Abstract
Here we show that blood-stage Plasmodium falciparum organisms accumulate a high mass of triacylglycerol and diacylglycerol. However, we failed to detect cholesterol esters, a second neutral lipid species reported to be important for a related apicomplexan, Toxoplasma gondii. Evidence for P. falciparum and T. gondii homologues of acyl coenzyme A:diacylglycerol acyltransferase suggests that acylglycerols may be the conserved neutral lipids in apicomplexans.
Plasmodium falciparum is a protozoan parasite belonging to the phylum Apicomplexa. During the blood stages of infection, its merozoite stage enters the red cell to become an intracellular ring-stage parasite. After 24 h, the ring develops through the trophozoite and multinucleate schizont stages. At the end of the intraerythrocytic cycle, as many as 16 daughter merozoites emerge to reinfect red cells and thus maintain the asexual cycle.
Parasite-induced synthesis of both sphingolipids and phospholipids as well as utilization of host cholesterol is critical for intraerythrocytic proliferation of P. falciparum (6, 8-10, 15). Fatty acid synthesis and host-derived cholesterol are known to be important for intracellular replication of a related apicomplexan, Toxoplasma gondii (3, 4). Studies with T. gondii have also shown the presence of neutral lipid droplets and the production of cholesterol esters (CE) that can be blocked by inhibitors of mammalian acyl coenzyme A cholesterol acyltransferase (ACAT); this inhibition is detrimental to parasite growth (16). However, the presence of a second major neutral lipid class, acylglycerols (triacylglycerol [TAG] and diacylglycerol [DAG]), was not examined in T. gondii. Further, CE, acylglycerols, their stage-dependent accumulation, and the genes underlying their biosynthesis remain poorly defined for P. falciparum.
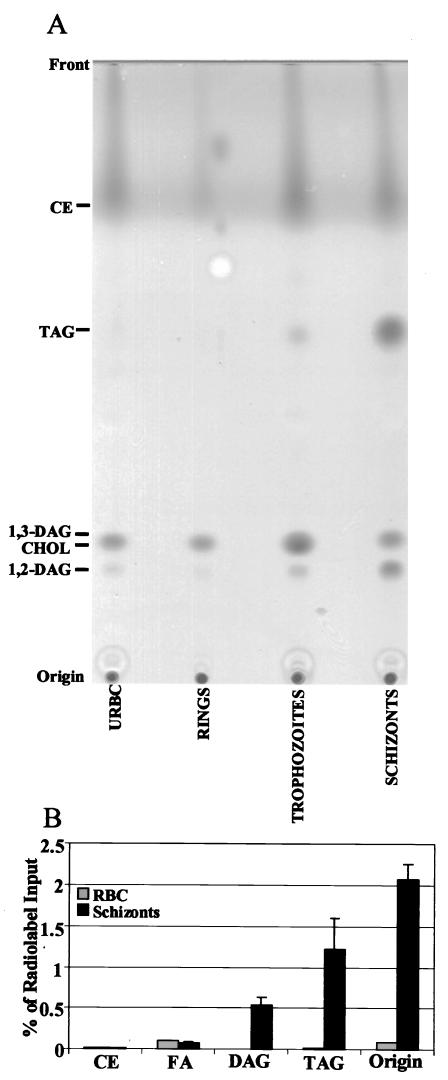
Uninfected erythrocytes, as well as P. falciparum-infected erythrocytes at the ring, trophozoite, and schizont stages of growth, isolated from in vitro culture were extracted, and neutral lipids were analyzed by thin-layer chromatography (TLC) (see materials and methods information available online at http://bigbird.pathology.northwestern.edu/Nawabi/supplemental_Methods). Uninfected erythrocytes contained no detectable levels of TAG. However, accumulation of high levels of TAG and DAG was detected in infected erythrocytes, with maximum levels seen at the terminal schizont stage (Fig. 1A). The identities of TAG and DAG were confirmed by two-dimensional TLC analysis (data not shown). Interestingly, there was no difference between levels of CE detected in infected erythrocytes and those in their uninfected counterparts (Fig. 1A). Since uninfected erythrocyte membranes do not contain CE, these data suggest that infected cells also fail to accumulate esters. To further investigate this, we used the more sensitive Amplex red cholesterol assay (see materials and methods information online). This kit converts CE to cholesterol by the action of an esterase activity. Thus, measurement of cholesterol in samples before and after esterase treatment indicates levels of esters present in them. Using this assay, we found no change in the levels of cholesterol, in either infected erythrocytes (6.89 ± 0.1 μg/5 × 106 cells) or uninfected erythrocytes (6.83 ± 0.1 μg/5 × 106 cells) before and after esterase treatment. Together, these data suggest that CE are absent and acylglycerols are the major neutral lipids found in P. falciparum-infected erythrocytes. Biosynthetic studies using [3H]oleic acid coupled to bovine serum albumin indicated active incorporation of radiolabel into TAG and DAG but not CE (Fig. 1B). This incorporation of radiolabel is not seen in uninfected erythrocytes, suggesting that acylglycerols are actively synthesized by P. falciparum. This may underlie the observed TAG and DAG accumulation in infected erythrocytes. In contrast, no new synthesis of CE is seen, suggesting that they are not produced upon infection. Staining infected erythrocytes with the dye Nile red (which binds lipid droplets) indicates that acylglycerols are present in large droplets within the parasite (see the figure available online at http://bigbird.pathology.northwestern.edu/Nawabi/supplemental_Figure1).
