Abstract
A 14-mer α-pheromone peptide of Candida albicans was chemically synthesized and used to analyze the role of white-opaque switching in the mating process. The α-pheromone peptide blocked cell multiplication and induced “shmooing” in a/a cells expressing the opaque-phase phenotype but not in a/a cells expressing the white-phase phenotype. The α-pheromone peptide induced these effects at 25°C but not at 37°C. An analysis of mating-associated gene expression revealed several categories of gene regulation, including (i) MTL-homozygous-specific, pheromone stimulated, switching-independent (CAG1 and STE4); (ii) mating type-specific, pheromone-induced, switching-independent (STE2); and (iii) pheromone-induced, switching-dependent (FIG1, KAR4, and HWP1). An analysis of switching-regulated genes revealed an additional category of opaque-phase-specific genes that are downregulated by α-pheromone only in a/a cells (OP4, SAP1, and SAP3). These results demonstrate that α-pheromone causes shmooing, the initial step in the mating process, only in a/a cells expressing the opaque phenotype and only at temperatures below that in the human host. These results further demonstrate that although some mating-associated genes are stimulated by the α-pheromone peptide in both white- and opaque-phase cells, others are stimulated only in opaque-phase cells, revealing a category of gene regulation unique to C. albicans in which α-pheromone induction requires the white-opaque transition. These results demonstrate that in C. albicans, the mating process and associated gene regulation must be examined within the context of white-opaque switching.
Approximately 97% of clinical isolates of Candida albicans are heterozygous (20) at the single mating-type locus (MTL) containing the gene MTLa1 in the MTLa copy of the locus on one homolog of chromosome 5 and the genes MTLα1 and MTLα2 in the MTLα copy on the other homolog (12). A minority of these a/α strains undergo homozygosis to both a/a and α/α at a high frequency (20, 33). Approximately 3% of the clinical isolates are homozygous (a/a or α/α) (20) and capable of mating (21). However, for MTL-homozygous cells to become mating competent, they must first switch from the white to the opaque phenotype (21, 26). White-opaque switching (36) is repressed in MTL heterozygotes by the Mtla1p-Mtlα2p complex and activated in MTL homozygotes (21, 26). When opaque-phase a/a and α/α cells are mixed, they progress through a sequence of mating stages that parallel those in the mating process of Saccharomyces cerevisiae, including “shmooing,” growth of the shmoo projection (conjugation tube), chemotropism, fusion of conjugation tubes to form a zygote, daughter cell formation from the conjugation bridge of the zygote, and nuclear migration from parent cells to the fusion junction (16, 26, 37).
The requirements of shmooing and growth of the conjugation tube, therefore, are (i) MTL homozygosity, (ii) a switch from the white to the opaque phase, and (iii) the presence of opaque-phase cells of the opposite mating type. Furthermore, mating in C. albicans has recently been demonstrated to be dependent upon a protein kinase cascade similar to that of the pheromone-activated kinase pathway in S. cerevisiae (5, 25). These requirements together suggest that mating in C. albicans, as in S. cerevisiae (39), is dependent on pheromone induction but that, in contrast to S. cerevisiae, pheromone induction may be dependent upon the white-opaque transition. To test these suggestions directly, a peptide was chemically synthesized based on the α-pheromone gene sequence (MFα1) identified in the C. albicans genome database. A corresponding a-pheromone gene was not similarly identified. The effects of the synthesized α-pheromone peptide on a/a cells was tested. It is demonstrated here that the α-pheromone peptide blocks cell multiplication and induces shmooing only in a/a cells expressing the opaque-phase phenotype, that the peptide elicits these effects at 25°C but not at a physiological temperature (37°C), and that the peptide activates or upregulates genes involved in mating, including a subset that requires the white-opaque transition for activation. It is also demonstrated here that three opaque-phase-specific genes are downregulated by α-pheromone. One of these genes has been implicated in skin colonization (15), which has recently been demonstrated to facilitate mating (16). These results demonstrate a novel dependency of pheromone induction on white-opaque switching in C. albicans and underscore the need to analyze C. albicans mating and associated gene expression within the context of the white-opaque transition.
MATERIALS AND METHODS
Strain maintenance and growth.
The strains used in the present study were the three α/α strains WO-1 (36), P78048 (20), and P57072 (20); the three a/a strains P37005 (20), P75063 (20), and L26 (20); and the a/α strain 3153A (35). All strains were maintained in 20% glycerol at −80°C and then plated on agar containing modified Lee's medium, supplemented with zinc and arginine (3), prior to use in experiments. For distinguishing white- and opaque-phase colonies and sectors, 5 μg of phloxine B/ml, which differentially stains opaque-phase cells red (1), was added to the agar growth medium. Opaque-phase and white-phase cells were verified microscopically (1, 36) prior to use.
Production of α-pheromone.
The α-pheromone peptide was custom synthesized for the present study by Alpha Diagnostic International (San Antonio, Tex.). The sequence used was GFRLTNFGYFEPGK, deduced from the MFα1 DNA sequence provided in the C. albicans genome database (http://genolist.pasteur.fr/CandidaDB/). The peptide was purified by high-pressure liquid chromatography and demonstrated to be 96% pure by mass spectroscopy.
Pheromone treatment.
Cells were grown in suspension in a rotary water bath shaker (250 rpm) at 25°C in liquid modified Lee's medium. Cells were harvested in either mid-log phase (5 × 106 to 1 × 107 cells/ml) or early stationary phase (7 × 107 cells/ml). Harvested cells were pelleted and resuspended in an equal volume of fresh liquid modified Lee's medium containing 10−6 M α-pheromone and incubated at 25°C in a rotary water bath shaker (250 rpm).
Northern analysis.
Northern blot hybridization was performed according to methods previously described (47). In brief, cells were harvested in the mid-log phase of growth and resuspended in fresh modified Lee's medium. Half of each sample was treated with α-pheromone for 2 h as described above, and the other half was incubated without pheromone under similar conditions. The formation of shmoos was monitored microscopically. Total RNA was isolated by using the RNeasy kit according to the manufacturer's instructions (Qiagen, Inc., Valencia, Calif.). RNA was separated on an agarose formaldehyde gel, transferred to Hybond-N+ nylon membrane (Amersham, Piscataway, N.J.) and hybridized with a 32P-labeled probe. Prehybridization, hybridization, and washing were performed according to the methods of Church and Gilbert (6). The hybridized membrane was exposed to Kodak XAR film. Blots were stripped and rehybridized up to three times. Probes were synthesized by PCR for CAG1 (34), STE2, STE4, FIG1, KAR4, SAP9, HWP1 (42), OP4 (30), SAP1 (29), SAP3 (44), and CDR3 (2) by using the relevant oligonucleotide pairs listed in Table 1. Homologs of S. cerevisiae genes STE2, STE4, FIG1, KAR4, and SAP9 were found in the Candida DB database (http://genolist.pasteur.fr/CandidaDB/) through links found in the Saccharomyces genome database (http://genome-www.stanford.edu/Saccharomyces).
