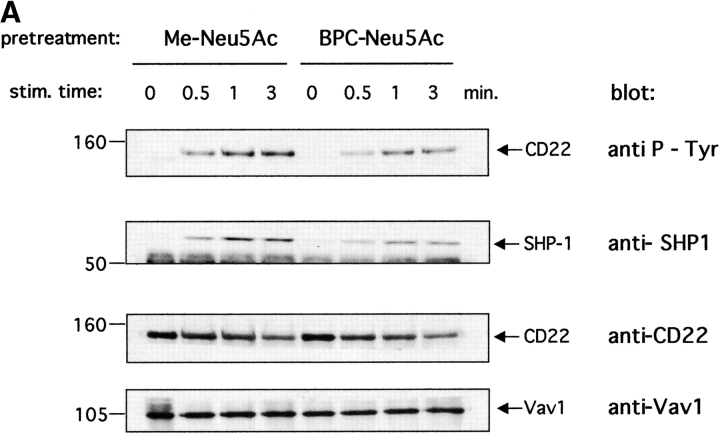
The next question was whether BPC-Neu5Ac treatment interferes with CD22 phosphorylation. Fig. 4 shows that anti-IgM treatment of Daudi cells in presence of BPC-Neu5Ac led to a reduced tyrosine phosphorylation of CD22, when compared with treatment with Me-Neu5Ac. Me-Neu5Ac pretreatment served as a control, because it gave identical CD22 activation as no pretreatment in several experiments (data not shown). The presence of BPC-Neu5Ac also led to less SHP-1 coprecipitation with CD22 (Fig. 4). The weaker inhibitor BPAc-Neu5Ac had no influence on tyrosine phosphorylation of hCD22 (data not shown). These results show that by disturbing the ligand-binding domain the inhibitory function of CD22 can be reduced.
Figure 4.
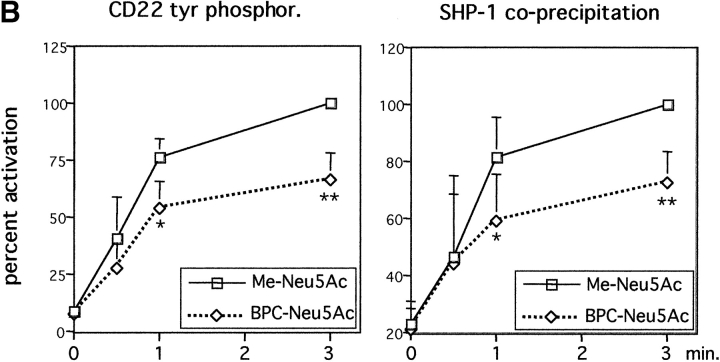
Lower tyrosine-phosphorylation of CD22 and less SHP-1 recruitment in presence of BPC-Neu5Ac. Daudi cells were stimulated with anti-IgM in presence of Me-Neu5Ac (control) or BPC-Neu5Ac (both 250 μM). Cells were lysed and immunoprecipitated with anti-CD22 plus anti-Vav1. The anti-Vav1 I.P. served as loading control. (A) Immunoprecipitates were separated on a gel, blotted, and probed with the indicated blotting antibodies. The membrane containing the largest fragments was reprobed with anti-CD22. (B) Densitometric analysis of band intensities of the gel. Western blots were scanned and quantified. Intensities of P-tyr bands, or SHP-1 bands were divided by intensities of Vav1 bands. Vav1 was used as loading control, because the anti-CD22 antibody showed interference with the first antibody. The calculated ratio of the highest activation (3 min, Me-Neu5Ac) was set at 100%. Mean results from four experiments are shown (±SD). *P < 0.05; **P < 0.01 in Student's t test.