FIG. 1.
Detection of TAG in P. falciparum-infected erythrocytes. (A) Uninfected red blood cells (RBC; 5 × 107) or RBCs containing 5 × 107 ring-, trophozoite-, or schizont-stage parasites were lysed, and lipids were processed for TLC as described in the material and methods information online (see the text). Migration of standards is indicated. CHOL, cholesterol. (B) RBCs and Percoll-purified schizont-infected red cells (5 × 107 each) were incubated with [3H]oleic acid for 2 h, and lipids were processed for TLC. The plate was scraped, and radioactivity was quantified by scintillation counting (see materials and methods information online). FA, fatty acid.
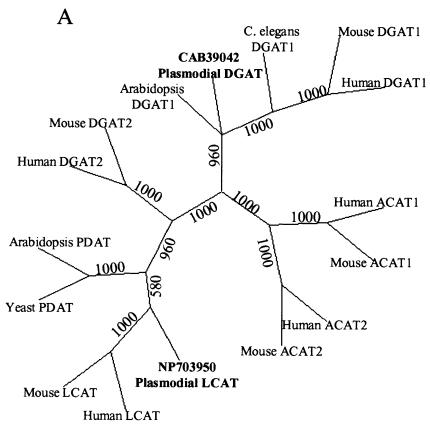
Since TAG was the major acylglycerol detected, we searched the P. falciparum genome for genes that may be involved in its formation. The best-characterized pathway for TAG biosynthesis is the Kennedy pathway, in which glycerol-3-phosphate is acylated three times, the final acyl transfer being mediated by the enzyme acyl coenzyme A:DAG acyltransferase (DGAT) (7, 11). We were able to identify a gene (GenBank accession no. CAB39042) with over 50% (the highest homology was within the conserved C terminus) homology to DGAT1 family members from other eukaryotes. Two very conserved regions with the consensus sequences FGDRXFY(R/K)DWWN and IERXLKL, unique to DGAT1 family members, are both present in CAB39042 (1). This consensus is not present in a second family of DGAT proteins, designated DGAT2. Moreover, the DGAT1 family is not closely related to the DGAT2 family. The DGAT1 family shows significant similarities with the ACAT1 family, but a phylogenetic tree (Fig. 2A) constructed by the method of parsimony (see materials and methods information online) reveals that despite the high homology between DGAT1 and ACAT families, CAB39042 (PfDGAT) is more closely related to the DGAT1 family of acyltransferases.
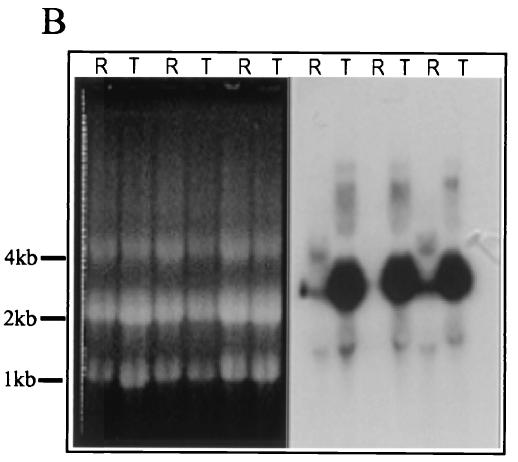
FIG. 2.