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′ to 3′) |
|---|---|
| CAG1SmaIF | TCCCCCGGGGTATTCAATCATGGGTTGT |
| CAG1SmaIR | TCCCCCGGGTATTCGCCTACTTCTTGC |
| STE2SmaIF | TCCCCCGGGTCAATGAATATCAATTCAACT |
| STE2SmaIR | TCCCCCGGGTATTTATTACACTCTTTTGA |
| STE4ShortF | ATCTTGCTCAATCGTTAA |
| STE4ShortR | AGACACTATACTGATCAA |
| FIG1F | ATGAATTTACCATTGAAATTC |
| FIG1R | CATAGCTTCTGCTCTACC |
| KAR4F | ATGTATACTTACAATAAGTTTGGG |
| KAR4R | TACCTCTGTAGCACCAGA |
| SAP9F | ATGAGACTCAATTCTGTT |
| SAP9R | TCGTCTTCATTAGATCCA |
| HWP1F | CACAGGTAGACGGTCAAGGT |
| HWP1R | GATCCAGAAGTAACTGGAACAGAACTT |
| OP4NDEIF | GGCATATGAAGTTTTCACAAGCC |
| OP4SPHIR | GGTGGTTGCTCTTCCGCACTAATAAAGTTT TCTTTT |
| SAP1-5′ | ATGGGAGTTGGATCTATAAC |
| SAP1-3′ | TTACCTTCTTGACCAGTAGC |
| SAP3-5′ | CCT TCTCTAAAATTATGGATTG |
| SAP3-3′ | TTGATTTCACCTTGGGGACC |
| CDR3E | ATACACTACCAATTGTAAGGA |
| CDR3BR | CATCAATGCTGCAAACTCAAG |
RESULTS
C. albicans α-pheromone gene.
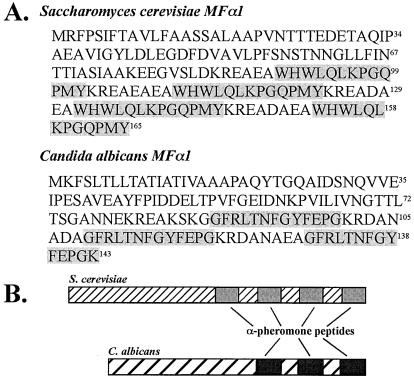
MFα1 of C. albicans encodes a protein precursor that contains three copies of the α-pheromone peptide. In S. cerevisiae, the peptides are released by posttranscriptional cleavage (39). Although the number and amino acid sequences of the S. cerevisiae and C. albicans pheromone peptides differ, the general arrangements of the pheromone peptide sequences are similar in the MFα1 genes of C. albicans and S. cerevisiae (Fig. 1). The S. cerevisiae MFα1 gene has four evenly spaced copies of the mature α-pheromone peptide sequence (39). The first three are flanked at their carboxy termini by the Kex2 cleavage site KR (Fig. 1A). The last copy terminates coincidently with the terminus of the deduced protein (Fig. 1A). The C. albicans MFα1 gene has three evenly spaced copies of the mature α-pheromone peptide sequence (Fig. 1A). The first two are flanked at their carboxy termini by the Kex2 cleavage site KR (Fig. 1A). The last copy terminates one amino acid in from the terminus of the deduced protein. The three deduced C. albicans α-pheromone peptides, therefore, consist of two 13-mers and one 14-mer. Since we would have to assume that the canonical cleavage sites at both ends of the 13-mer were functional, whereas we would only have to assume that the amino-terminal cleavage site of the 14-mer was functional given that the carboxyl terminus represented the end of the protein, we first tested the 14-mer for activity. Because the 14-mer proved active at the same concentrations as the α-pheromone peptide of Saccharomyces cerevisiae, we used the 14-mer in subsequent studies. We did not test whether the 13-mer was active.
FIG. 1.
The Candida albicans α-pheromone gene MFα1 contains three equally spaced sequences encoding α-pheromone peptides: two encoding 13-mers and one encoding a 14-mer. S. cerevisiae contains four sequences encoding 13-mers. (A) The deduced amino acid sequences of the C. albicans and S. cerevisiae MFα1 genes, with pheromone peptides shaded. (B) Models of protein configurations. The C. albicans 14-mer sequence was used to synthesize chemically a peptide for experimentation.
α-pheromone peptide induces shmoos only in opaque-phase a/a cells.
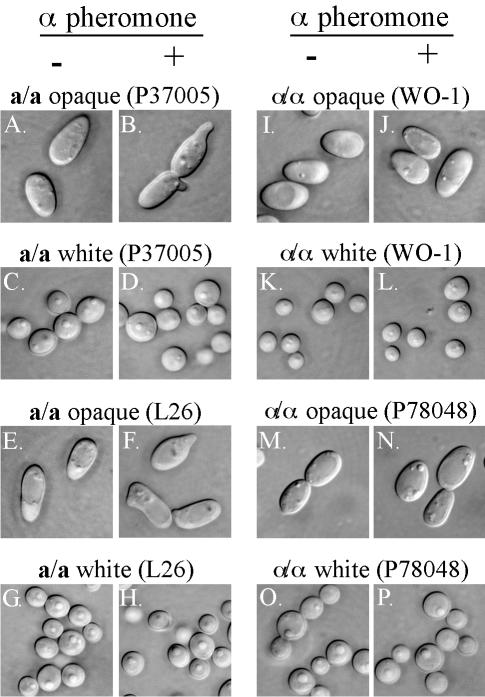
Three a/a strains (P37005, L26, and P75063) and three α/α strains (WO-1, P78048, and P57072) were tested for α-pheromone-induced shmooing at 25°C. Both white-phase cells and opaque-phase cells of each strain were tested. Since the results for each set of strains were similar, data for only the a/a strains P37005 and L26 and the α/α strains WO-1 and P78048 are shown. The α-pheromone peptide induced shmooing in opaque-phase cells of all three tested a/a strains (Fig. 2B and F). The induced projections formed on the sides as well as the ends of the bean-shaped opaque-phase cells (Fig. 2B and F). The α-pheromone peptide did not induce shmooing in white-phase cells of the three a/a strains (Fig. 2D and H). No shmoo morphologies were observed in the untreated cultures of either opaque- or white-phase cells of the three a/a strains (Fig. 2A, C, E, and G). The α-pheromone peptide did not induce shmooing in either opaque- or white-phase cells of the three α/α strains (Fig. 2J, L, N, and P) or the a/α strain 3153A (data not shown). Again, no shmoo morphologies were observed in the untreated cultures of both opaque- and white-phase cells of the three α/α strains (Fig. 2I, K, M, and O) or the a/α strain (data not shown). In all cultures, several thousand cells were examined over a 6-h period. These results demonstrate that shmooing is induced at 25°C by the α-pheromone peptide exclusively in opaque a/a cells.
FIG. 2.
The α-phermone peptide induces shmooing only in opaque-phase a/a cells. Two a/a strains (P37005 and L26) and two α/α strains (WO-1 and P78048) were tested as indicated in panels A to P. Cells were examined after 2 h of incubation. Untreated and α-pheromone-treated cells are noted by minus and plus signs. The α-pheromone peptide induced shmooing only in opaque-phase a/a cells (B and F). It did not induce shmooing in white-phase a/a cells (D and H) or in either opaque-phase (J and N) or white-phase (L and P) α/α cells (D and H). Incubation of up to 6 h for each sample did not change the results (data not shown). Scale bar, 2 μm.
Multiple additions of α-pheromone peptide.