Discussion
This work describes the newly developed sialoside BPC-Neu5Ac as a specific inhibitor for the ligand binding domain of hCD22. To unequivocally interpret our experiments on B cell signaling in presence of BPC-Neu5Ac, it was crucial to address the issue of specificity of this compound. The high specificity of BPC-Neu5Ac for hCD22 is supported by the following evidence: first, BPC-Neu5Ac inhibited binding of hCD22-Fc to Sia containing target cells very well, but not the binding of the closely related Siglec-Fc proteins, mCD22-Fc, Sn-Fc, and MAG-Fc (data not shown); second, BPC-Neu5Ac inhibited staining of sialidase-treated human B cells with the synthetic CD22 ligand NeuGc2,6-PAA, while it had no effect on staining of murine B cells. The specificity of the synthetic CD22 ligand for CD22 in this staining protocol was demonstrated with murine wild-type and CD22−/− B cells. A high specificity of the probe also for hCD22 is therefore very likely, although other probe-binding receptors on human B cells cannot be totally excluded; and third, a new crystal structure of BPC-Neu5Ac bound to Sn confirmed the predicted binding site to this Siglec (unpublished data). The higher affinity of BPC-Neu5Ac for hCD22 than for mSn can be explained by molecular modeling of the CD22 binding site. The Val-109 and Leu-107 of Sn which make contact to the biphenyl group of the sialoside are substituted by Arg-111 and Met-109 in hCD22. The biphenyl substituent could be sandwiched between these two side chains in hCD22 contributing a substantial binding affinity.
Together these data clearly suggest that the higher IgM triggered Ca2+ signal of BPC-Neu5Ac treated B cells is due to a specific inhibition of the ligand-binding domain of CD22. This interference with ligand-binding leads to an incomplete activation of the intracellular inhibitory domain of CD22. From the data presented it is obvious that the availability of 2,6Sia ligands on glycoproteins on the cellular surface is important for the function of this Siglec. B cells usually display high levels of 2,6Sia on the surface (13, 14). Upon in vitro activation, a subset of human peripheral B cells seems to downregulate surface expression of 2,6Sia (13). This could be due to downregulation of the α2,6 sialyltransferase ST6GalI which is highly regulated in several cell types (22) or activation of a sialidase (13). Thus, the inhibitory activity of CD22 could be regulated by the differential expression of 2,6Sia on the B cell surface. The inhibitor BPC-Neu5Ac most likely also affects the cellular distribution of CD22 on the B cell membrane.
All available structural data show that the ligand-binding domains of Siglecs are specific for the sialylated carbohydrate moieties with no involvement of the core protein in binding (16). Also, recent surface plasmon resonance experiments have shown that the affinity of CD22 for 2,6Sia, coupled to different carriers, is very similar, irrespective of whether the sugar is attached to different protein backbones or even polyacrylamide (Bakker, T., and A. van der Merwe, personal communication). Thus, any glycoprotein on the B cell surface containing 2,6Sia as terminal sugars could be a potential ligand for CD22. Our Ca2+ data argue against the model that ligand binding of CD22 by other surface glycoproteins sequesters CD22 away from the BCR and thereby releases the BCR from CD22 inhibition (18). In this case, interference with the ligand binding by sialosides would release CD22 from this sequestering and lead to its availability for BCR inhibition, resulting in a lower Ca2+ signal.