Phylogenetic analysis and stage-specific mRNA expression of PfDGAT. (A) The sequences used were retrieved from the National Center for Biotechnology Information. The sequences were aligned by using the ClustalW program. The alignments were reconciled and further adjusted by removing the amino and carboxy termini. For branch reliability, bootstrapping was carried out with 1,000 repetitions. (B) Northern blot indicating the presence of the DGAT transcript during ring (R) and trophozoite (T) stages. Data are shown in triplicate.
In our search we were also able to identify a putative plasmodial lecithin-cholesterol acyltransferase (LCAT) (GenBank accession no. NP_703950). LCAT is the enzyme that transfers an acyl chain from phosphatidylcholine (lecithin) to the 3-hydroxyl group of cholesterol, producing lysophosphatidylcholine and CE. The conserved C-terminal domain of NP_703950 is 90% homologous (∼25% identity) to the consensus domain of LCAT. Phospholipid:DAG acyltransferase (PDAT), an enzyme homologous to LCAT, is also able to synthesize TAG in plants and yeasts (5, 13). Thus, it was important to establish whether NP_703950 is more homologous to PDAT or LCAT. Our percent similarity analyses revealed that NP_703950 is more similar to LCAT (17 and 16.4% similar to human and mouse enzymes) than to PDAT (11.4 and 13.2% similar to Arabidopsis and yeast enzymes). Further, phylogenetic analysis revealed closest homology within the LCAT family (Fig. 2A). Thus, it is highly unlikely that the TAG found in P. falciparum-infected erythrocytes is synthesized by this putative LCAT.
Our phylogenetic analysis makes PfDGAT an attractive candidate for the enzyme responsible for TAG biosynthesis. Thus, Northern blot analysis was undertaken (see materials and methods information online) to determine whether PfDGAT is expressed during blood-stage infection. Shown in Fig. 2B is a blot obtained by using a 900-bp fragment of PfDGAT as a probe. Low levels of transcripts were seen in rings with a marked elevation in trophozoite stages. This expression closely parallels the stage-specific accumulation of TAG seen in Fig. 1. These data strongly suggest that the plasmodial genome encodes a DGAT that is expressed and may be responsible for the high levels of TAG present in infected erythrocytes.
Previous studies with T. gondii have shown that an inhibitor of mammalian ACAT, Sandoz 58-035, blocked T. gondii growth and the production of CE (16). Although we failed to detect cholesterol esterification activity, PfDGAT is highly homologous to the ACAT and DGAT families (2, 12), and therefore, we treated P. falciparum-infected red cells with Sandoz 58-035 for 2 h at a concentration (10 μg/ml) known to inhibit 99% of ACAT activity in mammalian cells (14, 17). However, we failed to induce any change in the incorporation of [3H]oleic acid into either TAG or its intermediates or show any deleterious effects on plasmodial growth (data not shown). This is consistent with the lack of CE production in P. falciparum. The absence of CE in P. falciparum-infected erythrocytes was surprising. It suggests that the putative plasmodial LCAT is not functional in blood stages of the parasite. Despite the lack of CE and the absence of parasite genes for cholesterol biosynthesis, cholesterol in the red cell membrane is essential for the establishment of infection, and vacuolar cholesterol (derived from the red cell) is critical to the stability of trophozoite-stage infected erythrocytes (8).
Our studies show that TAG is a major lipid species stored in lipid droplets in the late trophozoite and schizont stages of P. falciparum. It may be utilized to store acyl groups for phospholipid synthesis, glycosyl phosphatidyl inositol synthesis, and possibly for beta-oxidation (although evidence for the complete pathway in P. falciparum is still not available). Sonda et al. suggested that two expressed sequence tags may reflect the presence of ACAT homologues in T. gondii (16). However, with a higher level of completion of the T. gondii genome, we can now ascribe both to a single open reading frame (unique identifier, TGG_3996; toxodb.org). A BLAST search using human ACAT1 or DGAT1 as the query against the T. gondii database revealed that TGG_3996 is more homologous to human DGAT1 (3.6e−15) than to human ACAT1 (9.9e−6). Further, using this open reading frame as a query in BLAST resulted in hits for DGAT1 homologues but not ACAT homologues. This suggests that TAG may also be present in T. gondii. A separate T. gondii ACAT homologue underlying cholesterol esterification has yet to be identified. Given that TAG is produced at such high levels in P. falciparum, an organism that causes human malaria, it is tempting to speculate that blocking its synthesis may be detrimental to infection.
Acknowledgments
We thank Yvonne Lange (Rush Medical Center, Chicago, Ill.) for the generous gift of the mammalian ACAT inhibitor Sandoz 58-035 and for her critical reading of the manuscript.