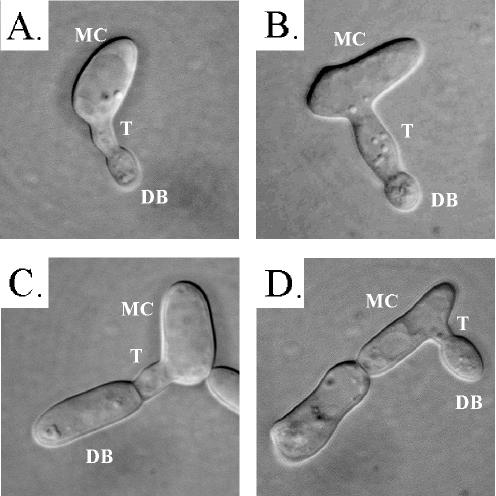
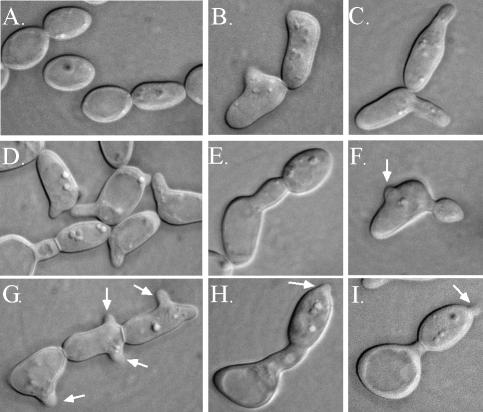
In a mixed a/a × α/α culture at 25°C, the projections formed by individual shmoos grew into tubes (referred to here as conjugation tubes) with lengths ranging from one to several cell diameters (21). Many of these tubes reverted to the budding growth form (i.e., formed buds) at the tube apices, presumably because no tube of opposite mating type was encountered in these cases. a/a cells treated with a single dose of the α-pheromone peptide formed shorter tubes than those formed in mixed cultures. A majority of these tubes reverted at their apices to the budding growth form when incubated for periods in excess of 2 h (Fig. 3A to D). Presumably, reversion was due to hydrolysis of the α-pheromone peptide by secreted aspartyl proteinases, as is the case in S. cerevisiae (19, 39). We therefore tested the effects of adding fresh α-pheromone at 2-h intervals over an 8-h period. At 0 h, the cell population was free of the shmoo morphology (Fig. 4A). At 2 h, ca. 70% of cells had shmooed (Fig. 4B). At 4 h, the majority of tubes formed by shmoos had grown to lengths ranging between one-half and two cell diameters (Fig. 4C and D) and, in a minority of cases, the tubes had reverted to a budding growth form at the end of the tube (Fig. 4D to F). Between 4 and 8 h, the proportion of tubes with apical buds had increased, and many of the original mother cells (Fig. 4F and G) and apical buds (Fig. 4H and I) had shmooed once again. At 8 h, a minority of apical daughter cells in turn budded, but there was no indication that any of the apical daughter cells had separated from the conjugation tubes. These results indicate that added α-pheromone peptide is rapidly deactivated or degraded. They also demonstrate that apical daughter cells formed in the reversion process remain in the opaque-phase phenotype, based not only on their shape but also on the capacity of α-pheromone to induce shmooing.
FIG. 3.
(A and B) Since the α-pheromone is rapidly degraded, the shmoo projections on opaque a/a cells revert apically to the budding growth form after 4 h, forming a daughter bud at the tube apex. (C and D) After 8 h, the daughter cell at the tube apex exhibited an elongate morphology, like that of the opaque-phase mother cell. MC, mother cell; T, conjugation tube (shmoo projection); DB, daughter bud. Scale bar, 2 μm.
FIG. 4.
Repeated addition of α-pheromone causes opaque-phase a/a mother cells to form multiple projections. The α-pheromone peptide was added every 2 h each time to a final concentration of 3 × 10−6 M. (A) Zero time; (B) 2 h; (C, D, E, and F); 4 h; (G, H, and I) 8 h. The arrows point to secondary rounds of shmooing. Note that secondary rounds of shmooing were not seen when α-pheromone was only added at 0 h. Note also the extension of two new projections from a single cell compartment in an apparent pseudohypha in panel G.
Shmoo induction by α-pheromone peptide is dose dependent.
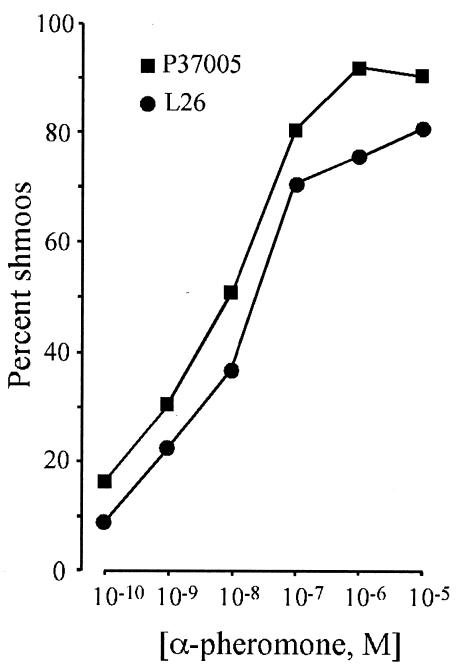
In S. cerevisiae, the proportion of a cells that shmoo in response to α-pheromone peptide is dose dependent (28). To test this characteristic in C. albicans, opaque-phase cells of a/a strains P37005 and L26 were treated with increasing concentrations of α-pheromone peptide in the range of 10−10 to 10−5 M. A total of 500 cells were scored for shmooing at each concentration after 2 h of incubation. The proportion of shmoos induced in P37005 cultures increased from 17 to 80% through the concentration range 10−10 to 10−7 M α-pheromone peptide and then approached a plateau of 90% between 10−7 and 10−5 M (Fig. 5). The dose dependency of shmoo formation in L26 cultures was similar (Fig. 5). These results demonstrate that, as is the case for S. cerevisiae, shmoo induction in a/a cells of C. albicans by α-pheromone is dose dependent and that the concentration of α-pheromone peptide eliciting a maximum effect is ca. 10−6 M under the conditions employed.
FIG. 5.
The percentage of the opaque-phase a/a cell population that shmoos is dependent on the dose of α-pheromone peptide. Opaque-phase cells of two a/a strains (P37005 and L26) were incubated in different concentrations of α-pheromone peptide for 2 h, and the proportion of cells forming shmoos was determined.
α-Pheromone causes cell multiplication arrest in opaque-phase, but not white-phase, a/a cells.
In S. cerevisiae, α-pheromone causes growth arrest in the G1 phase of the cell cycle (32). In mating experiments between opaque a/a and opaque α/α cells, the proportion of white-phase cell contaminants resulting from spontaneous opaque-to-white switching increased over an 18-h incubation period (data not shown), suggesting that opaque-phase cells, but not white-phase cells, were growth inhibited by pheromone from the opposite mating type. To test this characteristic in C. albicans, the cell number was monitored in white- and opaque-phase cultures of two a/a strains (P37005 and L26) in the absence or presence of α-pheromone peptide. In this case, an individual budded or unbudded cell, an individual shmoo, and an individual revertant cell attached still to the mother cell (Fig. 3A through D) were scored as single cells. Fresh peptide was added every 2 h to a final concentration of 4 × 10−6 M. Untreated and treated white-phase cells and untreated opaque-phase cells of both a/a strains exhibited an initial 2-h lag period, followed by exponential cell multiplication. However, α-pheromone-treated opaque-phase cells of both a/a strains did not increase in number through the 8-h treatment regime (Fig. 6A and B, respectively). These results demonstrate that α-pheromone causes an arrest in traditional cell multiplication in opaque-phase a/a cells. Treated cells did extend tubes, revert apically to the budding growth form, and shmoo again as previously described (Fig. 4). They did not cell separate. A significant number of α-pheromone-treated cells contained multiple nuclei, demonstrating that if a G1 block did occur, it was transient.