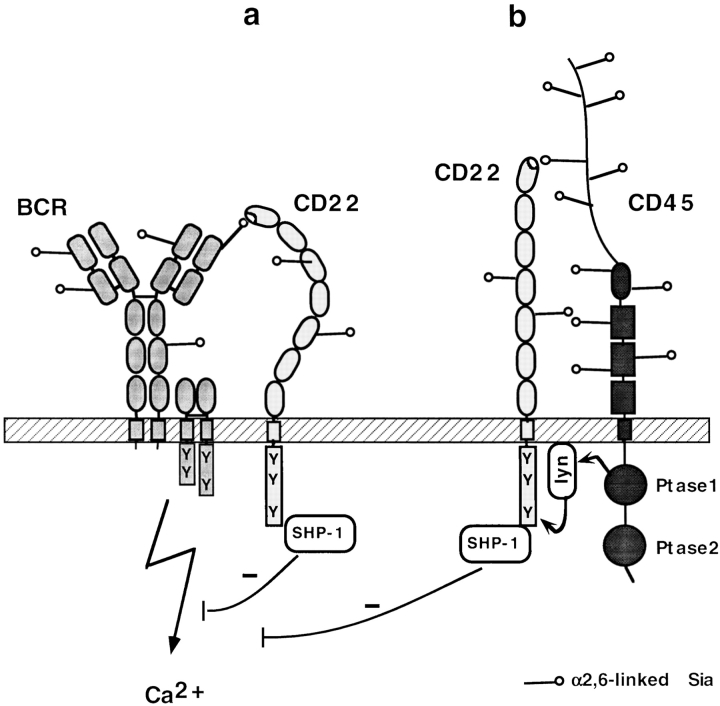
In contrast, our results support the model that the lectin domain mediates CD22 interaction to specific transmembrane glycoprotein ligands which are positively involved in BCR signaling. Such a specificity would be in contradiction to the only carbohydrate-based binding specificity mentioned above. But Sia-binding of CD22 could support interaction to certain transmembrane proteins to which a specific interaction is accomplished by other mechanisms. Alternatively, CD22 binds to many glycoproteins, but the ones which are abundant, structurally well accessible and carry the highest level of appropriately linked Sia win. There are two likely candidates as ligands for CD22: one is membrane Ig. It has been shown that a low stochiometric amount of CD22 can be coprecipitated with IgM from the B cell surface (23, 24). CD22 lectin binding to sialylated membraneIgM may contribute to this constitutive association (Fig. 5 A). Proximity to the BCR is likely to be crucial for CD22 because the recruited SHP-1 needs to be near its tyrosine phosphorylated substrates of the antigen receptor signaling complex. A second likely candidate as CD22 ligand is CD45, since CD45 is an abundant protein on the B cell surface, is highly glycosylated and has been detected as a prominent ligand by immunoprecipitation with CD22-Fc (25). Also, CD45 is known to activate lyn, the src-like kinase responsible for CD22 phosphorylation (26) (Fig. 5 B). After initial binding to CD45, CD22 could subsequently interact with the BCR or even recruit CD45 to the BCR. These possibilities will be directly tested in future experiments. Additionally to the discussed cis-binding, trans interactions of CD22 to ligands on adjacent cells cannot be excluded. Such interactions which may relieve the B cell from CD22 suppression, could be relevant when B cells are closely packed together such as in primary follicles (5, 18). Recently, it was shown that trans interactions of CD22 with ligands on target cells can also influence Ag-specific B cell activation (27).
Figure 5.
Model of possible ligands for CD22 on the B cell surface. (A) Interaction to surface Ig (BCR). (B) Interaction to CD45. Both CD22 interactions would be 2,6 Sia-dependent and could thus be inhibited by BPC-Neu5Ac, leading to impairment of CD22 activation, SHP-1 recruitment and a higher Ca2+ flux.
In summary, we have described a novel role for the CD22-lectin domain. Binding to ligands on the cell surface apparently supports the inhibitory function of CD22 on BCR-triggered Ca2+ flux. Our data are very well compatible with the study of Jin et al. (28) who have come to similar conclusions with a completely different experimental approach. In addition to addressing the biological role of the adhesion domain of CD22, the newly described sialoside could be potentially very important also therapeutically. Application of this structurally simple compound leads to an enhanced B cell response, which could be valuable for immunocompromised patients. Oligomerization of the sialoside can easily be used in the future to enhance affinity and avidity and improve the biological efficacy even further.