This work was supported by American Heart Association predoctoral fellowship 0215249Z (to P.N.) and NIH grant AI39075 (to K.H.)
REFERENCES
- 1.Bouvier-Nave, P., P. Benveniste, P. Oelkers, S. L. Sturley, and H. Schaller. 2000. Expression in yeast and tobacco of plant cDNAs encoding acyl CoA:diacylglycerol acyltransferase. Eur. J. Biochem. 267:85-96. [DOI] [PubMed] [Google Scholar]
- 2.Cases, S., S. J. Smith, Y. W. Zheng, H. M. Myers, S. R. Lear, E. Sande, S. Novak, C. Collins, C. B. Welch, A. J. Lusis, S. K. Erickson, and R. V. Farese, Jr. 1998. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 95:13018-13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charron, A. J., and L. D. Sibley. 2002. Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J. Cell Sci. 115:3049-3059. [DOI] [PubMed] [Google Scholar]
- 4.Coppens, I., A. P. Sinai, and K. A. Joiner. 2000. Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J. Cell Biol. 149:167-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlqvist, A., U. Stahl, M. Lenman, A. Banas, M. Lee, L. Sandager, H. Ronne, and S. Stymne. 2000. Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. USA 97:6487-6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dluzewski, A. R., K. Rangachari, R. J. Wilson, and W. B. Gratzer. 1985. Relation of red cell membrane properties to invasion by Plasmodium falciparum. Parasitology 91(Pt. 2):273-280. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy, E. 1961. Fed. Proc. 20:934-940. [PubMed] [Google Scholar]
- 8.Lauer, S., J. VanWye, T. Harrison, H. McManus, B. U. Samuel, N. L. Hiller, N. Mohandas, and K. Haldar. 2000. Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J. 19:3556-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauer, S. A., N. Ghori, and K. Haldar. 1995. Sphingolipid synthesis as a target for chemotherapy against malaria parasites. Proc. Natl. Acad. Sci. USA 92:9181-9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauer, S. A., P. K. Rathod, N. Ghori, and K. Haldar. 1997. A membrane network for nutrient import in red cells infected with the malaria parasite. Science 276:1122-1125. [DOI] [PubMed] [Google Scholar]
- 11.Lehner, R., and A. Kuksis. 1996. Biosynthesis of triacylglycerols. Prog. Lipid Res. 35:169-201. [DOI] [PubMed] [Google Scholar]
- 12.Oelkers, P., A. Behari, D. Cromley, J. T. Billheimer, and S. L. Sturley. 1998. Characterization of two human genes encoding acyl coenzyme A:cholesterol acyltransferase-related enzymes. J. Biol. Chem. 273:26765-26771. [DOI] [PubMed] [Google Scholar]
- 13.Oelkers, P., A. Tinkelenberg, N. Erdeniz, D. Cromley, J. T. Billheimer, and S. L. Sturley. 2000. A lecithin cholesterol acyltransferase-like gene mediates diacylglycerol esterification in yeast. J. Biol. Chem. 275:15609-15612. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez, A., P. S. Bachorik, and S. B. Wee. 1999. Novel effects of the acyl-coenzyme A:cholesterol acyltransferase inhibitor 58-035 on foam cell development in primary human monocyte-derived macrophages. Arterioscler. Thromb. Vasc. Biol. 19:2199-2206. [DOI] [PubMed] [Google Scholar]
- 15.Samuel, B. U., N. Mohandas, T. Harrison, H. McManus, W. Rosse, M. Reid, and K. Haldar. 2001. The role of cholesterol and glycosylphosphatidylinositol-anchored proteins of erythrocyte rafts in regulating raft protein content and malarial infection. J. Biol. Chem. 276:29319-29329. [DOI] [PubMed] [Google Scholar]
- 16.Sonda, S., L. M. Ting, S. Novak, K. Kim, J. J. Maher, R. V. Farese, Jr., and J. D. Ernst. 2001. Cholesterol esterification by host and parasite is essential for optimal proliferation of Toxoplasma gondii. J. Biol. Chem. 276:34434-34440. [DOI] [PubMed] [Google Scholar]
- 17.Warner, G. J., G. Stoudt, M. Bamberger, W. J. Johnson, and G. H. Rothblat. 1995. Cell toxicity induced by inhibition of acyl coenzyme A:cholesterol acyltransferase and accumulation of unesterified cholesterol. J. Biol. Chem. 270:5772-5778. [DOI] [PubMed] [Google Scholar]