FIG. 6.
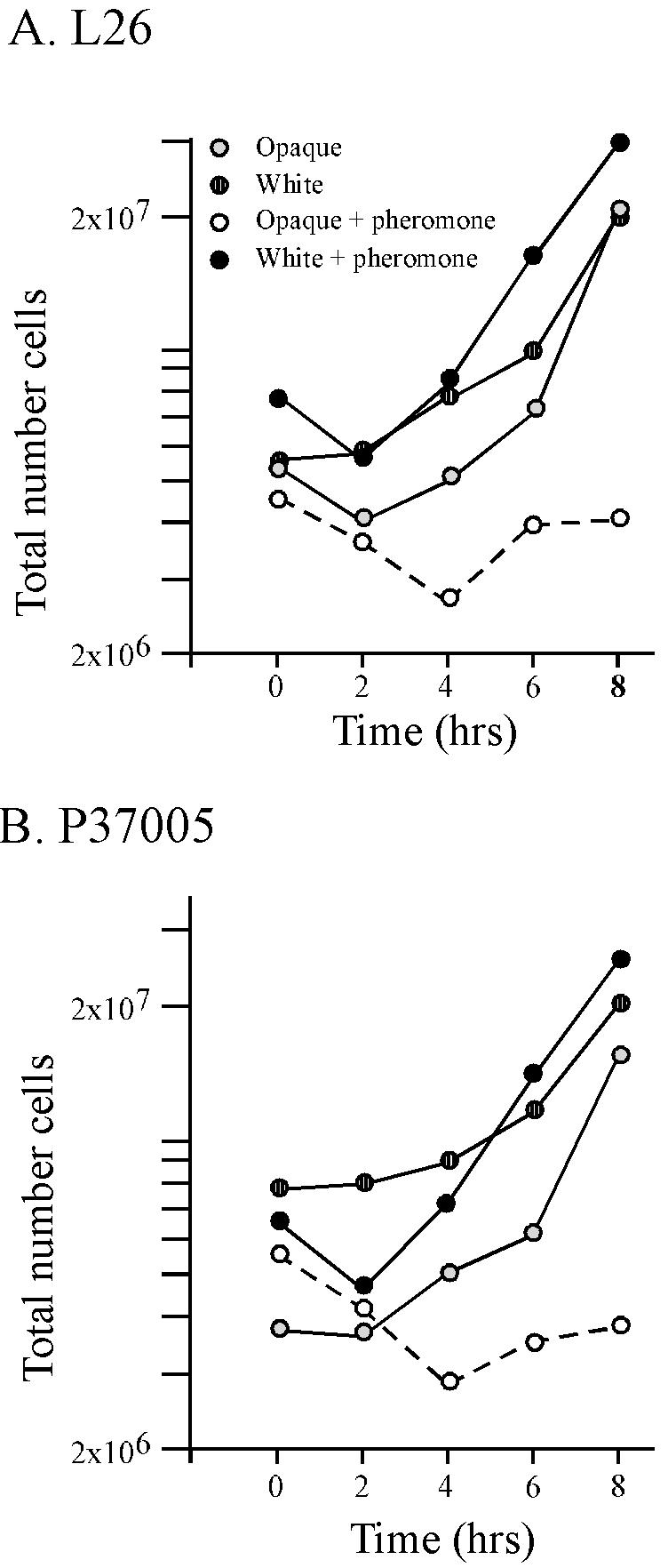
The α-pheromone peptide inhibits cell multiplication only in opaque-phase a/a cells. Two a/a strains, L26 and P37005, were tested. Cells were diluted into fresh growth medium and treated with repeated additions of α-pheromone peptide over an 8-h period. α-Pheromone peptide was added every 2 h to a final concentration of 4 × 10−6 M. Cells were removed, and cell density was determined at 2-h intervals. Single cells, shmoos, revertants, and budded cells that had not separated were considered single cells in computing the “total number of cells.” The symbol key in panel A applies also to panel B.
α-Pheromone peptide does not stimulate shmooing at 37°C.
When α-pheromone peptide was added to a culture of a/a cells in the opaque phase at 25°C and the temperature was immediately increased to 37°C, no shmoos formed, even after 6 h of incubation (data not shown). Opaque-phase cells, instead, formed daughter buds just as untreated opaque-phase cells treated similarly had done. Cell numbers increased in treated opaque-phase a/a cultures after 4 h at 37°C (data not shown). The addition of fresh α-pheromone every 2 h for a 6-h period failed to stimulate shmooing. When opaque-phase a/a cells were treated with α-pheromone for 1 h at 25°C and the temperature was then increased to 37°C, the cells formed short projections which then reverted to the budding growth form (i.e., formed a bud) at their apices (data not shown). When white-phase a/a cells were treated with α-pheromone peptide and the temperature was raised to 37°C immediately or after 1 h, the cells formed hyphae, just as untreated white-phase a/a cells treated similarly had done. Together, these results indicate that α-pheromone does not cause a block in cell multiplication or induce shmooing in opaque-phase a/a cells at physiological temperature (37°C).
Gene regulation.
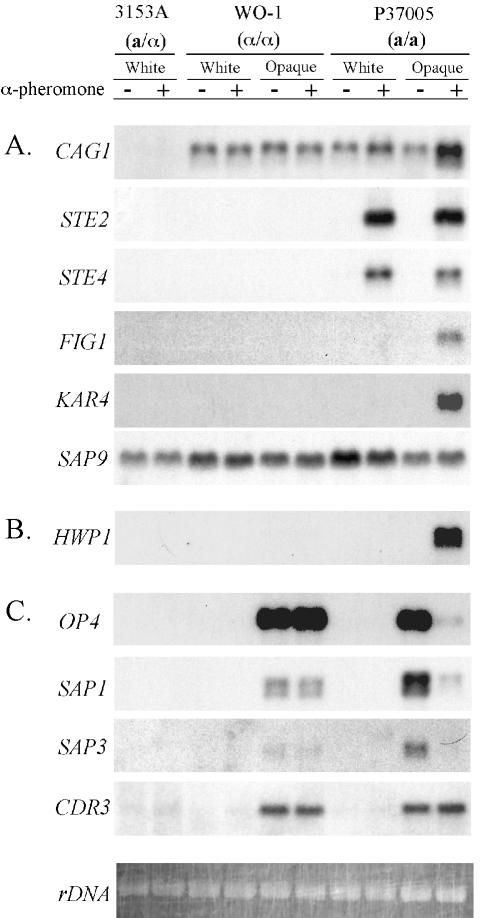
The dependencies of C. albicans mating on MTL homozygosis (13, 21, 24, 26) and on a mitogen-activated protein (MAP) kinase cascade (5, 25) are similar to that of S. cerevisiae, suggesting that the regulation of gene expression accompanying the mating process will also be similar. However, differences in gene regulation must exist because of the unique dependency of C. albicans mating on the white-opaque transition (37). This prediction was explored by Northern analysis of mating-associated gene expression and opaque-phase-specific gene expression in the absence or presence of α-pheromone. In the discussion that follows, we consider “induction” to mean an increase from a nondetectable to significant signal and “upregulation” to mean an increase from a detectable level to a higher level. The Northern analysis data presented in Fig. 7 are for strain P37005. All of these results were confirmed by independent Northern analysis of strain L26 (data not shown).
FIG. 7.