Acknowledgments
We thank Sonja Rotzoll for expert technical help with Ca2+ assays, Nadine Bock and Marlies Rusch for performing hapten inhibition assays, and Sibylle Geis for carrying out the intricate chemical syntheses. We thank all the colleagues from the HSFP grant, especially Drs. Yvonne Jones, Paul Crocker, Anton van der Merwe, and Mary Filbin for valuable discussions.
This work was supported by a HFSP research grant and by the Deutsche Forschungsgemeinschaft through SFB465 (to L. Nitschke).
S. Kelm and J. Gerlach contributed equally to this work.
References
- 1.Nitschke, L., R. Carsetti, B. Ocker, G. Köhler, and M.C. Lamers. 1997. CD22 is a negative regulator of B-cell receptor signalling. Curr. Biol. 7:133–143. [DOI] [PubMed] [Google Scholar]
- 2.O'Keefe, T.L., G.T. Williams, S.L. Davies, and M.S. Neuberger. 1996. Hyperresponsive B cells in CD22-deficient mice. Science. 274:798–801. [DOI] [PubMed] [Google Scholar]
- 3.Otipoby, K.L., K.B. Andersson, K.E. Draves, S.J. Klaus, A.G. Farr, J.D. Kerner, R.M. Perlmutter, C.L. Law, and E.A. Clark. 1996. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 384:634–637. [DOI] [PubMed] [Google Scholar]
- 4.Sato, S., A.S. Miller, M. Inaoki, C.B. Bock, P.J. Jansen, M.L. Tang, and T.F. Tedder. 1996. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 5:551–562. [DOI] [PubMed] [Google Scholar]
- 5.Doody, G.M., L.B. Justement, C.C. Delibrias, R.J. Matthews, J. Lin, M.L. Thomas, and D.T. Fearon. 1995. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 269:242–244. [DOI] [PubMed] [Google Scholar]
- 6.Cyster, J.G., and C.C. Goodnow. 1997. Tuning antigen receptor signaling by CD22: integrating cues from antigens and the microenvironment. Immunity. 6:509–517. [DOI] [PubMed] [Google Scholar]
- 7.Nitschke, L., H. Floyd, and P.R. Crocker. 2001. New functions for the sialic acid-binding adhesion molecule CD22, a member of the growing family of Siglecs. Scand. J. Immunol. 53:227–234. [DOI] [PubMed] [Google Scholar]
- 8.Crocker, P.R., and A. Varki. 2001. Siglecs, sialic acids and innate immunity. Trends Immunol. 22:337–342. [DOI] [PubMed] [Google Scholar]
- 9.Kelm, S., A. Pelz, R. Schauer, M.T. Filbin, S. Tang, M.E. de Bellard, R.L. Schnaar, J.A. Mahoney, A. Hartnell, P. Bradfield, et al. 1994. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr. Biol. 4:965–972. [DOI] [PubMed] [Google Scholar]
- 10.Powell, L.D., and A. Varki. 1994. The oligosaccharide binding specificities of CD22β, a sialic acid-specific lectin of B cells. J. Biol. Chem. 269:10628–10636. [PubMed] [Google Scholar]
- 11.Engel, P., Y. Nojima, D. Rothstein, L.J. Zhou, G.L. Wilson, J.H. Kehrl, and T.F. Tedder. 1993. The same epitope on CD22 of B lymphocytes mediates the adhesion of erythrocytes, T and B lymphocytes, neutrophils, and monocytes. J. Immunol. 150:4719–4732. [PubMed] [Google Scholar]
- 12.Hanasaki, K., A. Varki, and L.D. Powell. 1995. CD22-mediated cell adhesion to cytokine-activated human endothelial cells. Positive and negative regulation by α2-6-sialylation of cellular glycoproteins. J. Biol. Chem. 270:7533–7542. [DOI] [PubMed] [Google Scholar]