Northern analysis of gene transcription reveals the dependency of select mating-associated genes on the white-opaque transition and the downregulation of select opaque-phase-specific genes by α-pheromone. (A) Regulation of genes that are homologs of mating-associated genes in S. cerevisiae; (B) regulation of HWP1, a hypha-specific gene with no known homolog in S. cerevisiae; (C) regulation of four opaque-phase-specific genes. Three strains were tested: a/α strain 3153A, α/α strain WO-1, and a/a strain P37005. The former is blocked in the white phase, a characteristic of a/α cells (19, 25). The latter two were tested in the white and opaque phases. Untreated and α-pheromone-treated cells are indicated by minus and plus signs, respectively. Ethidium bromide-stained ribosomal DNA (rDNA) is presented at the bottom of the figure for an assessment of loading.
CAG1.
In S. cerevisiae, the expression of GPA1, which encodes the pheromone receptor-associated G-protein α subunit (27, 31), is haploid specific (i.e., it is expressed in both a and α cells but not in a/α cells) and upregulated by α-pheromone (27, 31). In C. albicans, CAG1, the C. albicans homolog, was expressed in white- and opaque-phase a/a and α/α cells but not in a/α cells. The α-pheromone peptide upregulated CAG1 expression in both white- and opaque-phase a/a cells but not in α/α cells (Fig. 7A). Expression of CAG1 was, therefore, MTL homozygous specific and upregulated by α-pheromone in C. albicans, as is expression of its homolog GPA1 in S. cerevisiae. Expression in C. albicans was independent of switching.
STE2.
In S. cerevisiae, expression of STE2, which encodes the α-factor receptor, is mating type specific (i.e., a specific) and stimulated by α-pheromone (4). In C. albicans, high levels of STE2 transcript were observed only in α-pheromone-treated white- and opaque-phase a/a cells (Fig. 7A). However, when the same Northern blots were exposed to X-ray film for extended periods of time, low levels of transcript were evident in untreated white- and opaque-phase a/a cells but not in untreated or treated white- or opaque-phase α/α cells or in a/α cells (data not shown). Expression of STE2 was, therefore, mating type specific (a specific) and upregulated by α-pheromone in C. albicans, as is the expression of its homolog in S. cerevisiae. Regulation in C. albicans was independent of switching.
STE4.
In S. cerevisiae, expression of STE4, which encodes the G protein β subunit, is haploid specific and neither induced nor stimulated by α-pheromone (7, 45). In C. albicans, high levels were observed only in α-pheromone-treated white- and opaque-phase a/a cells (Fig. 7A). When Northern blots were exposed to X-ray film for extended periods of time, low levels of transcript were evident in untreated and α-pheromone-treated white- and opaque-phase α/α cells and in untreated white- and opaque-phase a/a cells but not in a/α cells (data not shown). The expression of STE4, therefore, was MTL homozygous specific, as in S. cerevisiae but, in contrast to S. cerevisiae, it was α-pheromone induced. Expression in C. albicans was independent of switching.
FIG1.
In S. cerevisiae, the expression of FIG1, which encodes a putative membrane-associated protein required for efficient mating, is induced by α-pheromone in a cells (9). In C. albicans, FIG1 was expressed only in α-pheromone-treated opaque-phase a/a cells (Fig. 7A). Extended exposure time to X-ray film failed to reveal signals in the other cell types (data not shown). Therefore, expression of FIG1 in C. albicans was α-pheromone induced in a/a cells, as in S. cerevisiae, but it was also opaque phase specific.
KAR4.
In S. cerevisiae, the expression of KAR4, which encodes a protein required for efficient karyogamy, is haploid specific and stimulated by α-pheromone in a cells (14). In C. albicans, KAR4 was expressed only in α-pheromone-treated opaque-phase a/a cells (Fig. 7A). Extended exposure time to X-ray film failed to reveal signals in the other cell types (data not shown). Therefore, expression of KAR4 in C. albicans was α-pheromone induced in a/a cells, as in S. cerevisiae, but it was also, like FIG1, opaque phase specific (Fig. 7A).
SAP9.
In S. cerevisiae, BAR1 (SST1), which encodes an α-pheromone-degrading aspartyl proteinase (22, 23, 38), is mating type specific (i.e., a specific) and pheromone induced. In C. albicans, SAP9, a member of the family of secreted aspartyl proteinases, was most homologous to BAR1 of S. cerevisiae (http://genolist.pasteur.fr/CandidaDB). In C. albicans, the expression of SAP9 was constitutively expressed in a/α, a/a, and α/α cells. The level of expression was higher in white-phase a/a and α/α cells (Fig. 7). Lan et al. (17) also demonstrated in an expression array analysis that SAP9 was expressed at higher levels in white-phase cells than in opaque-phase cells. Therefore, if SAP9 is the homolog of BAR1, its regulation in the two species is markedly different.
HWP1.
In C. albicans, HWP1 encodes a hypha-specific adhesin (42) that acts as a substrate for a human transglutaminase that catalyzes a covalent linkage between Hwp1p and a molecule on human cells (43). HWP1 has no known S. cerevisiae homolog. As demonstrated elsewhere (7a), HWP1 was expressed only by opaque-phase a/a cells in response to α-pheromone (Fig. 7B). Extended exposure time of X-ray film failed to reveal signals in the other cell types (data not shown). Therefore, expression of HWP1 in C. albicans was α-pheromone induced in a/a cells and opaque phase specific, as for FIG1 and KAR4 (Fig. 7A).
OP4, SAP1, SAP3, and CDR3.
Since switching represents an essential step in the acquisition of mating competency (21, 26, 37), the possibility was entertained that select opaque-phase-specific genes may play roles in the mating process and/or may also be regulated by the mating process. OP4, an opaque-phase-specific gene with no identified S. cerevisiae homolog (30), was expressed in untreated and α-pheromone-treated opaque-phase α/α cells (Fig. 7C). It was also expressed in untreated opaque-phase a/a cells (Fig. 7). However, expression was downregulated by α-pheromone in opaque-phase a/a cells (Fig. 7C). SAP1 and SAP3, two opaque-phase-specific secreted aspartyl proteinases (11, 29, 44), were expressed in untreated and treated opaque-phase α/α cells and at higher levels in untreated opaque-phase a/a cells (Fig. 7C). However, expression of both was downregulated by α-pheromone in opaque-phase a/a cells (Fig. 7A). Finally, CDR3, an opaque-phase-specific gene encoding an ABC transporter (2), was expressed at similar levels in untreated and α-pheromone-treated opaque-phase α/α and a/a cells (Fig. 7C). It was not downregulated by α-pheromone, as was the case for OP4, SAP1, and SAP3 (Fig. 7C).
DISCUSSION
Even though C. albicans contains a single-mating-type locus, MTL, a configuration that contrasts markedly with the cassette configurations of S. cerevisiae (12, 37) and C. glabrata (41), it utilizes mating-type genes (12, 13, 24; R. Zhao, W. Wei, S. R. Lockhart, and D. R. Soll, unpublished observations) and a MAP kinase cascade (5, 25) similar to those in S. cerevisiae to regulate mating and the associated program of gene expression. It also proceeds through a sequence of cytological stages in the mating process similar to that in S. cerevisiae (21, 37, 37b). A major difference between the mating processes, however, is the inclusion of the white-opaque transition in the mating process of C. albicans (21, 26, 37). The addition of this complex, pleiotropic switch event in the acquisition of mating competence suggested first that the mating pheromones would induce MTL-homozygous cells to shmoo only after they switched from white- to opaque-phase and, second, that new classes of gene regulation would exist in the mating process of C. albicans that would include dependencies on the white-opaque transition. Here, we explored these suggestions by analyzing the responses of white- and opaque-phase cells to a chemically synthesized α-pheromone peptide.
α-Pheromone induces shmooing only in a/a cells in the opaque phase.
C. albicans possesses the α-pheromone gene MFα1, which contains three putative α-pheromone peptide sequences, two that are 13 amino acids long and defined at the carboxy-terminal end by consensus Kex2 and Kex1 cleavage sites, and one that is 14 amino acids long and defined at the carboxy-terminal end by the translation termination signal. We synthesized the third peptide, the longest of the three, which proved active. While the 14-amino-acid peptide induced shmooing in opaque-phase cells of the unrelated a/a strains P37005, L26, and P75063, it did not induce shmooing in white-phase cells of any of these strains, even though Northern analysis revealed selective α-pheromone-induced upregulation in both the white and the opaque phases of the mating-associated genes CAG1, STE2, and STE4 (Fig. 8), which play key roles in the initial transduction of the α-pheromone signal in S. cerevisiae. The fact that α-pheromone upregulates expression of a select group of mating-type-specific genes in both white- and opaque-phase cells, but stimulates shmooing and mating only in opaque-phase cells (Fig. 8), indicates that in white-phase cells either white-phase-specific mechanisms selectively suppress genes necessary for the full pheromone response or opaque-phase-specific mechanisms are required for the full pheromone response. In this regard, a number of white-phase-specific genes that are downregulated and a number of opaque-phase-specific genes that are upregulated when cells switch from the white to opaque phase (Soll, unpublished) are now under investigation for possible roles in mating competence.
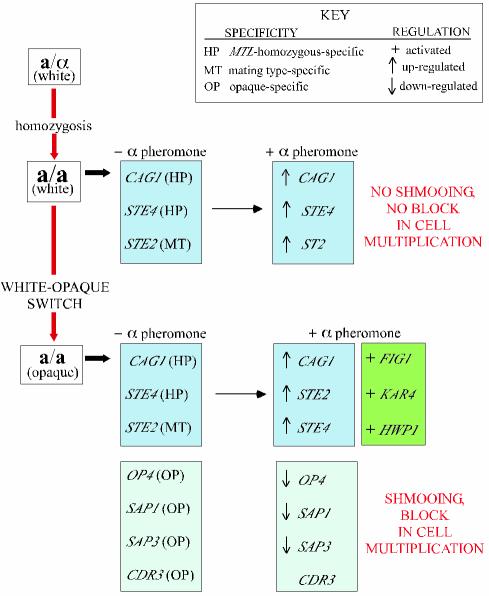
FIG. 8.
Model of the roles of switching and α-pheromone induction in the expression of genes during the mating process of C. albicans. A key to understanding the regulation of each gene is presented in the upper right-hand corner of the figure. Genes are grouped in three categories that are color-coded. Blue, haploid-specific (HP) and mating-type-specific (MT) genes upregulated by α-pheromone in both white- and opaque-phase cells; green, genes that are activated by the α-pheromone, exclusively in the opaque phase; orange, opaque-phase-specific genes, three of which are downregulated by α-pheromone.
As in S. cerevisiae (19, 39), the effect of a single dose of α-pheromone is short-lived, presumably because of rapid digestion by endoproteolysis. Hence, a single addition of α-pheromone peptide to opaque-phase a/a cells resulted in shmooing and a short period of tube elongation, followed in a majority of cases by apical reversion to the budding growth form. Repeated addition of the peptide to opaque-phase a/a cells at 2-h intervals caused cyclic shmooing of the original cells and apical buds, suggesting that the peptide was indeed short-lived and that the mating program could be repeatedly terminated and reinstated. In the process of reversion, we found that nuclei divided in some cells, demonstrating that the regime of α pheromone addition did not cause a permanent block in G1. However, we observed a significant difference between the α-pheromone-treated cultures and mixed a/a × α/α cultures. The propensity to revert in the former cultures was much greater. We assume that a G1 block does occur in a/a and α/α cells prior to mating, as demonstrated in S. cerevisiae, although we present no data in support of this.
α-Pheromone does not induce shmooing at a physiological temperature (37°C).
The experiments demonstrating that α-pheromone induces shmooing were performed at 25°C. When similar experiments were performed at 37°C, the opaque-phase a/a cells budded rather than shmooed, as they did in the absence of α-pheromone. It was previously demonstrated that when opaque-phase cells were transferred from 25 to 37°C, they immediately stopped expressing at least two opaque-phase-specific genes, OP4 and SAP1 (30). After two cell divisions at 37°C, the daughter buds that formed were in the white phase (37a, 40). Together, these results indicate that in order for α-pheromone to induce shmooing and undergo traditional mating, opaque-phase cells must express opaque-phase-specific genes. Miller and Johnson (26), however, demonstrated through complementation the formation of tetraploids at low frequency in a/− × α/− crosses in mice. These fusions would have to have occurred at 37°C. Our results suggest that these fusions may have been facilitated by the white-opaque transition through the following scenario. The white-phase a/− and white-phase α/− cells presumably used in these experiments switched to opaque-phase cells at a frequency between 10−2 and 10−3 in the mouse body. Opaque-phase a/− and opaque-phase α/− cells would then have a window of two cell divisions within which to mate. The failure to express opaque-phase genes may have reduced the efficiency but did not eliminate opaque-phase-facilitated fusion.
If mating of C. albicans opaque a/a and α/α cells cannot occur efficiently inside the human body, we considered an alternative body site, the skin, which has a slightly reduced temperature. We previously had demonstrated that skin is preferentially colonized by opaque-phase cells (15). We, therefore, recently tested whether mating occurs on skin. We found that mixtures of opaque-phase a/a and α/α cells fused at frequencies five times higher on mouse skin than they did in clumps of cells in suspension cultures (16). In some regions of the skin, 50% of all a/a and α/α cells fused. All of the cytological stages of the mating process were observed, including conjugation tube chemotropism. We suggest that skin provides a temperature that facilitates pheromone induction of shmooing and mating of C. albicans a/a and α/α strains.
Unique categories of gene regulation.
Northern analysis revealed that several mating-associated genes in C. albicans were regulated like their S. cerevisiae homologs. Expression of CAG1 was MTL homozygous specific, upregulated by α-pheromone, and independent of white-opaque switching (Fig. 8), a pattern similar to that of its homolog GPA1 in S. cerevisiae (27, 31). Expression of STE2 was mating type specific, upregulated by α-pheromone, and independent of white-opaque switching (Fig. 8), like its S. cerevisiae homolog (4). However, the regulation of other mating-associated genes either included a dependency on the white-opaque transition in addition to the dependencies exhibited by their S. cerevisiae homologs or was fundamentally different. Expression of FIG1 and KAR4, like their S. cerevisiae homologs (9, 14), were α-pheromone induced, but α-pheromone induction occurred only in opaque-phase a/a cells (Fig. 8). During the mating process, HWP1, a unique C. albicans adhesin gene (42), was regulated in a manner similar to that for FIG1 and KAR4 (Fig. 8). The regulation of STE4 differed markedly from that of its S. cerevisiae homolog. As in S. cerevisiae (7, 45), STE4 was specific for cells homozygous at the mating-type locus, but in contrast to its S. cerevisiae homolog it was upregulated by α-pheromone (Fig. 8).