- 13.Razi, N., and A. Varki. 1998. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc. Natl. Acad. Sci. USA. 95:7469–7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Floyd, H., L. Nitschke, and P.R. Crocker. 2000. A novel subset of murine B cells that expresses unmasked forms of CD22 is enriched in the bone marrow: implications for B-cell homing to the bone marrow. Immunology. 101:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nitschke, L., H. Floyd, D.J. Ferguson, and P.R. Crocker. 1999. Identification of CD22 ligands on bone marrow sinusoidal endothelium implicated in CD22-dependent homing of recirculating B cells. J. Exp. Med. 189:1513–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.May, A.P., R.C. Robinson, M. Vinson, P.R. Crocker, and E.Y. Jones. 1998. Crystal structure of the N-terminal domain of sialoadhesin in complex with 3′ sialyllactose at 1.85 A resolution. Mol. Cell. 1:719–728. [DOI] [PubMed] [Google Scholar]
- 17.van der Merwe, P.A., P.R. Crocker, M. Vinson, A.N. Barclay, R. Schauer, and S. Kelm. 1996. Localization of the putative sialic acid-binding site on the immunoglobulin superfamily cell-surface molecule CD22. J. Biol. Chem. 271:9273–9280. [PubMed] [Google Scholar]
- 18.Doody, G.M., P.W. Dempsey, and D.T. Fearon. 1996. Activation of B lymphocytes: integrating signals from CD19, CD22 and FcγRIIb1. Curr. Opin. Immunol. 8:378–382. [DOI] [PubMed] [Google Scholar]
- 19.Stamenkovic, I., D. Sgroi, A. Aruffo, M.S. Sy, and T. Anderson. 1991. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and α2-6 sialyltransferase, CD75, on B cells. Cell. 66:1133–1144. [DOI] [PubMed] [Google Scholar]
- 20.Brossmer, R., and H.J. Gross. 1964. Sialic acid analogs and application for preparation of neoglycoconjugates. Meth. Enzymol. 247B:153–176. [DOI] [PubMed] [Google Scholar]
- 21.Kelm, S., R. Brossmer, R. Isecke, H.J. Gross, K. Strenge, and R. Schauer. 1998. Functional groups of sialic acids involved in binding to siglecs (sialoadhesins) deduced from interactions with synthetic analogues. Eur. J. Biochem. 255:663–672. [DOI] [PubMed] [Google Scholar]
- 22.Lo, N.W., and J.T. Lau. 1996. Transcription of the β-galactoside α2,6-sialyltransferase gene in B lymphocytes is directed by a separate and distinct promoter. Glycobiology. 6:271–279. [DOI] [PubMed] [Google Scholar]
- 23.Leprince, C., K.E. Draves, R.L. Geahlen, J.A. Ledbetter, and E.A. Clark. 1993. CD22 associates with the human surface IgM-B-cell antigen receptor complex. Proc. Natl. Acad. Sci. USA. 90:3236–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peaker, C.J., and M.S. Neuberger. 1993. Association of CD22 with the B cell antigen receptor. Eur. J. Immunol. 23:1358–1363. [DOI] [PubMed] [Google Scholar]
- 25.Sgroi, D., G.A. Koretzky, and I. Stamenkovic. 1995. Regulation of CD45 engagement by the B-cell receptor CD22. Proc. Natl. Acad. Sci. USA. 92:4026–4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith, K.G.C., D.M. Tarlinton, G.M. Doody, M.L. Hibbs, and D.T. Fearon. 1998. Inhibition of the B cell by CD22: a requirement for Lyn. J. Exp. Med. 187:807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanoue, A., F.D. Batista, M. Stewart, and M.S. Neuberger. 2002. Interaction of CD22 with α2,6-linked sialoglyconjugates: innate recognition of self to dampen B cell autoreactivity? Eur. J. Immunol. 32:348–355. [DOI] [PubMed] [Google Scholar]
- 28.Jin, L., P.A. McLean, B.G. Neel, and H.H. Wortis. 2002. Sialic acid binding domains of CD22 are required for negative regulation of B cell receptor signaling. J. Exp. Med. 195:1199–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]