Since the phenotypic transition from the white to opaque phase is a prerequisite for mating in C. albicans, opaque-phase-specific genes must now be considered within the context of mating. Three of four opaque-phase-specific genes tested, OP4 (30), SAP1 (29), and SAP3 (11, 44) were downregulated by α-pheromone (Fig. 8). A fourth opaque-phase-specific gene, CDR3 (2), was not downregulated by α-pheromone (Fig. 8). In the case of the secreted aspartyl proteinases, one can speculate on the reason. It has been demonstrated through mutational analysis that Sap1 plays a role in opaque-phase-specific colonization of skin (15), which in turn facilitates mating (16). While secretion of proteinases is necessary for cavitation of the mother cell into the skin (15), it may be detrimental to mating tube migration along the skin surface. It is not immediately obvious why OP4 may be downregulated, since its function is not known, or why CDR3, an ABC transporter gene, is not downregulated.
Together, these results reveal an increase in the complexity of gene regulation during the mating process due to the addition of white-opaque switching (Fig. 8). In addition, they reveal for the first time that the expression of a select set of opaque-phase-specific genes is in turn regulated by α-pheromone. In S. cerevisiae, α-pheromone regulates genes through the activation of a MAP kinase cascade that in turn activates STE12, which encodes a transcription factor (18). Both Chen et al. (5) and Magee et al. (25) recently demonstrated through null and overexpression mutants that this same cascade is essential for mating in C. albicans, which they assessed by genetic complementation. Because α-pheromone upregulates a set of select genes in both white- and opaque-phase a/a cells, while selectively activating a second set of select genes only in opaque-phase a/a cells (Fig. 8), it is possible that the MAP kinase cascade is activated in both white- and opaque-phase a/a cells, but selectivity is achieved at the level of the promoter. In S. cerevisiae, Ste12p binds cooperatively with two or more PRE sites (TGAAACA) (8, 10, 46), as well as cooperatively with other factors. The promoters of the α-pheromone-stimulated or α-pheromone-induced C. albicans genes CAG1, STE2, STE4, FIG1, KAR4, and HWP1 contained on average 0.90 PRE sites with 0 mismatches and 6.8 PRE sites with 0 or 1 mismatches, supporting the proposal that PRE sites may play a role in the regulation of α-pheromone-induced genes in C. albicans. In support of this model, Chen et al. (5) recently demonstrated that overexpression of CPH1 in C. albicans caused upregulation of FIG1. It seems likely, then, that CPH1 selectively upregulates genes in both white- and opaque-phase a/a cells and activates others only in the opaque phase through selective interactions with phase-specific transcription factors, a possibility now under investigation.
Summary.
The results presented here first and foremost demonstrate that α-pheromone induces shmooing, the initial step in mating, only in opaque-phase a/a cells of C. albicans. Second, these results demonstrate that α-pheromone does not induce shmooing at 37°C, suggesting that mating-based fusions in animal models may not be traditional and that mating may occur normally between a/a and α/α cells outside the body, such as on skin. Third, our results demonstrate that although some mating-associated genes are induced by α-pheromone in both white- and opaque-phase a/a cells, others are induced only in opaque-phase a/a cells. This result suggests, first, that the capacity to fully respond to α-pheromone depends on a switch from white to opaque phase and, second, that differential expression of α-pheromone induced, opaque-specific genes is probably regulated by an opaque-phase-specific trans-acting factor that interacts cooperatively with Cph1p. Third, our results reveal that some, but not all, opaque-phase-specific genes are downregulated by α-pheromone. Finally, our results demonstrate that in C. albicans, gene regulation in the mating process must be examined within the context of white-opaque switching.
Acknowledgments
We thank Julie Collins for assembling the manuscript.
This work was supported by NIH grant AI2392.
REFERENCES
- 1.Anderson, J. M., and D. R. Soll. 1987. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J. Bacteriol. 169:5579-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balan, I., A. M. Alarco, and M. Raymond. 1997. The Candida albicans CDR3 gene codes for an opaque-phase ABC transporter. J. Bacteriol. 179:7210-7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedell, G. W., and D. R. Soll. 1979. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect. Immun. 26:348-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkholder, A. C., and L. H. Hartwell. 1985. The yeast alpha-factor receptor: structural properties deduced from the sequence of the STE2 gene. Nucleic Acids Res. 13:8463-8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, J., S. Lane, and H. Liu. 2002. A conserved mitogen-activated protein kinase pathway is required for mating in Candida albicans. Mol. Microbiol. 46:1335-1344. [DOI] [PubMed] [Google Scholar]
- 6.Church, G. M., and W. Gilbert. 1984. Genomic sequencing. Proc. Natl. Acad. Sci. USA 81:1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole, G. M., and S. I. Reed. 1991. Pheromone-induced phosphorylation of a G protein beta subunit in Saccharomyces cerevisiae is associated with an adaptive response to mating pheromone. Cell 64:703-716. [DOI] [PubMed] [Google Scholar]
- 7a.Daniels, K. J., S. R. Lockhart, J. F. Staab, P. Sundstrom, and D. R. Soll. The adhesin Hurp1 and the first daughter cell localize to the a/a portion of the conjugation bridge during Candida albicans mating. Mol. Biol. Cell, in press. [DOI] [PMC free article] [PubMed]
- 8.Dolan, J. W., C. Kirkman, and S. Fields. 1989. The yeast Ste12 protein binds to the DNA sequence mediating pheromone induction. Proc. Natl. Acad. Sci. USA 86:5703-5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdman, S., L. Lin, M. Malczynski, and M. Snyder. 1998. Pheromone-regulated genes required for yeast mating differentiation. J. Cell Biol. 140:461-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Errede, B., and G. Ammerer. 1989. Ste12, a protein involved in cell-type-specific transcription and signal transduction in yeast, is part of protein-DNA complexes. Genes Dev. 3:1349-1361. [DOI] [PubMed] [Google Scholar]
- 11.Hube, B., M. Monod, D. A. Schofield, A. J. Brown, and N. A. Gow. 1994. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol. Microbiol. 14:87-99. [DOI] [PubMed] [Google Scholar]
- 12.Hull, C. M., and A. D. Johnson. 1999. Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science 285:1271-1275. [DOI] [PubMed] [Google Scholar]
- 13.Hull, C. M., R. M. Raisner, and A. D. Johnson. 2000. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289:307-310. [DOI] [PubMed] [Google Scholar]
- 14.Kurihara, L. J., B. G. Stewart, A. E. Gammie, and M. D. Rose. 1996. Kar4p, a karyogamy-specific component of the yeast pheromone response pathway. Mol. Cell. Biol. 16:3990-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kvaal, C., S. A. Lachke, T. Srikantha, K. Daniels, J. McCoy, and D. R. Soll. 1999. Misexpression of the opaque-phase-specific gene PEP1 (SAP1) in the white phase of Candida albicans confers increased virulence in a mouse model of cutaneous infection. Infect. Immun. 67:6652-6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lachke, S. A., S. R. Lockhart, K. J. Daniels, and D. R. Soll. 2003. Skin facilitates Candida albicans mating. Infect. Immun. 71:4970-4976. [DOI] [PMC free article] [PubMed]
- 17.Lan, C. Y., G. Newport, L. A. Murillo, T. Jones, S. Scherer, R. W. Davis, and N. Agabian. 2002. Metabolic specialization associated with phenotypic switching in Candida albicans. Proc. Natl. Acad. Sci. USA 99:14907-14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lengeler, K. B., R. C. Davidson, C. D'souza, T. Harashima, W. C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipke, P. N., A. Taylor, and C. E. Ballou. 1976. Morphogenic effects of alpha-factor on Saccharomyces cerevisiae a cells. J. Bacteriol. 127:610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockhart, S. R., C. Pujol, K. J. Daniels, M. G. Miller, A. D. Johnson, M. A. Pfaller, and D. R. Soll. 2002. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics 162:737-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lockhart, S. R., K. J. Daniels, R. Zhao, D. Wessels, and D. R. Soll. 2003. Cell biology of mating in Candida albicans. Eukaryot. Cell 2:49-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacKay, V. L., J. Armstrong, C. Yip, S. Welch, K. Walker, S. Osborn, P. Sheppard, and J. Forstrom. 1991. Characterization of the Bar proteinase, an extracellular enzyme from the yeast Saccharomyces cerevisiae. Adv. Exp. Med. Biol. 306:161-172. [DOI] [PubMed] [Google Scholar]
- 23.MacKay, V. L., S. K. Welch, M. Y. Insley, T. R. Manney, J. Holly, G. C. Saari, and M. L. Parker. 1988. The Saccharomyces cerevisiae BAR1 gene encodes an exported protein with homology to pepsin. Proc. Natl. Acad. Sci. USA 85:55-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magee, B. B., and P. T. Magee. 2000. Induction of mating in Candida albicans by construction of MTLa and MTLα strains. Science 289:310-313. [DOI] [PubMed] [Google Scholar]
- 25.Magee, B. B., M. Legrand, A. M. Alarco, M. Raymond, and P. T. Magee. 2002. Many of the genes required for mating in Saccharomyces cerevisiae are also required for mating in Candida albicans. Mol. Microbiol. 46:1345-1351. [DOI] [PubMed] [Google Scholar]
- 26.Miller, M. G., and A. D. Johnson. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293-302. [DOI] [PubMed] [Google Scholar]
- 27.Miyajima, I., M. Nakafuku, N. Nakayama, C. Brenner, A. Miyajima, K. Kaibuchi, K. Arai, Y. Kaziro, and K. Matsumoto. 1987. GPA1, a haploid-specific essential gene, encodes a yeast homolog of mammalian G protein which may be involved in mating factor signal transduction. Cell 50:1011-1019. [DOI] [PubMed] [Google Scholar]
- 28.Moore, S. A. 1983. Comparison of dose-response curves for alpha factor-induced cell division arrest, agglutination, and projection formation of yeast cells. Implication for the mechanism of alpha factor action. J. Biol. Chem. 258:13849-13856. [PubMed] [Google Scholar]
- 29.Morrow, B., T. Srikantha, and D. R. Soll. 1992. Transcription of the gene for a pepsinogen, PEP1, is regulated by white-opaque switching in Candida albicans. Mol. Cell. Biol. 12:2997-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrow, B., T. Srikantha, J. Anderson, and D. R. Soll. 1993. Coordinate regulation of two opaque-phase-specific genes during white-opaque switching in Candida albicans. Infect. Immun. 61:1823-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakafuku, M., H. Itoh, S. Nakamura, and Y. Kaziro. 1987. Occurrence in Saccharomyces cerevisiae of a gene homologous to the cDNA coding for the alpha subunit of mammalian G proteins. Proc. Natl. Acad. Sci. USA 84:2140-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pringle, J. R., and L. H. Hartwell. 1981. The Saccharomyces cerevisiae cell cycle, p. 97-142. In J. N. Strathern et al. (ed.), Molecular biology of the yeast Saccharomyces: life cycle and inherence. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 33.Pujol, C., S. A. Messer, M. Pfaller, and D. R. Soll. 2003. Drug resistance is not directly affected by mating type locus zygosity in Candida albicans. Antimicrob. Agents Chemother. 47:1207-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadhu, C. D. Hoekstra, M. J. McEachern, S. I. Reed, and J. B. Hicks. 1992. A G-protein alpha subunit from asexual Candida albicans functions in the mating signal transduction pathway of Saccharomyces cerevisiae and is regulated by the a1-α2 repressor. Mol. Cell. Biol. 12:1977-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slutsky, B., J. Buffo, and D. R. Soll. 1985. High-frequency switching of colony morphology in Candida albicans. Science 230:666-669. [DOI] [PubMed] [Google Scholar]
- 36.Slutsky, B., M. Staebell, J. Anderson, L. Risen, M. Pfaller, and D. R. Soll. 1987. “White-opaque transition”: a second high-frequency switching system in Candida albicans. J. Bacteriol. 169:189-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soll, D. R., S. R. Lockhart, and R. Zhao. 2003. The unique relationship between zygosity, switching and mating in C. albicans. Eukaryot. Cell -397.2:390. [DOI] [PMC free article] [PubMed]
- 37a.Soll, D. R. 2003. Candida albicans, p. 165-201. In A. Craig and A. Scherf (ed.), Antigenic variation. Academic Press, San Diego, Calif.
- 37b.Soll, D. R. Mating type locus homozygosis, phenotypic switching and mating: a unique sequence of dependencies in Candida albicans. Bioassays, in press. [DOI] [PubMed]
- 38.Sprague, G. F., Jr., and I. Herskowitz. 1981. Control of yeast cell type by the mating type locus. I. Identification and control of expression of the a-specific gene, BAR1. J. Mol. Biol. 153:305-321. [DOI] [PubMed] [Google Scholar]
- 39.Sprague, G. F., Jr., and J. Thorner. 1992. Pheromone response and signal transduction during the mating process of Saccharomyces cerevisiae, p. 657-744. In J. R. Broach, J. R. Pringle, and E. W. Jones (ed.), The molecular biology of the yeast Saccharomyces. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 40.Srikantha, T., and D. R. Soll. 1993. A white-specific gene in the white-opaque switching system of Candida albicans. Gene 131:53-60. [DOI] [PubMed] [Google Scholar]
- 41.Srikantha, T., S. A. Lachke, and D. R. Soll. 2003. Three mating type-like loci in Candida glabrata. Eukaryot. Cell 2:328-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staab, J. F., C. A. Ferrer, and P. Sundstrom. 1996. Developmental expression of a tandemly repeated, proline-and glutamine-rich amino acid motif on hyphal surfaces on Candida albicans. J. Biol. Chem. 271:6298-6305. [DOI] [PubMed] [Google Scholar]
- 43.Staab, J. F., S. D. Bradway, P. L. Fidel, and P. Sundstrom. 1999. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 283:1535-1538. [DOI] [PubMed] [Google Scholar]
- 44.White, T. C., S. H. Miyasaki, and N. Agabian. 1993. Three distinct secreted aspartyl proteinases in Candida albicans. J. Bacteriol. 175:6126-6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whiteway, M., L. Hougan, D. Dignard, L. Bell, G. Saari, F. Grant, P. O'Hara, V. L. MacKay, and D. Y. Thomas. 1988. Function of the STE4 and STE18 genes in mating pheromone signal transduction in Saccharomyces cerevisiae. Cold Spring Harbor Symp. Quant. Biol. 53(Pt. 2):585-590. [DOI] [PubMed] [Google Scholar]
- 46.Yuan, Y. L., and S. Fields. 1991. Properties of the DNA-binding domain of the Saccharomyces cerevisiae Ste12 protein. Mol. Cell. Biol. 11:5910-5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao, R., S. R. Lockhart, K. Daniels, and D. R. Soll. 2002. Roles of TUP1 in switching, phase maintenance, and phase-specific gene expression in Candida albicans. Eukaryot. Cell 1:353-365. [DOI] [PMC free article] [PubMed] [Google Scholar]